Characterization of a Periplasmic D-Malate:Cytochrome c Oxidoreductase from Ectopseudomonas oleovorans CECT 5344 and Its Role in Extracytoplasmic Respiration and Cyanide Detoxification
Abstract
1. Introduction
2. Results
2.1. Identification and Subcellular Localization of the D-Malate:Cytochrome c Oxidoreductase Activity
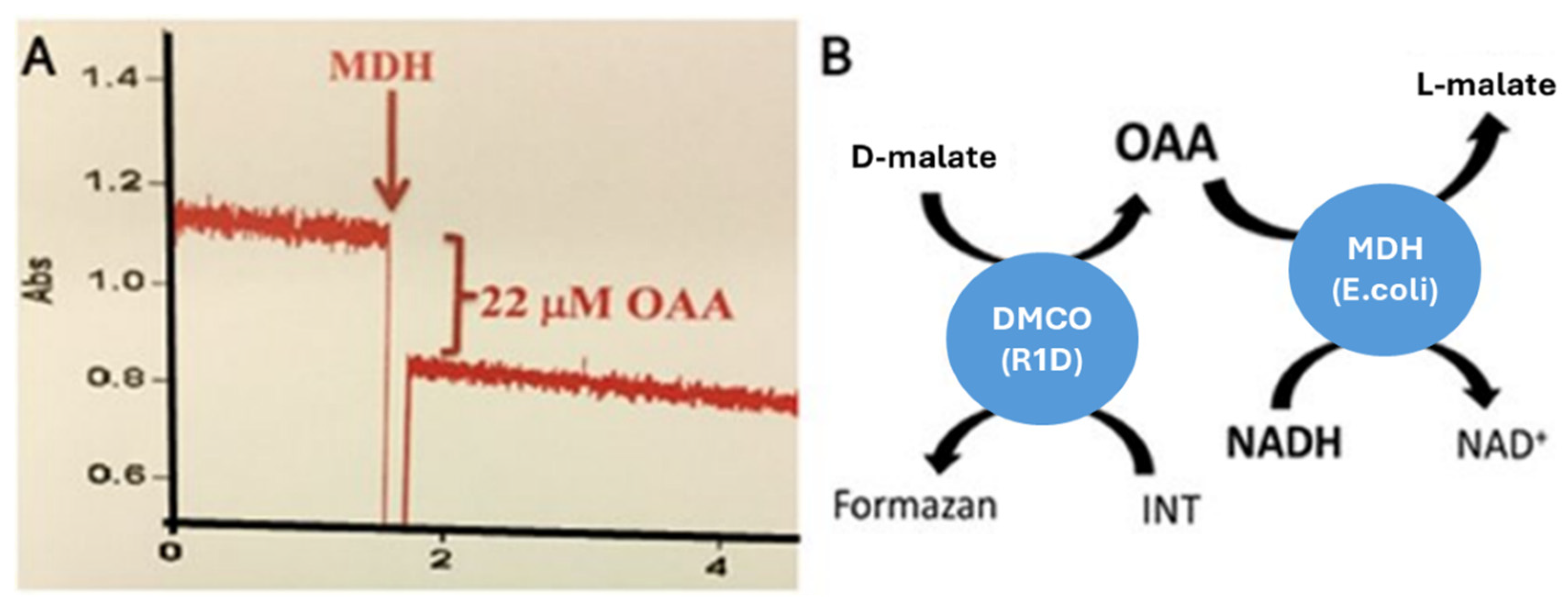
2.2. The Determination of the Reaction Product of D-Malate:Cytochrome c Oxidoreductase
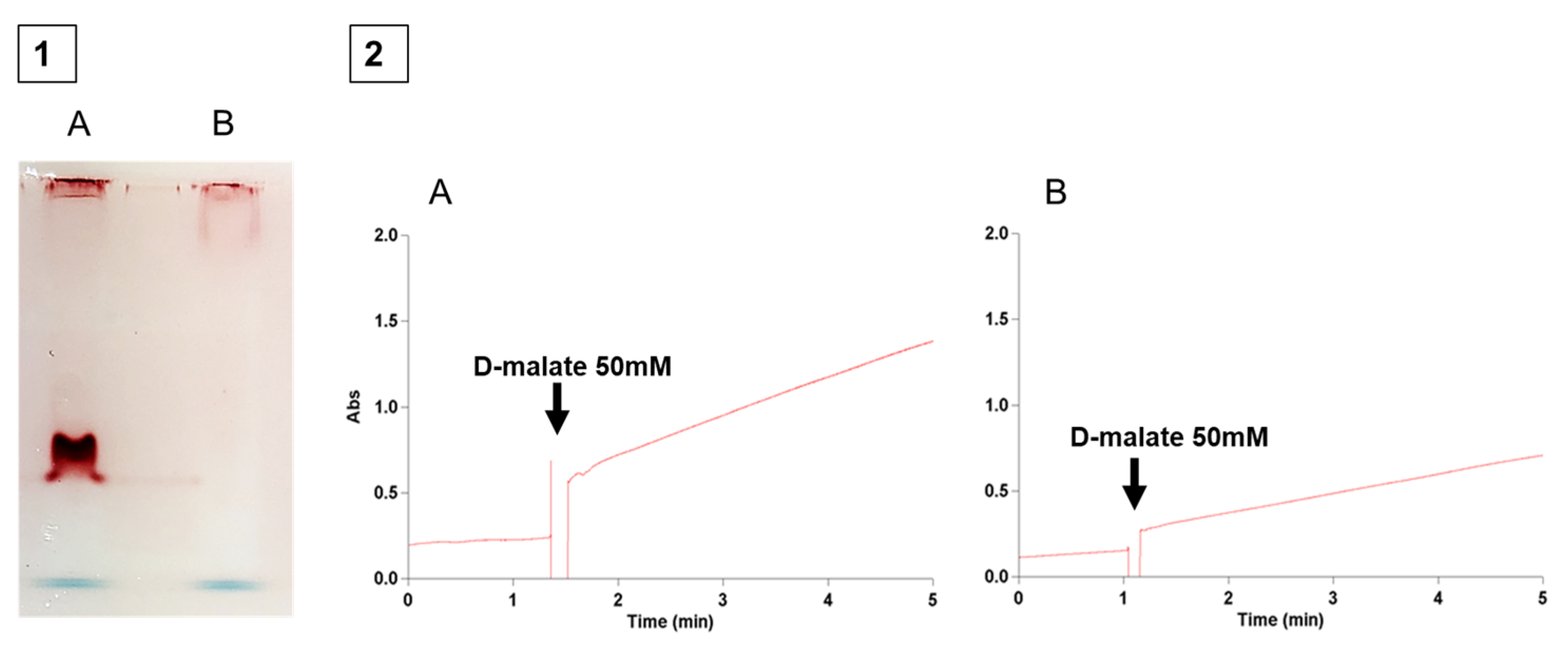
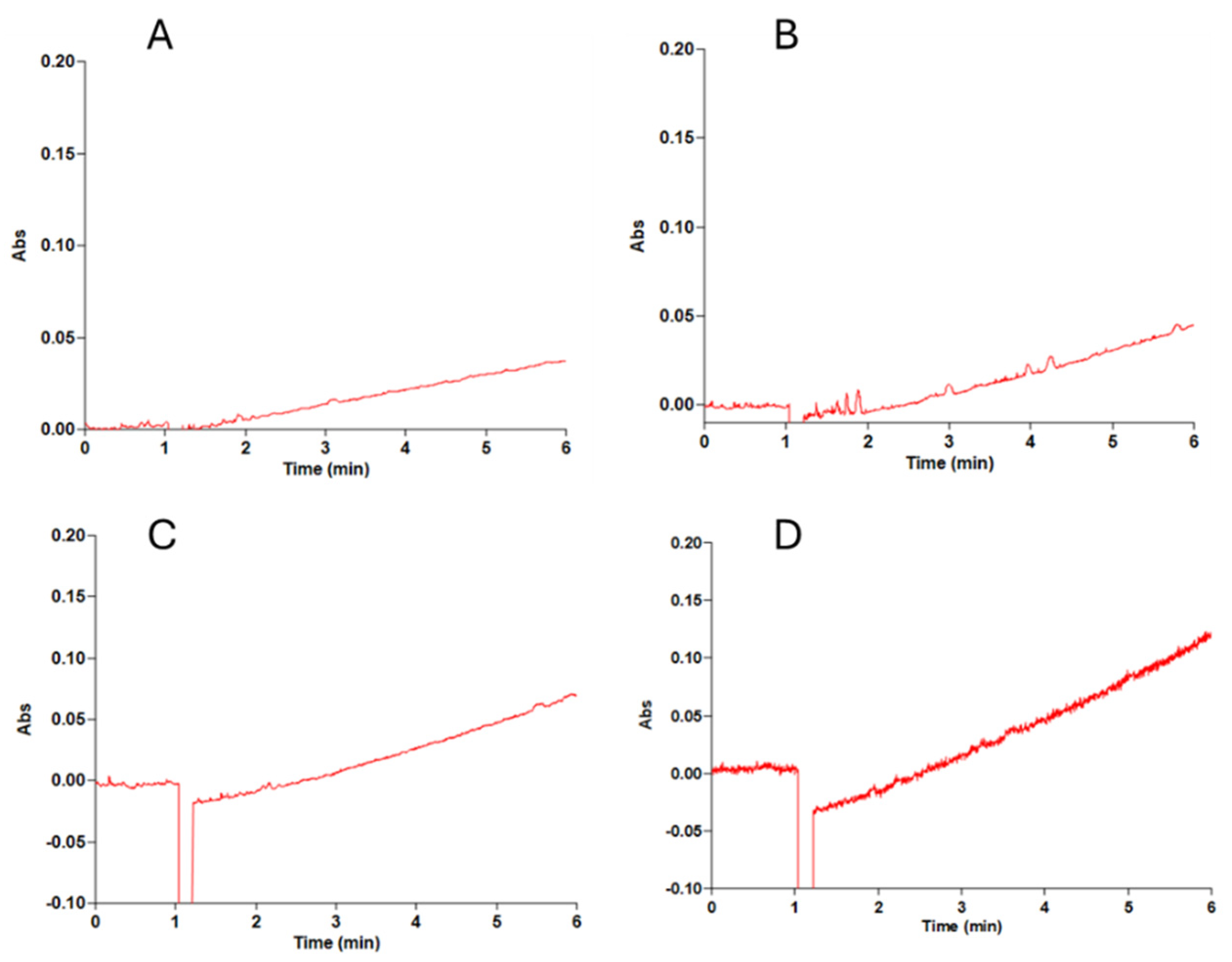
2.3. Spectrophotometric Determination of DMCO Activity
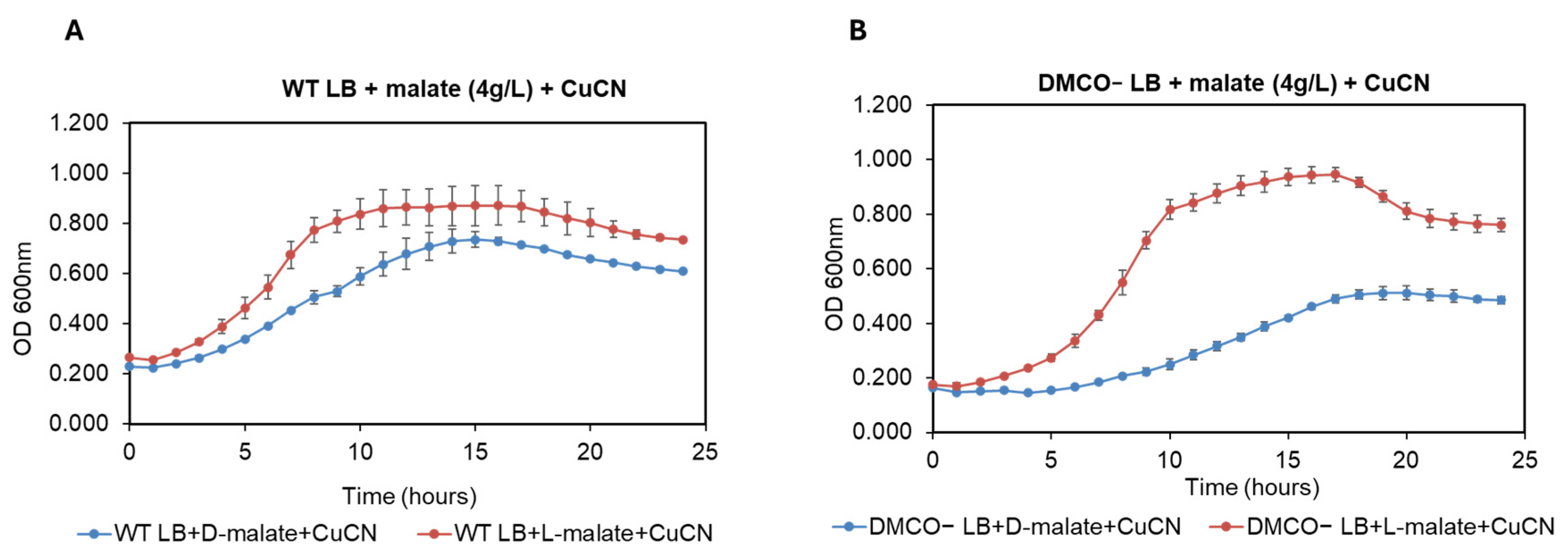
2.4. Mutagenesis of DMCO in Ectopseudomonas oleovorans CECT 5344 R1D
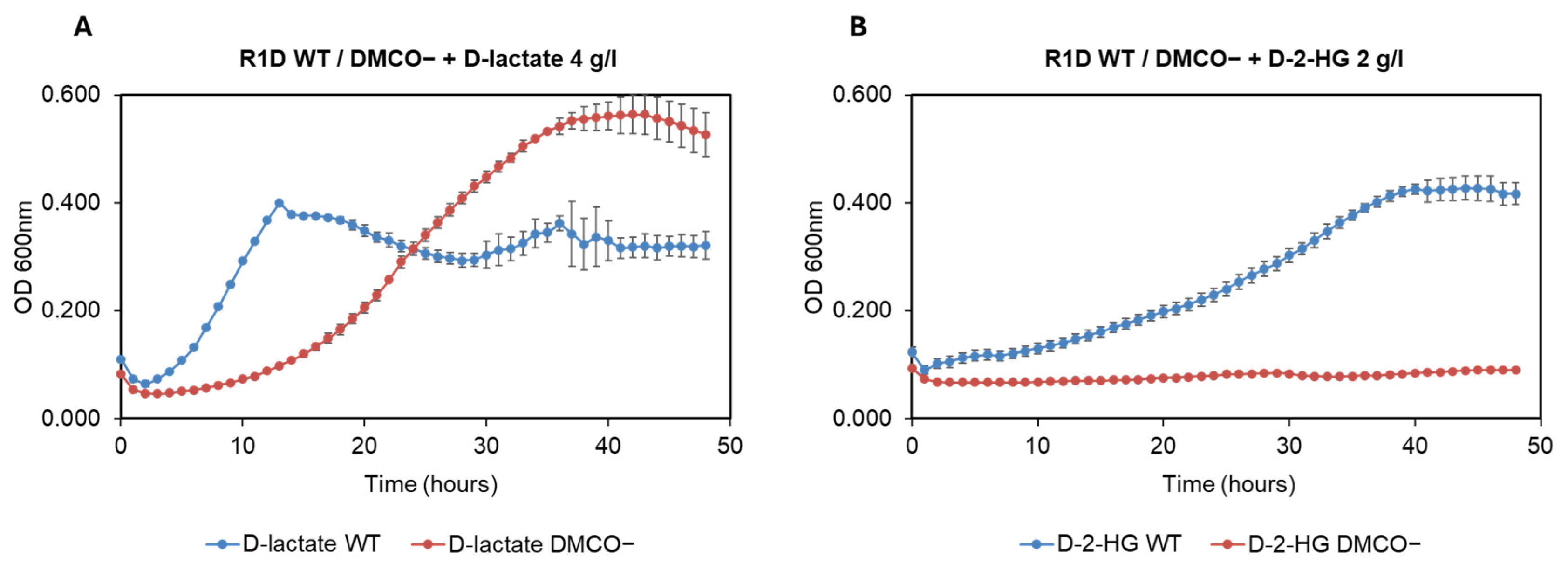
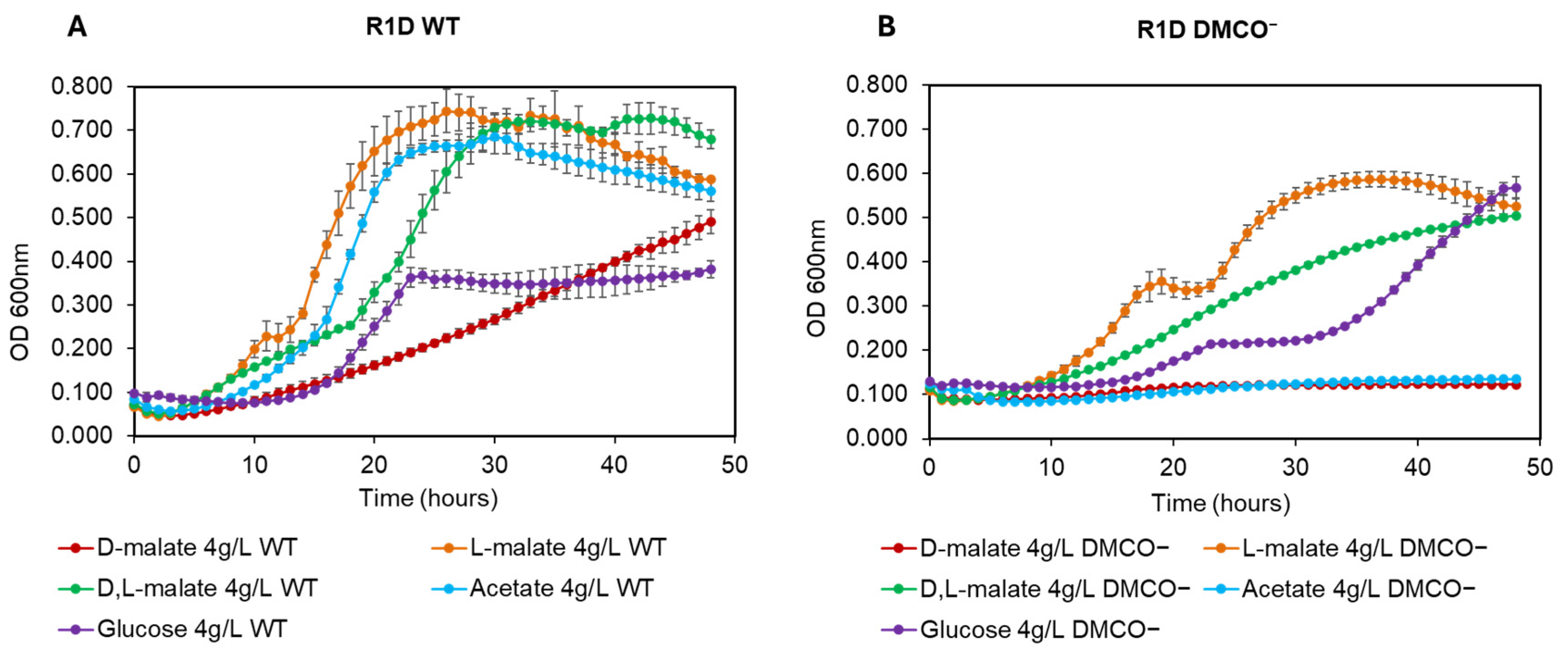
TPhenotype of the Mutant with Different Carbon Sources and the Influence of Cyanide
2.5. The Determination of Cytochrome c as the Physiological Substrate for DMCO
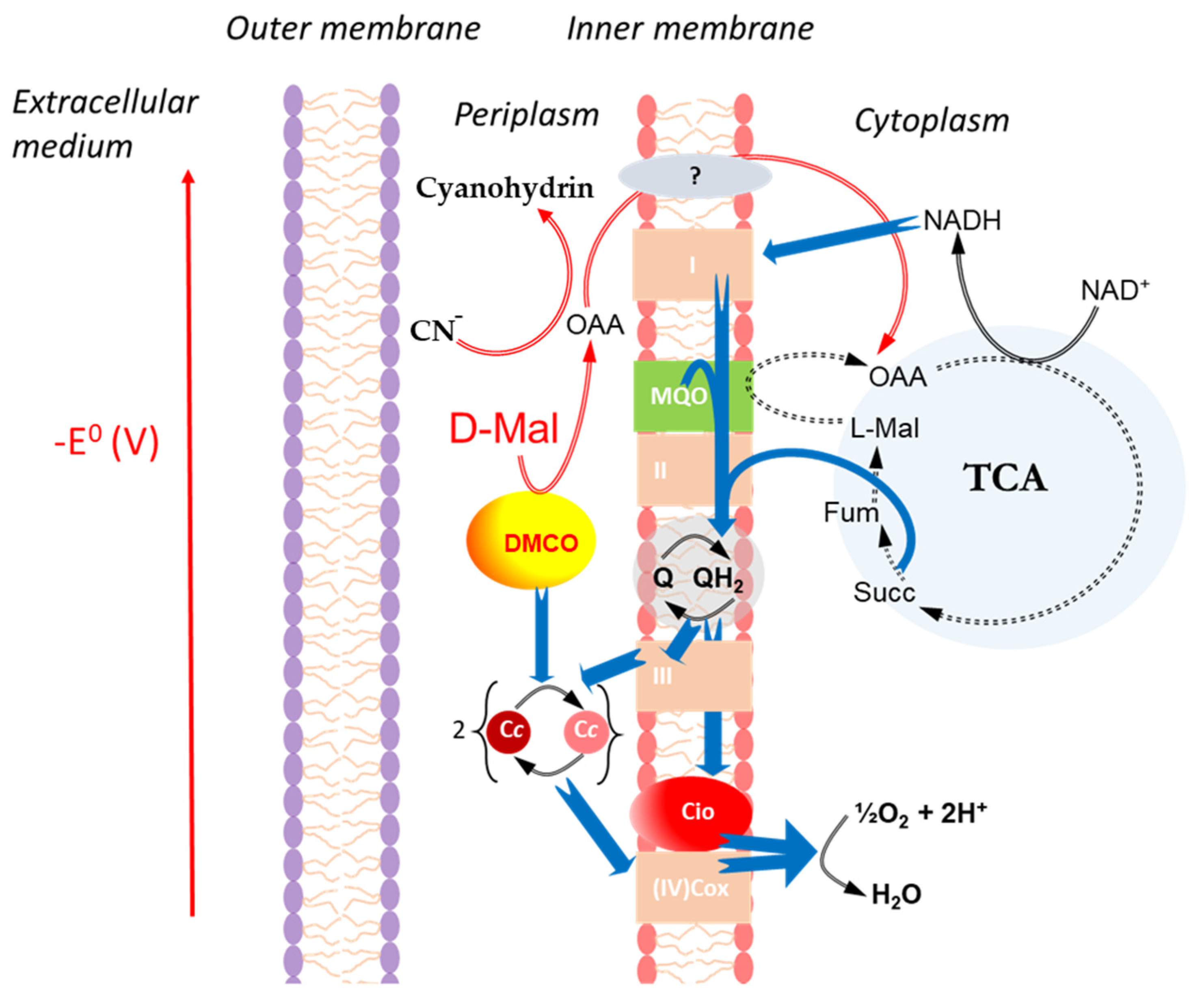
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Culture Media
4.3. Determinación of Cell Growth
4.4. Preparation of Cell-Free Extracts
4.5. Subcellular Fractionation
4.6. Analytical Determinations
4.6.1. Determination of Cyanide Concentration
4.6.2. Determination of Enzymatic Activities
Fumarase Activity: L-Malate Hydro-Lyase (EC 4.2.1.2)
Aconitase Activity (EC 4.2.1.3)
Nitrate Reductase Activity (EC 1.7.99.4)
Succinate Dehydrogenase Activity (EC 1.3.5.1)
Malate:Quinone Oxidoreductase Activity (EC 1.1.5.4)
Characterization of D-Malate Oxidase Activity (DMCO)
Native Polyacrylamide Gel Electrophoresis (Native-PAGE)
Spectrophotometric Method
Determination of DMCO Substrate Specificity
Physiological Reconstitution of D-Malate Oxidase Activity
Determination of the Reaction Product of D-Malate Oxidase
4.7. DNA Manipulation Techniques
4.7.1. Extraction and Purification of Nucleic Acids
4.7.2. Preparation of Electrocompetent Cells
4.7.3. Transformation of Electrocompetent Cells
4.7.4. DNA Amplification by Polymerase Chain Reaction (PCR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DHG | D-2-hydroxyglutarate |
| 2DHGDH | D-2-hydroxyglutarate dehydrogenase |
| 3-PHP | 3-phosphohydroxypyruvate |
| D3PG | D-3-phosphoglycerate |
| DMCO | D-malate:cytochrome c oxidoreductase |
| ETC | Electron Transport Chain |
| aKG | 2-Ketoglutarate acid |
| MDH | L-malate dehydrogenase |
| MQO | L-malate:quinone oxidoreductase |
| PAGE | Polyacrylamide Gel Electrophoresis |
| TCA | Tricarboxylic Acid Cycle |
References
- Hopper, D.J.; Chapman, P.J.; Dagley, S. Metabolism of l-Malate and d-Malate by a Species of Pseudomonas. J. Bacteriol. 1970, 104, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, Y.; Zhou, N.-Y. Identification of a Specific Maleate Hydratase in the Direct Hydrolysis Route of the Gentisate Pathway. Appl. Environ. Microbiol. 2015, 81, 5753–5760. [Google Scholar] [CrossRef]
- Asano, Y.; Ueda, M.; Yamada, H. Microbial production of D-malate from maleate. Appl. Environ. Microbiol. 1993, 59, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.R.; Hegre, C.S. Inducible D-Malic Enzyme in Escherichia coli. Nature 1966, 212, 1611–1612. [Google Scholar] [CrossRef]
- Martinez-Luque, M.; Castillo, F.; Blasco, R. Assimilation of D-malate by Rhodobacter capsulatus E1F1. Curr. Microbiol. 2001, 43, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, M.; Cao, M.; Zhang, W.; Kang, Z.; Xu, P.; Ma, C.; Gao, C. d-2-Hydroxyglutarate dehydrogenase plays a dual role in l-serine biosynthesis and d-malate utilization in the bacterium Pseudomonas stutzeri. J. Biol. Chem. 2018, 293, 15513–15523. [Google Scholar] [CrossRef]
- Anderson, C.P.; Dagley, S.; Hopper, D. Metabolic Pathways of Malate Enantiomers. Trans. Neb. Acad. Sci. Affil. Soc. 1972, 1, 42–51. [Google Scholar]
- Lukas, H.; Reimann, J.; Kim, O.B.; Grimpo, J.; Unden, G. Regulation of Aerobic and Anaerobic D-Malate Metabolism of Escherichia coli by the LysR-Type Regulator DmlR (YeaT). J. Bacteriol. 2010, 192, 2503–2511. [Google Scholar] [CrossRef]
- Michielsen, M.J.F.; Frielink, C.; Wijffels, R.H.; Tramper, J.; Beeftink, H.H. d-malate production by permeabilized Pseudomonas pseudoalcaligenes; optimization of conversion and biocatalyst productivity. J. Biotechnol. 2000, 79, 13–26. [Google Scholar] [CrossRef]
- Unden, G.; Strecker, A.; Kleefeld, A.; Kim, O.B. C4-Dicarboxylate Utilization in Aerobic and Anaerobic Growth. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Huertas, M.-J.; Martínez-Luque, M.; Moreno-Vivián, C.; Roldán, M.D.; García-Gil, L.J.; Castillo, F.; Blasco, R. Bacterial Degradation of Cyanide and Its Metal Complexes under Alkaline Conditions. Appl. Environ. Microbiol. 2005, 71, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Igeño, M.I.; Becerra, G.; Guijo, M.I.; Merchán, F.; Blasco, R. Metabolic adaptation of Pseudomonas pseudoalcaligenes CECT 5344 to cyanide: Role of malate–quinone oxidoreductases, aconitase and fumarase isoenzymes. Biochem. Soc. Trans. 2011, 39, 1849–1853. [Google Scholar] [CrossRef]
- Trevors, J.T.; Knowles, R. Electron Transport System Activity in Soil, Sediment, and Pure Cultures. CRC Crit. Rev. Microbiol. 1984, 11, 83–100. [Google Scholar] [CrossRef]
- Acera, F.; Carmona, M.I.; Castillo, F.; Quesada, A.; Blasco, R. A Cyanide-Induced 3-Cyanoalanine Nitrilase in the Cyanide-Assimilating Bacterium Pseudomonas pseudoalcaligenes Strain CECT 5344. Appl. Environ. Microbiol. 2017, 83, e00089-17. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Huertas, M.-J.; Sáez, L.P.; Luque-Romero, M.M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D.; Blasco, R. Characterization of the Pseudomonas pseudoalcaligenes CECT 5344 Cyanase, an Enzyme That Is Not Essential for Cyanide Assimilation. Appl. Environ. Microbiol. 2008, 74, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Quaye, J.A.; Gadda, G. Metal-Triggered FAD Reduction in d-2-Hydroxyglutarate Dehydrogenase from Pseudomonas aeruginosa PAO1. ACS Bio. Med. Chem. Au. 2025, 5, 204–214. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, H.; Zhang, T.; Ding, J. Structure, substrate specificity, and catalytic mechanism of human D-2-HGDH and insights into pathogenicity of disease-associated mutations. Cell Discov. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Quaye, J.A.; Gadda, G. The Pseudomonas aeruginosa PAO1 metallo flavoprotein d-2-hydroxyglutarate dehydrogenase requires Zn2 for substrate orientation and activation. J. Biol. Chem. 2023, 299, 103008. [Google Scholar] [CrossRef]
- Berks, B.C. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 1996, 22, 393–404. [Google Scholar] [CrossRef]
- Muesch, A.; Hartmann, E.; Rohde, K.; Rubartelli, A.; Sitia, R.; Rapoport, T.A. A novel pathway for secretory proteins? Trends Biochem. Sci. 1990, 15, 86–88. [Google Scholar] [CrossRef]
- Rubartelli, A.; Bajetto, A.; Allavena, G.; Wollman, E.; Sitia, R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 1992, 267, 24161–24164. [Google Scholar] [CrossRef] [PubMed]
- Rubartelli, A.; Cozzolino, F.; Talio, M.; Sitia, R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990, 9, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Moonlighting proteins. Trends Biochem. Sci. 1999, 24, 8–11. [Google Scholar] [CrossRef]
- Jeffery, C.J. Moonlighting proteins: Old proteins learning new tricks. Trends Genet. 2003, 19, 415–417. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Kiemer, L.; Fausbøll, A.; Brunak, S. Non-classical protein secretion in bacteria. BMC Microbiol. 2005, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, M.; Gao, C.; Zhang, Y.; Ge, Y.; Guo, S.; Guo, X.; Zhou, Z.; Liu, Q.; Zhang, Y.; et al. Coupling between d-3-phosphoglycerate dehydrogenase and d-2-hydroxyglutarate dehydrogenase drives bacterial l-serine synthesis. Proc. Natl. Acad. Sci. USA 2017, 114, E7574–E7582. [Google Scholar] [CrossRef]
- Grant, G.A. Contrasting catalytic and allosteric mechanisms for phosphoglycerate dehydrogenases. Arch. Biochem. Biophys. 2012, 519, 175–185. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Merchán, F.; Blasco, R.; Igeño, M.I.; Martínez-Luque, M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D. Cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 involves a malate:quinone oxidoreductase and an associated cyanide-insensitive electron transfer chain. Microbiology 2011, 157, 739–746. [Google Scholar] [CrossRef]
- van der Rest, M.E.; Frank, C.; Molenaar, D. Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Escherichia coli. J. Bacteriol. 2000, 182, 6892–6899. [Google Scholar] [CrossRef]
- Molenaar, D.; van der Rest, M.E.; Drysch, A.; Yücel, R. Functions of the membrane-associated and cytoplasmic malate dehydrogenases in the citric acid cycle of Corynebacterium glutamicum. J. Bacteriol. 2000, 182, 6884–6891. [Google Scholar] [CrossRef]
- Moreno, R.; Martinez-Gomariz, M.; Yuste, L.; Gil, C.; Rojo, F. The Pseudomonas putida Crc global regulator controls the hierarchical assimilation of amino acids in a complete medium: Evidence from proteomic and genomic analyses. Proteomics 2009, 9, 2910–2928. [Google Scholar] [CrossRef]
- Welchen, E.; Schmitz, J.; Fuchs, P.; García, L.; Wagner, S.; Wienstroer, J.; Schertl, P.; Braun, H.-P.; Schwarzländer, M.; Gonzalez, D.H.; et al. D-Lactate Dehydrogenase Links Methylglyoxal Degradation and Electron Transport through Cytochrome c. Plant Physiol. 2016, 172, 901–912. [Google Scholar] [PubMed]
- Palleroni, N.J. The Pseudomonas story. Environ. Microbiol. 2010, 12, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Silby, M.W.; Winstanley, C.; Godfrey, S.A.C.; Levy, S.B.; Jackson, R.W. Pseudomonas genomes: Diverse and adaptable. FEMS Microbiol. Rev. 2011, 35, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Wibberg, D.; Luque-Almagro, V.M.; Igeño, M.I.; Bremges, A.; Roldán, M.D.; Merchán, F.; Sáez, L.P.; Guijo, M.I.; Manso, M.I.; Macías, D.; et al. Complete genome sequence of the cyanide-degrading bacterium Pseudomonas pseudoalcaligenes CECT5344. J. Biotechnol. 2014, 175, 67–68. [Google Scholar] [CrossRef]
- Dvořák, P.; Nikel, P.I.; Damborský, J.; de Lorenzo, V. Bioremediation 3.0: Engineering pollutant-removing bacteria in the times of systemic biology. Biotechnol. Adv. 2017, 35, 845–866. [Google Scholar] [CrossRef]
- Kornberg, H.L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem. J. 1966, 99, 1–11. [Google Scholar] [CrossRef]
- Dolan, S.K.; Welch, M. The Glyoxylate Shunt, 60 Years On. Annu. Rev. Microbiol. 2018, 72, 309–330. [Google Scholar] [CrossRef]
- Estepa, J.; Luque-Almagro, V.M.; Manso, I.; Escribano, M.P.; Martínez-Luque, M.; Castillo, F.; Moreno-Vivián, C.; Roldán, M.D. The nit1C gene cluster of Pseudomonas pseudoalcaligenes CECT5344 involved in assimilation of nitriles is essential for growth on cyanide. Environ. Microbiol. Rep. 2012, 4, 326–334. [Google Scholar] [CrossRef]
- Sáez, L.P.; Rodríguez-Caballero, G.; Olaya-Abril, A.; Cabello, P.; Moreno-Vivián, C.; Roldán, M.D.; Luque-Almagro, V.M. Genomic Insights into Cyanide Biodegradation in the Pseudomonas Genus. Int. J. Mol. Sci. 2024, 25, 4456. [Google Scholar] [CrossRef]
- Janausch, I.G.; Zientz, E.; Tran, Q.H.; Kröger, A.; Unden, G. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta (BBA)—Bioenerg. 2002, 1553, 39–56. [Google Scholar] [CrossRef]
- LaPorte, D.C.; Koshland, D.E. Phosphorylation of isocitrate dehydrogenase as a demonstration of enhanced sensitivity in covalent regulation. Nature 1983, 305, 286–290. [Google Scholar] [CrossRef]
- Bennett, B.D.; Kimball, E.H.; Gao, M.; Osterhout, R.; van Dien, S.J.; Rabinowitz, J.D. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 2009, 5, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Unden, G.; Bongaerts, J. Alternative respiratory pathways of Escherichia coli: Energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta 1997, 1320, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6, 742. [Google Scholar] [CrossRef] [PubMed]
- Sazanov, L.A.; Jackson, J.B. Proton-translocating transhydrogenase and NAD- and NADP-linked isocitrate dehydrogenases operate in a substrate cycle which contributes to fine regulation of the tricarboxylic acid cycle activity in mitochondria. FEBS Lett. 1994, 344, 109–116. [Google Scholar] [CrossRef]
- Wolfe, A.J. The Acetate Switch. Microbiol. Mol. Biol. Rev. 2005, 69, 12–50. [Google Scholar] [CrossRef]
- Dunn, M.F.; Ramírez-Trujillo, J.A.; Hernández-Lucas, I. Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology 2009, 155, 3166–3175. [Google Scholar] [CrossRef]
- Rojo, F. Carbon catabolite repression in Pseudomonas: Optimizing metabolic versatility and interactions with the environment. FEMS Microbiol. Rev. 2010, 34, 658–684. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Acera, F.; Igeño, M.I.; Wibberg, D.; Roldán, M.D.; Sáez, L.P.; Hennig, M.; Quesada, A.; Huertas, M.J.; Blom, J.; et al. Draft whole genome sequence of the cyanide-degrading bacterium Pseudomonas pseudoalcaligenes CECT5344. Environ. Microbiol. 2013, 15, 253–270. [Google Scholar] [CrossRef]
- Quesada, A.; Guijo, M.I.; Merchan, F.; Blazquez, B.; Igeño, M.I.; Blasco, R. Essential role of cytochrome bd-related oxidase in cyanide resistance of Pseudomonas pseudoalcaligenes CECT5344. Appl. Environ. Microbiol. 2007, 73, 5118–5124. [Google Scholar] [CrossRef] [PubMed]
- Becerra, G.; Merchán, F.; Blasco, R.; Igeño, M.I. Characterization of a ferric uptake regulator (Fur)-mutant of the cyanotrophic bacterium Pseudomonas pseudoalcaligenes CECT5344. J. Biotechnol. 2014, 190, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Imperi, F.; Ciccosanti, F.; Perdomo, A.B.; Tiburzi, F.; Mancone, C.; Alonzi, T.; Ascenzi, P.; Piacentini, M.; Visca, P.; Fimia, G.M. Analysis of the periplasmic proteome of Pseudomonas aeruginosa, a metabolically versatile opportunistic pathogen. Proteomics 2009, 9, 1901–1915. [Google Scholar] [CrossRef]
- Stitt, M. Fumarase and citrate synthase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Vch Pub: Weinheim, Germany, 1984; pp. 353–362. [Google Scholar]
- Singh, R.; Chénier, D.; Bériault, R.; Mailloux, R.; Hamel, R.D.; Appanna, V.D. Blue native polyacrylamide gel electrophoresis and the monitoring of malate- and oxaloacetate-producing enzymes. J. Biochem. Biophys. Methods 2005, 64, 189–199. [Google Scholar] [CrossRef] [PubMed]






| Aconitase | Nitrate Reductase | Succinate Oxidase | DMCO | |||||
|---|---|---|---|---|---|---|---|---|
| S.A. (U/mg) | % S.A. | S.A. (U/mg) | % S.A. | S.A. (U/mg) | % S.A. | S.A. (U/mg) | % S.A. | |
| Periplasm | 9.40 × 108 | 20.8 | 4.81 × 105 | 97.8 | 0.00 | 0.00 | 82.83 | 83.6 |
| Cytoplasm | 3.52 × 109 | 77.8 | 9.73 × 103 | 1.98 | 0.00 | 0.00 | 14.32 | 14.4 |
| Membranes | 6.13 × 107 | 1.3 | 0.00 | 0.00 | 2.18 × 105 | 100.00 | 1.85 | 1.8 |
| ACONITASE | NITRATE REDUCTASE | SUCCINATE OXIDASE | DMCO | |||||
| S.A. (U/mg) | % S.A. | S.A. (U/mg) | % S.A. | S.A. (U/mg) | % S.A. | S.A. (U/mg) | % S.A. | |
| Periplasm | 9.40 × 108 | 20.8 | 4.81 × 105 | 97.8 | 0.00 | 0.00 | 82.83 | 83.6 |
| Cytoplasm | 3.52 × 109 | 77.8 | 9.73 × 103 | 1.98 | 0.00 | 0.00 | 14.32 | 14.4 |
| Membranes | 6.13 × 107 | 1.3 | 0.00 | 0.00 | 2.18 × 105 | 100.00 | 1.85 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchán, F.; Población, A.G.; Guijo, M.I.; Gómez-Ortega, M.; Morales-Durán, F.; Alonso-Ríos, I.; Sánchez-Clemente, R.; Blasco, R. Characterization of a Periplasmic D-Malate:Cytochrome c Oxidoreductase from Ectopseudomonas oleovorans CECT 5344 and Its Role in Extracytoplasmic Respiration and Cyanide Detoxification. Int. J. Mol. Sci. 2025, 26, 6575. https://doi.org/10.3390/ijms26146575
Merchán F, Población AG, Guijo MI, Gómez-Ortega M, Morales-Durán F, Alonso-Ríos I, Sánchez-Clemente R, Blasco R. Characterization of a Periplasmic D-Malate:Cytochrome c Oxidoreductase from Ectopseudomonas oleovorans CECT 5344 and Its Role in Extracytoplasmic Respiration and Cyanide Detoxification. International Journal of Molecular Sciences. 2025; 26(14):6575. https://doi.org/10.3390/ijms26146575
Chicago/Turabian StyleMerchán, Faustino, Ana G. Población, María Isabel Guijo, Mar Gómez-Ortega, Felipe Morales-Durán, Irene Alonso-Ríos, Rubén Sánchez-Clemente, and Rafael Blasco. 2025. "Characterization of a Periplasmic D-Malate:Cytochrome c Oxidoreductase from Ectopseudomonas oleovorans CECT 5344 and Its Role in Extracytoplasmic Respiration and Cyanide Detoxification" International Journal of Molecular Sciences 26, no. 14: 6575. https://doi.org/10.3390/ijms26146575
APA StyleMerchán, F., Población, A. G., Guijo, M. I., Gómez-Ortega, M., Morales-Durán, F., Alonso-Ríos, I., Sánchez-Clemente, R., & Blasco, R. (2025). Characterization of a Periplasmic D-Malate:Cytochrome c Oxidoreductase from Ectopseudomonas oleovorans CECT 5344 and Its Role in Extracytoplasmic Respiration and Cyanide Detoxification. International Journal of Molecular Sciences, 26(14), 6575. https://doi.org/10.3390/ijms26146575







