Mechanisms Underlying Radioresistance and Reversal Strategies in Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Mechanism of RT Resistance
2.1. DNA Damage Repair
2.2. Cell Cycle Dysregulation
2.3. Cellular Senescence
2.4. CSCs and EMT
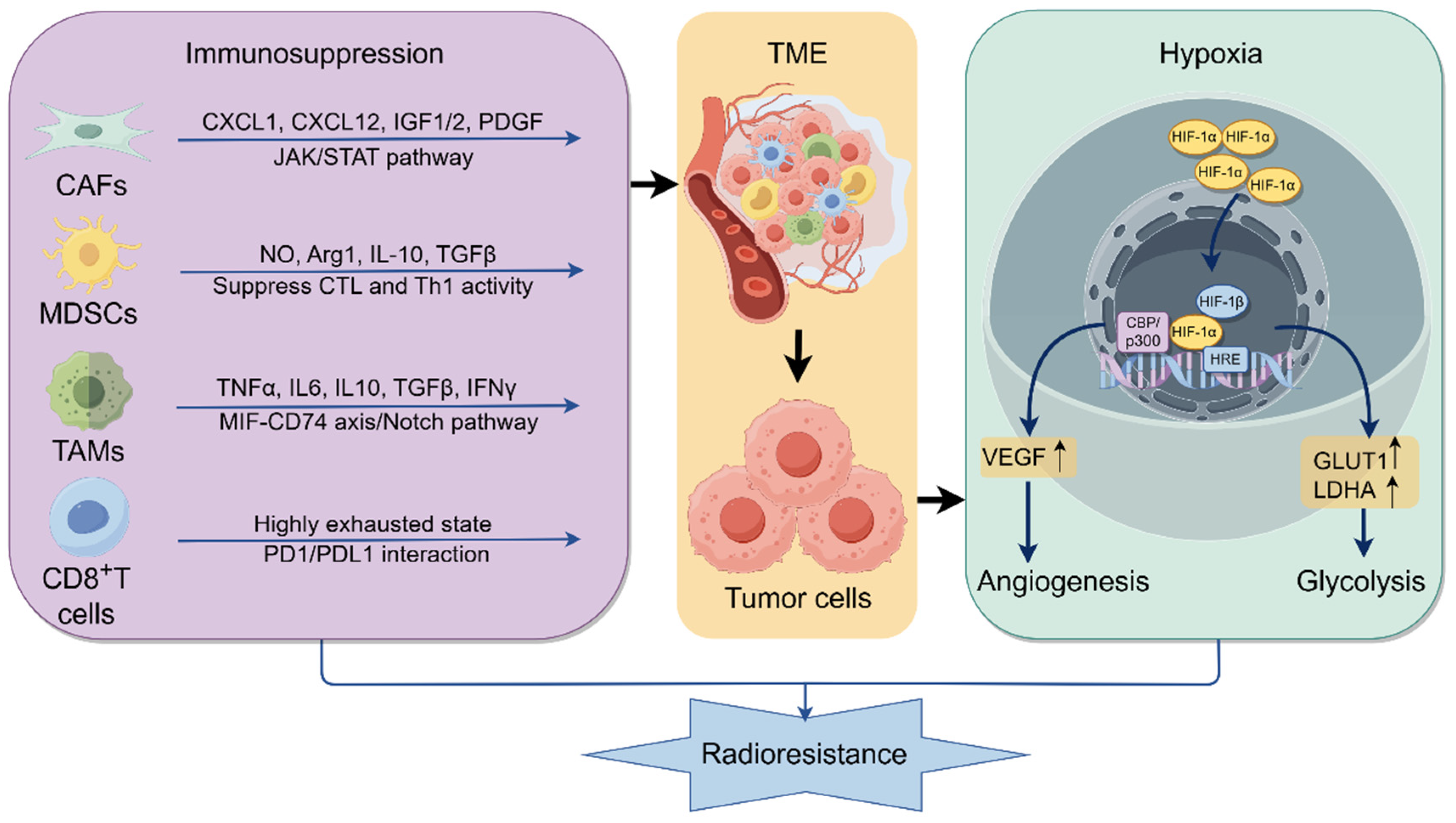
2.5. Hypoxia and Immunosuppressive TME
2.6. Abnormal Regulation of Cell Death
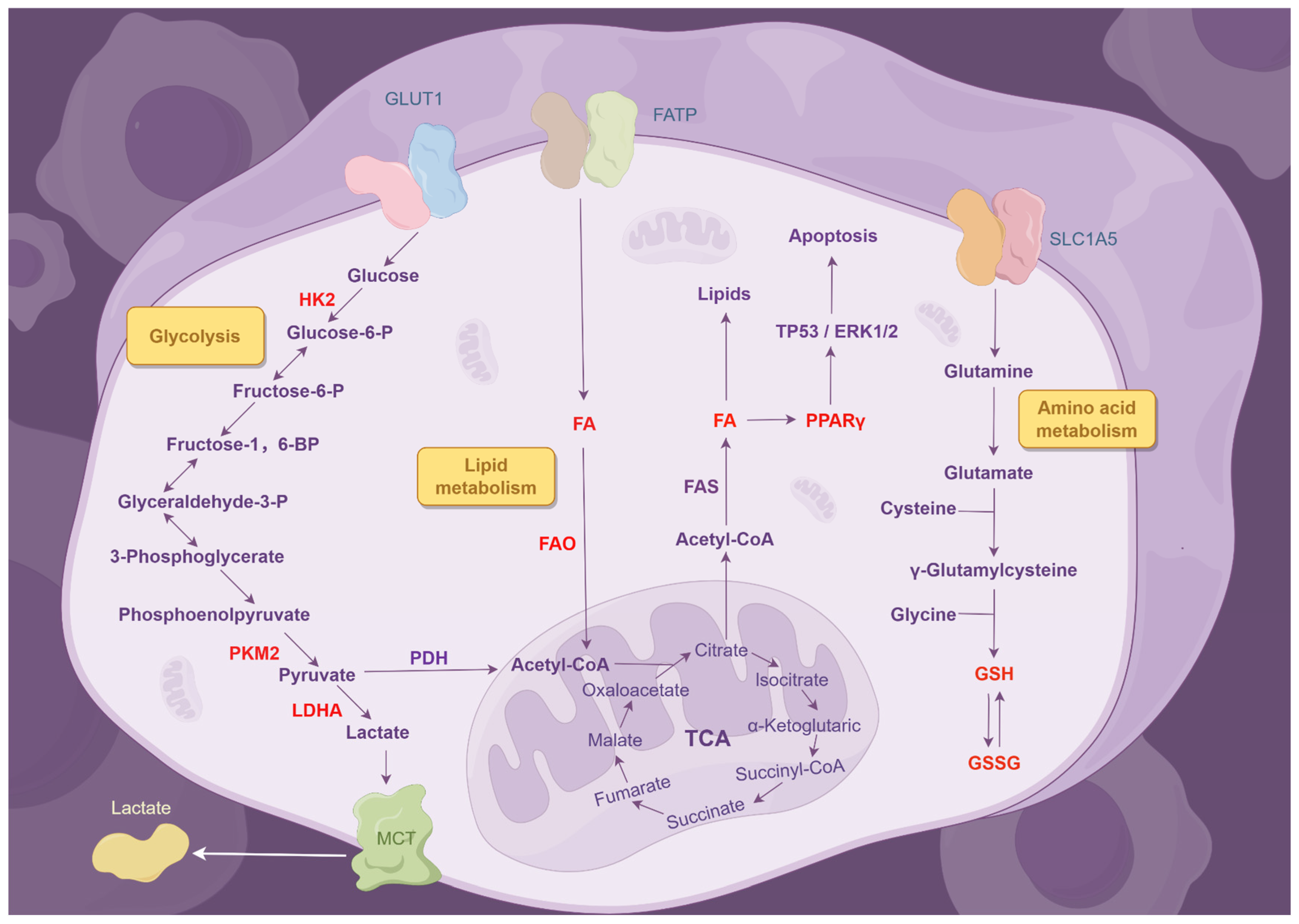
2.7. Metabolic Dysregulation
2.8. Exosomes
2.9. Gene Mutation and Aberrant Activation of Pro-Survival Signaling Pathways
2.10. Epigenetic Dysregulation
3. Reversal Strategies for RT Resistance
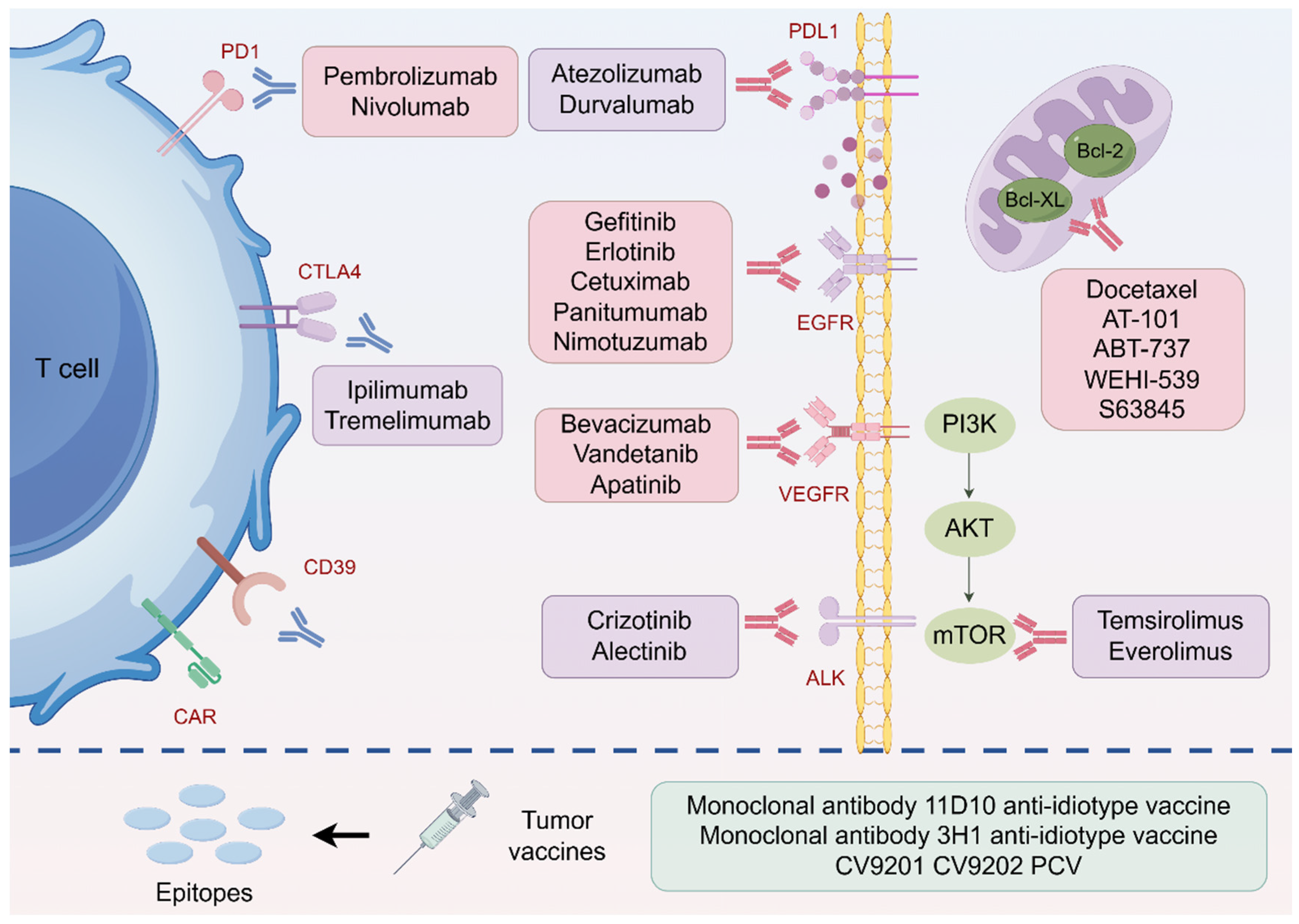
3.1. Immunotherapy for Radiosensitization
3.2. Targeted Therapy for Radiosensitization
3.3. Modulating DNA Damage Repair
3.4. Overcoming Hypoxia
3.5. Targeting Metabolic Processes
3.6. Targeting Exosomes
3.7. Regulating Epigenetics
3.8. Nanoradiosensitizers
4. Predictive Biomarkers of Radiosensitivity
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, B.; He, S. Advances and challenges in the treatment of lung cancer. Biomed. Pharmacother. 2023, 169, 115891. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Williams, M.C.; Stewart, C.; Weir, N.W.; Newby, D.E. Using radiation safely in cardiology: What imagers need to know. Heart 2019, 105, 798–806. [Google Scholar] [CrossRef]
- Walker, M.J.; Zhou, C.; Backen, A.; Pernemalm, M.; Williamson, A.J.K.; Priest, L.J.C.; Koh, P.; Faivre-Finn, C.; Blackhall, F.H.; Dive, C.; et al. Discovery and Validation of Predictive Biomarkers of Survival for Non-small Cell Lung Cancer Patients Undergoing Radical Radiotherapy: Two Proteins With Predictive Value. eBioMedicine 2015, 2, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, G.O.; Curtin, N.J. Targeting DNA Damage Response Pathways in Cancer. In Comprehensive Medicinal Chemistry III; Newcastle University: Newcastle upon Tyne, UK, 2017; pp. 104–133. [Google Scholar]
- Fok, J.H.L.; Ramos-Montoya, A.; Vazquez-Chantada, M.; Wijnhoven, P.W.G.; Follia, V.; James, N.; Farrington, P.M.; Karmokar, A.; Willis, S.E.; Cairns, J.; et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat. Commun. 2019, 10, 5065. [Google Scholar] [CrossRef]
- Ancel, J.; Dewolf, M.; Deslée, G.; Nawrocky-Raby, B.; Dalstein, V.; Gilles, C.; Polette, M. Clinical Impact of the Epithelial-Mesenchymal Transition in Lung Cancer as a Biomarker Assisting in Therapeutic Decisions. Cells Tissues Organs 2022, 211, 91–109. [Google Scholar] [CrossRef]
- Skvortsova, I.; Debbage, P.; Kumar, V.; Skvortsov, S. Radiation resistance: Cancer stem cells (CSCs) and their enigmatic pro-survival signaling. Semin. Cancer Biol. 2015, 35, 39–44. [Google Scholar] [CrossRef]
- Hanley, R.; Pagliari, F.; Garcia-Calderón, D.; Fernandes Guerreiro, J.; Genard, G.; Jansen, J.; Nisticò, C.; Marafioti, M.G.; Tirinato, L.; Seco, J. Radio-resistance of hypoxic tumors: Exploring the effects of oxygen and X-ray radiation on non-small lung cancer cell lines. Radiat. Oncol. 2023, 18, 81. [Google Scholar] [CrossRef]
- Huang, Z.; Xiao, Z.; Yu, L.; Liu, J.; Yang, Y.; Ouyang, W. Tumor-associated macrophages in non-small-cell lung cancer: From treatment resistance mechanisms to therapeutic targets. Crit. Rev. Oncol. Hematol. 2024, 196, 104284. [Google Scholar] [CrossRef]
- Sobol, B.; Azzam Nieto, O.; Eberlein, E.L.; Scherr, A.L.; Ismail, L.; Kessler, A.; Nader, L.; Schwab, M.; Hoffmeister, P.; Schmitt, N.; et al. Specific Targeting of Antiapoptotic Bcl-2 Proteins as a Radiosensitizing Approach in Solid Tumors. Int. J. Mol. Sci. 2022, 23, 7850. [Google Scholar] [CrossRef]
- Fang, J.; Rao, X.; Wang, C.; Wang, Y.; Wu, C.; Zhou, R. Role of exosomes in modulating non-small cell lung cancer radiosensitivity. Front. Pharmacol. 2024, 15, 1471476. [Google Scholar] [CrossRef]
- Jie, C.; Li, R.; Cheng, Y.; Wang, Z.; Wu, Q.; Xie, C. Prospects and feasibility of synergistic therapy with radiotherapy, immunotherapy, and DNA methyltransferase inhibitors in non-small cell lung cancer. Front. Immunol. 2023, 14, 1122352. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, K.; Ma, S.; Zhang, S. HDAC inhibitors: A new radiosensitizer for non-small-cell lung cancer. Tumori J. 2015, 101, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Liu, J.; Verma, V.; Wu, M.; Welsh, J.; Yu, J.; Chen, D. Combined treatment of non-small cell lung cancer using radiotherapy and immunotherapy: Challenges and updates. Cancer Commun. 2021, 41, 1086–1099. [Google Scholar] [CrossRef]
- Shibuya, K.; Komaki, R.; Shintani, T.; Itasaka, S.; Ryan, A.; Jürgensmeier, J.M.; Milas, L.; Ang, K.; Herbst, R.S.; O’Reilly, M.S. Targeted Therapy Against VEGFR and EGFR With ZD6474 Enhances the Therapeutic Efficacy of Irradiation in an Orthotopic Model of Human Non–Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Lee, N.K.; Saad, S.E.; Fong, I.L. Clinical translation for targeting DNA damage repair in non-small cell lung cancer: A review. Transl. Lung Cancer Res. 2024, 13, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.W.H.; Kaanders, J.H.A.M.; Span, P.N.; Bussink, J. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism to Improve Radiotherapy Efficacy. Clin. Cancer Res. 2012, 18, 5585–5594. [Google Scholar] [CrossRef]
- Tennant, D.A.; Duran, R.V.; Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef]
- Cabrera-Licona, A.; Perez-Anorve, I.X.; Flores-Fortis, M.; Moral-Hernandez, O.D.; Gonzalez-de la Rosa, C.H.; Suarez-Sanchez, R.; Chavez-Saldana, M.; Arechaga-Ocampo, E. Deciphering the epigenetic network in cancer radioresistance. Radiother. Oncol. 2021, 159, 48–59. [Google Scholar] [CrossRef]
- Zhen, W.; Weichselbaum, R.R.; Lin, W. Nanoparticle-Mediated Radiotherapy Remodels the Tumor Microenvironment to Enhance Antitumor Efficacy. Adv. Mater. 2023, 35, 2206370. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pelaez, M.; Young, L.; Vazquez-Chantada, M.; Nelson, N.; Durant, S.; Wilkinson, R.W.; Poon, E.; Gaspar, M.; Valge-Archer, V.; Smith, P.; et al. Targeting DNA damage response components induces enhanced STING-dependent type-I IFN response in ATM deficient cancer cells and drives dendritic cell activation. OncoImmunology 2022, 11, 2117321. [Google Scholar] [CrossRef]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 1867, 118659. [Google Scholar] [CrossRef]
- D’Andrea, F.P.; Safwat, A.; Kassem, M.; Gautier, L.; Overgaard, J.; Horsman, M.R. Cancer stem cell overexpression of nicotinamide N-methyltransferase enhances cellular radiation resistance. Radiother. Oncol. 2011, 99, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; White, S.D.; Frame, G.; Bosiljkov, A.; Khan, S.; Haas, R.; Yang, Q.; Sheng, M.; Huang, X.; Higgins, G.S.; et al. A Novel Primary Cell Line Model of Localized Prostate Cancer and Radioresistance—A Role for Nicotinamide N-Methyltransferase. Cells 2025, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59 (Suppl. S2), ii91–ii97. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Li, J.; Sun, Y.; Gao, Y.; Wang, Y.; Du, L.; Liu, Y.; Ji, K.; He, N.; et al. NRF2 promotes radiation resistance by cooperating with TOPBP1 to activate the ATR-CHK1 signaling pathway. Theranostics 2024, 14, 681–698. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. Nrf2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef]
- Gong, S.; Li, Y.; Lv, L.; Men, W. Restored microRNA-519a enhances the radiosensitivity of non-small cell lung cancer via suppressing EphA2. Gene Ther. 2021, 29, 588–600. [Google Scholar] [CrossRef]
- Chambers, C.R.; Ritchie, S.; Pereira, B.A.; Timpson, P. Overcoming the senescence-associated secretory phenotype (SASP): A complex mechanism of resistance in the treatment of cancer. Mol. Oncol. 2021, 15, 3242–3255. [Google Scholar] [CrossRef]
- Sentek, H.; Braun, A.; Budeus, B.; Klein, D. Non-small cell lung cancer cells and concomitant cancer therapy induce a resistance-promoting phenotype of tumor-associated mesenchymal stem cells. Front. Oncol. 2024, 14, 1406268. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, M.; Zhong, T.; Wang, M.; Wu, F.; Lu, J.; Sun, D.; Xiao, C.; Sun, Y.; Hu, Y.; et al. LILRB2 inhibition enhances radiation sensitivity in non-small cell lung cancer by attenuating radiation-induced senescence. Cancer Lett. 2024, 593, 216930. [Google Scholar] [CrossRef]
- Pustovalova, M.; Alhaddad, L.; Smetanina, N.; Chigasova, A.; Blokhina, T.; Chuprov-Netochin, R.; Osipov, A.N.; Leonov, S. The p53–53BP1-Related Survival of A549 and H1299 Human Lung Cancer Cells after Multifractionated Radiotherapy Demonstrated Different Response to Additional Acute X-ray Exposure. Int. J. Mol. Sci. 2020, 21, 3342. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Lee, J.-H.; Kim, R.-K.; Jung, U.; Kahm, Y.-J.; Cho, E.-W.; Kim, I.-G. HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription. Int. J. Mol. Sci. 2020, 21, 6957. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.I.; Kim, R.K.; Cho, E.W.; Kim, I.G. Tescalcin/c-Src/IGF1Rβ-mediated STAT3 activation enhances cancer stemness and radioresistant properties through ALDH1. Sci. Rep. 2018, 8, 10711. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-R.; Choi, Y.-J.; Kim, J.-Y.; Kim, I.-G.; Jung, U. Repeated Irradiation with γ-Ray Induces Cancer Stemness through TGF-β-DLX2 Signaling in the A549 Human Lung Cancer Cell Line. Int. J. Mol. Sci. 2021, 22, 4284. [Google Scholar] [CrossRef] [PubMed]
- Nisar, H.; Sanchidrián González, P.M.; Brauny, M.; Labonté, F.M.; Schmitz, C.; Roggan, M.D.; Konda, B.; Hellweg, C.E. Hypoxia Changes Energy Metabolism and Growth Rate in Non-Small Cell Lung Cancer Cells. Cancers 2023, 15, 2472. [Google Scholar] [CrossRef]
- Chaachouay, H.; Fehrenbacher, B.; Toulany, M.; Schaller, M.; Multhoff, G.; Rodemann, H.P. AMPK-independent autophagy promotes radioresistance of human tumor cells under clinical relevant hypoxia in vitro. Radiother. Oncol. 2015, 116, 409–416. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 2018, 15, 21–32. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Oh, S.H.; Woo, J.-K.; Hong, W.K.; Lee, H.-Y. Targeting Heat Shock Protein 90 Overrides the Resistance of Lung Cancer Cells by Blocking Radiation-Induced Stabilization of Hypoxia-Inducible Factor-1α. Cancer Res. 2009, 69, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Song, Z.; Su, J.; Li, L.; Zou, L.; Zou, K. The PRX-1/TLR4 axis promotes hypoxia-induced radiotherapy resistance in non-small cell lung cancer by targeting the NF-κB/p65 pathway. Cell. Signal. 2023, 110, 110806. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Tan, Y.; Wei, Q.; Yu, W. Cancer-associated fibroblasts in radiotherapy: Challenges and new opportunities. Cell Commun. Signal. 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, Y.; Wan, C.; Sun, Y.; Dai, X.; Huang, J.; Hu, Y.; Gao, Y.; Wu, B.; Zhang, Z.; et al. Targeting senescence-like fibroblasts radiosensitizes non–small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight 2021, 6, e146334. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.Y.; Hewitt, S.M. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J. Transl. Med. 2020, 18, 443. [Google Scholar] [CrossRef]
- Zhang, F.; Sang, Y.; Chen, D.; Wu, X.; Wang, X.; Yang, W.; Chen, Y. M2 macrophage-derived exosomal long non-coding RNA AGAP2-AS1 enhances radiotherapy immunity in lung cancer by reducing microRNA-296 and elevating NOTCH2. Cell Death Dis. 2021, 12, 467. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Hu, P.; Zhang, J. The Correlation Between Low-Dose Radiotherapy Area of the Mediastinum and CD8+T Cells and the Efficacy of Radiotherapy for Non-Small Cell Lung Cancer. Cancer Manag. Res. 2024, 16, 23–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Ji, K.; Jiang, S.; Dong, Y.; Sun, L.; Wang, J.; Hu, G.; Chen, D.; Chen, K.; et al. CD39 inhibition and VISTA blockade may overcome radiotherapy resistance by targeting exhausted CD8+ T cells and immunosuppressive myeloid cells. Cell Rep. Med. 2023, 4, 101151. [Google Scholar] [CrossRef]
- Liang, H.; Shen, X. LXR activation radiosensitizes non-small cell lung cancer by restricting myeloid-derived suppressor cells. Biochem. Biophys. Res. Commun. 2020, 528, 330–335. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Jiang, T.; Xie, H.; Zhu, Z.; Zhou, F.; Zhou, C. Combined Radiotherapy and Anti–PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 1085–1097. [Google Scholar] [CrossRef]
- Fischer, T.; Hartmann, O.; Reissland, M.; Prieto-Garcia, C.; Klann, K.; Pahor, N.; Schülein-Völk, C.; Baluapuri, A.; Polat, B.; Abazari, A.; et al. PTEN mutant non-small cell lung cancer require ATM to suppress pro-apoptotic signalling and evade radiotherapy. Cell Biosci. 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Yingzi, L.; Jie, G.; Guanxin, L.; Zimei, Z.; Zhen, C.; Zhi, L.; Yingjie, N.; Lunquan, S.; Tao, C.; et al. PPDPF Promotes the Progression and acts as an Antiapoptotic Protein in Non-Small Cell Lung Cancer. Int. J. Biol. Sci. 2022, 18, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, F.A.; Raji, A.; Ali, S.A.; Abdelbasset, W.K.; Alekhina, N.; Iswanto, A.H.; Terefe, E.M.; Jalil, A.T. Autophagy-related chemoradiotherapy sensitivity in non-small cell lung cancer (NSCLC). Pathol. Res. Pr. 2022, 233, 153823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, T.; Wang, Q.; Chen, R.; Xie, Y.; Chang, H.; Cheng, J. Repression of the AURKA-CXCL5 axis induces autophagic cell death and promotes radiosensitivity in non-small-cell lung cancer. Cancer Lett. 2021, 509, 89–104. [Google Scholar] [CrossRef]
- Wen, J.; Zheng, W.; Zeng, L.; Wang, B.; Chen, D.; Chen, Y.; Lu, X.; Shao, C.; Chen, J.; Fan, M. LTF Induces Radioresistance by Promoting Autophagy and Forms an AMPK/SP2/NEAT1/miR-214-5p Feedback Loop in Lung Squamous Cell Carcinoma. Int. J. Biol. Sci. 2023, 19, 1509–1527. [Google Scholar] [CrossRef]
- Han, F.; Chen, S.; Zhang, K.; Zhang, K.; Wang, M.; Wang, P. Targeting Nrf2/PHKG2 axis to enhance radiosensitivity in NSCLC. NPJ Precis. Oncol. 2024, 8, 183. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Yu, B.; Yang, T.; Liu, B.; Li, F.; Jin, X.; Li, Q. RSPO3 regulates the radioresistance of Non-Small cell lung cancer cells via NLRP3 Inflammasome-Mediated pyroptosis. Radiother. Oncol. 2024, 200, 110528. [Google Scholar] [CrossRef]
- Oike, T.; Komachi, M.; Ogiwara, H.; Amornwichet, N.; Saitoh, Y.; Torikai, K.; Kubo, N.; Nakano, T.; Kohno, T. C646, a selective small molecule inhibitor of histone acetyltransferase p300, radiosensitizes lung cancer cells by enhancing mitotic catastrophe. Radiother. Oncol. 2014, 111, 222–227. [Google Scholar] [CrossRef]
- Mirzaei, H.; Hamblin, M.R. Regulation of Glycolysis by Non-coding RNAs in Cancer: Switching on the Warburg Effect. Mol. Ther. Oncolytics 2020, 19, 218–239. [Google Scholar] [CrossRef]
- Meng, M.B.; Wang, H.H.; Guo, W.H.; Wu, Z.Q.; Zeng, X.L.; Zaorsky, N.G.; Shi, H.S.; Qian, D.; Niu, Z.M.; Jiang, B.; et al. Targeting pyruvate kinase M2 contributes to radiosensitivity of non-small cell lung cancer cells in vitro and in vivo. Cancer Lett. 2015, 356 Pt B, 985–993. [Google Scholar] [CrossRef]
- Shavit, R.; Ilouze, M.; Feinberg, T.; Lawrence, Y.R.; Tzur, Y.; Peled, N. Mitochondrial induction as a potential radio-sensitizer in lung cancer cells—A short report. Cell. Oncol. 2015, 38, 247–252. [Google Scholar] [CrossRef]
- Binkley, M.S.; Jeon, Y.J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S.; et al. KEAP1/NFE2L2 Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discov. 2020, 10, 1826–1841. [Google Scholar] [CrossRef]
- Sanchez-Castillo, A.; Heylen, E.; Hounjet, J.; Savelkouls, K.G.; Lieuwes, N.G.; Biemans, R.; Dubois, L.J.; Reynders, K.; Rouschop, K.M.; Vaes, R.D.W.; et al. Targeting serine/glycine metabolism improves radiotherapy response in non-small cell lung cancer. Br. J. Cancer 2024, 130, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, J.; Ge, S.; Su, Y.; Fan, X. Novel insight into metabolic reprogrammming in cancer radioresistance: A promising therapeutic target in radiotherapy. Int. J. Biol. Sci. 2023, 19, 811–828. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, T.; Wan, L.; Fa, P.; Kumar, P.; Cao, Y.; Prasad, C.B.; Qiu, Z.; Liu, J.; Wang, H.; et al. Targeting Squalene Epoxidase Interrupts Homologous Recombination via the ER Stress Response and Promotes Radiotherapy Efficacy. Cancer Res. 2022, 82, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Ostheimer, C.; Gunther, S.; Bache, M.; Vordermark, D.; Multhoff, G. Dynamics of Heat Shock Protein 70 Serum Levels As a Predictor of Clinical Response in Non-Small-Cell Lung Cancer and Correlation with the Hypoxia-Related Marker Osteopontin. Front. Immunol. 2017, 8, 1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhao, X.; Chen, Q.; Pan, Y.; Bai, Y.; Shao, C.; Zhang, J. Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment. Br. J. Cancer 2022, 127, 1760–1772. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Chen, Y.; Shi, Y.; Ze, Y.; Yao, Y. Irradiated Cell-Derived Exosomes Transmit Essential Molecules Inducing Radiation Therapy Resistance. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 192–202. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, Y.; Li, Z.; Jiao, Z.; Zhang, Y.; He, Y.; Chen, G.; Zhou, Q.; Wang, W.; Zhou, X.; et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 7. [Google Scholar] [CrossRef]
- Arrieta, O.; Ramirez-Tirado, L.A.; Caballe-Perez, E.; Mejia-Perez, A.; Zatarain-Barron, Z.L.; Cardona, A.F.; Lozano-Ruiz, F.; Segura-Gonzalez, M.; Cruz-Rico, G.; Maldonado, F.; et al. Response rate of patients with baseline brain metastases from recently diagnosed non-small cell lung cancer receiving radiotherapy according to EGFR, ALK and KRAS mutation status. Thorac. Cancer 2020, 11, 1026–1037. [Google Scholar] [CrossRef]
- Hellyer, J.A.; Padda, S.K.; Diehn, M.; Wakelee, H.A. Clinical Implications of KEAP1-NFE2L2 Mutations in NSCLC. J. Thorac. Oncol. 2021, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Pharaon, R.; Mambetsariev, I.; Nam, A.; Sattler, M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep. Med. 2021, 2, 100186. [Google Scholar] [CrossRef] [PubMed]
- Sitthideatphaiboon, P.; Galan-Cobo, A.; Negrao, M.V.; Qu, X.; Poteete, A.; Zhang, F.; Liu, D.D.; Lewis, W.E.; Kemp, H.N.; Lewis, J.; et al. STK11/LKB1 Mutations in NSCLC Are Associated with KEAP1/NRF2-Dependent Radiotherapy Resistance Targetable by Glutaminase Inhibition. Clin. Cancer Res. 2021, 27, 1720–1733. [Google Scholar] [CrossRef]
- Hu, S.; Fu, W.; Li, T.; Yuan, Q.; Wang, F.; Lv, G.; Lv, Y.; Fan, X.; Shen, Y.; Lin, F.; et al. Antagonism of EGFR and Notch limits resistance to EGFR inhibitors and radiation by decreasing tumor-initiating cell frequency. Sci. Transl. Med. 2017, 9, eaag0339. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.T.; Webb, A.; Shilo, K.; Robb, R.; Xu-Welliver, M.; Haglund, K.; Brownstein, J.; DeNicola, G.M.; Shen, C.; Williams, T.M. A PI3K gene expression signature predicts for recurrence in early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Cancer 2023, 129, 3971–3977. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Zeng, Z.; Wu, Q.; Jiang, X.; Li, S.; Sun, W.; Zhang, J.; Li, Y.; Li, J.; et al. LncRNA PCAT1 activates SOX2 and suppresses radioimmune responses via regulating cGAS/STING signalling in non-small cell lung cancer. Clin. Transl. Med. 2022, 12, e792. [Google Scholar] [CrossRef]
- Hoang, P.H.; Landi, M.T. DNA Methylation in Lung Cancer: Mechanisms and Associations with Histological Subtypes, Molecular Alterations, and Major Epidemiological Factors. Cancers 2022, 14, 961. [Google Scholar] [CrossRef]
- Wu, Z.; Qiu, M.; Guo, Y.; Zhao, J.; Liu, Z.; Wang, H.; Meng, M.; Yuan, Z.; Mi, Z. OTU deubiquitinase 4 is silenced and radiosensitizes non-small cell lung cancer cells via inhibiting DNA repair. Cancer Cell Int. 2019, 19, 99. [Google Scholar] [CrossRef]
- Moses, N.; Zhang, M.; Wu, J.Y.; Hu, C.; Xiang, S.; Geng, X.; Chen, Y.; Bai, W.; Zhang, Y.W.; Bepler, G.; et al. HDAC6 Regulates Radiosensitivity of Non-Small Cell Lung Cancer by Promoting Degradation of Chk1. Cells 2020, 9, 2237. [Google Scholar] [CrossRef]
- Li, Q.; Qin, K.; Tian, Y.; Chen, B.; Zhao, G.; Xu, S.; Wu, L. Inhibition of demethylase by IOX1 modulates chromatin accessibility to enhance NSCLC radiation sensitivity through attenuated PIF1. Cell Death Dis. 2023, 14, 817. [Google Scholar] [CrossRef]
- De, S.; Holvey-Bates, E.G.; Mahen, K.; Willard, B.; Stark, G.R. The ubiquitin E3 ligase FBXO22 degrades PD-L1 and sensitizes cancer cells to DNA damage. Proc. Natl. Acad. Sci. USA 2021, 118, e2112674118. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, Q.; Zhang, F.; Xian, X.; Wang, S.; Xia, J.; Li, J.; Tuo, Z.; Xiao, G.; Liu, L.; et al. SDH5 Depletion Enhances Radiosensitivity by Regulating p53: A New Method for Noninvasive Prediction of Radiotherapy Response. Theranostics 2019, 9, 6380–6395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, X.; Huang, L.; Zeng, J.; Ma, C.; Han, L.; Li, W.; Yu, J.; Yang, M. LINC00921 reduces lung cancer radiosensitivity by destabilizing NUDT21 and driving aberrant MED23 alternative polyadenylation. Cell Rep. 2023, 42, 113479. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Qiu, M.; Zhao, J.; Zou, F.; Meng, M.; Jiang, X.; Yuan, Z.; Mi, Z.; Wu, Z. MicroRNA-384 radiosensitizes human non-small cell lung cancer by impairing DNA damage response and repair signaling, which is inhibited by NF-κB. Cancer Biol. Med. 2024, 21, 1050–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Liu, X.; Li, F.; Chen, W.; Kuang, Y.; Zhao, X.; Li, L.; Yu, B.; Jin, X.; et al. CircZNF208 enhances the sensitivity to X-rays instead of carbon-ions through the miR-7-5p /SNCA signal axis in non-small-cell lung cancer cells. Cell. Signal. 2021, 84, 110012. [Google Scholar] [CrossRef]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Bernstein, M.B.; Krishnan, S.; Hodge, J.W.; Chang, J.Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Tachihara, M.; Tsujino, K.; Ishihara, T.; Hayashi, H.; Sato, Y.; Kurata, T.; Sugawara, S.; Shiraishi, Y.; Teraoka, S.; Azuma, K.; et al. Durvalumab Plus Concurrent Radiotherapy for Treatment of Locally Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2023, 9, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Riely, G.J.; Wood, D.E.; Ettinger, D.S.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. Non–Small Cell Lung Cancer, Version 4.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 249–274. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.F.; Morris, B.A.; Sethakorn, N.; Lang, J.M.; Schehr, J.L.; Zhao, S.G.; Morris, Z.S.; Buehler, D.; Eickhoff, J.C.; Harari, P.M.; et al. Combining Dual Checkpoint Immunotherapy with Ablative Radiation to All Sites of Oligometastatic Non-Small Cell Lung Cancer: Toxicity and Efficacy Results of a Phase 1b Trial. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 1481–1489. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Li, Z.; Xiao, Z.-F.; Li, D.; Liu, W.-Y. Case report: Radiotherapy plus pneumococcal conjugate vaccine stimulates abscopal immune response in a patient with ALK+ NSCLC. Front. Immunol. 2022, 13, 950252. [Google Scholar] [CrossRef]
- Zhuang, H.; zhao, l.; wang, p.; Pang, Q.; Zhao, X. Progress of clinical research on targeted therapy combined with thoracic radiotherapy for non-small-cell lung cancer. Drug Des. Dev. Ther. 2014, 8, 667–675. [Google Scholar] [CrossRef][Green Version]
- Xing, L.; Wu, G.; Wang, L.; Li, J.; Wang, J.; Yuan, Z.; Chen, M.; Xu, Y.; Fu, X.; Zhu, Z.; et al. Erlotinib Versus Etoposide/Cisplatin With Radiation Therapy in Unresectable Stage III Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer: A Multicenter, Randomized, Open-Label, Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1349–1358. [Google Scholar] [CrossRef]
- Lévy, C.; Allouache, D.; Lacroix, J.; Dugué, A.E.; Supiot, S.; Campone, M.; Mahe, M.; Kichou, S.; Leheurteur, M.; Hanzen, C.; et al. REBECA: A phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann. Oncol. 2014, 25, 2351–2356. [Google Scholar] [CrossRef]
- Wang, W.; Sun, X.; Hui, Z. Treatment Optimization for Brain Metastasis from Anaplastic Lymphoma Kinase Rearrangement Non-Small-Cell Lung Cancer. Oncol. Res. Treat. 2019, 42, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Robinson, C.; Bradley, J.; Goodgame, B.; Rooney, M.; Williams, K.; Gao, F.; Govindan, R. A Phase I Study of Temsirolimus and Thoracic Radiation in Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 119–123. [Google Scholar] [CrossRef]
- de Haan, R.; van Werkhoven, E.; van den Heuvel, M.M.; Peulen, H.M.U.; Sonke, G.S.; Elkhuizen, P.; van den Brekel, M.W.M.; Tesselaar, M.E.T.; Vens, C.; Schellens, J.H.M.; et al. Study protocols of three parallel phase 1 trials combining radical radiotherapy with the PARP inhibitor olaparib. BMC Cancer 2019, 19, 901. [Google Scholar] [CrossRef]
- Qiu, Z.; Oleinick, N.L.; Zhang, J. ATR/CHK1 inhibitors and cancer therapy. Radiother. Oncol. 2018, 126, 450–464. [Google Scholar] [CrossRef]
- Baschnagel, A.M.; Elnaggar, J.H.; VanBeek, H.J.; Kromke, A.C.; Skiba, J.H.; Kaushik, S.; Abel, L.; Clark, P.A.; Longhurst, C.A.; Nickel, K.P.; et al. ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non–Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts. Mol. Cancer Ther. 2021, 20, 2129–2139. [Google Scholar] [CrossRef]
- Zenke, F.T.; Zimmermann, A.; Sirrenberg, C.; Dahmen, H.; Kirkin, V.; Pehl, U.; Grombacher, T.; Wilm, C.; Fuchss, T.; Amendt, C.; et al. Pharmacologic Inhibitor of DNA-PK, M3814, Potentiates Radiotherapy and Regresses Human Tumors in Mouse Models. Mol. Cancer Ther. 2020, 19, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Sowers, A.; Choudhuri, R.; Wissler, M.; Gamson, J.; Mathias, A.; Cook, J.A.; Mitchell, J.B. Abemaciclib, a Selective CDK4/6 Inhibitor, Enhances the Radiosensitivity of Non–Small Cell Lung Cancer In Vitro and In Vivo. Clin. Cancer Res. 2018, 24, 3994–4005. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Asselin, M.-C.; Reymen, B.; Jackson, A.; Lambin, P.; West, C.M.L.; O’Connor, J.P.B.; Faivre-Finn, C. Targeting Hypoxia to Improve Non–Small Cell Lung Cancer Outcome. JNCI J. Natl. Cancer Inst. 2018, 110, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Gandara, D.R.; Lara, P.N.; Goldberg, Z.; Le, Q.T.; Mack, P.C.; Lau, D.H.M.; Gumerlock, P.H. Tirapazamine: Prototype for a novel class of therapeutic agents targeting tumor hypoxia. Semin. Oncol. 2002, 29, 102–109. [Google Scholar] [CrossRef]
- Choy, H.; Nabid, A.; Stea, B.; Scott, C.; Roa, W.; Kleinberg, L.; Ayoub, J.; Smith, C.; Souhami, L.; Hamburg, S.; et al. Phase II Multicenter Study of Induction Chemotherapy Followed by Concurrent Efaproxiral (RSR13) and Thoracic Radiotherapy for Patients With Locally Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 5918–5928. [Google Scholar] [CrossRef]
- Zannella, V.E.; Dal Pra, A.; Muaddi, H.; McKee, T.D.; Stapleton, S.; Sykes, J.; Glicksman, R.; Chaib, S.; Zamiara, P.; Milosevic, M.; et al. Reprogramming Metabolism with Metformin Improves Tumor Oxygenation and Radiotherapy Response. Clin. Cancer Res. 2013, 19, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Li, B.; Xu, X.; Xu, J.; Hu, S. Inhibition of FASN expression enhances radiosensitivity in human non-small cell lung cancer. Oncol. Lett. 2018, 15, 4578–4584. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.-P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471. [Google Scholar] [CrossRef]
- Shimada, Y.; Matsubayashi, J.; Kudo, Y.; Maehara, S.; Takeuchi, S.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Nagao, T.; Ikeda, N. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci. Rep. 2021, 11, 7830. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef]
- Shi, W.; Lawrence, Y.R.; Choy, H.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Judy, K.D.; Farrell, C.J.; Moshel, Y.; Berger, A.C.; et al. Vorinostat as a radiosensitizer for brain metastasis: A phase I clinical trial. J. Neurooncol 2014, 118, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Tang, J.; Chen, Q.; Yu, Y.; Dong, Y.; Hao, J.; Wu, W. Synergistic cerium oxide nanozymes: Targeting DNA damage and alleviating tumor hypoxia for improved NSCLC radiotherapy efficiency. J. Nanobiotechnology 2024, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, S.; Hong, B.-J.; Lee, C.-J.; Kim, Y.-E.; Bok, S.; Oh, J.-M.; Gwak, S.-H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806. [Google Scholar] [CrossRef]
- Ding, X.; Cheng, J.; Pang, Q.; Wei, X.; Zhang, X.; Wang, P.; Yuan, Z.; Qian, D. BIBR1532, a Selective Telomerase Inhibitor, Enhances Radiosensitivity of Non-Small Cell Lung Cancer Through Increasing Telomere Dysfunction and ATM/CHK1 Inhibition. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 861–874. [Google Scholar] [CrossRef]
- Mendoza-Munoz, P.L.; Gavande, N.S.; VanderVere-Carozza, P.S.; Pawelczak, K.S.; Dynlacht, J.R.; Garrett, J.E.; Turchi, J.J. Ku–DNA binding inhibitors modulate the DNA damage response in response to DNA double-strand breaks. NAR Cancer 2023, 5, zcad003. [Google Scholar] [CrossRef]
- van Haren, M.J.; Zhang, Y.; Thijssen, V.; Buijs, N.; Gao, Y.; Mateuszuk, L.; Fedak, F.A.; Kij, A.; Campagna, R.; Sartini, D.; et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT). RSC Chem. Biol. 2021, 2, 1546–1555. [Google Scholar] [CrossRef]
- van Haren, M.J.; Gao, Y.; Buijs, N.; Campagna, R.; Sartini, D.; Emanuelli, M.; Mateuszuk, L.; Kij, A.; Chlopicki, S.; Escudé Martinez de Castilla, P.; et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules 2021, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Le Blanc, J.M.; Wang, C.; Zhan, T.; Zhuang, H.; Wang, P.; Yuan, Z.; Lu, B. Coadministration of Trametinib and Palbociclib Radiosensitizes KRAS-Mutant Non–Small Cell Lung Cancers In Vitro and In Vivo. Clin. Cancer Res. 2016, 22, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Moretti, L.; Li, B.; Kim, K.W.; Chen, H.; Lu, B. AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Moretti, L.; Mitchell, L.R.; Jung, D.K.; Lu, B. Combined Bcl-2/Mammalian Target of Rapamycin Inhibition Leads to Enhanced Radiosensitization via Induction of Apoptosis and Autophagy in Non–Small Cell Lung Tumor Xenograft Model. Clin. Cancer Res. 2009, 15, 6096–6105. [Google Scholar] [CrossRef]
- Sun, H.; Du, Y.; Yao, M.; Wang, Q.; Ji, K.; Du, L.; Xu, C.; He, N.; Wang, J.; Zhang, M.; et al. cIAP1/2 are involved in the radiosensitizing effect of birinapant on NSCLC cell line in vitro. J. Cell. Mol. Med. 2021, 25, 6125–6136. [Google Scholar] [CrossRef]
- Bayo, J.; Tran, T.A.; Wang, L.; Peña-Llopis, S.; Das, A.K.; Martinez, E.D. Jumonji Inhibitors Overcome Radioresistance in Cancer through Changes in H3K4 Methylation at Double-Strand Breaks. Cell Rep. 2018, 25, 1040–1050.e5. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, L.; Qiang, Z.; Jiang, J.; Zhu, Z.; Ren, J. Enhancing Targeted Cancer Treatment by Combining Hyperthermia and Radiotherapy Using Mn–Zn Ferrite Magnetic Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 3550–3562. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Jin, X.; Liu, Y.; Li, P.; Shen, Z.; Wu, A.; Zheng, X.; Chen, W.; Li, Q. Radiosensitizing Effect of Gadolinium Oxide Nanocrystals in NSCLC Cells Under Carbon Ion Irradiation. Nanoscale Res. Lett. 2019, 14, 328. [Google Scholar] [CrossRef]
| Mechanisms | Targets | Radiosensitizers | Phases | Primary Endpoints | Registration |
|---|---|---|---|---|---|
| Targeting immune checkpoints | PD1 | Pembrolizumab | Phase I | ORR | NCT01295827 |
| PD1 | Pembrolizumab | Phase II | OS, PFS | NCT02343952 | |
| PDL1 | Durvalumab | Phase II | PFS | jRCT2080224763 | |
| CTLA4 | Ipilimumab | Phase II | OS, PFS | NCT02221739 | |
| PD1 + CTLA4 | Nivolumab + Ipilimumab | Phase I | ORR, PFS | NCT03223155 | |
| PDL1 + CTLA4 | Durvalumab + Tremelimumab | Phase II | OS, PFS | NCT05000710 | |
| Tumor vaccines | Tumor antigens | Monoclonal antibody 11D10 anti-idiotype vaccine and monoclonal antibody 3H1 anti-idiotype vaccine | Phase II | OS, PFS | NCT00006470 |
| Tumor antigens | CV9202 | Phase I | OS, PFS | NCT01915524 | |
| Targeting cell proliferation | EGFR | Osimertinib | Phase III | OS | NCT03521154 |
| EGFR | Gefitinib | Phase I | ORR, PFS | NCT00328562 | |
| EGFR | Erlotinib | Phase II | OS, PFS | NCT00563784 | |
| EGFR | Cetuximab | Phase III | ORR | NCT00115518 | |
| EGFR | Panitumumab | Phase II | OS | NCT00979212 | |
| EGFR | Nimotuzumab | Phase II | OS, PFS | NCT00872482 | |
| ALK | Ceritinib | Phase II | OS, PFS | NCT02336451 | |
| ALK | Crizotinib | Phase II | OS, PFS | NCT02314364 | |
| ALK | Alectinib | Phase I/II | PFS | NCT05724004 | |
| PI3K | Buparlisib | Phase I | MTD | NCT02128724 | |
| mTOR | Temsirolimus | Phase III | MTD | NCT00796796 | |
| mTOR | Everolimus | Phase I | MTD | NCT01063478 | |
| PKC | Enzastaurin | Phase II | PFS | NCT00415363 | |
| Targeting angiogenesis | VEGF | Endostar | Phase II | OS, PFS | NCT01733589 |
| VEGF | Bevacizumab | Phase II | PFS | NCT04345146 | |
| VEGF | Vandetanib | Phase I | MTD | NCT00807170 | |
| VEGFR | Anlotinib | Phase II | PFS | NCT03672136 | |
| Targeting cell apoptosis | Bcl-2/Bcl-xL | Docetaxel | Phase I | MTD | NCT00378404 |
| Targeting DNA damage repair | PARP | Olaparib | Phase I | ORR | NCT01562210 |
| PARP | Veliparib | Phase II | OS, ORR | NCT01657799 | |
| ATR | Berzosertib | Phase I | PFS | NCT02589522 | |
| ATR | Ceralasertib | Phase I | MTD | NCT04550104 | |
| CHK1 | Prexasertib | Phase II | OS, PFS | NCT02873975 | |
| DNA-PK | M3814 | Phase I | ORR | NCT02516813 | |
| Targeting hypoxia | HIF-1α | Nitroglycerin | Phase II | ORR | NCT06238882 |
| HIF-1α | Topotecan | Phase I | MTD | NCT00002537 | |
| Top II | Tirapazamine | Phase I | ORR | NCT00033410 | |
| Hb | Efaproxiral | Phase III | OS, PFS | NCT00055887 | |
| Mitochondrial complex I | Metformin | Phase II | OS, PFS | NCT02186847 | |
| Targeting inflammation | COX-2 | Celecoxib | Phase II | ORR | NCT00181532 |
| Targeting metabolism | Glutaminase | CB-839 | Phase I | ORR | NCT02071862 |
| Targeting epigenetics | HADC | Vorinostat | Phase I | MTD | NCT00946673 |
| Proteasome | Bortezomib | Phase I/II | OS | NCT00093756 |
| Mechanisms | Targets | Drugs | Conclusion | Reference |
|---|---|---|---|---|
| Immunosuppressive TME | CD39 | CD39i | Inhibition of CD39 combined with RT preferentially decreases the percentage of exhausted CD8+ T cells. | [49] |
| TAMs | Clodronate | Depletion of TAM by clodronate was sufficient to abrogate aerobic glycolysis and tumor hypoxia, thereby improving tumor response to anticancer therapies. | [118] | |
| DNA damage repair | CHK1 | MK-8776 | MK-8776 radiosensitized p53-defective NSCLC by abrogation of G2/M arrest and by inhibition of DSB repair. | [102] |
| ATM | BIBR1532 | BIBR1532 enhances radiosensitivity of NSCLC through increasing telomere dysfunction and ATM/CHK1 inhibition | [119] | |
| DNA-PK | AZD7648 | AZD7648 is an efficient sensitizer of radiation-induced DNA damage. | [7] | |
| DNA-PK | Ku-DBi | Ku-DBis inhibit cellular DNA-PK, NHEJ-catalyzed DSB repair and sensitize NSCLC cells to DSB-inducing agents. | [120] | |
| NNMT | Macrocyclic peptides, GYZ-319 | Macrocyclic peptides and GYZ-319 show potent inhibitory effects against NNMT. | [121,122] | |
| Cell cycle dysregula-tion | CDK4/6 | Abemaciclib | Abemaciclib combined with IR increases radiosensitivity in NSCLC in preclinical models. | [105] |
| CDK4/6 | Palbociclib | Palbociclib in combination with MEK inhibitor has significant anti-NSCLC activity and radiosensitizing effect in preclinical models. | [123] | |
| Hypoxia | GPX | Misonidazole | Misonidazole exhibits radiosensitizing effects in human LSCC. | [106] |
| Abnormal regulation of cell death | Bcl-2 | AT-101 | AT-101 inhibits Bcl-2 and leads to radiosensitization of NSCLC. | [124] |
| Bcl-2 | ABT-737 | Combined inhibition of Bcl-2 and mTOR amplifies radiosensitization in NSCLC xenografts by simultaneously inducing apoptosis and autophagy. | [125] | |
| Mcl-1/Bcl-xL | WEHI-539/ S63845 | Inhibition of Mcl-1 and Bcl-xL can result in increased radiation-induced cytotoxicity in NSCLC cell lines. | [12] | |
| cIAP1/2 | Birinapant | Birinapant-induced apoptosis and inhibited the proliferation of NSCLC cells after exposure to radiation. | [126] | |
| Metabolic dysregulation | Serine/glycine | Sertraline | The combination of sertraline and RT diminished the proliferation, clonogenicity, and self-renewal capacity of NSCLC stem cells. | [64] |
| SQLE | Terbinafine | SQLE inhibition increases radiation efficacy in NSCLC by impairing cholesterol synthesis and increasing squalene levels. | [66] | |
| Pyruvate | Dichloroacetate | Dichloroacetate radiosensitizes NSCLC by increasing the influx of pyruvate and promoting mitochondrial activation. | [62] | |
| Epigenetic dysregulation | DNMT | 5-aza-2’-deoxycytidine | 5-aza-2’-deoxycytidine promotes radiosensitivity by enhancing apoptosis and blocking oncogenic signaling. | [21] |
| Histone demethylase | IOX1 | IOX1-mediated inhibition of demethylase alters chromatin accessibility, thereby increasing radiation sensitivity in NSCLC. | [82] | |
| Histone demethylase | PBIT | The H3K4me3 demethylase inhibitor PBIT enhances the sensitivity of cancer cells to radiation. | [127] | |
| Nanoradiosensitizers | CD44 | Mn-Zn ferrite magnetic nanoparticles | Improving targeted cancer therapy through the integration of hyperthermia and RT utilizing Mn-Zn ferrite magnetic nanoparticles. | [128] |
| Hydroxyl radical | GONs | GONs augment hydroxyl radical generation and cellular damage during carbon ion irradiation. | [129] | |
| - | AuNP | AuNPs enhance radiation effects via physical, chemical and biological interactions with IR. | [116] | |
| QT/CeO2 | CeO2@ZIF-8-HA nanoparticles | The nanocomplexes catalyze the decomposition of H2O2 into O2, thereby markedly alleviating the hypoxia of the tumor microenvironment, while the radiosensitizer QT induces direct DNA damage post-radiotherapy. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Luo, S.; Shao, Q.; Li, P.; Huang, L.; Meng, L.; Cheng, H.; Zhang, A.; Gong, X. Mechanisms Underlying Radioresistance and Reversal Strategies in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2025, 26, 6559. https://doi.org/10.3390/ijms26146559
Zhao C, Luo S, Shao Q, Li P, Huang L, Meng L, Cheng H, Zhang A, Gong X. Mechanisms Underlying Radioresistance and Reversal Strategies in Non-Small Cell Lung Cancer. International Journal of Molecular Sciences. 2025; 26(14):6559. https://doi.org/10.3390/ijms26146559
Chicago/Turabian StyleZhao, Chenhui, Shilan Luo, Qing Shao, Peng Li, Litang Huang, Lu Meng, Hongxia Cheng, Anqi Zhang, and Xiaomei Gong. 2025. "Mechanisms Underlying Radioresistance and Reversal Strategies in Non-Small Cell Lung Cancer" International Journal of Molecular Sciences 26, no. 14: 6559. https://doi.org/10.3390/ijms26146559
APA StyleZhao, C., Luo, S., Shao, Q., Li, P., Huang, L., Meng, L., Cheng, H., Zhang, A., & Gong, X. (2025). Mechanisms Underlying Radioresistance and Reversal Strategies in Non-Small Cell Lung Cancer. International Journal of Molecular Sciences, 26(14), 6559. https://doi.org/10.3390/ijms26146559






