A High-Calorie Diet Aggravates Lipopolysaccharide-Induced Pulmonary Inflammation in Juvenile Rats via Hypothalamic-Pituitary-Adrenal Axis-Related Pathways
Abstract
1. Introduction
2. Results
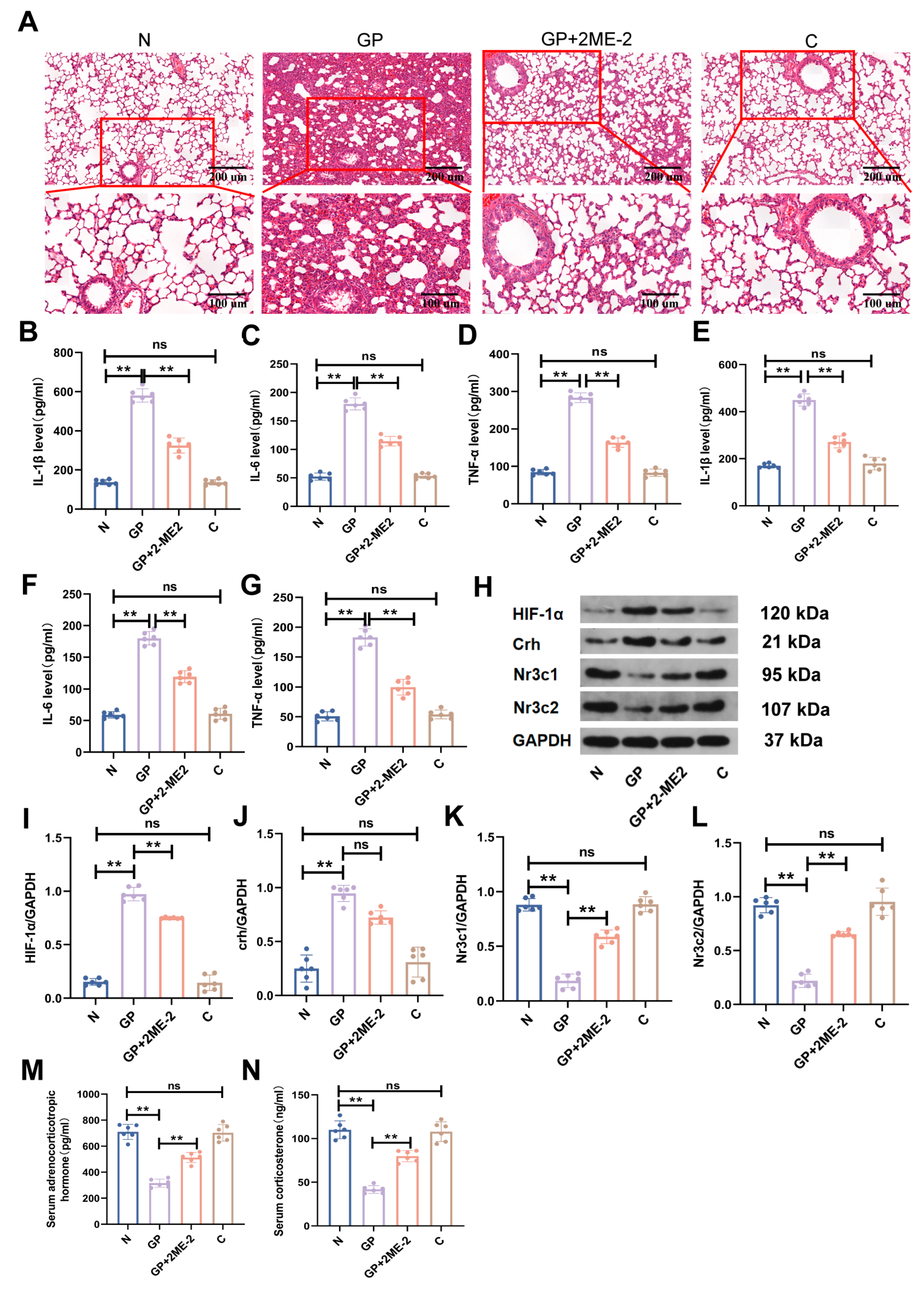
2.1. A High-Calorie Diet Significantly Aggravates LPS-Induced Pneumonia in Juvenile Rats
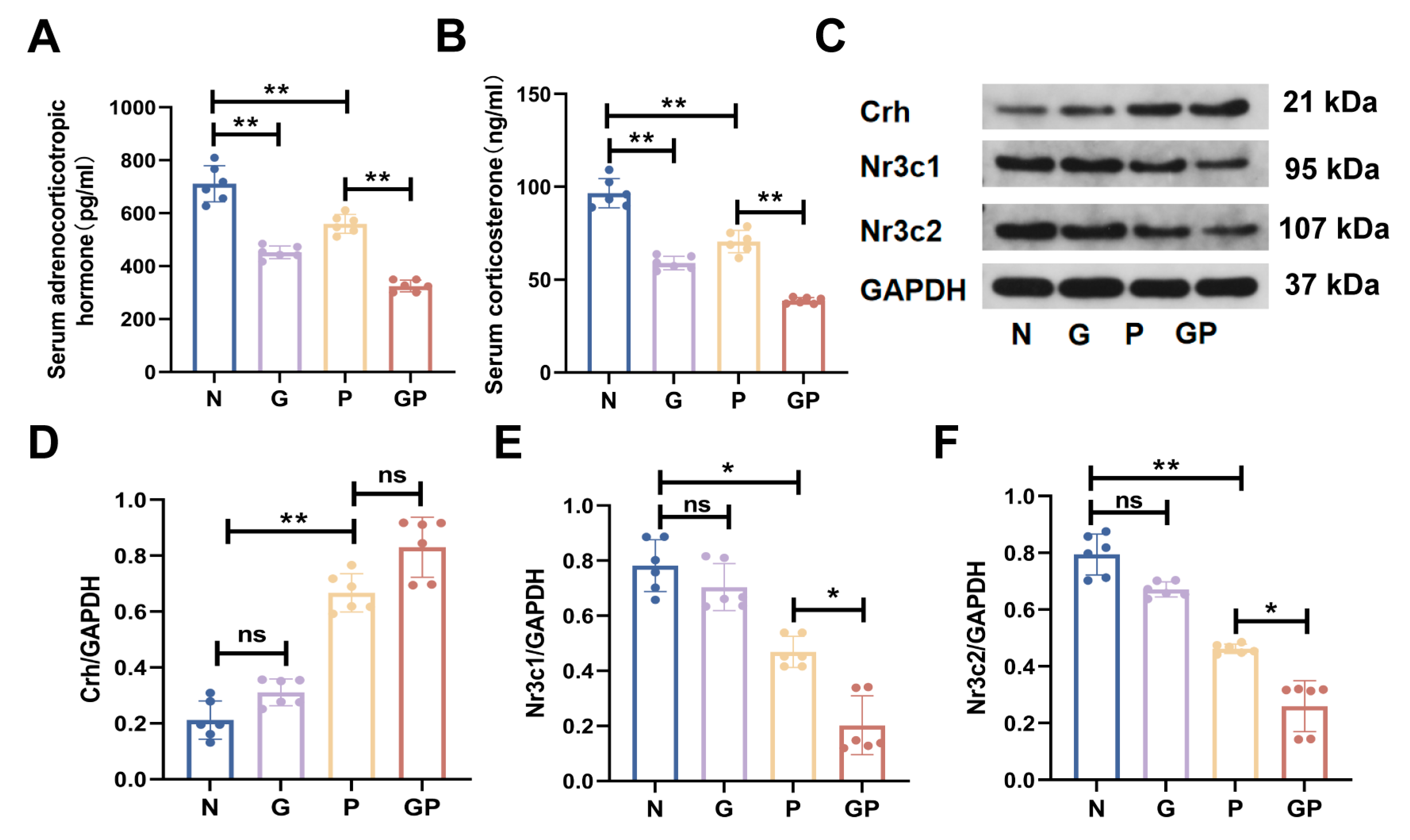
2.2. A High-Calorie Diet Disturbs the HPA Axis
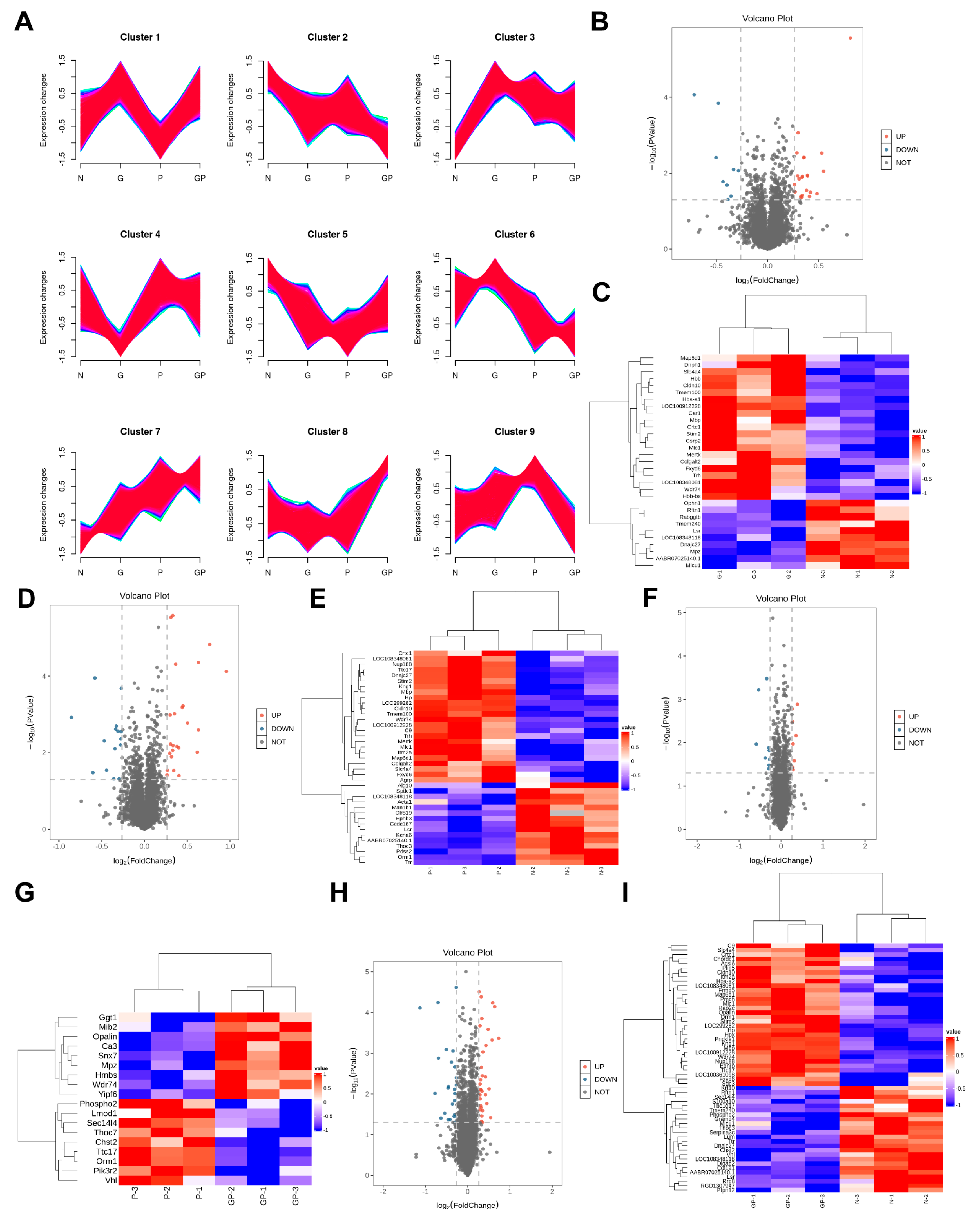
2.3. The Protein Expression in the Hypothalamic Tissue of GP Mice Is Different from That of Pure P Mice
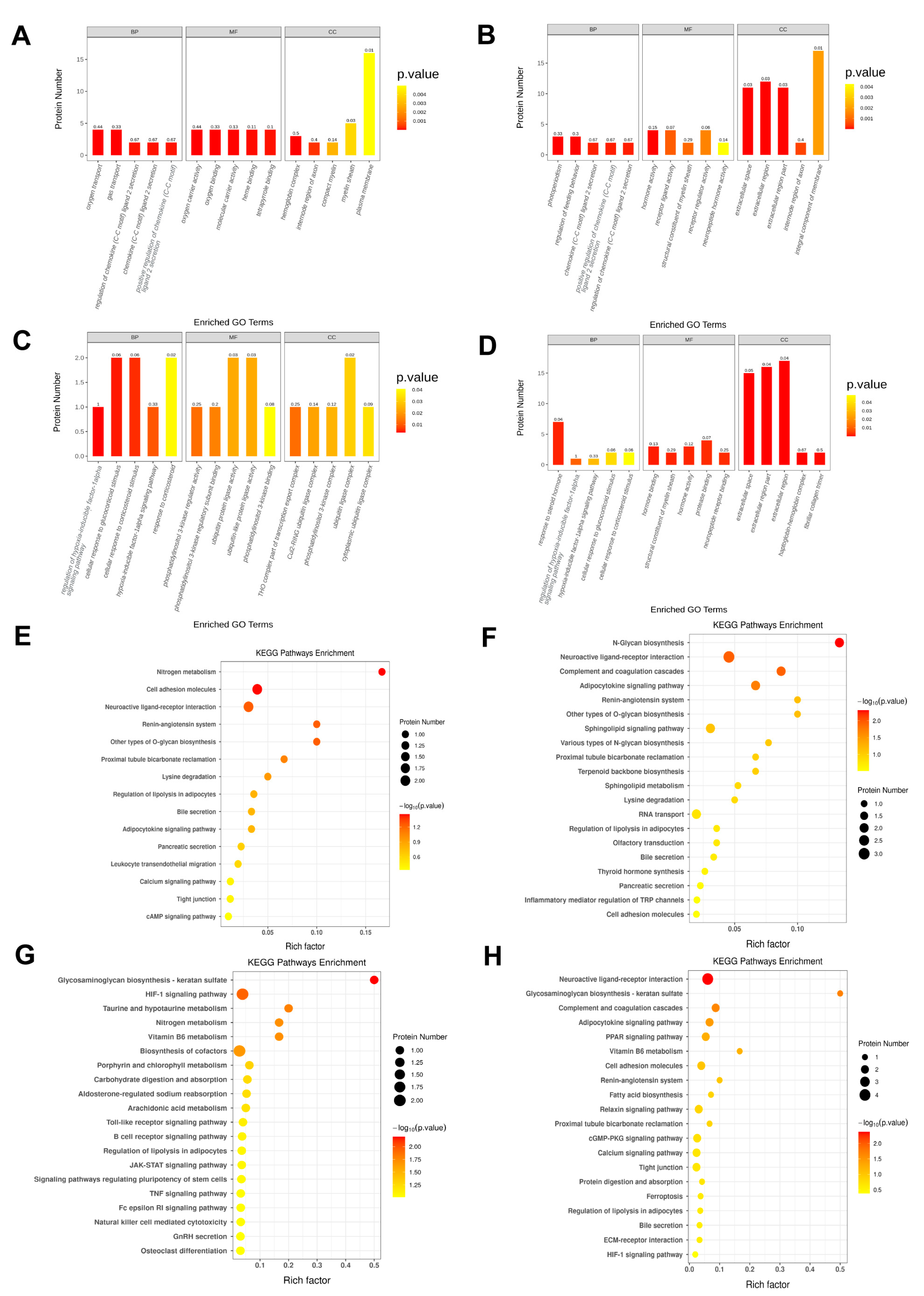
2.4. A High-Calorie Diet Is Associated with Cellular Response to Corticosteroid Stimulus in the Hypothalamic Tissue of Juvenile Rats with LPS-Induced Pneumonia
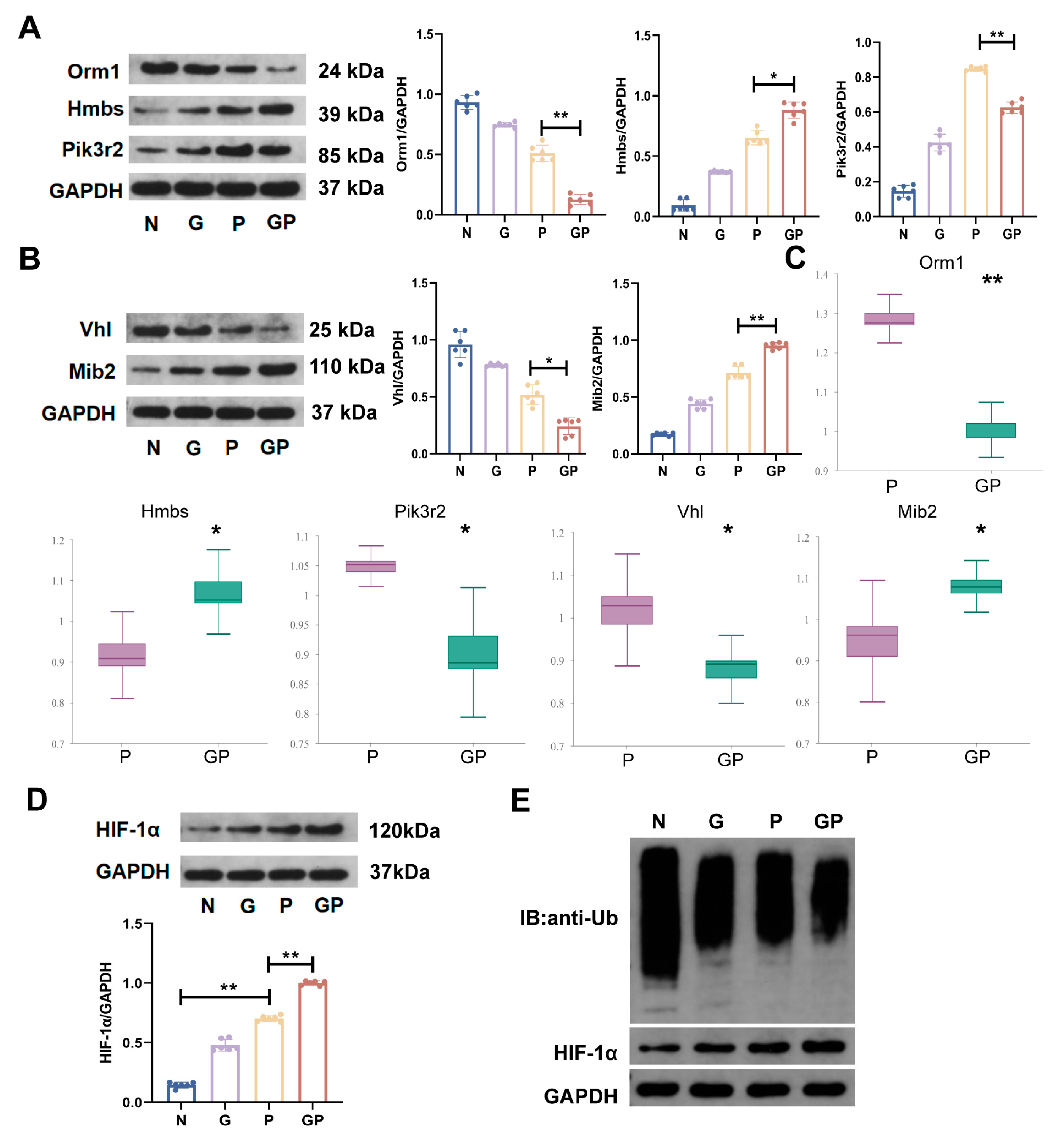
2.5. A High-Calorie Diet May Aggravate LPS-Induced Pneumonia by Activating the HIF-1α Pathway in the Hypothalamus
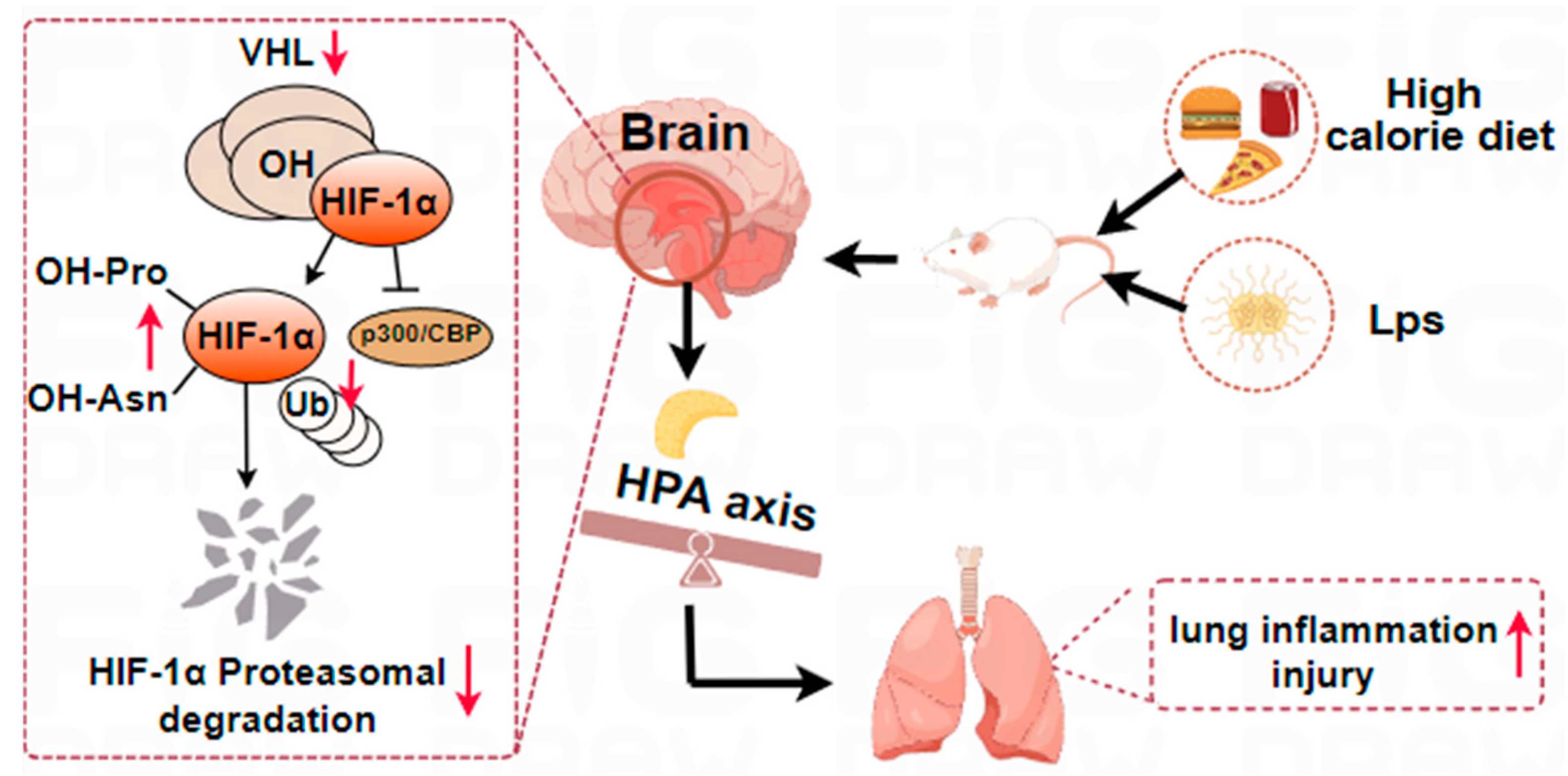
2.6. The Aggravation of LPS-Induced Pneumonia by a High-Calorie Diet Is Associated with the HIF-1α-Mediated HPA Axis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Experiments
4.3. Lung Histopathology
4.4. TMT Proteomic Analysis
4.5. Drug Administration
4.6. Western Blot Analysis
4.7. ELISA
4.8. Co-Immunoprecipitation (Co-IP)
4.9. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPA | Hypothalamic-pituitary-adrenal |
| LPS | Lipopolysaccharide |
| ACTH | Adrenocorticotropic hormone |
| CORT | Corticosterone |
| HIF-1α | Hypoxia-inducible factor-1alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha |
| Crh | Corticotropin-releasing hormone |
| Nr3c1 | Nuclear receptor subfamily 3 group C member 1 |
| Nr3c2 | Nuclear receptor subfamily 3 group C member 2 |
| VHL | Von Hippel-Lindau |
| MR | Mineralocorticoid receptors |
| GR | Glucocorticoid receptors |
| SPF | Specific Pathogen Free |
| iNOS | Inducible nitric oxide synthase |
| KEGG | Kyoto encyclopedia of genes and genomes |
| FC | Fold change |
| DEPs | Differentially expressed proteins |
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.E.; Hossain, A.T.; Nair, H.; Chisti, M.J.; Dockrell, D.; Arifeen, S.E.; Campbell, H. Prevalence of hypoxaemia in children with pneumonia in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Glob. Health 2022, 10, e348–e359. [Google Scholar] [CrossRef]
- Swedberg, E.; Shah, R.; Sadruddin, S.; Soeripto, J. Saving young children from forgotten killer: Pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L861–L862. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, T.; Zhao, Z.; Norback, D.; Wang, X.; Li, Y.; Deng, Q.; Lu, C.; Zhang, X.; Zheng, X.; et al. Prevalence, risk factors, impact and management of pneumonia among preschool children in Chinese seven cities: A cross-sectional study with interrupted time series analysis. BMC Med. 2023, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.T.; Chisti, M.J.; Pavia, A.T. Prevention and Control of Childhood Pneumonia and Diarrhea. Pediatr. Clin. N. Am. 2016, 63, 67–79. [Google Scholar] [CrossRef]
- King, C.; McCollum, E.D. Trends in the global burden of paediatric lower respiratory infections. Lancet Infect. Dis. 2020, 20, 4–5. [Google Scholar] [CrossRef]
- GBD 2019 LRI Collaborators. Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: Results from the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2022, 22, 1626–1647. [Google Scholar] [CrossRef]
- Dong, F.; Yu, H.; Ma, J.; Wu, L.; Liu, T.; Lv, G.; Zhen, J.; Li, X.; Lewith, G.; Gu, X. Exploring association between gastrointestinal heat retention syndrome and recurrent respiratory tract infections in children: A prospective cohort study. BMC Complement Altern. Med. 2016, 16, 82. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Khanh Hoang, N.; Maegawa, E.; Murakami, S.; Schaffer, S.W.; Ito, T. N-Chlorotaurine Reduces the Lung and Systemic Inflammation in LPS-Induced Pneumonia in High Fat Diet-Induced Obese Mice. Metabolites 2022, 12, 349. [Google Scholar] [CrossRef]
- Bai, C.; Liu, T.; Xu, J.; Ma, X.; Huang, L.; Liu, S.; Yu, H.; Chen, J.; Gu, X. Effect of High Calorie Diet on Intestinal Flora in LPS-Induced Pneumonia Rats. Sci. Rep. 2020, 10, 1701. [Google Scholar] [CrossRef]
- Liu, H.; Bai, C.; Xian, F.; Liu, S.; Long, C.; Hu, L.; Liu, T.; Gu, X. A high-calorie diet aggravates LPS-induced pneumonia by disturbing the gut microbiota and Th17/Treg balance. J. Leukoc. Biol. 2022, 112, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Bajinka, O.; Tang, Z.; Qiu, X.; Tan, Y. Lung-brain axis: Metabolomics and pathological changes in lungs and brain of respiratory syncytial virus-infected mice. J. Med. Virol. 2022, 94, 5885–5893. [Google Scholar] [CrossRef] [PubMed]
- Villalba, N.; Ma, Y.; Gahan, S.A.; Joly-Amado, A.; Spence, S.; Yang, X.; Nash, K.R.; Yuan, S.Y. Lung infection by Pseudomonas aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice. J. Neuroinflammation 2023, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Bajinka, O.; Simbilyabo, L.; Tan, T.; Jabang, J.; Saleem, S.A. Lung-brain axis. Crit. Rev. Microbiol. 2022, 48, 257–269. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, T.; Yu, Y.; Li, M.; Sun, X.; Song, W.; Wang, Y.; Zhang, C.; Fu, F. The etiology of poststroke-depression: A hypothesis involving HPA axis. Biomed. Pharmacother. 2022, 151, 113146. [Google Scholar] [CrossRef]
- Gil, N.L.; Azevedo, G.A.; Balbino, A.M.; Silva, M.M.; Carvalho, M.H.C.; Akamine, E.H.; Keller, A.C.; Landgraf, R.G.; Landgraf, M.A. Intrauterine growth restriction leads to a high-corticosterone producing offspring: An implication for pulmonary infection susceptibility. Life Sci. 2021, 281, 119764. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA—Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Rummel, C. Inflammatory transcription factors as activation markers and functional readouts in immune-to-brain communication. Brain Behav. Immun. 2016, 54, 1–14. [Google Scholar] [CrossRef]
- Mussa, B.M.; Srivastava, A.; Verberne, A.J.M. COVID-19 and Neurological Impairment: Hypothalamic Circuits and Beyond. Viruses 2021, 13, 498. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Ortiz-Valladares, M.; Pedraza-Medina, R.; Pinto-González, M.F.; Muñiz, J.G.; Gonzalez-Perez, O.; Moy-López, N.A. Neurobiological approaches of high-fat diet intake in early development and their impact on mood disorders in adulthood: A systematic review. Neurosci. Biobehav. Rev. 2021, 129, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Hryhorczuk, C.; Décarie-Spain, L.; Sharma, S.; Daneault, C.; Rosiers, C.D.; Alquier, T.; Fulton, S. Saturated high-fat feeding independent of obesity alters hypothalamus-pituitary-adrenal axis function but not anxiety-like behaviour. Psychoneuroendocrinology 2017, 83, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kosutova, P.; Mikolka, P.; Kolomaznik, M.; Balentova, S.; Calkovska, A.; Mokra, D. Effects of S-Nitroso-N-Acetyl-Penicillamine (SNAP) on Inflammation, Lung Tissue Apoptosis and iNOS Activity in a Rabbit Model of Acute Lung Injury. Adv. Exp. Med. Biol. 2016, 935, 13–23. [Google Scholar] [PubMed]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Z.J.; Yu, X.; Wang, P. Dietary regulation in health and disease. Signal Transduct. Target Ther. 2022, 7, 252. [Google Scholar] [CrossRef]
- Reedy, J.; Krebs-Smith, S.M. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J. Am. Diet Assoc. 2010, 110, 1477–1484. [Google Scholar] [CrossRef]
- Wambogo, E.A.; O’Connor, L.E.; Shams-White, M.M.; Herrick, K.A.; Reedy, J. Top sources and trends in consumption of total energy and energy from solid fats and added sugars among youth aged 2–18 years: United States 2009–2018. Am. J. Clin. Nutr. 2022, 116, 1779–1789. [Google Scholar] [CrossRef]
- Saavedra, J.M. The Changing Landscape of Children’s Diet and Nutrition: New Threats, New Opportunities. Ann. Nutr. Metab. 2022, 78, 40–50. [Google Scholar] [CrossRef]
- Cheng, J.; Pan, T.; Ye, G.H.; Liu, Q. Calorie controlled diet for chronic asthma. Cochrane Database Syst. Rev. 2005, 3, CD004674. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, W.; Lin, F.; Li, W.; Wang, P.; Liao, G.; Zhang, L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain-Lung Axis. Cell. Mol. Neurobiol. 2023, 43, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Rensen, N.; Gemke, R.J.; van Dalen, E.C.; Rotteveel, J.; Kaspers, G.J. Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database Syst. Rev. 2017, 11, CD008727. [Google Scholar] [CrossRef]
- Auvinen, H.E.; Romijn, J.A.; Biermasz, N.R.; Havekes, L.M.; Smit, J.W.; Rensen, P.C.; Pereira, A.M. Effects of high fat diet on the Basal activity of the hypothalamus-pituitary-adrenal axis in mice: A systematic review. Horm. Metab. Res. 2011, 43, 899–906. [Google Scholar] [CrossRef]
- McNeilly, A.D.; Stewart, C.A.; Sutherland, C.; Balfour, D.J. High fat feeding is associated with stimulation of the hypothalamic-pituitary-adrenal axis and reduced anxiety in the rat. Psychoneuroendocrinology 2015, 52, 272–280. [Google Scholar] [CrossRef]
- Auvinen, H.E.; Romijn, J.A.; Biermasz, N.R.; Pijl, H.; Havekes, L.M.; Smit, J.W.; Rensen, P.C.; Pereira, A.M. The effects of high fat diet on the basal activity of the hypothalamus-pituitary-adrenal axis in mice. J. Endocrinol. 2012, 214, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Keller-Wood, M. Hypothalamic-Pituitary-Adrenal Axis-Feedback Control. Compr. Physiol. 2015, 5, 1161–1182. [Google Scholar] [CrossRef]
- Öztürk, K.H.; Ünal, G.Ö.; Doğuç, D.K.; Toğay, V.A.; Koşar, P.A.; Sezik, M. Hypothalamic NR3C1 DNA methylation in rats exposed to prenatal stress. Mol. Biol. Rep. 2022, 49, 7921–7928. [Google Scholar] [CrossRef]
- Chalfun, G.; Reis, M.M.; de Oliveira, M.B.G.; de Araújo Brasil, A.; Dos Santos Salú, M.; da Cunha, A.J.L.A.; Prata-Barbosa, A.; de Magalhães-Barbosa, M.C. Perinatal stress and methylation of the NR3C1 gene in newborns: Systematic review. Epigenetics 2022, 17, 1003–1019. [Google Scholar] [CrossRef]
- Ferraù, F.; Ceccato, F.; Cannavò, S.; Scaroni, C. What we have to know about corticosteroids use during Sars-Cov-2 infection. J. Endocrinol. Investig. 2021, 44, 693–701. [Google Scholar] [CrossRef]
- Gaspar, J.M.; Mendes, N.F.; Corrêa-da-Silva, F.; Lima-Junior, J.C.; Gaspar, R.C.; Ropelle, E.R.; Araujo, E.P.; Carvalho, H.M.; Velloso, L.A. Downregulation of HIF complex in the hypothalamus exacerbates diet-induced obesity. Brain Behav. Immun. 2018, 73, 550–561. [Google Scholar] [CrossRef]
- Voit, R.A.; Sankaran, V.G. Stabilizing HIF to Ameliorate Anemia. Cell 2020, 180, 6. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhang, Y.; Zhu, C.; Chen, H.; Sun, J. Metabolic Regulation of Hypoxia-Inducible Factors in Hypothalamus. Front. Endocrinol. 2021, 12, 650284. [Google Scholar] [CrossRef]
- Marchi, D.; Santhakumar, K.; Markham, E.; Li, N.; Storbeck, K.H.; Krone, N.; Cunliffe, V.T.; van Eeden, F.J.M. Bidirectional crosstalk between Hypoxia-Inducible Factor and glucocorticoid signalling in zebrafish larvae. PLoS Genet. 2020, 16, e1008757. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Ma, Z.Q.; Tang, J.W.; Zhao, Y.; Wang, X.; Liu, Q.; Wang, P.P.; John, C.; Chen, X.Q.; Du, J.Z. The integration of multiple signaling pathways provides for bidirectional control of CRHR1 gene transcription in rat pituitary cell during hypoxia. Mol. Cell Endocrinol. 2017, 454, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Bai, C.; Zhu, J.; Su, C.; Wang, Y.; Liu, H.; Li, Q.; Qin, X.; Gu, X.; Liu, T. Pharmacological mechanisms of Ma Xing Shi Gan Decoction in treating influenza virus-induced pneumonia: Intestinal microbiota and pulmonary glycolysis. Front. Pharmacol. 2024, 15, 1404021. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Taheri, S.; Liu, K.J.; Shi, H. Hypoxia-inducible factor 1 contributes to N-acetylcysteine’s protection in stroke. Free Radic. Biol. Med. 2014, 68, 8–21. [Google Scholar] [CrossRef]
- Liu, X.; Ding, H.; Li, X.; Deng, Y.; Liu, X.; Wang, K.; Wen, M.; Chen, S.; Jiang, W.; Zeng, H. Hypercapnia Exacerbates the Blood-Brain Barrier Disruption Via Promoting HIF-1a Nuclear Translocation in the Astrocytes of the Hippocampus: Implication in Further Cognitive Impairment in Hypoxemic Adult Rats. Neurochem. Res. 2020, 45, 1674–1689. [Google Scholar] [CrossRef]

| Rat Maintenance Fodder | High-Calorie Fodder | |
|---|---|---|
| Energy, kJ/100 g | 340 | 1828.12 |
| Moisture | 9.7% | 10.10% |
| Crude protein | 16.26% | 13.73% |
| Crude fat | 4.62% | 16.10% |
| Carbohydrate | 5.25% | 58.80% |
| Sodium | 0.22% | 0.44% |
| Fiber | 2.3% | 1.8% |
| Crude ash | 6.2% | 0.83% |
| Calcium | 1–1.8% | 0.95% |
| Total phosphorus | 0.6–1.2% | 0.38% |
| Group | N | Feed (1–6 Days) | Atomization (4–6 Days) |
|---|---|---|---|
| N | 6 | Rat maintenance fodder | Physiologic saline |
| P | 6 | Rat maintenance fodder | LPS solution |
| G | 6 | High-calorie fodder | Physiologic saline |
| GP | 6 | High-calorie fodder | LPS solution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, H.; Bai, C.; Jiang, L.; Su, C.; Qin, X.; Liu, T.; Gu, X. A High-Calorie Diet Aggravates Lipopolysaccharide-Induced Pulmonary Inflammation in Juvenile Rats via Hypothalamic-Pituitary-Adrenal Axis-Related Pathways. Int. J. Mol. Sci. 2025, 26, 6554. https://doi.org/10.3390/ijms26146554
Li Q, Liu H, Bai C, Jiang L, Su C, Qin X, Liu T, Gu X. A High-Calorie Diet Aggravates Lipopolysaccharide-Induced Pulmonary Inflammation in Juvenile Rats via Hypothalamic-Pituitary-Adrenal Axis-Related Pathways. International Journal of Molecular Sciences. 2025; 26(14):6554. https://doi.org/10.3390/ijms26146554
Chicago/Turabian StyleLi, Qianqian, Hui Liu, Chen Bai, Lin Jiang, Chen Su, Xueying Qin, Tiegang Liu, and Xiaohong Gu. 2025. "A High-Calorie Diet Aggravates Lipopolysaccharide-Induced Pulmonary Inflammation in Juvenile Rats via Hypothalamic-Pituitary-Adrenal Axis-Related Pathways" International Journal of Molecular Sciences 26, no. 14: 6554. https://doi.org/10.3390/ijms26146554
APA StyleLi, Q., Liu, H., Bai, C., Jiang, L., Su, C., Qin, X., Liu, T., & Gu, X. (2025). A High-Calorie Diet Aggravates Lipopolysaccharide-Induced Pulmonary Inflammation in Juvenile Rats via Hypothalamic-Pituitary-Adrenal Axis-Related Pathways. International Journal of Molecular Sciences, 26(14), 6554. https://doi.org/10.3390/ijms26146554






