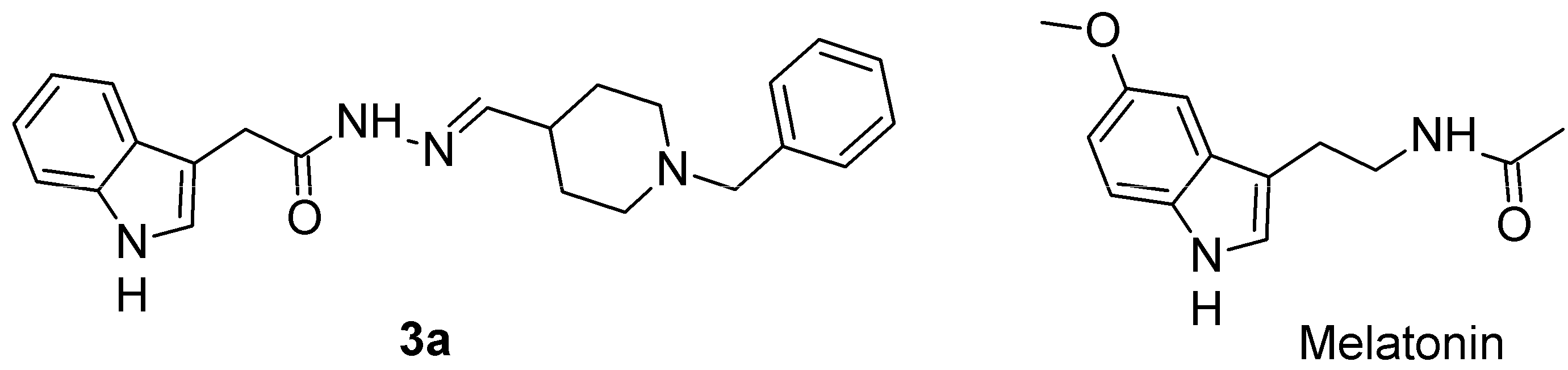
The Novel Melatonin Analog Containing Donepezil Fragment Prevents Cognitive Impairments and Associated Oxidative Stress in a Hybrid Rat Model of Melatonin Deficiency and icvAβ1-42
Abstract
1. Introduction
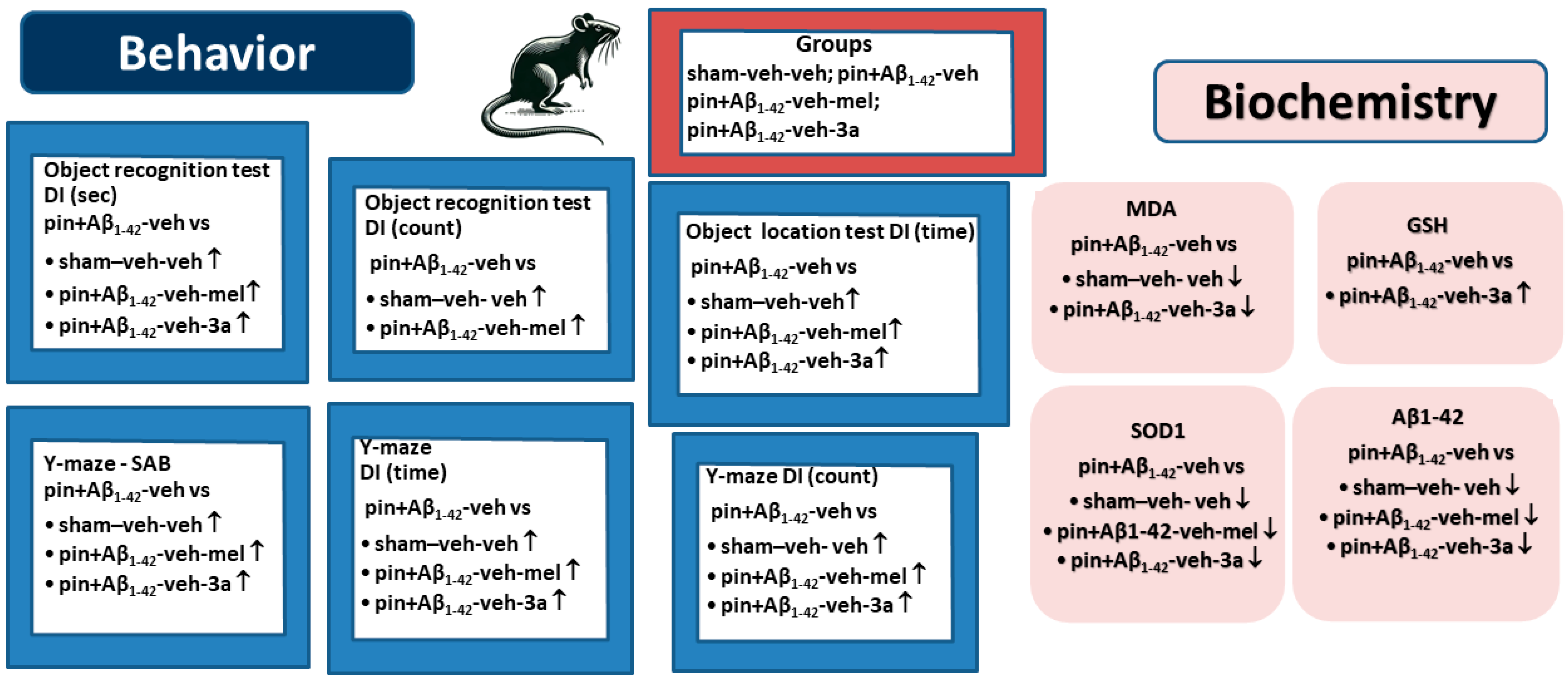
2. Results
2.1. The Compound 3a Prevented Impairment of Working and Short-Term Memory Induced by Concomitant Melatonin Deficiency and Aβ1-42 Infusion
2.1.1. Working and Short-Term Spatial Memory, Measured in Two Protocols of Y Maze
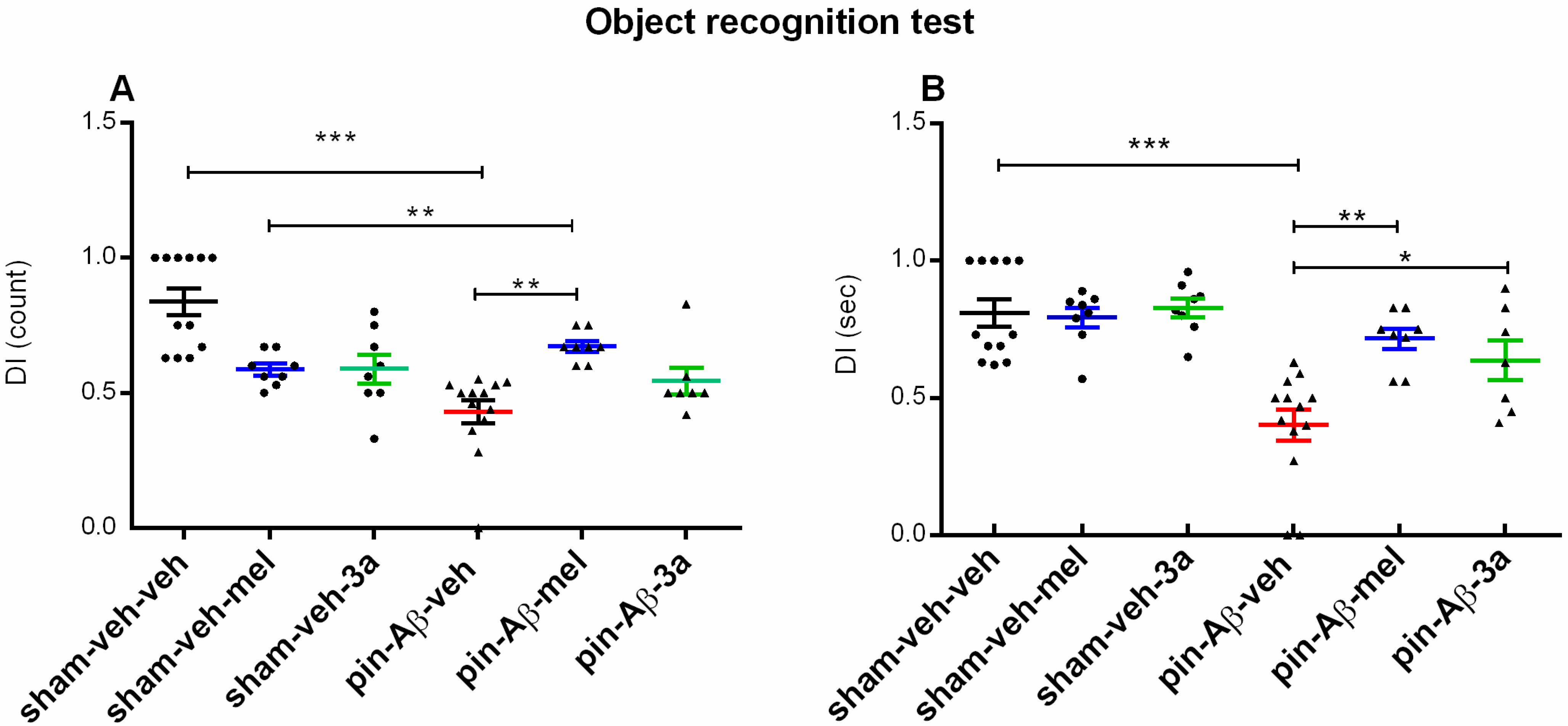
2.1.2. Short-Term Recognition Memory, Evaluated in the Object Recognition Test (ORT)
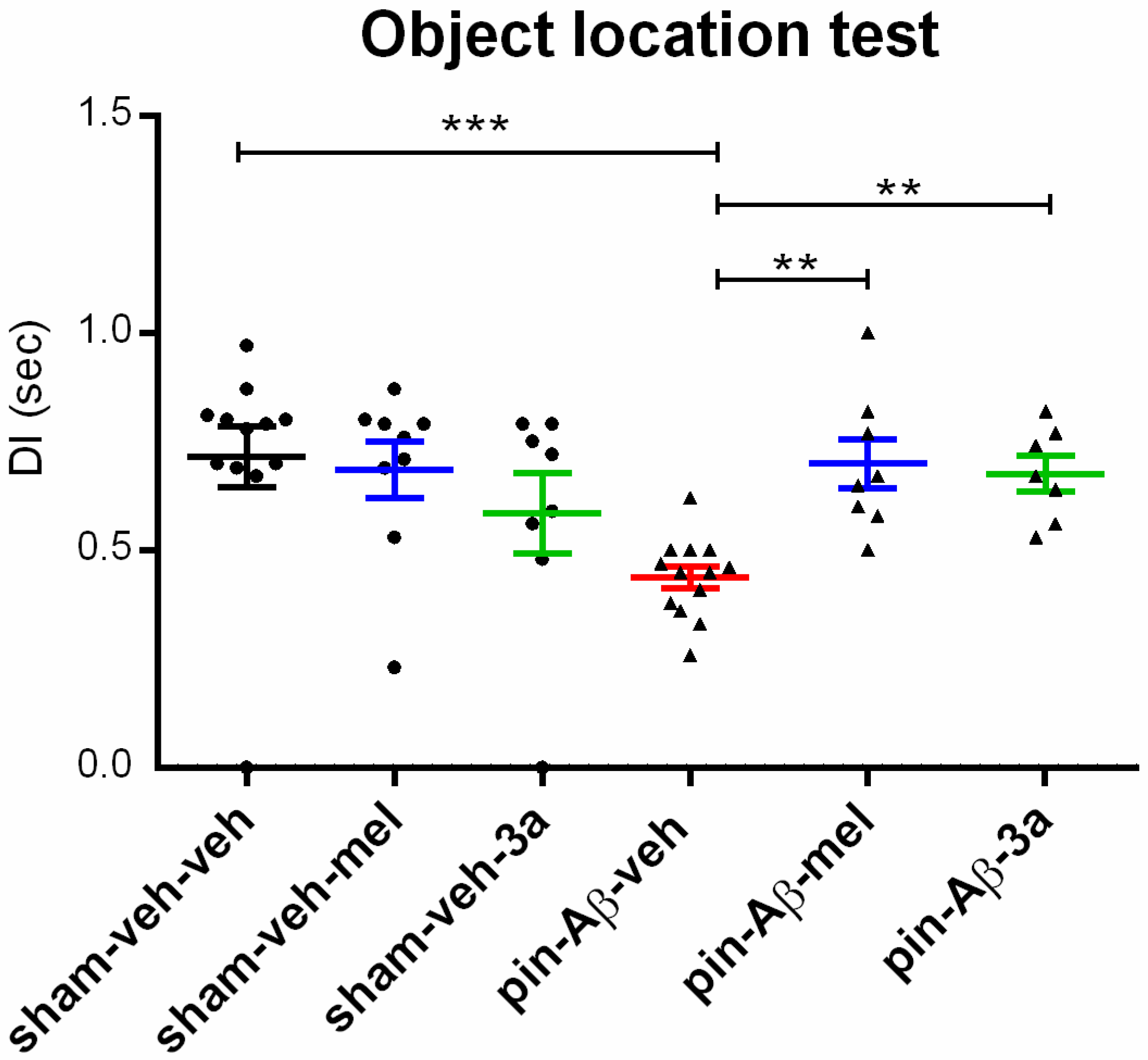
2.1.3. Short-Term Spatial Memory, Assessed in the Object Location Test (OLT)
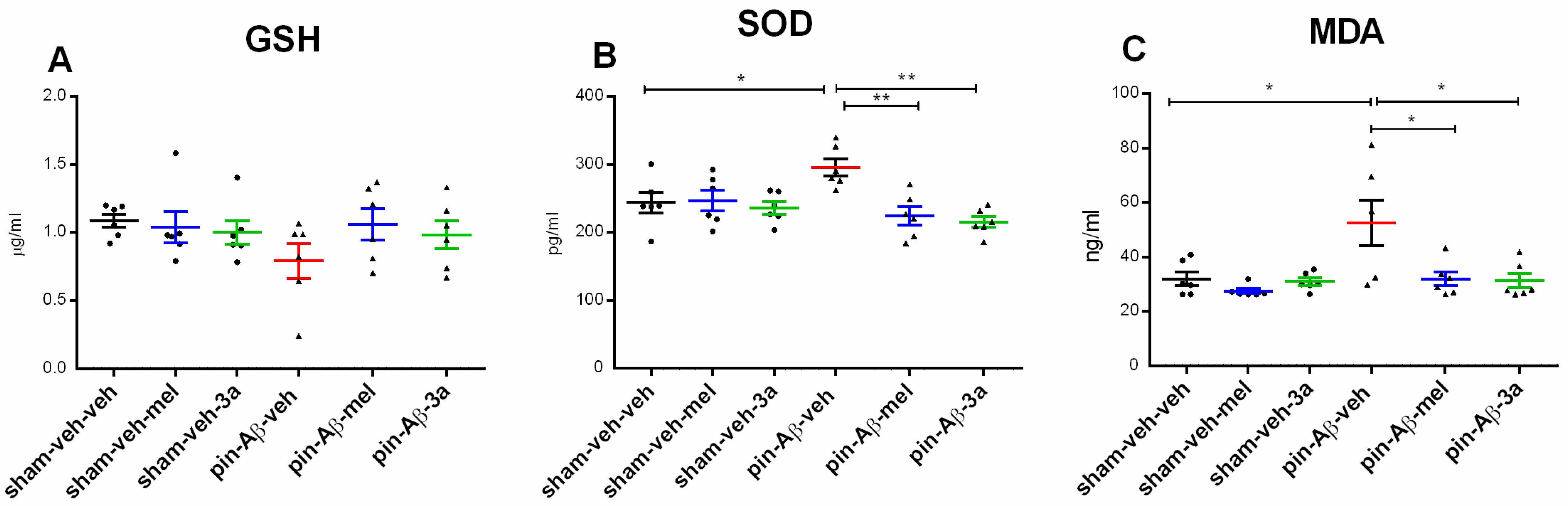
2.2. Markers of Oxidative Stress, Glutathione (GSH), Superoxide Dismutase (SOD) and Malondialdehyde (MDA), Measured by ELISA
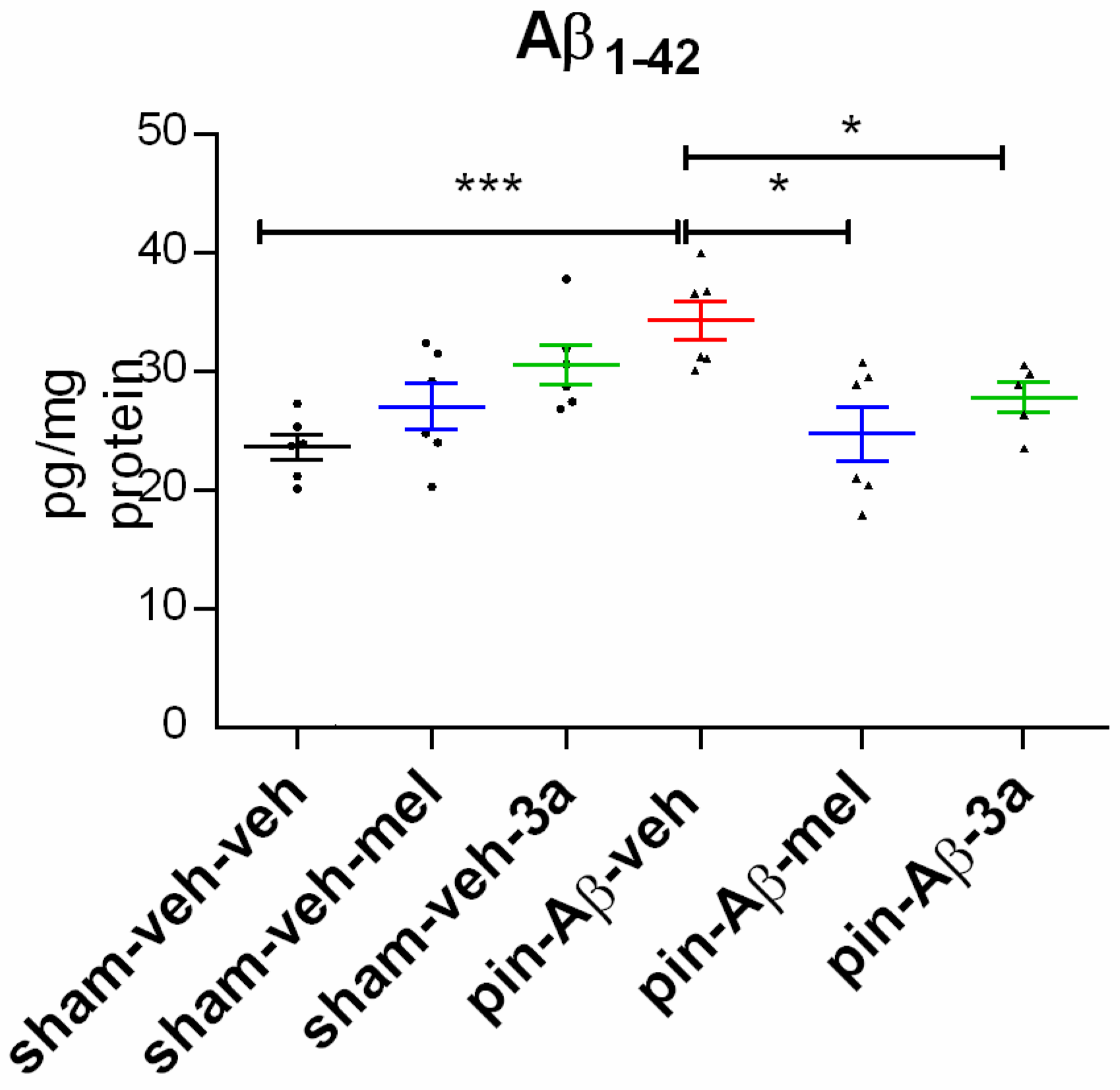
2.3. Expression of Amyloid β1-42, Measured in the Hippocampus by ELISA
3. Discussion
Limitations of the Study and the Future Directions
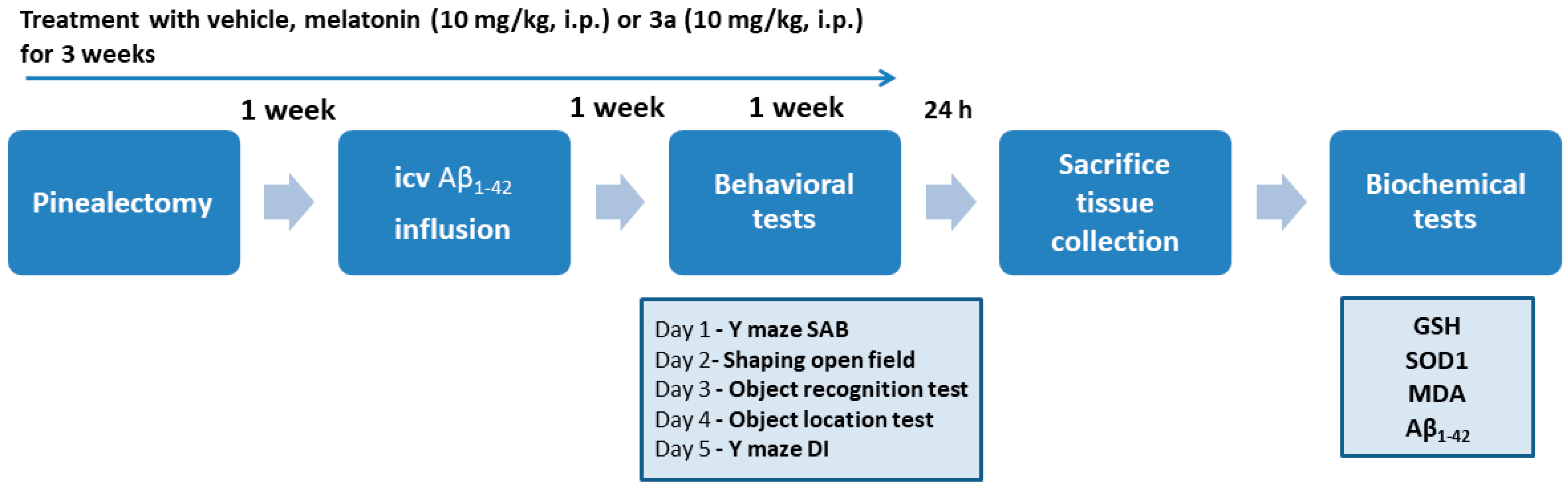
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animals
4.3. Experimental Groups
4.4. Surgery Procedure and Icv Infusion of Aβ1-42
4.5. Behavioral Tests
4.5.1. Y-Maze
Protocol 1
Protocol 2
4.5.2. Object Recognition Test
4.5.3. Object Location Test
4.6. Tissue Homogenization and Biochemistry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid beta |
| Aβ1-42 | Amyloid beta peptide 1–42 |
| BBB | Blood-brain barrier |
| BChE | Butyrylcholinesterase |
| CCL-1 | Continuous cell line 1 (normal murine fibroblasts) |
| DI | Discrimination Index |
| ELISA | Enzyme-linked immunosorbent assay |
| FO | Familiar object/location |
| FRAP | Ferric reducing antioxidant power |
| GSH | Glutathione |
| icv | intracerebroventricular |
| i.p. | intraperitoneal |
| MDA | Malondialdehyde |
| MTDs | Multi-target drugs |
| NFTs | Neurofibrillary tangles |
| NO | Novel object/location |
| NMDA | N-methyl-D-aspartate |
| ORT | Object recognition test |
| OLT | Object location test |
| PAMPA-BBB | Parallel artificial membrane permeability assay-blood-brain barrier |
| ROS | Reactive oxygen species |
| SAB | Spontaneous alternation behavior |
| SOD | Superoxide dismutase |
| SM | Sphingomyelin |
References
- Mak, W. Impacts of Alzheimer’s Disease on Identity and Potential Therapeutic Approaches for Mitigating Identity Loss. IJHSR 2024, 6, 93–104. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. Alzheimer’s Disease: Exploring the Landscape of Cognitive Decline. ACS Chem. Neurosci. 2024, 15, 3800–3827. [Google Scholar] [CrossRef]
- Testo, A.A.; Roundy, G.; Dumas, J.A. Cognitive Decline in Alzheimer’s Disease. In Neurobiology of Alzheimer’s Disease; Kidd, E.J., Newhouse, P.A., Eds.; Current Topics in Behavioral Neurosciences; Springer Nature: Cham, Switzerland, 2024; Volume 69, pp. 181–195. ISBN 978-3-031-84919-0. [Google Scholar]
- Zvěřová, M. Clinical Aspects of Alzheimer’s Disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Y.; Fan, H. Review on Pathogenesis and Treatment of Alzheimer’s Disease. Dev. Dyn. 2025, 254, 296–309. [Google Scholar] [CrossRef]
- Engelhardt, E.; Resende, E.D.P.F.; Gomes, K.B. Physiopathological Mechanisms Underlying Alzheimer’s Disease: A Narrative Review. Dement. Neuropsychol. 2024, 18, e2024VR01. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Chen, G. Loss of Cholinergic and Monoaminergic Afferents in APPswe/PS1ΔE9 Transgenic Mouse Model of Cerebral Amyloidosis Preferentially Occurs Near Amyloid Plaques. Int. J. Mol. Sci. 2024, 25, 5004. [Google Scholar] [CrossRef]
- Moore, A.H.; O’Banion, M.K. Neuroinflammation and Anti-Inflammatory Therapy for Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2002, 54, 1627–1656. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative Damage in Neurodegeneration: Roles in the Pathogenesis and Progression of Alzheimer Disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef]
- Anitha, K.; Singh, M.K.; Kohat, K.; Sri Varshini, T.; Chenchula, S.; Padmavathi, R.; Amerneni, L.S.; Vishnu Vardhan, K.; Mythili Bai, K.; Chavan, M.R.; et al. Recent Insights into the Neurobiology of Alzheimer’s Disease and Advanced Treatment Strategies. Mol. Neurobiol. 2025, 62, 2314–2332. [Google Scholar] [CrossRef]
- Bang, S. Diagnosis and Treatment of Alzheimer’s Disease Targeting the Amyloid Beta (Aβ). IJHSR 2025, 7, 15–19. [Google Scholar] [CrossRef]
- Panza, F.; Dibello, V.; Sardone, R.; Zupo, R.; Castellana, F.; Leccisotti, I.; Moretti, M.C.; Altamura, M.; Bellomo, A.; Daniele, A.; et al. Successes and Failures: The Latest Advances in the Clinical Development of Amyloid–β–Targeting Monoclonal Antibodies for Treating Alzheimer’s Disease. Expert Opin. Biol. Ther. 2025, 25, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.T.; Stoyanov, B.P.; Simeonova, R. New Insights into the Development of Donepezil-Based Hybrid and Natural Molecules as Multi-Target Drug Agents for Alzheimer’s Disease Treatment. Molecules 2024, 29, 5314. [Google Scholar] [CrossRef]
- Sirimaharaj, N.; Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. Unveiling the Protective Roles of Melatonin on Glial Cells in the Battle Against Alzheimer’s Disease—Insights from In Vivo and In Vitro Studies. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, P.; Bendlin, B.B.; Zetterberg, H.; De Felice, F.; Tan, X.; Benedict, C. Melatonin: A Potential Nighttime Guardian against Alzheimer’s. Mol. Psychiatry 2025, 30, 237–250. [Google Scholar] [CrossRef]
- Breitner, C.S. The Role of Anti-Inflammatory Drugs in the Prevention and Treatment of Alzheimer’s Disease. Annu. Rev. Med. 1996, 47, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Shabani Sadr, N.K.; Bakhtiarzadeh, F.; Shahpasand, K.; Mirnajafi-Zadeh, J.; Behmanesh, M. Improving Effects of Melatonin on Memory and Synaptic Potentiation in a Mouse Model of Alzheimer’s-like Disease: The Involvement of Glutamate Homeostasis and mGluRs Receptors. Behav. Brain Funct. 2025, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Tzoneva, R.; Georgieva, I.; Ivanova, N.; Uzunova, V.; Nenchovska, Z.; Apostolova, S.; Stoyanova, T.; Tchekalarova, J. The Role of Melatonin on Behavioral Changes and Concomitant Oxidative Stress in icvAβ1-42 Rat Model with Pinealectomy. Int. J. Mol. Sci. 2021, 22, 12763. [Google Scholar] [CrossRef]
- Angelova, V.T.; Georgiev, B.; Pencheva, T.; Pajeva, I.; Rangelov, M.; Todorova, N.; Zheleva-Dimitrova, D.; Kalcheva-Yovkova, E.; Valkova, I.V.; Vassilev, N.; et al. Design, Synthesis, In Silico Studies and In Vitro Evaluation of New Indole- and/or Donepezil-like Hybrids as Multitarget-Directed Agents for Alzheimer’s Disease. Pharmaceuticals 2023, 16, 1194. [Google Scholar] [CrossRef]
- Polis, B.; Samson, A.O. Addressing the Discrepancies Between Animal Models and Human Alzheimer’s Disease Pathology: Implications for Translational Research. JAD 2024, 98, 1199–1218. [Google Scholar] [CrossRef]
- Serrano, C.; Bolea, R.; Lyahyai, J.; Filali, H.; Varona, L.; Marcos-Carcavilla, A.; Acín, C.; Calvo, J.H.; Serrano, M.; Badiola, J.J.; et al. Changes in HSP Gene and Protein Expression in Natural Scrapie with Brain Damage. Vet. Res. 2011, 42, 13. [Google Scholar] [CrossRef]
- Nous, A.; Engelborghs, S.; Smolders, I. Melatonin Levels in the Alzheimer’s Disease Continuum: A Systematic Review. Alzheimers Res. Ther. 2021, 13, 52. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Furio, A.M.; Brusco, L.I. Clinical Aspects of Melatonin Intervention in Alzheimers Disease Progression. Curr. Neuropharmacol. 2010, 8, 218–227. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Nenchovska, Z.; Kortenska, L.; Uzunova, V.; Georgieva, I.; Tzoneva, R. Impact of Melatonin Deficit on Emotional Status and Oxidative Stress-Induced Changes in Sphingomyelin and Cholesterol Level in Young Adult, Mature, and Aged Rats. Int. J. Mol. Sci. 2022, 23, 2809. [Google Scholar] [CrossRef]
- Georgieva, I.; Tchekalarova, J.; Nenchovska, Z.; Kortenska, L.; Tzoneva, R. Melatonin Supplementation Alleviates Impaired Spatial Memory by Influencing Aβ1-42 Metabolism via γ-Secretase in the icvAβ1-42 Rat Model with Pinealectomy. Int. J. Mol. Sci. 2024, 25, 10294. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Ivanova, P.; Krushovlieva, D.; Kortenska, L.; Angelova, V.T. Protective Effect of the Novel Melatonin Analogue Containing Donepezil Fragment on Memory Impairment via MT/ERK/CREB Signaling in the Hippocampus in a Rat Model of Pinealectomy and Subsequent Aβ1-42 Infusion. Int. J. Mol. Sci. 2024, 25, 1867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yau, H.-Y.; Wong, W.-Y.; Li, R.A.; Huang, Y.; Yao, X. Role of TRPM2 in H2O2-Induced Cell Apoptosis in Endothelial Cells. PLoS ONE 2012, 7, e43186. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin Prevents Cognitive Impairments by Ameliorating Oxidative Stress and Neuroinflammation in Rat Model of Sporadic Dementia of Alzheimer Type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. Oxidative Stress in the Progression of Alzheimer Disease in the Frontal Cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Franco-Bocanegra, D.; Toral Rios, D.; Campos-Peña, V. Early Onset Alzheimer’s Disease and Oxidative Stress. Oxidative Med. Cell. Longev. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Lee, H.-P.; Pancholi, N.; Esposito, L.; Previll, L.A.; Wang, X.; Zhu, X.; Smith, M.A.; Lee, H. Early Induction of Oxidative Stress in Mouse Model of Alzheimer Disease with Reduced Mitochondrial Superoxide Dismutase Activity. PLoS ONE 2012, 7, e28033. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.; Moreira, P.I.; Proença, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain Oxidative Stress in a Triple-Transgenic Mouse Model of Alzheimer Disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Hrischev, P.; Ivanova, P.; Boyadjiev, N.; Georgieva, K. Metabolic Footprint in Young, Middle-Aged and Elderly Rats with Melatonin Deficit. Physiol. Behav. 2022, 250, 113786. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, P.; Kortenska, L.; Angelova, V.T.; Tchekalarova, J. The Novel Melatonin Analog Containing Donepezil Fragment Prevents Cognitive Impairments and Associated Oxidative Stress in a Hybrid Rat Model of Melatonin Deficiency and icvAβ1-42. Int. J. Mol. Sci. 2025, 26, 6553. https://doi.org/10.3390/ijms26146553
Ivanova P, Kortenska L, Angelova VT, Tchekalarova J. The Novel Melatonin Analog Containing Donepezil Fragment Prevents Cognitive Impairments and Associated Oxidative Stress in a Hybrid Rat Model of Melatonin Deficiency and icvAβ1-42. International Journal of Molecular Sciences. 2025; 26(14):6553. https://doi.org/10.3390/ijms26146553
Chicago/Turabian StyleIvanova, Petya, Lidia Kortenska, Violina T. Angelova, and Jana Tchekalarova. 2025. "The Novel Melatonin Analog Containing Donepezil Fragment Prevents Cognitive Impairments and Associated Oxidative Stress in a Hybrid Rat Model of Melatonin Deficiency and icvAβ1-42" International Journal of Molecular Sciences 26, no. 14: 6553. https://doi.org/10.3390/ijms26146553
APA StyleIvanova, P., Kortenska, L., Angelova, V. T., & Tchekalarova, J. (2025). The Novel Melatonin Analog Containing Donepezil Fragment Prevents Cognitive Impairments and Associated Oxidative Stress in a Hybrid Rat Model of Melatonin Deficiency and icvAβ1-42. International Journal of Molecular Sciences, 26(14), 6553. https://doi.org/10.3390/ijms26146553







