Overcoming the Reversibility in the Diels–Alder Reaction of Bio-Based Electron-Poor Furans with Maleimides Through Liquid-to-Solid Phase Transition
Abstract
1. Introduction
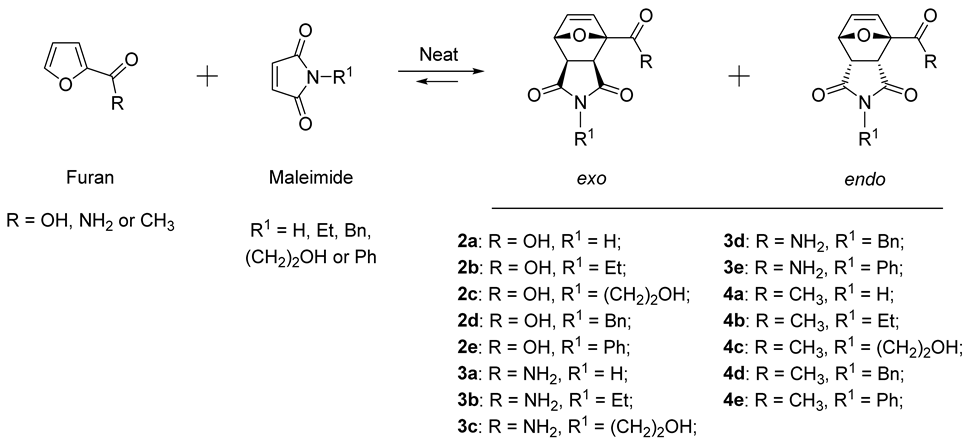
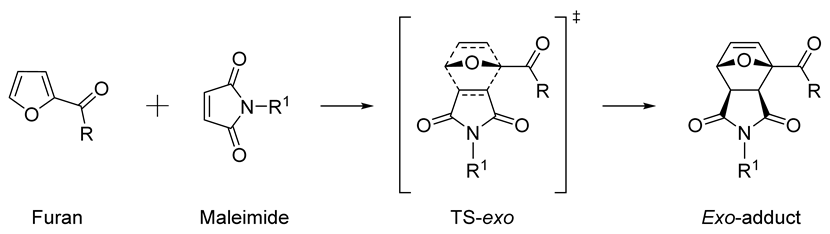
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Optimization of the fmDA Reaction Conditions
3.3. Typical Solvent-Free Protocol for Synthesis of Exo-Adducts
3.4. Study of the Retro-DA Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DA | Diels–Alder |
| DFT | density functional theory |
| DMSO | dimethyl sulfoxide |
| DSC | differential scanning calorimetry |
| fmDA | furan–maleimide Diels–Alder |
| HEM | N-(2-hydroxyethyl)maleimide |
| HPM | N-(4-hydroxyphenyl)maleimide |
| NMR | nuclear magnetic resonance |
| PXRD | powder X-ray diffraction |
| ROMP | ring-opening metathesis polymerization |
| ssNMR | solid-state nuclear magnetic resonance |
References
- Briou, B.; Ameduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Funel, J.A.; Abele, S. Industrial applications of the Diels-Alder reaction. Angew. Chem. Int. Ed. 2013, 52, 3822–3863. [Google Scholar] [CrossRef]
- Shukla, H.; Promcharoen, P.; Poonsawat, T.; Chakarawet, K.; Chumkaeo, P.; Somsook, E. Diels-Alder Cycloaddition of 2,5-Bis(hydroxymethyl)furan (BHMF) and N-Phenylmaleimide Derivatives. ACS Omega 2024, 9, 36380–36388. [Google Scholar] [CrossRef]
- Skolia, E.; Kokotos, C.G. Direct Diels-Alder Reaction of Biomass-Derived Furfurol with Maleimides in a Bio-Based Green Solvent. Eur. J. Org. Chem. 2024, 27, e202400105. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Y.; Cui, H. Recent advances in the synthesis of 2-Furoic Acid and 2,5-furandicarboxylic acid from furfural. ChemSusChem 2024, 18, e202401390. [Google Scholar]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(hydroxymethyl)furfural. ChemistryOpen 2020, 9, 1135–1148. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Mitrev, Y.N.; Simeonov, S.P.; Afonso, C.A.M. Going Beyond the Limits of the Biorenewable Platform: Sodium Dithionite-Promoted Stabilization of 5-Hydroxymethylfurfural. ChemSusChem 2018, 11, 1612–1616. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent Advances in Catalytic Conversion of Biomass to 2,5-Furandicarboxylic Acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Thoma, C.; Konnerth, J.; Sailer-Kronlachner, W.; Solt, P.; Rosenau, T.; van Herwijnen, H.W.G. Current situation of the challenging scale-up development of hydroxymethylfurfural production. ChemSusChem 2020, 13, 3544–3564. [Google Scholar]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, eaap9722. [Google Scholar] [CrossRef]
- Galkin, K.I.; Krivodaeva, E.A.; Romashov, L.V.; Zalesskiy, S.S.; Kachala, V.V.; Burykina, J.V.; Ananikov, V.P. Critical Influence of 5-Hydroxymethylfurfural Aging and Decomposition on the Utility of Biomass Conversion in Organic Synthesis. Angew. Chem. Int. Ed. 2016, 55, 8338–8342. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. Furan Polymers: State of the Art and Perspectives. Macromol. Mater. Eng. 2022, 307, 2100902. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Li, Y.; Zhang, H.; Li, H.; Yang, S. Advances in Diels–Alder/aromatization of biomass furan derivatives towards renewable aromatic hydrocarbons. Catal. Sci. Technol. 2022, 12, 1902–1921. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Averochkin, G.M.; Ananikov, V.P. Biobased C6-Furans in Organic Synthesis and Industry: Cycloaddition Chemistry as a Key Approach to Aromatic Building Blocks. ACS Sustain. Chem. Eng. 2021, 9, 3011–3042. [Google Scholar] [CrossRef]
- Ravasco, J.; Gomes, R.F.A. Recent Advances on Diels-Alder-Driven Preparation of Bio-Based Aromatics. ChemSusChem 2021, 14, 3047. [Google Scholar] [CrossRef]
- Liu, D.-H.; He, H.-L.; Zhang, Y.-B.; Li, Z. Oxidative Aromatization of Biobased Chemicals to Benzene Derivatives through Tandem Catalysis. ACS Sustain. Chem. Eng. 2020, 8, 14322–14329. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Genuino, H.C.; van der Waal, J.C.; de Jong, E.; Weckhuysen, B.M.; van Haveren, J.; Bruijnincx, P.C.; van Es, D.S. A Facile Solid-Phase Route to Renewable Aromatic Chemicals from Biobased Furanics. Angew. Chem. Int. Ed. 2016, 55, 1368–1371. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Genuino, H.C.; Sliwa, M.; van der Waal, J.C.; de Jong, E.; van Haveren, J.; Weckhuysen, B.M.; Bruijnincx, P.C.; van Es, D.S. Substituted Phthalic Anhydrides from Biobased Furanics: A New Approach to Renewable Aromatics. ChemSusChem 2015, 8, 3052–3056. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, A.; Fraile, J.M.; Gómez, M.V.; Mayoral, J.A.; Moreno, A.; Saiz, A.; Vázquez, E. Synergy between Heterogeneous Catalysis and Microwave Irradiation in an Efficient One-Pot Synthesis of Benzene Derivatives via Ring-Opening of Diels-Alder Cycloadducts of Substituted Furans. Synlett 2001, 2001, 0753–0756. [Google Scholar] [CrossRef]
- Santos, C.S.; Rodini Mattioli, R.; Soares Baptista, J.; Menezes da Silva, V.H.; Browne, D.L.; Pastre, J.C. Nitrogenated aromatics from chitin. Green Chem. 2023, 25, 5059–5067. [Google Scholar] [CrossRef]
- Settle, A.E.; Berstis, L.; Rorrer, N.A.; Román-Leshkov, Y.; Beckham, G.T.; Richards, R.M.; Vardon, D.R. Heterogeneous Diels–Alder catalysis for biomass-derived aromatic compounds. Green Chem. 2017, 19, 3468–3492. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, Z.; He, S.; Yang, H.; Atia, H.; Abdel-Mageed, A.M.; Wohlrab, S.; Barath, E.; Tin, S.; Heeres, H.J.; et al. Benzenoid Aromatics from Renewable Resources. Chem. Rev. 2024, 124, 10701–10876. [Google Scholar] [CrossRef]
- Chang, H.; Huber, G.W.; Dumesic, J.A. Chemical-Switching Strategy for Synthesis and Controlled Release of Norcantharimides from a Biomass-Derived Chemical. ChemSusChem 2020, 13, 5213–5219. [Google Scholar] [CrossRef]
- Naz, F.; Wu, Y.; Zhang, N.; Yang, Z.; Yu, C. Anticancer Attributes of Cantharidin: Involved Molecular Mechanisms and Pathways. Molecules 2020, 25, 3279. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Galkin, K.I.; Gordeev, E.G.; Ananikov, V.P. Efficient route for the construction of polycyclic systems from bioderived HMF. Green Chem. 2017, 19, 4858–4864. [Google Scholar] [CrossRef]
- Galkin, K.I.; Kucherov, F.A.; Markov, O.N.; Egorova, K.S.; Posvyatenko, A.V.; Ananikov, V.P. Facile Chemical Access to Biologically Active Norcantharidin Derivatives from Biomass. Molecules 2017, 22, 2210. [Google Scholar] [CrossRef]
- Yasir, M.; Liu, P.; Markwart, J.C.; Suraeva, O.; Wurm, F.R.; Smart, J.; Lattuada, M.; Kilbinger, A.F.M. One-Step Ring Opening Metathesis Block-Like Copolymers and their Compositional Analysis by a Novel Retardation Technique. Angew. Chem. Int. Ed. 2020, 59, 13597–13601. [Google Scholar] [CrossRef]
- Yasir, M.; Liu, P.; Tennie, I.K.; Kilbinger, A.F.M. Catalytic living ring-opening metathesis polymerization with Grubbs’ second- and third-generation catalysts. Nat. Chem. 2019, 11, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yasir, M.; Kilbinger, A.F.M. Catalytic Living Ring Opening Metathesis Polymerisation: The Importance of Ring Strain in Chain Transfer Agents. Angew. Chem. Int. Ed. 2019, 58, 15278–15282. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Galkin, K.I.; Sandulenko, I.V.; Polezhaev, A.V. Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems. Processes 2021, 10, 30. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Mondal, P.; Jana, G.; Pal, T.S.; Chattaraj, P.K.; Singha, N.K. Self-healable functional polymers based on Diels–Alder ‘click chemistry’ involving substituted furan and triazolinedione derivatives: A simple and very fast approach. Polym. Chem. 2021, 12, 6283–6290. [Google Scholar] [CrossRef]
- Cioc, R.C.; Crockatt, M.; van der Waal, J.C.; Bruijnincx, P.C.A. The Interplay between Kinetics and Thermodynamics in Furan Diels-Alder Chemistry for Sustainable Chemicals Production. Angew. Chem. Int. Ed. 2022, 61, e202114720. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes. Int. J. Mol. Sci. 2021, 22, 11856. [Google Scholar] [CrossRef]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Boutelle, R.C.; Northrop, B.H. Substituent effects on the reversibility of furan-maleimide cycloadditions. J. Org. Chem. 2011, 76, 7994–8002. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, J.; Faba, L.; Ordóñez, S. Role of Reactant Alkylation Grade in the Selectivity and Stability of Furan–Alkene Diels–Alder Reactions. ACS Sustain. Chem. Eng. 2022, 10, 3057–3065. [Google Scholar] [CrossRef]
- Burton, R.R.; Tam, W. Ruthenium-catalyzed [2 + 2] cycloadditions between C1-substituted 7-oxanorbornadienes and alkynes. Tetrahedron Lett. 2006, 47, 7185–7189. [Google Scholar] [CrossRef]
- Raheem, M.-A.; Tam, W. Synthesis of C1-Substituted 7-Oxanorbornadienes. Synth. Commun. 2013, 43, 260–267. [Google Scholar] [CrossRef]
- Burton, R.R.; Tam, W. Study on the reactivity of oxabicyclic alkenes in ruthenium-catalyzed [2 + 2] cycloadditions. J. Org. Chem. 2007, 72, 7333–7336. [Google Scholar] [CrossRef]
- Koehler, K.C.; Durackova, A.; Kloxin, C.J.; Bowman, C.N. Kinetic and thermodynamic measurements for the facile property prediction of diels–alder-conjugated material behavior. AIChE J. 2012, 58, 3545–3552. [Google Scholar] [CrossRef]
- Cioc, R.C.; Smak, T.J.; Crockatt, M.; van der Waal, J.C.; Bruijnincx, P.C.A. Furoic acid and derivatives as atypical dienes in Diels-Alder reactions. Green Chem. 2021, 23, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.L.; Wood, J.M.; Almeida, R.G.; Berry, N.G.; da Silva Junior, E.N.; Bower, J.F. The Synthesis and Reactivity of Naphthoquinonynes. Angew. Chem. Int. Ed. 2024, 63, e202400188. [Google Scholar] [CrossRef]
- Lucke, D.; Campbell, A.S.; Petzold, M.; Sarpong, R. Access to Naphthoic Acid Derivatives through an Oxabenzonorbornadiene Rearrangement. Org. Lett. 2023, 25, 7349–7353. [Google Scholar] [CrossRef]
- Serum, E.M.; Selvakumar, S.; Zimmermann, N.; Sibi, M.P. Valorization of 2,5-furandicarboxylic acid. Diels–Alder reactions with benzyne. Green Chem. 2018, 20, 1448–1454. [Google Scholar] [CrossRef]
- Serum, E.M.; Sutton, C.A.; Renner, A.C.; Dawn, D.; Sibi, M.P. New AB type monomers from lignocellulosic biomass. Pure Appl. Chem. 2019, 91, 389–396. [Google Scholar] [CrossRef]
- van Scodeller, I.; De Oliveira Vigier, K.; Muller, E.; Ma, C.; Guegan, F.; Wischert, R.; Jerome, F. A Combined Experimental-Theoretical Study on Diels-Alder Reaction with Bio-Based Furfural: Towards Renewable Aromatics. ChemSusChem 2021, 14, 313–323. [Google Scholar] [CrossRef]
- Higson, S.; Subrizi, F.; Sheppard, T.D.; Hailes, H.C. Chemical cascades in water for the synthesis of functionalized aromatics from furfurals. Green Chem. 2016, 18, 1855–1858. [Google Scholar] [CrossRef]
- Potts, K.T.; Walsh, E.B. Furfural dimethylhydrazone: A versatile diene for arene cycloaromatization. J. Org. Chem. 2002, 49, 4099–4101. [Google Scholar] [CrossRef]
- Cioc, R.C.; Lutz, M.; Pidko, E.A.; Crockatt, M.; van der Waal, J.C.; Bruijnincx, P.C.A. Direct Diels–Alder reactions of furfural derivatives with maleimides. Green Chem. 2021, 23, 367–373. [Google Scholar] [CrossRef]
- Rammohan, A.; Krinochkin, A.P.; Khasanov, A.F.; Kopchuk, D.S.; Zyryanov, G.V. Sustainable Solvent-Free Diels–Alder Approaches in the Development of Constructive Heterocycles and Functionalized Materials: A Review. Top. Curr. Chem. 2022, 380, 43. [Google Scholar] [CrossRef]
- Jegat, C.; Mignard, N. Effect of the polymer matrix on the thermal behaviour of a furan-maleimide type adduct in the molten state. Polym. Bull. 2008, 60, 799–808. [Google Scholar] [CrossRef][Green Version]
- Bastin, L.D.; Nigam, M.; Martinus, S.; Maloney, J.E.; Benyack, L.L.; Gainer, B. Synthesis of substituted N-phenylmaleimides and use in a Diels-Alder reaction: A green multi-step synthesis for an undergraduate organic chemistry laboratory. Green Chem. Lett. Rev. 2019, 12, 127–135. [Google Scholar] [CrossRef]
- Pereira, J.G.; Ravasco, J.M.J.M.; Vale, J.R.; Queda, F.; Gomes, R.F.A. A direct Diels–Alder reaction of chitin derived 3-acetamido-5-acetylfuran. Green Chem. 2022, 24, 7131–7136. [Google Scholar] [CrossRef]
- Kang, K.H.; Chang, Y.-W.; Sabzi, M. Reprocessable and healable ethylene copolymer/f-rGO nanocomposites crosslinked by Diels-Alder adducts with infrared- and thermo-responsive behavior. Polym. Test. 2021, 104, 107383. [Google Scholar] [CrossRef]
- Gaina, V.; Ursache, O.; Gaina, C.; Timpu, D.; Tanasa, F. New Bismaleimide-Silica Hybrid Materials: A Critical Assessment of Properties in Correlation with the Method of Synthesis. Polym.-Plast. Technol. Eng. 2016, 55, 784–801. [Google Scholar] [CrossRef]
- Ax, J.; Wenz, G. Thermoreversible Networks by Diels–Alder Reaction of Cellulose Furoates With Bismaleimides. Macromol. Chem. Phys. 2012, 213, 182–186. [Google Scholar] [CrossRef]
- Berson, J.A.; Swidler, R. A Synthesis of Maleimide. J. Am. Chem. Soc. 1954, 76, 2835–2836. [Google Scholar] [CrossRef]
- Smyth, D.G.; Nagamatsu, A.; Fruton, J.S. Some Reactions of N-Ethylmaleimide1. J. Am. Chem. Soc. 1960, 82, 4600–4604. [Google Scholar] [CrossRef]
- Heath, W.H.; Palmieri, F.; Adams, J.R.; Long, B.K.; Chute, J.; Holcombe, T.W.; Zieren, S.; Truitt, M.J.; White, J.L.; Willson, C.G. Degradable Cross-Linkers and Strippable Imaging Materials for Step-and-Flash Imprint Lithography. Macromolecules 2008, 41, 719–726. [Google Scholar] [CrossRef]
- Tawney, P.O.; Snyder, R.H.; Conger, R.P.; Leibbrand, K.A.; Stiteler, C.H.; Williams, A.R. The Chemistry of Maleimide and Its Derivatives. II. Maleimide and N-Methylolmaleimide. J. Org. Chem. 1961, 26, 15–21. [Google Scholar] [CrossRef]
- Cava, M.P.; Deana, A.A.; Muth, K.; Mitchell, M.J. N-Phenylmaleimide. Org. Synth. 1961, 41, 93. [Google Scholar]
- Mohammed, I.A.; Mustapha, A. Synthesis of new azo compounds based on N-(4-hydroxypheneyl)maleimide and N-(4-methylpheneyl)maleimide. Molecules 2010, 15, 7498–7509. [Google Scholar] [CrossRef]
- Clavier, H.; Broggi, J.; Nolan, S.P. Ring-Rearrangement Metathesis (RRM) Mediated by Ruthenium-Indenylidene Complexes. Eur. J. Org. Chem. 2010, 2010, 937–943. [Google Scholar] [CrossRef]
- Román, E.; Gil, M.; Luque-Agudo, V.; Serrano, J. Expeditious ‘On-Water’ Cycloaddition between N-Substituted Maleimides and Furans. Synlett 2014, 25, 2179–2183. [Google Scholar] [CrossRef]
- Oakley, J.H.; Hughes, T.J.; Graham, B.F.; Marsh, K.N.; May, E.F. Determination of melting temperatures in hydrocarbon mixtures by differential scanning calorimetry. J. Chem. Thermodyn. 2017, 108, 59–70. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, S.L. Advances in simultaneous DSC-FTIR microspectroscopy for rapid solid-state chemical stability studies: Some dipeptide drugs as examples. Adv. Drug Delivery Rev. 2012, 64, 461–478. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K.; Benelmir, R.; Dahmani, A. DSC measurement and prediction of phase diagrams for binary mixtures of energetic materials’ stabilizers. Thermochim. Acta 2013, 565, 8–16. [Google Scholar] [CrossRef]
- Canadell, J.; Fischer, H.; De With, G.; van Benthem, R.A.T.M. Stereoisomeric effects in thermo-remendable polymer networks based on Diels-Alder crosslink reactions. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Rickborn, B. The Retro-Diels-Alder Reaction Part I. C–C Dienophiles. Org. React. 1998, 52, 1–393. [Google Scholar]
- Sanchez, A.; Pedroso, E.; Grandas, A. Maleimide-dimethylfuran exo adducts: Effective maleimide protection in the synthesis of oligonucleotide conjugates. Org. Lett. 2011, 13, 4364–4367. [Google Scholar] [CrossRef]
- Ma, X.-Y.; He, Y.; Lu, T.-T.; Lu, M. Conversion of aldoximes into nitriles catalyzed by simple transition metal salt of the fourth period in acetonitrile. Tetrahedron 2013, 69, 2560–2564. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef]
- van Wüllen, C. Molecular density functional calculations in the regular relativistic approximation: Method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys. 1998, 109, 392–399. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
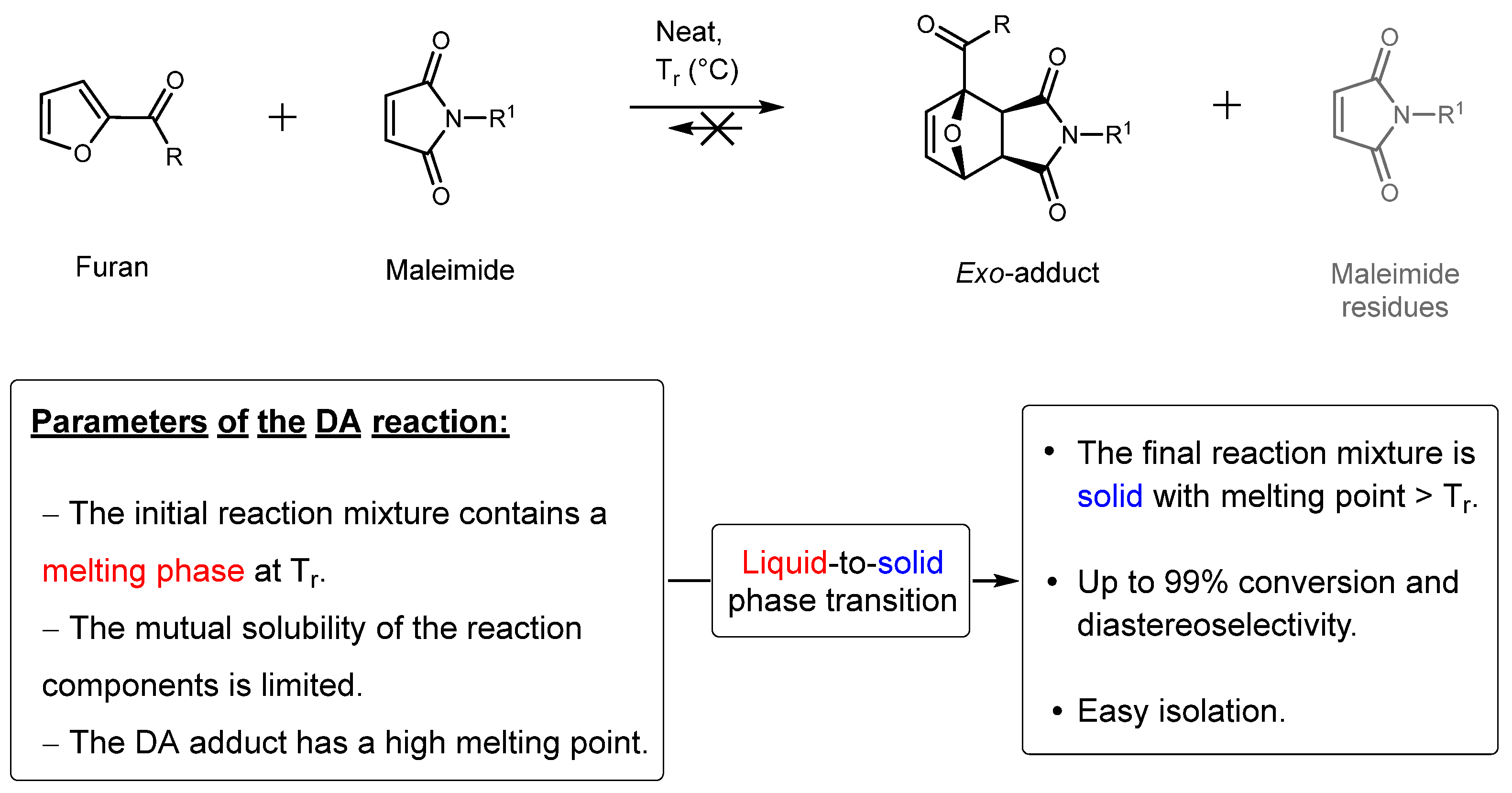
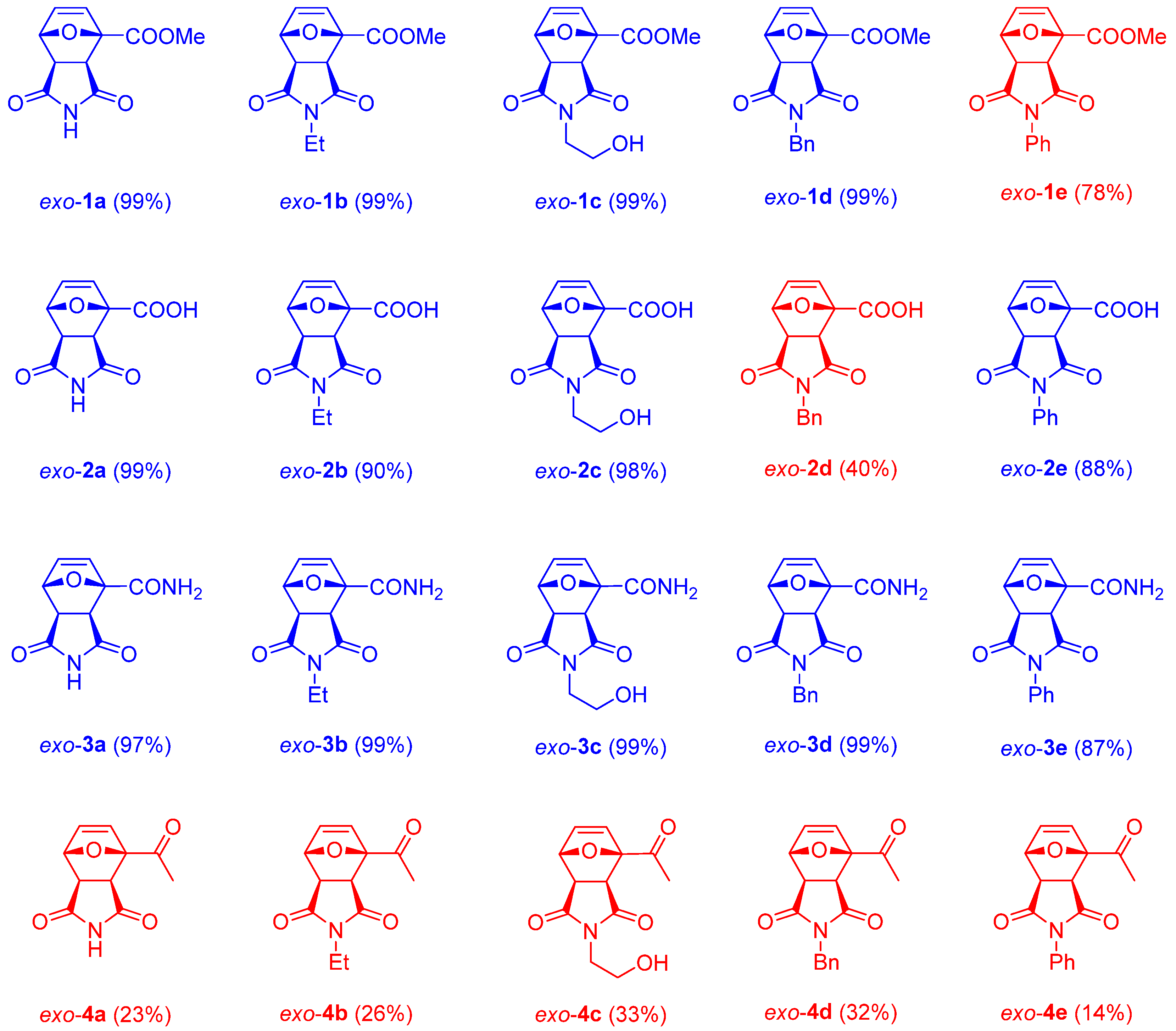
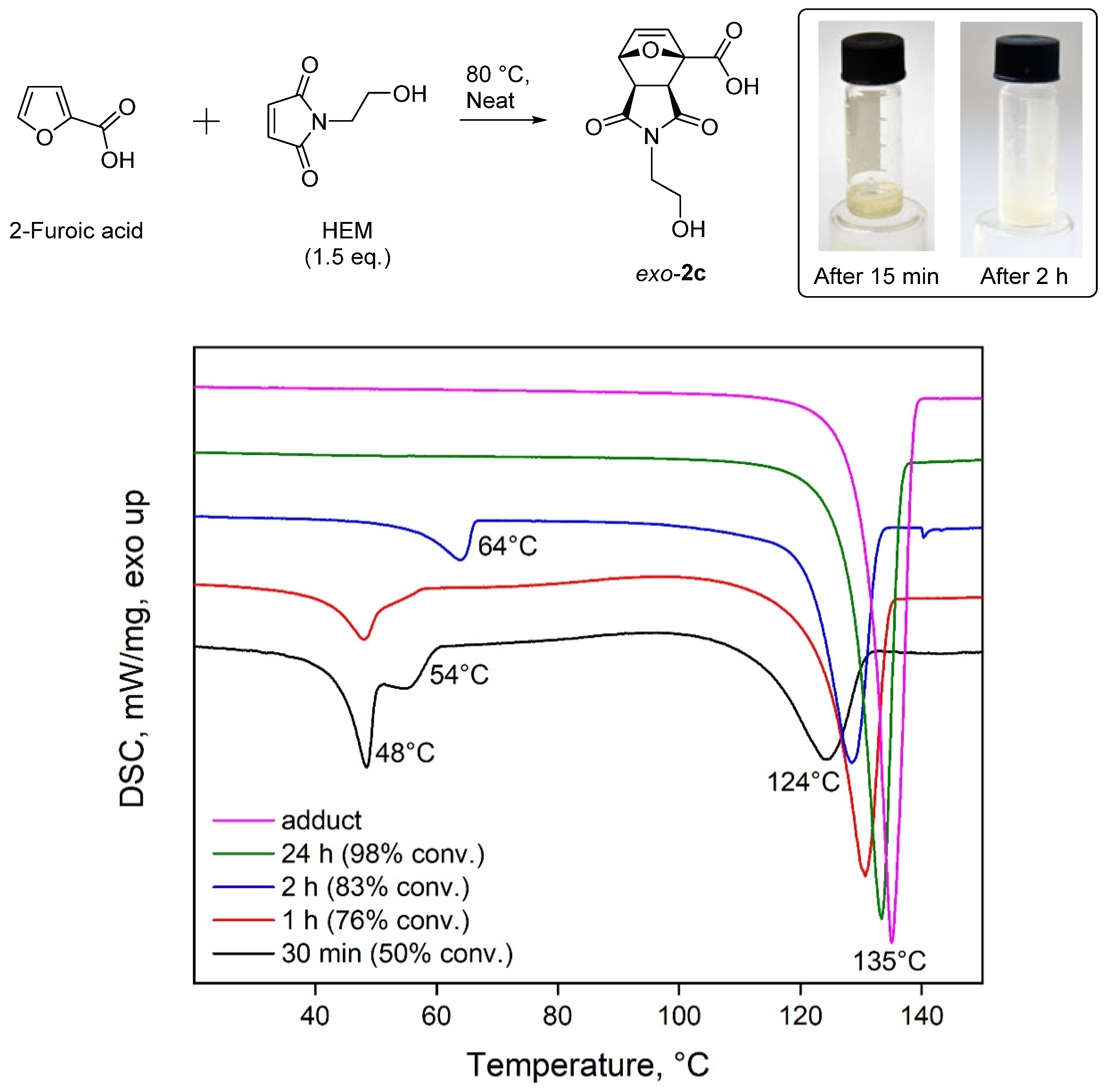
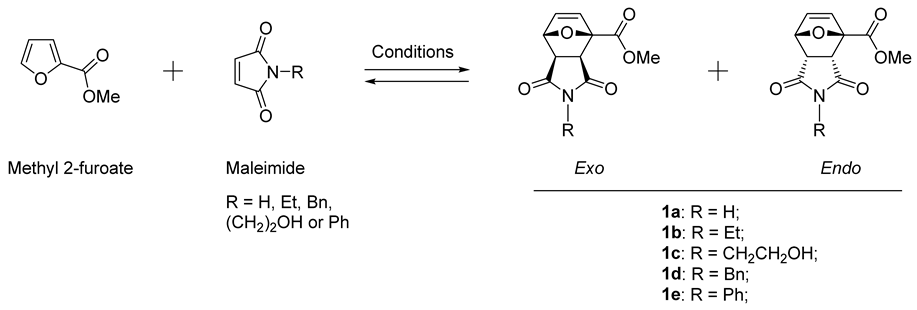 | |||||||
|---|---|---|---|---|---|---|---|
| № | R | Eq. of Maleimide | T, °C | Time, h | Solvent | Product (Initial Furan/endo/exo Ratio, %) 1 | Aggregate State 2 |
| 1 | H | 1.5 | 60 | 24 | Acetone | 1a (92.5/0.5/7) | - |
| 2 | Et | 1.5 | 60 | 24 | Acetone | 1b (98/trace/2) | - |
| 3 | H | 1.5 | 60 | 24 | H2O | 1a (65/3/32) | - |
| 4 | Et | 1.5 | 60 | 24 | H2O | 1b (61/4/35) | - |
| 5 | H | 1.5 | 50 | 24 | - | 1a (42/1/57) | Solid + melt |
| 6 | H | 1.1 | 60 | 24 | - | 1a (1/0/99)/87 3 | Solid |
| 7 | H | 1.25 | 60 | 24 | - | 1a (15/0/85) | Solid + melt |
| 8 | H | 1.25 | 60 | 72 | - | 1a (2/0/98) | Solid |
| 9 | H | 1.5 | 60 | 24 | - | 1a (3/0/97) | Solid |
| 10 | H | 1.5 | 80 | 24 | - | 1a (70/1/29) | Melt |
| 11 | Et | 1.1 | 60 | 72 | - | 1b (1/0/99)/86 3 | Solid |
| 12 | Et | 1.25 | 60 | 24 | - | 1b (18.5/0.5/81) | Melt |
| 13 | Et | 1.25 | 60 | 72 | - | 1b (1/0/98) | Solid |
| 14 | Et | 1.5 | 60 | 24 | - | 1b (39/2/59) | Melt |
| 15 | Et | 1.5 | 60 | 72 | - | 1b (2/0/98) | Solid |
| 16 | Et | 1.5 | 80 | 24 | - | 1b (58/2/40) | Melt |
| 17 | (CH2)2OH | 1.25 | 60 | 72 | - | 1c (1/0/99)/79 3 | Solid |
| 18 | (CH2)2OH | 1.25 | 60 | 72 | - | 1c (31/2/67) | Melt |
| 19 | Bn | 1.25 | 60 | 72 | - | 1d (1/0/99)/91 3 | Solid |
| 20 | Ph | 1.25 | 60 | 72 | - | 1e (21/1/78)/67 3 | Melt |
 | ||||||
|---|---|---|---|---|---|---|
| № | R | R 1 | Eq. of Maleimide | Conditions | Product (Initial Furan/endo/exo Ratio, %) 1 | Aggregate State 2 |
| 1 | OH | H | 1.5 | 80 °C, 24 h | 2a (1/0/99)/78 3 | Solid |
| 2 | OH | Et | 1.25 | 60 °C, 24 h | 2b (46/2/51) | Melt |
| 3 | OH | Et | 1.25 | 60 °C, 72 h | 2b (10/0/90)76 3 | Solid +melt |
| 4 | OH | Et | 1.5 | 60 °C, 72 h | 2b (42/2/55) | Melt |
| 5 | OH | Et | 1.5 | 80 °C, 24 h | 2b (65/2/32) | Melt |
| 6 | OH | (CH2)2OH | 1.5 | 80 °C, 24 h | 2c (2/0/98)/92 3 | Solid |
| 7 | OH | Bn | 1.5 | 80 °C, 24 h | 2d (60/0/40) 4 | Melt |
| 8 | OH | Ph | 1.25 | 80 °C, 24 h | 2e (22/0/88)77 3 | Solid |
| 9 | NH2 | H | 1.1 | 80 °C, 24 h | 3a (3/0/97)/85 3 | Solid |
| 10 | NH2 | Et | 1.25 | 80 °C, 24 h | 3b (3/0/97) | Solid |
| 11 | NH2 | Et | 1.5 | 80 °C, 24 h | 3b (1/0/99)/88 3 | Solid |
| 12 | NH2 | Et | 1.5 | 100 °C, 6 h | 3b (2/0/98) | Solid |
| 13 | NH2 | (CH2)2OH | 1.25 | 80 °C, 24 h | 3c (1/0/99)/87 3 | Solid |
| 14 | NH2 | Bn | 1.25 | 80 °C, 24 h | 3d (1/0/99)/87 3 | Solid |
| 15 | NH2 | Ph | 1.25 | 80 °C, 24 h | 3e (11/0/89)/81 3 | Solid |
| 16 | CH3 | H | 1.5 | 60 °C, 72 h | 4a (76/1/23)/16 3 | Melt |
| 17 | CH3 | Et | 1.5 | 60 °C, 72 h | 4b (72/2/26)/18 3 | Melt |
| 18 | CH3 | (CH2)2OH | 1.25 | 60 °C, 72 h | 4c (66/1/33) 4 | Melt |
| 19 | CH3 | Bn | 1.25 | 60 °C, 72 h | 4d (67/1/32) 4 | Melt |
| 20 | CH3 | Ph | 1.25 | 60 °C, 72 h | 4e (85.5/0.5/14) 4 | Melt |
 | ||||
|---|---|---|---|---|
| № | Reaction Participant | ΔE, kcal/mol | ΔHo298K, kcal/mol | ΔGo298K, kcal/mol |
| 1 | Methyl 2-furoate | 0.0 | 0.0 | 0.0 |
| 2 | TS-exo-1b | 10.5 | 10.0 | 25.2 |
| 3 | Exo-1b | −8.0 | −8.9 | 7.1 |
| 4 | TS-exo-1e | 10.3 | 9.9 | 25.3 |
| 5 | Exo-1e | −7.1 | −7.9 | 8.2 |
| 6 | 2-Acetylfuran | 0.0 | 0.0 | 0.0 |
| 7 | TS-exo-4b | 11.5 | 11.0 | 25.9 |
| 8 | Exo-4b | −7.1 | −7.9 | 7.9 |
| 9 | TS-exo-4e | 10.7 | 10.3 | 25.5 |
| 10 | Exo-4e | −6.1 | −6.9 | 9.1 |
| № | Adduct | Solubility in Ethyl Acetate | Solubility in Tetrahydrofuran | Solubility in Acetone |
|---|---|---|---|---|
| 1 | exo-1a | − | − | − |
| 2 | exo-1d | − | + | + |
| 3 | exo-1e | − | − | + |
| 4 | exo-2a | − | − | − |
| 5 | exo-2b | + | + | + |
| 6 | exo-2e | − | + | + |
| 7 | exo-3d | − | − | − |
| 8 | exo-3e | − | − | − |
| 9 | exo-4a | + | + | + |
| 10 | exo-4b | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galkin, K.I.; Zakharova, D.V.; Aysin, R.R.; Danshina, A.A.; Pak, A.M.; Sandulenko, I.V.; Novikov, R.A.; Egorova, K.S. Overcoming the Reversibility in the Diels–Alder Reaction of Bio-Based Electron-Poor Furans with Maleimides Through Liquid-to-Solid Phase Transition. Int. J. Mol. Sci. 2025, 26, 6550. https://doi.org/10.3390/ijms26146550
Galkin KI, Zakharova DV, Aysin RR, Danshina AA, Pak AM, Sandulenko IV, Novikov RA, Egorova KS. Overcoming the Reversibility in the Diels–Alder Reaction of Bio-Based Electron-Poor Furans with Maleimides Through Liquid-to-Solid Phase Transition. International Journal of Molecular Sciences. 2025; 26(14):6550. https://doi.org/10.3390/ijms26146550
Chicago/Turabian StyleGalkin, Konstantin I., Daria V. Zakharova, Rinat R. Aysin, Anastasia A. Danshina, Alexandra M. Pak, Irina V. Sandulenko, Roman A. Novikov, and Ksenia S. Egorova. 2025. "Overcoming the Reversibility in the Diels–Alder Reaction of Bio-Based Electron-Poor Furans with Maleimides Through Liquid-to-Solid Phase Transition" International Journal of Molecular Sciences 26, no. 14: 6550. https://doi.org/10.3390/ijms26146550
APA StyleGalkin, K. I., Zakharova, D. V., Aysin, R. R., Danshina, A. A., Pak, A. M., Sandulenko, I. V., Novikov, R. A., & Egorova, K. S. (2025). Overcoming the Reversibility in the Diels–Alder Reaction of Bio-Based Electron-Poor Furans with Maleimides Through Liquid-to-Solid Phase Transition. International Journal of Molecular Sciences, 26(14), 6550. https://doi.org/10.3390/ijms26146550







