Brain and Immune System: Intercellular Communication During Homeostasis and Neuroimmunomodulation upon Dysfunction
Abstract
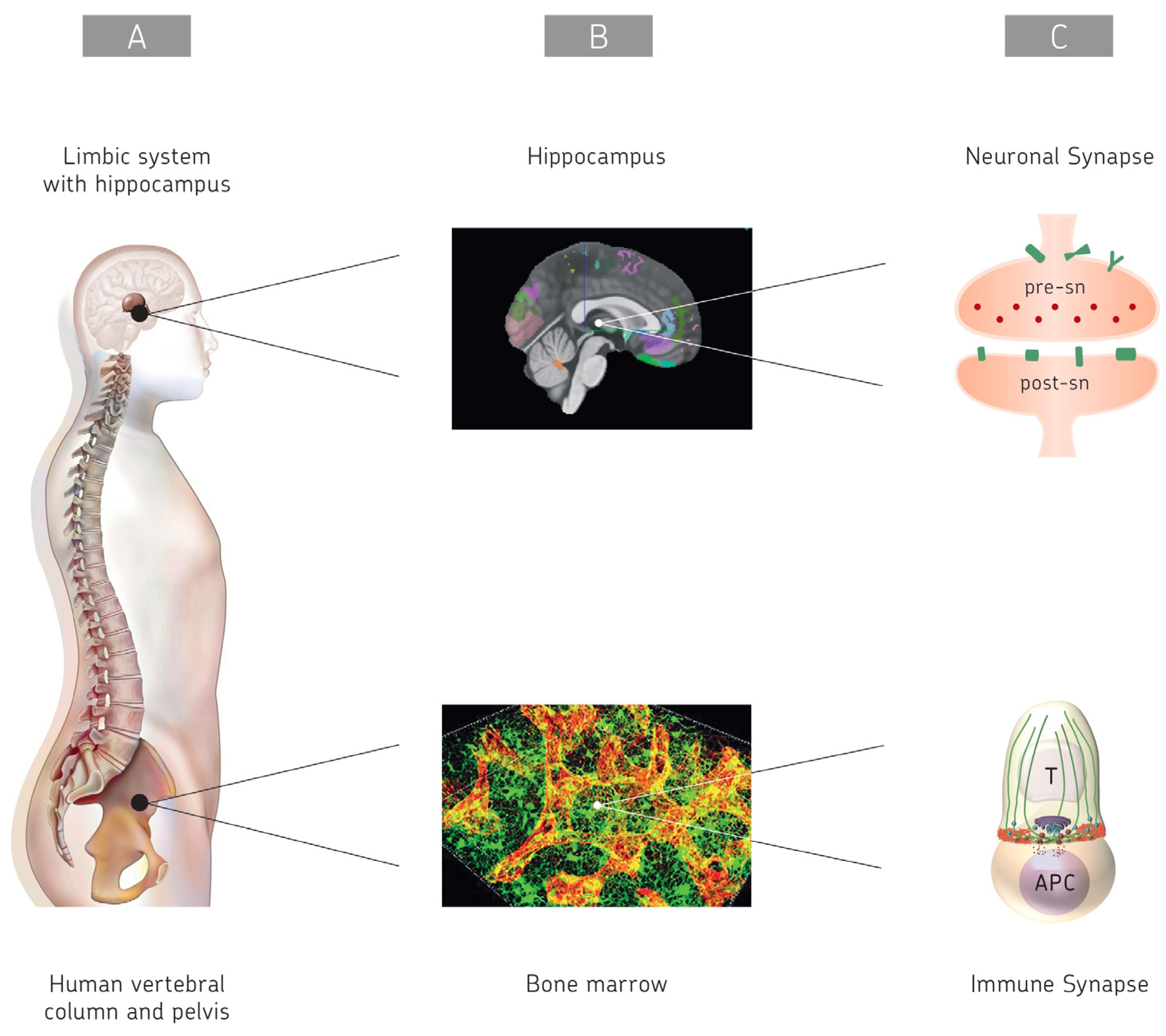
1. Introduction
2. Intercellular Communication
2.1. The Brain
2.1.1. Basic Information
2.1.2. Neuronal Synapses
2.1.3. Neuronal Network Visualization
2.1.4. CNS Versus PNS
2.1.5. Neuronal Intercellular Signal Communication
2.1.6. New Discoveries
2.1.7. Intercellular Communication in the Brain by TNTs and EVs
2.1.8. Summary
2.2. The Immune System
2.2.1. Basic Information
2.2.2. Cognate Antigen Recognition by B and T Lymphocytes
2.2.3. Immune Regulation for the Prevention of Autoimmune Reactivity
2.2.4. Antigen Presentation to T Cells by Dendritic Cells
2.2.5. Immunogenic T-APC Interaction
2.2.6. Formation of an Immunological Synapse
2.2.7. Cytoskeletal Reorganization and T Cell Polarization
2.2.8. Priming of Mitochondria of Importance for MTC Differentiation
2.2.9. Tripartite T-APC-T Cell Interactions
2.2.10. Intercellular Communication via Cytokines
2.2.11. The CIS and PIS
2.2.12. Intercellular Communication by TNTs and EVs
2.2.13. Similarities Between the Brain and the Immune System
2.2.14. Summary
3. Neuroimmune Interfaces and Network Communication
3.1. Basic Information
3.2. Communication via SLYM
3.3. Communication via Gateway-Specific Blood Vessels
- (i)
- Gravity gateway reflex: Gravity activates sensory nerves in the soleus muscle, whose cell bodies are located at the dorsal root ganglion of the fifth lumbar spinal cord (L5). Soleus muscles are anti-gravity muscles and are necessary to maintain posture for weight-bearing. The gateway reflex is created via the sympathetic pathway from L5 sympathetic ganglions. Characteristic mediators at the L5 vertebra are autoreactive CD4 T cells, norepinephrine (NE), and chemokine CCL20, an IL-6 amplifier [61]. CCL20 attracts CCR6-expressing immune cells in an EAE mouse model, such as Th17 cells, which are also involved in MS (see Section 4.6).
- (ii)
- Pain-induced gateway reflex: Pain stimuli are delivered to the anterior cingulate cortex (ACC), which has neurons related to pain sensation. This activation finally reaches the L5 vertebrate via sympathetic nerves to induce the release of CX3CL1 from ventral blood vessels. Characteristic components of the reflex include autoreactive CD4 T cells, NE, CX3CR1 + monocytes, and CX3CL1 chemokines [61].
- (iii)
- Stress gateway reflex: Chronic mental stresses sequentially activate neurons in the paraventricular nucleus of the hypothalamus and other neurons connecting specific vessels adjacent to the third ventricle, dentate gyrus, and thalamus. This neural activation induces the CCL5-expression-dependent accumulation of CD4 T cells and MHC class IIhi monocytes. The induced microinflammation in specific brain vessels activates the dorsomedial nucleus of hypothalamus, propagates activation signals to dorsal motor nucleus of the vagus nerve, and finally causes upper severe gastrointestinal (GI) tract failure. In the investigated mouse model, the stomach epithelial cell damage induced bleeding, and the acute elevation of cytosolic potassium ions caused sudden cardiac dysfunction and death [61].
- (iv)
- Light gateway reflex: This reflex [62] is novel in that it negatively regulates injured vascular endothelial cells to provide a protective effect in the retina of experimental autoimmune uveitis (EAU) mice. The renal inflammation by photoreceptor peptide-specific CD4 T cells could be reduced by photopic (visual) light, which simulates strongly neurons in retina tissue [62].
3.4. Communication Between the Bone Marrow and Brain
3.5. Communication in the Hippocampus and Choroid Plexus
3.6. Neurogenesis in the Hippocampus and Crosstalk with BM-MNCs
3.7. Neuroimmune–Cutaneous Crosstalk in the Skin and Nose
3.8. Gut Microbiota–Brain Interactions
3.9. Summary of Neuroimmune Interfaces
3.10. A Unifying Hypothesis of CNS-CIS Neuroimmune Homeostasis
4. Neuroimmunomodulation
4.1. Basic Information
4.2. Stimulation of the Vagus Nerve
4.3. Neurofeedback
4.4. Transcranial Magnetic Stimulation (TMS)
4.5. Migraine
4.6. Multiple Sclerosis
4.7. Glioblastoma: Neuroimmunomodulation and Immunotherapy
- (i)
- Inhibition of neuron–glioma interaction. With regard to intercellular communication there is one key feature which is unique to GBM in comparison to other cancers, namely neuron–glioma interaction. Interactions between presynaptic neurons and postsynaptic gliomas drive GBM tumor development. The interactions involve paracrine signaling factors (e.g., neuroligin-3, brain-derived neurotropic factor 1–3), GABAergic synaptic communication, and α-amino-3-hydroxy-5-methyl-4-isoxalol-propionic acid (AMPA) postsynaptic currents [121]. GABAergic synaptic communication can be inhibited by levetiracatam and AMPA postsynaptic currents can be inhibited by by perampanel [121].
- (ii)
- Antidepressants. Neuroimmunomodulation and the treatment of GBM is complex and influenced in a dynamic way by tumor-host interactions, in particular in the tumor microenvironment (TME). For instance, GSCs home in on a specific TME niche consisting of stromal and immune cells with many reciprocal intercellular communications. GSCs communicate with their TME by cell–cell interaction via TNTs [121]. Antidepressants, such as imipramine, amitryptyline, fluoxetine, mirtazapine. agomelatine, and escitalopram, are prescribed to inhibit GSC plasticity and to combat the side effects of chemotherapy [121].
- (iii)
- Anti-hypoxia treatment. Hypoxia leads to autophagy which inhibits the effects of radio- and chemotherapy. Chloroquine treatment can inhibit autophagy. The monoclonal antibody bevacizumab can inhibit the angiogenesis factor vascular endothelial growth factor (VEGF). Hypoxia also upregulates the hypoxia-inducible factor HIF-1α which drives cellular metabolism toward anaerobic fermentation. Mebendazole and melatonin can be used to normalize HIF-1α expression levels [121]. Interestingly, oncolytic Newcastle disease virus (NDV) was demonstrated to be capable of breaking hypoxia and other cancer resistances [122].
- (iv)
- Anti-immunosuppression. Due to metabolic reprogramming and TME acidification, M2 macrophages and glia-associated macrophages (GAMs) are upregulated, which inhibits CTL responses. The upregulation of TGF-ß by both tumor-associated macrophages (TAMs) and tricarbonic acid (TCA) cycle mutations further inhibit the innate and adaptive immune system. TCA cycle mutations can be inhibited with ONC201 treatment, isoelectric inhibitors, and peptide vaccines. Anti-PDL1 checkpoint inhibitors can inhibit immunosuppression exerted through PDL1+ M2 macrophages [121].
- (v)
- Active-specific immune stimulation/immunotherapy (ASI). The Immune-Oncological Center Cologne, Germany (IOZK), has developed an individualized multimodal immunotherapy (IMI) for cancer patients in which oncolytic NDV and cancer-derived EVs play an important role. The scientific rationale and clinical experience has recently been summarized [50]. The strategy involves repeated cancer-immunity cycles evoked in cancer patients by systemic NDV exposure combined with modulated electrohyperthermia (mEHT) pretreatment to induce immunogenic cell death (ICD). This “ICD immunotherapy” generates cancer cell debris, including EVs and apoptotic cell bodies that accumulate in blood plasma. These immunogenic components, carrying important information about the individuality of the patient’s cancer (e.g., neoantigens and shared TAs), are harvested and loaded onto patient-derived DCs to generate the dendritic cell vaccine IO-VACR. This is then used for ASI by intradermal vaccination. The IMI strategy involves three treatment phases: (I) Anticancer treatment (resection, radio- and chemotherapy, and ICD immunotherapy), (II) immunization (ICD immunotherapy, ASI, and neuroimmunomodulation), and (III) maintenance and immune protection (ICD immunotherapy, peptide vaccines, boosted DC vaccines). Such a combined treatment strategy should be the aim for future GBM treatment approaches [121].
4.8. Summary
5. Conclusions and Perspectives
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Henley, C. Foundations of Neuroscience; Open Edition; Michigan State University: East Lansing, MI, USA, 2021; pp. 1–395. [Google Scholar]
- Galizia, C.G.; Lledo, P.-M. (Eds.) Neurosciences—From Molecules to Behavior: A University Textbook; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Mak, T.W.; Saunders, M.E.; Jett, B.D. (Eds.) Academic Cell. In Primer to the Immune Response, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. (Eds.) Cellular and Molecular Immunolog, 10th ed; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Schirrmacher, V. Mitochondria at work: New insights into regulation and dysregulation of cellular energy supply and metabolism. Biomedicines 2020, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of glia cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef]
- Farhy-Tselnicker, I.; Allen, N.J. Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Dev. 2018, 13, 7. [Google Scholar] [CrossRef]
- Rasia-Filho, A.A.; Calcagnotto, M.E.; von Bohlen und Halbach, O. Glial cell modulation of dendritic spine structure and synaptic function. Adv. Neurobiol. 2023, 34, 255–310. [Google Scholar] [CrossRef]
- Brunger, A.; Leitz, J. The core complex of the Ca2+-triggered presynaptic fusion machinery. J. Mol. Biol. 2023, 435, 167853. [Google Scholar] [CrossRef]
- Qiu, Y.; Peng, Y.; Wang, J. Immunoregulatory role of neurotransmitters. Adv. Neuroimmunol. 1996, 6, 223–231. [Google Scholar] [CrossRef]
- Mamah, D.; Ji, A.; Rutlin, J.; Shimony, J.S. White matter integrity in schizophrenia and bipolar disorder: Tract- and voxel-based analyses of diffusion data from the Connectom scanner. Neuroimage Clin. 2019, 21, 101649. [Google Scholar] [CrossRef]
- Leybaert, L.; Cabooter, L.; Braet, K. Calcium signal communication between glial and vascular brain cells. Acta Neurol. Belg. 2004, 104, 51–56. [Google Scholar]
- Goenaga, J.; Araque, A.; Kofuji, P.; Moro Chao, D.H. Calcium signaling in astrocytes and gliotransmitter release. Front. Synaptic Neurosci. 2023, 15, 1138577. [Google Scholar] [CrossRef]
- Chahinian, H.; Yahi, N.; Fantini, J. Glutamate, gangliosides, and the synapse: Electrostatics at work in the brain. Int. J. Mol. Sci. 2024, 25, 8583. [Google Scholar] [CrossRef]
- Chen, J.; Ding, X.; Zhang, D. Challenges and strategies faced in the electrochemical biosensing analysis of neurochemicals In Vivo: A review. Talanta 2024, 266, 124933. [Google Scholar] [CrossRef] [PubMed]
- Lawn, T.; Howard, M.A.; Turkheimer, F.; Misic, B.; Deco, G.; Martins, D.; Dipasquale, O. From neurotransmitters to networks: Transcending organisational hierarchies with molecular-informed functional imaging. Neurosci. Biobehav. Rev. 2023, 150, 105193. [Google Scholar] [CrossRef] [PubMed]
- van Oostrum, M.; Blok, T.M.; Giandomenico, S.L.; Dieck, S.T.; Tushev, G.; Fürst, N.; Langer, J.D.; Schuman, E.M. The proteomic landscape of synaptic diversity across brain regions and cell types. Cell 2023, 186, 5411–5427.e23. [Google Scholar] [CrossRef] [PubMed]
- Magee, J.C.; Grienberger, C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Moriano, J.A.; Chen, S.; Zuo, G.; Cebrian-Silla, A.; Zhang, S.; Mukhtar, T.; Wang, S.; Song, M.; et al. Molecular and cellular dynamics of the developing human neocortex at single-cell resolution. bioRxiv 2024. [CrossRef]
- Smalley, J.L.; Kontou, G.; Choi, C.; Ren, Q.; Albrecht, D.; Abiraman, K.; Rodriguez Santos, M.A.; Bope, C.E.; Deeb, T.Z.; Davies, P.A.; et al. Isolation and characterization of multi-protein complexes enriched in the K-Cl co-transporter 2 from brain plasma membranes. Front. Mol. Neurosci. 2020, 13, 563091. [Google Scholar] [CrossRef]
- Wang, X.; Liang, J.; Sun, H. The network of tumor microtubes: An improperly reactivated neural cell network with stemness feature for resistance and recurrence in gliomas. Front. Oncol. 2022, 12, 921975. [Google Scholar] [CrossRef]
- Dagar, S.; Pathak, D.; Oza, H.V.; Mylavarapu, S.V.S. Tunneling nanotubes and related structures: Molecular mechanisms of formation and function. Biochem. J. 2021, 478, 3977–3998. [Google Scholar] [CrossRef]
- Chen, J.; Cao, J. Astrocyte-to-neuron transportation of enhanced green fluorescent protein in cerebral cortex requires F-actin dependent tunneling nanotubes. Sci. Rep. 2021, 11, 16798. [Google Scholar] [CrossRef]
- Chinnery, H.R.; Keller, K.E. Tunneling nanotubes and the eye: Intercellular communication and implications for ocular health and disease. BioMed Res. Int. 2020, 2020, 7246785. [Google Scholar] [CrossRef]
- Scheiblich, H.; Eikens, F.; Wischhof, L.; Opitz, S.; Jüngling, K.; Cserep, C.; Schmidt, S.V.; Lambertz, J.; Bellande, T.; Posfai, B.; et al. Microglia rescue neurons from aggregate-induced neuronal dysfunction and death through tunneling nanotubes. Neuron 2024, 112, 3106–3125.e8. [Google Scholar] [CrossRef] [PubMed]
- Gousset, K.; Schiff, E.; Langevin, C.; Marijanovic, Z.; Caputo, A.; Browman, D.T.; Chenouard, N.; de Chaumont, F.; Martino, A.; Enninga, J.; et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 2009, 11, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Di Liegro, C.M.; Di Liegro, I. Cell-to-cell communication in learning and memory: From neuro- and glio-transmission to information exchange mediated by extracellular vesicles. Int. J. Mol. Sci. 2019, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Chen, A.; Su, Y.; You, M.; Guo, H.; Tan, S.; He, Q.; Hu, B. Extracellular vesicles: A new communication paradigm of complement in neurological diseases. Brain Res. Bull. 2023, 199, 110667. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, A. Adipose extracellular vesicles in intercellular and inter-organ crosstalk in metabolic health and diseases. Front. Immunol. 2021, 12, 608680. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A. Extracellular vesicles as mediators of neuroinflammation in intercellular and inter-organ crosstalk. Int. J. Mol. Sci. 2024, 25, 7041. [Google Scholar] [CrossRef]
- Vignais, M.-L.; Caicedo, A.; Brondello, J.-M.; Jorgensen, C. Cell connections by tunneling nanotubes: Effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017, 2017, 6917941. [Google Scholar] [CrossRef]
- Khalilzad, M.A.; Mohammadi, J.; Amirsaadat, S.; Najafi, S.; Zare, S.; Nilforoushzadeh, M.A.; Khaliza, M.; Amirkham, M.A.; Peyrovan, A.; Khalili, S.F.S.; et al. Therapeutic potential of apoptotic vesicles in modulating inflammation, immune responses, and tissue regeneration. J. Nano Biotechnol. 2025, 23, 260. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef]
- Schirrmacher, V. Bone marrow: The central immune system. Immuno 2023, 3, 289–329. [Google Scholar] [CrossRef]
- Feuerer, M.; Beckhove, P.; Garbi, N.; Mahnke, Y.; Limmer, A.; Hommel, M.; Hämmerling, G.J.; Kyewski, B.; Hamann, A.; Umansky, V.; et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 2003, 9, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Sedwick, C.E.; Morgan, M.M.; Jusino, L.; Cannon, J.L.; Miller, J.; Burkhardt, J.K. TCR, LFA-1, and CD28 play unique and complementary roles in signalling T cell cytoskeletal reorganization. J. Immunol. 1999, 162, 1367–1375. [Google Scholar] [CrossRef]
- Lanzavecchia, A.; Sallusto, F. From synapses to immunological memory: The role of sustained T cell stimulation. Curr. Opin. Immunol. 2000, 12, 92–98. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Valpuesta, J.M.; Sanchez-Madrid, F. Folding for the immunological synapse: CCT chaperonin and the cytoskeleton. Front. Cell Dev. Biol. 2021, 9, 658460. [Google Scholar] [CrossRef]
- Cassioli, C.; Baldari, C.T. Lymphocyte polarization during immune synapse assembly: Centrosomal actin joins the game. Front. Immunol. 2022, 13, 830835. [Google Scholar] [CrossRef]
- Mastrogiovanni, M.; Juzanz, M.; Alcover, A.; Di Bartolo, V. Coordinating cytoskeleton and molecular traffick in T cell migration, activation and effector functions. Front. Cell Dev. Biol. 2020, 8, 591348. [Google Scholar] [CrossRef]
- Dustin, M.L.; Choudhuri, K. Signaling and polarized communication across the T cell immune synapse. Annu. Rev. Cell Dev. Biol. 2016, 32, 303–325. [Google Scholar] [CrossRef]
- Saliba, D.G.; Cespedes-Donoso, P.F.; Balint, S.; Compeer, E.B.; Korobchevskaya, K.; Valvo, S.; Mayya, V.; Kvalvaag, A.; Peng, Y.; Dong, T.; et al. Composition and structure of synaptic ectosomes exporting antigen receptor linked to functional CD40 ligand from helper T cells. Elife 2019, 8, e47528. [Google Scholar] [CrossRef]
- Gonzalez-Mancha, N.; Rodriguez-Rodriguez, C.; Alcover, A.; Merida, I. Sorting Nexin 27 enables MTOC and secretory machinery translocation to the immune synapse. Front. Immunol. 2022, 12, 814570. [Google Scholar] [CrossRef]
- Geltink, R.I.K.; O’Sullivan, D.; Corrado, M.; Bremser, A.; Buck, M.D.; Buescher, J.M.; Firat, E.; Zhu, X.; Niedermann, G.; Caputa, G.; et al. Mitochondrial priming by CD28. Cell 2017, 171, 385–397.e11. [Google Scholar] [CrossRef]
- Williama, M.A.; Tyznik, A.J.; Bevon, M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8 memory T cells. Nature 2006, 441, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Aigner, M.; Schirrmacher, V. Transcriptome analysis and cytokine profiling of naive T cells stimulated by a tumor vaccine via CD3 and CD25. Int. J. Oncol. 2010, 37, 1439–1452. [Google Scholar] [PubMed][Green Version]
- Goodman, S.; Naphade, S.; Khan, M.; Sharma, J.; Cherqui, S. Macrophage polarization impacts tunneling nanotube formation and intercellular organelle trafficking. Sci. Rep. 2019, 9, 14529. [Google Scholar] [CrossRef] [PubMed]
- Rainy, N.; Chetrit, D.; Rouger, V.; Vernitsky, H.; Rechavi, O.; Marguet, D.; Goldstein, I.; Ehrlich, M.; Kloog, Y. H-Ras transfers from B to T cells via tunneling nanotubes. Cell Death Dis. 2013, 4, e726. [Google Scholar] [CrossRef]
- Watkins, S.C.; Salter, R.D. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 2005, 23, 309–318. [Google Scholar] [CrossRef]
- Schirrmacher, V.; Van Gool, S.; Stuecker, W. Individualized multimodal immunotherapy (IMI): Scientific rationale and clinical experience from a single institution. Biomedicines 2024, 12, 754. [Google Scholar] [CrossRef]
- Kim, H.-R.; Jun, C.-D. T cell microvilli: Sensors or senders? Front. Immunol. 2019, 10, 1753. [Google Scholar] [CrossRef]
- Armada, G.; Roque, S.; Serre-Miranda, C.; Ferreira, L.; Vale, A.; Rodrigues, A.J.; Hong, W.; Correia-Neves, M.; Vieira, N. SNX27: A trans-species cognitive modulator with implications for anxiety and stress susceptibility. Neurobiol. Stress 2024, 30, 100619. [Google Scholar] [CrossRef]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef]
- Herz, J.; Fu, Z.; Kim, K.; Dykstra, T.; Wall, M.; Li, H.; Salvador, A.F.; Zou, B.; Yan, N.; Blackburn, S.M.; et al. GABAergic neuronal IL-4R mediates T cell effect on memory. Neuron 2021, 109, 3609–3618.e9. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Kipnis, J. Brain borders at the central stage of neuroimmunology. Nature 2022, 612, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.W.; McGavern, D.B. Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol. Rev. 2022, 306, 58–75. [Google Scholar] [CrossRef]
- Mollgard, K.; Beinlich, F.R.M.; Kusk, P.; Miyakoshi, L.M.; Delle, C.; Pla, V.; Hauglund, N.L.; Esmail, T.; Rasmussen, M.K.; Gomolka, R.S.; et al. A mesothelium divides the subarachnoid space into functional compartments. Science 2023, 379, 84–88. [Google Scholar] [CrossRef]
- Mills, W.A., 3rd; Coburn, M.A.; Eyo, U.B. The emergence of the calvarial hematopoietic niche in health and disease. Immunol. Rev. 2022, 311, 26–38. [Google Scholar] [CrossRef]
- Ghanizada, H.; Nedergaard, M. The glymphatic system. Handb. Clin. Neurol. 2025, 209, 161–170. [Google Scholar] [CrossRef]
- Tanaka, H.; Hasebe, R.; Murakami, K.; Sugawara, T.; Yamasaki, T.; Murakami, M. Gateway reflexes describe novel neuro-immune communications that establish immune cell gateways at specific vessels. Bioelectron. Med. 2023, 9, 24. [Google Scholar] [CrossRef]
- Stofkova, A.; Kamimura, D.; Ohki, T.; Ota, M.; Arima, Y.; Murakami, M. Photopic light-mediated down-regulation of local α1A-adrenergic signaling protects blood-retina barrier in experimental autoimmune uveoretinitis. Sci. Rep. 2019, 9, 2353. [Google Scholar] [CrossRef]
- Schirrmacher, V. Interconnection and communication between bone marrow—The central immune system and the central nervous system. J. Neurosci. Neurol. Disord. 2023, 7, 090–093. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Manz, M.G. Quantification and three-dimensional microanatomical organization of the bone marrow. Blood Adv. 2017, 1, 407–416. [Google Scholar] [CrossRef]
- Wu, K.; Li, R.; Zhang, Y.; Liu, Y.; Wang, M.; Huang, J.; Zhu, C.; Zhang, J.; Yuan, X.; Liu, Q. The discovery of a new type of innervation in lymphoid organs. Physiol. Rep. 2023, 11, e15604. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, S.; Li, S.; Liang, B.; Han, X.; Liang, Y.; Wei, X. Nerves within bone and their application in tissue engineering of bone regeneration. Front. Neurol. 2023, 13, 1085560. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Mi, B.; Hu, Y.; Lin, S.; Xiong, Y.; Lu, X.; Panayi, A.C.; Li, G.; Liu, G. Hallmarks of peripheral nerve function in bone regeneration. Bone Res. 2023, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Milo, I.; Sapoznikov, A.; Kalchenko, V.; Tal, O.; Krauthgamer, R.; van Rooijen, N.; Dudziak, D.; Jung, S.; Shakhar, G. Dynamic imaging reveals promiscous crosspresentation of blood-borne antigens to naïve CD8+ T cells in the bone marrow. Blood 2013, 122, 193–208. [Google Scholar] [CrossRef]
- Feuerer, M.; Beckhove, P.; Bai, L.; Solomayer, E.F.; Bastert, G.; Diel, I.J.; Pedain, C.; Oberniedermayr, M.; Schirrmacher, V.; Umansky, V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat. Med. 2001, 7, 452–458. [Google Scholar] [CrossRef]
- Schirrmacher, V. Cancer-reactive memory T cells from bone marrow: Spontaneous induction and therapeutic potential (Review). Int. J. Oncol. 2015, 47, 2005–2016. [Google Scholar] [CrossRef]
- Sommerfeldt, N.; Schütz, F.; Sohn, C.; Förster, J.; Schirrmacher, V.; Beckhove, P. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res. 2006, 66, 8258–8265. [Google Scholar] [CrossRef]
- Rolando, C.; Taylor, V. Neural stem cell of the hippocampus: Development, physiology regulation, and dysfunction in disease. Curr. Top. Dev. Biol. 2014, 107, 183–206. [Google Scholar] [CrossRef]
- Baruch, K.; Schwartz, M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav. Immun. 2013, 34, 11–16. [Google Scholar] [CrossRef]
- Matsuda, T.; Kakashima, K. Bidirectional communication between the innate immune and nervous systems for homeostatic neurogenesis in the adult hippocampus. Neurogenesis 2015, 2, e1081714. [Google Scholar] [CrossRef]
- Okinaka, Y.; Maeda, M.; Kataoka, Y.; Nakagomi, T.; Doi, A.; Boltze, J.; Claussen, C.; Gul, S.; Taguchi, A. Direct water-soluble molecules transfer from transplanted bone marrow mononuclear cell to hippocampal neural stem cells. Stem Cells Dev. 2024, 33, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Yasui, T.; Kimura, T.; Gul, S.; Claussen, C.; et al. Bone marrow mononuclear cells activate angiogenesis via gap-junction-mediated cell-cell interaction. Stroke 2020, 51, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zeng, M.; Su, B.; Zhang, Y.; Wu, K.; Zhou, T.; Wu, C.; Sun, J.; Fan, H. Bioactive cell niche mediating uniform thermal stimulus for BMSC neural differentiation through TRPV1 channel activation. J. Mater. Chem. B 2023, 11, 6567–6580. [Google Scholar] [CrossRef]
- Saglam-Metiner, P.; Duran, E.; Sabour-Takanlou, L.; Biray-Avci, C.; Yesil-Celiktas, O. Differentiation of neurons, astrocytes, oligodendrocytes and microglia from human induced pluripotent stem cells to form neural tissue-on-chip: A neuroinflammation model to evaluate the therapeutic potential of extracellular vesicles derived from mesenchymal stem cells. Stem Cell Rev. Rep. 2024, 20, 413–436. [Google Scholar] [CrossRef]
- Bueno, C.; Garcia-Bernal, D.; Martinez, S.; Blanquer, M.; Moraleda, J.M. The nuclei of human adult stem cells can move within the cell and generate cellular protrusions to contact other cells. Stem Cell Res. Ther. 2024, 15, 32. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Fan, Y.; Zhao, Z.; Li, L.; Okano, H.; Ouchi, T. Dissecting calvarial bones and sutures at single-cell resolution. Biol. Rev. 2023, 98, 1749–1767. [Google Scholar] [CrossRef]
- Talagas, M. Anatomical contacts between sensory neurons and epidermal cells: An unrecognized anatomical network for neuro-immuno-cutaneous crosstalk. Br. J. Dermatol. 2023, 188, 176–185. [Google Scholar] [CrossRef]
- Klimov, V.; Cherevko, N.; Klimov, A.; Novikov, P. Neuronal-immune cell units in allergic inflammation in the nose. Int. J. Mol. Sci. 2022, 23, 6938. [Google Scholar] [CrossRef]
- Ratsika, A.; Pereira, J.S.C.; Lynch, C.M.K.; Clarke, G.; Cryan, J.F. Microbiota-immune-brain interactions: A lifespan perspective. Curr. Opin. Neurobiol. 2023, 78, 102652. [Google Scholar] [CrossRef]
- Wang, I.-C.; Buffington, S.A.; Salas, R. Microbiota-gut-brain axis in psychiatry: Focus on depressive disorders. Curr. Epidemiol. Rep. 2024, 11, 222–232. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Ao, H. Corticosterone effects induced by stress and immunity and inflammation: Mechanisms of communication. Front. Endocrinol. 2025, 16, 1448750. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G.; Ali, S.A. IL-33’s role in the gut immune system: A comprehensive review of its crosstalk and regulation. Life Sci. 2023, 327, 121868. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, G.K.; D’Cruz, L.M.; Turnquist, H.R. Emerging functions of IL-33 in homeostasis and immunity. Annu. Rev. Immunol. 2022, 40, 15–43. [Google Scholar] [CrossRef]
- Saba, J.B.; Turnquist, H.R. The reparative roles of IL-33. Transplantation 2023, 107, 1069–1078. [Google Scholar] [CrossRef]
- Collins, N.; Han, S.-J.; Enamorado, M.; Link, V.M.; Huang, B.; Moseman, E.A.; Kishton, R.J.; Shannon, J.P.; Dixit, D.; Schwab, S.R.; et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 2019, 178, 1088–1101. [Google Scholar] [CrossRef]
- Krsek, A.; Ostojic, L.; Zivalj, D.; Baticic, L. Navigating the neuroimmunomodulation frontier: Pioneering approaches and promising horizons—A comprehensive review. Int. J. Mol. Sci. 2024, 25, 9695. [Google Scholar] [CrossRef]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.-J.; Wang, X. Melatonin and autophagy in aging-related neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef]
- Liang, C.; Song, R.; Zhang, J.; Yao, J.; Guan, Z.; Zeng, X. Melatonin enhances NK cell function in aged mice by increasing T-bet expression via the JAK3-STAT5 signaling pathway. Immun. Ageing 2024, 21, 59. [Google Scholar] [CrossRef]
- Rehman, M.U.; Sehar, N.; Rasool, I.; Aldossari, R.M.; Wani, A.B.; Rashid, S.M.; Wali, A.F.; Ali, A.; Arafah, A.; Khan, A. Glymphatic pathway: An emerging perspective in the pathophysiology of neurodegenerative diseases. Int. J. Geriatr. Psychiatry 2024, 39, e6104. [Google Scholar] [CrossRef]
- Kopec, K.; Szleszkowski, S.; Koziorowski, D.; Szlufik, S. Glymphatic system and mitochondrial dysfunction as two crucial players in pathophysiology of neurodegenerative disorders. Int. J. Mol. Sci. 2023, 24, 10366. [Google Scholar] [CrossRef]
- Klinkovskij, A.; Shepelev, M.; Isaakyan, Y.; Aniskin, D.; Ulasov, I. Advances of genome editing with CRISPR/Cas9 in neurodegeneration: The right path towards therapy. Biomedicines 2023, 11, 3333. [Google Scholar] [CrossRef] [PubMed]
- Cantone, M.; Di Pino, G.; Capone, F.; Piombo, M.; Chiarello, D.; Cheeran, B.; Pennisi, G.; Di Lazzaro, V. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin. Neurophysiol. 2014, 125, 1509–1532. [Google Scholar] [CrossRef] [PubMed]
- Nelson-Maney, N.P.; Balint, L.; Beeson, A.L.; Serafin, D.S.; Kistner, B.M.; Douglas, E.S.; Siddiqui, A.H.; Tauro, A.M.; Caron, K.M. Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J. Clin. Investig. 2024, 134, e175616. [Google Scholar] [CrossRef]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef]
- Hartung, H.P.; Rieckmann, P. Pathogenesis of immune-mediated demyelination in the CNS. J. Neural Transm. Suupl. 1997, 50, 173–181. [Google Scholar] [CrossRef]
- Jelcic, I.; Naghavian, R.; Fanaswala, I.; Macnair, W.; Esposito, C.; Calini, D.; Han, Y.; Marti, Z.; Raposo, C.; Del Castillo, J.S.; et al. T-bet+ CXCR3+ B cells drive hyperreactive B-T cell interactions in multiple sclerosis. Cell Rep. Med. 2025, 6, 102027. [Google Scholar] [CrossRef]
- Bergner, C.G.; van der Meer, F.; Franz, J.; Vakrakou, A.; Würfel, T.; Nessler, S.; Schäfer, L.; Nau-Gietz, C.; Winkler, A.; Lagumersindez-Denis, N.; et al. BCAS1-positive oligodendrocytes enable efficient cortical remyelination in multiple sclerosis. Brain 2024, 148, 908–920. [Google Scholar] [CrossRef]
- Stevanovic, I.; Ninkovic, M.; Mancic, B.; Milivojevic, M.; Stojanovic, I.; Ilic, T.; Vujovic, M.; Djukic, M. Compensatory neuroprotective response of thioredoxin reductase against oxidative-nitrosative stress induced by experimental autoimmune encephalomyelitis in rats: Modulation by theta burst stimulation. Molecules 2020, 25, 3922. [Google Scholar] [CrossRef]
- Pennisi, G.; Cornelius, C.; Cavallaro, M.M.; Trovato Salinaro, A.; Cambria, M.T.; Pennisi, M.; Bella, R.; Milone, P.; Ventimiglia, B.; Migliore, M.R.; et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem. Pharmacol. 2011, 82, 1490–1499. [Google Scholar] [CrossRef]
- Cwiklinska, H.; Cichalewska-Studzinska, M.; Selmaj, K.W.; Mycko, M.P. The heat shock protein HSP70 promotes Th17 genes’ expression via specific regulation of microRNA. Int. J. Mol. Sci. 2020, 21, 2823. [Google Scholar] [CrossRef]
- Larochelle, C.; Wasser, B.; Jamann, H.; Löffel, J.T.; Cui, Q.-L.; Tastet, O.; Schillner, M.; Luchtman, D.; Birkenstock, J.; Stroh, A.; et al. Pro-inflammatory T helper 17 directly harms oligodendrocytes in neuroinflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025813118. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-Y.; Zheng, Y.; Pecsok, M.K.; Wang, K.; Li, W.; Gong, M.-J.; Wu, F.; Zhang, L. C-reactive protein suppresses the Th17 response indirectly by attenuating the antigen presentation ability of monocyte derived dendritic cells in experimental autoimmune encephalomyelitis. Front. Immunol. 2021, 12, 589200. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Jin, L.; Fang, Z.; Wenig, Y.; Zhang, Y.; Zhang, J.; Xie, D.; Tang, Y.; Guo, S.; Huang, Y.; et al. TIA1-mediated stress granules promote the neuroinflammation and demyelination in experimental autoimmune encephalomyelitis through upregulating IL-31RA signaling. Adv. Sci. 2025, 12, e2409086. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, J.; Yang, R.; Yang, H.; Guo, M.; Ji, B.; Du, G.; Li, L. Panobinostat attenuates experimental autoimmune encephalomyelitis in mice via suppressing oxidative stress-related neuroinflammation and mitochondrial dysfunction. Int. J. Mol. Sci. 2024, 25, 12035. [Google Scholar] [CrossRef]
- Mao, P.; Manczak, M.; Shirendeb, U.P.; Hemachandra Reddy, P. MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochim. Biophys. Acta BBA) Mol. Basis Dis. 2013, 1832, 2322–2331. [Google Scholar] [CrossRef]
- Gurrea-Rubio, M.; Wang, Q.; Mills, E.A.; Wu, Q.; Pitt, D.; Tsou, P.-S.; Fox, D.A.; Mao-Draayer, Y. Siponimod attenuates neuronal cell death triggered by neuroinflammation via NfκB and mitochondrial pathways. Int. J. Mol. Sci. 2024, 25, 2454. [Google Scholar] [CrossRef]
- Higashi, C.; Kawaji, A.; Tsuda, N.; Hayashi, M.; Saito, R.; Yagishita, Y.; Suzuki, T.; Uruno, A.; Nakamura, M.; Nakao, K.; et al. The novel Nrf2 inducer TFM-735 ameliorates experimental autoimmune encephalomyelitis in mice. Eur. J. Pharmacol. 2017, 802, 76–84. [Google Scholar] [CrossRef]
- Long, T.; Yang, Y.; Peng, L.; Li, Z. Neuroprotective effects of melatonin on experimental allergic encephalomyelitis mice via anti-oxidative stress activity. J. Mol. Neurosci. 2018, 64, 233–241. [Google Scholar] [CrossRef]
- Amirinejad, R.; Shirvani-Farsani, Z.; Gargari, B.N.; Sahraian, M.A.; Soltani, B.M.; Behmanesh, M. Vitamin D changes expression of DNA repair genes in the patients with multiple sclerosis. Gene 2021, 781, 145488. [Google Scholar] [CrossRef]
- Li, B.; Tan, G.-J.; Lin, H.-Q.; Zhang, J.-N.; Guo, L.; Chen, L.-P. Neuroprotective effects of alpha-lipoic acid on long-term experimental autoimmune encephalomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6517–6528. [Google Scholar] [CrossRef]
- Wang, M.-R.; Zhang, X.-J.; Liu, H.-C.; Ma, W.-D.; Zhang, M.-L.; Zhang, Y.; Li, X.; Dou, M.-M.; Jing, Y.-L.; Chu, Y.-J.; et al. Matrine protects oligodendrocytes by inhibiting their apoptosis and enhancing mitochondrial autophagy. Brain Res. Bull. 2019, 153, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kassis, I.; Ben-Zwi, M.; Petrou, P.; Halimi, M.; Karussis, D. Synergistic neuroprotective effects of Fingolimod and mesenchymal stem cells (MSC) in experimental autoimmune encephalomyelitis. Immuno. Lett. 2021, 233, 11–19. [Google Scholar] [CrossRef]
- Oechterring, J.; Schaedelin, S.A.; Stein, K.; Maceski, A.M.; Melie-Garcia, L.; Benkert, P.; Cagol, A.; Leber, S.; Galbusera, R.; Ruberte, E.; et al. Aberrant complement activation is associated with structural brain damage in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200361. [Google Scholar] [CrossRef]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.P.; et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef]
- Michailidou, I.; Willems, J.G.; Kooi, E.J.; van Eden, C.; Gold, S.M.; Geurts, J.J.; Baas, F.; Huitinga, I.; Ramaglia, V. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol. 2015, 77, 1007–1026. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Kampers, L.F.C.; Metselaar, D.S.; Vinci, M.; Scirocchi, F.; van Zanten, S.V.; Eyrich, M.; Biassoni, V.; Hulleman, E.; Karremann, M.; Stücker, W.; et al. The complexity of malignant glioma treatment. Cancers 2025, 17, 879. [Google Scholar] [CrossRef]
- Schirrmacher, V.; van Gool, S.; Stuecker, W. Breaking therapy resistance: An update on oncolytic Newcastle disease virus for improvements of cancer therapy. Biomedicines 2019, 7, 66. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Brem, S.; Campian, J.L.; Trusheim, J.E.; Iwamoto, F.M.; Tran, D.D.; Ansstas, G.; Cobbs, C.S.; Heth, J.A.; et al. Association of autologous tumor-lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma. JAMA Oncol. 2023, 9, 112–121. [Google Scholar] [CrossRef]
| Feature | Description | Ref | Year |
|---|---|---|---|
| Synapses in the brain | One neuron can make up to ten thousand synapses. | [6] | 2023 |
| The brain comprises more than 1 × 1014 synapses. | |||
| Neurogenesis | Tri-IPC cells lead to neuronal development. | [19] | 2024 |
| Immune synapses | The repertoire of α and β T cell receptors is about 1016, while that of γ and δ T cells is about 1018. The B cell receptor repertoire is about 1011. | [4] | 2021 |
| Repertoire of antigen-specific immune receptors | These numbers give an impression of the diversity of immune synapses. | ||
| Lymphopoiesis | Lymphocyte receptor rearrangement occurs in CLP cells. | [33] | 2010 |
| Neurotransmitters | Serotonin, epinephrine, dopamine, acetylcholine, and gamma-aminobutyric acid | [1] | 2021 |
| [10] | 1996 | ||
| Gliotransmitters | Glutamate, d-serine, ATP | [2] | 2016 |
| Cytokines | Il-1 to Il-33 | [4] | 2021 |
| Interferons | IFN-α, β, and γ | [4] | 2021 |
| Synaptic ectosomes | Exocytosis via Sorting nexin 27 | [43] | 2022 |
| [52] | 2024 | ||
| Gap junctions | Transmission of small molecules, calcium flux | [1] | 2021 |
| TNT | F-actin containing thin nanoprotrusions | [22] | 2021 |
| EV | Extracellular vesicle | [28] | 2023 |
| Interorgan crosstalk | [27] | 2019 |
| Feature | Description | Ref | Year |
|---|---|---|---|
| SLYM | Permits in-brain exchange of solutes between CSF and venous blood; lymphatic-like membranes and vascular channels; glymphatic system | [58] | 2023 |
| [60] | 2025 | ||
| Gateway reflexes | Specific vessels at distinct sites | [61] | 2023 |
| Brain and bone marrow | Adrenergic and cholinergic nerves running in BM adjacent to arteries and arterioles | [65] | 2023 |
| [34] | 2023 | ||
| A neuro-osteogenic network | [66] | 2023 | |
| Bone regeneration | [67] | 2023 | |
| Choroid plexus | An active neuro-immunological interface | [73] | 2013 |
| Hippocampus | Bidirectional neuroimmune communication for homeostatic neurogenesis | [74] | 2015 |
| Crosstalk with BM-MNCs | [75] | 2024 | |
| Intraepidermal free nerve endings in skin | Neuroimmune–cutaneous crosstalk | [81] | 2023 |
| Network in nose allergic inflammation | [82] | 2022 | |
| Gut–brain interactions | Gut microbiota–immune system–brain interactions | [83] | 2023 |
| Depressive disorders | [84] | 2024 | |
| Role of IL-33 | [86] | 2023 | |
| CNS-CIS neuroimmune homeostasis hypothesis | Regulatory control over three types of stem cells, namely HSCs, NSCs and MSCs | This review | 2025 |
| The supporting role of TNTs and EVs | |||
| CIS: Storage and refuge for immune memory | [89] | 2019 | |
| [34] | 2023 |
| Feature | Description | Ref | Year |
|---|---|---|---|
| Vagus nerve stimulation | Modulation of enteroendocrine functions and mental and emotional processes | [95] | 2023 |
| Transcranial magnetic stimulation | Modulation of major depressive disorders, dementia, or degenerative diseases | [96] | 2014 |
| Active–specific immune stimulation (ASI) | Cancer: GBM immunotherapy (IMI) | [50] | 2024 |
| GBM | ICD immunotherapy | [121] | 2025 |
| Inhibition of neuron-glioma interaction | [121] | 2025 | |
| [121] | 2025 | ||
| Antidepressants | [121] | 2025 | |
| Anti-immunosuppression | |||
| Anti-hypoxia treatment | [121] | 2025 | |
| Breaking therapy resistance | [122] | 2019 | |
| Phase 3 clinical study | [123] | 2023 | |
| Multiple sclerosis: | Th17 cells directly harming oligodendrocytes | [105] | 2021 |
| -Aberrant T cell response | |||
| -Immunomodulation | T cell targets: a number of myelin and non-myelin antigens. T cell–B cell interactions | [100] | 2025 |
| BCAS1+ oligodendrocytes for remyelination | [101] | 2024 | |
| TIA1-mediated stress granules | [107] | 2025 | |
| Redox regulation of cellular stress in MS | [103] | 2011 | |
| -Aberrant complement activation | Association with structural brain damage | [117] | 2025 |
| Migraine | MLV dysfunction | [97] | 2024 |
| Triptans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirrmacher, V. Brain and Immune System: Intercellular Communication During Homeostasis and Neuroimmunomodulation upon Dysfunction. Int. J. Mol. Sci. 2025, 26, 6552. https://doi.org/10.3390/ijms26146552
Schirrmacher V. Brain and Immune System: Intercellular Communication During Homeostasis and Neuroimmunomodulation upon Dysfunction. International Journal of Molecular Sciences. 2025; 26(14):6552. https://doi.org/10.3390/ijms26146552
Chicago/Turabian StyleSchirrmacher, Volker. 2025. "Brain and Immune System: Intercellular Communication During Homeostasis and Neuroimmunomodulation upon Dysfunction" International Journal of Molecular Sciences 26, no. 14: 6552. https://doi.org/10.3390/ijms26146552
APA StyleSchirrmacher, V. (2025). Brain and Immune System: Intercellular Communication During Homeostasis and Neuroimmunomodulation upon Dysfunction. International Journal of Molecular Sciences, 26(14), 6552. https://doi.org/10.3390/ijms26146552






