Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental condition frequently associated with gastrointestinal symptoms, gut dysbiosis, and metabolic dysfunctions such as insulin resistance (IR). Recent evidence suggests that the gut microbiota may influence both metabolic and neurological processes through the gut–brain–metabolic axis. This review explores the molecular mechanisms linking dysbiosis, IR, and ASD, focusing on pathways such as TLR/NF-κB activation, PI3K/Akt/mTOR disruption, and the action of microbial metabolites, like short-chain fatty acids (SCFAs), lipopolysaccharide (LPS), and γ-aminobutyric acid (GABA). We discuss how dysbiosis may contribute to increased intestinal permeability, systemic inflammation, and neuroimmune activation, ultimately affecting brain development and behavior. Common microbial alterations in ASD and IR—including increased Clostridium, Desulfovibrio, and Alistipes, and reduced Bifidobacterium and butyrate-producing genera—suggest a shared pathophysiology. We also highlight potential therapeutic strategies, such as microbiota modulation, insulin-like growth factor 1 (IGF-1) treatment, and dietary interventions. Understanding these interconnected mechanisms may support the development of microbiota-targeted approaches for individuals with ASD metabolic comorbidities.
1. Introduction
Autism spectrum disorder is a complex neurodevelopmental condition that typically manifests in early childhood and persists throughout life [1]. It is characterized by a wide range of symptoms that vary between individuals and are generally grouped into behavioral and medical categories [1]. People with ASD often exhibit deficits in social communication and interaction, accompanied by restricted and repetitive patterns of behavior, interests, or activities [2]. This broad heterogeneity poses significant challenges for diagnosis, management, and the development of effective interventions [1].
Epidemiological data reflects a rising prevalence of ASD worldwide. According to the most recent estimates from the U.S. Centers for Disease Control and Prevention (CDC), approximately 1 in 31 children aged 8 years were identified with ASD in the United States in 2022 [3]. A systematic review covering data from 2008 to 2024 reported a global prevalence of 0.77%, with regional variations: 0.28% in Asia, 1.1% in America, 0.71% in Europe, and 1.51% in Africa and Australia [4]. Although discrepancies exist among prevalence studies, such as the one published in 2022 [5], the observed increase is likely attributable to improved awareness and diagnostic practices [4].
Gastrointestinal problems have been reported in approximately one-third of the children with ASD, with common symptoms including constipation, excessive abdominal gas, and diarrhea [1]. These symptoms not only contribute to increased morbidity but are also associated with greater severity of core ASD features. The high prevalence of gastrointestinal issues has drawn significant attention to the gut–brain axis [6]. This axis is a bidirectional communication network linking the gut microbiota, the central nervous system (CNS), and metabolic processes [7].
Growing evidence indicates a strong correlation between the gut microbiota and brain function, suggesting that gut dysbiosis may play a critical role in the development and regulation of the CNS, as well as in the pathogenesis of neurodevelopmental disorders [8]. Several pathways mediate this communication, including the vagus nerve and a range of endocrine, immune, and biochemical signaling mechanisms [8]. Dysbiosis has been linked to neurological disorders through mechanisms such as activation of the hypothalamic–pituitary–adrenal (HPA) axis, imbalances in neurotransmitter production, systemic inflammation, and increased permeability of the intestinal and blood–brain barriers [8].
Furthermore, recent studies have proposed an expanded gut–brain–metabolic axis that integrates metabolic processes, such as glucose regulation, into the microbiota–neurodevelopment framework [7]. Notably, insulin resistance, a metabolic state in which tissues fail to respond adequately to insulin, has emerged as a relevant factor in neurodevelopmental disorders [9]. However, the molecular mechanisms linking insulin resistance, the gut microbiota, and ASD remain poorly understood.
This article aims to explore, from a molecular perspective, the emerging evidence linking insulin resistance and alterations in the gut microbiota within the context of ASD. By integrating current scientific evidence, this article seeks to provide an integrated view of the potential molecular pathways that may underlie this triad. This perspective may offer new insights for targeted interventions and promising directions for future research.
2. The Effects of Dysbiosis in ASD
In recent years, the study of the gut microbiome has emerged as a central focus in understanding the biological factors underlying ASD. Several studies have documented specific alterations in the gut microbiome composition of individuals with ASD, generating significant interest in its potential role in the manifestation of both gastrointestinal and neurobehavioral symptoms [10,11]. These findings suggest that the gut microbiota plays a fundamental role in the function and regulation of the CNS through the gut–brain axis. In this context, dysbiosis not only exacerbates gastrointestinal disorders but may also influence neuroinflammation, neurotransmission, and brain metabolism, positioning itself as a significant modulating factor in the pathophysiology of ASD [12].
Dysbiosis can influence neurodevelopment through multiple interrelated pathways, particularly via the synthesis and signaling of neuroactive compounds. Gut microorganisms can produce neurotransmitters such as serotonin (5-HT), γ-aminobutyric acid, and dopamine, as well as key metabolites like short-chain fatty acids and aromatic derivatives, which can directly or indirectly modulate brain circuits [13,14]. Approximately 90% of serotonin is synthesized in the gut from tryptophan by enterochromaffin cells, a process regulated by the gut microbiota through modulation of tryptophan hydroxylase 1 (TPH1) [15]. In ASD, peripheral hyperserotonemia accompanied by central serotonin deficiency has been reported, potentially reflecting impaired serotonergic signaling associated with social and behavioral deficits [16].
Similarly, the gut microbiota contributes to the production of GABA, a key inhibitory neurotransmitter. Bacteria such as Bacteroides, Bifidobacterium, and Lactobacillus produce GABA via glutamate fermentation. Disruptions in their abundance or function may reduce GABA bioavailability in the CNS [17,18]. Altered GABAergic signaling has been implicated in the excitatory/inhibitory imbalance observed in ASD, which may underlie neuronal hyperexcitability, anxiety, and repetitive behaviors [19,20]. Dopaminergic pathways may also be indirectly modulated by microbial activity through effects on tyrosine metabolism, inflammation, and neurotransmitter transporters, such as the dopamine transporter (DAT), with implications for motivation and executive functioning in ASD [21].
Moreover, microbial fermentation of dietary fibers produces SCFAs, such as butyrate and propionate, which exert neuroactive effects by modulating synaptic plasticity, microglial activation, and gene expression. Reduced butyrate levels, commonly observed in the fecal microbiota of children with ASD, may compromise intestinal barrier integrity, alter CNS immune responses, and affect serotonergic and dopaminergic pathways. In mouse models, gut dysbiosis was associated with reduced social behavior, increased fecal butyrate levels, and neutrophil infiltration, suggesting a role for altered microbiota in autism-related behaviors [22].
In contrast, in animal models, excessive propionate levels have been shown to induce ASD-like behaviors, mitochondrial dysfunction, and oxidative stress. Other microbial metabolites, such as 4-ethylphenyl sulfate (4EPS), derived from Clostridium species, have demonstrated neurotoxic potential by disrupting myelination and altering oligodendrocyte maturation, which can contribute to emotional dysregulation and social impairments [15,23].
These findings underscore that microbial dysbiosis in ASD is not merely a gastrointestinal manifestation but may act as a critical modulator of neurochemical signaling, neuroimmune responses, and brain development. Understanding the precise microbial and metabolic pathways involved may offer promising directions for novel diagnostic and therapeutic strategies.
3. The Role of Insulin Resistance in Neurodevelopment
Insulin and insulin-like growth factor 1 signaling play crucial roles in maintaining brain homeostasis and regulating neurodevelopmental processes [9]. Insulin is implicated in two major signaling cascades, AKT/mTOR and RAS/ERK, which regulate cellular growth, metabolism, and survival. Disruptions in these pathways have been strongly associated with various neurodevelopmental disorders, particularly ASD [24,25]. IGF-1, a neurotrophic factor critical for proper CNS development, is involved in neuronal growth, synaptogenesis, survival, and migration [26]. Through its widely expressed receptor, IGF1R, it acts via endocrine, paracrine, and autocrine mechanisms [26].
Multiple factors can contribute to insulin signaling dysfunction in neurodevelopmental disorders such as ASD, including inflammation, oxidative stress, and mitochondrial dysfunction, all of which can lead to IR [27,28]. Genetic predisposition and environmental factors during brain development may exacerbate these conditions [28].
An additional mechanism of interest involves the receptor for advanced glycation end products (RAGE), which activates the PI3K/Akt/mTOR signaling pathway and promotes inflammatory neurodegeneration [29,30]. Disruption of this pathway has been linked to defects in neuronal translation, resulting in decreased levels of proteins involved in synapse formation or neuronal structure [31]. Similar disruptions are observed in IR, which promotes defects in neuronal autophagy and apoptosis, contributing to neurodegenerative disorders such as Parkinson’s disease [9]. Furthermore, IR has been associated with an exaggerated immune response due to the loss of insulin’s anti-inflammatory effects, leading to increased production of pro-inflammatory transcription factors (NF-κB) and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), as well as Toll-like receptor (TLR) overexpression [32].
Emerging research has identified a notable association between IR and ASD. One study found that 16.85% of children and adolescents with ASD, treated with risperidone, exhibited IR, independent of pharmacogenetic gene polymorphisms or drug plasma level [33]. Another investigation comparing individuals with ASD to neurotypical controls revealed that Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) scores were 0.31 units higher in the ASD group, even after adjusting for variables such as sex, age, BMI z-score category, and lipids [34]. Moreover, a systematic review explored insulin resistance in children with autism, revealing no conclusive link with prenatal IR, but highlighting postnatal risk factors such as poor diet, inactivity, and antipsychotic use. Elevated HOMA-IR levels in adolescents with ASD suggest altered glucose metabolism, underscoring the need for further research on metabolic–neurodevelopmental interactions [35].
4. Gut Dysbiosis Induces Low-Grade Inflammation and Insulin Resistance
Gut dysbiosis has been implicated in low-grade chronic inflammation through the recognition of pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide or flagellin, by pattern recognition receptors (PRRs), primarily those of the TLR family. This recognition triggers inflammatory responses in the host [36]. Dysbiosis can arise from various environmental and lifestyle factors, including an unhealthy diet [37], antibiotic use [38], and exposure to toxic compounds, microplastics, and pollution, among others [39,40,41].
The gut microbiota plays a crucial role in host immunity modulation, and its dysbiosis is associated with abnormal production of inflammatory biomarkers, such as C-reactive protein (CRP) or interleukin-6 (IL-6) [42]. Inter-individual differences in cytokine profiles often reflect specific microbial compositions that influence host immune responses [43]. For instance, microbial tryptophan metabolism produces the metabolite tryptophol, which has demonstrated inhibitory effects on TNF-α responses [43].
A state of low-grade chronic inflammation is also frequently observed in individuals with type 2 diabetes or IR [44]. Microbial alterations in overweight individuals include a reduction in sulfate-reducing bacteria and Bacteroides, alongside increased production of branched-chain fatty acids, phenolics, valeric acid, and hydroxy acids, all of which contribute to systemic inflammation [45]. Dysbiosis may also impair gut barrier function, facilitating metabolic endotoxemia and exacerbating complications such as retinopathy and nephropathy in obese individuals [46]. Thus, gut dysbiosis, through the overproduction of intestinal pro-inflammatory cytokines, may promote the migration of these inflammatory mediators and bacterial antigens to various host organs, such as the pancreas, inducing local inflammatory processes that could contribute to the development of IR.
4.1. Immune Dysregulation and Cytokine Imbalance in ASD
Immune dysregulation and elevated pro-inflammatory cytokine levels have been implicated in the early onset of ASD. For instance, increased interleukin-8 (IL-8) and decreased IL-10 levels have been detected in children with ASD compared to controls [47]. The activation of inflammasomes has also been described in individuals with ASD, promoting the overexpression of IL-1β and IL-18, and a reduction in the anti-inflammatory IL-33 cytokine [48]. Additional evidence has identified increased levels of inflammatory cytokines, including TNF-α, IL-4, and IL-21, in the cerebrospinal fluid of individuals with ASD, further highlighting the role of immune response in ASD pathogenesis and the potential utility of cytokine profiles for differential diagnosis from other neurological disorders [49]. In ASD, the production of these altered cytokines has been linked to gut dysbiosis and gut permeability [48,50]. Another study linked gut dysbiosis to ASD through a microbiota-driven “TNFα–sphingolipid–st–steroid hormone” axis. Children with ASD showed elevated TNF-α, enrichment of Bifidobacterium bifidum and Segatella copri, and upregulation of sphingolipid metabolism. Metabolomics revealed reduced steroid hormones, including estriol and deoxycorticosterone. TNF-α correlated positively with microbial toxin pathways and negatively with steroid biosynthesis. These findings highlight a potential mechanism by which microbial and immune disruptions contribute to neurodevelopmental alterations in ASD [51].
Although definitive evidence is still lacking, it could be hypothesized that gut dysbiosis may promote the translocation of bacterial components, such as LPSs, from the intestine to the bloodstream, ultimately reaching neuronal tissues. Once there, LPSs may trigger several pro-inflammatory signaling cascades, mainly via TLR pathways, leading to IL overproduction, which could contribute to ASD pathogenesis [48,52] (Figure 1).
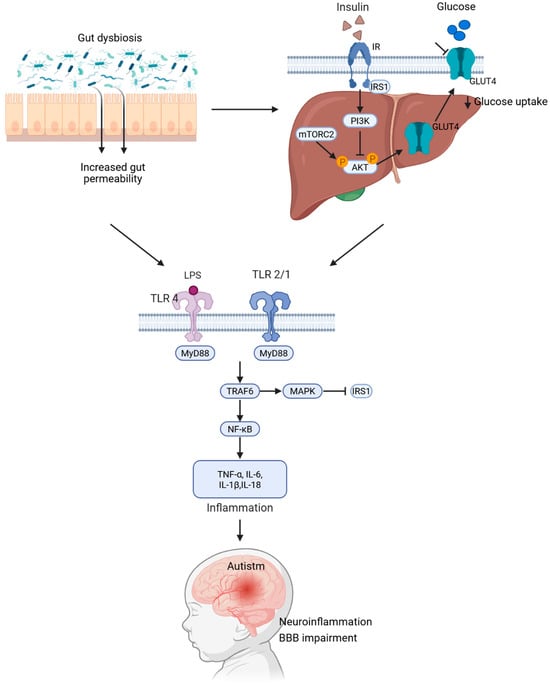
Figure 1.
Mechanistic model linking gut dysbiosis, insulin resistance, and autism spectrum disorder. Gut dysbiosis leads to increased intestinal permeability, allowing lipopolysaccharides to enter the bloodstream. This triggers the activation of TLR4 and TLR2/1 through MyD88. Consequently, this initiates the TRAF6–MAPK–NF-κB signaling pathway, resulting in an elevation in pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1β, and IL-18. These cytokines induce inhibitory phosphorylation of IRS1, which dampens the PI3K/AKT-GLUT4 pathway and contributes to systemic insulin resistance. The resulting chronic inflammation and disruption of the blood–brain barrier promote neuroinflammation and neurodevelopmental changes associated with ASD.
4.2. TLR-Mediated Inflammatory Signaling Triggered by LPS: A Mechanistic Link Between Intestinal Permeability and ASD Pathogenesis
TLR signaling may potentially be involved with LPS recognition in ASD associated with increased intestinal permeability [48]. This pathway may begin with the recognition of LPSs by TLR2, TLR4, and TLR9 expressed in neuronal cells. This recognition leads to receptor dimerization and the recruitment of adaptor proteins, such as Toll/interleukin 1 receptor domain-containing adapter interferon-β (TRIF) and myeloid differentiation primary-response protein 88 (MyD88). Subsequently, MyD88 binds to IL-1R-associated kinases, which activate tumor necrosis factor receptor-associated factor 6 (TRAF6). Activated TRAF6 then triggers the mitogen-activated protein kinase (MAPK) pathway and nuclear factor kappa B (NF-κB), initiating inflammatory responses. Moreover, TLR3 and 4 promote the translocation of NF-κB to the nucleus and induce the synthesis of interferon-beta (IFN-β), chemokines such as CCL5 and CXCL10, and the expression of IL-1β and IL-18 [29,53].
Thus, the proposed role of increased intestinal permeability in ASD development could be supported by the possibility that LPSs could trigger this inflammatory pathway. Although further research is required, exploring this pathway may reveal new therapeutic and diagnostic opportunities for individuals with ASD.
Consequently, gut dysbiosis that increases intestinal permeability may contribute to the development of ASD by enabling the migration and dissemination of bacterial antigens, such as LPSs, into neuronal tissue, potentially inducing local inflammatory responses that promote neurological dysfunction associated with ASD. Moreover, under conditions of enhanced internal permeability, the translocation of LPSs into pancreatic islets may decrease insulin production, thereby diminishing insulin’s anti-inflammatory effects in the brain, which could further contribute to ASD symptom onset. These hypotheses could be assessed in pregnant animal models exhibiting insulin signaling dysfunction to evaluate whether maternal gut dysbiosis promotes the migration of maternal bacterial antigens into the fetal neuronal tissue, potentially triggering neurodevelopmental disorders such as ASD.
4.3. Gut Microbiota-Derived Metabolites and Tissue-Specific Impacts on Insulin Resistance
In parallel, the gut microbiota has gained recognition as a metabolically active “organ” capable of producing a wide variety of bioactive metabolites. These gut bacteria-derived compounds interact with metabolically active organs, playing a central role in regulating glucose and lipid homeostasis, inflammation, and insulin sensitivity [54,55]. Under the conditions of IR, gut dysbiosis leads to altered microbial metabolism, characterized by a decrease in beneficial metabolites, such as SCFAs, and an increase in detrimental compounds, such as trimethylamine N-oxide (TMAO), hydrogen sulfide, and phenylacetic acid [56].
Insulin regulates glucose homeostasis across skeletal muscle, the liver, and adipose tissue [56,57]. In skeletal muscle, insulin promotes glucose uptake. However, under IR conditions, this uptake is compromised by disruptions in the insulin signaling pathway, particularly in the translocation of the GLUT4 transporter. Consequently, glucose not utilized by muscle is redirected to the liver, favoring de novo lipogenesis and ectopic lipid accumulation, thereby exacerbating IR [57,58]. In this context, a study revealed that extracellular vesicles derived from bacteria such as Pseudomonas panacis can block insulin signaling in skeletal muscle and adipose tissue, reducing glucose uptake and worsening metabolic dysfunction [59].
The liver plays a central role in glucose metabolism, including gluconeogenesis, glycogenolysis, glycogen synthesis, and de novo lipogenesis [60]. Under healthy conditions, insulin suppresses hepatic glucose production. However, in IR, this suppression fails, leading to a phenomenon called selective hepatic insulin resistance [61]. For instance, hydrogen sulfide, from sulfate-reducing bacteria, enhances gluconeogenesis and inhibits glycogen synthesis [62,63], while phenylacetic acid from Bacteroides spp. has been associated with non-alcoholic fatty liver disease (NAFLD), promoting hepatic triglyceride accumulation by inhibiting AKT phosphorylation, a key event in insulin signaling [56,64]. TMAO has also been shown to exert direct effects on hepatocytes and has been linked to insulin resistance and alterations in glucose metabolism [65,66]. In animal models, TMAO activates cellular pathways, such as the endoplasmic reticulum kinase PKR, and induces the expression of gluconeogenic genes (G6pc and Pck1) via the transcription factor FOXO1, contributing to the development of hyperglycemia [67].
Adipose tissue, recognized as both an endocrine organ and an energy reservoir, is also highly responsive to insulin action. Under normal conditions, insulin stimulates glucose and fatty acid uptake and inhibits lipolysis. In IR, these processes are impaired: glucose uptake is reduced, and the release of free fatty acids and glycerol is increased, contributing to lipid accumulation in the liver and muscle and favoring hepatic gluconeogenesis [68,69]. This metabolic dysfunction is further exacerbated by chronic inflammation, driven by macrophage infiltration and the release of pro-inflammatory cytokines [70,71]. Microbial metabolites such as TMAO, SCFAs, and indole derivatives influence adipokine signaling, including leptin and adiponectin, thereby altering metabolic pathways, promoting oxidative stress, and contributing to the progression of IR [70,72].
Among microbial-derived metabolites, SCFAs—particularly acetate, propionate, and butyrate—exert protective effects on host metabolism. These metabolites, produced through dietary fiber fermentation, activate the AMPK pathway, stimulate fatty acid oxidation, and reduce lipid accumulation in the liver. In skeletal muscle, they promote the expression of genes involved in lipid oxidation and glucose uptake [73,74]. In adipose tissue, propionate and butyrate reduce inflammation, and propionate also increases GLUT4 expression, thereby enhancing insulin-induced glucose uptake. Overall, these findings establish SCFAs as essential mediators in maintaining insulin sensitivity and highlight their potential as therapeutic agents for addressing dysbiosis and metabolic diseases [56,64,74].
4.4. Differential Abundance of the Gut Microbiota in ASD and Insulin Resistance
In individuals with IR, a distinctive gut microbial profile has been identified compared to metabolically healthy subjects. This profile is characterized by a lower abundance of symbiotic bacteria, such as Akkermansia muciniphila [75], Bifidobacterium spp. [76], and Clostridium coccoides [76], whose presence is associated with increased gut barrier integrity, reduced inflammation, and improved insulin sensitivity. In contrast, species such as Prevotella copri and Bacteroides vulgatus have been identified as key drivers of the microbial biosynthesis of branched-chain amino acids (BCAAs) [77]. The accumulation of BCAAs in the circulation can interfere with insulin signaling through the mTOR and IRS1 pathways. In addition, this dysbiosis favors the expansion of LPS-producing Gram-negative bacteria, contributing to chronic metabolic endotoxaemia that activates the TLR4/NF-κB pathway. This, in turn, leads to systemic inflammation and dysfunction of insulin-dependent signaling in peripheral tissues [77,78,79].
Complementing this evidence, a recent multi-omics analysis identified functional microbial signatures linked to IR. In insulin-resistant individuals, increased levels of genera such as Blautia and Dorea (family Lachnospiraceae), as well as certain Actinobacteria, were observed, correlating positively with elevated levels of fecal monosaccharides, such as fructose and glucose. This association suggests increased degradation of complex carbohydrates into simple, absorbable sugars. The accumulation of these sugars has been linked to elevated levels of pro-inflammatory cytokines. In contrast, microbial profiles associated with insulin sensitivity (IS) displayed a higher abundance of Alistipes, Bacteroides, and Faecalibacterium, which correlated negatively with HOMA-IR and fecal sugar levels. Furthermore, they favored the production of fermentative metabolites, such as SCFAs. These findings were experimentally validated by the oral administration of Alistipes indistinctus in murine models, which significantly improved insulin sensitivity and reduced intestinal monosaccharide load [55].
Studies in obese pediatric populations have reinforced the association between IR and gut dysbiosis. In this group, a significant reduction in the phylum Firmicutes and a relative increase in Bacteroidetes were observed, resulting in a decreased (F/B) ratio compared to insulin-sensitive children. At the taxonomic level, the highest abundance of Coriobacteriales, Turicibacterales, Pasteurellales, and Turicibacteraceae was associated with the IS group, while Peptococcaceae predominated in subjects with IR. In addition, genera such as Butyricimonas, Alistipes, and Anaerostipes showed significant correlations with key biomarkers, such as ANGPTL4 and adropine, suggesting that gut microbial composition not only reflects metabolic status but actively participates in its regulation through cytokine-mediated mechanisms and metabolite production [80]. These findings position the gut microbiota not only as a biomarker of metabolic status but as an active regulator of insulin sensitivity and a potential target for precision clinical interventions.
Another study reported that individuals with IR exhibited an enrichment in the bacterial genera of Lachnospiraceae, i.e., Dorea and Blautia, as well as Actinobacteria, which were correlated with the metabolism of disaccharides and oligosaccharides [55]. Moreover, gut dysbiosis induced by the insulin receptor antagonist S961 led to a reduction in bacterial diversity, increased intestinal permeability, a higher abundance of pro-inflammatory Proteobacteria (Enterobacteriaceae), decreased abundances of Bacteroidetes, Actinobacteria, and Firmicutes, hyperglycemia, and insulin resistance, which were related to impaired insulin signaling that leads to chronic low-grade inflammation [81].
Furthermore, multiple studies have documented significant alterations in the gut microbiota of individuals with ASD, characterized by a state of intestinal dysbiosis involving imbalances in microbial composition and function. This dysbiosis often manifests as an inverted F/B ratio and consistent variations in the abundance of key bacterial genera. Among the most increased taxa in individuals with ASD are Clostridium spp., Alistipes [55,82], Desulfovibrio [82,83,84], Sutterella [82], Akkermansia muciniphila [50,75,82], and Prevotella spp. [77,84]. In contrast, genera considered beneficial, such as Bacteroides, Bifidobacterium spp., Lactobacillus spp., Faecalibacterium prausnitzii, Roseburia spp., and Subdoligranulum spp., tend to be decreased [50,83]. These alterations impact essential functions, such as the production of neuroactive metabolites (propionic acid, butyrate, and p-cresol), mucin degradation, the regulation of neurotransmitters, such as GABA and glutamate, and the maintenance of intestinal barrier integrity [85,86].
Notably, recent multi-omics studies have identified an elevated fecal GABA/glutamate ratio as a metabolic signature of mild ASD, linked to an overrepresentation of Escherichia spp. Moreover, behavioral alterations observed in mice colonized with E. coli from ASD donors suggest a functional link between microbial neurotransmitter imbalance and ASD symptomatology, pointing to promising diagnostic and therapeutic avenues targeting microbial GABAergic signaling [87].
The loss of butyrate-producing bacteria, such as Faecalibacterium prausnitzii and Roseburia spp., can compromise epithelial integrity, increase intestinal permeability, and promote the activation of neuro-inflammatory and immune responses. At the same time, the increase in potentially pro-inflammatory bacteria, such as Clostridium spp. or Desulfovibrio spp., could contribute to the exacerbation of gastrointestinal and neurobehavioral symptoms characteristic of ASD. Taken together, these findings reinforce the modulatory role of the gut microbiota in the pathophysiology of ASD, operating through the microbiota–gut–brain axis, with relevant clinical implications for the neurological, immunological, and digestive homeostasis of these patients [88,89].
Although no specific studies have examined dysbiosis profiles exclusively in the context of IR and ASD, indirect comparisons have been made using research focused on related metabolic conditions, such as obesity and type 2 diabetes. This correlation is especially relevant given that individuals with ASD have been reported to be at increased risk of developing obesity, more so than diabetes, because of factors such as selective food intake, sedentary lifestyle, and prolonged use of antipsychotics [90].
A comparison of the microbial profiles observed in ASD and in conditions associated with IR reveals common patterns of bacterial alterations, highlighting increases in genera such as Alistipes, Desulfovibrio, and Prevotella, as well as decreases in Bifidobacterium spp. and butyrate-producing bacteria (Figure 2) (Table 1). This convergence suggests that manipulating the gut microbiota through dietary interventions and prebiotic or probiotic strategies could represent a promising therapeutic approach to improve not only ASD symptoms but also metabolic alterations associated with IR in this vulnerable population [55,82].
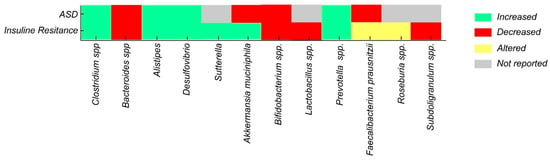
Figure 2.
Alterations in gut bacterial taxa in ASD vs. IR.
Additionally, another study reported the presence of specific Clostridium species, non-spore-forming anaerobes, and microaerophilic bacteria in children with ASD compared to controls, further supporting the potential role of dysbiosis in the onset of autism [91]. Moreover, a large metagenomic study of 1627 children revealed that ASD is associated with alterations not only in bacteria but also in archaea, fungi, viruses, and microbial functions. A multikingdom marker panel achieved high diagnostic accuracy, suggesting the gut microbiome’s potential as a non-invasive tool for ASD detection [92].

Table 1.
Differential abundance and functional implications of key gut microbial taxa in IR and ASD.
Table 1.
Differential abundance and functional implications of key gut microbial taxa in IR and ASD.
| Bacteria | Alteration in ASD | Functional Role or Effect | Alteration in IR | References |
|---|---|---|---|---|
| Clostridium spp. | Increased | Includes species affecting immunity and metabolism; p-cresol producers | Increased | [82,93,94] |
| Bacteroides spp. | Decreased | Beneficial commensal; reduction may impair intestinal barrier function | Decreased | [82,93,95] |
| Alistipes | Increased | May disrupt cognition via propionic acid production | Increased | [55,82] |
| Desulfovibrio | Increased | Sulfate-reducing bacteria; may induce mucosal damage and neuroinflammation | Increased | [82,83,84] |
| Sutterella | Increased | Associated with gastrointestinal symptoms in ASD | - | [82] |
| Akkermansia muciniphila | Increased | Mucin-degrading bacteria; affects mucus barrier integrity | Decreased | [50,75,82] |
| Bifidobacterium spp. | Decreased | Psychobiotic and SCFA producer; modulates GABA and glutamate | Decreased | [76,82] |
| Lactobacillus spp. | Decreased | Psychobiotic; modulates gut–brain axis communication | - | [95] |
| Prevotella spp. | Increased | Fiber-degrading bacteria; increased after microbiota transfer therapy (MTT) | Increased | [77,84] |
| Faecalibacterium prausnitzii | Altered | Butyrate producer; regulates immune function | Decreased | [50,83] |
| Roseburia spp. | Altered | Butyrate producer; supports epithelial tight junction integrity | - | [50] |
| Subdoligranulum spp. | Decreased | Butyrate producer; reduced in ASD | - | [50] |
| Bacteroidetes | Decreased | Important for polysaccharide digestion; reduction may allow overgrowth of other bacteria | Increase | [80,96,97] |
| Firmicutes | Decreased | F/B ratio inversion; implications for neurodevelopment and inflammation | Decreased | [80,98] |
5. Therapeutic and Research Implications
IGF-1 is a neurotrophic molecule essential for CNS development, promoting neuronal proliferation, migration, survival, and synapse formation. It has emerged as a promising therapeutic candidate for both syndromic and non-syndromic forms of ASD due to its critical role in CNS development and function [26,99]. Preclinical models of Rett syndrome have demonstrated that IGF-1 can ameliorate respiratory and behavioral abnormalities [100]. Moreover, a study on the impact of IGF-1 treatment on neurons derived from ASD patients compared to controls revealed heterogeneous responses among ASD patients, depending on the levels of IGF-1 receptor expression [26]. These findings suggest that IGF-1 could represent a targeted therapy to address core neural deficits in ASD.
Building on these findings, a double-blind, placebo-controlled Phase II trial (ClinicalTrials.gov Identifiers: NCT01970345) was initiated to evaluate the safety and feasibility of IGF-1 in children with non-syndromic ASD [101]. Although the trial was terminated in April 2023 because of drug supply issues, the convergence of molecular evidence on IGF1R signaling underscores a shared mechanistic axis in ASD and opens new avenues for targeted metabolic therapies.
In parallel, the convergence of immunometabolic mechanisms suggests microbiota-targeted therapies as a promising therapeutic option for neurodevelopmental disorders such as ASD. For example, Bacteroides fragilis BF839 showed efficacy in a controlled clinical trial, improving gastrointestinal symptoms and behavior in children with ASD [102]. Another study, involving microbiota transfer therapy in children diagnosed with ASD, reported beneficial changes in the gut environment, including increased abundance of Bifidobacterium, Prevotella, and Desulfovibrio, leading to improvements in both gastrointestinal and behavioral symptoms of ASD that persisted for up to two years post-treatment [103]. Complementary interventions with probiotics aimed at enhancing SCFA-producing bacteria (Bifidobacterium, Lactobacilllus) have also shown benefits for both insulin resistance via metabolic anti-inflammation and ASD symptomatology [104,105]. Collectively, these findings support therapeutic strategies that restore gut barrier function, thereby reducing central neuroinflammation.
6. Future Directions
There is increasing evidence that gut dysbiosis is linked to IR, systemic low-grade chronic inflammation, and neurodevelopmental disorders such as ASD. Future case–control studies should assess the underlying molecular pathways involved in microbiota–host interactions. A promising approach would be the characterization of the specific microbial signatures and metabolites that modulate host immune responses through TLRs and other PRRs. High-throughput metagenomics and metabolomics could facilitate the identification of bacterial taxa and microbial-derived metabolites that either attenuate or exacerbate pro-inflammatory cytokine production in both peripheral and central tissues. These findings could help to classify patients based on their microbial profiles and inflammatory signatures, enabling the development of microbiota-targeted therapeutic interventions.
Another area of research could be the investigation of the developmental and maternal origins of gut–brain axis disruption, particularly during gestation and early life. Given that the maternal microbiome could play a pivotal role in fetal immune and neural development, studies using animal models should explore whether maternal insulin resistance or gut dysbiosis promotes the translocation of bacterial endotoxins, such as LPSs, into fetal brain tissue. This research could clarify whether inflammatory signaling cascades (TLR4-MyD88-NF-κB, RAGE-PI3K/Akt/mTOR) are initiated in utero, potentially predisposing offspring to ASD-like neurodevelopmental phenotypes. These findings could be critical for initiating maternal monitoring and early preventive strategies.
Moreover, strategies of intervention through dietary and microbial modulation should be assessed in animal models with a risk of metabolic or neurodevelopmental disorders. These studies could evaluate the impact of prebiotics, probiotics, postbiotics, or specific dietary interventions on microbial composition, intestinal permeability, and systemic inflammation, as well as their effect on IR and ASD pathogenesis.
Finally, the identification of specific inflammatory biomarkers of ASD could enhance the personalized medicine approach. Biomarkers, such as serum LPS, IL-6, or TNF-α, and microbial metagenomic and metabolite profiling, could be integrated into diagnostic frameworks to detect early-stage dysbiosis to predict disease risk. Furthermore, longitudinal studies are needed to assess whether changes in the gut microbiota, and systemic inflammation are related to the onset of neurological symptoms in a gut–immune–brain axis mode, potentially guiding the development of novel diagnostic and therapeutic strategies.
7. Conclusions
This review consolidates emerging evidence supporting a functional convergence between gut microbiota dysbiosis, insulin resistance, and autism spectrum disorder. The changes in the gut microbiota are characterized by an increase in pro-inflammatory bacteria and a decrease in beneficial, anti-inflammatory genera. This suggests that the gut microbiota plays a significant role in both metabolic and neurodevelopmental disorders. Bacterial metabolites, such as lipopolysaccharides, trimethylamine N-oxide, and phenylacetic acid, may act as mediators that trigger immune activation and disrupt insulin signaling.
The identified parallels between autism spectrum disorder and insulin resistance in terms of microbial signatures and inflammatory pathways highlight the importance of investigating the gut–brain–metabolic axis. These insights open new avenues for microbiota-targeted interventions, including probiotics, dietary modulation, and anti-inflammatory strategies, particularly in individuals with autism spectrum disorder who have metabolic comorbidities, such as obesity or insulin resistance.
Author Contributions
R.T.-T., P.G.-R. and A.K.Z.: conceptualization and writing—original draft. S.C.-U., E.P.-C., R.T.-T. and V.A.R.-P.: investigation and writing—review and editing. A.K.Z.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this article was funded by Universidad UTE-Ecuador. The funder had no role in the study design, bibliographic analysis, decision to publish, or preparation of this manuscript.
Institutional Review Board Statement
Not applicable.
Acknowledgments
The authors are grateful to Universidad UTE for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nadeem, M.S.; Murtaza, B.N.; Al-Ghamdi, M.A.; Ali, A.; Zamzami, M.A.; Khan, J.A.; Ahmad, A.; Rehman, M.U.; Kazmin, I. Autism—A Comprehensive Array of Prominent Signs and Symptoms. Curr. Pharm. Des. 2021, 27, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Signs and Symptoms of Autism Spectrum Disorders. 2023. Available online: https://www.cdc.gov/autism/ (accessed on 14 May 2025).
- Shaw, K.A.; Williams, S.; Patrick, M.E.; Valencia-Prado, M.; Durkin, M.S.; Howerton, E.M.; Ladd-Acosta, C.M.; Pas, E.T.; Bakian, A.V.; Bartholomew, P.; et al. Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years—Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. MMWR Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Issac, A.; Halemani, K.; Shetty, A.; Thimmappa, L.; Vijay, V.; Koni, K.; Mishra, P.; Kapoor, V. The global prevalence of autism spectrum disorder in children: A systematic review and meta-analysis. Osong Public Health Res. Perspect. 2025, 16, 3–27. [Google Scholar] [CrossRef]
- Salari, N.; Rasoulpoor, S.; Rasoulpoor, S.; Shohaimi, S.; Jafarpour, S.; Abdoli, N.; Khaledi-Paveh, B.; Mohammadi, M. The global prevalence of autism spectrum disorder: A comprehensive systematic review and meta-analysis. Ital. J. Pediatr. 2022, 48, 112. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.C.; Montgomery, J.M.; Taylor, M.W. The Gut-Microbiota-Brain Axis in Autism Spectrum Disorder. In Autism Spectrum Disorders; Exon Publications: Brisbane, AU, USA, 2021; pp. 95–114. [Google Scholar] [CrossRef]
- Abildinova, G.Z.; Benberin, V.V.; Vochshenkova, T.A.; Afshar, A.; Mussin, N.M.; Kaliyev, A.A.; Zhussupova, Z.; Tamadon, A. The gut-brain-metabolic axis: Exploring the role of microbiota in insulin resistance and cognitive function. Front. Microbiol. 2024, 15, 1463958. [Google Scholar] [CrossRef]
- Naufel, M.F.; Truzzi Gde, M.; Ferreira, C.M.; Coelho, F.M.S. The brain-gut-microbiota axis in the treatment of neurologic and psychiatric disorders. Arq. De Neuro-Psiquiatr. 2023, 81, 670–684. [Google Scholar] [CrossRef]
- Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Cadena-Ullauri, S.; Frias-Toral, E.; Guevara-Ramírez, P.; Paz-Cruz, E.; Chapela, S.; Montalván, M.; Morales-López, T.; Simancas-Racines, D.; et al. The Molecular Mechanisms of the Relationship between Insulin Resistance and Parkinson’s Disease Pathogenesis. Nutrients 2023, 15, 3585. [Google Scholar] [CrossRef]
- Lewandowska-Pietruszka, Z.; Figlerowicz, M.; Mazur-Melewska, K. Microbiota in Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 16660. [Google Scholar] [CrossRef]
- El Mazouri, S.; Aanniz, T.; Bouyahya, A.; Jaoudi REl Rahmadi, M.; Ardianto, C.; Ouadghiri, M. Gut Microbiota in Autism Spectrum Disorder: A Systematic Review. Prog. Microbes Mol. Biol. 2024, 7. [Google Scholar] [CrossRef]
- Zarimeidani, F.; Rahmati, R.; Mostafavi, M.; Darvishi, M.; Khodadadi, S.; Mohammadi, M.; Shamlou, F.; Bakhtiyari, S.; Alipourfard, I. Gut Microbiota and Autism Spectrum Disorder: A Neuroinflammatory Mediated Mechanism of Pathogenesis? Inflammation 2024, 48, 501–519. [Google Scholar] [CrossRef]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef] [PubMed]
- Damiani, F.; Cornuti, S.; Tognini, P. The gut-brain connection: Exploring the influence of the gut microbiota on neuroplasticity and neurodevelopmental disorders. Neuropharmacology 2023, 231, 109491. [Google Scholar] [CrossRef]
- Zhou, M.; Niu, B.; Ma, J.; Ge, Y.; Han, Y.; Wu, W.; Yue, C. Intervention and research progress of gut microbiota-immune-nervous system in autism spectrum disorders among students. Front. Microbiol. 2025, 16, 1535455. [Google Scholar] [CrossRef]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2015, 321, 24. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From bugs to brain: Unravelling the GABA signalling networks in the brain–gut–microbiome axis. Brain 2024, 148, 1479. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Alabdali, A.; Ben Bacha, A.; Alonazi, M.; Al-Ayadhi, L.Y.; Alanazi, A.S.J.; El-Ansary, A. Comparative evaluation of certain biomarkers emphasizing abnormal GABA inhibitory effect and glutamate excitotoxicity in autism spectrum disorders. Front. Psychiatry 2025, 16, 1562631. [Google Scholar] [CrossRef] [PubMed]
- Pizzarelli, R.; Cherubini, E. Alterations of GABAergic Signaling in Autism Spectrum Disorders. Neural Plast. 2011, 2011, 297153. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- de Theije, C.G.M.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen , J.; Kraneveld, A.D.; Oozeer , R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Pascual, F.; Camilli, S.; Lockey, R.F.; Kolliputi, N. Mind-body connection: Metabolite 4-ethylphenyl linked to anxiety behavior and oligodendrocyte modification in autism spectrum disorder. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G422–G425. [Google Scholar] [CrossRef] [PubMed]
- Vasic, V.; Jones, M.S.O.; Haslinger, D.; Knaus, L.S.; Schmeisser, M.J.; Novarino, G.; Chiocchetti, A.G. Translating the Role of mTOR- and RAS-Associated Signalopathies in Autism Spectrum Disorder: Models, Mechanisms and Treatment. Genes 2021, 12, 1746. [Google Scholar] [CrossRef]
- Réthelyi, J.M.; Vincze, K.; Schall, D.; Glennon, J.; Berkel, S. The role of insulin/IGF1 signalling in neurodevelopmental and neuropsychiatric disorders—Evidence from human neuronal cell models. Neurosci. Biobehav. Rev. 2023, 153, 105330. [Google Scholar] [CrossRef] [PubMed]
- Linker, S.B.; Mendes, A.P.D.; Marchetto, M.C.; Marchetto, M.C. IGF-1 treatment causes unique transcriptional response in neurons from individuals with idiopathic autism. Mol. Autism 2020, 11, 55. [Google Scholar] [CrossRef]
- Rasgon, N.L.; McEwen, B.S. Insulin resistance—A missing link no more. Mol. Psychiatry 2016, 21, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. Insulin signaling in the control of glucose and lipid homeostasis. Handb. Exp. Pharmacol. 2016, 233, 51–71. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Derk, J.; MacLean, M.; Juranek, J.; Schmidt, A.M. The Receptor for Advanced Glycation Endproducts (RAGE) and Mediation of Inflammatory Neurodegeneration. J. Alzheimers Dis. Park. 2018, 8, 421. [Google Scholar] [CrossRef]
- Sato, A.; Ikeda, K. Genetic and Environmental Contributions to Autism Spectrum Disorder Through Mechanistic Target of Rapamycin. Biol. Psychiatry Glob. Open Sci. 2021, 2, 95. [Google Scholar] [CrossRef]
- Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Tamayo-Trujillo, R.; Guevara-Ramírez, P.; Paz-Cruz, E.; Castañeda Cataña, M.A.; Zambrano, A.K. Interplay between endogenous hormones and immune systems in human metapneumovirus pathogenesis and management. Front. Pharmacol. 2025, 16, 1568828. [Google Scholar] [CrossRef]
- Sukasem, C.; Vanwong, N.; Srisawasdi, P.; Ngamsamut, N.; Nuntamool, N.; Hongkaew, Y.; Puangpetch, A.; Chamkrachangpada, B.; Limsila, P. Pharmacogenetics of Risperidone-Induced Insulin Resistance in Children and Adolescents with Autism Spectrum Disorder. Basic. Clin. Pharmacol. Toxicol. 2018, 123, 42–50. [Google Scholar] [CrossRef]
- Manco, M.; Guerrera, S.; Ravà, L.; Ciofi degli Atti, M.; Di Vara, S.; Valeri, G.; Vicari, S. Cross-sectional investigation of insulin resistance in youths with autism spectrum disorder. Any role for reduced brain glucose metabolism? Transl. Psychiatry 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Braykova, R.; Toneva, A. Aspects of Insulin Resistance in Children with Autism. J. IMAB-Annu. Proceeding Sci. Pap. 2025, 31, 6111–6115. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinction in the gut microbiota compounds over generations. Nature 2016, 529, 212. [Google Scholar] [CrossRef]
- Lathakumari, R.H.; Vajravelu, L.K.; Satheesan, A.; Ravi, S.; Thulukanam, J. Antibiotics and the gut microbiome: Understanding the impact on human health. Med. Microecol. 2024, 20, 100106. [Google Scholar] [CrossRef]
- Kaur, R.; Rawal, R. Influence of heavy metal exposure on gut microbiota: Recent advances. J. Biochem. Mol. Toxicol. 2023, 37, e23485. [Google Scholar] [CrossRef]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef]
- Sen, P.; Fan, Y.; Schlezinger, J.J.; Ehrlich, S.D.; Webster, T.F.; Hyötyläinen, T.; Pedersen, O.; Orešič, M. Exposure to environmental toxicants is associated with gut microbiome dysbiosis, insulin resistance and obesity. Environ. Int. 2024, 186, 108569. [Google Scholar] [CrossRef]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 1773. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Effect of overweight on gastrointestinal microbiology and immunology: Correlation with blood biomarkers. Br. J. Nutr. 2010, 103, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, J.L.; Cao, X.Y.; Zhang, H.P.; Tan, Y.J.; Chu, T.; Zhao, L.L.; Liu, Z.; Ren, Y.S. Gut microbiota dysbiosis as an inflammaging condition that regulates obesity-related retinopathy and nephropathy. Front. Microbiol. 2022, 13, 1040846. [Google Scholar] [CrossRef] [PubMed]
- Bryn, V.; Aass, H.C.D.; Skjeldal, O.H.; Isaksen, J.; Saugstad, O.D.; Ormstad, H. Cytokine Profile in Autism Spectrum Disorders in Children. J. Mol. Neurosci. 2017, 61, 1–7. [Google Scholar] [CrossRef]
- Saresella, M.; Piancone, F.; Marventano, I.; Zoppis, M.; Hernis, A.; Zanette, M.; Trabattoni, D.; Chiappedi, M.; Ghezzo, A.; Canevini, M.P.; et al. Multiple inflammasome complexes are activated in autistic spectrum disorders. Brain Behav. Immun. 2016, 57, 125–133. [Google Scholar] [CrossRef]
- Than, U.T.T.; Nguyen, L.T.; Nguyen, P.H.; Nguyen, X.H.; Trinh, D.P.; Hoang, D.H.; Nguyen, P.A.T.; Dang, V.D. Inflammatory mediators drive neuroinflammation in autism spectrum disorder and cerebral palsy. Sci. Rep. 2023, 13, 22587. [Google Scholar] [CrossRef]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal Barrier Dysfunction and Microbiota–Gut–Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef]
- Shao, L.; Cai, G.; Fu, J.; Zhang, W.; Ye, Y.; Ling, Z.; Ye, S. Gut microbial ‘TNFα-sphingolipids-steroid hormones’ axis in children with autism spectrum disorder: An insight from meta-omics analysis. J. Transl. Med. 2024, 22, 1165. [Google Scholar] [CrossRef]
- Wei, H.; Alberts, I.; Li, X. Brain IL-6 and autism. Neuroscience 2013, 252, 320–325. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Lee, H.Y.; Korea, S.; Jt, C.; Xc, P. Mechanisms linking gut microbial metabolites to insulin resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785. [Google Scholar] [CrossRef]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Defective Insulin-Induced GLUT4 Translocation in Skeletal Muscle of High Fat-Fed Rats Is Associated with Alterations in Both Akt/Protein Kinase B and Atypical Protein Kinase C (ζ/λ) Activities. Diabetes 2001, 50, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kwon, Y.; Kim, D.K.; Jeon, J.; Jang, S.C.; Wang, T.; Ban, M.; Kim, M.H.; Jeon, S.G.; Kim, M.S.; et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci. Rep. 2015, 5, 15878. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell Mol. Gastroenterol. Hepatol. 2018, 7, 447. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Rotariu, M.; Poștaru, M.; Turnea, M.; Galaction, A.I. Role of Microbiota-Derived Hydrogen Sulfide (H2S) in Modulating the Gut–Brain Axis: Implications for Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines 2024, 12, 2670. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; Zhao, Y.; Yang, G.; Wang, C.; Zhao, Z.; Li, S. Chondroitin sulfate stimulates the secretion of H2S by Desulfovibrio to improve insulin sensitivity in NAFLD mice. Int. J. Biol. Macromol. 2022, 213, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; McCarty, M.; Okeefe, J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019, 6, 890. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Jin, S.; Lv, J.; Li, M.; Feng, N. The gut microbiota derived metabolite trimethylamine N-oxide: Its important role in cancer and other diseases. Biomed. Pharmacother. 2024, 177, 117031. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e5. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- May, K.S.; den Hartigh, L.J. Gut Microbial-Derived Short Chain Fatty Acids: Impact on Adipose Tissue Physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Joon, A.; Sharma, A.; Jalandra, R.; Bayal, N.; Dhar, R.; Karmakar, S. Nonalcoholic Fatty Liver Disease and Gut-liver Axis: Role of Intestinal Microbiota and Therapeutic Mechanisms. J. Transl. Gastroenterol. 2024, 2, 38–51. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Targeting the Adipose Tissue–Liver–Gut Microbiota Crosstalk to Cure MASLD. Biology 2023, 12, 1471. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.S.; Grześkowiak, Ł.M.; Salminen, S.; Laitinen, K.; Bressan, J.; Gouveia Peluzio Mdo, C. Faecal levels of Bifidobacterium and Clostridium coccoides but not plasma lipopolysaccharide are inversely related to insulin and HOMA index in women. Clin. Nutr. 2013, 32, 1017–1022. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Yoon, M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X.; McCormick, K.L. Gut Microbiota of Chinese Obese Children and Adolescents With and Without Insulin Resistance. Front. Endocrinol. 2021, 12, 636272. [Google Scholar] [CrossRef]
- Gueddouri, D.; Caüzac, M.; Fauveau, V.; Benhamed, F.; Charifi, W.; Beaudoin, L.; Rouland, M.; Sicherre, F.; Lehuen, A.; Postic, C.; et al. Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction. Mol. Metab. 2022, 57, 101438. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Liu, C.; Liu, T.; Gao, J.; Cai, Y.; Fan, X. Alteration of Gut Microbiota: New Strategy for Treating Autism Spectrum Disorder. Front. Cell Dev. Biol. 2022, 10, 792490. [Google Scholar] [CrossRef]
- Xuan, W.; Ou, Y.; Chen, W.; Huang, L.; Wen, C.; Huang, G.; Tang, W.; Zeng, D.; Huang, S.; Xiao, L.; et al. Faecalibacterium prausnitzii Improves Lipid Metabolism Disorder and Insulin Resistance in Type 2 Diabetic Mice. Br. J. Biomed. Sci. 2023, 80, 10794. [Google Scholar] [CrossRef]
- Nirmalkar, K.; Qureshi, F.; Kang, D.W.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Shotgun Metagenomics Study Suggests Alteration in Sulfur Metabolism and Oxidative Stress in Children with Autism and Improvement after Microbiota Transfer Therapy. Int. J. Mol. Sci. 2022, 23, 13481. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-żydecka, K.; Jakubczyk, K.; Maciejewska-Markiewicz, D.; Janda, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, W. Gut Biofactory—Neurocompetent Metabolites within the Gastrointestinal Tract. A Scoping Review. Nutrients 2020, 12, 3369. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut–brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Jiang, J.; Pan, Y.; Yang, Y.; Fang, X.; Liang, L.; Li, H.; Dong, Z.; Fan, S.; et al. Gut microbial GABA imbalance emerges as a metabolic signature in mild autism spectrum disorder linked to overrepresented Escherichia. Cell Rep. Med. 2025, 6, 101919. [Google Scholar] [CrossRef]
- Mohebali, N.; Weigel, M.; Hain, T.; Sütel, M.; Bull, J.; Kreikemeyer, B.; Breitrück, A. Faecalibacterium prausnitzii, Bacteroides faecis and Roseburia intestinalis attenuate clinical symptoms of experimental colitis by regulating Treg/Th17 cell balance and intestinal barrier integrity. Biomed. Pharmacother. 2023, 167, 115568. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Mehra, A.; Arora, G.; Sahni, G.; Kaur, M.; Singh, H.; Singh, B.; Kaur, S. Gut microbiota and Autism Spectrum Disorder: From pathogenesis to potential therapeutic perspectives. J. Tradit. Complement. Med. 2023, 13, 135–149. [Google Scholar] [CrossRef]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal Microflora Studies in Late-Onset Autism. Clin. Infect. Dis. 2002, 35 (Suppl. S1), S6–S16. [Google Scholar] [CrossRef]
- Su, Q.; Wong, O.W.H.; Lu, W.; Wan, Y.; Zhang, L.; Xu, W.; Li, M.K.T.; Liu, C.; Cheung, C.P.; Ching, J.Y.L.; et al. Multikingdom and functional gut microbiota markers for autism spectrum disorder. Nat. Microbiol. 2024, 9, 2344–2355. [Google Scholar] [CrossRef]
- Del Chierico, F.; Manco, M.; Gardini, S.; Guarrasi, V.; Russo, A.; Bianchi, M.; Tortosa, V.; Quagliariello, A.; Shashaj, B.; Fintini, D.; et al. Fecal microbiota signatures of insulin resistance, inflammation, and metabolic syndrome in youth with obesity: A pilot study. Acta Diabetol. 2021, 58, 1009–1022. [Google Scholar] [CrossRef]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Luo, X.; Meng, Y.; Zhong, Z.; Zheng, H.; Yang, Y. The fecal microbiota from children with autism impact gut metabolism and learning and memory abilities of honeybees. Front. Microbiol. 2023, 14, 1278162. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Xu, X.J.; Lang, J.D.; Yang, J.; Long, B.; Liu, X.D.; Zeng, X.F.; Tian, G.; You, X. Differences of gut microbiota and behavioral symptoms between two subgroups of autistic children based on γδT cells-derived IFN-γ Levels: A preliminary study. Front. Immunol. 2023, 14, 1100816. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Zhang, J.; He, Y.; Wang, J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci. Rep. 2018, 8, 13981. [Google Scholar] [CrossRef]
- Marchetto, M.C.; Belinson, H.; Tian, Y.; Freitas, B.C.; Fu, C.; Vadodaria, K.C.; Beltrao-Braga, P.; Trujillo, C.A.; Mendes, A.P.D.; Padmanabhan, K.; et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatry 2017, 22, 820–835. [Google Scholar] [CrossRef]
- Khwaja, O.S.; Ho, E.; Barnes, K.V.; O’Leary, H.M.; Pereira, L.M.; Finkelstein, Y.; Nelson, C.A.; Vogel-Farley, V.; DeGregorio, G.; Holm, I.A.; et al. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4596–4601. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Study Details|A Pilot Treatment Study of Insulin-like Growth Factor-1 (IGF-1) in Autism Spectrum Disorder|ClinicalTrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT01970345?utm_source=chatgpt.com (accessed on 26 June 2025).
- Lin, C.H.; Zeng, T.; Lu, C.W.; Li, D.Y.; Liu, Y.Y.; Li, B.M.; Chen, S.Q.; Deng, Y.H. Efficacy and safety of Bacteroides fragilis BF839 for pediatric autism spectrum disorder: A randomized clinical trial. Front. Nutr. 2024, 11, 1447059. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Salles, B.I.M.; Cioffi, D.; Ferreira, S.R.G. Probiotics supplementation and insulin resistance: A systematic review. Diabetol. Metab. Syndr. 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Liu, Y.; Rhoads, J.M. Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 2016, 22, 10093–10102. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).