Mapping Morphine’s Antinociceptive Impact on the Ventral Tegmental Area During Nociceptive Stimulation: A Novel Microimaging Approach in a Neuropathic Pain Model
Abstract
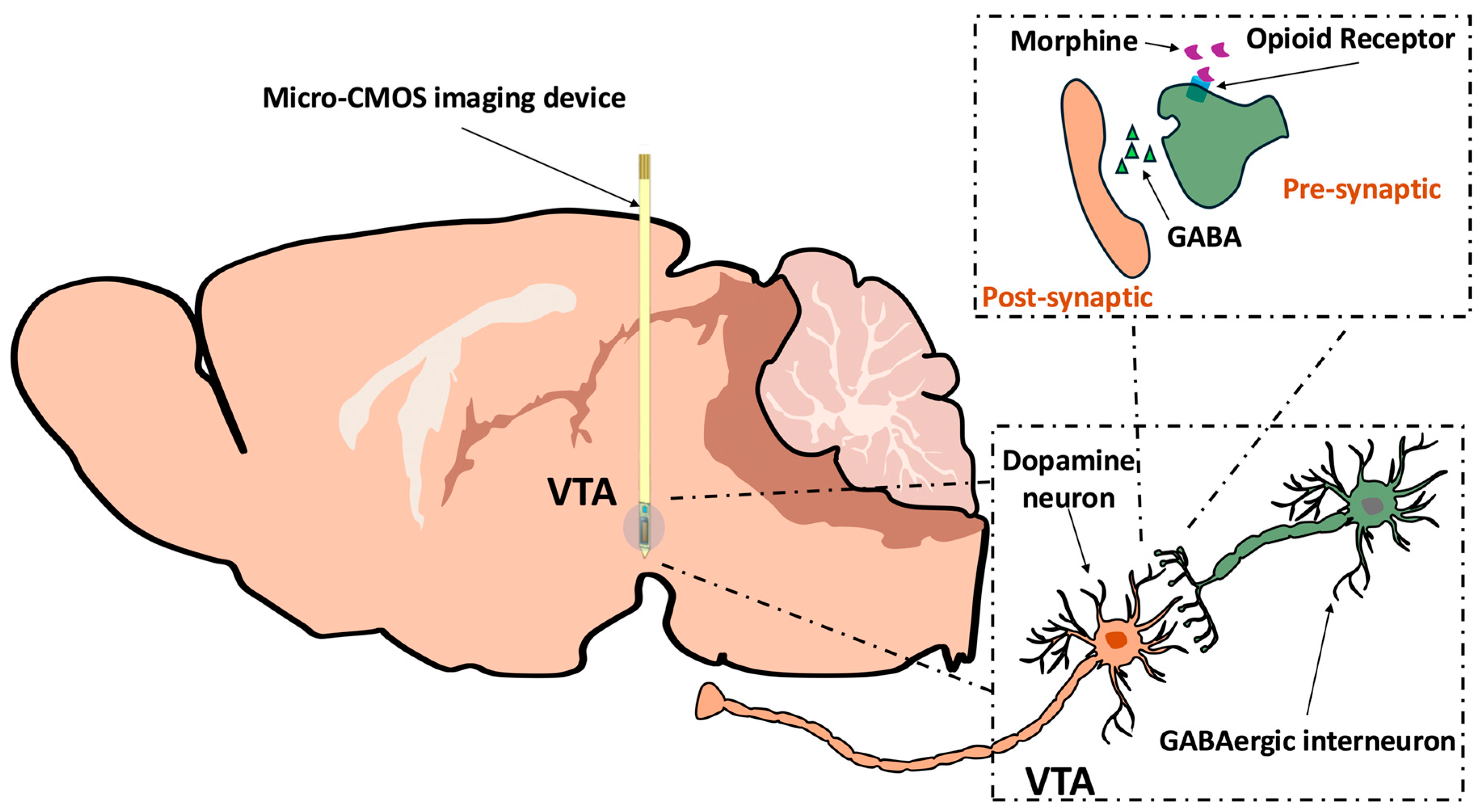
1. Introduction
2. Results
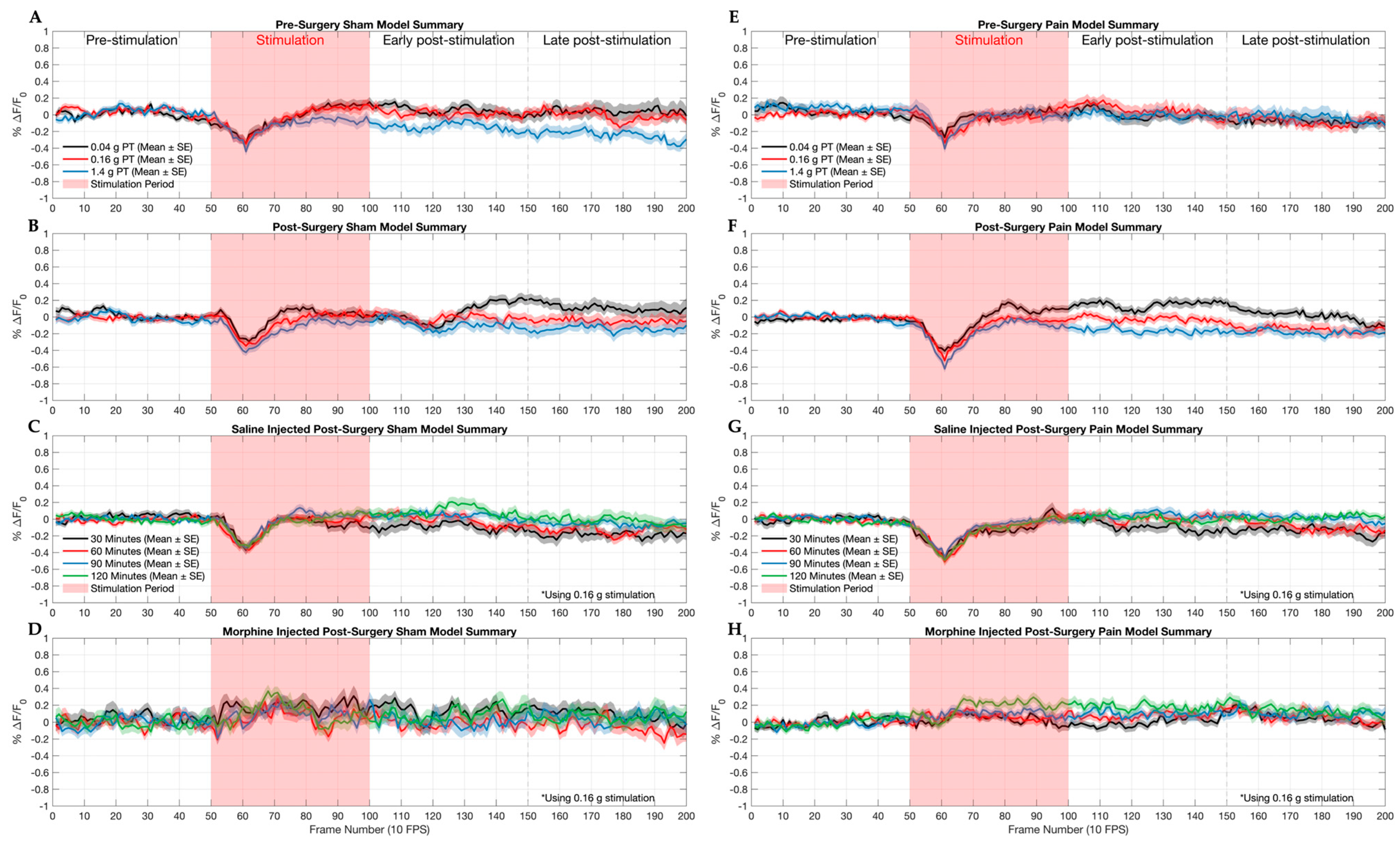
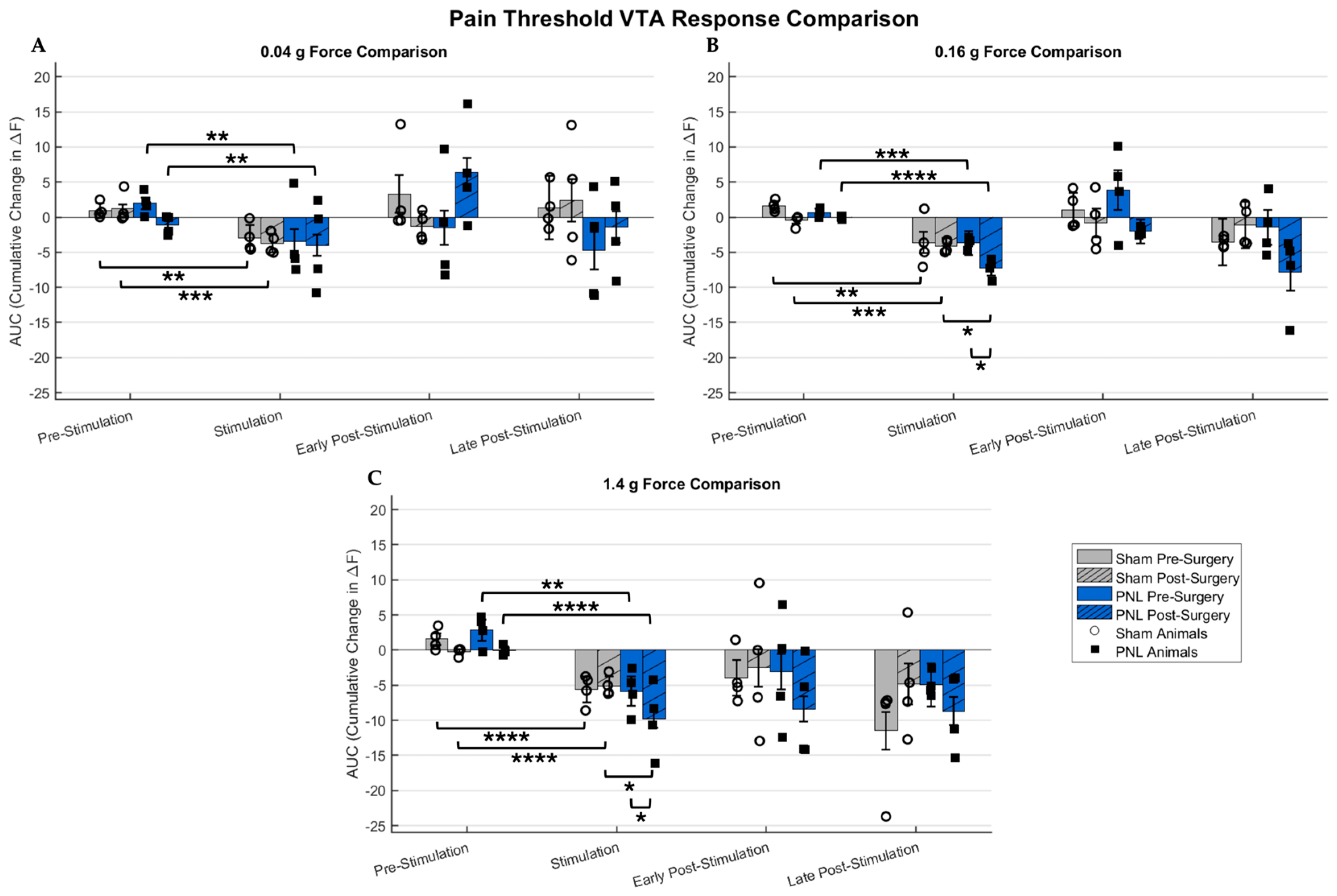
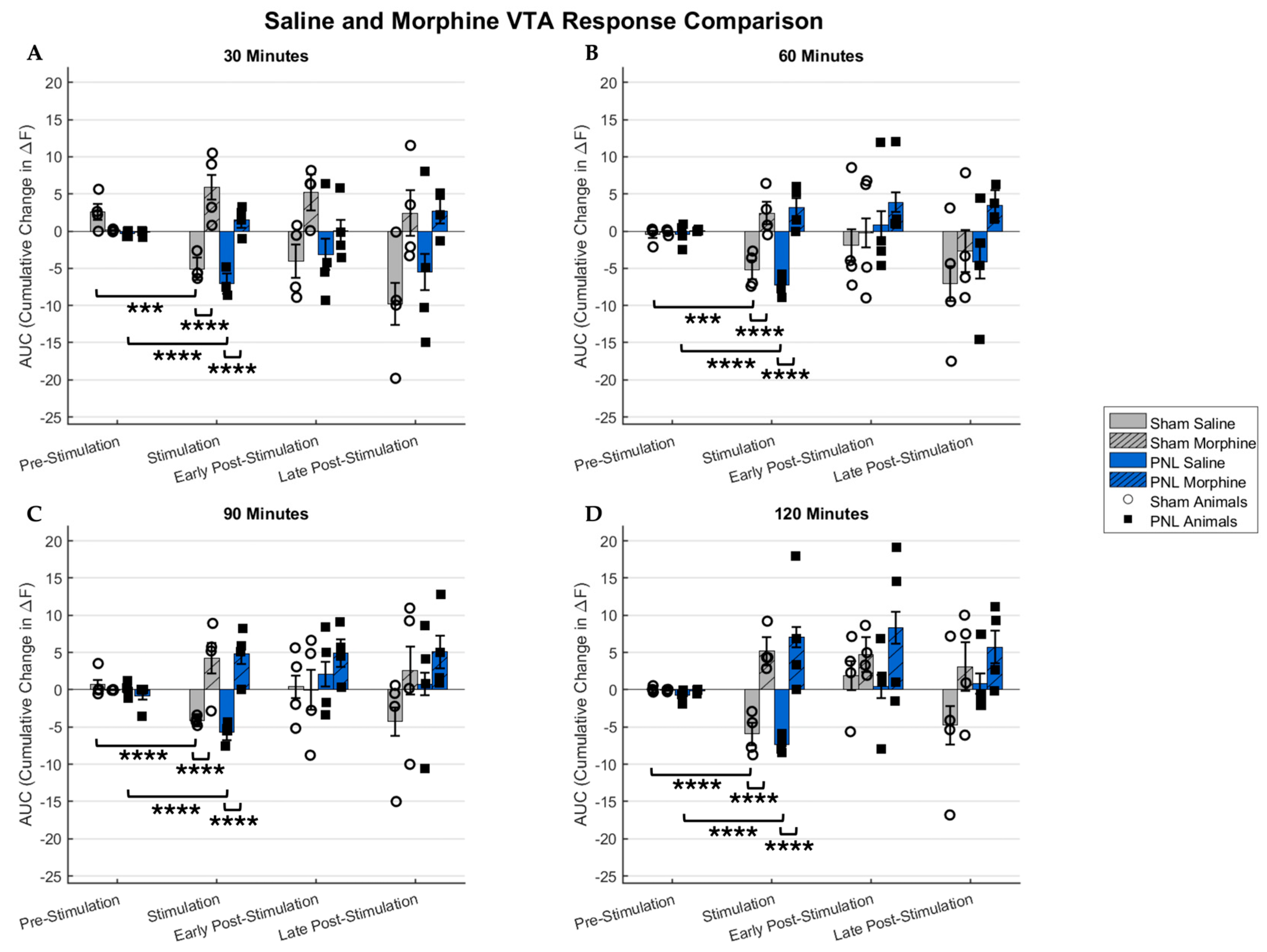
2.1. Sham and Pain Model Mice Responses to Graded Stimulation and Analgesic Condition
2.2. Confirmation of GCaMP6s Virus Expression and CMOS Imaging Device Position
3. Discussion
3.1. CMOS Imaging and Alternative Methods
3.2. Imaging Acute Nociception in a Chronic Pain Model with Therapeutic Treatment
4. Materials and Methods
4.1. Ethics Statement and Animal Care
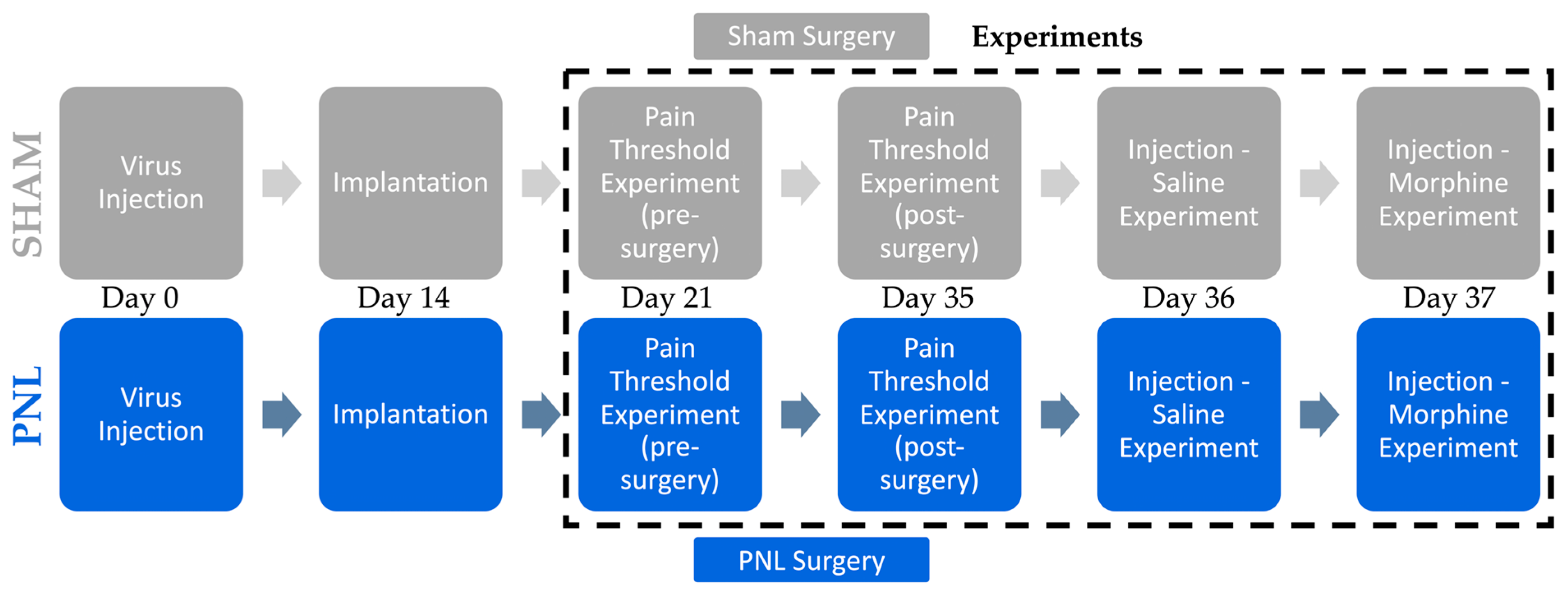
4.2. Experimental Design
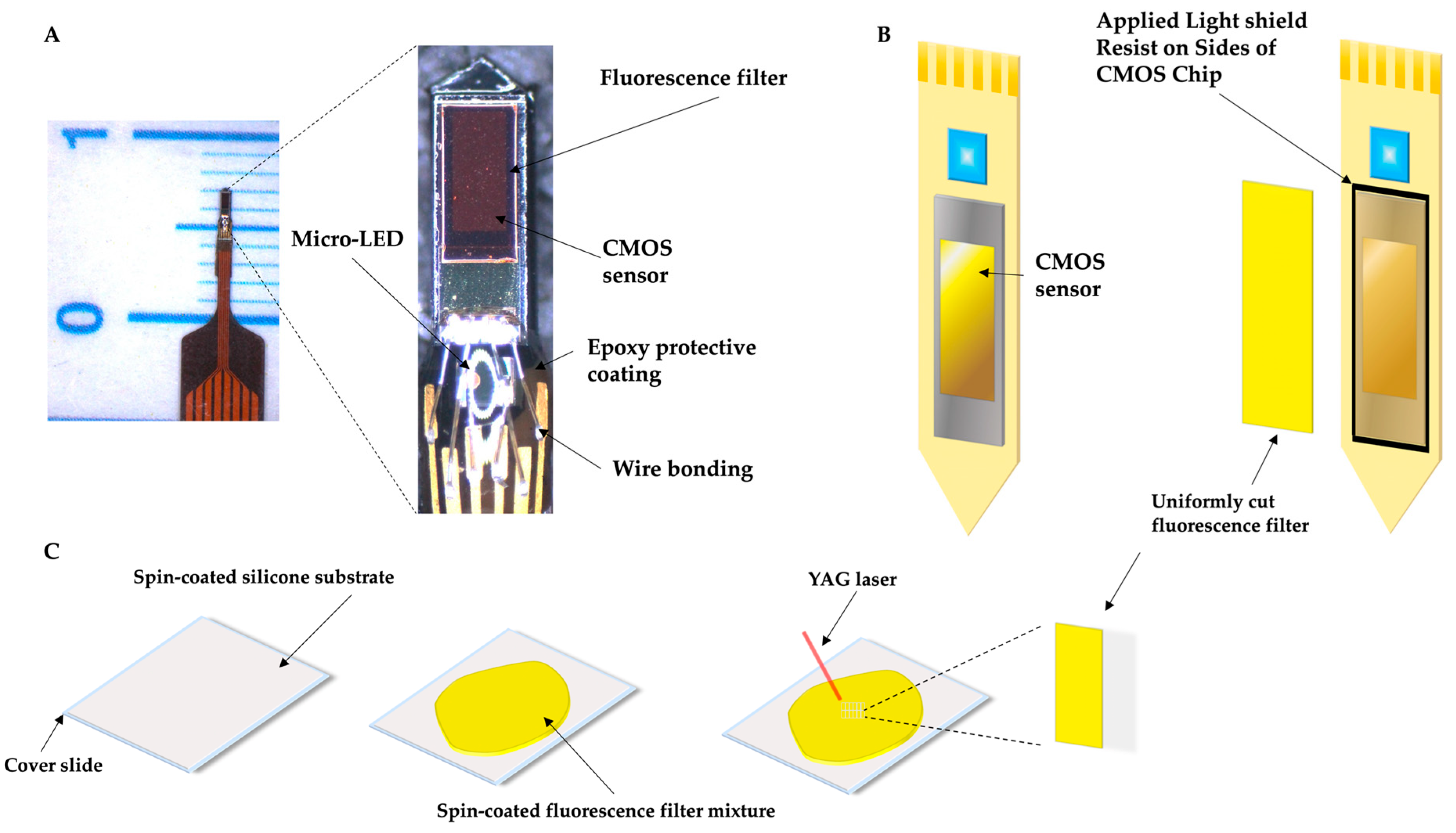
4.3. Imaging Device and Fabrication
4.4. Stereotaxis Surgery
4.5. GCaMP6s AAV Injection
4.6. CMOS Imaging Device Implantation
4.7. Sham and Partial Ligation of the Sciatic Nerve Surgery
4.8. Imaging and ROI Analysis
4.9. Implantation Site Confirmation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DA | Dopamine |
| VTA | Ventral tegmental area |
| NAc | Nucleus accumbens |
| GABA | γ-aminobutyric acid |
| PNL | Partial nerve ligation |
| PFC | Prefrontal cortex |
| CMOS | Complementary metal–oxide–semiconductor |
| CRF | Corticotropin-releasing factor |
| AAV | Adeno-associated virus |
| ROI | Region of interest |
| CNS | Central nervous system |
References
- Kauer, J.A.; Malenka, R.C. Synaptic Plasticity and Addiction. Nat. Rev. Neurosci. 2007, 8, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, W.; Ma, Y.; Tossell, K.; Harris, J.J.; Harding, E.C.; Ba, W.; Miracca, G.; Wang, D.; Li, L.; et al. GABA and Glutamate Neurons in the VTA Regulate Sleep and Wakefulness. Nat. Neurosci. 2019, 22, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Moulin, T.C.; Schiöth, H.B. Excitability, Synaptic Balance, and Addiction: The Homeostatic Dynamics of Ionotropic Glutamatergic Receptors in VTA after Cocaine Exposure. Behav. Brain Funct. 2020, 16, 6. [Google Scholar] [CrossRef]
- Ziółkowska, B. The Role of Mesostriatal Dopamine System and Corticostriatal Glutamatergic Transmission in Chronic Pain. Brain Sci. 2021, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Mauderli, A.P.; Vierck, C.J.; Cannon, R.L.; Rodrigues, A.; Shen, C. Relationships Between Skin Temperature and Temporal Summation of Heat and Cold Pain. J. Neurophysiol. 2003, 90, 100–109. [Google Scholar] [CrossRef]
- Klaphake, E. Bacterial and Parasitic Diseases of Selected Invertebrates. Vet. Clin. North Am. Exot. Anim. Pract. 2009, 12, 639–648. [Google Scholar] [CrossRef]
- Markovic, T.; Pedersen, C.E.; Massaly, N.; Vachez, Y.M.; Ruyle, B.; Murphy, C.A.; Abiraman, K.; Shin, J.H.; Garcia, J.J.; Yoon, H.J.; et al. Pain Induces Adaptations in Ventral Tegmental Area Dopamine Neurons to Drive Anhedonia-like Behavior. Nat. Neurosci. 2021, 24, 1601–1613. [Google Scholar] [CrossRef]
- Nguyen, A.; Wang, J.; Holland, K.M.; Ehlman, D.C.; Welder, L.E.; Miller, K.D.; Stone, D.M. Trends in Drug Overdose Deaths by Intent and Drug Categories, United States, 1999–2022. Am. J. Public Health 2024, 114, 1081–1085. [Google Scholar] [CrossRef]
- Cooper, T.E.; Chen, J.; Wiffen, P.J.; Derry, S.; Carr, D.B.; Aldington, D.; Cole, P.; Moore, R.A. Morphine for Chronic Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2017, 5, CD011669. [Google Scholar] [CrossRef]
- Galaj, E.; Han, X.; Shen, H.; Jordan, C.J.; He, Y.; Humburg, B.; Bi, G.-H.; Xi, Z.-X. Dissecting the Role of GABA Neurons in the VTA versus SNr in Opioid Reward. J. Neurosci. 2020, 40, 8853–8869. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Zhao, X.; Cui, R.; Li, X. Relationship between the Role of Muscarinic M3 Receptors in Morphine-Induced Conditioned Place Preference and the Mesolimbic Dopamine System. Neurosci. Lett. 2022, 786, 136774. [Google Scholar] [CrossRef] [PubMed]
- Akbar, L.; Castillo, V.C.G.; Olorocisimo, J.P.; Ohta, Y.; Kawahara, M.; Takehara, H.; Haruta, M.; Tashiro, H.; Sasagawa, K.; Ohsawa, M.; et al. Multi-Region Microdialysis Imaging Platform Revealed Dorsal Raphe Nucleus Calcium Signaling and Serotonin Dynamics during Nociceptive Pain. Int. J. Mol. Sci. 2023, 24, 6654. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, Y.; Ohta, Y.; Akay, Y.M.; Ohta, J.; Akay, M. Monitoring Neural Activities in the VTA in Response to Nicotine Intake Using a Novel Implantable Microimaging Device. IEEE J. Mag. 2020, 8, 68013–68020. Available online: https://ieeexplore.ieee.org/document/9057587 (accessed on 8 January 2025). [CrossRef]
- Sunaga, Y.; Ohta, Y.; Murakami, T.E.; Akay, Y.M.; Ohta, J.; Akay, M. Monitoring Neuronal Dynamics in the Ventral Tegmental Area Using an Implantable Microimaging Device with Microdialysis System. IEEE J. Mag. 2021, 9, 55871–55878. Available online: https://ieeexplore.ieee.org/document/9398866 (accessed on 8 January 2025). [CrossRef]
- Ganaway, A.; Tatsuta, K.; Castillo, V.C.G.; Okada, R.; Sunaga, Y.; Ohta, Y.; Ohta, J.; Ohsawa, M.; Akay, M.; Akay, Y.M. Investigating the Influence of Morphine and Cocaine on the Mesolimbic Pathway Using a Novel Microimaging Platform. Int. J. Mol. Sci. 2023, 24, 16303. [Google Scholar] [CrossRef]
- Olorocisimo, J.P.; Ohta, Y.; Regonia, P.R.; Castillo, V.C.G.; Yoshimoto, J.; Takehara, H.; Sasagawa, K.; Ohta, J. Brain-Implantable Needle-Type CMOS Imaging Device Enables Multi-Layer Dissection of Seizure Calcium Dynamics in the Hippocampus. J. Neural Eng. 2024, 21, 046022. [Google Scholar] [CrossRef]
- Ohta, J.; Ohta, Y.; Takehara, H.; Noda, T.; Sasagawa, K.; Tokuda, T.; Haruta, M.; Kobayashi, T.; Akay, Y.M.; Akay, M. Implantable Microimaging Device for Observing Brain Activities of Rodents. Proc. IEEE 2017, 105, 158–166. [Google Scholar] [CrossRef]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole Animal Perfusion Fixation for Rodents. J. Vis. Exp. 2012, 65, 3564. [Google Scholar] [CrossRef]
- Xu, C.; Nedergaard, M.; Fowell, D.J.; Friedl, P.; Ji, N. Multiphoton Fluorescence Microscopy for In Vivo Imaging. Cell 2024, 187, 4458–4487. [Google Scholar] [CrossRef]
- Chen, T.; Xue, Z.; Wang, C.; Qu, Z.; Wong, K.K.; Wong, S.T.C. Motion Correction for Cellular-Resolution Multi-Photon Fluorescence Microscopy Imaging of Awake Head-Restrained Mice Using Speed Embedded HMM. Comput. Med. Imaging Graph. 2012, 36, 171–182. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Cocker, E.D.; Piyawattanametha, W.; Jung, J.C.; Cheung, E.L.M.; Schnitzer, M.J. Fiber-Optic Fluorescence Imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef]
- Kielbinski, M.; Bernacka, J. Fiber Photometry in Neuroscience Research: Principles, Applications, and Future Directions. Pharmacol. Rep. 2024, 76, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Beacher, N.J.; Washington, K.A.; Zhang, Y.; Li, Y.; Lin, D.-T. GRIN Lens Applications for Studying Neurobiology of Substance Use Disorder. Addict. Neurosci. 2022, 4, 100049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, B.; Barbera, G.; Hawes, S.; Zhang, Y.; Stump, K.; Baum, I.; Yang, Y.; Li, Y.; Lin, D.-T. Miniscope GRIN Lens System for Calcium Imaging of Neuronal Activity from Deep Brain Structures in Behaving Animals. Curr. Protoc. Neurosci. 2019, 86, e56. [Google Scholar] [CrossRef]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of Brain Microdialysis. Curr. Protoc. Neurosci. 2009, 47, 7.1.1–7.1.28. [Google Scholar] [CrossRef]
- Brischoux, F.; Chakraborty, S.; Brierley, D.I.; Ungless, M.A. Phasic Excitation of Dopamine Neurons in Ventral VTA by Noxious Stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 4894–4899. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jia, W.-B.; Xu, X.; Chen, R.; Wang, L.-B.; Su, X.-J.; Xu, P.-F.; Liu, X.-Q.; Wen, J.; Song, X.-Y.; et al. A Glutamatergic DRN–VTA Pathway Modulates Neuropathic Pain and Comorbid Anhedonia-like Behavior in Mice. Nat. Commun. 2023, 14, 5124. [Google Scholar] [CrossRef]
- Leknes, S.; Tracey, I. A Common Neurobiology for Pain and Pleasure. Nat. Rev. Neurosci. 2008, 9, 314–320. [Google Scholar] [CrossRef]
- Matsui, A.; Williams, J.T. Opioid-Sensitive GABA Inputs from Rostromedial Tegmental Nucleus Synapse onto Midbrain Dopamine Neurons. J. Neurosci. 2011, 31, 17729–17735. [Google Scholar] [CrossRef]
- Ganaway, A.; Sunaga, Y.; Ohta, Y.; Ohta, J.; Akay, M.; Akay, Y.M. Investigating the Modulation of the VTA Neurons in Nicotine-Exposed Pups during Early Maturation Using Optogenetics. Int. J. Mol. Sci. 2023, 24, 2280. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.-C.; Xu, Z.; Gan, J.; Yu, Y.-M.; Tang, H.-M.; Zhu, Y.; Yang, J.-X.; Ding, H.-L.; Cao, J.-L. Acute Ongoing Nociception Delays Recovery of Consciousness from Sevoflurane Anesthesia via a Midbrain Circuit. J. Neurosci. 2024, 44, e0740242024. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.A.; Mazei-Robison, M.S. Opioid-Induced Molecular and Cellular Plasticity of Ventral Tegmental Area Dopamine Neurons. Cold Spring Harb. Perspect. Med. 2021, 11, a039362. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganaway, A.; Kamata, A.; Yao, D.; Sakoori, K.; Okada, R.; Chen, T.; Ohta, Y.; Ohta, J.; Ohsawa, M.; Akay, M.; et al. Mapping Morphine’s Antinociceptive Impact on the Ventral Tegmental Area During Nociceptive Stimulation: A Novel Microimaging Approach in a Neuropathic Pain Model. Int. J. Mol. Sci. 2025, 26, 6526. https://doi.org/10.3390/ijms26136526
Ganaway A, Kamata A, Yao D, Sakoori K, Okada R, Chen T, Ohta Y, Ohta J, Ohsawa M, Akay M, et al. Mapping Morphine’s Antinociceptive Impact on the Ventral Tegmental Area During Nociceptive Stimulation: A Novel Microimaging Approach in a Neuropathic Pain Model. International Journal of Molecular Sciences. 2025; 26(13):6526. https://doi.org/10.3390/ijms26136526
Chicago/Turabian StyleGanaway, Austin, Airi Kamata, Dunyan Yao, Kazuto Sakoori, Ryoma Okada, Ting Chen, Yasumi Ohta, Jun Ohta, Masahiro Ohsawa, Metin Akay, and et al. 2025. "Mapping Morphine’s Antinociceptive Impact on the Ventral Tegmental Area During Nociceptive Stimulation: A Novel Microimaging Approach in a Neuropathic Pain Model" International Journal of Molecular Sciences 26, no. 13: 6526. https://doi.org/10.3390/ijms26136526
APA StyleGanaway, A., Kamata, A., Yao, D., Sakoori, K., Okada, R., Chen, T., Ohta, Y., Ohta, J., Ohsawa, M., Akay, M., & Akay, Y. M. (2025). Mapping Morphine’s Antinociceptive Impact on the Ventral Tegmental Area During Nociceptive Stimulation: A Novel Microimaging Approach in a Neuropathic Pain Model. International Journal of Molecular Sciences, 26(13), 6526. https://doi.org/10.3390/ijms26136526









