Urinary Inflammatory and Oxidative Stress Biomarkers as Indicators for the Clinical Management of Benign Prostatic Hyperplasia
Abstract
1. Introduction
2. Results
2.1. Baseline Clinical Characteristics and Urinary Biomarker Profiles
2.2. Treatment Outcomes and Changes in Urinary Biomarkers
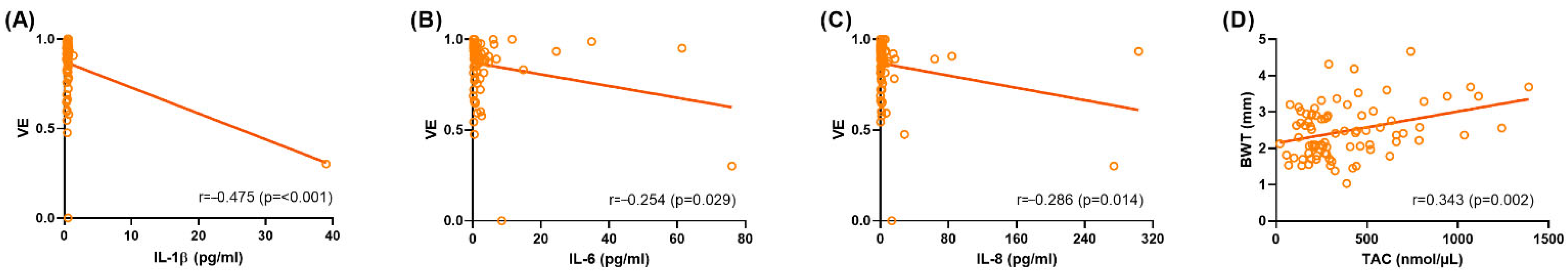
2.3. Correlation Analyses Between Clinical Characteristics and Urinary Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Clinical Assessment and Follow-Up
4.3. Assessment of Urinary Biomarker Levels
4.4. Quantification of Urinary Oxidative Stress Biomarkers
4.5. Quantification of Urinary Inflammatory Biomarkers
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.P.; Chuang, Y.C.; Sumarsono, B.; Chang, H.C. The prevalence and bother of lower urinary tract symptoms in men and women aged 40 years or over in Taiwan. J. Formos. Med. Assoc. 2019, 118, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.T. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)? World J. Urol. 2019, 37, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Kuo, H.-C. Videourodynamic analysis in men with lower urinary tract symptoms: Correlation between age and prostate size with lower urinary tract dysfunction. Urol. Sci. 2016, 27, 21–25. [Google Scholar] [CrossRef]
- Mirone, V.; Imbimbo, C.; Longo, N.; Fusco, F. The detrusor muscle: An innocent victim of bladder outlet obstruction. Eur. Urol. 2007, 51, 57–66. [Google Scholar] [CrossRef]
- Fusco, F.; Creta, M.; De Nunzio, C.; Iacovelli, V.; Mangiapia, F.; Li Marzi, V.; Finazzi Agro, E. Progressive bladder remodeling due to bladder outlet obstruction: A systematic review of morphological and molecular evidences in humans. BMC Urol. 2018, 18, 15. [Google Scholar] [CrossRef]
- Miyata, Y.; Matsuo, T.; Mitsunari, K.; Asai, A.; Ohba, K.; Sakai, H. A Review of Oxidative Stress and Urinary Dysfunction Caused by Bladder Outlet Obstruction and Treatments Using Antioxidants. Antioxidants 2019, 8, 132. [Google Scholar] [CrossRef]
- Bostanci, Y.; Kazzazi, A.; Momtahen, S.; Laze, J.; Djavan, B. Correlation between benign prostatic hyperplasia and inflammation. Curr. Opin. Urol. 2013, 23, 5–10. [Google Scholar] [CrossRef]
- Inamura, S.; Terada, N. Chronic inflammation in benign prostatic hyperplasia: Pathophysiology and treatment options. Int. J. Urol. 2024, 31, 968–974. [Google Scholar] [CrossRef]
- Kaltsas, A.; Giannakas, T.; Stavropoulos, M.; Kratiras, Z.; Chrisofos, M. Oxidative Stress in Benign Prostatic Hyperplasia: Mechanisms, Clinical Relevance and Therapeutic Perspectives. Diseases 2025, 13, 53. [Google Scholar] [CrossRef]
- Tyagi, P.; Wang, Z.; Yoshimura, N. Urinary Biomarkers and Benign Prostatic Hyperplasia. Curr. Bladder Dysfunct. Rep. 2019, 14, 31–40. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Malkov, M.I.; Lee, C.T.; Taylor, C.T. Regulation of the Hypoxia-Inducible Factor (HIF) by Pro-Inflammatory Cytokines. Cells 2021, 10, 2340. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.R.; Jiang, Y.H.; Jhang, J.F.; Kuo, H.C. Use of Urinary Biomarkers in Discriminating Interstitial Cystitis/Bladder Pain Syndrome from Male Lower Urinary Tract Dysfunctions. Int. J. Mol. Sci. 2023, 24, 12055. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Motley, S.S.; Koyama, T.; Kashyap, M.; Gingrich, J.; Yoshimura, N.; Fowke, J.H. Molecular correlates in urine for the obesity and prostatic inflammation of BPH/LUTS patients. Prostate 2018, 78, 17–24. [Google Scholar] [CrossRef]
- Tyagi, P.; Motley, S.S.; Kashyap, M.; Pore, S.; Gingrich, J.; Wang, Z.; Yoshimura, N.; Fowke, J.H. Urine chemokines indicate pathogenic association of obesity with BPH/LUTS. Int. Urol. Nephrol. 2015, 47, 1051–1058. [Google Scholar] [CrossRef][Green Version]
- Komninos, C.; Mitsogiannis, I. Obstruction-induced alterations within the urinary bladder and their role in the pathophysiology of lower urinary tract symptomatology. Can. Urol. Assoc. J. 2014, 8, E524–E530. [Google Scholar] [CrossRef]
- Akshay, A.; Gheinani, A.H.; Besic, M.; Braga, S.; Uldry, A.C.; Heller, M.; Rehrauer, H.; Fournier, C.A.; Burkhard, F.C.; Monastyrskaya, K. De-obstruction of bladder outlet in humans reverses organ remodelling by normalizing the expression of key transcription factors. BMC Urol. 2024, 24, 33. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lin, Y.P.; Levin, R.M.; Chen, M.L. The relevance of immune responses to partial bladder outlet obstruction and reversal. Neurourol. Urodyn. 2017, 36, 1306–1312. [Google Scholar] [CrossRef]
- Lin, W.Y.; Wu, S.B.; Lin, Y.P.; Chang, P.J.; Levin, R.M.; Wei, Y.H. Reversing bladder outlet obstruction attenuates systemic and tissue oxidative stress. BJU Int. 2012, 110, 1208–1213. [Google Scholar] [CrossRef]
- Di Minno, A.; Aveta, A.; Gelzo, M.; Tripodi, L.; Pandolfo, S.D.; Crocetto, F.; Imbimbo, C.; Castaldo, G. 8-Hydroxy-2-Deoxyguanosine and 8-Iso-Prostaglandin F2alpha: Putative Biomarkers to assess Oxidative Stress Damage Following Robot-Assisted Radical Prostatectomy (RARP). J. Clin. Med. 2022, 11, 6102. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hanai, T.; Matsui, T.; Oka, M.; Tanaka, M.; Uemura, H. Eviprostat suppresses urinary oxidative stress in a rabbit model of partial bladder outlet obstruction and in patients with benign prostatic hyperplasia. Phytother. Res. 2010, 24, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y.; Majima, T.; Matsukawa, Y.; Yamamoto, T.; Yoshida, M.; Gotoh, M. Intraprostatic Reflux of Urine Induces Inflammation in a Rat. Prostate 2017, 77, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, W.; Liu, X.; Wang, H.; Fang, Z.; Liang, C.; Wang, T.; Xu, K. Nerve Growth Factor Levels are Associated with Overactive Bladder Symptoms and Long-Term Treatment Outcome after Transurethral Resection of the Prostate in Patients with Benign Prostatic Hyperplasia. J. Urol. 2018, 200, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Manieri, C.; Carter, S.S.; Romano, G.; Trucchi, A.; Valenti, M.; Tubaro, A. The diagnosis of bladder outlet obstruction in men by ultrasound measurement of bladder wall thickness. J. Urol. 1998, 159, 761–765. [Google Scholar] [CrossRef]
- Nickel, J.C.; O’Leary, M.P.; Lepor, H.; Caramelli, K.E.; Thomas, H.; Hill, L.A.; Hoel, G.E. Silodosin for men with chronic prostatitis/chronic pelvic pain syndrome: Results of a phase II multicenter, double-blind, placebo controlled study. J. Urol. 2011, 186, 125–131. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Ho, H.C.; Hsu, Y.H.; Kuo, H.C. Diagnostic and prognostic value of urine biomarkers among women with dysfunctional voiding. Sci. Rep. 2022, 12, 6608. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine cytokines as biomarkers for diagnosing interstitial cystitis/bladder pain syndrome and mapping its clinical characteristics. Am. J. Physiol. Ren. Physiol. 2020, 318, F1391–F1399. [Google Scholar] [CrossRef]
- Chen, S.F.; Jiang, Y.H.; Kuo, H.C. Urinary biomarkers in patients with detrusor underactivity with and without bladder function recovery. Int. Urol. Nephrol. 2017, 49, 1763–1770. [Google Scholar] [CrossRef]

| Clinical BPH | |||||||
|---|---|---|---|---|---|---|---|
| Medical Treatment Group N = 33 | Surgical Treatment Group N = 29 | Overall N = 62 | Control N = 20 | p-Value $ | p-Value # | ||
| Age | 64.28 ± 7.12 | 66.88 ± 6.19 | 65.33 ± 6.84 | 38.0 ± 7.9 | <0.001 | 0.087 | |
| PSA (ng/mL) | 2.41 ± 1.95 | 2.95 ± 2.66 | 2.63 ± 2.26 | 0.287 | 0.287 | ||
| TPV (mL) | 41.4 ± 13.05 | 44.76 ± 16.41 | 42.76 ± 14.5 | 0.300 | 0.300 | ||
| TZI | 0.41 ± 0.11 | 0.44 ± 0.11 | 0.43 ± 0.11 | 0.230 | 0.230 | ||
| DWT (mm) | 1.17 ± 0.54 | 1.08 ± 0.38 | 1.13 ± 0.48 | 0.69 ± 0.20 | <0.001 | 0.410 | |
| BWT (mm) | 2.53 ± 0.81 | 2.42 ± 0.57 | 2.48 ± 0.72 | 1.58 ± 0.33 | <0.001 | 0.467 | |
| Symptom scores | |||||||
| IPSS-V | Baseline | 11.08 ± 5.31 | 12.35 ± 4.91 | 11.60 ± 5.16 | 0.50 ± 0.89 | <0.001 | 0.270 |
| Δ at 3mo | −3.48 ± 5.96 * | −9.62 ± 6.55 * | −6.35 ± 6.92 * | <0.001 | |||
| IPSS-S | Baseline | 5.20 ± 3.84 | 6.74 ± 4.19 | 5.82 ± 4.04 | 0.70 ± 1.030 | <0.001 | 0.087 |
| Δ at 3mo | −1.91 ± 3.6 * | −3.17 ± 4.18 * | −2.50 ± 3.90 * | 0.206 | |||
| IPSS | Baseline | 16.28 ± 7 | 19.09 ± 7.03 | 17.42 ± 7.11 | 1.20 ± 1.20 | <0.001 | 0.075 |
| Δ at 3mo | −5.39 ± 7.16 * | −12.79 ± 9.50 * | −8.85 ± 9.06 * | 0.001 | |||
| QoL | Baseline | 3.62 ± 1.1 | 3.97 ± 1.27 | 3.76 ± 1.18 | 0.65 ± 0.75 | <0.001 | 0.182 |
| Δ at 3mo | −1.09 ± 1.65 * | −2.31 ± 1.51 * | −1.66 ± 1.69 * | 0.004 | |||
| OABSS | Baseline | 4.52 ± 3.52 | 5.32 ± 3.20 | 4.85 ± 3.40 | 0.65 ± 0.67 | <0.001 | 0.290 |
| Δ at 3mo | −2.06 ± 3.77 * | −1.69 ± 3.63 * | −1.89 ± 3.68 * | 0.696 | |||
| Uroflowmetry | |||||||
| BC (mL) | Baseline | 301.15 ± 179.27 | 263.83 ± 119.17 | 286.59 ± 158.78 | 441.71 ± 156.22 | <0.001 | 0.302 |
| Δ at 3mo | 25.55 ± 173.73 | −14.17 ± 85.88 | 6.4 ± 138.76 | 0.280 | |||
| Qmax (mL/s) | Baseline | 9.72 ± 3.43 | 8.05 ± 4.14 | 9.07 ± 3.79 | 24.72 ± 7.33 | <0.001 | 0.051 |
| Δ at 3mo | 1.09 ± 5.41 | 6.06 ± 5.68 * | 3.49 ± 6.04 * | 0.001 | |||
| cQmax | Baseline | 0.6 ± 0.21 | 0.53 ± 0.28 | 0.57 ± 0.24 | 1.30 ± 0.72 | <0.001 | 0.225 |
| Δ at 3mo | 0.03 ± 0.28 | 0.4 ± 0.37 * | 0.21 ± 0.37 * | <0.001 | |||
| Vol (mL) | Baseline | 259.86 ± 131.37 | 213.81 ± 97.49 | 241.89 ± 120.78 | 425.1 ± 153.52 | <0.001 | 0.092 |
| Δ at 3mo | 13.1 ± 116.06 | 14.7 ± 80.43 | 13.88 ± 99.58 | 0.953 | |||
| PVR (mL) | Baseline | 41.3 ± 105.39 | 50.03 ± 54.45 | 44.71 ± 88.72 | 16.65 ± 21.62 | 0.342 | 0.667 |
| Δ at 3mo | 12.45 ± 198.92 | −28.85 ± 60.24 | −7.46 ± 149.31 | 0.305 | |||
| VE | Baseline | 0.89 ± 0.11 | 0.83 ± 0.16 | 0.87 ± 0.13 | 0.95 ± 0.08 | 0.005 | 0.086 |
| Δ at 3mo | −0.03 ± 0.21 | 0.08 ± 0.18 * | 0.02 ± 0.2 | 0.054 |
| Clinical BPH | |||||||
|---|---|---|---|---|---|---|---|
| Medical Treatment Group N = 33 | Surgical Treatment Group N = 29 | Overall N = 62 | Control N = 20 | p-Value $ | p-Value # | ||
| Urine biomarkers @ | |||||||
| 8-OHdG | Baseline | 79.83 (29.65, 110.22) | 102.65 (39.43, 132.08) | 85.31 (36.51, 118.61) | 65.1 (54.95, 89.19) | 0.136 | 0.505 |
| Δ at 3mo | −1.05 (−41.38, 13.44) | 9.45 (−26.23, 47.85) | 1.75 (−40.18, 31.69) | 0.121 | |||
| 8-isoprostane | Baseline | 11.88 (7.59, 26.73) | 16.6 (9.56, 30.2) | 14.16 (8.98, 29.82) | 20.72 (12.86, 30.68) | 0.057 | 0.266 |
| Δ at 3mo | −2.88 (−8.18, 2.73) | −4.12 (−21.24, 12.31) | −4.11 (−14.03, 4.66) * | 1.000 | |||
| TAC | Baseline | 262.65 (179.62, 446.77) | 316.41 (198.45, 585.2) | 282.56 (195, 489.78) | 167.03 (103.82, 416.68) | 0.045 | 0.266 |
| Δ at 3mo | −23.18 (−136.51, 65.94) | −64.7 (−443.65, 65.08) * | −47.81 (−207.97, 64.13) * | 0.605 | |||
| PGE2 | Baseline | 166.95 (119.72, 301.06) | 271.32 (185.2, 409.52) | 218.92 (124.77, 339.98) | 128.58 (87.85, 367.99) | 0.003 | 0.004 |
| Δ at 3mo | −29.6 (−110.92, 75.02) | −64.71 (−223.72, 49.56) * | −39.84 (−157.77, 57.78) | 0.605 | |||
| IL-1β | Baseline | 0.55 (0.41, 0.61) | 0.48 (0.36, 0.55) | 0.54 (0.39, 0.58) | 0.5 (0.44, 0.51) | 0.002 | 0.009 |
| Δ at 3mo | −0.06 (−0.14, 0.02) * | −0.05 (−0.1, 0.06) | −0.06 (−0.11, 0.04) * | 1.000 | |||
| IL-6 | Baseline | 0.64 (0.35, 1.88) | 1.17 (0.37, 3.39) | 0.73 (0.35, 2.36) | 0.3 (0.16, 0.73) | 0.045 | 0.266 |
| Δ at 3mo | −0.09 (−0.57, 0.1) | 0.4 (−1.24, 6.73) | −0.04 (−0.61, 1.79) | 0.121 | |||
| IL-8 | Baseline | 1.28 (0.48, 3.15) | 1.31 (0.28, 5.56) | 1.28 (0.36, 3.63) | 0.27 (0.14, 1.12) | 0.136 | 0.824 |
| Δ at 3mo | −0.37 (−1.65, 0.38) * | 1.07 (−0.85, 15.94) | −0.05 (−1.34, 1.54) | 0.121 | |||
| TNF-α | Baseline | 1.08 (0.95, 1.25) | 1.06 (0.31, 1.22) | 1.08 (0.69, 1.24) | 1.17 (1.11, 1.17) | 0.234 | 0.824 |
| Δ at 3mo | −0.1 (−0.22, 0.04) * | 0 (−0.16, 0.13) | −0.05 (−0.16, 0.09) | 0.121 |
| Medical Treatment Group of Clinical BPH Patients | |||||
|---|---|---|---|---|---|
| (A) GRA < 2 (N = 12, 36.4%) | (B) GRA ≥ 2 (N = 21, 63.6%) | Overall N = 33 | p-Value | ||
| Age | 65.58 ± 7.65 | 66 ± 6.46 | 65.85 ± 6.8 | 0.869 | |
| PSA (ng/mL) | 3.79 ± 2.78 | 2.27 ± 1.68 | 2.82 ± 2.23 | 0.059 | |
| TPV (mL) | 47.51 ± 18.42 | 42.19 ± 11.28 | 44.12 ± 14.25 | 0.310 | |
| TZI | 0.43 ± 0.12 | 0.43 ± 0.13 | 0.43 ± 0.12 | 0.944 | |
| DWT (mm) | 1.04 ± 0.5 | 1.38 ± 0.61 | 1.26 ± 0.59 | 0.117 | |
| BWT (mm) | 2.46 ± 0.81 | 2.76 ± 0.79 | 2.65 ± 0.8 | 0.300 | |
| Symptom scores | |||||
| IPSS-V | Baseline | 9.08 ± 5.14 | 12.05 ± 5.55 | 10.97 ± 5.51 | 0.140 |
| Δ at 3mo | −0.75 ± 4.03 | −5.05 ± 6.39 * | −3.48 ± 5.96 * | 0.044 | |
| IPSS-S | Baseline | 6.08 ± 3.5 | 6.48 ± 3.97 | 6.33 ± 3.76 | 0.778 |
| Δ at 3mo | −0.25 ± 3.17 | −2.86 ± 3.55 * | −1.91 ± 3.6 * | 0.043 | |
| IPSS | Baseline | 15.17 ± 7.53 | 18.52 ± 7.15 | 17.3 ± 7.35 | 0.212 |
| Δ at 3mo | −1 ± 4.82 | −7.9 ± 7.14 * | −5.39 ± 7.16 * | 0.006 | |
| QoL | Baseline | 3.92 ± 1.31 | 3.67 ± 1.15 | 3.76 ± 1.2 | 0.573 |
| Δ at 3mo | −0.33 ± 1.5 | −1.52 ± 1.6 * | −1.09 ± 1.65 * | 0.044 | |
| OABSS | Baseline | 5.75 ± 4.14 | 5.1 ± 3.39 | 5.33 ± 3.63 | 0.626 |
| Δ at 3mo | −0.58 ± 3.32 | −2.9 ± 3.83 * | −2.06 ± 3.77 * | 0.089 | |
| Uroflowmetry | |||||
| BC (mL) | Baseline | 250.3 ± 126.14 | 286.19 ± 199.48 | 273.14 ± 175.06 | 0.579 |
| Δ at 3mo | 26.22 ± 214.13 | 25.25 ± 158.66 | 25.55 ± 173.73 | 0.989 | |
| Qmax (mL/s) | Baseline | 8.68 ± 3.36 | 9.6 ± 4.19 | 9.26 ± 3.88 | 0.520 |
| Δ at 3mo | −1.47 ± 2.9 | 2.24 ± 5.92 | 1.09 ± 5.41 | 0.088 | |
| cQmax | Baseline | 0.58 ± 0.19 | 0.6 ± 0.25 | 0.59 ± 0.23 | 0.723 |
| Δ at 3mo | −0.04 ± 0.25 | 0.06 ± 0.29 | 0.03 ± 0.28 | 0.402 | |
| Vol (mL) | Baseline | 229.33 ± 119.81 | 224.05 ± 86.25 | 225.97 ± 97.93 | 0.884 |
| Δ at 3mo | −64.78 ± 89.44 | 48.15 ± 110.98 | 13.1 ± 116.06 | 0.012 | |
| PVR (mL) | Baseline | 21 ± 15.7 | 62.14 ± 158.54 | 47.18 ± 127.27 | 0.380 |
| Δ at 3mo | 91 ± 238.18 | −22.9 ± 173.74 | 12.45 ± 198.92 | 0.157 | |
| VE | Baseline | 0.91 ± 0.08 | 0.85 ± 0.15 | 0.87 ± 0.13 | 0.205 |
| Δ at 3mo | −0.11 ± 0.21 | 0.01 ± 0.2 | −0.03 ± 0.21 | 0.147 | |
| Urine biomarkers @ | |||||
| 8-OHdG | Baseline | 66.09 (25.61, 93.36) | 80.46 (29.1, 107.14) | 79.83 (29.65, 110.22) | 0.818 |
| Δ at 3mo | 31.61 (0.3, 79.45) | −3.93 (−46.81, 3.89) | −1.05 (−41.38, 13.44) | 0.017 | |
| 8-isoprostane | Baseline | 11.14 (3.37, 21.78) | 11.34 (8.17, 20.74) | 11.88 (7.59, 26.73) | 0.818 |
| Δ at 3mo | −0.78 (−6.31, 12.72) | −4.63 (−13.31, −0.16) * | −2.88 (−8.18, 2.73) | 0.376 | |
| TAC | Baseline | 219.84 (151.79, 408.17) | 249.88 (197.86, 457.04) | 262.65 (179.62, 446.77) | 0.818 |
| Δ at 3mo | 32.71 (−99.69, 315.95) | −52.84 (−179.49, 22.5) | −23.18 (−136.51, 65.94) | 0.378 | |
| PGE2 | Baseline | 147.56 (105.38, 298.02) | 187.41 (133.92, 396.23) | 166.95 (119.72, 301.06) | 0.340 |
| Δ at 3mo | 75.02 (−32.71, 338.89) | −60.04 (−128.32, 1.19) * | −29.6 (−110.92, 75.02) | 0.102 | |
| IL-1β | Baseline | 0.59 (0.42, 0.63) | 0.55 (0.48, 0.64) | 0.57 (0.45, 0.62) | 0.611 |
| Δ at 3mo | −0.09 (−0.11, 0.02) | −0.06 (−0.17, 0.03) * | −0.06 (−0.14, 0.02) * | 0.894 | |
| IL-6 | Baseline | 0.7 (0.23, 3.82) | 0.69 (0.37, 2.14) | 0.69 (0.37, 2.02) | 0.795 |
| Δ at 3mo | −0.07 (−1.39, 1.44) | −0.15 (−0.57, 0.05) | −0.09 (−0.57, 0.1) | 0.894 | |
| IL-8 | Baseline | 1.73 (0.29, 4.07) | 1.22 (0.64, 9.47) | 1.43 (0.55, 3.53) | 0.611 |
| Δ at 3mo | −0.11 (−1.65, 0.67) | −0.39 (−2.91, 0.29) * | −0.37 (−1.65, 0.38) * | 0.894 | |
| TNF-α | Baseline | 1.19 (0.65, 1.3) | 1.06 (0.96, 1.31) | 1.16 (1, 1.29) | 0.990 |
| Δ at 3mo | −0.11 (−0.22, 0.04) * | −0.06 (−0.36, 0.03) | −0.1 (−0.22, 0.04) * | 0.537 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-H.; Lee, J.; Kuo, H.-C.; Wu, Y.-H. Urinary Inflammatory and Oxidative Stress Biomarkers as Indicators for the Clinical Management of Benign Prostatic Hyperplasia. Int. J. Mol. Sci. 2025, 26, 6516. https://doi.org/10.3390/ijms26136516
Jiang Y-H, Lee J, Kuo H-C, Wu Y-H. Urinary Inflammatory and Oxidative Stress Biomarkers as Indicators for the Clinical Management of Benign Prostatic Hyperplasia. International Journal of Molecular Sciences. 2025; 26(13):6516. https://doi.org/10.3390/ijms26136516
Chicago/Turabian StyleJiang, Yuan-Hong, Jimmy Lee, Hann-Chorng Kuo, and Ya-Hui Wu. 2025. "Urinary Inflammatory and Oxidative Stress Biomarkers as Indicators for the Clinical Management of Benign Prostatic Hyperplasia" International Journal of Molecular Sciences 26, no. 13: 6516. https://doi.org/10.3390/ijms26136516
APA StyleJiang, Y.-H., Lee, J., Kuo, H.-C., & Wu, Y.-H. (2025). Urinary Inflammatory and Oxidative Stress Biomarkers as Indicators for the Clinical Management of Benign Prostatic Hyperplasia. International Journal of Molecular Sciences, 26(13), 6516. https://doi.org/10.3390/ijms26136516







