Psychopharmacological Therapy Positively Modulates Disease Activity in Inflammatory Bowel Disease: A Systematic Review
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Collection Process
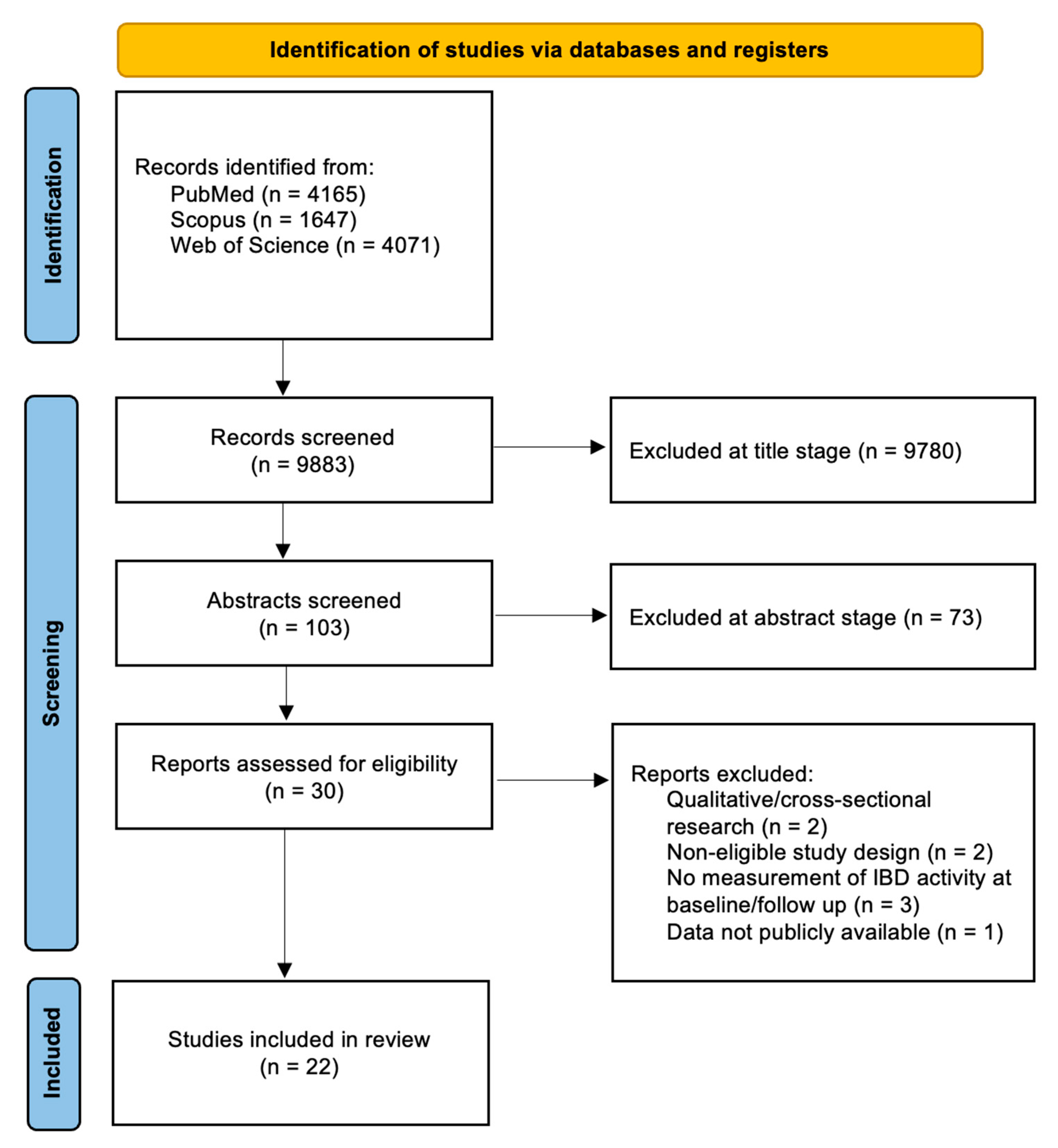
3. Results
3.1. Antidepressants
3.2. Antiepileptics
3.3. Hypnoinducers and Anxiolytics
3.4. Mood Stabilizers
3.5. Others
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodhand, J.R.; Wahed, M.; Mawdsley, J.E.; Farmer, A.D.; Aziz, Q.; Rampton, D.S. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm. Bowel. Dis. 2012, 18, 2301–2309. [Google Scholar] [CrossRef]
- Roderburg, C.; Yaqubi, K.; Konrad, M.; May, P.; Luedde, T.; Kostev, K.; Loosen, S.H. Association between inflammatory bowel disease and subsequent depression or anxiety disorders—A retrospective cohort study of 31,728 outpatients. J. Psychiatr. Res. 2024, 169, 231–237. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Fisk, J.D.; Dolovich, C.; Hitchon, C.A.; Graff, L.A.; El-Gabalawy, R.; Lix, L.M.; Bolton, J.M.; Patten, S.B.; Marrie, R.A. Understanding Predictors of Fatigue Over Time in Persons With Inflammatory Bowel Disease: The Importance of Depressive and Anxiety Symptoms. Am. J. Gastroenterol. 2024, 119, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Neuendorf, R.; Harding, A.; Stello, N.; Hanes, D.; Wahbeh, H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Maunder, R.G.; Levenstein, S. The role of stress in the development and clinical course of inflammatory bowel disease: Epidemiological evidence. Curr. Mol. Med. 2008, 8, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Fairbrass, K.M.; Lovatt, J.; Barberio, B.; Yuan, Y.; Gracie, D.J.; Ford, A.C. Bidirectional brain-gut axis effects influence mood and prognosis in IBD: A systematic review and meta-analysis. Gut 2022, 71, 1773–1780. [Google Scholar] [CrossRef]
- Jain, A.; Marrie, R.A.; Shafer, L.A.; Graff, L.A.; Patten, S.B.; El-Gabalawy, R.; Sareen, J.; Bolton, J.M.; Fisk, J.D.; Bernstein, C.N. Incidence of Adverse Psychiatric Events During Treatment of Inflammatory Bowel Disease With Biologic Therapies: A Systematic Review. Crohn’s Colitis 360 2020, 2, otz053. [Google Scholar] [CrossRef]
- Menichetti, J.; Fiorino, G.; Vegni, E. Personalizing Psychological Treatment Along the IBD Journey: From Diagnosis to Surgery. Curr. Drug Targets 2018, 19, 722–728. [Google Scholar] [CrossRef]
- Buckley, J.P.; Kappelman, M.D.; Allen, J.K.; Van Meter, S.A.; Cook, S.F. The burden of comedication among patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2725–2736. [Google Scholar] [CrossRef]
- Thorkelson, G.; Bielefeldt, K.; Szigethy, E. Empirically Supported Use of Psychiatric Medications in Adolescents and Adults with IBD. Inflamm. Bowel Dis. 2016, 22, 1509–1522. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Andrews, J.M. Attitudes towards antidepressants among people living with inflammatory bowel disease: An online Australia-wide survey. J. Crohn’s Colitis 2014, 8, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, I.; Regazzi, M.G.; Bonucci, G.; Rizzello, F.; Carini, G.; Muratori, R.; Poggioli, G.; Campieri, M.; EspriMici Study Group. Prevalence and effectiveness of psychiatric treatments for patients with IBD: A systematic literature review. J. Psychosom. Res. 2017, 101, 68–95. [Google Scholar] [CrossRef] [PubMed]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12. [Google Scholar] [CrossRef]
- Kim, D.B.; Lee, K.-M.; Lee, J.M.; Chung, Y.Y.; Sung, H.J.; Paik, C.N.; Chung, W.C.; Jung, J.-H.; Choi, H.J. Correlation between Histological Activity and Endoscopic, Clinical, and Serologic Activities in Patients with Ulcerative Colitis. Gastroenterol. Res. Pract. 2016, 2016, 32051. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Sandborn, W.J.; Dubois, C.; Rutgeerts, P. Correlation Between the Crohn’s Disease Activity and Harvey–Bradshaw Indices in Assessing Crohn’s Disease Severity. Clin. Gastroenterol. Hepatol. 2010, 8, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Yarlas, A.; Maher, S.; Bayliss, M.; Lovley, A.; Cappelleri, J.C.; Bushmakin, A.G.; DiBonaventura, M.D. The Inflammatory Bowel Disease Questionnaire in Randomized Controlled Trials of Treatment for Ulcerative Colitis: Systematic Review and Meta-Analysis. J. Patient Cent. Res. Rev. 2020, 7, 189–205. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- Turner, D.; Seow, C.H.; Greenberg, G.R.; Griffiths, A.M.; Silverberg, M.S.; Steinhart, A.H. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1081–1088. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bianchi Porro, G. A practical guide to the management of distal ulcerative colitis. Drugs 1998, 55, 519–542. [Google Scholar] [CrossRef]
- Hall, B.J.; Hamlin, P.J.; Gracie, D.J.; Ford, A.C. The Effect of Antidepressants on the Course of Inflammatory Bowel Disease. Can J. Gastroenterol. Hepatol. 2018, 2018, 2047242. [Google Scholar] [CrossRef]
- Kristensen, M.S.; Kjærulff, T.M.; Ersbøll, A.K.; Green, A.; Hallas, J.; Thygesen, L.C. The Influence of Antidepressants on the Disease Course Among Patients With Crohn’s Disease and Ulcerative Colitis—A Danish Nationwide Register–Based Cohort Study. Inflammatory Bowel Diseases 2019, 25, 886–893. [Google Scholar] [CrossRef]
- Yanartas, O.; Kani, H.T.; Bicakci, E.; Kilic, I.; Banzragch, M.; Acikel, C.; Atug, O.; Kuscu, K.; Imeryuz, N.; Akin, H. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr. Dis. Treat. 2016, 12, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Goodhand, J.R.; Greig, F.I.S.; Koodun, Y.; McDermott, A.; Wahed, M.; Langmead, L.; Rampton, D.S. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm. Bowel Dis. 2012, 18, 1232–1239. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Hughes, P.A.; Bampton, P.; Gordon, A.; Campaniello, M.A.; Mavrangelos, C.; Stewart, B.J.; Esterman, A.; Andrews, J.M. Fluoxetine for Maintenance of Remission and to Improve Quality of Life in Patients with Crohn’s Disease: A Pilot Randomized Placebo-Controlled Trial. J. Crohn’s Colitis 2017, 11, 509–514. [Google Scholar] [CrossRef]
- Daghaghzadeh, H.; Naji, F.; Afshar, H.; Sharbafchi, M.R.; Feizi, A.; Maroufi, M.; Tabatabaeeyan, M.; Adibi, P.; Tavakoli, H. Efficacy of duloxetine add on in treatment of inflammatory bowel disease patients: A double-blind controlled study. J. Res. Med. Sci. 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Liang, C.; Chen, P.; Tang, Y.; Zhang, C.; Lei, N.; Luo, Y.; Duan, S.; Zhang, Y. Venlafaxine as an Adjuvant Therapy for Inflammatory Bowel Disease Patients With Anxious and Depressive Symptoms: A Randomized Controlled Trial. Front. Psychiatry 2022, 13, 880058. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, H.N.; Cassell, B.; Kanuri, N.; Gyawali, C.P.; Gutierrez, A.; Dassopoulos, T.; Ciorba, M.A.; Sayuk, G.S. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J. Clin. Gastroenterol. 2014, 48, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Walecka-Kapica, E.; Klupinska, G.; Pawłowicz, M.; Florkowski, A.; Wachowska-Kelly, P.; Chojnacki, J. Evaluation of the influence of tianeptine on the psychosomatic status of patients with ulcerative colitis in remission. Pol. Merkur. Lekarski 2011, 31, 92–96. [Google Scholar]
- Kane, S.; Altschuler, E.L.; Kast, R.E. Crohn’s disease remission on bupropion. Gastroenterology 2003, 125, 1290. [Google Scholar] [CrossRef]
- Kast, R.E.; Altschuler, E.L. Remission of Crohn’s disease on bupropion. Gastroenterology 2001, 121, 1260–1261. [Google Scholar] [CrossRef]
- Kast, R.E. Crohn’s disease remission with phenelzine treatment. Gastroenterology 1998, 115, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, N.; Blackwell, J.; Saxena, S.; Bottle, A.; Petersen, I.; Creese, H.; Hotopf, M.; Pollok, R.C.G.; POP-IBD study group. Antidepressant medication use in Inflammatory Bowel Disease: A nationally representative population-based study. Aliment. Pharmacol. Ther. 2022, 55, 1330–1341. [Google Scholar] [CrossRef]
- Crockett, S.D.; Schectman, R.; Stürmer, T.; Kappelman, M.D. Topiramate use does not reduce flares of inflammatory bowel disease. Dig. Dis. Sci. 2014, 59, 1535–1543. [Google Scholar] [CrossRef]
- D’Onofrio, A.M.; Di Vincenzo, F.; Ferrajoli, G.F.; Scaldaferri, F.; Camardese, G. Low Dose Pregabalin Improves Gastrointestinal Symptoms of Crohn’s Disease. Case Rep. Gastrointest. Med. 2024, 2024, 3744500. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Fisk, J.D.; Walld, R.; Bolton, J.M.; Sareen, J.; Patten, S.B.; Singer, A.; Lix, L.M.; Hitchon, C.A.; El-Gabalawy, R.; et al. Use of Benzodiazepines and Z-Drugs in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2022, 117, 2046–2054. [Google Scholar] [CrossRef]
- Stokes, D.K. Lorazepam in anxiety associated with chronic enteritis and ulcerative colitis. J. Clin. Psychiatry 1978, 39, 53–57. [Google Scholar]
- Zisook, S. Ulcerative colitis: Case responding to treatment with lithium carbonate. JAMA 1972, 219, 755. [Google Scholar] [CrossRef]
- Lie, M.R.K.L.; Van Der Giessen, J.; Fuhler, G.M.; de Lima, A.; Peppelenbosch, M.P.; Van Der Ent, C.; Van Der Woude, C.J. Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. J. Transl. Med. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Bingaman, S.I.; Ruggiero, F.; Mauger, D.T.; Mukherjee, A.; McGovern, C.O.; Zagon, I.S. Therapy with the Opioid Antagonist Naltrexone Promotes Mucosal Healing in Active Crohn’s Disease: A Randomized Placebo-Controlled Trial. Dig. Dis. Sci. 2011, 56, 2088–2097. [Google Scholar] [CrossRef]
- Smith, J.P.; Stock, H.; Bingaman, S.; Mauger, D.; Rogosnitzky, M.; Zagon, I.S. Low-dose naltrexone therapy improves active Crohn’s disease. Am. J. Gastroenterol. 2007, 102, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Field, D.; Bingaman, S.I.; Evans, R.; Mauger, D.T. Safety and tolerability of low-dose naltrexone therapy in children with moderate to severe Crohn’s disease: A pilot study. J. Clin. Gastroenterol. 2013, 47, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lechin, F.; Van Der Dijs, B.; Insausti, C.L.; Gómez, F.; Villa, S.; Lechin, A.E.; Arocha, L.; Oramas, O. Treatment of Ulcerative Colitis With Clonidine. J. Clin. Pharma 1985, 25, 219–226. [Google Scholar] [CrossRef]
- Furlan, R.; Ardizzone, S.; Palazzolo, L.; Rimoldi, A.; Perego, F.; Barbic, F.; Bevilacqua, M.; Vago, L.; Porro, G.B.; Malliani, A. Sympathetic overactivity in active ulcerative colitis: Effects of clonidine. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R224–R232. [Google Scholar] [CrossRef]
- Paulides, E.; Lie, M.R.K.L.; Van Der Woude, C.J. Low-dose naltrexone for the induction of remission in patients with mild to moderate Crohn’s disease: Protocol for the randomised, double-blinded, placebo-controlled, multicentre LDN Crohn study. BMJ Open 2022, 12, e058358. [Google Scholar] [CrossRef]
- Fava, G.A.; Gatti, A.; Belaise, C.; Guidi, J.; Offidani, E. Withdrawal Symptoms after Selective Serotonin Reuptake Inhibitor Discontinuation: A Systematic Review. Psychother. Psychosom. 2015, 84, 72–81. [Google Scholar] [CrossRef]
- Fuller-Thomson, E.; Sulman, J. Depression and inflammatory bowel disease: Findings from two nationally representative Canadian surveys. Inflamm. Bowel Dis. 2006, 12, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Haapamäki, J.; Tanskanen, A.; Roine, R.P.; Blom, M.; Turunen, U.; Mäntylä, J.; Färkkilä, M.A.; Arkkila, P.E.T. Medication use among inflammatory bowel disease patients: Excessive consumption of antidepressants and analgesics. Scand. J. Gastroenterol. 2013, 48, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Mikocka-Walus, A.; Prady, S.L.; Pollok, J.; Esterman, A.J.; Gordon, A.L.; Knowles, S.; Andrews, J.M. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst. Rev. 2019, 4, CD012680. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.A.; Turnbull, D.A.; Moulding, N.T.; Wilson, I.G.; Andrews, J.M.; Holtmann, G.J. Antidepressants and inflammatory bowel disease: A systematic review. Clin. Pract. Epidemiol. Ment. Health 2006, 2, 24. [Google Scholar] [CrossRef]
- Macer, B.J.D.; Prady, S.L.; Mikocka-Walus, A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2017, 23, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Vallerand, I.A.; Shaheen, A.-A.; Lowerison, M.W.; Swain, M.G.; Barnabe, C.; Patten, S.B.; Kaplan, G.G. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2019, 68, 1606–1612. [Google Scholar] [CrossRef]
- Ghia, J.-E.; Blennerhassett, P.; Collins, S.M. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J. Clin. Investig. 2008, 118, 2209–2218. [Google Scholar] [CrossRef]
- Ghia, J.; Blennerhassett, P.; Deng, Y.; Verdu, E.F.; Khan, W.I.; Collins, S.M. Reactivation of Inflammatory Bowel Disease in a Mouse Model of Depression. Gastroenterology 2009, 136, 2280–2288.e4. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef]
- Martino, M.; Rocchi, G.; Escelsior, A.; Fornaro, M. Immunomodulation Mechanism of Antidepressants: Interactions between Serotonin/Norepinephrine Balance and Th1/Th2 Balance. Curr. Neuropharmacol. 2012, 10, 97–123. [Google Scholar] [CrossRef]
- Varghese, A.K.; Verdú, E.F.; Bercik, P.; Khan, W.I.; Blennerhassett, P.A.; Szechtman, H.; Collins, S.M. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology 2006, 130, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Guemei, A.A.; El Din, N.M.N.; Baraka, A.M.; El Said Darwish, I. Do desipramine [10,11-dihydro-5-[3-(methylamino) propyl]-5H-dibenz[b,f]azepine monohydrochloride] and fluoxetine [N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]-propan-1-amine] ameliorate the extent of colonic damage induced by acetic acid in rats? J. Pharmacol. Exp. Ther. 2008, 327, 846–850. [Google Scholar] [CrossRef]
- Teng, S.; Yang, Y.; Zhang, W.; Li, X.; Li, W.; Cui, Z.; Min, L.; Wu, J. Antidepressant fluoxetine alleviates colitis by reshaping intestinal microenvironment. Cell Commun. Signal. 2024, 22, 176. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef]
- Zhu, J.; Smith, K.; Hsieh, P.N.; Mburu, Y.K.; Chattopadhyay, S.; Sen, G.C.; Sarkar, S.N. High-throughput screening for TLR3-IFN regulatory factor 3 signaling pathway modulators identifies several antipsychotic drugs as TLR inhibitors. J. Immunol. 2010, 184, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Branco-de-Almeida, L.S.; Kajiya, M.; Cardoso, C.R.; Silva, M.J.B.; Ohta, K.; Rosalen, P.L.; Franco, G.C.N.; Han, X.; Taubman, M.A.; Kawai, T. Selective serotonin reuptake inhibitors attenuate the antigen presentation from dendritic cells to effector T lymphocytes. FEMS Immunol. Med. Microbiol. 2011, 62, 283–294. [Google Scholar] [CrossRef][Green Version]
- Vollmar, P.; Nessler, S.; Kalluri, S.R.; Hartung, H.-P.; Hemmer, B. The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int. J. Neuropsychopharmacol. 2009, 12, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Rocchi, G.; Escelsior, A.; Contini, P.; Colicchio, S.; de Berardis, D.; Amore, M.; Fornaro, P.; Fornaro, M. NGF serum levels variations in major depressed patients receiving duloxetine. Psychoneuroendocrinology 2013, 38, 1824–1828. [Google Scholar] [CrossRef]
- Fornaro, M.; Martino, M.; Battaglia, F.; Colicchio, S.; Perugi, G. Increase in IL-6 levels among major depressive disorder patients after a 6-week treatment with duloxetine 60 mg/day: A preliminary observation. Neuropsychiatr. Dis. Treat. 2011, 7, 51–56. [Google Scholar] [CrossRef]
- Başterzi, A.D.; Yazici, K.; Buturak, V.; Cimen, B.; Yazici, A.; Eskandari, G.; Tot Acar, S.; Taşdelen, B. Effects of venlafaxine and fluoxetine on lymphocyte subsets in patients with major depressive disorder: A flow cytometric analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 70–75. [Google Scholar] [CrossRef]
- Arnone, D.; Saraykar, S.; Salem, H.; Teixeira, A.L.; Dantzer, R.; Selvaraj, S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018, 92, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Nettis, M.A.; Mondelli, V.; Pariante, C.M. Inflammation in cancer and depression: A starring role for the kynurenine pathway. Psychopharmacology 2019, 236, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Numata, S.; Watanabe, S.-Y.; Hatakeyama, Y.; Kinoshita, M.; Tomioka, Y.; Nakahara, K.; Nikawa, T.; Ohmori, T. Altered KYN/TRP, Gln/Glu, and Met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder. Sci. Rep. 2017, 7, 4855. [Google Scholar] [CrossRef]
- Xu, F.; Xie, Q.; Kuang, W.; Dong, Z. Interactions Between Antidepressants and Intestinal Microbiota. Neurotherapeutics 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- Getachew, B.; Aubee, J.I.; Schottenfeld, R.S.; Csoka, A.B.; Thompson, K.M.; Tizabi, Y. Ketamine interactions with gut-microbiota in rats: Relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018, 18, 222. [Google Scholar] [CrossRef]
- Birkinshaw, H.; Friedrich, C.M.; Cole, P.; Eccleston, C.; Serfaty, M.; Stewart, G.; White, S.; Moore, R.A.; Phillippo, D.; Pincus, T. Antidepressants for pain management in adults with chronic pain: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 5, CD014682. [Google Scholar] [CrossRef]
- Pan, H.-L.; Wu, Z.-Z.; Zhou, H.-Y.; Chen, S.-R.; Zhang, H.-M.; Li, D.-P. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol. Ther. 2008, 117, 141–161. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Stiglbauer, V.; Brasanac, J.; Chae, W.R.; Wagener, F.; Zimbalski, K.; Jefsen, O.H.; Liu, S.; Seals, M.R.; Gamradt, S.; et al. Efficacy and Safety of Antidepressants in Patients With Comorbid Depression and Medical Diseases: An Umbrella Systematic Review and Meta-Analysis. JAMA Psychiatry 2023, 80, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Oliva, V.; Lippi, M.; Paci, R.; Del Fabro, L.; Delvecchio, G.; Brambilla, P.; De Ronchi, D.; Fanelli, G.; Serretti, A. Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110266. [Google Scholar] [CrossRef]
- Barros, L.L.; Farias, A.Q.; Rezaie, A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: Prevalence, diagnosis and treatment. World. J. Gastroenterol. 2019, 25, 4414–4426. [Google Scholar] [CrossRef]
- Tack, J.; Broekaert, D.; Corsetti, M.; Fischler, B.; Janssens, J. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment. Pharmacol. Ther. 2006, 23, 265–274. [Google Scholar] [CrossRef]
- Kenna, H.A.; Poon, A.W.; de los Angeles, C.P.; Koran, L.M. Psychiatric complications of treatment with corticosteroids: Review with case report. Psychiatry Clin. Neurosci. 2011, 65, 549–560. [Google Scholar] [CrossRef]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef]
- Brown, E.S.; Suppes, T. Mood symptoms during corticosteroid therapy: A review. Harv. Rev. Psychiatry 1998, 5, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Vanner, S.; Kashyap, P.C.; Nasser, Y. Chronic Visceral Pain: New Peripheral Mechanistic Insights and Resulting Treatments. Gastroenterology 2024, 166, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Patierno, S.; Selmer, I.-S.; Kirchgessner, A. The opioid system in the gastrointestinal tract. Neurogastroenterol. Motil. 2004, 16, 3–16. [Google Scholar] [CrossRef]
- Ramanathan, S.; Panksepp, J.; Johnson, B. Is Fibromyalgia An Endocrine/Endorphin Deficit Disorder? Is Low Dose Naltrexone a New Treatment Option? Psychosomatics 2012, 53, 591–594. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Peng, Y.; Hutchinson, M.R.; Rice, K.C.; Yin, H.; Watkins, L.R. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol. 2016, 173, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Wang, X.; Mustafa, S.; Hutchinson, M.R. In vivo veritas: (+)-Naltrexone’s actions define translational importance. Trends Pharmacol. Sci. 2014, 35, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Deuring, J.J.; Fuhler, G.M.; Konstantinov, S.R.; Peppelenbosch, M.P.; Kuipers, E.J.; De Haar, C.; Van Der Woude, C.J. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn’s disease. Gut 2014, 63, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, A.; Nabavizadeh, F.; Zekri, A.; Amiri, F. Naltrexone changes the expression of lipid metabolism—Related proteins in the endoplasmic reticulum stress induced hepatic steatosis in mice. Clin. Exp. Pharma. Physio. 2017, 44, 207–212. [Google Scholar] [CrossRef]
- Moslehi, A.; Nabavizadeh, F.; Dehpou, A.R.; Tavanga, S.M.; Hassanzadeh, G.; Zekri, A.; Nahrevanian, H.; Sohanaki, H. Naltrexone attenuates endoplasmic reticulum stress induced hepatic injury in mice. Acta Physiol. Hung. 2014, 101, 341–352. [Google Scholar] [CrossRef]
- Tawfik, D.I.; Osman, A.S.; Tolba, H.M.; Khattab, A.; Abdel-Salam, L.O.; Kamel, M.M. Evaluation of therapeutic effect of low dose naltrexone in experimentally-induced Crohn’s disease in rats. Neuropeptides 2016, 59, 39–45. [Google Scholar] [CrossRef]
- Alcaro, A.; Huber, R.; Panksepp, J. Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective. Brain Res. Rev. 2007, 56, 283–321. [Google Scholar] [CrossRef]
- Brown, N.; Panksepp, J. Low-dose naltrexone for disease prevention and quality of life. Med. Hypotheses 2009, 72, 333–337. [Google Scholar] [CrossRef]
- Ghia, J.E.; Blennerhassett, P.; Kumar–Ondiveeran, H.; Verdu, E.F.; Collins, S.M. The Vagus Nerve: A Tonic Inhibitory Influence Associated With Inflammatory Bowel Disease in a Murine Model. Gastroenterology 2006, 131, 1122–1130. [Google Scholar] [CrossRef]
- Bernik, T.R.; Friedman, S.G.; Ochani, M.; DiRaimo, R.; Ulloa, L.; Yang, H.; Sudan, S.; Czura, C.J.; Ivanova, S.M.; Tracey, K.J. Pharmacological Stimulation of the Cholinergic Antiinflammatory Pathway. J. Exp. Med. 2002, 195, 781–788. [Google Scholar] [CrossRef]
- Pullan, R.D.; Rhodes, J.; Ganesh, S.; Mani, V.; Morris, J.S.; Williams, G.T.; Newcombe, R.G.; Russell, M.; Feyerabend, C.; Thomas, G.; et al. Transdermal Nicotine for Active Ulcerative Colitis. N. Engl. J. Med. 1994, 330, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Motavallian, A.; Zamani, E.; Bouzari, S.; Rezaeyan, F.; Karimian, P.; Evazalipour, M. Anti-inflammatory effect of pregabalin on acetic acid-induced colitis in the rats. Res. Pharm. Sci. 2022, 17, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hu, S.; Liu, S.; Tang, B.; Liu, Y.; Tang, L.; Lei, Y.; Zhong, L.; Yang, S.; He, S. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol. Res. 2022, 175, 105992. [Google Scholar] [CrossRef]
- Allergan USA, Inc. Highlights of Prescribing Information Celexa® (Citalopram). Ref. ID 4933291. Published Online February 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf (accessed on 30 March 2025).
- Abbvie Inc. Highlights of Prescribing Information Lexapro® (Escitalopram). Reference ID: 5367836. Published Online April 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/021323s058lbl.pdf (accessed on 30 March 2025).
- Jazz Pharmaceuticals, Inc. Highlights of Prescribing Information Luvox® (Fluvoxamine Maleate). Published Online 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022235lbl.pdf (accessed on 30 March 2025).
- Apotex Inc. Highlights of Prescribing Information Paxil (Paroxetine). Reference ID: 5274678. Published Online August 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/020031s082lbl.pdf (accessed on 30 March 2025).
- Viatris Specialty LLC. Highlights of Prescribing Information ZOLOFT (Sertraline Hydrochloride). Reference ID: 5229497. Published Online August 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/019839s108,20990s062lbl.pdf (accessed on 30 March 2025).
- Lilly USA, LLC. Highlights of Prescribing Information Cymbalta® (Duloxetine Delayed-Release Capsules). Reference ID: 5229383. Published Online August 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021427s055s057lbl.pdf (accessed on 30 March 2025).
- Viatris Specialty LLC. Highlights of Prescribing Information Effexor XR® (Venlafaxine Extended-Release). Reference ID: 5229419. Published Online August 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/020699s118lbl.pdf (accessed on 30 March 2025).
- GlaxoSmithKline. Highlights of Prescribing Information Wellbutrin (Bupropion Hydrochloride). Reference ID: 5376823. Published Online April 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/018644s061lbl.pdf (accessed on 30 March 2025).
- Merck & Co Inc. Highlights of Prescribing Information Remeron® (Mirtazapine). Reference ID: 4878026. Published Online November 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020415s038,021208s028lbl.pdf (accessed on 30 March 2025).
- Norman, T.R.; Olver, J.S. Agomelatine for depression: Expanding the horizons? Expert Opin Pharmacother. 2019, 20, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Moraczewski, J.; Awosika, A.O.; Aedma, K.K. Tricyclic Antidepressants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557791/ (accessed on 24 July 2024).
- Sabri, M.A.; Saber-Ayad, M.M. MAO Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557395/ (accessed on 24 July 2024).
- Janssen Ortho LLC. Highlights of Prescribing Information Topamax® (Topiramate). Reference ID: 5167873. Published Online May 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/020505s065,020844s056lbl.pdf (accessed on 30 March 2025).
- West-Ward Pharmaceuticals. Highlights of Prescribing Information Lithium and Lithium Carbonate. Reference ID: 5060222. Published Online October 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/017812s036,018421s035,018558s030lbl.pdf (accessed on 30 March 2025).
- Duramed Pharmaceuticals, Inc. Highlights of Prescribing Information Revia® (Naltrexone Hydrochloride). Reference ID: 3383348. Published Online October 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018932s017lbl.pdf (accessed on 30 March 2025).
- Peng, L.; Morford, K.L.; Levander, X.A. Benzodiazepines and Related Sedatives. Med. Clin. N. Am. 2022, 106, 113–129. [Google Scholar] [CrossRef]
- Siriwardena, A.N.; Qureshi, Z.; Gibson, S.; Collier, S.; Latham, M. GPs’ attitudes to benzodiazepine and “Z-drug” prescribing: A barrier to implementation of evidence and guidance on hypnotics. Br. J. Gen. Pract. 2006, 56, 964–967. [Google Scholar]


| Measurement and Assessment | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Author (Year), Ref. | Drug | Study Population | Study Design | IBD | Psychiatric Condition | IBD | Psychiatric Condition | Follow-Up |
| Daghaghzadeh, et al. (2015) [30] | Duloxetina | 35 adult IBD patients (no flare up in the previous 6 months) CD = 13 UC = 22 | Placebo-controlled RCT: Study group (n = 17) treated with duloxetine 30–60 mg daily for 12 weeks. Control group (n = 18) treated with placebo. Both groups also received a stable dose of mesalazine | The severity of symptoms was assessed with LCAI | Anxiety and depression were assessed with HADS. The QOL was assessed with WHOQOL-BREF. | Severity of symptoms was significantly decreased in the duloxetine group compared to the placebo group. | Depression and anxiety were significantly decreased in the duloxetin group compared to the placebo group. Physical, psychological, and social QoL were significantly increased after treatment with duloxetine. | 12 weeks |
| Yanartas et al. (2016) [27] | Sertraline 21.0%, escitalopram 15.8%, bupropion 12.3%, mirtazapine 12.3%, paroxetine 10.6%, venlafaxine 5.2%, duloxetine 3.5%, fluvoxamine 1.8%, antidepressant combination treatment | 67 patients with IBD and anxiety and/or a depression disorder CD = 31 UC = 36 | Prospective cohort study: Patients were divided into 2 groups: Group A (47 patients who had completely adhered to the 6-month drug treatment Group B (20 patients who were nonadherent) | CDAI and modified MMS were used for the assessment of disease activity in patients with CD or UC, respectively, along with CRP, complete blood count, and routine blood biochemistry | Anxiety and depression were assessed with HADS. SF-36 and ASEX tests were used to assess QoL and sexual dysfunction | Hemoglobin plus CDAI parameters were statistically improved at the final visit in group A | HAD-Anxiety (HAD-A), HAD-Depression (HAD-D), all domains of SF-36, ASEX were statistically improved at the final visit in group A. | 6 months |
| Iskandar et al. (2014) [32] | TCA | 81 patients with IBD in clinical remission/mild activity and IBS UC = 23 CD = 58 | Retrospective study: IBD patients in clinical remission/with mild inflammation but with persistent GI symptoms were treated with TCA (10–150 mg). | Established Likert scales were used to score baseline symptom severity (0 = no symptoms, 3 = severe symptoms) and TCA response (0 = no improvement; 3 = complete satisfaction). | N/A | Baseline severity scores (CD: 2.07 ± 0.03, UC: 2.03 ± 0.04, p = 0.67), UC patients responded significantly better to TCA therapy, with a Likert response score of 1.86 ± 0.13 for UC and 1.26 ± 0.11 for CD (p = 0.003). 83% of UC patients had at least a moderate symptomatic improvement on TCA, compared with 50% of CD patients (p = 0.01). | N/A | 11 years |
| Goodhand et al. (2012) [28] | Citalopram 20 mg (20–60 mg) and fluoxetine 20 mg (20–60 mg) were the most used. Others were SSRIs, TCA, NaSSa, and SNRIs. | 58 IBD patients divided into 2 groups (n = 29 IBD patients on antidepressant, and n = 29 IBD control patients not on antidepressant). Patients were treated also with corticosteroids, 5-ASA, immunosuppressive agents and anti-TNF-α. UC = 14 CD = 15 (in each group) | Retrospective case–control study: Comparison of the course of IBD patients during the year before (year 1) and after (year 2) they were started on an antidepressant to that of controls matched for age, sex, disease type, medication at baseline, and relapse rate in year 1. | The outcome measures were the number of relapses, number of endoscopic procedures, number of hospital admissions and outpatient attendances, numbers of courses of steroids and relapse-related use of other IBD medications. Disease activity was defined as the presence of diarrhea and rectal bleeding in UC, the presence of abdominal pain and/or diarrhea and/or a CRP >10 mg/L in CD, a relapse was defined as the presence of these symptoms with a step-up in IBD medication. | N/A | Patients had fewer relapses and courses of steroids in the year after starting an antidepressant than in the year before (1 [0–4] (median [range]) vs. 0 [0–4], p = 0.002; 1 [0–3] vs. 0 [0–4], p < 0.001, respectively); the controls showed no changes between years 1 and 2 in relapses or courses of steroids | N/A | 2 years |
| Mikocka-Walus et al. (2017) [29] | Fluoxetina 20 mg daily | 26 adult patients with clinically established diagnosis of CD, in clinical remission but who flared CD in the last 12 month. | Pilot Randomized Placebo-Controlled Trial: Study group: 14 patients treated with fluoxetine Placebo group: 12 patients. Patients in both treatment arms remained on their current IBD medication. Participants provided blood and stool samples and complete questionnaires on four occasions [baseline, 3, 6 and 12 months] | The primary outcome measures were a significant group difference in the CD remission rate as measured on the CDAI [cut-off < 150]. The secondary measure was difference on remission rates as measured by fecal calprotectin; | The primary outcome was the difference in means on the WHOQo. Secondary measures were differences between HADS. | There was no statistically significant difference in the proportion of participants in remission at any time point, using either the CDAI [F(3, 27.5) = 0.064, p = 0.978] or fecal calprotectin [F (3, 32.5) = 1.08, p = 0.371], | There was no significant group difference in physical QoL, psychological QoL, social relationships QoL or environmental QoL over the 12 months. There was no significant group difference in anxiety or depression over the 12 months. | 12 months |
| Kane et al. (2003) [34] | Buproprion (100 mg) | 4 CD patients | Case series: Bupropion (100 mg) was prescribed for depression or smoking cessation | Outcome measurements: CDAI. | Two patients were diagnosed with depression | CDAI decreased to 150 within 6 weeks without a change in other Crohn’s medications | N/A | 6 weeks |
| Kast and Altschuler et al. (2001) [35] | Bupropion | 44-year-old woman with CD, taking fluoxetine 40 mg every day for depression, and mesalamine 500 mg twice a day. 45-year-old-man with CD, taking azathioprine and fluoxetine to help with pain control (then switched to bupropion) | Case series: Female: Bupropion 150 mg 3 times daily for depression and dysthymia. Fluoxetine was stopped and mesalamine was tapered off. Male: Bupropion 150 mg 3 times daily for smoking cessation | Outcome measurements: CDAI Woman: CDAI was 202. Man: CDAI was 275. | Woman had an episode of major depression, superimposed on a chronic mild depressed state (dysthymia). | Female: 19-month remission, no CD flares since starting bupropion. She stopped buproprion on her own but had a relapse and was forced to restart buproprion. CDAI = 0. Male: CDAI = 45. | Female: major depression remitted; dysthymia remained. | Woman: 19 months Man: not reported |
| Kast et al. (1998) [36] | Phenelzine | 33-year-old woman with CD. | Case report: Phenelzine 15 mg three times daily for one month, then 30 mg 3 times daily for 2 years. | Outcome measurements: clinical symptoms, such as diarrhea and abdominal pain. | She had an anxiety-prominent major depressive episode. | Seven days after phenelzine intake, bowel movements dropped from 10 to 3 or 4 daily without cramping. One month later, after increasing phenelzine 30 mg three times daily, she had 1 well-formed bowel movement daily and no cramping. Azathioprine and prednisone were tapered off. Two years after she switched to nortriptyline and after 6 weeks was admitted to the hospital with CD relapse. | Depression responded well. | 2 years |
| Liang et al. (2022) [31] | Venlafaxine | 45 patients with IBD were included UC = 21 CD = 25 | Prospective, randomized, double-blind, and placebo-controlled clinical trial. IBD patients with symptoms of anxiety or depression were randomly assigned to receive either venlafaxine 150 mg daily or equivalent placebo and followed for 6 months (25 received venlafaxine and 20 placebo). IBDQ, Mayo score, CDAI, HADS and blood examination were completed before the enrollment, during, and after the follow-up. | The primary outcome measures were as follows: the IBDQ score and CDAI for CD and Mayo score for UC at the onset, 3 months, and 6 months, which were analyzed in the complete case population. The secondary outcome measures were the disease course, SES-CD, UCEIS, relapse rate, frequency of corticosteroids/biologics use, and laboratory parameters (WBC, ALB, CRP, ESR, TNF-α, IL-10) between the venlafaxine and placebo groups | Psychiatric outcome measures were the means on the Hospital Anxiety Depression Scale (HADS, measured on three occasions. | IBDQ scores were significantly higher in the venlafaxine group compared with placebo group (3 months: comparison of effect size:0.59, p = 0.005; 6 months: comparison of effect size: 1.19, p < 0.001. UC patients with venlafaxine had lower Mayo score than that in placebo group after the 6-month assessment (comparison of effect size: −1.47, p < 0.001). Venlafaxine showed significant decrease in CDAI scores compared with placebo at 6 months (comparison of effect size: −0.87, p = 0.006). No significant differences were observed between the 2 groups in 3 months. UCEIS (1.9 vs. 1.7, p = 0.249) and SES-CD (4.6 vs. 8.6, p = 0.071) showed no significant differences between the venlafaxine group and placebo group at 6 months. | A significant reduction in the HADS depression scores was observed between the two groups both at 3 months (6.41 vs. 8.81, p < 0.001) and at 6 months | 6 months |
| Kristensen et al. (2019) [26] | Antidepressants (SNRI, SSRI, TCA, Mirtazapine and others) | 42,890 IBD patients were included (UC: 69.5%; CD: 30.5%). | Population-based cohort study Patients with CD or UC were recruited from the Danish National Patient Register (2000–2017). Information on antidepressant use and proxy measures of disease activity (healthcare and drug utilization) was extracted. Disease activity rates by antidepressant use adjusted for confounders were estimated with Poisson regression. The analyses were performed stratified by IBD subtype and type of antidepressants. | As surrogate markers of disease relapse the primary outcomes were defined as either (1) hospitalization with IBD as the primary diagnosis; (2) surgery associated with IBD or (3) step-up medication in terms of a redeemed prescription of corticosteroids. | N/A | The antidepressant group had a significantly lower relapse rate (IRR, 0.85; 95% CI, 0.81–0.90) compared with the other patients. The association was more pronounced in patients with CD compared with UC patients. Patients with no prior use of antidepressants before IBD onset had a favorable influence on the disease course when exposed to antidepressants compared with nonusers. | N/A | 17 years (2000–2017) |
| Chojnacki et al. (2011) [33] | Tianeptine | 60 UC patients in remission. | Clinical trial: Patients were divided in 2 groups of 30 subjects: the first (group 1) received aminosalicylates (2.0 g daily) and tianeptine (3 × 12.5 mg) and the second (group 2) received placebo. Symptoms of anxiety, depression and IBD clinical activity were assessed and compared between the two groups every 3 months for 1 year. | MCDAI, hemoglobin and CRP were evaluated to assess disease activity. | Anxiety and depression were assessed, respectively, with Hamilton Anxiety Rating Scale-HARS and Back Depression Inventory-BDI. | At 12 months, a significant decrease in disease activity index (respectively, 3.05 ± 1.36 and 4.65 ± 1.69), insignificantly lower level of CRP (7.00 5.65 and 9.41 ± 10.12) and higher level of hemoglobin (11.93 ± 0.83 and 11.0 ± 0.70) were observed in the tianeptine group compared with placebo group. | After 12 months, significant decreases in anxiety (from 20.35 ± 4.03 to 12.65 ± 3.78 points) and depression (from 19.95 ± 4.49 points to 9.60 ± 2.76 points) were observed in the tianeptine group compared with placebo group. | 12 months |
| Hall et al. (2018) [25] | 59.3% SSRI 31.5% TCA 3.7% SNRI 3.7% both SNRI and TCA 1.9% trazodone | 331 patients with an established radiological, endoscopic or histological diagnosis of CD or UC. | Longitudinal cohort study: Cohort 1: 54 patients treated with antidepressant at baseline. Cohort 2: 277 patients not treated with antidepressant Participants were followed for a minimum period of 2 years to assess the occurrence of 4 clinical endpoints. | Longitudinal disease activity was assessed with 4 outcomes:
| Anxiety and depression data were collected at baseline using the hospital anxiety and depression scale (HADS). Somatization data were collected at baseline using the patient health questionnaire-15 (PHQ-15). | During longitudinal follow-up, there was a trend towards lower rates of any of the four endpoints of IBD activity in patients with abnormal anxiety scores at baseline and who were receiving an antidepressant (42.3% versus 64.6%, p = 0.05). Based on univariate Cox regression analysis, there was a trend towards lower rates of escalation of medical therapy among patients receiving antidepressants at baseline (HR = 0.59; 95% CI 0.35–1.00, p = 0.05). | N/A | 2-year follow-up |
| Outcomes | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Author (Year), Ref. | Drug | Study Population | Study Design | IBD | Psychiatric Condition | IBD | Psychiatric Condition | Follow-Up |
| Crockett et al. (2014), [38] | Topiramate | 1733 IBD subjects CD = 955 UC = 755 IBD-U = 23 | Retrospective cohort study Study group (n = 775) treated with Topiramate (≤50 mg/day and ≥100 mg/day dose) Control group (n = 958) treated with comparator drugs (other anticonvulsant and anti-migraine medications) | Primary: new prescription for an oral steroid (≥14 days). Secondary: initiation of biologic agents, abdominal surgery, and hospitalization | N/A | Topiramate use was not associated with the primary outcome of steroid prescriptions (HR 1.14, 95% CI 0.74, 1.73). Results did not differ significantly by IBD subtype. There is no difference between the cohorts respect to post-exposure initiation of biologic agents (HR 0.93, 95% CI 0.35, 2.52), abdominal surgery (HR 1.22, 95% CI 0.70, 2.12), or hospitalization (HR 0.78, 95% CI 0.49, 1.26). | N/A | Median follow-up of 2.8 months |
| D’Onofrio et al. (2024) [39] | Pregbalin | 1 CD patient | Case Report | Assessment of HBI, SES-CD and CRP after therapy with pregabalin | N/A | After 2 months of treatment: HBI dropped from 9 to 4 and CRP normalized. After 6 months of treatment SES-CD dropped from 17 to 6. | N/A | 6 months |
| Outcomes | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (Year), Ref. | Drug | Disease Type | Study Population | Study Design | IBD | Psychiatric Condition | IBD | Psychiatric Condition | Follow-Up |
| Zisook et al. (1972) [42] | Lithium carbonate | UC | Case report 67 years old man with cyclic manic-depressive psychosis and UC | Administration of lithium carbonate in the manic phase of the patient with also frequent, bloody stools with recent endoscopic findings of “severe, active ulcerative colitis, already on salicosulfapiridine (azulfadine) therapy”. | N/A | N/A | After 5 days of therapy evidence of marked improvement in frequency of evacuations and bleeding. After two months endoscopic evidence of revealed marked improvement with only a few small bleeding points. | No more overtly psychotic | 16 months |
| Outcomes | Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Author (Year), Ref. | Drug | Study Population | Study Design | IBD | Psychiatric Condition | IBD | Psychiatric Condition | Follow-Up |
| Furlan et al. (2006) [48] | Clonidine | 23 UC patients 20 controls | Clinical Trial First part: UC patients were compared with 20 healthy controls regarding the neural autonomic profile. Secondo part: a subgroup of 16 patients randomly assigned to 8-wk transdermal clonidine (15 mg/weeks, 9 subjects), or placebo (7 patients). | DAI and endoscopic pattern before and after clonidine/placebo. | N/A | Changes in the autonomic profile after clonidine were associated with a reduction in the DAI score. Normalization of the autonomic profile with clonidine was accompanied by an improvement of the disease and a general increase in sympathetic activity characterized active UC. | N/A | 8 weeks |
| Lechin et al. (1985) [47] | Clonidine | 45 UC patients with at least one year of disease duration and severe activity (10 or more bloody stools per day for 15 or more days, not treated with steroids or sulfasalazine in the previous three months) | Double-blind Clinical Trial Prednisone (n = 15) 20 mg tid. Sulfasalazine (n = 15) 1.5 mg tid. Clonidine (n = 15) 0.3 mg tid. Each group was treated for three six-week periods, separated by two six-week placebo periods. | Assessment of rating scales for clinical, endoscopic, histologic, and radiologic changes. biochemical parameters. Distal colon motility changes. | N/A | Clonidine and prednisone were effective in treating idiopathic UC. Both drugs were more effective than sulfasalazine. Furthermore, clonidine potentiated prednisone and sulfasalazine effects. | N/A | 30 weeks followed by an open evaluation of at least one year |
| Lie et al. (2018) [43] | Naltrexone | 47 IBD patients (CD = 28 UC = 19) | Clinical trial: participants were steroid dependent or steroid refractory with clinical active disease at initiation of LDN (4.5 mg Naltrexone once daily) therapy. 87.2% previously received at least one anti-TNFα agent. 40.4% had been treated with two anti-TNFα agent. | Clinical response = self-assessed disease activity decreased within the first 4 weeks of therapy and lasted for at least 4 weeks. Endoscopic findings were scored from 0 to 3, (no inflammation to severe inflammation) Consecutive endoscopies were performed at baseline and at 12 weeks or at time of relapse, whichever occurred earlier. | N/A |
| N/A | 12 weeks |
| Paulides et al. (2022) [49] | Naltrexone | 122 patients with mild to moderately active CD, defined by endoscopy SES-CD of 3–15. | Randomized, double-blinded, placebo-controlled multicenter clinical trial:
| Primary objective defined as SES-CD ≤2 and an ulcerated surface subscore ≤1 in all five segments at week 12. Secondary:
|
| ongoing study | ongoing study | 52 weeks |
| Smith et al. (2011) [44] | Naltrexone | 40 CD patients with moderate to severe activity (CDAI of ≥220) | Randomized double-blind placebo-controlled study.
| Primary outcome: clinical response (70-point decline in CDAI scores from baseline values at 12 weeks). Secondary outcome:
| N/A |
| N/A | 12 weeks (+additional 12 weeks) |
| Smith et al. (2007) [45] | Naltrexone | 17 = CD treated with 4.5 mg naltrexone/day. | Open-labeled pilot prospective trial including CD patients with activity index (CDAI) score of 220–450, with no active biological therapy treated with naltrexone. | CDAI scores were assessed pretreatment, every 4 weeks on therapy and 4 weeks after completion of the study drug. | IBDQ and the short-form (SF-36) quality of life surveys | CDAI scores decreased significantly (p = 0.01) with LDN, from baseline to 4 weeks after completing therapy. 89% of response (p < 0.001) 67% of remission. | Improvement in both quality-of-life surveys with LDN compared with baseline | 12 weeks |
| Smith et al. (2013) [46] | Naltrexone | CD = 12 (pediatric patients mean age of 12.3 years) moderate to severe Crohn’s disease (PCDAI of >30), not in therapy with steroids greater than 10 mg daily, anti-TNFα agents | Pilot clinical trial Study group: 6 CD treated with naltrexone (0.1 mg/kg) Placebo: 6 CD 11 CD = open-labeled treatment with 8 additional weeks of naltrexone. | Primary outcome: safety and tolerability compared to placebo. secondary outcome: clinical response with 10-point decline in PCDAI scores compared to baseline/pretreatment values. | Quality of life was monitored by the Impact III survey. |
| Systemic and social quality of life improved with naltrexone treatment (p = 0.035). | 16 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vincenzo, F.; D’Onofrio, A.M.; Del Gaudio, A.; Chiera, E.; Ferrajoli, G.F.; Pesaresi, F.; Simonetti, A.; Mazza, M.; Kotzalidis, G.D.; Pettorruso, M.; et al. Psychopharmacological Therapy Positively Modulates Disease Activity in Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 6514. https://doi.org/10.3390/ijms26136514
Di Vincenzo F, D’Onofrio AM, Del Gaudio A, Chiera E, Ferrajoli GF, Pesaresi F, Simonetti A, Mazza M, Kotzalidis GD, Pettorruso M, et al. Psychopharmacological Therapy Positively Modulates Disease Activity in Inflammatory Bowel Disease: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(13):6514. https://doi.org/10.3390/ijms26136514
Chicago/Turabian StyleDi Vincenzo, Federica, Antonio Maria D’Onofrio, Angelo Del Gaudio, Elena Chiera, Gaspare Filippo Ferrajoli, Francesco Pesaresi, Alessio Simonetti, Marianna Mazza, Georgios Demetrios Kotzalidis, Mauro Pettorruso, and et al. 2025. "Psychopharmacological Therapy Positively Modulates Disease Activity in Inflammatory Bowel Disease: A Systematic Review" International Journal of Molecular Sciences 26, no. 13: 6514. https://doi.org/10.3390/ijms26136514
APA StyleDi Vincenzo, F., D’Onofrio, A. M., Del Gaudio, A., Chiera, E., Ferrajoli, G. F., Pesaresi, F., Simonetti, A., Mazza, M., Kotzalidis, G. D., Pettorruso, M., Martinotti, G., Lopetuso, L. R., Gasbarrini, A., Sani, G., Fiorino, G., Scaldaferri, F., & Camardese, G. (2025). Psychopharmacological Therapy Positively Modulates Disease Activity in Inflammatory Bowel Disease: A Systematic Review. International Journal of Molecular Sciences, 26(13), 6514. https://doi.org/10.3390/ijms26136514












