NK Cells: A Powerful Squad Versus SARS-CoV-2
Abstract
1. Introduction
2. Briefly, Where Do They Come from?
3. What Are Their Main Characteristics and How Do They Function?
4. The Fight of NK Cells Against Pathogens
5. NK Fights Against SARS-CoV-2
5.1. Activation, Functional Dynamics, and Exhaustion of NK Cells in SARS-CoV-2
5.2. Impact of SARS-CoV-2 Variants on NK Cell Responses
5.3. New Insights in Cellular Therapies Using NK Cells
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taborda, N.A.; Montoya, C.J. Las Células Natural Killer y Su Papel En La Respuesta Inmunitaria Durante La Infección Por El Virus de La Inmunodeficiencia Humana Tipo-1. Inmunología 2014, 33, 11–20. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Cai, S.; Peng, H.; Huyan, T.; Yang, H. The Role of NK Cells in Fighting the Virus Infection and Sepsis. Int. J. Med. Sci. 2021, 18, 3236–3248. [Google Scholar] [CrossRef] [PubMed]
- Agresti, N.; Lalezari, J.P.; Amodeo, P.P.; Mody, K.; Mosher, S.F.; Seethamraju, H.; Kelly, S.A.; Pourhassan, N.Z.; Sudduth, C.D.; Bovinet, C.; et al. Disruption of CCR5 Signaling to Treat COVID-19-Associated Cytokine Storm: Case Series of Four Critically Ill Patients Treated with Leronlimab. J. Transl. Autoimmun. 2021, 4, 100083. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, C.; Khan, L.; Osman, M.S.; Cohen Tervaert, J.W. Natural Killer Cell Dysfunction and Its Role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351. [Google Scholar] [CrossRef]
- Maucourant, C.; Filipovic, I.; Ponzetta, A.; Aleman, S.; Cornillet, M.; Hertwig, L.; Strunz, B.; Lentini, A.; Reinius, B.; Brownlie, D.; et al. Natural Killer Cell Immunotypes Related to COVID-19 Disease Severity. Sci. Immunol. 2020, 5, eabd6832. [Google Scholar] [CrossRef]
- Almehmadi, M.; Allahyani, M.; Aljuaid, A.; Alsuwat, M.A.; Halawi, M. Elevated Levels of CD56+ T Cells, CD16+ CD56+ T Cells, and CD56dim NK Cells in Herpes Simplex Virus Type 1 Seropositive Healthy Individuals. Saudi Med. J. 2024, 45, 1312–1317. [Google Scholar] [CrossRef]
- Kenney, A.D.; Dowdle, J.A.; Bozzacco, L.; McMichael, T.M.; St Gelais, C.; Panfil, A.R.; Sun, Y.; Schlesinger, L.S.; Anderson, M.Z.; Green, P.L.; et al. Human Genetic Determinants of Viral Diseases. Annu. Rev. Genet. 2017, 51, 241–263. [Google Scholar] [CrossRef]
- Björkström, N.K.; Ponzetta, A. Natural Killer Cells and Unconventional T Cells in COVID-19. Curr. Opin. Virol. 2021, 49, 176–182. [Google Scholar] [CrossRef]
- Hellwig, D.; Voigt, J.; Bouzani, M.; Löffler, J.; Albrecht-Eckardt, D.; Weber, M.; Brunke, S.; Martin, R.; Kurzai, O.; Hünniger, K. Candida Albicans Induces Metabolic Reprogramming in Human NK Cells and Responds to Perforin with a Zinc Depletion Response. Front. Microbiol. 2016, 7, 750. [Google Scholar] [CrossRef]
- Ferreira, C. Pandemics and epidemics: Emphasis on COVID-19. Int. J. Infect. 2025, 9, 11–14. [Google Scholar]
- Jaiswal, S.R.; Malhotra, P.; Mitra, D.K.; Chakrabarti, S. Focusing on a Unique Innate Memory Cell Population of Natural Killer Cells in the Fight against COVID-19: Harnessing the Ubiquity of Cytomegalovirus Exposure. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020047. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Dong, X.; Zhou, X.; Zhao, L.; Wang, X.; Rashu, R.; Zhao, W.; Yang, X. NK Cells Contribute to Protective Memory T Cell Mediated Immunity to Chlamydia Muridarum Infection. Front. Cell. Infect. Microbiol. 2020, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.J.W.; Long, E.O. The Peptide Selectivity Model: Interpreting NK Cell KIR-HLA-I Binding Interactions and Their Associations to Human Diseases. Trends Immunol. 2024, 45, 959–970. [Google Scholar] [CrossRef] [PubMed]
- De Louche, C.D.; Roghanian, A. Human Inhibitory Leukocyte Ig-like Receptors: From Immunotolerance to Immunotherapy. JCI Insight 2022, 7, e151553. [Google Scholar] [CrossRef]
- Lewis Marffy, A.L.; McCarthy, A.J. Leukocyte Immunoglobulin-Like Receptors (LILRs) on Human Neutrophils: Modulators of Infection and Immunity. Front. Immunol. 2020, 11, 857. [Google Scholar] [CrossRef]
- Abdallah, F.; Coindre, S.; Gardet, M.; Meurisse, F.; Naji, A.; Suganuma, N.; Abi-Rached, L.; Lambotte, O.; Favier, B. Leukocyte Immunoglobulin-Like Receptors in Regulating the Immune Response in Infectious Diseases: A Window of Opportunity to Pathogen Persistence and a Sound Target in Therapeutics. Front. Immunol. 2021, 12, 717998. [Google Scholar] [CrossRef]
- Ghaffarpour, S.; Ghazanfari, T.; Ardestani, S.K.; Naghizadeh, M.M.; Vaez Mahdavi, M.R.; Salehi, M.; Majd, A.M.M.; Rashidi, A.; Chenary, M.R.; Mostafazadeh, A.; et al. Cytokine Profiles Dynamics in COVID-19 Patients: A Longitudinal Analysis of Disease Severity and Outcomes. Sci. Rep. 2025, 15, 14209. [Google Scholar] [CrossRef]
- Han, E.; Youn, S.; Kwon, K.T.; Kim, S.C.; Jo, H.-Y.; Jung, I. Disease Progression Associated Cytokines in COVID-19 Patients with Deteriorating and Recovering Health Conditions. Sci. Rep. 2024, 14, 24712. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive Snapshots of Natural Killer Cells Functions, Signaling, Molecular Mechanisms and Clinical Utilization. Signal Transduct. Target. Ther. 2024, 9, 302. [Google Scholar] [CrossRef]
- Shen, Z.; Meng, X.; Rautela, J.; Chopin, M.; Huntington, N.D. Adjusting the Scope of Natural Killer Cells in Cancer Therapy. Cell. Mol. Immunol. 2025, 22, 699–711. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.M.; Rothman, P.B.; Yu, J.; et al. Tissue-Resident Natural Killer (NK) Cells Are Cell Lineages Distinct from Thymic and Conventional Splenic NK Cells. eLife 2014, 3, e01659. [Google Scholar] [CrossRef]
- Cichocki, F.; Grzywacz, B.; Miller, J.S. Human NK Cell Development: One Road or Many? Front. Immunol. 2019, 10, 2078. [Google Scholar] [CrossRef]
- Garand, M.; Goodier, M.; Owolabi, O.; Donkor, S.; Kampmann, B.; Sutherland, J.S. Functional and Phenotypic Changes of Natural Killer Cells in Whole Blood during Mycobacterium Tuberculosis Infection and Disease. Front. Immunol. 2018, 9, 257. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human Natural Killer Cells: A Unique Innate Immunoregulatory Role for the CD56(Bright) Subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef]
- Caligiuri, M.A. Human Natural Killer Cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Scoville, S.D.; Freud, A.G.; Caligiuri, M.A. Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front. Immunol. 2017, 8, 360. [Google Scholar] [CrossRef]
- Borrego, F.; Masilamani, M.; Marusina, A.I.; Tang, X.; Coligan, J.E. The CD94/NKG2 Family of Receptors: From Molecules and Cells to Clinical Relevance. Immunol. Res. 2006, 35, 263–277. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. The C-Type Lectin-like Domain Superfamily. FEBS J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.C.; Pende, D.; Moretta, L. Human NK Cells, Their Receptors and Function. Eur. J. Immunol. 2021, 51, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Stern-Ginossar, N.; Mandelboim, O. Receptors on NK Cells. In Natural Killer Cells: Basic Science and Clinical Application; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; p. 168. ISBN 978-0-12-370454-2. [Google Scholar]
- Pituch-Noworolska, A.M. NK Cells in SARS-CoV-2 Infection. Cent. Eur. J. Immunol. 2022, 47, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Alrubayyi, A. NK Cells in COVID-19: Protectors or Opponents? Nat. Rev. Immunol. 2020, 20, 520. [Google Scholar] [CrossRef]
- Brusilovsky, M.; Rosental, B.; Shemesh, A.; Appel, M.Y.; Porgador, A. Human NK Cell Recognition of Target Cells in the Prism of Natural Cytotoxicity Receptors and Their Ligands. J. Immunotoxicol. 2012, 9, 267–274. [Google Scholar] [CrossRef]
- Vitale, M.; Cantoni, C.; Della Chiesa, M.; Ferlazzo, G.; Carlomagno, S.; Pende, D.; Falco, M.; Pessino, A.; Muccio, L.; De Maria, A.; et al. An Historical Overview: The Discovery of How NK Cells Can Kill Enemies, Recruit Defense Troops, and More. Front. Immunol. 2019, 10, 1415. [Google Scholar] [CrossRef]
- Dębska-Zielkowska, J.; Moszkowska, G.; Zieliński, M.; Zielińska, H.; Dukat-Mazurek, A.; Trzonkowski, P.; Stefańska, K. Kir Receptors as Key Regulators of Nk Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Hammer, Q.; Cuapio, A.; Bister, J.; Björkström, N.K.; Ljunggren, H.-G. NK Cells in COVID-19—From Disease to Vaccination. J. Leukoc. Biol. 2023, 114, 507–512. [Google Scholar] [CrossRef]
- Bjorgen, J.C.; Dick, J.K.; Cromarty, R.; Hart, G.T.; Rhein, J. NK Cell Subsets and Dysfunction during Viral Infection: A New Avenue for Therapeutics? Front. Immunol. 2023, 14, 1267774. [Google Scholar] [CrossRef]
- Masselli, E.; Vaccarezza, M.; Carubbi, C.; Pozzi, G. NK Cells: A Double Edge Sword against SARS-CoV-2. Adv. Biol. Regul. 2020, 77, 100737. [Google Scholar] [CrossRef]
- Moretta, L.; Pietra, G.; Montaldo, E.; Vacca, P.; Pende, D.; Falco, M.; Del Zotto, G.; Locatelli, F.; Moretta, A.; Mingari, M.C. Human NK Cells: From Surface Receptors to the Therapy of Leukemias and Solid Tumors. Front. Immunol. 2014, 5, 87. [Google Scholar] [CrossRef]
- Wauquier, N.; Padilla, C.; Becquart, P.; Leroy, E.; Vieillard, V. Association of KIR2DS1 and KIR2DS3 with Fatal Outcome in Ebola Virus Infection. Immunogenetics 2010, 62, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.; Silva, A.M.; Carvalho, C.; Leal, B.; Santos, E.; Costa, P.P.; Silva, B.M. The Role of KIR2DS1 in Multiple Sclerosis—KIR in Portuguese MS Patients. J. Neuroimmunol. 2014, 269, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.; Mihaylova, A.; Ivanova, M.; Mihailova, S. Impact of KIR/HLA Ligand Combinations on Immune Responses in Malignant Melanoma. Cancer Immunol. Immunother. 2007, 56, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, J.; Rozemuller, E.H.; Petersen, E.J.; van den Tweel, J.G.; Verdonck, L.F.; Tilanus, M.G.J. Activating KIRs Exert a Crucial Role on Relapse and Overall Survival after HLA-Identical Sibling Transplantation. Mol. Immunol. 2008, 45, 2255–2261. [Google Scholar] [CrossRef]
- Martin, M.P.; Gao, X.; Lee, J.H.; Nelson, G.W.; Detels, R.; Goedert, J.J.; Buchbinder, S.; Hoots, K.; Vlahov, D.; Trowsdale, J.; et al. Epistatic Interaction between KIR3DS1 and HLA-B Delays the Progression to AIDS. Nat. Genet. 2002, 31, 429–434. [Google Scholar] [CrossRef]
- Hajeer, A.; Jawdat, D.; Massadeh, S.; Aljawini, N.; Abedalthagafi, M.S. Association of KIR Gene Polymorphisms with COVID-19 Disease. Clin. Immunol. 2022, 234, 108911. [Google Scholar] [CrossRef]
- Dizaji Asl, K.; Mazloumi, Z.; Majidi, G.; Kalarestaghi, H.; Sabetkam, S.; Rafat, A. NK Cell Dysfunction Is Linked with Disease Severity in SARS-CoV-2 Patients. Cell Biochem. Funct. 2022, 40, 559–568. [Google Scholar] [CrossRef]
- Wang, C.; Xia, C.Q. The Involvement of Natural Killer Cells in the Pathogenesis of Severe Acute Respiratory Syndrome. Am. J. Clin. Pathol. 2004, 121, 507–511. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Tukwasibwe, S.; Nakimuli, A.; Traherne, J.; Chazara, O.; Jayaraman, J.; Trowsdale, J.; Moffett, A.; Jagannathan, P.; Rosenthal, P.J.; Cose, S.; et al. Variations in Killer-Cell Immunoglobulin-like Receptor and Human Leukocyte Antigen Genes and Immunity to Malaria. Cell. Mol. Immunol. 2020, 17, 799–806. [Google Scholar] [CrossRef]
- Lee, D.J.; Sieling, P.A.; Ochoa, M.T.; Krutzik, S.R.; Guo, B.; Hernandez, M.; Rea, T.H.; Cheng, G.; Colonna, M.; Modlin, R.L. LILRA2 Activation Inhibits Dendritic Cell Differentiation and Antigen Presentation to T Cells. J. Immunol. 2007, 179, 8128–8136. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Hernández, D.L.; Benítez-Sánchez, A.; Rodríguez-Cuevas, J.S.; Rosales-Saavedra, T.; Guerra-Palomares, S.E.; Comas-García, A.; Noyola, D.E.; García-Sepúlveda, C.A. Killer-Cell Immunoglobulin-like Receptors and Cytomegalovirus Reactivation during Late Pregnancy. Int. J. Immunogenet. 2016, 43, 189–199. [Google Scholar] [CrossRef] [PubMed]
- de Souza Perce-Da-Silva, D.; Joaquim, T.E.; Do Couto Aleixo, A.L.Q.; Motta, J.P.R.; da Costa Lima-Junior, J.; Ribeiro-Alves, M.; de Oliveira-Ferreira, J.; de Moraes Sobrino Porto, L.C.; Banic, D.M.; Amendoeira, M.R.R. Influence of Killer Immunoglobulin-like Receptors Genes on the Recurrence Rate of Ocular Toxoplasmosis in Brazil. Mem. Inst. Oswaldo Cruz 2023, 118, e220203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Zhou, X.; Pan, Q.; Zhao, W.; Yang, X.; Wang, H. Natural Killer Cells Regulate Pulmonary Macrophages Polarization in Host Defense Against Chlamydial Respiratory Infection. Front. Cell. Infect. Microbiol. 2022, 11, 775663. [Google Scholar] [CrossRef]
- Guo, H.; Kumar, P.; Malarkannan, S. Evasion of Natural Killer Cells by Influenza Virus. J. Leukoc. Biol. 2011, 89, 189–194. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.-D. Innate Immunity: The First Line of Defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Zafarani, A.; Razizadeh, M.H.; Pashangzadeh, S.; Amirzargar, M.R.; Taghavi-Farahabadi, M.; Mahmoudi, M. Natural Killer Cells in COVID-19: From Infection, to Vaccination and Therapy. Future Virol. 2023, 18, 177–191. [Google Scholar] [CrossRef]
- de los Rios Kobara, I.; Jayewickreme, R.; Lee, M.J.; Wilk, A.J.; Blomkalns, A.L.; Nadeau, K.C.; Yang, S.; Rogers, A.J.; Blish, C.A. Interferon-Mediated NK Cell Activation Is Associated with Limited Neutralization Breadth during SARS-CoV-2 Infection. bioRxiv 2024. [Google Scholar] [CrossRef]
- Bao, C.; Tao, X.; Cui, W.; Hao, Y.; Zheng, S.; Yi, B.; Pan, T.; Young, K.H.; Qian, W. Natural Killer Cells Associated with SARS-CoV-2 Viral RNA Shedding, Antibody Response and Mortality in COVID-19 Patients. Exp. Hematol. Oncol. 2021, 10, 5. [Google Scholar] [CrossRef]
- Moll-Bernardes, R.; Fortier, S.C.; Sousa, A.S.; Lopes, R.D.; Vera, N.; Conde, L.; Feldman, A.; Arruda, G.; Cabral-Castro, M.; Albuquerque, D.C.; et al. NKG2A Expression among CD8 Cells Is Associated with COVID-19 Progression in Hypertensive Patients: Insights from the BRACE CORONA Randomized Trial. J. Clin. Med. 2022, 11, 3713. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Schauner, R.; Burke, J.; Borocz, J.; Vasana, S.; Sobieraj, L.; Giraudo, M.; Jackson, Z.; Ansari, Q.; Navas, M.; et al. NK Cells from COVID-19 Positive Patients Exhibit Enhanced Cytotoxic Activity upon NKG2A and KIR2DL1 Blockade. Front. Immunol. 2023, 14, 1022890. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Duan, X.; Dong, J.Z.; Chang, Y.; Han, Y.; Li, Y.; Jiang, W.; Fan, H.; Hou, X.; Cao, W.; et al. The Unreversible Reduced but Persistent Activated NK and CD8+ T Cells in Severe/Critical COVID-19 during Omicron Pandemic in China. Emerg. Microbes Infect. 2023, 12, 2208679. [Google Scholar] [CrossRef] [PubMed]
- Trimbake, D.; Singh, D.; K., Y.G.; Babar, P.; S., V.D.; Tripathy, A.S. Durability of Functional SARS-CoV-2-Specific Immunological Memory and T Cell Response up to 8–9 Months Postrecovery from COVID-19. J. Immunol. Res. 2025, 2025, 9743866. [Google Scholar] [CrossRef]
- Voogd, L.; Van Den Broek, R.L.; Van Wolfswinkel, M.; Franken, K.L.M.C.; Ruibal, P.; Jespers, W.; Leitner, J.; Steinberger, P.; Van Westen, G.J.P.; Ottenhoff, T.H.M.; et al. HLA-E/Peptide Complexes Differentially Interact with NKG2A/CD94 and T Cell Receptors. J. Immunol. 2025, 214, 595–605. [Google Scholar] [CrossRef]
- Vietzen, H.; Zoufaly, A.; Traugott, M.; Aberle, J.; Aberle, S.W.; Puchhammer-Stöckl, E. Deletion of the NKG2C Receptor Encoding KLRC2 Gene and HLA-E Variants Are Risk Factors for Severe COVID-19. Genet. Med. 2021, 23, 963–967. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Claus, M.; Krüger, N.; Reusing, S.; Gall, E.; Bade-Döding, C.; Braun, A.; Watzl, C.; Uhrberg, M.; Walter, L. SARS-CoV-2 Infection Induces Adaptive NK Cell Responses by Spike Protein-Mediated Induction of HLA-E Expression. Emerg. Microbes Infect. 2024, 13, 2361019. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Yamaguchi, K.; Shofiudin, M.A.; Mimura, M.; Takata, M.; Mihara, S.; Kawakami, T.; Doi, A.; Matsuda, R.; Kato, H.; et al. Distinct Immunity Dynamics of Natural Killer Cells in Mild and Moderate COVID-19 Cases during the Omicron Variant Phase. Front. Immunol. 2025, 16, 1594296. [Google Scholar] [CrossRef]
- Ma, M.T.; Jiang, Q.; Chen, C.-H.; Badeti, S.; Wang, X.; Zeng, C.; Evans, D.; Bodnar, B.; Marras, S.A.E.; Tyagi, S.; et al. S309-CAR-NK Cells Bind the Omicron Variants in Vitro and Reduce SARS-CoV-2 Viral Loads in Humanized ACE2-NSG Mice. J. Virol. 2024, 98, e0003824. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Zhou, M.; Mi, J.; Liu, J.; Han, L.; Yu, X.; Zhang, X. Characteristics of SARS-CoV-2 Variants and Potential Co-Infected Pathogens in Hospitalized Patients Based on Metagenomic next-Generation Sequencing. Sci. Rep. 2025, 15, 18923. [Google Scholar] [CrossRef]
- Majed, S.O.; Mustafa, S.A.; Jalal, P.J.; Fatah, M.H.; Karim, A.Y.; Hassannejad, S.; Miasko, M.H. Genome Analysis of SARS-CoV-2 Delta Variant in the Kurdistan Region of Iraq. J. Infect. Dev. Ctries. 2024, 18, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Varchetta, S.; Mele, D.; Oliviero, B.; Mantovani, S.; Ludovisi, S.; Cerino, A.; Bruno, R.; Castelli, A.; Mosconi, M.; Vecchia, M.; et al. Unique Immunological Profile in Patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Terunuma, H.; Nieda, M. Exploring the Utility of NK Cells in COVID-19. Biomedicines 2022, 10, 1002. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.; Ghasemzadeh, A.; Hosseini, E. Exhausted NK Cells and Cytokine Storms in COVID-19: Whether NK Cell Therapy Could Be a Therapeutic Choice. Hum. Immunol. 2022, 83, 86–98. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus Disease 2019 (COVID-19): An Overview of the Immunopathology, Serological Diagnosis and Management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- D’Andrea, P.; Tauro, L.; Qin, S. Pulmonary Inflammation Is Mediated by Cyotkines in COVID-19. Int. J. Infect. 2025, 8, 62–67. [Google Scholar]
- Andrea, A.E.; Chiron, A.; Sarrabayrouse, G.; Bessoles, S.; Hacein-Bey-Abina, S. A Structural, Genetic and Clinical Comparison of CAR-T Cells and CAR-NK Cells: Companions or Competitors? Front. Immunol. 2024, 15, 1459818. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-Cell Therapy in the Era of Solid Tumor Treatment: Current Challenges and Emerging Therapeutic Advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Wibowo, T.; Kogue, Y.; Ikeda, S.; Yaga, M.; Tachikawa, M.; Suga, M.; Kida, S.; Shibata, K.; Tsutsumi, K.; Murakami, H.; et al. CAR-NK Cells Derived from Cord Blood Originate Mainly from CD56−CD7+CD34−HLA-DR−Lin− NK Progenitor Cells. Mol. Ther. Methods Clin. Dev. 2024, 32, 101374. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, Y.; Sun, G.; Zhang, X.; Cao, J.; Shao, M.; Liang, X.; Wang, L. Clinical Application and Prospect of Immune Checkpoint Inhibitors for CAR-NK Cell in Tumor Immunotherapy. Front. Immunol. 2023, 13, 1081546. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, J.; Moraru, M.; Al-Akioui-Sanz, K.; Altadill, M.; Muntasell, A.; López-Botet, M.; Vilches, C. NKG2C Sequence Polymorphism Modulates the Expansion of Adaptive NK Cells in Response to Human CMV. HLA 2024, 104, e15764. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhao, X. Natural Killer Cells Play an Important Role in Virus Infection Control: Antiviral Mechanism, Subset Expansion and Clinical Application. Clin. Immunol. 2021, 227, 108727. [Google Scholar] [CrossRef] [PubMed]

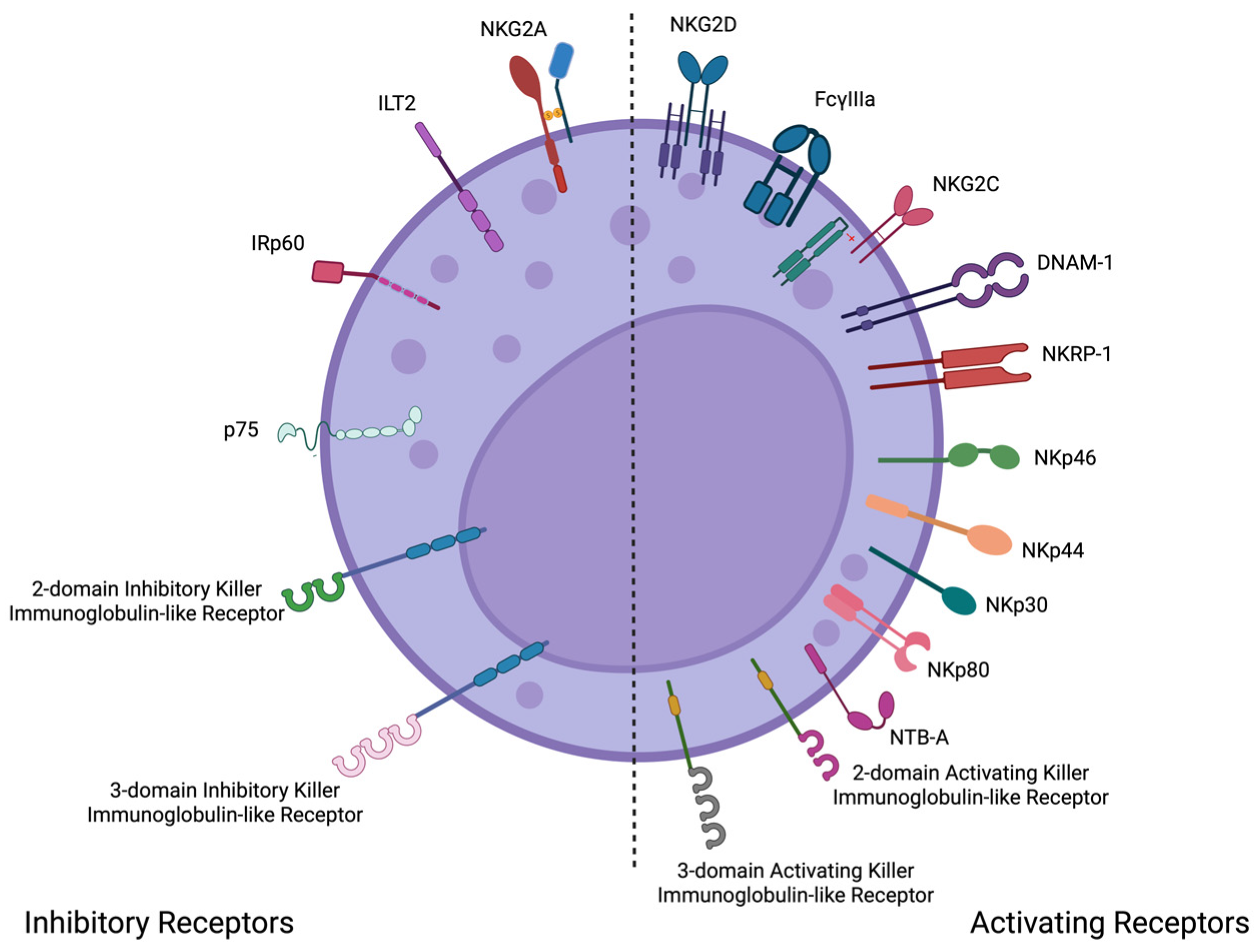
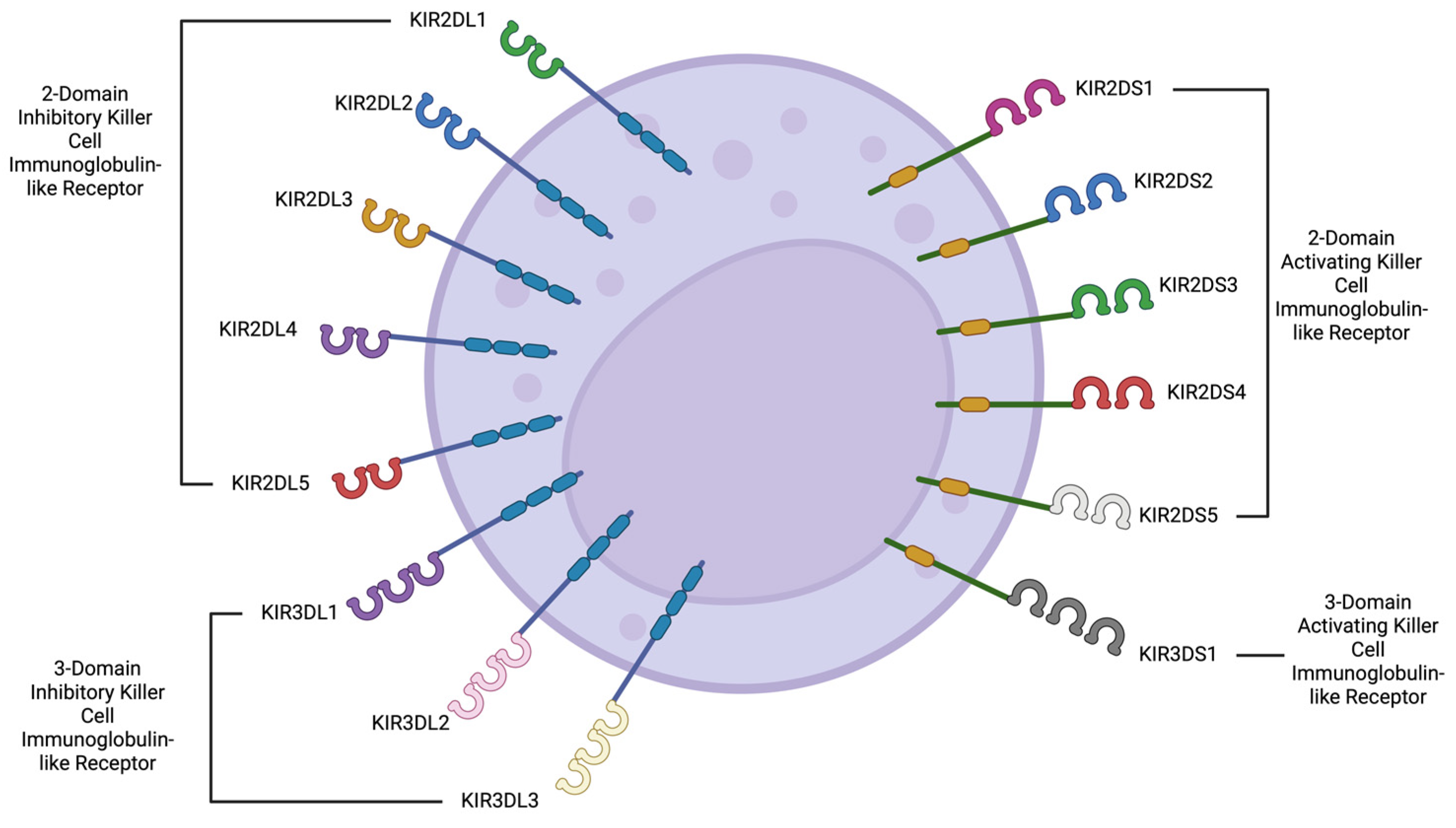
| NK Cell Receptor Type | Main Roles on Viral Infections | Main Roles on Non-Viral Infectious Processes |
|---|---|---|
| Natural Cytotoxicity Receptors (NCR) | NKp46, NKp30 and NKp44 are underexpressed in patients infected with HIV [2]. NKp46 recognizes influenza hemagglutinin triggering the elimination of the infected cell [3]. NKp44 serves as a ligand to the flavivirus West Nile virus, triggering its activation and elimination of the infected cell [4]. | NKp44 has been showed to serve as a direct binding site for several pathogens, such as Mycobacterium bovis and tuberculosis, as well as Pseudomonas aeuroginosa [4,5,8]. |
| Leukocyte Immunoglobulin-Like Receptors (LILRs) | LILRB1: upregulation has been associated with HCMV reactivation, after lung transplantation [13]. | LILRA2: augmented expression has been observed in patients suffering from leprosy [52]. |
| Killer Immunoglobulin-like Receptors (KIRs) | KIR2DS1/2DS3 are found more frequently in the patients who died due to the Ebola virus [14]. KIR2DL3/2DL5 are found more frequently in patients infected by hepatitis C with a more rapid onset of the chronic phase of the disease. Statistically lower frequencies of the haplotype cB03|tA01, containing the inhibitory gene KIR2DL5 and the pair KIR2DS3/2DS5, were observed among the pregnant women infected with CMV [53]. | KIR2DL2/2DS2: an increased frequency in patients who did not develop ocular toxoplasmosis [54]. |
| Studied Population | Involved Receptors | Proposed Mechanism |
|---|---|---|
| 27 Norwegian SARS-CoV-2-positive patients with moderate and severe forms of the disease [29] | In general, the number of NK cells observed in both the moderate and the severe groups is decreased; however, an especially activated profile either for CD56dim or CD56bright is also observed, responsible for the typical aggressive symptomatology due to the cytokine storm. | The NK cells found in the lung tissue show a strong immune activation and thus increased interferon response, especially in the patients with the moderate version of the disease. On the other hand, the patients with the severe form of the disease showed an increased population of neutrophils and monocytes. |
| 424 Saudi patients SARS-CoV-2 positive with different degrees of severity ranging from asymptomatic to severe forms of the disease [30] | KIR2DS4 and KIR3DL1 were observed to be in a higher frequency in patients with the severe form of the disease. KIR3DL1, 2DL5, 2DS1, 2DS5, and 3DS1 were associated with a protective effect. | KIR2DS4 and KIR3DL1 are part of the inhibitory KIR A haplotype, which could be stopping the NK cell from interacting with their HLA ligand exert a cytotoxic mechanism against the infected cell. |
| 100 US American patients SARS-CoV-2 positive with different degrees of severity ranging from asymptomatic to severe forms of the disease [31] | The expression of NKG2D was observed to be decreased in the studied groups with different degrees of the disease (from asymptomatic to severe). | A specific phenotypic profile was observed for the healthy controls as well as for the patients with the severe form of the disease. The observed signature describes a NK cell population with decreased cytotoxicity in the severely ill patients, which could explain the increase in the symptomatology. |
| 28 Italian patients SARS-CoV-2 positive with different degrees of severity, and grouped according to their need of oxygen support [37] | A generalized decrease in the number of lymphocytes (including NK cells) was observed in all three categories. The phenotypic profile of the circulating NK cells was mainly inhibitory, with an increased expression of NKG2A and KIR2DL1, as well as a decrease in the number of CD16+/CD56bright NK cells, mainly cytokine producers. | There seems to be an increased turnover of inflammatory NK cells in the patients who required oxygen support and mechanical ventilation, suggesting an imbalance and a lack of regulation between the cytotoxic and cytokine-producing NK cells with an activating profile. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Hernández, D.L.; Noyola, M.V.; Martínez-Rider, R.; Bernal-Silva, S.; Comas-Garcia, A. NK Cells: A Powerful Squad Versus SARS-CoV-2. Int. J. Mol. Sci. 2025, 26, 6500. https://doi.org/10.3390/ijms26136500
Alvarado-Hernández DL, Noyola MV, Martínez-Rider R, Bernal-Silva S, Comas-Garcia A. NK Cells: A Powerful Squad Versus SARS-CoV-2. International Journal of Molecular Sciences. 2025; 26(13):6500. https://doi.org/10.3390/ijms26136500
Chicago/Turabian StyleAlvarado-Hernández, Diana Lorena, Marlen Vitales Noyola, Ricardo Martínez-Rider, Sofía Bernal-Silva, and Andreu Comas-Garcia. 2025. "NK Cells: A Powerful Squad Versus SARS-CoV-2" International Journal of Molecular Sciences 26, no. 13: 6500. https://doi.org/10.3390/ijms26136500
APA StyleAlvarado-Hernández, D. L., Noyola, M. V., Martínez-Rider, R., Bernal-Silva, S., & Comas-Garcia, A. (2025). NK Cells: A Powerful Squad Versus SARS-CoV-2. International Journal of Molecular Sciences, 26(13), 6500. https://doi.org/10.3390/ijms26136500







