Fulminant and Slowly Progressive Type 1 Diabetes Associated with Pregnancy
Abstract
1. Introduction
2. Literature Search Strategy
3. Classification of Impaired Glucose Tolerance in Pregnancy
3.1. Pregestational Diabetes
3.2. Overt Diabetes in Pregnancy
3.3. Gestational Diabetes
4. Classification of Type 1 Diabetes
4.1. Acute-Onset Type 1 Diabetes
4.2. Slowly Progressive Type 1 Diabetes (SPIDDM)
4.3. Fulminant Type 1 Diabetes
5. Genetic Factors
5.1. Genetic Factors of Gestational Diabetes
5.2. Genetic Factors of Type 1 Diabetes
6. Predictive Markers for Type 1 Diabetes
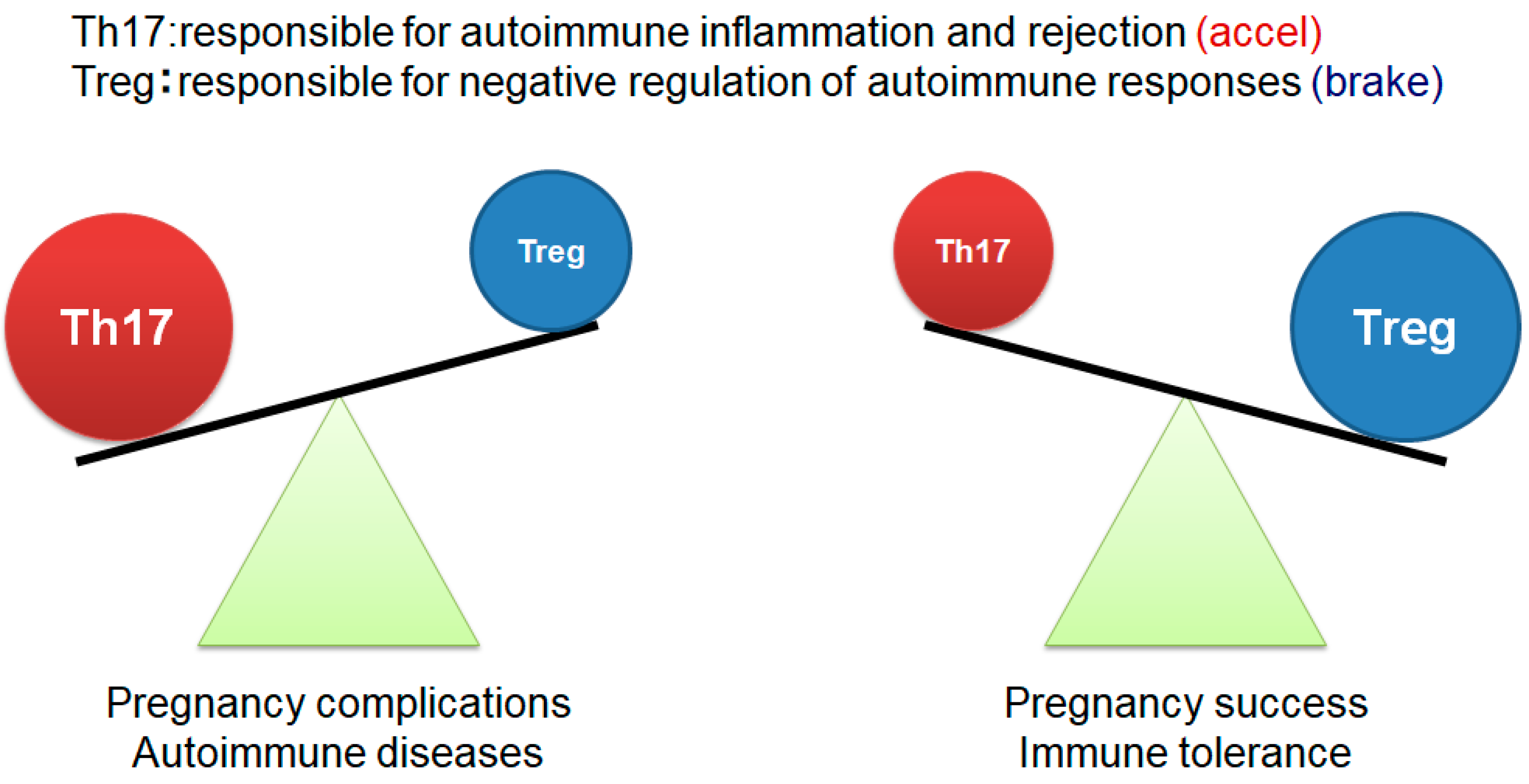
7. Pregnancy and Immunity
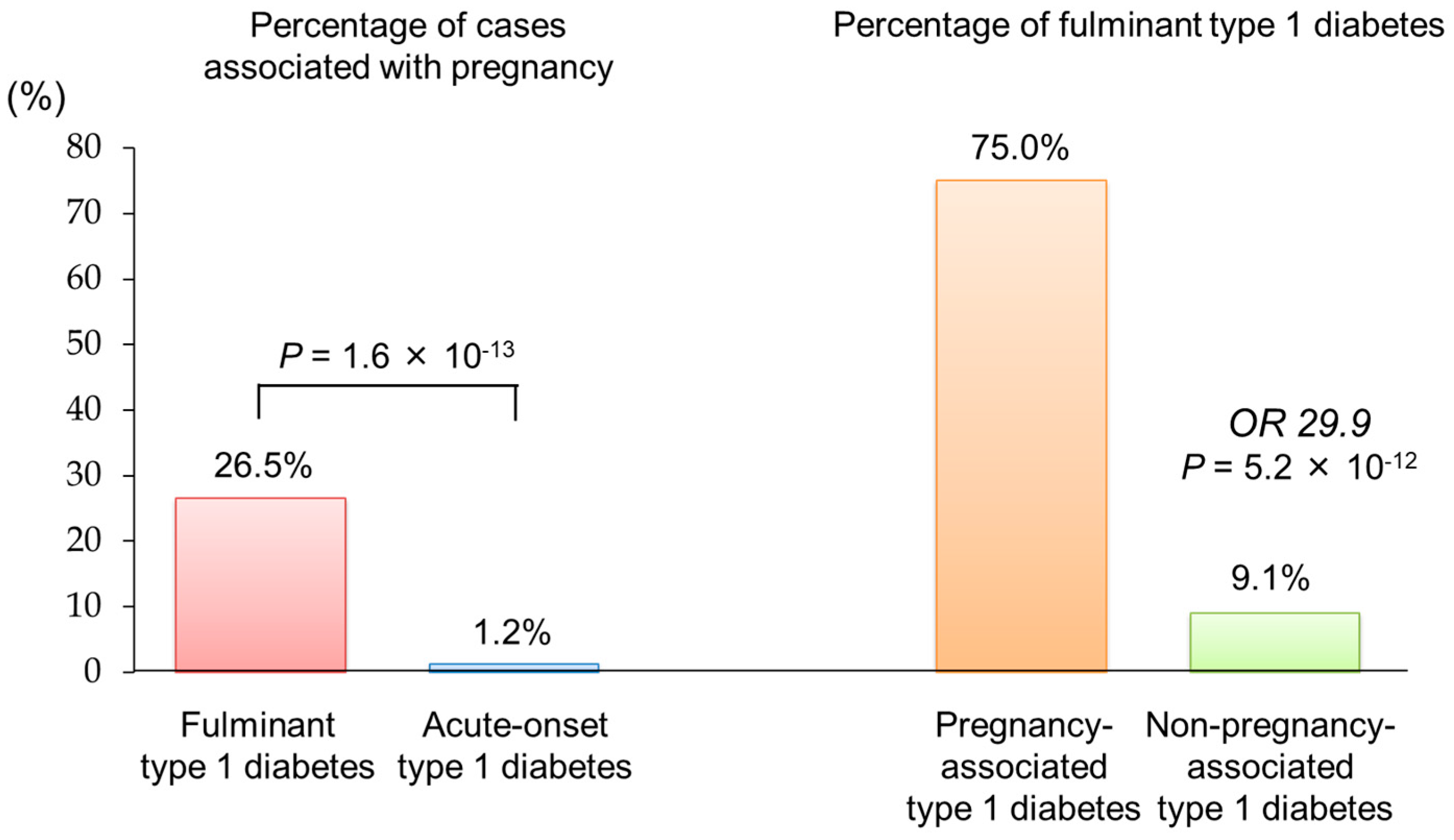
8. Pregnancy-Associated Fulminant Type 1 Diabetes
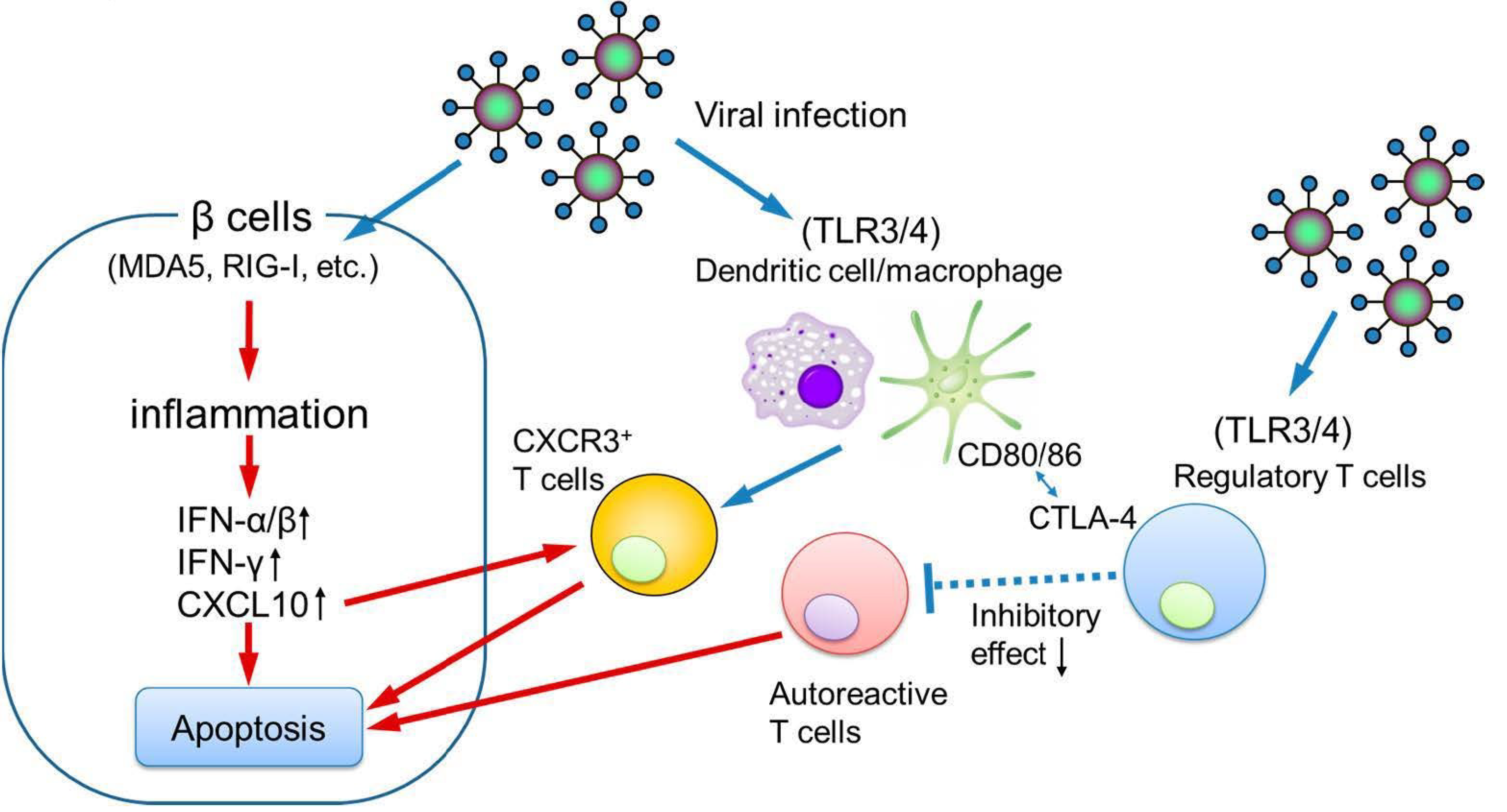
9. Etiology of Pregnancy-Associated Fulminant Type 1 Diabetes
10. Etiology of Autoimmune Gestational Diabetes
11. Autoimmune Gestational Diabetes as a Predictor of Type 1 Diabetes Development
12. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ADIPOQ | adiponectin |
| BMI | body mass index |
| CDKAL1 | CDK5 regulatory subunit-associated protein 1-like 1 |
| CDKN2A/2B | cyclin-dependent kinase inhibitor 2A/2B |
| CTLA-4 | cytotoxic T lymphocyte antigen-4 |
| GADA | glutamic acid decarboxylase autoantibodies |
| HAPO | hyperglycemia and adverse pregnancy outcomes |
| HLA | human leukocyte antigen |
| IA-2A | insulinoma-associated antigen-2 autoantibodies |
| IAA | insulin autoantibodies |
| IADPSG | International Association of Diabetes and Pregnancy Study Groups |
| ICA | islet cell antibody |
| IL2RA | interleukin 2 receptor subunit alpha |
| LADA | latent autoimmune diabetes in adults |
| LGA | large-for-gestational-age |
| MAD5 | melanoma differentiation–associated gene-5 |
| MDFIC2 | MyoD family inhibitor domain containing 2 |
| MTNR1B | melatonin receptor 1B |
| OGTT | oral glucose tolerance test |
| PTPN22 | protein tyrosine phosphatase non-receptor type 22 |
| RIG-I | retinoic acid–inducible gene I |
| SPIDDM | slowly progressive type 1 diabetes |
| SSR1-RREB1 | signal sequence receptor subunit 1-Ras-responsive element binding protein 1 |
| TCF7L2 | transcription factor 7 like 2 |
| Th1 | T-helper 1 |
| Th2 | T-helper 2 |
| Th17 | T-helper 17 |
| TLR | toll-like receptor |
| Tregs | regulatory T cells |
| VNTR | variable number of tandem repeats |
| ZnT8A | zinc transporter 8 autoantibodies |
References
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; Hod, M.; Kitzmiler, J.L.; et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Miller, D.; Galaz, J.; Liu, T.N.; Romero, R.; Gomez-Lopez, N. The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation. J. Steroid Biochem. Mol. Biol. 2023, 229, 106254. [Google Scholar] [CrossRef] [PubMed]
- Stathi, D.; Lee, F.N.; Dhar, M.; Bobotis, S.; Arsenaki, E.; Agrawal, T.; Triantafyllidis, K.K.; Kechagias, K.S. Diabetic ketoacidosis in pregnancy: A systematic review of the reported cases. Clin. Med. Insights Endocrinol. Diabetes 2025, 18, 11795514241312849. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Matsuura, N.; Eguchi, K. Type 1 diabetes in Japan. Diabetologia 2006, 49, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, Y.; Hashimoto, K.; Hara, K.; Morimoto, J.; Namai, K.; Tanaka, A.; Tanaka, S.; Shimada, A. Current clinical state of type 1 diabetes in Saitama prefecture. Diabetol. Int. 2021, 13, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Takaike, H.; Miura, J.; Takagi, S.; Mochizuki, S.; Babazono, T. Clinical features among adult-onset type 1 diabetes.; distribution of subtypes.; and differences in probable and definite slowly progressive insulin-dependent diabetes mellitus: A single hospital-based study over a 13-year period. J. Diabetes Investig. 2025, 16, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Siebert, E.; Raja, V.; Mehrotra, C.; Richards, J.; Khan, J.; Graham, D.F. Determinants of progression of diabetic retinopathy in pregnancy. Diabetes Res. Clin. Pract. 2024, 214, 111784. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa-Shingu, K.; Waguri, M.; Takahara, M.; Katakami, N.; Shimomura, I. Clinical features of overt versus diagnosed pre-existing diabetes in pregnancy. J. Diabetes Investig. 2025, 16, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Modzelewski, R.; Stefanowicz-Rutkowska, M.M.; Matuszewski, W.; Bandurska-Stankiewicz, E.M. Gestational diabetes mellitus-Recent literature review. J. Clin. Med. 2022, 11, 5736. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E. Anti-islet autoantibodies in type 1 diabetes. Int. J. Mol. Sci. 2023, 24, 10012. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Noda, T.; Okada, S.; Myojin, T.; Kubo, S.; Higashino, T.; Ishii, H.; Imamura, T. Incidence and seasonality of type 1 diabetes: A population-based 3-year cohort study using the National Database in Japan. BMJ Open Diabetes Res. Care 2020, 8, e001262. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Kawasaki, E.; Abiru, N.; Awata, T.; Oikawa, Y.; Osawa, H.; Kajio, H.; Kozawa, J.; Takahashi, K.; Chujo, D.; et al. New diagnostic criteria (2023) for slowly progressive type 1 diabetes (SPIDDM): Report from Committee on Type 1 Diabetes of the Japan Diabetes Society (English version). J. Diabetes Investig. 2024, 15, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, A.; Hanafusa, T. Fulminant type 1 diabetes-East and West. J. Clin. Endocrinol. Metab. 2023, 108, e1473–e1478. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Tohyama, M.; Kawamura, R.; Takata, Y.; Osawa, H.; Onuma, H. Fulminant type 1 diabetes caused by DIHS could be affected by the reactivation of HHV-6. J. Clin. Endocrinol. Metab. 2024, 109, e2024–e2030. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Imagawa, A.; Abiru, N.; Awata, T.; Ikegami, H.; Uchigata, Y.; Oikawa, Y.; Osawa, H.; Kajio, H.; Kawasaki, E.; et al. consultation of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol. Int. 2018, 10, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; Walters, R.K.; Pirinen, M.; Kurki, M.; Junna, N.; Goldstein, J.I.; Reeve, M.P.; Siirtola, H.; Lemmelä, S.M.; Turley, P.; et al. Distinct and shared genetic architectures of gestational diabetes mellitus and type 2 diabetes. Nat. Genet. 2024, 56, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, Y.; Hata, K.; Tajima, A.; Ochiai, D.; Saisho, Y.; Matsumoto, T.; Arata, N.; Miyakoshi, K.; Tanaka, M. Association of common polymorphisms with gestational diabetes mellitus in Japanese women: A case-control study. Endocr. J. 2017, 64, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Nawa, N.; Murakami, K.; Tanaka, T.; Ohseto, H.; Takahashi, I.; Narita, A.; Obara, T.; Ishikuro, M.; Orui, M.; et al. Genetic effects on gestational diabetes mellitus and their interactions with environmental factors among Japanese women. J. Hum. Genet. 2025, 70, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, H.; Noso, S. Genetics of type-1 diabetes. Diabetol. Int. 2024, 15, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Onengut-Gumuscu, S.; Concannon, P.; Akolkar, B.; Erlich, H.A.; Julier, C.; Morahan, G.; Nierras, C.R.; Pociot, F.; Todd, J.A.; Rich, S.S. Type 1 diabetes genetics consortium. J. Clin. Endocrinol. Metab. 2025, 110, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, H.; Zhang, Y.; Fatunbi, J.; Luu, T.; Kwak-Kim, J. The impact of reproductive hormones on T cell immunity; normal and assisted reproductive cycles. J. Reprod. Immunol. 2024, 165, 104295. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Fenizia, C.; Introini, A.; Zavatta, A.; Scaccabarozzi, C.; Biasin, M.; Savasi, V. The pathophysiological role of estrogens in the initial stages of pregnancy: Molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum. Reprod. Update 2023, 29, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, C.; Wu, D.; Yao, L.; Geng, M.; Li, S.; Guo, Y.; Wang, Q.; Wei, Z.; Li, W. Th17/Treg cell imbalance may contribute to spontaneous preterm labor. J. Immunol. Res. 2025, 2025, 8405365. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chi, H.; Qiao, J. Role of regulatory T cells in regulating fetal-maternal immune tolerance in healthy pregnancies and reproductive diseases. Front. Immunol. 2020, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Starosz, A.; Jamiołkowska-Sztabkowska, M.; Głowińska-Olszewska, B.; Moniuszko, M.; Bossowski, A.; Grubczak, K. Immunological balance between Treg and Th17 lymphocytes as a key element of type 1 diabetes progression in children. Front. Immunol. 2022, 13, 958430. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, G.; Kokudeva, M.; Siliogka, E.; Padilla, N.; Shumnalieva, R.; Della-Morte, D.; Ricordi, C.; Mihova, A.; Infante, M.; Velikova, T. T helper 17 cells and interleukin-17 immunity in type 1 diabetes: From pathophysiology to targeted immunotherapies. World J. Diabetes 2025, 16, 99936. [Google Scholar] [CrossRef] [PubMed]
- Tatovic, D.; Marwaha, A.; Taylor, P.; Hanna, S.J.; Carter, K.; Cheung, W.Y.; Luzio, S.; Dunseath, G.; Hutchings, H.A.; Holland, G.; et al. USTEKID Study Group. Ustekinumab for type 1 diabetes in adolescents: A multicenter.; double-blind.; randomized phase 2 trial. Nat. Med. 2024, 30, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; Socci, C.; Stabilini, A.; Valle, A.; Monti, P.; Piemonti, L.; Nano, R.; Olek, S.; Maffi, P.; Scavini, M.; et al. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 2011, 60, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Teniente-Serra, A.; Pizarro, E.; Quirant-Sánchez, B.; Fernández, M.A.; Vives-Pi, M.; Martinez-Caceres, E.M. Identifying changes in peripheral lymphocyte subpopulations in adult onset type 1 diabetes. Front. Immunol. 2021, 12, 784110. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Shimizu, I.; Hanafusa, T.; Imagawa, A.; Iwahashi, H.; Uchigata, Y.; Kanatsuna, A.; Kobayashi, T.; Shimada, A.; Maruyama, T.; et al. Nationwide survey on the prevalence of type 1 diabetes associated with pregnancy. Diabetes Pregnancy 2006, 6, 104–107. (In Japanese) [Google Scholar]
- Imagawa, A.; Hanafusa, T.; Awata, T.; Ikegami, H.; Uchigata, Y.; Osawa, H.; Kawasaki, E.; Kawabata, Y.; Kobayashi, T.; Shimada, A.; et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J. Diabetes Investig. 2012, 3, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Maruyama, T.; Imagawa, A.; Awata, T.; Ikegami, H.; Uchigata, Y.; Osawa, H.; Kawabata, Y.; Kobayashi, T.; Shimada, A.; et al. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus. J. Diabetes Investig. 2014, 5, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Makino, H.; Osawa, H.; Kounoue, E.; Imagawa, A.; Hanafusa, T.; Kawasaki, E.; Fujii, Y. Association of fulminant type 1 diabetes with pregnancy. Diabetes Res. Clin. Pract. 2003, 62, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Makino, H.; Imagawa, A.; Iwahashi, H.; Uchigata, Y.; Kanatsuka, A.; Kawasaki, E.; Kobayashi, T.; Shimada, A.; Maruyama, T.; et al. Clinical and immunogenetic characteristics of fulminant type 1 diabetes associated with pregnancy. J. Clin. Endocrinol. Metab. 2006, 91, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.; Nishida, Y.; Tanaka, S.; Maruyama, T.; Shimada, A.; Awata, T.; Suzuki, M.; Shimura, H.; Takizawa, S.; Ichijo, M.; et al. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates β-cell death in fulminant type 1 diabetes. Diabetes 2011, 60, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Imagawa, A.; Makino, H.; Uga, M.; Abiru, N.; Hanafusa, T.; Uchigata, Y.; Eguchi, K. Differences in the contribution of the CTLA4 gene to susceptibility to fulminant and type 1A diabetes in Japanese patients. Diabetes Care 2008, 31, 1608–1610. [Google Scholar] [CrossRef] [PubMed]
- Haseda, F.; Imagawa, A.; Murase-Mishiba, Y.; Sano, H.; Hirano-Kuwata, S.; Ueda, H.; Terasaki, J.; Hanafusa, T. Low CTLA-4 expression in CD4+ helper T-cells in patients with fulminant type 1 diabetes. Immunol. Lett. 2011, 139, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yin, W.; Wang, R.; Luo, S.; Zhou, Z. Fulminant type 1 diabetes: Focusing on triggering factors. Diabetes Metab. Res. Rev. 2024, 40, e3731. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, D.; de Leiva, A. Autoimmune gestational diabetes mellitus: A distinct clinical entity? Diabetes Metab. Res. Rev. 2001, 17, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Beunen, K.; Vercauter, L.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; et al. Type 1 diabetes-related autoimmune antibodies in women with gestational diabetes mellitus and the long-term risk for glucose intolerance. Front. Endocrinol. 2022, 13, 973820. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, A. Defining and classifying new subgroups of diabetes. Annu. Rev. Med. 2021, 72, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Luo, S.; Xiao, Z.; Zhang, Z.; Liu, B.; Zhou, Z. Latent autoimmune diabetes in adults: A focus on β-cell protection and therapy. Front. Endocrinol. 2022, 13, 959011. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.; Sun, Y.; Wei, Y.; Wang, S.; Song, G.; Yan, J.; Zhou, P.; Yang, H. Progression to type 2 diabetes mellitus after gestational diabetes mellitus diagnosed by IADPSG criteria: Systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1012244. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, R.; Zou, H.; Xie, L.; Zhou, Z.; Xiao, Y. Latent autoimmune diabetes in adults (LADA): From immunopathogenesis to immunotherapy. Front. Endocrinol. 2022, 13, 917169. [Google Scholar] [CrossRef] [PubMed]
- Horie, I.; Kawasaki, E.; Shimomura, A.; Satoh, T.; Ueki, I.; Kuwahara, H.; Ando, T.; Abiru, N.; Usa, T.; Eguchi, K. Emergence of anti-islet autoantibodies in Japanese patients with type 1 diabetes. Endocr. J. 2010, 57, 623–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Füchtenbusch, M.; Ferber, K.; Standl, E.; Ziegler, A.G. Prediction of type 1 diabetes postpartum in patients with gestational diabetes mellitus by combined islet cell autoantibody screening: A prospective multicenter study. Diabetes 1997, 46, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Ursing, D.; Törn, C.; Aberg, A.; Landin-Olsson, M. Presence of GAD antibodies during gestational diabetes mellitus predicts type 1 diabetes. Diabetes Care 2007, 30, 1968–1971. [Google Scholar] [CrossRef] [PubMed]
- Dereke, J.; Nilsson, C.; Landin-Olsson, M.; Hillman, M. Prevalence of zinc transporter 8 antibodies in gestational diabetes mellitus. Diabet. Med. 2012, 29, e436–e439. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Hillman, M.; Ursing, D.; Strevens, H.; Landin-Olsson, M. Clinical use of C-peptide and β-cell specific autoantibodies during gestational diabetes mellitus. Pract. Diabetes 2012, 29, 105–108. [Google Scholar] [CrossRef]
- Rudland, V.L.; Pech, C.; Harding, A.J.; Tan, K.; Lee, K.; Molyneaux, L.; Yue, D.K.; Wong, J.; Ross, G.P. Zinc transporter 8 autoantibodies: What is their clinical relevance in gestational diabetes? Diabet. Med. 2015, 32, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, T.P.; Højlund, K.; Snogdal, L.S.; Jensen, D.M. Glutamic acid decarboxylase autoantibody-positivity post-partum is associated with impaired β-cell function in women with gestational diabetes mellitus. Diabet. Med. 2015, 32, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, S.; Miyakoshi, K.; Endo, T.; Oishi, M.; Saisho, Y.; Tanaka, M. Perinatal outcomes of glutamic acid decarboxylase antibody-positive women with gestational diabetes mellitus. Pregnancy Diabetes 2019, 19, S84–S85. (In Japanese) [Google Scholar]
- Suzuki, M.; Ichikawa, R.; Koizumi, S.; Takano, K.; Shichiri, M. The prevalence of anti-GAD antibody positive women with gestational diabetes mellitus and overt diabetes in pregnancy. Pregnancy Diabetes 2019, 19, 37–41. (In Japanese) [Google Scholar]
- Luiro, K.; Auvinen, A.M.; Auvinen, J.; Jokelainen, J.; Järvelä, I.; Knip, M.; Tapanainen, J.S. Autoantibodies predict type 1 diabetes after gestational diabetes—A 23-year cohort study. Front. Endocrinol. 2023, 14, 1286375. [Google Scholar] [CrossRef] [PubMed]


| Acute-Onset Type 1 Diabetes | SPIDDM | Fulminant Type 1 Diabetes |
|---|---|---|
| Clinical symptoms and the need for insulin treatment | ||
| 1. Occurrence of diabetic ketosis or ketoacidosis around <3 months after the onset of hyperglycemic symptoms 2. Need for continuous insulin therapy after the diagnosis of diabetes mellitus. A temporary honeymoon period may occur. | 1. The absence of ketosis or ketoacidosis at the diagnosis of diabetes and the lack of need for insulin treatment to correct hyperglycemia immediately after diagnosis in principle | 1. Occurrence of diabetic ketosis or ketoacidosis soon (around 7 days) after the onset of hyperglycemic symptoms |
| Blood glucose‧HbA1c‧anti-islet autoantibodies (Note 1) | ||
| 3. Positive test result for anti-islet autoantibodies | 2. Positive test result for anti-islet autoantibodies at some time point during the disease course | 2. Plasma glucose level ≥ 288 mg/dL and HbA1c level < 8.7% at first visit (Note 2) |
| Endogenous insulin secretion | ||
| 4. Presence of endogenous insulin deficiency (fasting serum C-peptide < 0.6 ng/mL) without verifiable anti-islet autoantibodies | 3. Gradual decline in insulin secretion over time, no requirement for insulin treatment for ≥3 months (typically ≥ 6 months) after diagnosis of diabetes, and severe endogenous insulin deficiency (fasting serum C-peptide < 0.6 ng/mL) at last observed time point | 3. Urinary C-peptide excretion < 10 µg/day or fasting serum C-peptide level < 0.3 ng/mL and <0.5 ng/mL after intravenous glucagon (or after meal) load at onset |
| Diagnosis | ||
| “Acute-onset type 1 diabetes mellitus (autoimmune)”: fulfilled criteria 1, 2, and 3 “Acute-onset type 1 diabetes mellitus”: fulfilled criteria 1, 2, and 4 | “SPIDDM (definite)”: fulfilled criteria 1, 2, and 3 “SPIDDM (probable)”: fulfilled criteria 1 and 2 only, but not 3 | “Fulminant type 1 diabetes”: fulfilled criteria 1, 2, and 3 |
| Country | Number of Cases | GADA | IA-2A | ZnT8A | Reference |
|---|---|---|---|---|---|
| Germany | 437 | 9.5% | 6.2% | [52] | |
| Sweden | 385 | 6.0% | 1.8% | [53] | |
| Sweden | 193 | 6.2% | 2.6% | 2.6% | [54] |
| Sweden | 281 | 11.4% | 3.6% | [55] | |
| Denmark | 407 | 5.4% | [56] | ||
| Australia | 302 | 2.3% | 2.0% | 4.8% | [57] |
| Total | 2005 | 6.8% | 3.4% | 3.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, E. Fulminant and Slowly Progressive Type 1 Diabetes Associated with Pregnancy. Int. J. Mol. Sci. 2025, 26, 6499. https://doi.org/10.3390/ijms26136499
Kawasaki E. Fulminant and Slowly Progressive Type 1 Diabetes Associated with Pregnancy. International Journal of Molecular Sciences. 2025; 26(13):6499. https://doi.org/10.3390/ijms26136499
Chicago/Turabian StyleKawasaki, Eiji. 2025. "Fulminant and Slowly Progressive Type 1 Diabetes Associated with Pregnancy" International Journal of Molecular Sciences 26, no. 13: 6499. https://doi.org/10.3390/ijms26136499
APA StyleKawasaki, E. (2025). Fulminant and Slowly Progressive Type 1 Diabetes Associated with Pregnancy. International Journal of Molecular Sciences, 26(13), 6499. https://doi.org/10.3390/ijms26136499






