Levels of Growth Differentiation Factor 15 Correlated with Metabolic Dysfunction-Associated Steatotic Liver Disease in Children
Abstract
1. Introduction
2. Results
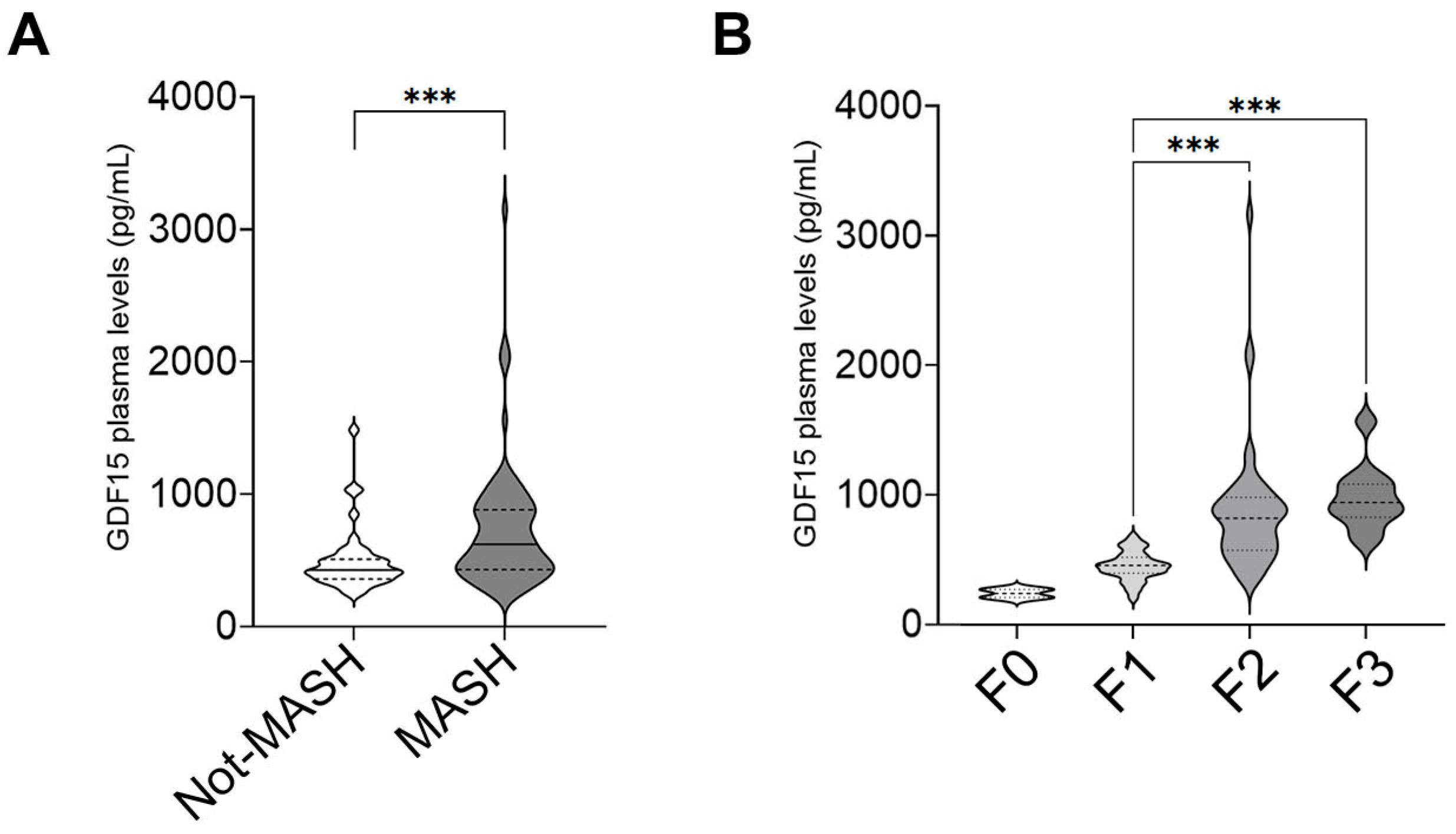
2.1. GDF15 Plasma Levels Increase in Children with MASLD
2.2. GDF15 Plasma Levels Are Associated with a More Severe Pattern of MASLD in Children
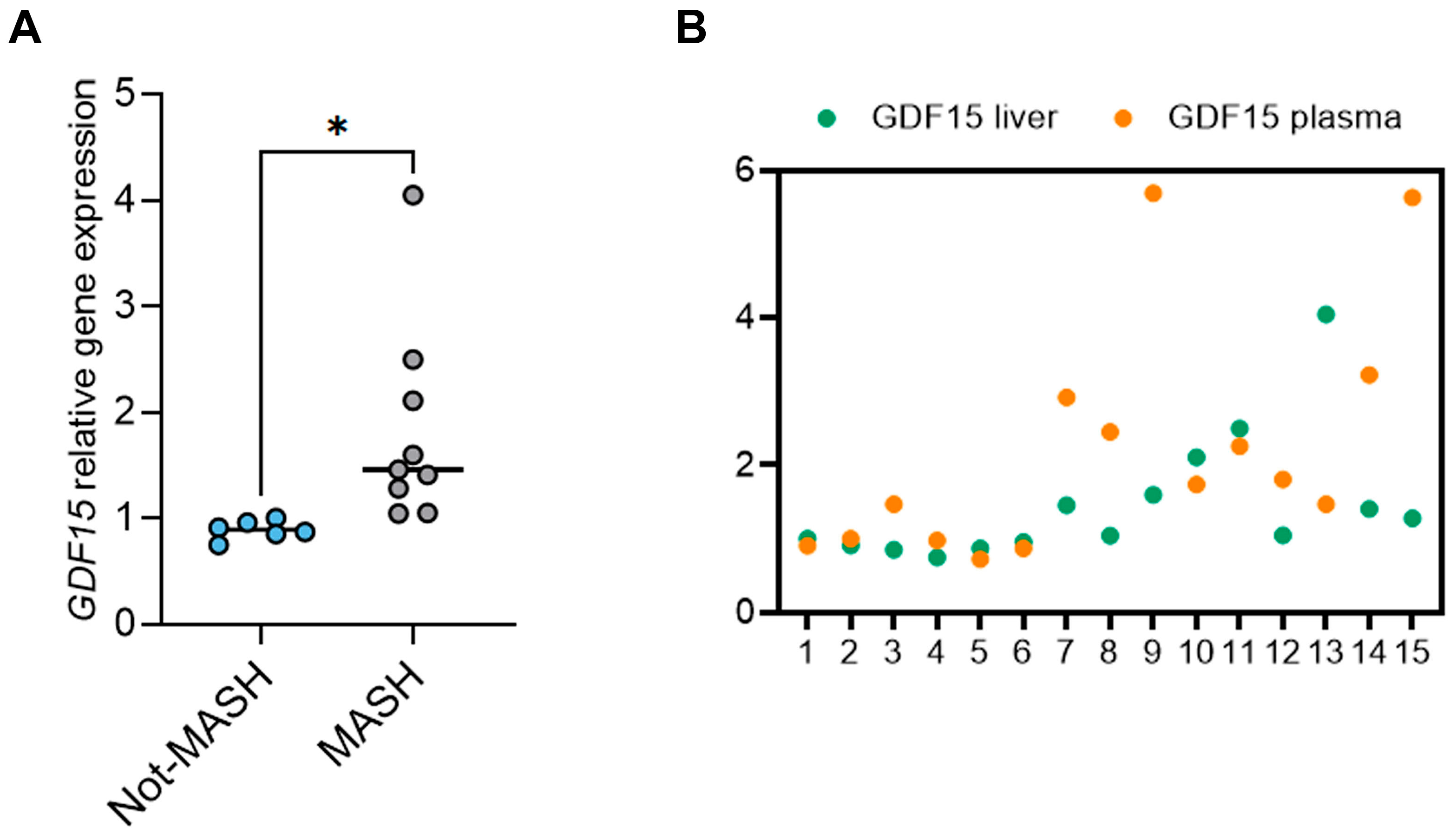
2.3. The Circulating Levels of GDF15 Correlate with Its Hepatic Levels in Children with MASLD
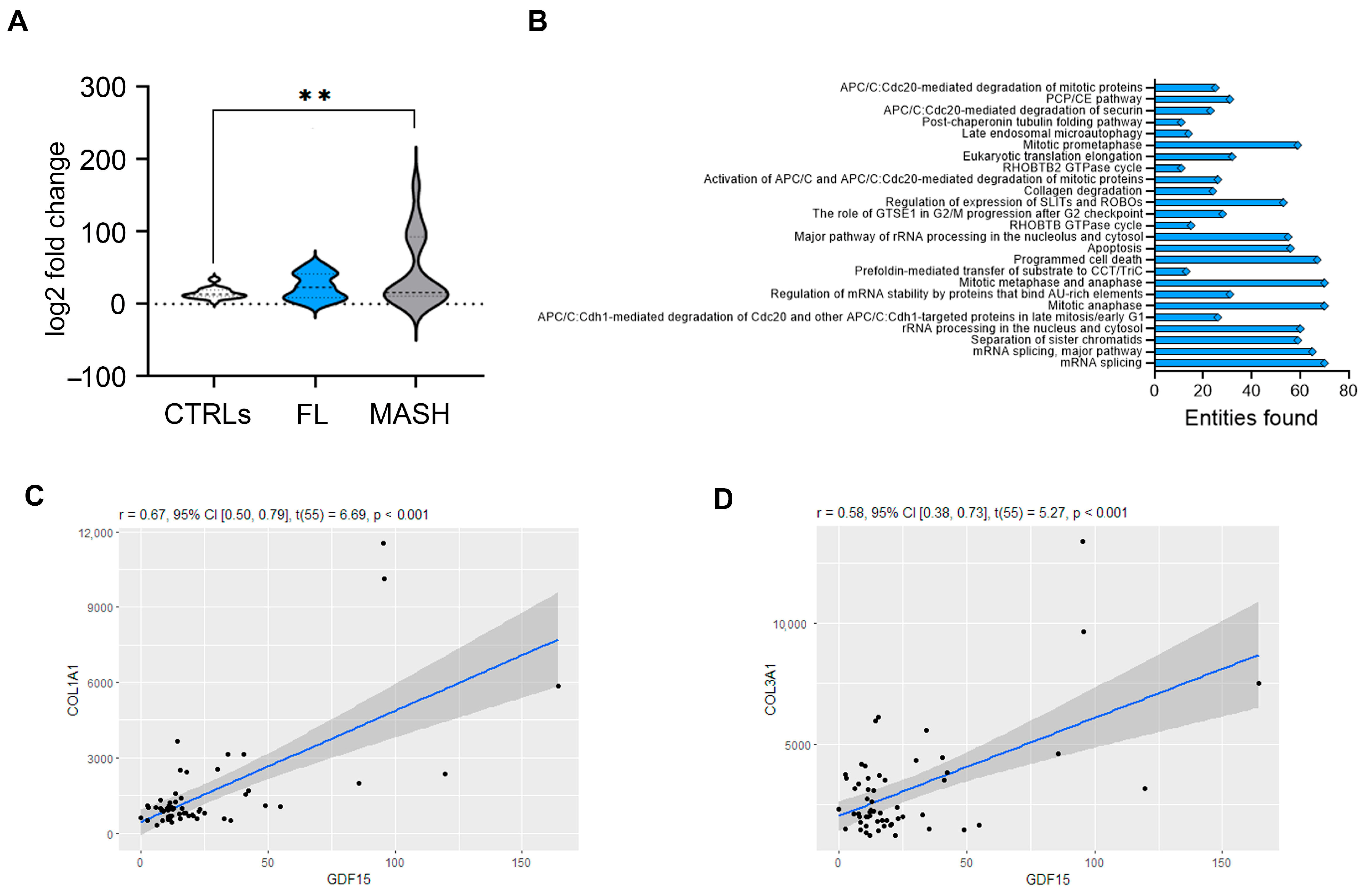
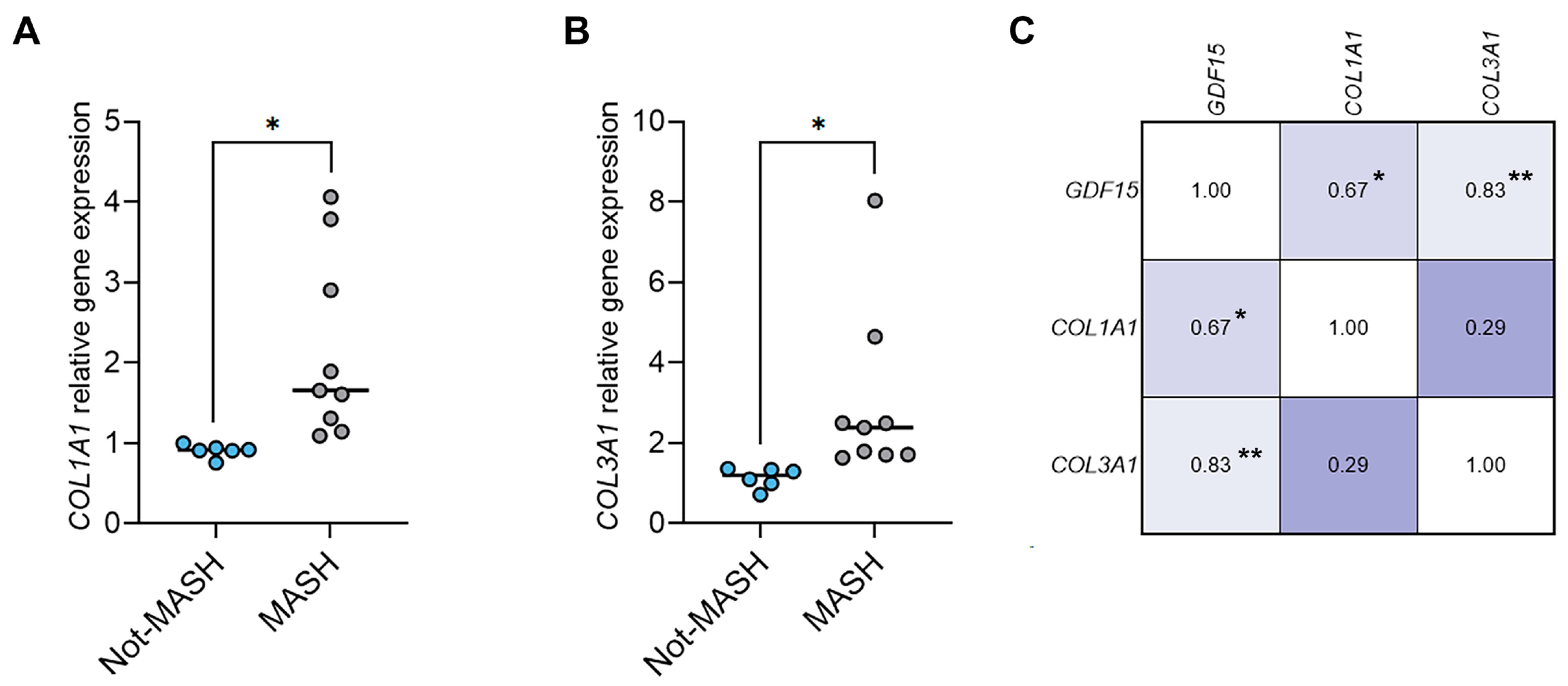
2.4. The Hepatic Levels of GDF15 Transcripts Positively Correlate with the Levels of Fibrotic Genes in Humans with MASLD and in a Murine MASLD Model
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Anthropometric Measurements
4.3. Biochemical Measurements
4.4. Histological Features
4.5. GDF15 Quantification in Human Plasma
4.6. Gene Dataset Analysis
4.7. Animal Model
4.8. Gene Expression in Human and Mouse Liver Samples
4.9. Sample Size Calculation
4.10. Statistical Analysis
4.11. GenAI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| COL | Collagen |
| GDF15 | Growth differentiation factor 15 |
| GGT | Gamma-glutamyl transferase |
| HDL-C | High-density lipoprotein cholesterol |
| HOMA-IR | Homeostasis model assessment for insulin resistance |
| LPS | Lipopolysaccharides |
| LDL-C | Low-density lipoprotein cholesterol |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| ND | Normal diet |
| TC | Total cholesterol |
| WC | Waist circumference |
| WD | Western diet |
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Zhang, X.; Loomba, R.; Schnabl, B. Global and national prevalence of nonalcoholic fatty liver disease in adolescents: An analysis of the global burden of disease study 2019. Hepatology 2023, 78, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Doycheva, I.; Issa, D.; Watt, K.D.; Lopez, R.; Rifai, G.; Alkhouri, N. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in young adults in the United States. J. Clin. Gastroenterol. 2018, 52, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vali, Y.; Lee, J.; Boursier, J.; Petta, S.; Wonders, K.; Tiniakos, D.; Bedossa, P.; Geier, A.; Francque, S.; Allison, M.; et al. Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS) consortium investigators. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): A comparative diagnostic accuracy study. Lancet Gastroenterol. Hepatol. 2023, 8, 714–725. [Google Scholar] [CrossRef]
- Chang, E.; Chang, J.S.; Kong, I.D.; Baik, S.K.; Kim, M.Y.; Park, K.S. Multidimensional Biomarker Analysis Including Mitochondrial Stress Indicators for Nonalcoholic Fatty Liver Disease. Gut Liver 2022, 16, 171–189. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Pothuraju, R.; Khan, P.; Sharma, G.; Muniyan, S.; Seshacharyulu, P.; Jain, M.; Nasser, M.W.; Batra, S.K. Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia. Cytokine Growth Factor Rev. 2022, 64, 71–83. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, S.; Ke, Y.; Li, Y.; Chen, Y. Growth differentiation factor 15: Emerging role in liver diseases. Cytokine 2024, 182, 156727. [Google Scholar] [CrossRef] [PubMed]
- Girona, J.; Guardiola, M.; Barroso, E.; García-Altares, M.; Ibarretxe, D.; Plana, N.; Ribalta, J.; Amigó, N.; Correig, X.; Vázquez-Carrera, M.; et al. GDF15 Circulating Lev-els Are Associated with Metabolic-Associated Liver Injury and Atherosclerotic Cardio-vascular Disease. Int. J. Mol. Sci. 2025, 26, 2039. [Google Scholar] [CrossRef]
- Koo, B.K.; Um, S.H.; Seo, D.S.; Joo, S.K.; Bae, J.M.; Park, J.H.; Chang, M.S.; Kim, J.H.; Lee, J.; Jeong, W.I.; et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int. 2018, 38, 695–705. [Google Scholar] [CrossRef]
- Galuppo, B.; Agazzi, C.; Pierpont, B.; Chick, J.; Li, Z.; Caprio, S.; Santoro, N. Growth differentiation factor 15 (GDF15) is associated with non-alcoholic fatty liver disease (NAFLD) in youth with overweight or obesity. Nutr. Diabetes 2022, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Murali, A.; Vandevender, A.M.; Chen, J.; Silva, A.G.; Bello, F.M.; Chuan, B.; Bahudhanapati, H.; Sipula, I.; Dedousis, N.; et al. Hepatocyte-derived GDF15 suppresses feeding and improves insulin sensitivity in obese mice. iScience 2022, 25, 105569. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Panera, N.; Crudele, A.; Alisi, A. Noninvasive diagnostic tools for pediatric NAFLD: Where are we now? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Govaere, O.; Cockell, S.; Tiniakos, D.; Queen, R.; Younes, R.; Vacca, M.; Alexander, L.; Ravaioli, F.; Palmer, J.; Petta, S.; et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 2020, 12, eaba4448. [Google Scholar] [CrossRef]
- Asrih, M.; Wei, S.; Nguyen, T.T.; Yi, H.S.; Ryu, D.; Gariani, K. Overview of growth differentiation factor 15 in metabolic syndrome. J. Cell. Mol. Med. 2023, 27, 1157–1167. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Jin, X.; Hsiao, E.C.; Perez, E.A.; Pierce, R.H.; Chavin, K.D.; Koniaris, L.G. Growth differentiation factor-15: Induction in liver injury through p53 and tumor necrosis factor-independent mechanisms. J. Surg. Res. 2006, 130, 45–51. [Google Scholar] [CrossRef]
- Bilson, J.; Scorletti, E.; Bindels, L.B.; Afolabi, P.R.; Targher, G.; Calder, P.C.; Sethi, J.K.; Byrne, C.D. Growth differentiation factor-15 and the association between type 2 diabetes and liver fibrosis in NAFLD. Nutr. Diabetes 2021, 11, 32. [Google Scholar] [CrossRef]
- Valenzuela-Vallejo, L.; Chrysafi, P.; Kouvari, M.; Guatibonza-Garcia, V.; Mylonakis, S.C.; Katsarou, A.; Verrastro, O.; Markakis, G.; Eslam, M.; Papatheodoridis, G.; et al. Circulating hormones in biopsy-proven steatotic liver disease and steatohepatitis: A Multicenter Observational Study. Metabolism 2023, 148, 155694. [Google Scholar] [CrossRef]
- Werge, M.P.; Grandt, J.; Thing, M.; Hetland, L.E.; Rashu, E.B.; Jensen, A.H.; Junker, A.E.; Richter, M.M.; Møller, S.; Bendtsen, F.; et al. Circulating and hepatic levels of growth differentiation factor 15 in patients with metabolic dysfunction-associated steatotic liver disease. Hepatol. Res. 2025, 55, 492–504. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.H.; Han, D.H.; Jo, Y.S.; Lee, Y.H.; Lee, M.S. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci. Rep. 2018, 8, 6789. [Google Scholar] [CrossRef]
- Patel, S.; Haider, A.; Alvarez-Guaita, A.; Bidault, G.; El-Sayed Moustafa, J.S.; Guiu-Jurado, E.; Tadross, J.A.; Warner, J.; Harrison, J.; Virtue, S.; et al. Combined genetic deletion of GDF15 and FGF21 has modest effects on body weight, hepatic steatosis and insulin resistance in high fat fed mice. Mol. Metab. 2022, 65, 101589. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Ichim, C.; Sasu, S.M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Patel, A.R.; Frikke-Schmidt, H.; Bezy, O.; Sabatini, P.V.; Rittig, N.; Jessen, N.; Myers, M.G., Jr.; Seeley, R.J. LPS induces rapid increase in GDF15 levels in mice, rats, and humans but is not required for anorexia in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G247–G255. [Google Scholar] [CrossRef] [PubMed]
- Kokkorakis, M.; Folkertsma, P.; Forte, J.C.; Wolffenbuttel, B.H.R.; van Dam, S.; Mantzoros, C.S. GDF-15 improves the predictive capacity of steatotic liver disease non-invasive tests for incident morbidity and mortality risk for cardio-renal-metabolic diseases and malignancies. Metabolism 2025, 163, 156047. [Google Scholar] [CrossRef]
- Xiong, Y.; Walker, K.; Min, X.; Hale, C.; Tran, T.; Komorowski, R.; Yang, J.; Davda, J.; Nuanmanee, N.; Kemp, D.; et al. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci. Transl. Med. 2017, 9, eaan8732. [Google Scholar] [CrossRef]
- European Society for Paediatric Gastroenterology Hepatology (ESPGHAN) and European Association for the Study of the Liver (EASL), on behalf of co-authors. Diagnosis of fatty liver in children should occur in parallel to investigation for other causes of liver disease. Lancet Gastroenterol. Hepatol. 2023, 8, 598–600. [Google Scholar] [CrossRef]
- Štěpánek, L.; Horáková, D.; Štěpánek, L.; Cibičková, Ľ.; Karásek, D.; Vaverková, H.; Nakládalová, M.; Juríčková, Ľ.; Kollárová, H. Associations Between Homeostasis Model Assessment (HOMA) and Routinely Examined Parameters in Individuals with Metabolic Syndrome. Physiol. Res. 2019, 68, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 2024, 29, 101133. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhu, R.; Wang, F.; Cheng, Y.; Liu, Y. Three Differential Expression Analysis Methods for RNA Sequencing: Limma, EdgeR, DESeq2. J. Vis. Exp. 2021, 175, e62528. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome pathway analysis: A high-performance in-memory approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.J.; Tarantino, G.; Iezzi, A.; Alisi, A.; Balsano, C. Copper-catalyzed dicarbonyl stress in NAFLD mice: Protective effects of Oleuropein treatment on liver damage. Nutr. Metab. 2022, 19, 9. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Pearson r | 95% Confidence Interval | p Value |
|---|---|---|---|
| Weight | 0.09 | −0.1053 to 0.2881 | 0.351 |
| Height | 0.04 | −0.1606 to 0.2357 | 0.702 |
| BMI | 0.14 | −0.0596 to 0.3297 | 0.168 |
| WC | 0.18 | −0.0164 to 0.3695 | 0.718 |
| TC | 0.17 | −0.0319 to 0.3542 | 0.099 |
| HDL-C | 0.05 | −0.0148 to 0.2479 | 0.610 |
| LDL-C | 0.06 | −0.1439 to 0.2518 | 0.583 |
| Triglycerides | 0.22 | 0.02343 to 0.4016 | 0.003 |
| ALT | 0.18 | −0.0201 to 0.3645 | 0.077 |
| AST | 0.06 | −0.1370 to 0.2583 | 0.537 |
| GGT | 0.19 | −0.0051 to 0.3774 | 0.056 |
| Fasting glucose | 0.07 | −0.1364 to 0.2649 | 0.519 |
| Fasting insulin | 0.04 | −0.1693 to 0.2537 | 0.686 |
| HOMA-IR | 0.13 | −0.0675 to 0.3225 | 0.192 |
| Steatosis | 0.06 | −0.1351 to 0.2602 | 0.524 |
| Ballooning | −0.01 | −0.2062 to 0.1906 | 0.936 |
| Lobular inflammation | 0.11 | −0.0998 to 0.2932 | 0.324 |
| NAS | 0.07 | −0.1291 to 0.2658 | 0.846 |
| Fibrosis | 0.49 | 0.3209 to 0.3626 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, A.; Braghini, M.R.; Andolina, G.; De Stefanis, C.; Cesarini, L.; Pastore, A.; Comparcola, D.; Monti, L.; Francalanci, P.; Balsano, C.; et al. Levels of Growth Differentiation Factor 15 Correlated with Metabolic Dysfunction-Associated Steatotic Liver Disease in Children. Int. J. Mol. Sci. 2025, 26, 6486. https://doi.org/10.3390/ijms26136486
Mosca A, Braghini MR, Andolina G, De Stefanis C, Cesarini L, Pastore A, Comparcola D, Monti L, Francalanci P, Balsano C, et al. Levels of Growth Differentiation Factor 15 Correlated with Metabolic Dysfunction-Associated Steatotic Liver Disease in Children. International Journal of Molecular Sciences. 2025; 26(13):6486. https://doi.org/10.3390/ijms26136486
Chicago/Turabian StyleMosca, Antonella, Maria Rita Braghini, Giulia Andolina, Cristiano De Stefanis, Lucia Cesarini, Anna Pastore, Donatella Comparcola, Lidia Monti, Paola Francalanci, Clara Balsano, and et al. 2025. "Levels of Growth Differentiation Factor 15 Correlated with Metabolic Dysfunction-Associated Steatotic Liver Disease in Children" International Journal of Molecular Sciences 26, no. 13: 6486. https://doi.org/10.3390/ijms26136486
APA StyleMosca, A., Braghini, M. R., Andolina, G., De Stefanis, C., Cesarini, L., Pastore, A., Comparcola, D., Monti, L., Francalanci, P., Balsano, C., Pietrobattista, A., Alisi, A., & Panera, N. (2025). Levels of Growth Differentiation Factor 15 Correlated with Metabolic Dysfunction-Associated Steatotic Liver Disease in Children. International Journal of Molecular Sciences, 26(13), 6486. https://doi.org/10.3390/ijms26136486









