A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis
Abstract
1. Introduction
2. Pathophysiologic Aspects of Biocompatibility of Hemodialysis: Recent Advances and Clinical Significance
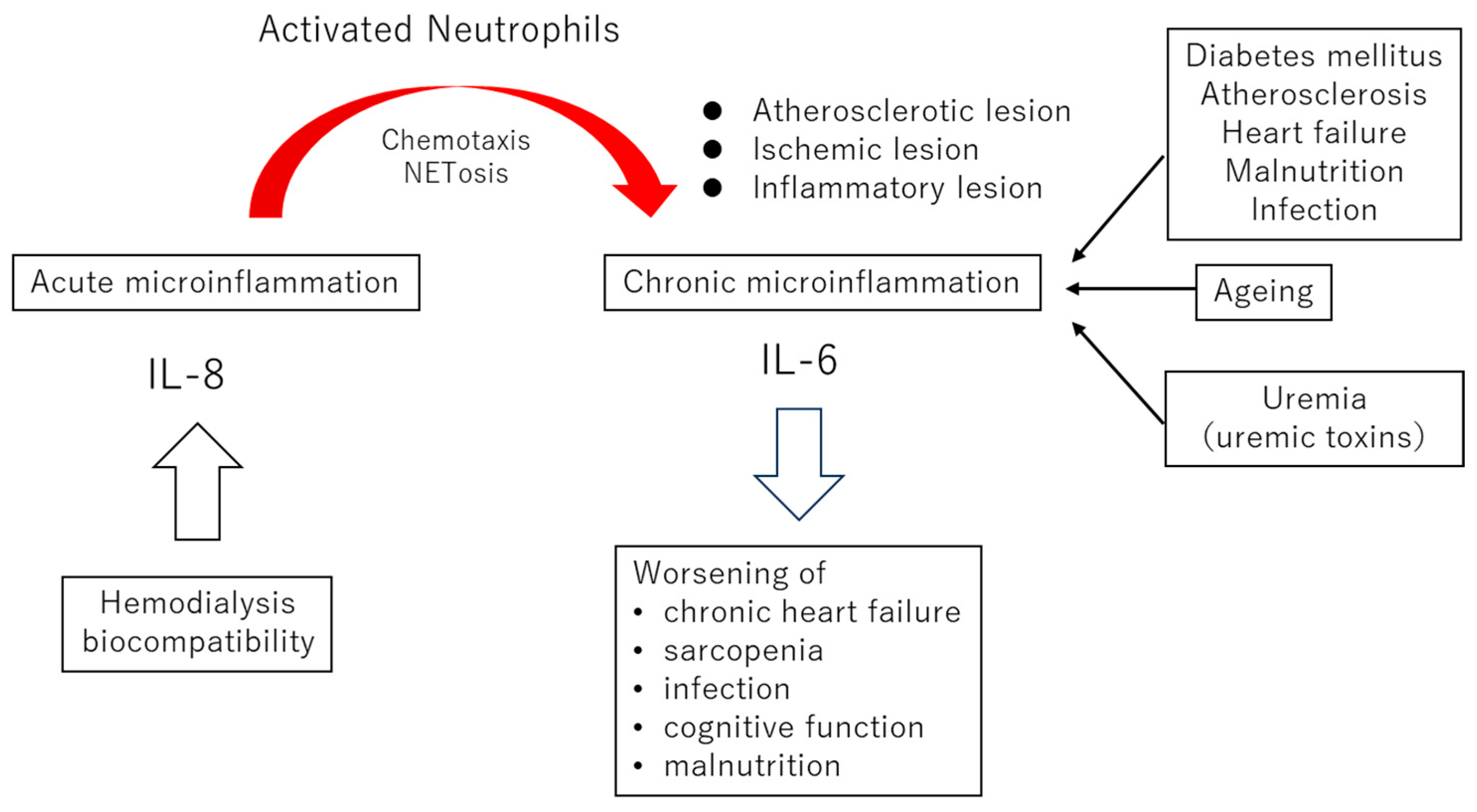
2.1. Commonalities of Chronic Microinflammation in the General Population and Dialysis Patients
2.2. The Roles of Neutrophil NETosis in Microinflammation Associated with Atherosclerosis
2.3. The Clinical Significance of Biocompatibilities
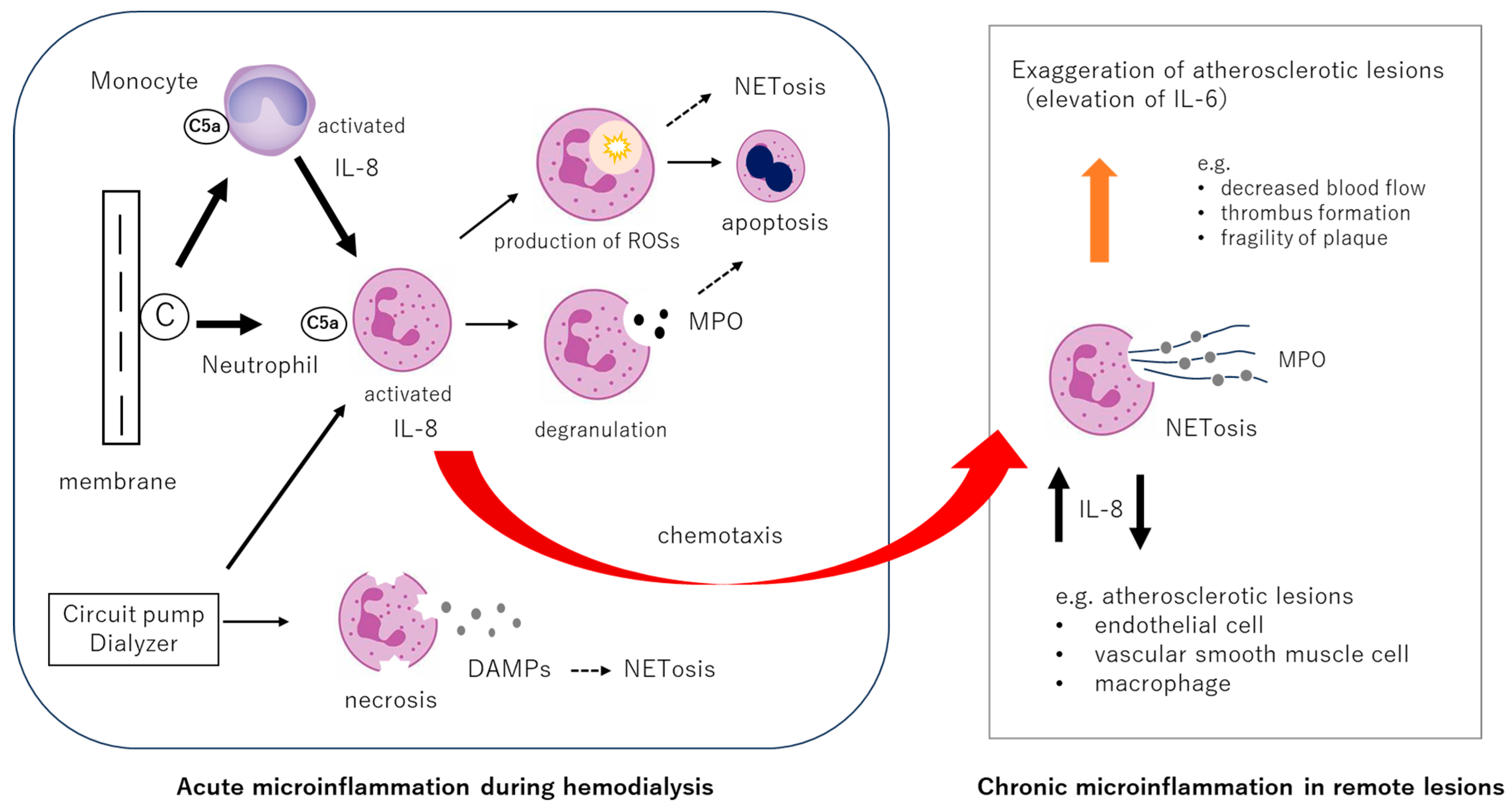
3. Core Mechanisms of Biocompatibility: Complement Activation, Neutrophil Stimulation, and the Interleukin-8 Pathway
3.1. Fundamental Biological Responses in Biocompatibility: The Roles of Complements and Neutrophil Activations
3.2. Unresolved Issues: Complement Activation by Hemodialysis Membranes
3.3. Neutrophil Responses During Hemodialysis
3.4. Neutrophil NETosis and Interleukin-8 (IL-8)
4. Impact of MPO, NETosis, and IL-8 on Patient Prognosis
4.1. Myeloperoxidase (MPO)
4.2. NETosis
4.3. Interleukin-8 (IL-8)
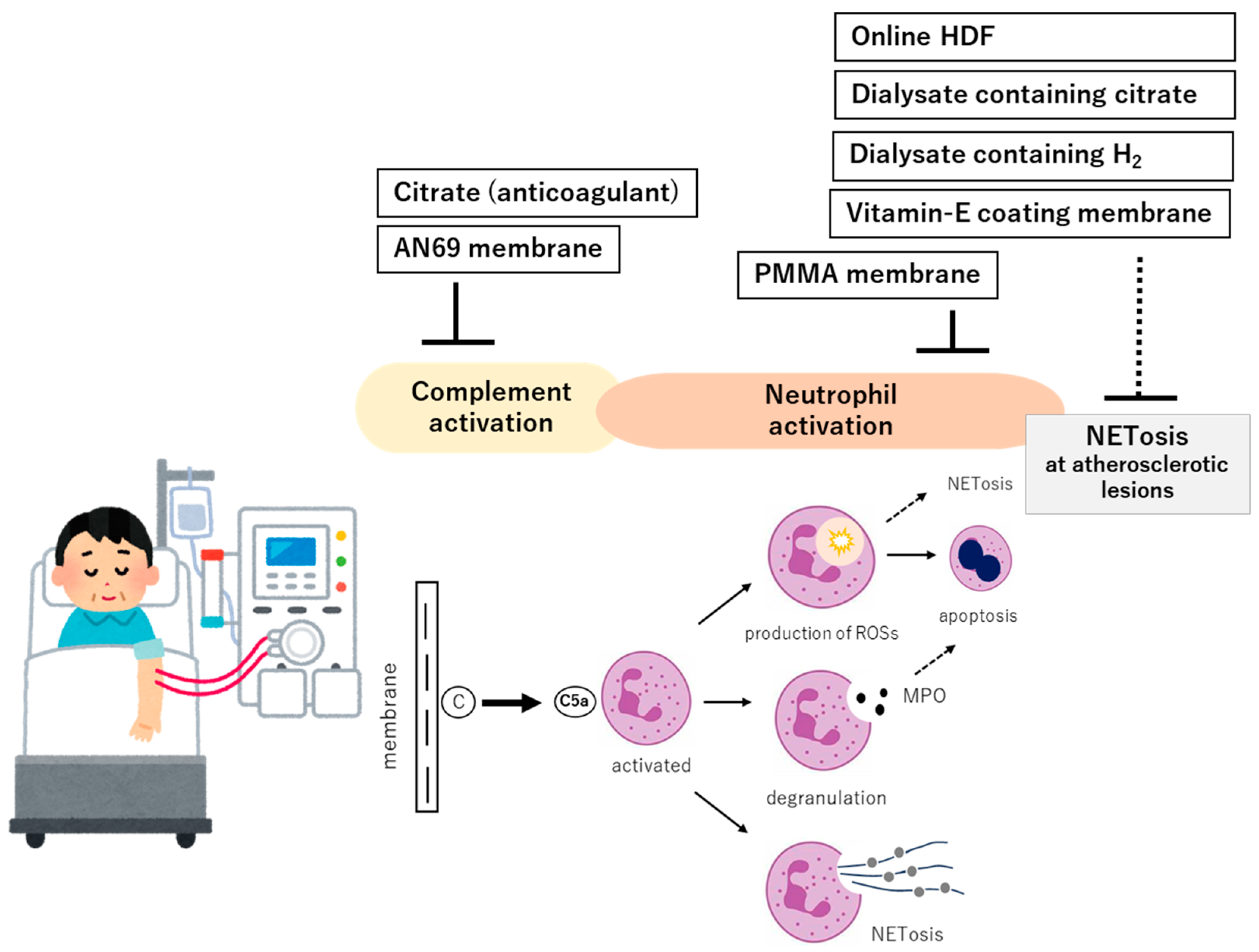
5. Therapeutic Strategies: Current Approaches and Future Possibilities
5.1. Dialyzers with Complement Adsorptive or Inhibitory Properties
5.2. Dialysis Modality
5.2.1. Online Hemodiafiltration
5.2.2. Extended-Hour Hemodialysis
5.2.3. Cool Dialysate Hemodialysis
5.2.4. Electrolyzed Water Hemodialysis
5.2.5. Expanded HD and Daily HD
5.3. Citrate as Anticoagulant
6. Future Perspectives on Improving Biocompatibility
6.1. Potential for Improvement Through Pharmacological Agents: Citrate-Based Anticoagulation
6.2. Development and Dissemination of Biocompatible Membranes and Bioactive Dialysates
6.2.1. Development of Highly Biocompatible Membranes
6.2.2. Citrate-Containing and H2-Enriched Dialysates
6.3. Utilization of Technology
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bowry, S.K.; Kircelli, F.; Himmele, R.; Nigwekar, S.U. Blood-incompatibility in haemodialysis: Alleviating inflammation and effects of coagulation. Clin. Kidney J. 2021, 14 (Suppl. 4), i59–i71. [Google Scholar] [CrossRef] [PubMed]
- Hörl, W.H. Hemodialysis membranes: Interleukins, biocompatibility, and middle molecules. J. Am. Soc. Nephrol. 2002, 13 (Suppl. 1), S62–S71. [Google Scholar] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Mehta, N.N.; Degoma, E.; Shapiro, M.D. IL-6 and Cardiovascular Risk: A Narrative Review. Curr. Atheroscler. Rep. 2024, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; O’mAhony, M.S.; Savva, G.M.; Calver, B.L.; Woodhouse, K.W. Inflammation and frailty measures in older people. J. Cell. Mol. Med. 2009, 13, 3103–3109. [Google Scholar] [CrossRef]
- Sepe, V.; Libetta, C.; Gregorini, M.; Rampino, T. The innate immune system in human kidney inflammaging. J. Nephrol. 2021, 35, 381–395. [Google Scholar] [CrossRef]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef]
- Hanafusa, N.; Nakai, S.; Iseki, K.; Tsubakihara, Y. Japanese society for dialysis therapy renal data registry—A window through which we can view the details of Japanese dialysis population. Kidney Int. Suppl. 2015, 5, 15–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitkauskaitė, M.; Mačionienė, E.; Stankevičius, R.; Miglinas, M.; Ix, J.H.; Brunström, M. Body Mass Index in Late Adolescence and Later Life Kidney Outcomes: A Population-Based Cohort Study in Swedish Men. Kidney Med. 2025, 7, 100982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Himmelfarb, J.; Stenvinkel, P.; Ikizler, T.A.; Hakim, R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002, 62, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H. Interleukin-6 levels can be used to estimate cardiovascular and all-cause mortality risk in dialysis patients: A meta-analysis and a systematic review. Immun. Inflamm. Dis. 2023, 11, e818. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Shi, Z.; Gong, S.; Li, Y.; Yan, K.; Bao, Y.; Ning, K. Neutrophil Extracellular Traps in Atherosclerosis: Research Progress. Int. J. Mol. Sci. 2025, 26, 2336. [Google Scholar] [CrossRef]
- Mázló, A.; Tang, Y.; Jenei, V.; Brauman, J.; Yousef, H.; Bácsi, A.; Koncz, G. Resolution Potential of Necrotic Cell Death Pathways. Int. J. Mol. Sci. 2022, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-B.; Chou, A.-H.; Lin, S.-I.; Chen, I.-H.; Lien, S.-P.; Liu, C.-C.; Chong, P.; Liu, S.-J.; Diamond, M.S. Toll-Like Receptor 9-Mediated Protection of Enterovirus 71 Infection in Mice Is Due to the Release of Danger-Associated Molecular Patterns. J. Virol. 2014, 88, 11658–11670. [Google Scholar] [CrossRef]
- Atamaniuk, J.; Ruzicka, K.; Stuhlmeier, K.M.; Karimi, A.; Eigner, M.; Mueller, M.M. Cell-Free Plasma DNA: A Marker for Apoptosis during Hemodialysis. Clin. Chem. 2006, 52, 523–526. [Google Scholar] [CrossRef]
- Moreira, V.G.; Martínez, T.d.l.C.; González, E.G.; García, B.P.; Menéndez, F.V.A. Increase in and clearance of cell-free plasma DNA in hemodialysis quantified by real-time PCR. Clin. Chem. Lab. Med. 2006, 44, 1410–1415. [Google Scholar] [CrossRef]
- Korabecna, M.; Opatrna, S.; Wirth, J.; Rulcova, K.; Eiselt, J.; Sefrna, F.; Horinek, A. Cell-Free Plasma DNA during Peritoneal Dialysis and Hemodialysis and in Patients with Chronic Kidney Disease. Ann. N. Y. Acad. Sci. 2008, 1137, 296–301. [Google Scholar] [CrossRef]
- Cichota, L.; Bochi, G.; Tatsch, E.; Torbitz, V.; Agnol, P.; Zanardo, J.; Barbisan, F.; Cruz, I.; Vaucher, R.; Moresco, R. Circulating Double-Stranded DNA in Plasma of Hemodialysis Patients and its Association with Iron Store. Clin. Lab. 2015, 61, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Bieber, S.; Muczynski, K.A.; Lood, C. Neutrophil Activation and Neutrophil Extracellular Trap Formation in Dialysis Patients. Kidney Med. 2020, 2, 692–698.e1. [Google Scholar] [CrossRef] [PubMed]
- Klopf, J.; Brostjan, C.; Eilenberg, W.; Neumayer, C. Neutrophil Extracellular Traps and Their Implications in Cardiovascular and Inflammatory Disease. Int. J. Mol. Sci. 2021, 22, 559. [Google Scholar] [CrossRef]
- Canaud, B.; Stenvinkel, P.; Scheiwe, R.; Steppan, S.; Bowry, S.; Castellano, G. The Janus-faced nature of complement in hemodialysis: Interplay between complement, inflammation, and bioincompatibility unveiling a self-amplifying loop contributing to organ damage. Front. Nephrol. 2024, 4, 1455321. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Barany, P.; Heimbürger, O.; Pecoits-Filho, R.; Lindholm, B. Mortality, malnutrition, and atherosclerosis in ESRD: What is the role of interleukin-6? Kidney Int. Suppl. 2002, 61, S103–S108. [Google Scholar] [CrossRef] [PubMed]
- Narita, I.; Alchi, B.; Omori, K.; Sato, F.; Ajiro, J.; Saga, D.; Kondo, D.; Skatsume, M.; Maruyama, S.; Kazama, J.; et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006, 69, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Fukuda, S.; Shoji, T.; Inaba, M.; Tsujimoto, Y.; Tabata, T.; Okuno, S.; Yamakawa, T.; Okada, S.; Okamura, M.; et al. Fatigue Is a Predictor for Cardiovascular Outcomes in Patients Undergoing Hemodialysis. Clin. J. Am. Soc. Nephrol. 2010, 5, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Yatim, K.M.; Lakkis, F.G. A Brief Journey through the Immune System. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poppelaars, F.; Faria, B.; da Costa, M.G.; Franssen, C.F.M.; van Son, W.J.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The Complement System in Dialysis: A Forgotten Story? Front. Immunol. 2018, 9, 71. [Google Scholar] [CrossRef]
- Ward, R.A.; McLeish, K.R. Hemodialysis with Cellulose Membranes Primes the Neutrophil Oxidative Burst. Artif. Organs 1995, 19, 801–807. [Google Scholar] [CrossRef]
- Inoshita, H.; Ohsawa, I.; Onda, K.; Tamano, M.; Horikoshi, S.; Ohi, H.; Tomino, Y. An analysis of functional activity via the three complement pathways during hemodialysis sessions: A new insight into the association between the lectin pathway and C5 activation. Clin. Kidney J. 2012, 5, 401–404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melchior, P.; Erlenkötter, A.; Zawada, A.M.; Delinski, D.; Schall, C.; Stauss-Grabo, M.; Kennedy, J.P. Complement activation by dialysis membranes and its association with secondary membrane formation and surface charge. Artif. Organs 2021, 45, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.C.; Grooteman, M.P.; van Houte, A.J.; Schoorl, M.; van Limbeek, J.; Nube, M.J. Low polymorphonuclear cell degranulation during citrate anticoagulation: A comparison between citrate and heparin dialysis. Nephrol. Dial. Transplant. 1997, 12, 1387–1393. [Google Scholar] [CrossRef]
- Schmaldienst, S.; Oberpichler, A.; Tschesche, H.; Hörl, W.H. Angiogenin: A Novel Inhibitor of Neutrophil Lactoferrin Release during Extracorporeal Circulation. Kidney Blood Press. Res. 2003, 26, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Damage-associated molecular patterns derived from mitochondria may contribute to the hemodialysis-associated inflammation. Int. Urol. Nephrol. 2014, 46, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Craddock, P.R.; Fehr, J.; Dalmasso, A.P.; Brighan, K.L.; Jacob, H.S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J. Clin. Investig. 1977, 59, 879–888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukushi, T.; Yamamoto, T.; Yoshida, M.; Fujikura, E.; Miyazaki, M.; Nakayama, M. Enhanced neutrophil apoptosis accompanying myeloperoxidase release during hemodialysis. Sci. Rep. 2020, 10, 21747. [Google Scholar] [CrossRef]
- Hou, Y.; Huttenlocher, A. Advancing chemokine research: The molecular function of CXCL8. J. Clin. Investig. 2024, 134, e180984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakanishi, I.; Moutabarrik, A.; Okada, N.; Kitamura, E.; Hayashi, A.; Syouji, T.; Namiki, M.; Ishibashi, M.; Zaid, D.; Tsubakihara, Y. Interleukin-8 in chronic renal failure and dialysis patients. Nephrol. Dial. Transplant. 1994, 9, 1435–1442. [Google Scholar]
- Pertosa, G.; Grandaliano, G.; Gesualdo, L.; Ranieri, E.; Monno, R.; Schena, F.P. Interleukin-6, interleukin-8 and monocyte chemotactic peptide-1 gene expression and protein synthesis are independently modulated by hemodialysis membranes. Kidney Int. 1998, 54, 570–579. [Google Scholar] [CrossRef]
- Tarakçıoğlu, M.; Erbağci, A.B.; Usalan, C.; Deveci, R.; Kocabaş, R. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediat. Inflamm. 2003, 12, 15–19. [Google Scholar] [CrossRef]
- Rysz, J.; Banach, M.; Cialkowska-Rysz, A.; Stolarek, R.; Barylski, M.; Drozdz, J.; Okonski, P. Blood serum levels of IL-2, IL-6, IL-8, TNF-alpha and IL-1beta in patients on maintenance hemodialysis. Membranes 2006, 3, 151–154. [Google Scholar]
- Niwa, T.; Miyazaki, T.; Sato, M.; Kambe, F.; Tsuzuki, T.; Uema, K.; Maeda, K.; Seo, H. Interleukin 8 and Biocompatibility of Dialysis Membranes. Am. J. Nephrol. 1995, 15, 181–185. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Lim, W.; Kireta, S.; Leedham, E.; Russ, G.; Coates, P. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007, 72, 1138–1148. [Google Scholar] [CrossRef]
- Ratner, B.D. The Biocompatibility of Implant Materials. In Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Hoffman, A.S., Ratner, B.D., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 383–399. [Google Scholar]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Gould, T.J.; Lysov, Z.; Liaw, P.C. Extracellular DNA and histones: Double-edged swords in immunothrombosis. J. Thromb. Haemost. 2015, 13 (Suppl. 1), S82–S91. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, Y.; Yang, X.; Yan, C.; Feng, Y. Recent Insights into Neutrophil Extracellular Traps in Cardiovascular Diseases. J. Clin. Med. 2022, 11, 6662. [Google Scholar] [CrossRef]
- An, Z.; Li, J.; Yu, J.; Wang, X.; Gao, H.; Zhang, W.; Wei, Z.; Zhang, J.; Zhang, Y.; Zhao, J.; et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle 2019, 18, 2928–2938. [Google Scholar] [CrossRef]
- Polinder-Bos, H.A.; García, D.V.; Kuipers, J.; Elting, J.W.J.; Aries, M.J.; Krijnen, W.P.; Groen, H.; Willemsen, A.T.; van Laar, P.J.; Strijkert, F.; et al. Hemodialysis Induces an Acute Decline in Cerebral Blood Flow in Elderly Patients. J. Am. Soc. Nephrol. 2018, 29, 1317–1325. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Brennan, M.-L.; Hazen, S.L. Serum Myeloperoxidase and Mortality in Maintenance Hemodialysis Patients. Am. J. Kidney Dis. 2006, 48, 59–68. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Lam, C.W.-K.; Chan, I.H.-S.; Wang, M.; Lui, S.-F.; Sanderson, J.E. Prognostic Value of Plasma Myeloperoxidase in ESRD Patients. Am. J. Kidney Dis. 2010, 56, 937–946. [Google Scholar] [CrossRef]
- Zuo, J.; Chaykovska, L.; Chu, C.; Chen, X.; Hasan, A.A.; Krämer, B.K.; Tepel, M.; Hocher, B. Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients. Antioxidants 2022, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Torino, C.; Pizzini, P.; Mezzatesta, S.; D’ARrigo, G.; Gori, M.; Carbone, F.; Schiavetta, E.; Cugno, V.; Cabri, M.; et al. Plasma levels of myeloperoxidase and resistin independently predict mortality in dialysis patients. Eur. J. Intern. Med. 2024, 129, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tovbin, D.; Novack, V.; Wiessman, M.P.; Elkadir, A.A.; Zlotnik, M.; Douvdevani, A. Circulating cell-free DNA in hemodialysis patients predicts mortality. Nephrol. Dial. Transplant. 2012, 27, 3929–3935. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Lee, H.W.; Joo, N.; Lee, H.S.; Song, Y.R.; Kim, H.J.; Kim, S.G. Prognostic role of circulating neutrophil extracellular traps levels for long-term mortality in new end-stage renal disease patients. Clin. Immunol. 2020, 210, 108263. [Google Scholar] [CrossRef]
- Einbinder, Y.; Shnaider, A.; Ghanayem, K.; Basok, A.; Rogachev, B.; Lior, Y.; Haviv, Y.S.; Cohen-Hagai, K.; Nacasch, N.; Rozenberg, I.; et al. Elevated Circulating Cell-Free DNA in Hemodialysis-Treated Patients Is Associated with Increased Mortality. Am. J. Nephrol. 2020, 51, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Taccola, D.; Rizza, G.M.; Consani, C.; Ghiadoni, L.; Filippi, C.; Cristofani, R.; Panicucci, E.; Migliori, M.; Sidoti, A.; et al. Interleukin-8 is a powerful prognostic predictor of all-cause and cardiovascular mortality in dialytic patients. Nephron. Clin. Pract. 2006, 102, c51–c58. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-H.; Glerup, R.I.; Svensson, M.H.S.; Eriksson, N.; Christensen, J.H.; de Laval, P.; Soveri, I.; Westerlund, M.; Linde, T.; Ljunggren, Ö.; et al. Novel Biomarkers Detected by Proteomics Predict Death and Cardiovascular Events in Hemodialysis Patients. Biomedicines 2022, 10, 740. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Obinger, C.; Hsuanyu, Y.; Dunford, H.B. Mechanism of reaction of myeloperoxidase with hydrogen peroxide and chloride ion. Eur. J. Biochem. 2000, 267, 5858–5864. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Pena-Esparragoza, J.K.; Scovner, K.M.; Waikar, S.S.; Mc Causland, F.R. Myeloperoxidase and the Risk of CKD Progression, Cardiovascular Disease, and Death in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020, 76, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.C.; Kim, J.-E.; Gu, J.-Y.; Yoo, H.J.; Ryu, J.W.; Kim, D.K.; Joo, K.W.; Kim, H.K. Significance of the DNA-Histone Complex Level as a Predictor of Major Adverse Cardiovascular Events in Hemodialysis Patients: The Effect of Uremic Toxin on DNA-Histone Complex Formation. Blood Purif. 2016, 41, 64–71. [Google Scholar] [CrossRef]
- Coimbra, S.; Rocha, S.; Nascimento, H.; Valente, M.J.; Catarino, C.; Rocha-Pereira, P.; Sameiro-Faria, M.; Oliveira, J.G.; Madureira, J.; Fernandes, J.C.; et al. Cell-free DNA as a marker for the outcome of end-stage renal disease patients on haemodialysis. Clin. Kidney J. 2020, 14, 1371–1378. [Google Scholar] [CrossRef]
- Said, N.; Lau, W.J.; Ho, Y.-C.; Lim, S.K.; Abidin, M.N.Z.; Ismail, A.F. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kokubo, K.; Kurihara, Y.; Kobayashi, K.; Tsukao, H.; Kobayashi, H. Evaluation of the Biocompatibility of Dialysis Membranes. Blood Purif. 2015, 40, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Stasi, A.; Caggiano, G.; Squiccimarro, E.; Losappio, V.; Fiorentino, M.; Alfieri, C.; Stallone, G.; Gesualdo, L.; Castellano, G. Enhancing Immune Protection in Hemodialysis Patients: Role of the Polymethyl Methacrylate Membrane. Blood Purif. 2023, 52 (Suppl. 1), 49–61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, A.K. Biocompatibility of hemodialysis membranes. J. Am. Soc. Nephrol. 1990, 1, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Moriyama, K.; Ledebo, I. AN69: Evolution of the world’s first high permeability membrane. Contrib. Nephrol. 2011, 173, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Higuchi, C.; Demura, R.; Sanaka, T.; Nihei, H. Reduction of Neutrophil Activation by Vitamin E Modified Dialyzer Membranes. Nephron 2000, 85, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Ominato, M.; Toyama, K.; Yasuda, T.; Sato, T.; Maeba, T.; Owada, S.; Ishida, M. Effects of a vitamin E-modified dialysis membrane on neutrophil superoxide anion radical production. Kidney Int. Suppl. 2001, 59, S137–S143. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Naso, A.; D’ANgelo, A.; Pagnin, E.; Zanardo, M.; Puato, M.; Rebeschini, M.; Landini, S.; Feriani, M.; Perego, A.; et al. Molecular Biology-Based Assessment of Vitamin E-Coated Dialyzer Effects on Oxidative Stress, Inflammation, and Vascular Remodeling. Artif. Organs 2011, 35, E33–E39. [Google Scholar] [CrossRef] [PubMed]
- Girndt, M.; Lengler, S.; Kaul, H.; Sester, U.; Sester, M.; Köhler, H. Prospective crossover trial of the influence of vitamin E–coated dialyzer membranes on T-cell activation and cytokine induction. Am. J. Kidney Dis. 2000, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M. Development of vitamin E-modified polysulfone membrane dialyzers. J. Artif. Organs 2006, 9, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, X.; Yang, M.; Su, B. Alleviation of Oxidative Stress during Hemodialysis Sessions by Hemodialysis Membrane Innovation: A Multidisciplinary Perspective. Blood Purif. 2023, 52, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H.; Dede, F.; Piskinpasa, S.; Falay, M.Y.; Odabas, A.R. Impact of Low- or High-Flux Haemodialysis and Online Haemodiafiltration on Inflammatory Markers and Lipid Profile in Chronic Haemodialysis Patients. Blood Purif. 2013, 35, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Filiopoulos, V.; Hadjiyannakos, D.; Metaxaki, P.; Sideris, V.; Takouli, L.; Anogiati, A.; Vlassopoulos, D. Inflammation and Oxidative Stress in Patients on Hemodiafiltration. Am. J. Nephrol. 2008, 28, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.; Chieti, A.; Pontrelli, P.; Rascio, F.; Castellano, G.; Stallone, G.; Infante, B.; Gesualdo, L.; Grandaliano, G.; Pertosa, G. On-line hemodiafiltration modulates atherosclerosis signaling in peripheral lymphomonocytes of hemodialysis patients. J. Nephrol. 2021, 34, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carracedo, J.; Merino, A.; Nogueras, S.; Carretero, D.; Berdud, I.; Rami, R.; Tetta, C.; Rodri, M.; Marti, A.; Aljama, P. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J. Am. Soc. Nephrol. 2006, 17, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.E.; Ahrenholz, P.; Paetow, W.; Waitz, G.; Wolf, H. Reduction of Heparin and Oxidative Potential by Means of Citrasate® in High-Flux Dialysis (HFD) and Online Hemodiafiltration (olHDF) in Pre and Postdilution. In Hemodialysis; Suzuki, H., Ed.; IntechOpen: London, UK, 2013; Chapter 24. [Google Scholar][Green Version]
- Sultan, A.M.; Hashem, N.U.; Kasem, M.F.; Mohammed, N.R.; Said, R.M. Effect of an Online Hemodiafiltration (OL-HDF) Session in Children with Chronic Kidney Disease Stage 5 on Circulating Neutrophil Extracellular Traps (NETs). Qjm Int. J. Med. 2024, 117 (Suppl. 2), hcae175.752. [Google Scholar] [CrossRef]
- Kruzel-Davila, E.; Vdovich, O.; Remez, L. (TH-PO237) Exploring the Impact of Hemodialysis Modalities on NETosis in Diabetic and Nondiabetic Patients. J. Am. Soc. Nephrol. 2024, 35, 10–1681. [Google Scholar] [CrossRef]
- Penne, E.L.; Blankestijn, P.J.; Bots, M.L.; Dorpel, M.A.v.D.; Grooteman, M.P.; Nubé, M.J.; van der Tweel, I.; ter Wee, P.M.; the CONTRAST study group. Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients—The Dutch CONvective TRAnsport STudy (CONTRAST): Rationale and design of a randomised controlled trial [ISRCTN38365125]. Curr. Control. Trials Cardiovasc. Med. 2005, 6, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macià, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 487–497, Erratum in J. Am. Soc. Nephrol. 2014, 25, 1130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blankestijn, P.J.; Vernooij, R.W.; Hockham, C.; Strippoli, G.F.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Hull, K.L.; March, D.S.; Churchward, D.R.; Graham-Brown, M.P.; Burton, J.O. The effect of extended-hours hemodialysis on outcomes: A systematic review and meta-analysis. Hemodial. Int. 2020, 24, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Chander, S.; Latif, R.; Bin Aamir, A.; Sorath, F.; Lohana, A.C.; Nadeem, M.Y.; Parkash, O. Clinical outcomes of patients undergoing hemodialysis with cool versus standard dialysate: A systematic review and meta-analysis. Am. J. Nephrol. 2025, 3, 1–16, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Presta, P.; Mazzitello, G.; Fuiano, L.; Andreucci, M.; Piraina, V.; Laratta, E.; Riccio, M.; Comi, N.; Fuiano, G. Cool temperature hemodialysis and biocompatibility in chronic hemodialysis patients: A preliminary study. J. Nephrol. 2009, 22, 760–765. [Google Scholar] [PubMed]
- Otte, K.; Jensen, P.; Svendsen, P.; Gram, J.; Starklint, H.; Jørgensen, K. Heparin-Free Hypothermal Hemodialysis at 20 °C Improves Biocompatibility. Blood Purif. 1997, 15, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Watanabe, K.; Sato, E.; Ito, Y.; Kadota, N.; Konishi, K.; Aizawa, C.; Maruyama, Y.; Fujimaru, T.; Nagahama, M.; et al. Hemodialysis employing molecular hydrogen (H2) enriched dialysis solution may improve dialysis related fatigue through impact on energy metabolism. Sci. Rep. 2025, 15, 5039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakayama, M.; Kabayama, S.; Miyazaki, M. Application of Electrolyzed Hydrogen Water for Management of Chronic Kidney Disease and Dialysis Treatment—Perspective View. Antioxidants 2024, 13, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakayama, M.; Itami, N.; Suzuki, H.; Hamada, H.; Yamamoto, R.; Tsunoda, K.; Osaka, N.; Nakano, H.; Maruyama, Y.; Kabayama, S.; et al. Novel haemodialysis (HD) treatment employing molecular hydrogen (H2)-enriched dialysis solution improves prognosis of chronic dialysis patients: A prospective observational study. Sci. Rep. 2018, 8, 254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, R.; Alcazar-Arroyo, R.; de Sequera-Ortiz, P. What is the role of expanded hemodialysis in renal replacement therapy in 2020? Nefrología 2021, 41, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, T.; Li, Y.; Li, J.; Yang, Q.; Wang, L.; Jiang, L.; Su, B. Effects of Expanded Hemodialysis with Medium Cut-Off Membranes on Maintenance Hemodialysis Patients: A Review. Membranes 2022, 12, 253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raimann, J.G.; Chan, C.T.; Daugirdas, J.T.; Depner, T.; Greene, T.; Kaysen, G.A.; Kliger, A.S.; Kotanko, P.; Larive, B.; Beck, G.; et al. The Predialysis Serum Sodium Level Modifies the Effect of Hemodialysis Frequency on Left-Ventricular Mass: The Frequent Hemodialysis Network Trials. Kidney Blood Press. Res. 2021, 46, 768–776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borawski, J. Myeloperoxidase as a Marker of Hemodialysis Biocompatibility and Oxidative Stress: The Underestimated Modifying Effects of Heparin. Am. J. Kidney Dis. 2006, 47, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Rudolph, V.; Kubala, L.; Berglund, L.; Schrepfer, S.; Deuse, T.; Haddad, M.; Risius, T.; Klemm, H.; Reichenspurner, H.C.; et al. Heparins Increase Endothelial Nitric Oxide Bioavailability by Liberating Vessel-Immobilized Myeloperoxidase. Circulation 2006, 113, 1871–1878. [Google Scholar] [CrossRef]
- Huang, S.; Sandholm, K.; Jonsson, N.; Nilsson, A.; Wieslander, A.; Grundström, G.; Hancock, V.; Ekdahl, K.N. Low concentrations of citrate reduce complement and granulocyte activation in vitro in human blood. Clin. Kidney J. 2015, 8, 31–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gritters, M.; Grooteman, M.P.; Schoorl, M.; Schoorl, M.; Bartels, P.C.; Scheffer, P.G.; Teerlink, T.; Schalkwijk, C.G.; Spreeuwenberg, M.; Nubé, M.J. Citrate anticoagulation abolishes degranulation of polymorphonuclear cells and platelets and reduces oxidative stress during haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Broseta, J.J.; López-Romero, L.C.; Cerveró, A.; Devesa-Such, R.; Soldevila, A.; Bea-Granell, S.; Sánchez-Pérez, P.; Hernández-Jaras, J. Improvements in Inflammation and Calcium Balance of Citrate versus Acetate as Dialysate Buffer in Maintenance Hemodialysis: A Unicentric, Cross-Over, Prospective Study. Blood Purif. 2021, 50, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Kim, D.R.; Cho, K.S.; Seo, J.-W.; Moon, H.; Park, E.J.; Kim, J.S.; Lee, T.W.; Ihm, C.G.; Jeong, D.W.; et al. Effects of Dialysate Acidification With Citrate Versus Acetate on Cell Damage, Uremic Toxin Levels, and Inflammation in Patients Receiving Maintenance Hemodialysis. Am. J. Kidney Dis. 2019, 73, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Talal, S.; Mona, K.; Karem, A.; Yaniv, L.; Reut, H.-M.; Ariel, S.; Moran, A.-K.; Harel, E.; Campisi-Pinto, S.; Mahmoud, A.-A.; et al. Neutrophil degranulation and severely impaired extracellular trap formation at the basis of susceptibility to infections of hemodialysis patients. BMC Med. 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarbock, A.; Küllmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mülling, N.; et al. Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury: A Randomized Clinical Trial. JAMA 2020, 324, 1629–1639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Source | Products | Influence/Symptoms |
|---|---|---|
| Ethylene oxide gas sterilization | First use syndrome, asthma | |
| DEHP, a phthalate plasticizer | endocrine disruptor | endocrine disruption |
| Complements | C3a, C5a, Membrane Attack Complex | activation of neutrophil and monocyte |
| Neutrophil | ROSs (hydroge peroxide, superoxide anion) | endothelial cell damage, oxidative stress |
| degradation (myeloperoxidase, elastase, et al.) | ||
| Platelet | thromboxane A2, Platelet-Derived Growth Factor, prostaglandins | platelet aggregation, thrombosis, atherosclerosis |
| Basophil·Mast cell·Eosinophil | histamine, leukotriene | hypotension, bronchospasm |
| Monocyte | Interleukin (IL)-1, IL-6, IL-8, | dialysis-related amyloidosis, microinflammation |
| Tumor Necrosis Factor-α, Interferon | ||
| Coagulation–Fibrinolysis system | Factor XIIa | blood chamber coagulation |
| Kinin–Kallikrein System | bradykinin | anaphylactoid reaction |
| Author (Year) Reference | Number of Patients (Observation Periods) | Results |
|---|---|---|
| (a) Myeloperoxidase (MPO) | ||
| Kalantar-Zadeh (2006) [56] | 356 (retrospective analysis) | Hazard Ratios (HRs) for death |
| 1.14 (1.03–1.26: 95%CI); P 0.01 each 1000-pmol/L of plasma MPO, 1.82 (1.07–3.10: 95%CI) in the highest as compared with the middle tertile | ||
| Wang (2010) [57] | 236 (3 years) | A doubling in plasma MPO level: increases of 46% (1.02–2.08; 95%CI) in mortality, and 60% (1.17–2.18) in cardiovascular events. |
| Zuo (2022) [58] | 347 (60 months) | HRs for death: 1.000035 (1.000020–1.000051: 95%CI) by univariate, and 1.000033 (1.000018–1.000049: 95%CI) by multivariate analysis |
| Liberale (2024) [59] | 1182 (median 2.9years) | HRs for all cause for mortality: 1.26 (1.11–1.42: 95%CI), and for cardiovascular death: 1.19 (1.01–1.41: 95%CI) |
| (b) NETosis | ||
| Tovbin (2012) [60] | 31 (42 months) | HR for all-cause of death of cell free DNA higher than 850 ng/mL: 8.0 (2.3–28.5: 95%CI) |
| Kim (2020) [61] | 281 | the nucleosome Q4 group had significantly increased all-cause and cardiovascular mortality compared to the Q1–3 groups |
| Einbinder (2020) [62] | 153 (46 months) | HR for mortality of post-HD cfDNA: 1.92 (1.03–3.56: 95%CI), |
| OR for mortality of post-HD cfDNA: 4.61(1.45–14.66: 95%CI) by 1 year, 4.36 (1.63–11.66) by 2 years, and 6.22 (2.2–17.59) by 3 years. | ||
| (c) Interleukin-8 | ||
| Panichi (2006) [63] | 76 (18 months) | the strongest independent predictor of all-cause and cardiovascular by stepwise regression analysis |
| Wu (2022) [64] | 331 (5 years) | HRs for all-cause mortality: 1.29 (1.11–1.59: 95%CI), for cardiovascular disease-mortality: 1.34 (1.02–1.76), and all vascular events: 1.33 (1.11–1.59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, M.; Miyakawa, H.; Ohama, K.; Kimura, H. A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis. Int. J. Mol. Sci. 2025, 26, 6472. https://doi.org/10.3390/ijms26136472
Nakayama M, Miyakawa H, Ohama K, Kimura H. A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis. International Journal of Molecular Sciences. 2025; 26(13):6472. https://doi.org/10.3390/ijms26136472
Chicago/Turabian StyleNakayama, Masaaki, Hiroyuki Miyakawa, Kazuya Ohama, and Hirokazu Kimura. 2025. "A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis" International Journal of Molecular Sciences 26, no. 13: 6472. https://doi.org/10.3390/ijms26136472
APA StyleNakayama, M., Miyakawa, H., Ohama, K., & Kimura, H. (2025). A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis. International Journal of Molecular Sciences, 26(13), 6472. https://doi.org/10.3390/ijms26136472







