Design and Characterization of Aromatic Copolyesters Containing Furan and Isophthalic Rings with Suitable Properties for Vascular Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Characterization
2.2. Thermal Analysis
2.3. Wide Angle X-Ray Scattering (WAXS)
2.4. Water Contact Angle (WCA) Measurements
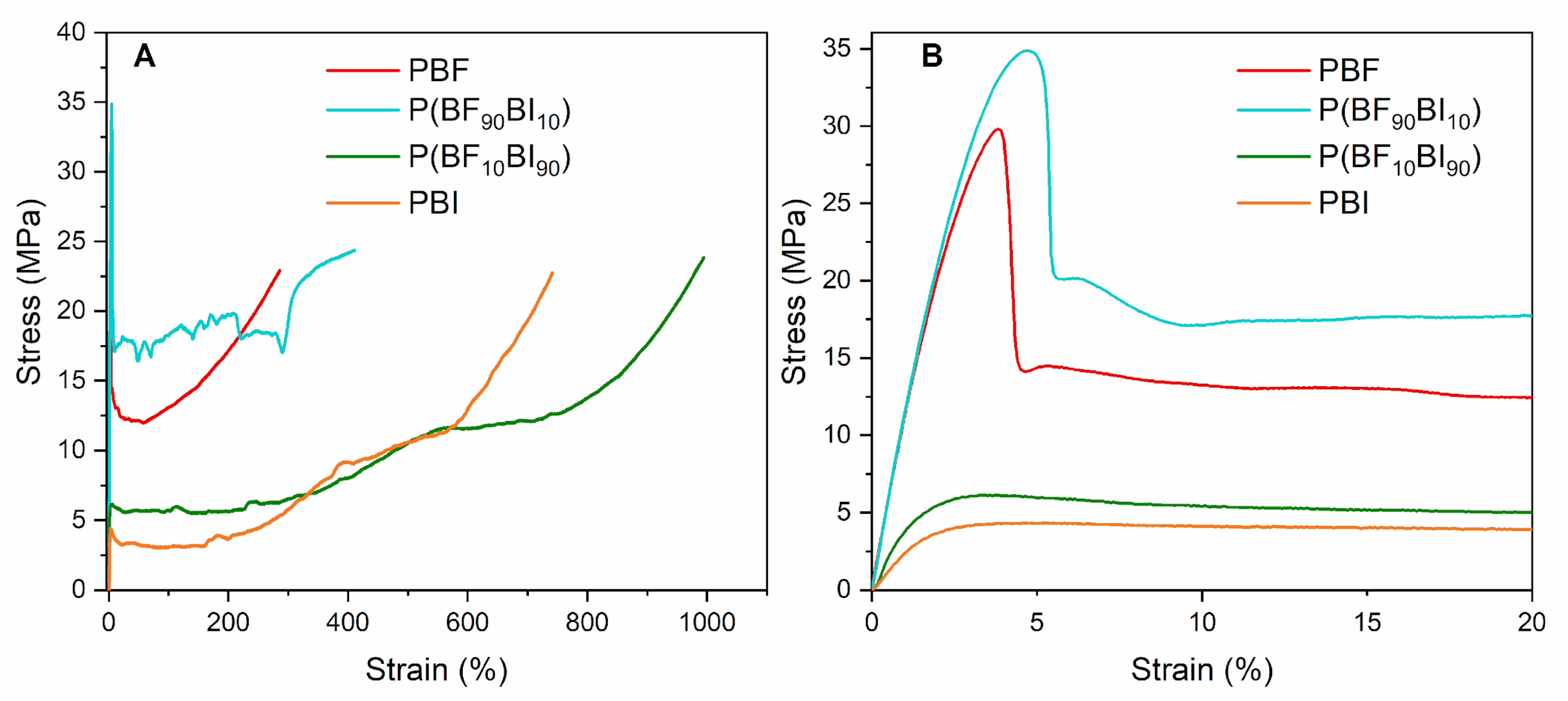
2.5. Mechanical Characterization
2.6. Hydrolytic Degradation Tests
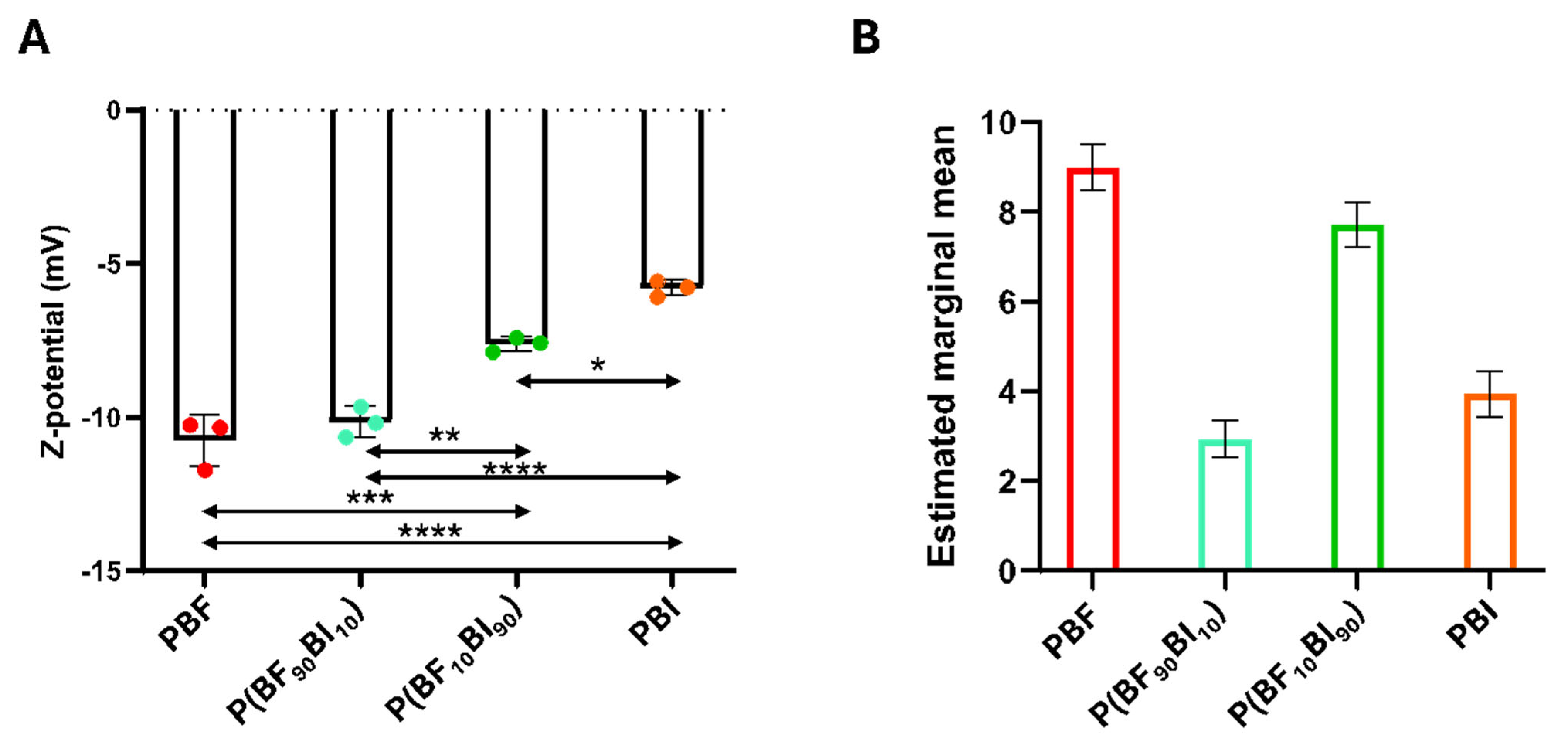
2.7. Surface Properties: Z-Potential and Texture Analysis
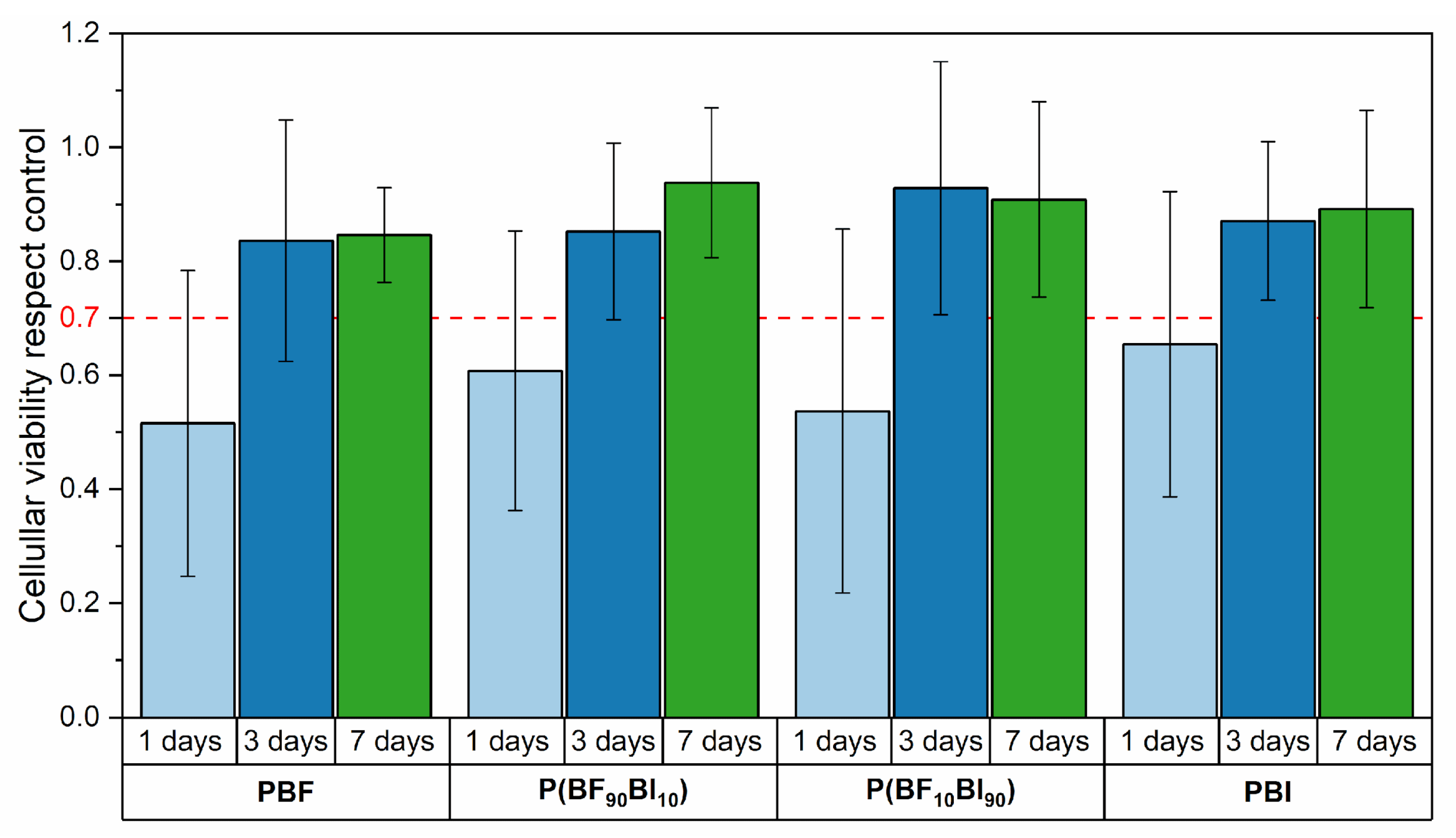
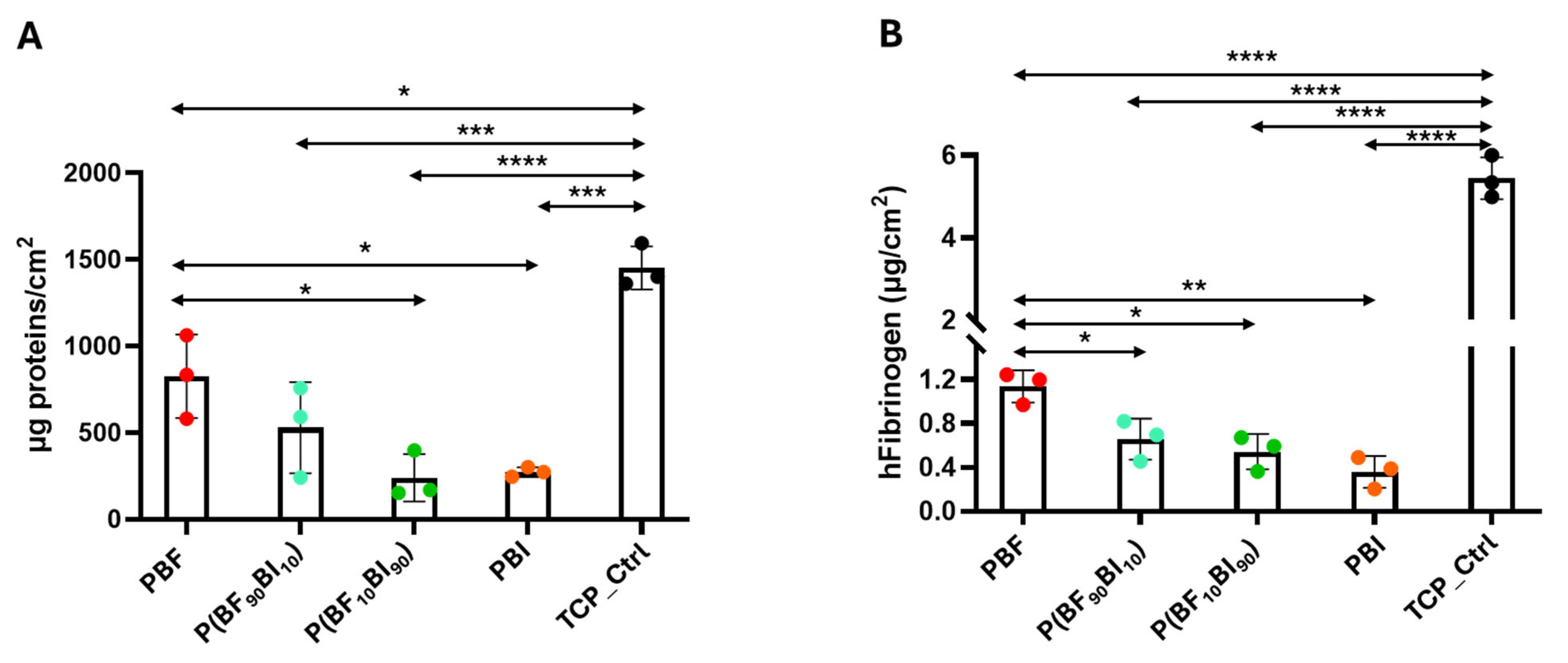
2.8. In Vitro Biological Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Molecular Characterization
3.4. Film Preparation
3.5. Thermal Analysis
3.6. Wide Angle X-Ray Scattering (WAXS)
3.7. Water Contact Angle (WCA) Measurements
3.8. Mechanical Characterization
3.9. Hydrolytic Degradation Tests
3.10. Surface Zeta Potential
3.11. Texture Features
3.12. In Vitro Biological Evaluation
3.12.1. Cytotoxicity Tests
3.12.2. Platelet Adhesion
3.12.3. Protein Quantification
Human Plasma Proteins Absorption
Human Fibrinogen Absorption
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, A.; Aboyans, V.; Vardas, P.; Townsend, N.; Torbica, A.; Kavousi, M.; Boriani, G.; Huculeci, R.; Kazakiewicz, D.; Scherr, D.; et al. European Society of Cardiology: The 2023 Atlas of Cardiovascular Disease Statistics. Eur. Heart J. 2024, 45, 4019–4062. [Google Scholar] [CrossRef]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef]
- Durán-Rey, D.; Crisóstomo, V.; Sánchez-Margallo, J.A.; Sánchez-Margallo, F.M. Systematic Review of Tissue-Engineered Vascular Grafts. Front. Bioeng. Biotechnol. 2021, 9, 771400. [Google Scholar] [CrossRef]
- Greenhalgh, R.M.; Becquemin, J.-P. Vascular and Endovascular Surgical Techniques, 4th ed.; W.B. Saunders: London, UK, 2001. [Google Scholar]
- Li, M.-X.; Wei, Q.-Q.; Mo, H.-L.; Ren, Y.; Zhang, W.; Lu, H.-J.; Joung, Y.K. Challenges and advances in materials and fabrication technologies of small-diameter vascular grafts. Biomater. Res. 2023, 27, 58. [Google Scholar] [CrossRef]
- Weekes, A.; Bartnikowski, N.; Pinto, N.; Jenkins, J.; Meinert, C.; Klein, T.J. Biofabrication of small diameter tissue-engineered vascular grafts. Acta Biomater. 2022, 138, 92–111. [Google Scholar] [CrossRef]
- Zizhou, R.; Wang, X.; Houshyar, S. Review of Polymeric Biomimetic Small-Diameter Vascular Grafts to Tackle Intimal Hyperplasia. ACS Omega 2022, 7, 22125–22148. [Google Scholar] [CrossRef]
- Ward, A.O.; Caputo, M.; Angelini, G.D.; George, S.J.; Zakkar, M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis 2017, 265, 266–274. [Google Scholar] [CrossRef]
- Friedman, S.G.; Lazzaro, R.S.; Spier, L.N.; Moccio, C.; Tortolani, A.J. A prospective randomized comparison of Dacron and polytetrafluoroethylene aortic bifurcation grafts. Surgery 1995, 117, 7–17. [Google Scholar] [CrossRef]
- Nevelsteen, A.; Wouters, L.; Suy, R. Aortofemoral dacron reconstruction for aorto-iliac occlusive disease: A 25-year survey. Eur. J. Vasc. Surg. 1991, 5, 179–186. [Google Scholar] [CrossRef]
- Ratner, B. Vascular Grafts: Technology Success/Technology Failure. BME Front. 2023, 4, 0003. [Google Scholar] [CrossRef]
- Sarkar, S.; Sales, K.M.; Hamilton, G.; Seifalian, A.M. Addressing thrombogenicity in vascular graft construction. J. Biomed. Mater. Res. 2007, 82B, 100–108. [Google Scholar] [CrossRef]
- Pu, F. Effects of plasma treated PET and PTFE on expression of adhesion molecules by human endothelial cells in vitro. Biomaterials 2002, 23, 2411–2428. [Google Scholar] [CrossRef]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef]
- Werpy, T.A.; Holladay, J.E.; White, J.F. Top Value Added Chemicals From Biomass: I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Xu, J.; Pang, Y. Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 2012, 53, 4145–4151. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.-H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furan dicarboxylate), a Biobased Alternative to PBT: Synthesis, Physical Properties, and Crystal Structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Soccio, M.; Martínez-Tong, D.E.; Alegría, A.; Munari, A.; Lotti, N. Molecular dynamics of fully biobased poly(butylene 2,5-furanoate) as revealed by broadband dielectric spectroscopy. Polymer 2017, 128, 24–30. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G. Solid-state structure and thermal characteristics of a sustainable biobased copolymer: Poly(butylene succinate-co-furanoate). Thermochim. Acta 2017, 656, 112–122. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Kowalczyk, I.; Kwiatkowski, K.; Zubkiewicz, A. Microstructure and Mechanical/Elastic Performance of Biobased Poly (Butylene Furanoate)–Block–Poly (Ethylene Oxide) Copolymers: Effect of the Flexible Segment Length. Polymers 2020, 12, 271. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Guigo, N.; Sbirrazzuoli, N.; Papageorgiou, D.G.; Bikiaris, D.N.; Nikolaidis, G.N.; Papageorgiou, G.Z. Towards increased sustainability for aromatic polyesters: Poly(butylene 2,5-furandicarboxylate) and its blends with poly(butylene terephthalate). Polymer 2021, 212, 123157. [Google Scholar] [CrossRef]
- Thanh, T.V.; Hao, L.T.; Cho, H.-Y.; Kim, H.; Park, S.-A.; Lee, M.; Kim, H.J.; Jeon, H.; Hwang, S.Y.; Park, J.; et al. Sustainable Poly(butylene adipate- co -furanoate) Composites with Sulfated Chitin Nanowhiskers: Synergy Leading to Superior Robustness and Improved Biodegradation. ACS Sustain. Chem. Eng. 2022, 10, 8411–8422. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. New Random Aromatic/Aliphatic Copolymers of 2,5-Furandicarboxylic and Camphoric Acids with Tunable Mechanical Properties and Exceptional Gas Barrier Capability for Sustainable Mono-Layered Food Packaging. Molecules 2023, 28, 4056. [Google Scholar] [CrossRef]
- He, Y.-C.; Wu, Y.-D.; Pan, X.-H.; Ma, C.-L. Biosynthesis of terephthalic acid, isophthalic acid and their derivatives from the corresponding dinitriles by tetrachloroterephthalonitrile-induced Rhodococcus sp. Biotechnol. Lett. 2014, 36, 341–347. [Google Scholar] [CrossRef]
- Chiorboli, E.; Pizzoli, M. Effect of crystallinity on the dynamic mechanical relaxations of poly(butylene isophthalate). Polym. Bull. 1989, 21, 77–84. [Google Scholar] [CrossRef]
- Pilati, F.; Munari, A.; Manaresi, P.; Milani, G.; Bonora, V. Linear and branched poly(butylene isophthalate): Synthesis and molecular characterization. Eur. Polym. J. 1987, 23, 265–271. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Zhao, X.; Zhu, D.; You, J. Self-Segregation Behavior of N-Ethyl-pentadecafluorooctanamide-Terminated Polybutylene Isophthalate and Its Effects on Film Morphology and Wettability. J. Phys. Chem. B 2009, 113, 15204–15211. [Google Scholar] [CrossRef]
- Quattrosoldi, S.; Lotti, N.; Soccio, M.; Schick, C.; Androsch, R. Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature. Polymers 2020, 12, 1099. [Google Scholar] [CrossRef]
- Gonçalves, F.A.M.M.; Costa, C.S.M.F.; Fabela, I.G.P.; Farinha, D.; Faneca, H.; Simões, P.N.; Serra, A.C.; Bártolo, P.J.; Coelho, J.F.J. 3D printing of new biobased unsaturated polyesters by microstereo-thermal-lithography. Biofabrication 2014, 6, 035024. [Google Scholar] [CrossRef]
- Sokołowska, M.; Zarei, M.; El Fray, M. Enzymatic synthesis of furan-based copolymers: Material characterization and potential for biomedical applications. Polym. Med. 2024, 54, 59–69. [Google Scholar] [CrossRef]
- Di Francesco, D.; Pigliafreddo, A.; Casarella, S.; Di Nunno, L.; Mantovani, D.; Boccafoschi, F. Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements. Biomolecules 2023, 13, 1389. [Google Scholar] [CrossRef]
- Edelman, E.R. Vascular Tissue Engineering: Designer Arteries. Circ. Res. 1999, 85, 1115–1117. [Google Scholar] [CrossRef]
- Mo, Y. The Resonance Energy of Benzene: A Revisit. J. Phys. Chem. A 2009, 113, 5163–5169. [Google Scholar] [CrossRef]
- Zhussupova, G.E.; Zhussupova, A.I. Impact of “Organic Chemistry” Course on Subsequent Courses, Studying Biologically Active Compounds. Procedia—Soc. Behav. Sci. 2015, 191, 1247–1254. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Y.; Wang, J.; Wang, S.; Zhuang, H.; Liu, J.; Huang, L.; Wang, Y.; Wang, W.; Belfiore, L.; et al. Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate). Materials 2018, 11, 587. [Google Scholar] [CrossRef]
- Sreelatha, K.; Predeep, P. Thermal Stability, Crystallization, and Melting Behaviours of Iodine Doped Semiconducting Polyethylene Terephthalate (PET) Thin Films. J. Macromol. Sci. Part B 2025, 64, 215–226. [Google Scholar] [CrossRef]
- Gilbert, M. Relation of Structure to Thermal and Mechanical Properties. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–73. [Google Scholar] [CrossRef]
- Mallet, C.; Allain, M.; Leriche, P.; Frère, P. Competition between π–π or furan–perfluorophenyl stacking interactions in conjugated compounds prepared from azomethine connections. CrystEngComm 2011, 13, 5833. [Google Scholar] [CrossRef]
- Welsh, G.E.; Blundell, D.J.; Windle, A.H. A transient mesophase on drawing polymers based on polyethylene terephthalate (PET) and polyethylene naphthoate (PEN). J. Mater. Sci. 2000, 35, 5225–5240. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, Y.; Shan, L.; Xu, M.; Wang, J.-G.; Zhang, Y.; Wang, W. A Robust Supramolecular Heterosynthon Assembled by a Hydrogen Bond and a Chalcogen Bond. Crystals 2021, 11, 1309. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.-C.; Gutiérrez-Fernández, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-Ordered Structure in Biobased Poly(pentamethylene furanoate) Responsible for Its Outstanding Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Guidotti, G.; Burzotta, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Munari, A.; Lotti, N. Chemical Modification of Poly(butylene trans-1,4-cyclohexanedicarboxylate) by Camphor: A New Example of Bio-Based Polyesters for Sustainable Food Packaging. Polymers 2021, 13, 2707. [Google Scholar] [CrossRef]
- Stoclet, G.; Seguela, R.; Lefebvre, J.-M.; Rochas, C. New Insights on the Strain-Induced Mesophase of Poly(D, L -lactide): In Situ WAXS and DSC Study of the Thermo-Mechanical Stability. Macromolecules 2010, 43, 7228–7237. [Google Scholar] [CrossRef]
- Lv, R.; Na, B.; Tian, N.; Zou, S.; Li, Z.; Jiang, S. Mesophase formation and its thermal transition in the stretched glassy polylactide revealed by infrared spectroscopy. Polymer 2011, 52, 4979–4984. [Google Scholar] [CrossRef]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(butylene 2,5-thiophenedicarboxylate): An Added Value to the Class of High Gas Barrier Biopolyesters. Polymers 2018, 10, 167. [Google Scholar] [CrossRef]
- Joshi, A.S.; Lawrence, J.G.; Coleman, M.R. Effect of Biaxial Orientation on Microstructure and Properties of Renewable Copolyesters of Poly(ethylene terephthalate) with 2,5-Furandicarboxylic Acid for Packaging Application. ACS Appl. Polym. Mater. 2019, 1, 1798–1810. [Google Scholar] [CrossRef]
- Wei, P.; Li, L.; Wang, L.; Yan, J.; Li, J.; Chen, C.; Zhang, Y. Synthesis and properties of high performance biobased liquid crystal polyester based on furandicarboxylic acid and sebacic acid. Eur. Polym. J. 2023, 183, 111738. [Google Scholar] [CrossRef]
- Cai, R.; Samulski, E.T. Liquid-crystalline aromatic polyesters containing isophthalic acid. Macromolecules 1994, 27, 135–140. [Google Scholar] [CrossRef]
- Ciobanu, C.I.; Berladean, I.; Epure, E.-L.; Simion, A.; Lisa, G.; Boussoualem, Y.; Carlescu, I. Mesomorphic and Thermal Behavior of Symmetric Bent-Core Liquid Crystal Compounds Derived from Resorcinol and Isophthalic Acid. Crystals 2021, 11, 1215. [Google Scholar] [CrossRef]
- Gotoh, K.; Shohbuke, E.; Kobayashi, Y.; Yamada, H. Wettability control of PET surface by plasma-induced polymer film deposition and plasma/UV oxidation in ambient air. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 1–10. [Google Scholar] [CrossRef]
- Fu, Y.; Soldera, M.; Wang, W.; Milles, S.; Deng, K.; Voisiat, B.; Nielsch, K.; Lasagni, A.F. Wettability control of polymeric microstructures replicated from laser-patterned stamps. Sci. Rep. 2020, 10, 22428. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, K.; Park, J.; Hwang, B.G.; Ko, Y.; Kim, H.; Han, J.; Seo, E.; Park, Y.; Lee, S.J. Nearly Perfect Durable Superhydrophobic Surfaces Fabricated by a Simple One-Step Plasma Treatment. Sci. Rep. 2017, 7, 1981. [Google Scholar] [CrossRef]
- Kang, H.; Lee, S.H.; Kim, K. Wettability of polytetrafluoroethylene surfaces by plasma etching modifications. PLoS ONE 2023, 18, e0282352. [Google Scholar] [CrossRef]
- Lyu; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and Time−Temperature Equivalence of Polymer Degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef]
- Kolská, Z.; Řezníčková, A.; Švorčík, V. Surface characterization of polymer foils. E-Polymers 2012, 12, 083. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2010.
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Shin, E.-K.; Park, H.; Noh, J.-Y.; Lim, K.-M.; Chung, J.-H. Platelet Shape Changes and Cytoskeleton Dynamics as Novel Therapeutic Targets for Anti-Thrombotic Drugs. Biomol. Ther. 2017, 25, 223–230. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.; Song, J.; Cheong, J.-W.; Shin, J.W.; Yang, W.I.; Kim, H.O. Platelet storage induces accelerated desialylation of platelets and increases hepatic thrombopoietin production. J. Transl. Med. 2018, 16, 199. [Google Scholar] [CrossRef]
- Apte, G.; Börke, J.; Rothe, H.; Liefeith, K.; Nguyen, T.-H. Modulation of Platelet-Surface Activation: Current State and Future Perspectives. ACS Appl. Bio Mater. 2020, 3, 5574–5589. [Google Scholar] [CrossRef]
- Sivaraman, B.; Latour, R.A. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials 2010, 31, 832–839. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, Y.M.; Kim, D.M. Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of PEO-containing block copolymers. Biomaterials 2000, 21, 683–691. [Google Scholar] [CrossRef]
- Koc, Y.; De Mello, A.J.; McHale, G.; Newton, M.I.; Roach, P.; Shirtcliffe, N.J. Nano-scale superhydrophobicity: Suppression of protein adsorption and promotion of flow-induced detachment. Lab Chip 2008, 8, 582. [Google Scholar] [CrossRef]
- Massa, T.M.; Yang, M.L.; Ho, J.Y.C.; Brash, J.L.; Santerre, J.P. Fibrinogen surface distribution correlates to platelet adhesion pattern on fluorinated surface-modified polyetherurethane. Biomaterials 2005, 26, 7367–7376. [Google Scholar] [CrossRef]
- Zhang, L.; Casey, B.; Galanakis, D.K.; Marmorat, C.; Skoog, S.; Vorvolakos, K.; Simon, M.; Rafailovich, M.H. The influence of surface chemistry on adsorbed fibrinogen conformation, orientation, fiber formation and platelet adhesion. Acta Biomater. 2017, 54, 164–174. [Google Scholar] [CrossRef]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The blood compatibility challenge. Part 2: Protein adsorption phenomena governing blood reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Weitz, J.I. The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Grunkemeier, J.M.; Horbett, T.A. Human plasma fibrinogen adsorption and platelet adhesion to polystyrene. J. Biomed. Mater. Res. 1999, 44, 130–139. [Google Scholar] [CrossRef]
- Motlagh, D.; Allen, J.; Hoshi, R.; Yang, J.; Lui, K.; Ameer, G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J. Biomed. Mater. Res. 2007, 82A, 907–916. [Google Scholar] [CrossRef]
- Zia, F.; Kendall, M.; Watson, S.P.; Mendes, P.M. Platelet aggregation induced by polystyrene and platinum nanoparticles is dependent on surface area. RSC Adv. 2018, 8, 37789–37794. [Google Scholar] [CrossRef]
- Mehrizi, T.Z.; Ardestani, M.S.; Rezayat, S.M.; Javanmard, A. A review study of the use of modified chitosan as a new approach to increase the preservation of blood products (erythrocytes, platelets, and plasma products): 2010–2022. Nanomed. J. 2023, 10, 16–32. [Google Scholar] [CrossRef]
- Wilson, A.C.; Chou, S.-F.; Lozano, R.; Chen, J.Y.; Neuenschwander, P.F. Thermal and Physico-Mechanical Characterizations of Thromboresistant Polyurethane Films. Bioengineering 2019, 6, 69. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices—Part 13: Identification and Quantification of Degradation Products from Polymeric Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2010.
- Budai-Szűcs, M.; Ruggeri, M.; Faccendini, A.; Léber, A.; Rossi, S.; Varga, G.; Bonferoni, M.C.; Vályi, P.; Burián, K.; Csányi, E.; et al. Electrospun Scaffolds in Periodontal Wound Healing. Polymers 2021, 13, 307. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man. Cybern. SMC 1973, 3, 610–621. [Google Scholar] [CrossRef]
- Löfstedt, T.; Brynolfsson, P.; Asklund, T.; Nyholm, T.; Garpebring, A. Gray-level invariant Haralick texture features. PLoS ONE 2019, 14, e0212110. [Google Scholar] [CrossRef]
- Restivo, E.; Peluso, E.; Bloise, N.; Bello, G.L.; Bruni, G.; Giannaccari, M.; Raiteri, R.; Fassina, L.; Visai, L. Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. J. Funct. Biomater. 2024, 15, 24. [Google Scholar] [CrossRef]
- Dapporto, M.; Tavoni, M.; Restivo, E.; Carella, F.; Bruni, G.; Mercatali, L.; Visai, L.; Tampieri, A.; Iafisco, M.; Sprio, S. Strontium-doped apatitic bone cements with tunable antibacterial and antibiofilm ability. Front. Bioeng. Biotechnol. 2022, 10, 969641. [Google Scholar] [CrossRef]
- Restivo, E.; Pugliese, D.; Gallichi-Nottiani, D.; Sammartino, J.C.; Bloise, N.; Peluso, E.; Percivalle, E.; Janner, D.; Milanese, D.; Visai, L. Effect of Low Copper Doping on the Optical, Cytocompatible, Antibacterial, and SARS-CoV-2 Trapping Properties of Calcium Phosphate Glasses. ACS Omega 2023, 8, 42264–42274. [Google Scholar] [CrossRef]



| Sample | 1H-NMR | Viscosimetry | GPC | ||
|---|---|---|---|---|---|
| BF Feed Mol% | BF Actual Mol% | I.V. dL/g | Mn g/mol | PDI - | |
| PBF | 100 | 100 | 1.13 | / | / |
| P(BF90BI10) | 90 | 91 | 0.97 | / | / |
| P(BF10BI90) | 10 | 10 | 0.77 | 50,000 | 1.7 |
| PBI | / | / | 0.89 | 55,000 | 1.6 |
| Sample | TGA | DSC | WAXS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I SCAN | II SCAN | |||||||||||
| Tid | Tmax | Tm | ΔHm | Tg | ΔCp | Tcc | ΔHcc | Tm | ΔHm | Xc | C.S. | |
| °C | °C | °C | J/g | °C | J/g °C | °C | J/g | °C | J/g | % | nm | |
| PBF | 378 | 392 | 170 | 40 | 34 | 0.364 | 95 | 34 | 169 | 40 | 36 | 5 |
| P(BF90BI10) | 375 | 392 | 156 | 36 | 33 | 0.432 | 110 | 28 | 155 | 28 | 29 | 4 |
| P(BF10BI90) | 387 | 409 | 125 | 31 | 24 | 0.354 | / | / | / | / | 34 | 10 |
| PBI | 389 | 409 | 142 | 38 | 22 | 0.381 | / | / | / | / | 37 | 10 |
| Sample | DSC—I Scan | WCA | |||||
|---|---|---|---|---|---|---|---|
| Tg | ΔCp | Tcc | ΔHcc | Tm | ΔHm | ° | |
| °C | J/g °C | °C | J/g | °C | J/g | ||
| PBF | 34 | 0.352 | 92 | 40 | 170 | 40 | 92 ± 3 |
| P(BF90BI10) | 33 | 0.245 | 102 | 34 | 154 | 34 | 92 ± 3 |
| P(BF10BI90) | 24 | 0.437 | / | / | / | / | 91 ± 3 |
| PBI | 22 | 0.367 | / | / | / | / | 96 ± 2 |
| Sample | E | σY | εY | σB | εB |
|---|---|---|---|---|---|
| MPa | MPa | % | MPa | % | |
| PBF | 1186 ± 99 | 26 ± 3 | 4.1 ± 0.2 | 28 ± 4 | 290 ± 99 |
| P(BF90BI10) | 1223 ± 127 | 34 ± 4 | 4.1 ± 0.4 | 32 ± 6 | 498 ± 99 |
| P(BF10BI90) | 449 ± 13 | 5.9 ± 0.2 | 2.4 ± 0.2 | 25 ± 6 | 926 ± 98 |
| PBI | 426 ± 20 | 4.3 ± 0.5 | 3.9 ± 0.8 | 23 ± 4 | 795 ± 38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondi, E.; Restivo, E.; Soccio, M.; Guidotti, G.; Bloise, N.; Motta, I.; Gazzano, M.; Ruggeri, M.; Fassina, L.; Visai, L.; et al. Design and Characterization of Aromatic Copolyesters Containing Furan and Isophthalic Rings with Suitable Properties for Vascular Tissue Engineering. Int. J. Mol. Sci. 2025, 26, 6470. https://doi.org/10.3390/ijms26136470
Bondi E, Restivo E, Soccio M, Guidotti G, Bloise N, Motta I, Gazzano M, Ruggeri M, Fassina L, Visai L, et al. Design and Characterization of Aromatic Copolyesters Containing Furan and Isophthalic Rings with Suitable Properties for Vascular Tissue Engineering. International Journal of Molecular Sciences. 2025; 26(13):6470. https://doi.org/10.3390/ijms26136470
Chicago/Turabian StyleBondi, Edoardo, Elisa Restivo, Michelina Soccio, Giulia Guidotti, Nora Bloise, Ilenia Motta, Massimo Gazzano, Marco Ruggeri, Lorenzo Fassina, Livia Visai, and et al. 2025. "Design and Characterization of Aromatic Copolyesters Containing Furan and Isophthalic Rings with Suitable Properties for Vascular Tissue Engineering" International Journal of Molecular Sciences 26, no. 13: 6470. https://doi.org/10.3390/ijms26136470
APA StyleBondi, E., Restivo, E., Soccio, M., Guidotti, G., Bloise, N., Motta, I., Gazzano, M., Ruggeri, M., Fassina, L., Visai, L., Pasquinelli, G., & Lotti, N. (2025). Design and Characterization of Aromatic Copolyesters Containing Furan and Isophthalic Rings with Suitable Properties for Vascular Tissue Engineering. International Journal of Molecular Sciences, 26(13), 6470. https://doi.org/10.3390/ijms26136470









