Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation
Abstract
1. Introduction
- oxidative stress;
- endothelial dysfunction;
- inflammation.
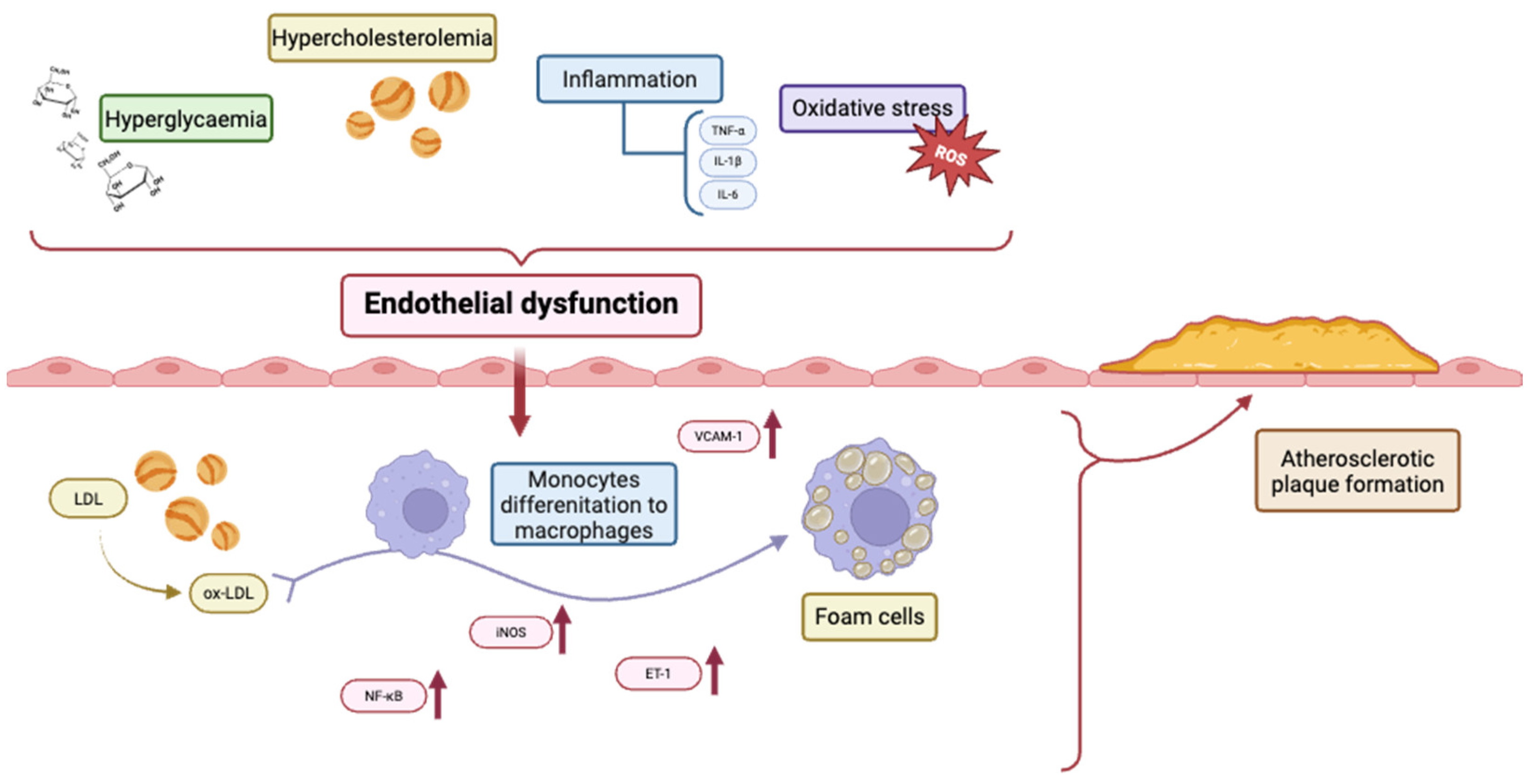
2. Factors Involved in Endothelial Dysfunction in Atherosclerosis
2.1. Oxidative Stress
2.2. Inflammation
2.3. NO and Shear Stress
2.4. Hypercholesterolemia
2.5. Hyperglycaemia
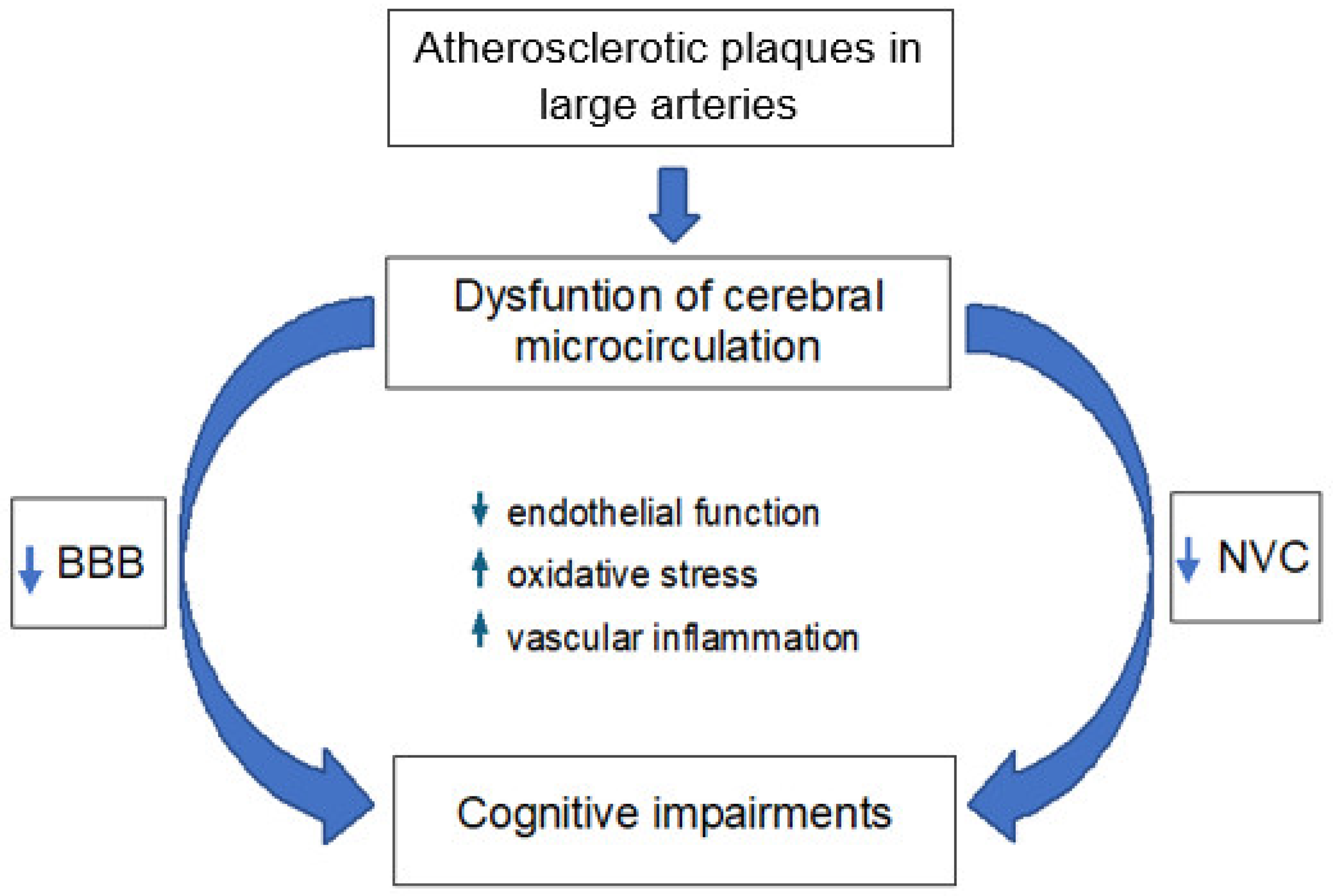
3. Cerebral Microcirculation in Atherosclerosis
3.1. Neurovascular Coupling
3.2. Blood–Brain Barrier
3.3. Cognitive Impairments
4. Final Remarks
Funding
Conflicts of Interest
References
- Zhong, C.; Deng, K.; Lang, X.; Shan, D.; Xie, Y.; Pan, W.; Yu, J. Therapeutic potential of natural flavonoids in atherosclerosis through endothelium-protective mechanisms: An update. Pharmacol. Ther. 2025, 271, 108864. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Chen, R.; Zheng, Q.; Yao, M.; Li, K.; Cao, Y.; Jiang, L. Oxidative stress disrupts vascular microenvironmental homeostasis affecting the development of atherosclerosis. Cell Biol. Int. 2024, 48, 1781–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar] [PubMed]
- Durante, A.; Mazzapicchi, A.; Baiardo Redaelli, M. Systemic and Cardiac Microvascular Dysfunction in Hypertension. Int. J. Mol. Sci. 2024, 25, 13294. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. A review about biomarkers for the investigation of vascular function and impairment in diabetes mellitus. Vasc. Health Risk Manag. 2016, 12, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. The pathophysiology of diabetic foot: A narrative review. J. Yeungnam Med. Sci. 2023, 40, 328–334. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Rai, V. Current and future role of biomarkers in the monitoring and prognosis of coronary artery disease. Future Cardiol. 2025, 21, 331–333. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Ni, L.; Lin, Y. Treatment of endothelial cell dysfunction in atherosclerosis: A new perspective integrating traditional and modern approaches. Front. Physiol. 2025, 16, 1555118. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Faraci, F.M. Reactive oxygen species: Influence on cerebral vascular tone. J. Appl. Physiol. 2006, 100, 739–743. [Google Scholar] [CrossRef]
- Cassuto, J.; Dou, H.; Czikora, I.; Szabo, A.; Patel, V.S.; Kamath, V.; Belin de Chantemele, E.; Feher, A.; Romero, M.J.; Bagi, Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes 2014, 63, 1381–1393. [Google Scholar] [CrossRef]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D.J. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. Cereb. Blood Flow. Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef]
- Park, S.J.; Shin, J.I.; Korean, J. Inflammation and hyponatremia: An underrecognized condition? Korean J. Pediatr. 2013, 56, 519–522. [Google Scholar] [CrossRef]
- Vila, E.; Salaices, M. Cytokines and vascular reactivity in resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1016–H1021. [Google Scholar] [CrossRef]
- Kofler, S.; Nickel, T.; Weis, M. Role of cytokines in cardiovascular diseases: A focus on endothelial responses to inflammation. Clin. Sci. 2005, 108, 205–213. [Google Scholar] [CrossRef]
- De Palma, C.; Meacci, E.; Perrotta, C.; Bruni, P.; Clementi, E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: A novel pathway relevant to the pathophysiology of endothelium. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Perrella, M.A.; Burnett, J.C., Jr.; Lee, M.E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ. Res. 1993, 73, 205–209. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Miller, F.J., Jr.; Gutterman, D.D.; Rios, C.D.; Heistad, D.D.; Davidson, B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998, 82, 1298–1305. [Google Scholar] [CrossRef]
- Collin, B.; Busseuil, D.; Zeller, M.; Perrin, C.; Barthez, O.; Duvillard, L.; Vergely, C.; Bardou, M.; Dumas, M.; Cottin, Y.; et al. Increased superoxide anion production is associated with early atherosclerosis and cardiovascular dysfunctions in a rabbit model. Mol. Cell. Biochem. 2007, 294, 225–235. [Google Scholar] [CrossRef]
- White, C.R.; Brock, T.A.; Chang, L.Y.; Crapo, J.; Briscoe, P.; Ku, D.; Bradley, W.A.; Gianturco, S.H.; Gore, J.; Freeman, B.A.; et al. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA 1994, 91, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, R.; Unnikrishnan, J.; Fu, A.; Nygren, J.; Short, K.R.; Schimke, J.; Barazzoni, R.; Nair, K.S. Impact of high-fat diet and antioxidant supplement on mitochondrial functions and gene transcripts in rat muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1055–E1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ya, B.; Yang, P.; Sun, F.; Zhang, L.; Li, Y.; Li, L. Impact of carotid atherosclerosis combined with hypercholesterolemia on cerebral microvessels and brain parenchyma in a new complex rat model. Neurochem. Res. 2014, 39, 653–660. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Lerman, A.; Edwards, B.S.; Hallett, J.W.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C., Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N. Engl. J. Med. 1991, 325, 997–1001. [Google Scholar] [CrossRef]
- Fan, J.; Unoki, H.; Iwasa, S.; Watanabe, T. Role of endothelin-1 in atherosclerosis. Ann. N. Y Acad. Sci. 2000, 902, 84–93; discussion 93–94. [Google Scholar] [CrossRef]
- Kawashima, S.; Yokoyama, M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Freiman, P.C.; Mitchell, G.G.; Heistad, D.D.; Armstrong, M.L.; Harrison, D.G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ. Res. 1986, 58, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Kawashima, S.; Yamashita, T.; Hirase, T.; Namiki, M.; Inoue, N.; Hirata, K.; Yasui, H.; Sakurai, H.; Yoshida, Y.; et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J. Clin. Investig. 2002, 110, 331–340. [Google Scholar] [CrossRef][Green Version]
- Ignarro, L.J.; Buga, G.M.; Wei, L.H.; Bauer, P.M.; Wu, G.; del Soldato, P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, J.; Lai, R.; Li, Q.; Ju, J.; Xu, H. Chinese Herbal Medicines and Active Metabolites: Potential Antioxidant Treatments for Atherosclerosis. Front. Pharmacol. 2021, 12, 675999. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Sheng, L.; Xu, X.; Zhou, B.; Shen, L.; Wu, M. Tongnao Decoction (TND) Alleviated Atherosclerosis by Playing Lowering Lipid, Anti-Inflammatory, and Antioxidant Roles. Oxid. Med. Cell. Longev. 2022, 2022, 6061197. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Li, X.; Guo, D.; Hu, Y.; Chen, Y. Oxidative Stress and Inflammation Are Associated with Coexistent Severe Multivessel Coronary Artery Stenosis and Right Carotid Artery Severe Stenosis in Elderly Patients. Oxid. Med. Cell. Longev. 2021, 2021, 2976447. [Google Scholar] [CrossRef]
- Tabaei, S.; Tabaee, S.S. DNA methylation abnormalities in atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Gertz, K.; Dirnagl, U.; Boehm, M.; Nickenig, G.; Endres, M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002, 942, 23–30. [Google Scholar] [CrossRef]
- Thomas, R.G.; Kim, J.H.; Kim, J.H.; Yoon, J.; Choi, K.H.; Jeong, Y.Y. Treatment of ischemic stroke by atorvastatin-loaded PEGylated liposome. Transl. Stroke Res. 2023, 15, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Choi, S.-H.; Fang, L.; Tsimikas, S. Lipoprotein modification and macrophage uptake: Role of pathologic cholesterol transport in atherogenesis. In Cholesterol Binding and Cholesterol Transport Proteins: Structure and Function in Health and Disease; Harris, J.R., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 229–251. [Google Scholar]
- Nowak, W.N.; Deng, J.; Ruan, X.Z.; Xu, Q. Reactive oxygen species generation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e41–e52. [Google Scholar] [CrossRef]
- Sen-Banerjee, S.; Mir, S.; Lin, Z.; Hamik, A.; Atkins, G.B.; Das, H.; Banerjee, P.; Kumar, A.; Jain, M.K. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005, 112, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Kostka, B.; Marczyk, I.; Krajewska, U.; Chałubiński, M.; Broncel, M. Effect of statins on platelet function in patients with hyperlipidemia. Arch. Med. Sci. 2013, 9, 622–628. [Google Scholar] [CrossRef]
- Choudhary, A.; Rawat, U.; Kumar, P.; Mittal, P. Pleotropic effects of statins: The dilemma of wider utilization of statin. Egypt. Heart J. 2023, 75, 1. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Haigl, B.; Basílio, J.; Hochreiter, B.; Gleitsmann, V.; Moser, B.; Hoesel, B.; Suur, B.E.; Puhm, F.; et al. Ikk2-mediated inflammatory activation of arterial endothelial cells promotes the development and progression of atherosclerosis. Atherosclerosis 2020, 307, 21–31. [Google Scholar] [CrossRef]
- Ebert, R.; Cumbana, R.; Lehmann, C.; Kutzner, L.; Toewe, A.; Ferreirós, N.; Parnham, M.J.; Schebb, N.H.; Steinhilber, D.; Kahnt, A.S. Long-term stimulation of toll-like receptor-2 and -4 upregulates 5-LO and 15-LO-2 expression thereby inducing a lipid mediator shift in human monocyte-derived macrophages. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158702. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.; Li, X.; Mehta, J.L.; Wang, X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res. Cardiol. 2018, 113, 5. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Buhlin, K.; Ekstrand, E.; de Faire, U.; Gustafsson, A.; Holmer, J.; Kjellström, B.; Lindahl, B.; Norhammar, A.; Klinge, B. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report From the PAROKRANK Study. Circulation 2016, 133, 576–583. [Google Scholar] [CrossRef]
- von Rossum, A.; Laher, I.; Choy, J.C. Immune-mediated vascular injury and dysfunction in transplant arteriosclerosis. Front. Immunol. 2015, 5, 684. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Lim, S.; Park, S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, T.; Pi, S.; Huang, L.; Liu, Y. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR-149-5p/MMP9 axis. Biochem. Biophys. Res. Commun. 2020, 529, 753–759. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2021, 42, 3230–3242. [Google Scholar] [CrossRef]
- Rui, W.; Guan, L.; Zhang, F.; Zhang, W.; Ding, W. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J. Appl. Toxicol. 2016, 36, 48–59. [Google Scholar] [CrossRef]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef]
- Khan, M.S.; Talha, K.M.; Maqsood, M.H.; Rymer, J.A.; Borlaug, B.A.; Docherty, K.F.; Pandey, A.; Kahles, F.; Cikes, M.; Lam, C.S.P.; et al. Interleukin-6 and Cardiovascular Events in Healthy Adults: MESA. JACC Adv. 2024, 3, 101063. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Sabzwari, S.R.A.; Vargova, V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus 2017, 9, e1144. [Google Scholar] [CrossRef]
- Pujades-Rodriguez, M.; Morgan, A.W.; Cubbon, R.M.; Wu, J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: A population-based cohort study. PLoS Med. 2020, 17, e1003432. [Google Scholar] [CrossRef]
- Singh, S.; Fumery, M.; Singh, A.G.; Singh, N.; Prokop, L.J.; Dulai, P.S.; Sandborn, W.J.; Curtis, J.R. Comparative Risk of Cardiovascular Events With Biologic and Synthetic Disease-Modifying Antirheumatic Drugs in Patients With Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2020, 72, 561–576. [Google Scholar] [CrossRef]
- Diamantis, E.; Kyriakos, G.; Quiles-Sanchez, L.V.; Farmaki, P.; Troupis, T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Curr. Cardiol. Rev. 2017, 13, 209–216. [Google Scholar] [CrossRef]
- Bonaventura, A.; Abbate, A. Colchicine for cardiovascular prevention: The dawn of a new era has finally come. Eur. Heart J. 2023, 44, 3303–3304. [Google Scholar] [CrossRef] [PubMed]
- Perera, B.; Wu, Y.; Nguyen, N.T.; Ta, H.T. Advances in drug delivery to atherosclerosis: Investigating the efficiency of different nanomaterials employed for different type of drugs. Mater. Today Bio 2023, 22, 100767. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kim, Y.H.; Lee, J.S.; Lee, J.W.; Oh, E.J.; Kim, H.M.; Lee, S.J.; Lee, J.; Lee, S.Y.; Huh, S.; et al. Oscillatory shear stress promotes angiogenic effects in arteriovenous malformations endothelial cells. Mol. Med. 2021, 27, 31. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Zhou, H.L.; Jiang, X.Z.; Ventikos, Y. Role of blood flow in endothelial functionality: A review. Front. Cell Dev. Biol. 2023, 11, 1259280. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Traub, O.; Berk, B.C. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Pan, S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid. Redox Signal 2009, 11, 1669–1682. [Google Scholar] [CrossRef]
- Man, M.Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory Role of Nitric Oxide in Cutaneous Inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef]
- Boveris, A.; Alvarez, S.; Navarro, A. The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radic. Biol. Med. 2002, 33, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.W.; Dixit, V.D. Mechanisms of disease: Inflammasome activation and the development of type 2 diabetes. Front. Immunol. 2013, 4, 50. [Google Scholar] [CrossRef]
- Shrishrimal, S.; Chatterjee, A.; Kosmacek, E.A.; Davis, P.J.; McDonald, J.T.; Oberley-Deegan, R.E. Manganese porphyrin, MnTE-2-PyP, treatment protects the prostate from radiation-induced fibrosis (RIF) by activating the NRF2 signaling pathway and enhancing SOD2 and sirtuin activity. Free Radic. Biol. Med. 2020, 152, 255–270. [Google Scholar] [CrossRef]
- Watts, G.F.; Catapano, A.L.; Masana, L.; Zambon, A.; Pirillo, A.; Tokgözoğlu, L. Hypercholesterolemia and cardiovascular disease: Focus on high cardiovascular risk patients. Atheroscler. Suppl. 2020, 42, e30–e34. [Google Scholar] [CrossRef]
- Stokes, K.Y.; Cooper, D.; Tailor, A.; Granger, D.N. Hypercholesterolemia promotes inflammation and microvascular dysfunction: Role of nitric oxide and superoxide. Free Radic. Biol. Med. 2002, 33, 1026–1036. [Google Scholar] [CrossRef]
- Stoll, G.; Bendszus, M. Inflammation and atherosclerosis: Novel insights into plaque formation and destabilization. Stroke 2006, 37, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C. Oxidation of LDL, atherogenesis, and apoptosis. Ann. N. Y. Acad. Sci. 2003, 1010, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Prasad, K.; Mishra, M. Mechanism of Hypercholesterolemia-Induced Atherosclerosis. Rev. Cardiovasc. Med. 2022, 23, 212. [Google Scholar] [CrossRef]
- Esmon, C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003, 1, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Schuhmann, M.K.; Garz, C.; Jandke, S.; Urlaub, D.; Mencl, S.; Zernecke, A.; Heinze, H.J.; Carare, R.O.; Kleinschnitz, C.; et al. Hypercholesterolemia induced cerebral small vessel disease. PLoS ONE 2017, 12, e0182822. [Google Scholar] [CrossRef]
- Ishikawa, M.; Stokes, K.Y.; Zhang, J.H.; Nanda, A.; Granger, D.N. Cerebral microvascular responses to hypercholesterolemia: Roles of NADPH oxidase and P-selectin. Circ. Res. 2004, 94, 239–244. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.H.; Lee, J.H.; Yun, Y.M.; Choi, D.H.; Kim, H.Y. Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 20. [Google Scholar] [CrossRef]
- Lopes, F.G.; Bottino, D.A.; Oliveira, F.J.; Mecenas, A.S.; Clapauch, R.; Bouskela, E. In elderly women moderate hypercholesterolemia is associated to endothelial and microcirculatory impairments. Microvasc. Res. 2013, 85, 99–103. [Google Scholar] [CrossRef]
- Todate, Y.; Ishigaki, Y. Effect of Hypercholesterolemia on the Characteristics of Cerebral Microvasculature. Diabetes 2018, 67 (Suppl. S1), 463-P. [Google Scholar] [CrossRef]
- Kitayama, J.; Faraci, F.M.; Lentz, S.R.; Heistad, D.D. Cerebral vascular dysfunction during hypercholesterolemia. Stroke 2007, 38, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Ebrahim, S. HDL-Cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke 2000, 31, 1882–1888. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk factors for ischemic stroke: Differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Demchuk, A.M.; Hess, D.C.; Brass, L.M.; Yatsu, F.M. Is cholesterol a risk factor for stroke?: Yes. Arch. Neurol. 1999, 56, 1518–1520, discussion 1524. [Google Scholar] [CrossRef]
- Kannel, W.B.; Dawber, T.R.; McNamara, P.M. Vascular Disease of the Brain—Epidemiologic Aspects: The Framingham Study. Am. J. Public Health Nations Health 1965, 55, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839, Erratum in Lancet 2008, 372, 292. [Google Scholar] [CrossRef]
- Beheshti, S.; Madsen, C.M.; Varbo, A.; Benn, M.; Nordestgaard, B.G. Relationship of Familial Hypercholesterolemia and High Low-Density Lipoprotein Cholesterol to Ischemic Stroke: Copenhagen General Population Study. Circulation 2018, 138, 578–589. [Google Scholar] [CrossRef]
- Amarenco, P.; Labreuche, J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009, 8, 453–463. [Google Scholar] [CrossRef]
- Joubert, J.; Lemmer, L.B.; Fourie, P.A.; van Gelder, A.L.; Darazs, B. Are clinical differences between black and white stroke patients caused by variations in the atherosclerotic involvement of the arterial tree? S. Afr. Med. J. 1990, 77, 248–251. [Google Scholar]
- Vaughan, C.J.; Delanty, N.; Basson, C.T. Statin therapy and stroke prevention. Curr. Opin. Cardiol. 2001, 16, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1. [Google Scholar] [CrossRef]
- Bornfeldt, K.E. Does Elevated Glucose Promote Atherosclerosis? Pros and Cons. Circ. Res. 2016, 119, 190–193. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Kanter, J.E.; Kramer, F.; Barnhart, S.; Shen, X.; Vivekanandan-Giri, A.; Wall, V.Z.; Kowitz, J.; Devaraj, S.; O’Brien, K.D.; et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014, 7, 356–365. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Nakahata, K.; Kinoshita, H.; Azma, T.; Matsuda, N.; Hama-Tomioka, K.; Haba, M.; Hatano, Y. Propofol restores brain microvascular function impaired by high glucose via the decrease in oxidative stress. Anesthesiology 2008, 108, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Lu, W.; Jia, C.; Li, H.; Wang, Z.; Jia, W. Relationships between glucose excursion and the activation of oxidative stress in patients with newly diagnosed type 2 diabetes or impaired glucose regulation. Endocrine 2010, 37, 201–208. [Google Scholar] [CrossRef]
- Karpen, C.W.; Cataland, S.; O’Dorisio, T.M.; Panganamala, R.V. Production of 12-hydroxyeicosatetraenoic acid and vitamin E status in platelets from type I human diabetic subjects. Diabetes 1985, 34, 526–531. [Google Scholar] [CrossRef]
- Chen, M.S.; Hutchinson, M.L.; Pecoraro, R.E.; Lee, W.Y.; Labbé, R.F. Hyperglycemia-induced intracellular depletion of ascorbic acid in human mononuclear leukocytes. Diabetes 1983, 32, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.D.; Chen, X.; Schmidt, A.M.; Brett, J.; Godman, G.; Zou, Y.S.; Scott, C.W.; Caputo, C.; Frappier, T.; Smith, M.A.; et al. Glycated tau protein in Alzheimer disease: A mechanism for induction of oxidant stress. Proc. Natl. Acad. Sci. USA 1994, 91, 7787–7791. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Matsui, T. Role of Ligands of Receptor for Advanced Glycation End Products (RAGE) in Peripheral Artery Disease. Rejuvenation Res. 2018, 21, 456–463. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hori, O.; Brett, J.; Yan, S.D.; Wautier, J.L.; Stern, D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. A J. Vasc. Biol. 1994, 14, 1521–1528. [Google Scholar] [CrossRef]
- Brett, J.; Schmidt, A.M.; Yan, S.D.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A.; et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar] [PubMed]
- Esposito, C.; Gerlach, H.; Brett, J.; Stern, D.; Vlassara, H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J. Exp. Med. 1989, 170, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G.; Simmons, L.K.; Sharpe, G.M. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am. J. Physiol. 1991, 260 Pt 2, H319–H326. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J.; Godfrey, J.A. Effect of hyperglycemia on brain penetrating arterioles and cerebral blood flow before and after ischemia/reperfusion. Transl. Stroke Res. 2010, 1, 127–134. [Google Scholar] [CrossRef]
- Ginsberg, M.D.; Prado, R.; Dietrich, W.D.; Busto, R.; Watson, B.D. Hyperglycemia reduces the extent of cerebral infarction in rats. Stroke 1987, 18, 570–574. [Google Scholar] [CrossRef]
- Uyttenboogaart, M.; Koch, M.W.; Stewart, R.E.; Vroomen, P.C.; Luijckx, G.J.; De Keyser, J. Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain 2007, 130 Pt 6, 1626–1630. [Google Scholar] [CrossRef]
- Nair, S.S.; Sylaja, P.N.; Sreedharan, S.E.; Sarma, S. Maintenance of normoglycemia may improve outcome in acute ischemic stroke. Ann. Indian. Acad. Neurol. 2017, 20, 122–126. [Google Scholar] [PubMed]
- Prospective Studies Collaboration and Asia Pacific Cohort Studies Collaboration. Sex-specific relevance of diabetes to occlusive vascular and other mortality: A collaborative meta-analysis of individual data from 980,793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018, 6, 538–546. [Google Scholar] [CrossRef]
- Nyúl-Tóth, Á.; Patai, R.; Csiszar, A.; Ungvari, A.; Gulej, R.; Mukli, P.; Yabluchanskiy, A.; Benyo, Z.; Sotonyi, P.; Prodan, C.I.; et al. Linking peripheral atherosclerosis to blood-brain barrier disruption: Elucidating its role as a manifestation of cerebral small vessel disease in vascular cognitive impairment. Geroscience 2024, 46, 6511–6536. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, A.; Patai, R.; Gulej, R.; Yabluchanskiy, A.; Benyo, Z.; Kovacs, I.; Sotonyi, P.; Kirkpartrick, A.C.; Prodan, C.I.; et al. Atherosclerotic burden and cerebral small vessel disease: Exploring the link through microvascular aging and cerebral microhemorrhages. Geroscience 2024, 46, 5103–5132. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Safar, M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005, 46, 200–2004. [Google Scholar] [CrossRef] [PubMed]
- van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.; van der Kuip, D.A.; Hofman, A.; Witteman, J.C. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef]
- Che, J.; Sun, Y.; Deng, Y.; Zhang, J. Blood-brain barrier disruption: A culprit of cognitive decline? Fluids Barriers CNS 2024, 21, 63. [Google Scholar] [CrossRef]
- Tarantini, S.; Tran, C.H.T.; Gordon, G.R.; Ungvari, Z.; Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 2017, 94, 52–58. [Google Scholar] [CrossRef]
- Bøthun, M.L.; Haaland, Ø.A.; Moen, G.; Logallo, N.; Svendsen, F.; Thomassen, L.; Helland, C.A. Impaired cerebrovascular reactivity may predict delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2019, 407, 116539. [Google Scholar] [CrossRef]
- Izawa, Y.; Gu, Y.H.; Osada, T.; Kanazawa, M.; Hawkins, B.T.; Koziol, J.A.; Papayannopoulou, T.; Spatz, M.; Del Zoppo, G.J. β1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J. Cereb. Blood Flow. Metab. 2018, 38, 641–658. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Ashpole, N.M.; Tucsek, Z.; Milne, G.L.; Valcarcel-Ares, N.M.; Menyhart, A.; Farkas, E.; Sonntag, W.E.; Csiszar, A.; et al. IGF-1 deficiency impairs neurovascular coupling in mice: Implications for cerebromicrovascular aging. Aging Cell 2015, 14, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, C.; Lu, X.; Moeini, M.; Thorin, E.; Lesage, F. Impact of atherosclerotic disease on cerebral microvasculature and tissue oxygenation in awake LDLR-/-hApoB+/+ transgenic mice. Neurophotonics 2019, 6, 045003. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Moeini, M.; Sakadžić, S.; Thorin, E.; Lesage, F. Atherosclerosis is associated with a decrease in cerebral microvascular blood flow and tissue oxygenation. PLoS ONE 2019, 14, e0221547. [Google Scholar] [CrossRef]
- Eyre, B.; Shaw, K.; Drew, D.; Rayson, A.; Shabir, O.; Lee, L.; Francis, S.; Berwick, J.; Howarth, C. Characterizing vascular function in mouse models of Alzheimer’s disease, atherosclerosis, and mixed Alzheimer’s and atherosclerosis. Neurophotonics 2025, 12 (Suppl. S1), S14610. [Google Scholar] [CrossRef]
- Vilar-Bergua, A.; Riba-Llena, I.; Nafría, C.; Bustamante, A.; Llombart, V.; Delgado, P.; Montaner, J. Blood and CSF biomarkers in brain subcortical ischemic vascular disease: Involved pathways and clinical applicability. J. Cereb. Blood Flow. Metab. 2016, 36, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Chimowitz, M.I. Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circ. Res. 2017, 120, 502–513. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y.; Ali, F. Atherosclerotic cardiovascular disease: A review of initiators and protective factors. Inflammopharmacology 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Komura, S.; Nomura, T.; Imaizumi, T.; Inamura, S.; Kanno, A.; Honda, O.; Hashimoto, Y.; Mikami, T.; Nonaka, T. Asymptomatic cerebral findings on 3-Tesla MRI in patients with severe carotid artery stenoses. J. Clin. Neurosci. 2022, 101, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, H.; Dahlstrom, K.A.; Culleton, S.; Sarrami, A.H.; McFarland, M.M.; Romero, J.R. Association between Extracranial Carotid Artery Plaque and Cognitive Dysfunction: A Systematic Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2022, 51, 377–385. [Google Scholar] [CrossRef]
- Bezerra, D.C.; Sharrett, A.R.; Matsushita, K.; Gottesman, R.F.; Shibata, D.; Mosley, T.H., Jr.; Coresh, J.; Szklo, M.; Carvalho, M.S.; Selvin, E. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology 2012, 78, 102–108. [Google Scholar] [CrossRef]
- Krueger, M.; Härtig, W.; Reichenbach, A.; Bechmann, I.; Michalski, D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS ONE 2013, 8, e56419. [Google Scholar] [CrossRef] [PubMed]
- Van Skike, C.E.; Jahrling, J.B.; Olson, A.B.; Sayre, N.L.; Hussong, S.A.; Ungvari, Z.; Lechleiter, J.D.; Galvan, V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H693–H703. [Google Scholar] [CrossRef]
- Roberts, J.M.; Maniskas, M.E.; Bix, G.J. Bilateral carotid artery stenosis causes unexpected early changes in brain extracellular matrix and blood-brain barrier integrity in mice. PLoS ONE 2018, 13, e0195765. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Z.; Ge, M.; Tang, O.; Cheng, Y.; Zhou, H.; Shen, Y.; Qin, F. Monocytic cell junction proteins serve important roles in atherosclerosis via the endoglin pathway. Mol. Med. Rep. 2017, 16, 6750–6756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, X.; Wan, Y.; Zhou, Q.; Zhu, H.; Wang, Y. Myosin light chain kinase inhibitor ML7 improves vascular endothelial dysfunction via tight junction regulation in a rabbit model of atherosclerosis. Mol. Med. Rep. 2015, 12, 4109–4116. [Google Scholar] [CrossRef]
- Kisucka, J.; Chauhan, A.K.; Zhao, B.Q.; Patten, I.S.; Yesilaltay, A.; Krieger, M.; Wagner, D.D. Elevated levels of soluble P-selectin in mice alter blood-brain barrier function, exacerbate stroke, and promote atherosclerosis. Blood 2009, 113, 6015–6022. [Google Scholar] [CrossRef] [PubMed]
- Gyanwali, B.; Tan, C.S.; Petr, J.; Escobosa, L.L.T.; Vrooman, H.; Chen, C.; Mutsaerts, H.J.; Hilal, S. Arterial Spin-Labeling Parameters and Their Associations with Risk Factors, Cerebral Small-Vessel Disease, and Etiologic Subtypes of Cognitive Impairment and Dementia. AJNR Am. J. Neuroradiol. 2022, 43, 1418–1423. [Google Scholar] [CrossRef]
- Lin, Z.; Sur, S.; Liu, P.; Li, Y.; Jiang, D.; Hou, X.; Darrow, J.; Pillai, J.J.; Yasar, S.; Rosenberg, P.; et al. Blood-Brain Barrier Breakdown in Relationship to Alzheimer and Vascular Disease. Ann. Neurol. 2021, 90, 227–238. [Google Scholar] [CrossRef]
- Kalayci, R.; Kaya, M.; Uzun, H.; Bilgic, B.; Ahishali, B.; Arican, N.; Elmas, I.; Küçük, M. Influence of hypercholesterolemia and hypertension on the integrity of the blood-brain barrier in rats. Int. J. Neurosci. 2009, 119, 1881–1904. [Google Scholar] [CrossRef]
- Cong, X.; Kong, W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell. Signal. 2020, 66, 109485. [Google Scholar] [CrossRef]
- El Ali, A.; Doeppner, T.R.; Zechariah, A.; Hermann, D.M. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: Role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 2011, 42, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow. Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef]
- Enciu, A.M.; Gherghiceanu, M.; Popescu, B.O. Triggers and effectors of oxidative stress at blood-brain barrier level: Relevance for brain ageing and neurodegeneration. Oxid. Med. Cell. Longev. 2013, 2013, 297512. [Google Scholar] [CrossRef]
- Milej, D.; Abdalmalak, A.; Desjardins, L.; Ahmed, H.; Lee, T.Y.; Diop, M.; Lawrence, K.S. Quantification of blood-brain barrier permeability by dynamic contrast-enhanced NIRS. Sci. Rep. 2017, 7, 1702. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Walker, L.; Rauchmann, B.S.; Perneczky, R. Dysfunction of the blood-brain barrier in Alzheimer’s disease: Evidence from human studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef]
- Ihara, M.; Yamamoto, Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke 2016, 47, 554–560. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Tarumi, T. Cerebral blood flow regulation and cognitive function: A role of arterial baroreflex function. J. Physiol. Sci. 2019, 69, 813–823. [Google Scholar] [CrossRef]
- Taheri, S.; Gasparovic, C.; Huisa, B.N.; Adair, J.C.; Edmonds, E.; Prestopnik, J.; Grossetete, M.; Shah, N.J.; Wills, J.; Qualls, C.; et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke 2011, 42, 2158–2163. [Google Scholar] [CrossRef]
- Suri, M.F.K.; Zhou, J.; Qiao, Y.; Chu, H.; Qureshi, A.I.; Mosley, T.; Gottesman, R.F.; Wruck, L.; Sharrett, A.R.; Alonso, A.; et al. Cognitive impairment and intracranial atherosclerotic stenosis in general population. Neurology 2018, 90, e1240–e1247. [Google Scholar] [CrossRef]
- Rossetti, H.C.; Weiner, M.; Hynan, L.S.; Cullum, C.M.; Khera, A.; Lacritz, L.H. Subclinical atherosclerosis and subsequent cognitive function. Atherosclerosis 2015, 241, 36–41. [Google Scholar] [CrossRef]
- Sabayan, B.; Goudarzi, R.; Ji, Y.; Borhani-Haghighi, A.; Olson-Bullis, B.A.; Murray, A.M.; Sedaghat, S. Intracranial Atherosclerosis Disease Associated With Cognitive Impairment and Dementia: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2023, 12, e032506. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Pan, Y.; Zhang, W.; Gao, D.; Ma, J.; Zhang, Y.; Ji, M.; Dai, Y.; Liu, Y.; Wang, Y.; et al. Associations Between Atherosclerosis and Subsequent Cognitive Decline: A Prospective Cohort Study. J. Am. Heart Assoc. 2024, 13, e036696. [Google Scholar] [CrossRef] [PubMed]
- Ihle-Hansen, H.; Ihle-Hansen, H.; Sandset, E.C.; Hagberg, G. Subclinical Carotid Artery Atherosclerosis and Cognitive Function: A Mini-Review. Front. Neurol. 2021, 12, 705043. [Google Scholar] [CrossRef]
- Auperin, A.; Berr, C.; Bonithon-Kopp, C.; Touboul, P.J.; Ruelland, I.; Ducimetiere, P.; Alperovitch, A. Ultrasonographic assessment of carotid wall characteristics and cognitive functions in a community sample of 59- to 71-year-olds. The EVA Study Group. Stroke 1996, 27, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Anbar, R.; Sultan, S.R.; Al Saikhan, L.; Alkharaiji, M.; Chaturvedi, N.; Hardy, R.; Richards, M.; Hughes, A. Is carotid artery atherosclerosis associated with poor cognitive function assessed using the Mini-Mental State Examination? A systematic review and meta-analysis. BMJ Open 2022, 12, e055131. [Google Scholar] [CrossRef]
- Gardener, H.; Caunca, M.R.; Dong, C.; Cheung, Y.K.; Elkind, M.S.V.; Sacco, R.L.; Rundek, T.; Wright, C.B. Ultrasound Markers of Carotid Atherosclerosis and Cognition: The Northern Manhattan Study. Stroke 2017, 48, 1855–1861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrowicz, M.; Konop, M.; Rybka, M.; Mazurek, Ł.; Stradczuk-Mazurek, M.; Kciuk, M.; Bądzyńska, B.; Dobrowolski, L.; Kuczeriszka, M. Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation. Int. J. Mol. Sci. 2025, 26, 6467. https://doi.org/10.3390/ijms26136467
Aleksandrowicz M, Konop M, Rybka M, Mazurek Ł, Stradczuk-Mazurek M, Kciuk M, Bądzyńska B, Dobrowolski L, Kuczeriszka M. Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation. International Journal of Molecular Sciences. 2025; 26(13):6467. https://doi.org/10.3390/ijms26136467
Chicago/Turabian StyleAleksandrowicz, Marta, Marek Konop, Mateusz Rybka, Łukasz Mazurek, Monika Stradczuk-Mazurek, Mateusz Kciuk, Bożena Bądzyńska, Leszek Dobrowolski, and Marta Kuczeriszka. 2025. "Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation" International Journal of Molecular Sciences 26, no. 13: 6467. https://doi.org/10.3390/ijms26136467
APA StyleAleksandrowicz, M., Konop, M., Rybka, M., Mazurek, Ł., Stradczuk-Mazurek, M., Kciuk, M., Bądzyńska, B., Dobrowolski, L., & Kuczeriszka, M. (2025). Dysfunction of Microcirculation in Atherosclerosis: Implications of Nitric Oxide, Oxidative Stress, and Inflammation. International Journal of Molecular Sciences, 26(13), 6467. https://doi.org/10.3390/ijms26136467






