Effects of 1-N-Naphthylphthalamic Acid on Root and Leaf Development of Muscari armeniacum and the Related Metabolic and Physiological Features
Abstract
1. Introduction
2. Results
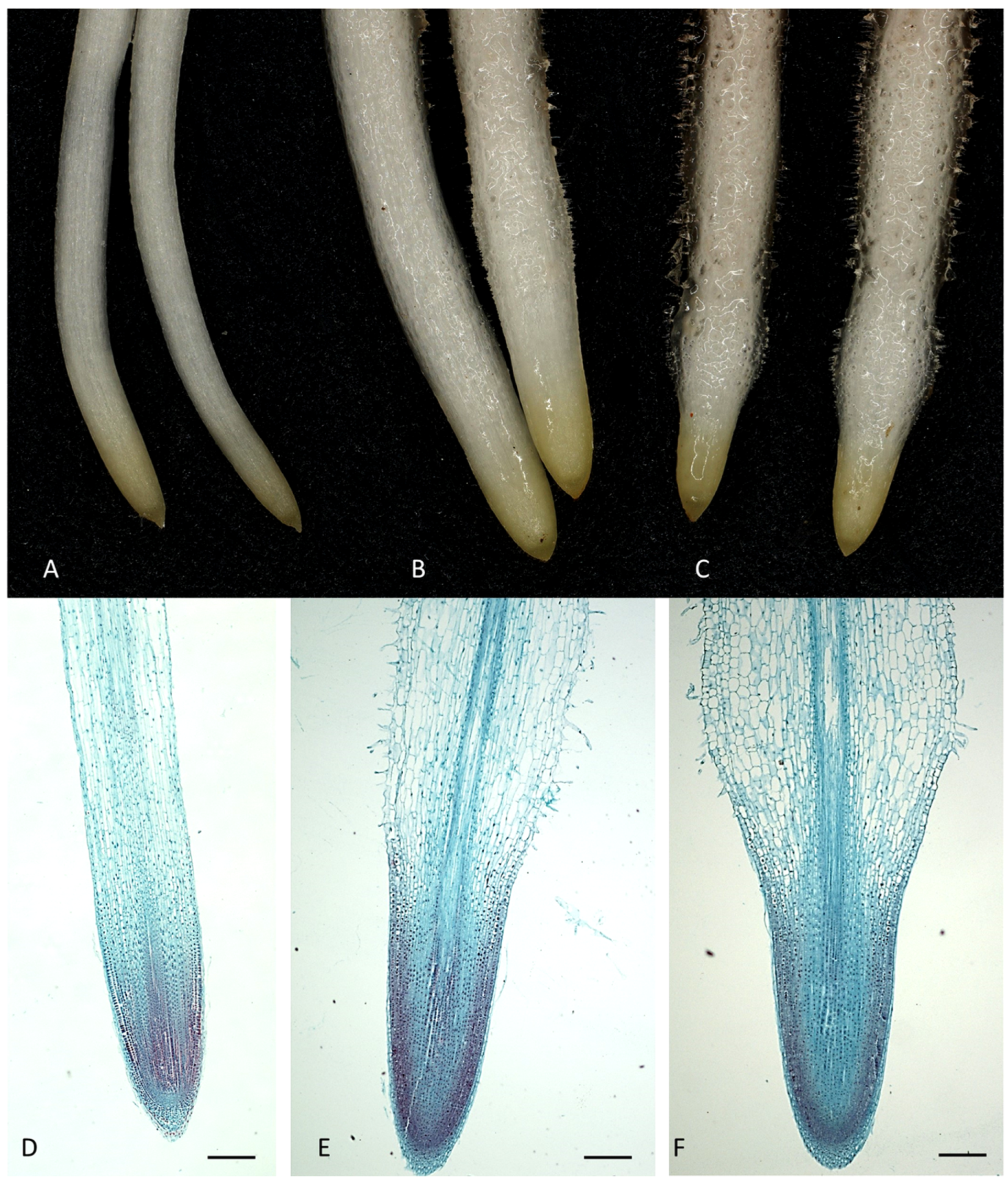
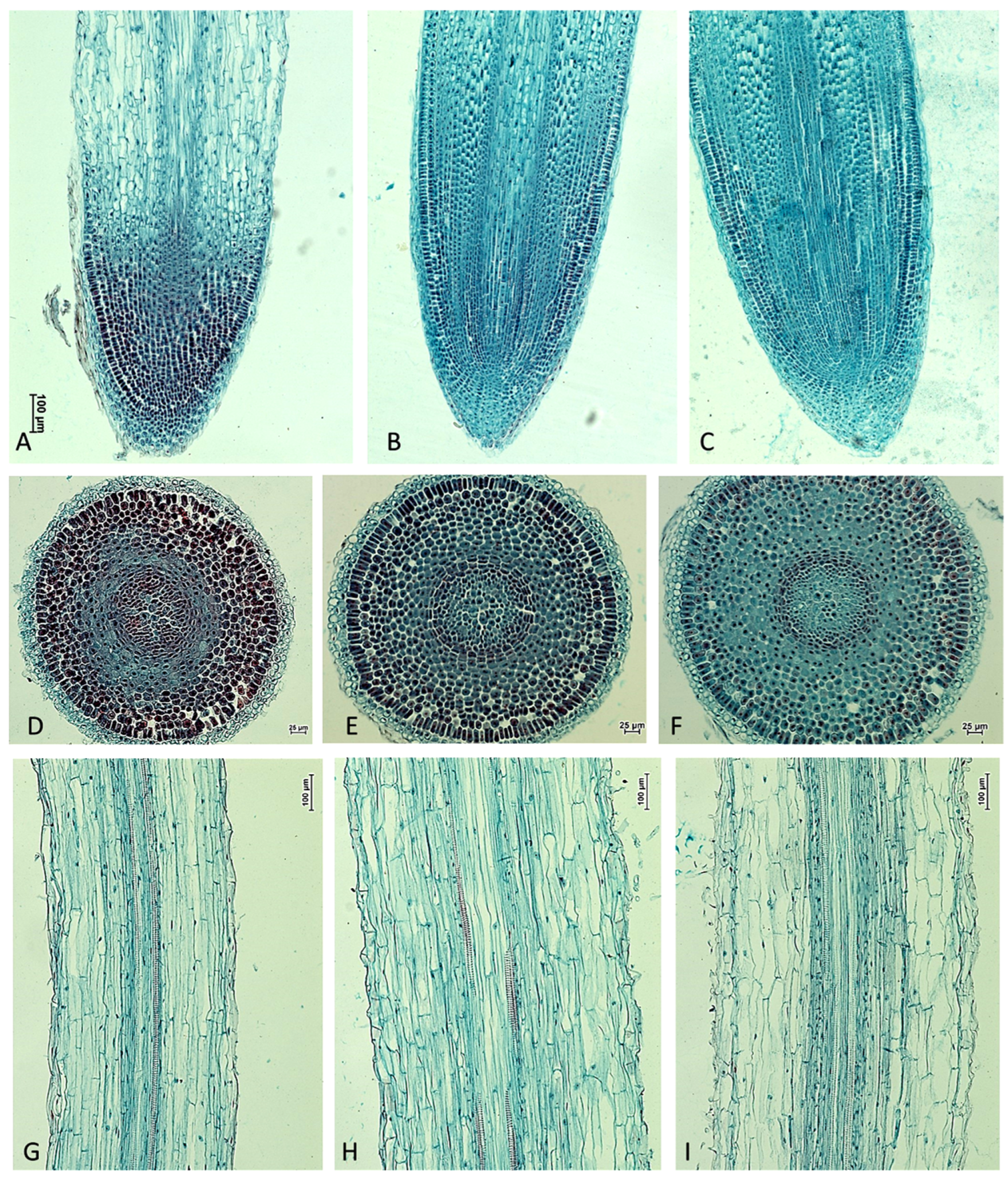
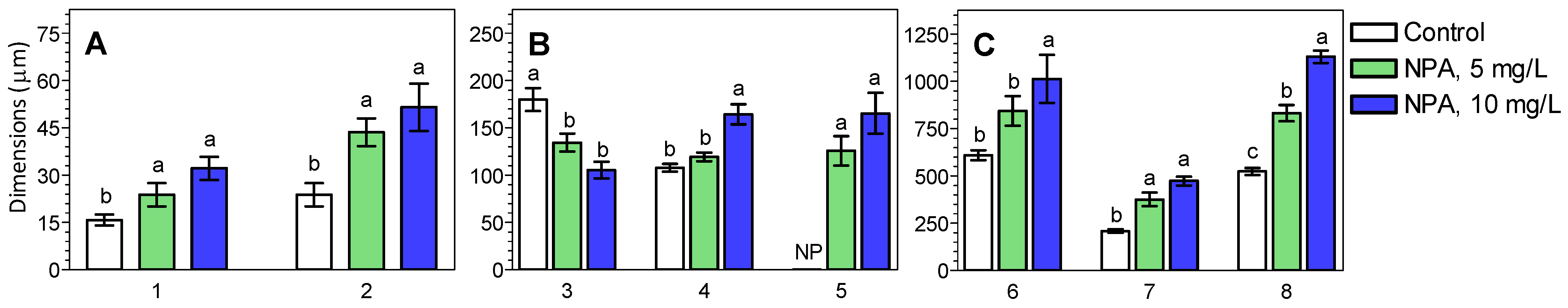
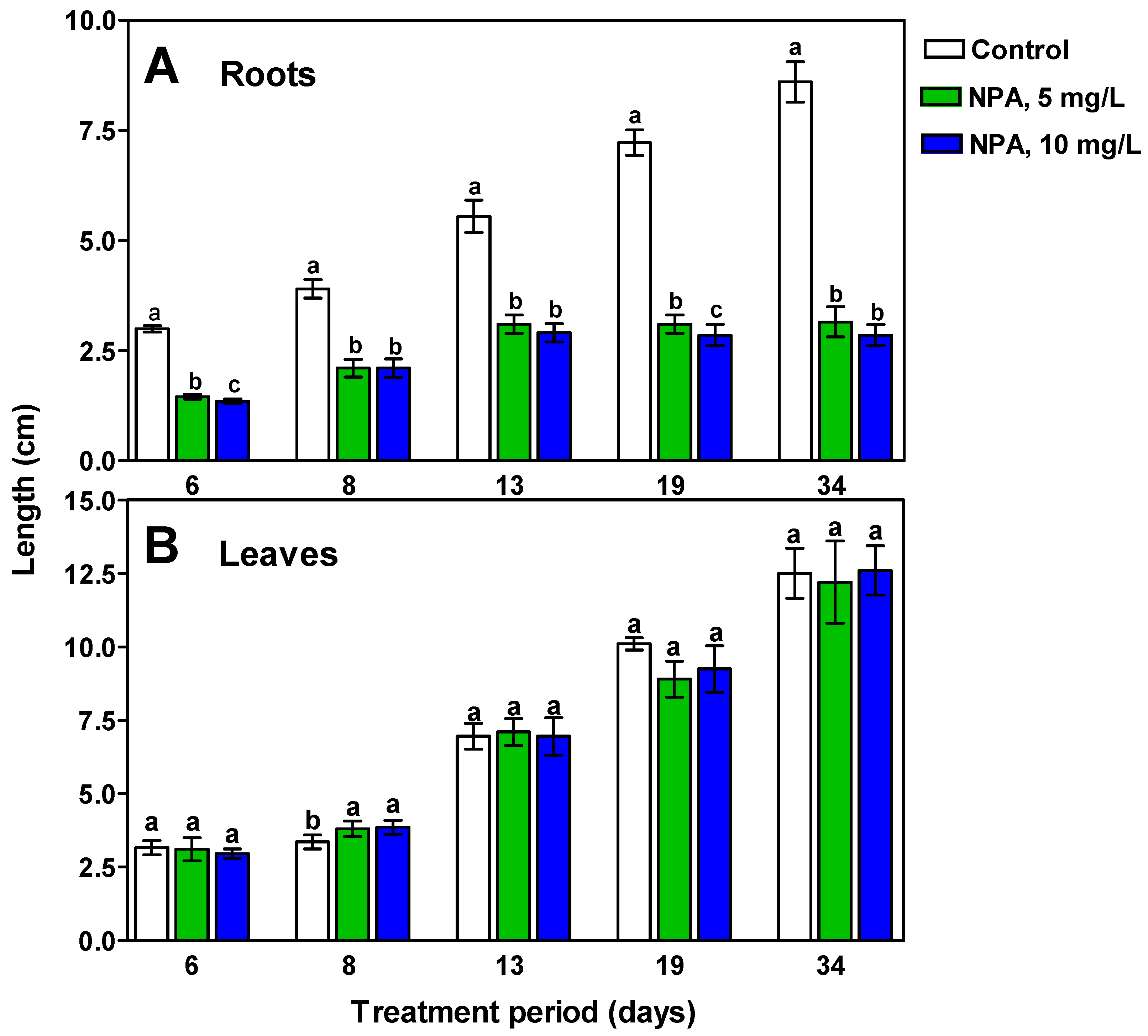
2.1. The Morphology and Histological Measurements of M. Armeniacum Roots
2.2. Content of Polar Compounds in M. armeniacum Roots
2.3. Content of Phenolic Compounds in M. armeniacum Roots
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Histological Observations and Measurements
4.3. Determination of Organic Acids, Amino Acids, and Soluble Carbohydrates
4.4. Determination of Phenolic Compounds
4.5. Determination of Anthocyanins
4.6. Statistical Evaluation of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPA | 1-N-naphthylphthalamic acid |
| ABA | Abscisic acid |
| IAA | Indolyl-3-acetic acid |
| GA | Gibberellic acid |
| BA | Benzyladenine |
| PIN | Proteins that facilitate auxin efflux |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC-MS | High-performance liquid chromatography-mass spectrometry |
| SD | Standard deviation |
References
- Yücesan, B.B.; Çiçek, F.; Gürel, E. Somatic embryogenesis and encapsulation of immature bulblets of an ornamental species, grape hyacinths (Muscari armeniacum Leichtlin ex Baker). Turk. J. Agric. For. 2014, 38, 716–722. [Google Scholar] [CrossRef]
- Faruq, M.O.; Shahinozzaman, M.; Azad, M.A.K.; Amin, M.N. In vitro propagation of a cut flower variety Muscari armeniacum Leichtlin ex Baker through direct bulblet proliferation pathways. GSC Biol. Pharm. Sci. 2018, 5, 67–75. [Google Scholar] [CrossRef]
- Saniewski, M.; Góraj-Koniarska, J.; Węgrzynowicz-Lesiak, J.; Gabryszewska, E. Hormonal regulation of the growth of leaves and inflorescence stalk in Muscari armeniacum Leichtl. Acta Agrobot. 2016, 69, 1654. [Google Scholar] [CrossRef]
- Kapusta, G. Seedbed tillage and herbicide influence on soybean (Glycine max) weed control and yield. Weed Sci. 1979, 27, 520–526. [Google Scholar] [CrossRef]
- Knerr, L.D.; Hopen, H.J.; Balke, N.E. Effect of Naptalam on Chloramben Toxicity, Uptake, Translocation, and Metabolism in Cucumber (Cucumis sativus). Weed Sci. 1991, 39, 27–32. [Google Scholar] [CrossRef]
- Harger, T.R.; King, P.A.; Barcel, D.J. Commercial Potential of Naptalam (NPA) for Ornamentals. In Proceedings of the Plant Growth Regulation Society of America-Annual Meeting, San Antonio, TX, USA, 28 July–1 August 2002; p. 192. [Google Scholar]
- Al-Khatib, K.; Kadir, S.; Libbey, C. Broadleaf weed control with clomazone in pickling cucumber (Cucumis sativus). Weed Technol. 2002, 9, 166–172. [Google Scholar] [CrossRef]
- Brandenberger, L.P.; Shrefler, J.W.; Webber, C.L.; Talbert, R.E.; Payton, M.E.; Wells, L.K.; McClelland, M. Preemergence Weed Control in Direct-Seeded Watermelon. Weed Technol. 2005, 19, 706–712. [Google Scholar] [CrossRef]
- Abas, L.; Kolb, M.; Stadlmann, J.; Janacek, D.P.; Lukic, K.; Schwechheimer, C.; Sazanov, L.A.; Mach, L.; Friml, J.; Hammes, U.Z. Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc. Natl. Acad. Sci. USA 2020, 118, e2020857118. [Google Scholar] [CrossRef]
- Johnston, C.R.; Malladi, A.; Vencill, W.K.; Grey, T.L.; Culpepper, S.; Henry, G.; Czarnota, M.A.; Randell, T.M. Investigation of physiological and molecu lar mechanisms conferring diurnal variation in auxinic herbicide efficacy. PLoS ONE 2020, 15, e0238144. [Google Scholar] [CrossRef]
- Kong, M.; Liu, X.; Sun, L.; Tan, S. Molecular mechanisms of N-1-naphthylphthalamic acid, a chemical tool in plant biology and agriculture. Mol. Hortic. 2022, 2, 22. [Google Scholar] [CrossRef]
- Teale, W.; Palme, K. Naphthylphthalamic acid and the mechanism of polar auxin transport. J. Exp. Bot. 2018, 69, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Marasek-Ciołakowska, A.; Dziurka, M.; Góraj-Koniarska, J.; Kowalska, U.; Szablińska-Piernik, J.; Horbowicz, M.; Wiczkowski, W.; Miyamoto, K.; Ueda, J.; Saniewski, M. Histological, hormonal and metabolic response triggered by N-1-naphthylphthalamic acid-induced stem swelling in Solidago canadensis L. Acta Sci. Pol. Hortorum Cultus 2024, 23, 25–40. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Saniewski, M.; Ueda, J.; Miyamoto, K. Mode of action of 1-N-naphthylphthalamic acid in conspicuous local stem swelling of succulent plant, Bryophyllum calycinum: Relevance to the aspects of its histological observation and comprehensive analyses of plant hormones. Int. J. Mol. Sci. 2021, 22, 3118. [Google Scholar] [CrossRef]
- Nongmaithem, S.; Devulapalli, S.; Sreelakshmi, Y.; Sharma, R. Is naphthylphthalamic acid a specific phytotropin? It elevates ethylene and alters metabolic homeostasis in tomato. Plant Sci. 2020, 291, 110358. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Rosen, E.; Masson, P.H. Gravitropism in higher plants. Plant Physiol. 1999, 120, 343–350. [Google Scholar] [CrossRef]
- Young, L.M.; Evans, M.L. Patterns of auxin and abscisic acid movement in the tips of gravistimulated primary roots of maize. Plant Growth Regul. 1996, 20, 253–258. [Google Scholar] [CrossRef]
- Ruegger, M.; Dewey, E.; Hobbie, L.; Brown, D.; Bernasconi, P.; Turner, J.; Muday, G.; Estelle, M. Reduced naphthylphthalamic acid binding in the tir3mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 1997, 9, 745–757. [Google Scholar] [CrossRef]
- Liu, J.H.; Reid, D.M. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings. IV. The role of changes in endogenous free and conjugated indole-3-acetic acid. Physiol. Plant. 1992, 86, 285–292. [Google Scholar] [CrossRef]
- Garrido, G.; Ramón Guerrero, J.; Angel Cano, E.; Acosta, M.; Sánchez-Bravo, J. Origin and basipetal transport of the IAA responsible for rooting of carnation cuttings. Physiol. Plant. 2002, 114, 303–312. [Google Scholar] [CrossRef]
- Marks, T.R.; Ford, Y.Y.; Cameron, R.W.F.; Goodwin, C.; Myers, P.E.; Judd, H.L. A role for polar auxin transport in rhizogenesis. Plant Cell Tiss. Org. 2002, 70, 189–198. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef]
- Chang, X.Y.; Zhang, K.; Yuan, Y.; Ni, P.; Ma, J.; Liu, H.; Gong, S.; Yang, G.; Bai, M. A simple, rapid, and quantifiable system for studying adventitious root formation in grapevine. Plant Growth Regul. 2022, 98, 117–126. [Google Scholar] [CrossRef]
- Gong, W.; Long, J.; Wu, J.; Du, C.; Zhang, X.; Zhang, J. Application of NPA restrained leaf expansion by reduced cell division in soybean under shade stress. J. Plant Growth Regul. 2022, 41, 3345–3358. [Google Scholar] [CrossRef]
- Rashotte, A.M.; Brady, S.R.; Reed, R.C.; Ante, S.J.; Muday, G.K. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000, 122, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Makigawa, S.; Sun, J.; Kodama, K.; Sugiyama, H.; Matsumoto, K.; Iwata, T.; Wasano, N.; Kano, A.; Morita, M.T.; et al. Design and synthesis of strong root gravitropism inhibitors with no concomitant growth inhibition. Sci. Rep. 2023, 13, 5173. [Google Scholar] [CrossRef] [PubMed]
- Blancaflor, E.; Fasano, J.; Gilroy, S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998, 116, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.; Strohm, A.; Masson, P. Gravity sensing and signal transduction in vascular plant primary roots. Am. J. Bot. 2013, 100, 126–142. [Google Scholar] [CrossRef]
- Ranwala, A.P.; Miller, W.B. Analysis of nonstructural carbohydrates in storage organs of 30 ornamental geophytes by high-performance anion-exchange chromatography with pulsed amperometric detection. New Phytol. 2008, 180, 421–433. [Google Scholar] [CrossRef]
- Sheikh, F.R.; Jose-Santhi, J.; Kalia, D.; Singh, K.; Singh, R.K. Sugars as the regulators of dormancy and sprouting in geophytes. Ind. Crop. Prod. 2022, 189, 115817. [Google Scholar] [CrossRef]
- Bancal, P.; Carpita, N.C.; Gaudillère, J.P. Differences in fructan accumulation in induced and field-grown wheat plants: An elongation-trimming pathway for their synthesis. New Phytol. 1992, 120, 313–321. [Google Scholar] [CrossRef]
- Shiomi, N.; Benkeblia, N. The metabolism of the fructooligosaccharides in onion bulbs: A comprehensive review. J. Appl. Glycosci. 2005, 52, 121–127. [Google Scholar] [CrossRef]
- Barbakadze, V.V.; Targamadze, I.L.; Usov, A.I. Glucofructan from bulbs of grape hyacinth Muscari szovitsianum Baker (Liliaceae). Bioorg. Khim. 1996, 22, 441–445. [Google Scholar]
- Bokov, D.O. Muscari armeniacum Leichtlin (grape hyacinth): Phytochemistry and biological activities review. Asian J. Pharm. Clin. Res. 2019, 12, 68–72. [Google Scholar] [CrossRef]
- Barbakadze, V.V.; Gakhokidze, R.A.; Shengeliya, Z.S.; Usov, A.I. Preliminary investigation of water-soluble polysaccharides from Georgian plants. Chem. Nat. Compd. 1989, 25, 281–286. [Google Scholar] [CrossRef]
- Mancuso, S.; Barlow, P.W.; Volkmann, D.; Baluška, F. Actin turnover-mediated gravity response in maize root apices: Gravitropism of decapped roots implicates gravisensing outside of the root cap. Plant Signal. Behav. 2006, 1, 52–58. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Zhou, Y.; He, J.; Kang, B.H. Root Cap-Specific Transcriptomics Identify the NAC Transcription Factor SOMBRERO as a Versatile Regulator of Auxin Gradients. bioRxiv 2025, 22, 634243. [Google Scholar] [CrossRef]
- Su, S.-H.; Gibbs, N.M.; Jancewicz, A.L.; Masson, P.H. Molecular mechanisms of root gravitropism. Curr. Biol. 2017, 27, R964–R972. [Google Scholar] [CrossRef]
- Barlow, P.W. Gravity perception in plants: A multiplicity of systems derived by evolution? Plant Cell Environ. 1995, 18, 951–962. [Google Scholar] [CrossRef]
- Muday, G.K.; Haworth, P. Tomato root growth, gravitropism, and lateral root development. Correlation with auxin transport. Plant Physiol. Biochem. 1994, 32, 193–203. [Google Scholar]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar] [CrossRef]
- Ciereszko, I. Regulatory roles of sugars in plant growth and development. Acta Soc. Bot. Pol. 2018, 87, 3583. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiol. Plant. 2021, 174, 13546. [Google Scholar] [CrossRef] [PubMed]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruan, Y.L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef]
- Hartig, K.; Beck, E. Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol. 2006, 8, 389–396. [Google Scholar] [CrossRef]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thevenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 2004, 16, 1191–1205. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Buer, C.S.; Kordbacheh, F.; Truong, T.T.; Hocart, C.H.; Djordjevic, M.A. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 2013, 238, 171–189. [Google Scholar] [CrossRef]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Jacobs, M.; Rubery, P.H. Naturally occurring auxin transport regulators. Science 1988, 241, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.S.; Hoogner, K.R.; Peer, W.A.; Taiz, L. Identification, purification, and molecular cloning of N-1-naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol. 2002, 128, 935–950. [Google Scholar] [CrossRef]
- Gayomba, S.R.; Muday, G.K. Flavonols regulate root hair development by modulating accumulation of reactive oxygen species in the root epidermis. Development 2020, 147, dev185819. [Google Scholar] [CrossRef]
- Maloney, G.S.; DiNapoli, K.T.; Muday, G.K. The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development. Plant Physiol. 2014, 166, 614–631. [Google Scholar] [CrossRef] [PubMed]
- Konings, H. Gravitropism of roots: An evaluation of progress during the last three decades. Acta Bot. Neerl. 1995, 44, 195–223. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.; Shukla, L.I. Recent advances in auxin biosynthesis and homeostasis. 3 Biotech 2023, 13, 290. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Saniewski, M.; Dziurka, M.; Kowalska, U.; Góraj-Koniarska, J.; Ueda, J.; Miyamoto, K. Formation of the secondary abscission zone induced by the interaction of methyl jasmonate and auxin in Bryophyllum calycinum: Relevance to auxin status and histology. Int. J. Mol. Sci. 2020, 21, 2784. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Changes in Polar Metabolites during Seed Germination and Early Seedling Development of Pea, Cucumber, and Wheat. Agriculture 2023, 13, 2278. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef] [PubMed]
- Lahuta, L.B.; Górecki, R.J. Raffinose in seedlings of winter vetch (Vicia villosa Roth.) under osmotic stress and followed by recovery. Acta Physiol. Plant. 2011, 33, 725–733. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Horbowicz, M. Effect of elicitation with iron chelate and sodium metasili-cate on phenolic compounds in legume sprouts. Molecules 2021, 26, 1345. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef]

| Analyzed Compound | 7-Day Treatment Period | 17-Day Treatment Period | ||||
|---|---|---|---|---|---|---|
| Control, Water | NPA, 5 mg/L | NPA, 10 mg/L | Control, Water | NPA, 5 mg/L | NPA, 10 mg/L | |
| Succinate | 0.43 ± 0.05 a* | 0.43 ± 0.05 a | 0.42 ± 0.01 a | 0.24 ± 0.01 b | 0.26 ± 0.03 b | 0.24 ± 0.03 b |
| Fumarate | 0.12 ± 0.03 a | 0.13 ± 0.05 a | 0.16 ± 0.03 a | 0.06 ± 0.01 a | 0.10 ± 0.01 a | 0.08 ± 0.02 a |
| Malate | 33.07 ± 4.46 a | 34.65 ± 3.58 a | 33.81 ± 0.25 a | 21.05 ± 0.95 b | 22.64 ± 0.72 b | 22.09 ± 0.50 b |
| Citrate | 10.81 ± 2.55 a | 7.99 ± 2.15 a | 9.77 ± 0.34 a | 7.49 ± 0.67 a | 7.34 ± 0.80 a | 8.68 ± 4.05 a |
| Total acids | 44.4 3± 6.95 ab | 43.20 ± 4.47 ab | 44.16 ± 0.56 a | 28.84 ± 0.80 c | 30.33 ± 1.45 bc | 31.09 ± 3.62 bc |
| Phosphate | 4.69 ± 0.69 a | 4.94 ± 0.53 a | 4.70 ± 0.26 a | 3.96 ± 0.17 a | 4.56 ± 0.16 a | 4.23 ± 0.15 a |
| Valine | 0.19 ± 0.07 a | 0.21 ± 0.09 a | 0.16 ± 0.10 a | 0.13 ± 0.01 a | 0.07 ± 0.02 a | 0.07 ± 0.02 a |
| Alanine | 1.33 ± 0.19 a | 1.32 ± 0.13 a | 1.20 ± 0.09 a | 0.73 ± 0.05 b | 0.90 ± 0.01 b | 0.85 ± 0.31 ab |
| Proline | 1.09 ± 0.05 a | 1.29 ± 0.27 ab | 1.22 ± 0.13 ab | 0.72 ± 0.09 b | 0.88 ± 0.01 b | 0.64 ± 0.23 ab |
| Serine | 0.80 ± 0.14 a | 0.73 ± 0.14 a | 0.70 ± 0.09 a | 0.44 ± 0.04 a | 0.46 ± 0.05 a | 0.39 ± 0.09 a |
| Aspartate | 0.51 ± 0.07 a | 0.44 ± 0.05 a | 0.37 ± 0.05 ab | 0.29 ± 0.03 ab | 0.26 ± 0.01 b | 0.28 ± 0.01 b |
| Glutamate | 0.49 ± 0.13 ab | 0.88 ± 0.11 a | 0.67 ± 0.01 a | 0.31 ± 0.02 b | 0.57 ± 0.04 ab | 0.44 ± 0.13 ab |
| Asparagine | 6.67 ± 1.14 ab | 7.83 ± 1.49 ab | 7.41 ± 0.44 a | 4.54 ± 0.29 b | 5.34 ± 0.73 ab | 4.56 ± 1.31 ab |
| iso-Leucine | 0.28 ± 0.05 ab | 0.32 ± 0.05 ab | 0.27 ± 0.01 a | 0.17 ± 0.01 b | 0.22 ± 0.03 ab | 0.19 ± 0.05 ab |
| OH-Proline | 0.55 ± 0.19 abc | 0.68 ± 0.09 a | 0.48 ± 0.08 ab | 0.34 ± 0.04 b | 0.21 ± 0.02 b | 0.10 ± 0.01 c |
| GABA | 0.24 ± 0.04 a | 0.29 ± 0.01 a | 0.24 ± 0.02 a | 0.25 ± 0.03 a | 0.33 ± 0.01 a | 0.33 ± 0.09 a |
| Glutamine | 0.05± 0.02 a | 0.10 ± 0.03 a | 0.11 ± 0.02 a | 0.04 ± 0.01 a | 0.10 ± 0.02 a | 0.09 ± 0.04 a |
| Total amino acids | 12.19 ± 1.92 ab | 14.10 ± 2.13 ab | 12.84 ± 0.82 a | 7.98 ± 0.55 b | 9.34 ± 0.89 ab | 7.93 ± 1.79 ab |
| Analyzed Carbohydrate | 7-Day Treatment Period | 17-Day Treatment Period | ||||
|---|---|---|---|---|---|---|
| Control, Water | NPA, 5 mg/L | NPA, 10 mg/L | Control, Water | NPA, 5 mg/L | NPA, 10 mg/L | |
| Fructose | 50.12 ± 2.86 c* | 36.67 ± 1.34 d | 39.28 ± 2.42 cd | 80.75 ± 2.14 a | 84.24 ± 2.42 a | 70.83 ± 1.51 b |
| Glucose | 68.61 ± 2.29 b | 62.04 ± 9.13 b | 60.08 ± 4.32 b | 91.49 ± 2.72 a | 98.32 ± 2.56 a | 107.0 ± 8.34 a |
| myo-Inositol | 9.79 ± 0.61 a | 9.27 ± 0.60 a | 9.65 ± 0.72 a | 4.58 ± 0.20 b | 5.15 ± 0.18 b | 5.38 ± 0.22 b |
| Sucrose | 49.89± 3.94 c | 57.59 ± 4.02 c | 54.93 ± 3.98 c | 60.36 ± 0.84 c | 73.31 ± 2.03 b | 82.91 ± 1.51 a |
| Unk 1 | 3.41 ± 0.19 ab | 2.80 ± 0.11 b | 2.85 ± 0.17 b | 3.49 ± 0.03 ab | 4.00 ± 0.24 a | 4.21 ± 0.62 a |
| Unk 2 (GF2) | 45.54 ± 3.28 abc | 49.97 ± 3.72 a | 50.27 ± 4.52 abc | 38.71 ± 0.46 c | 44.72 ± 1.57 b | 55.58 ± 1.94 a |
| Unk 3 (GF3) | 16.53 ± 1.47 bc | 20.68 ± 2.00 ab | 21.18 ± 2.25 ab | 14.34 ± 0.14 c | 17.62 ± 0.64 b | 22.11 ± 0.63 a |
| Unk 4 (GF3) | 20.30 ± 1.68 a | 20.58 ± 2.09 a | 21.19 ± 2.17 a | 11.81 ± 0.20 c | 11.72 ± 0.49 c | 14.54 ± 0.41 b |
| Unk 5 (GF4) | 5.84 ± 0.69 ab | 8.49 ± 1.07 a | 8.84 ± 1.17 a | 4.76 ± 0.06 b | 5.85 ± 0.23 a | 7.10 ± 0.10 a |
| Unk 6 (GF4) | 0.79 ± 0.26 b | 1.55 ± 0.24 ab | 1.50 ± 0.26 ab | 1.24 ± 0.03 b | 2.26 ± 0.15 a | 2.56 ± 0.05 a |
| Unk 7 (GF4) | 14.88 ± 1.09 ab | 17.28 ± 1.98 a | 18.75 ± 2.42 a | 9.13 ± 0.13 c | 9.76 ± 0.33 c | 11.98 ± 0.19 b |
| Total unk 1–7 | 107.3 ± 8.01 a | 121.4 ± 10.9 a | 124.6 ± 12.6 a | 83.47 ± 0.73 b | 95.9 ± 3.6 ab | 118.1 ± 3.0 a |
| Total carbohydrates | 285.7 ± 16.4 b | 286.9 ± 24.6 b | 288.5 ± 23.5 b | 320.6 ± 4.1 b | 356.9 ± 10.7 ab | 384.2 ± 12.0 a |
| Acid Forms | Control, Water | NPA, 5 mg/L | NPA, 10 mg/L |
|---|---|---|---|
| Ferulic acid (3-methoxy-4-hydroxycinnamic acid) | |||
| Free | 10.75 ± 0.02 a* | 6.68 ± 0.46 b | 5.26 ± 0.54 b |
| Esters | 5.79 ± 0.05 a | 3.28 ± 0.15 c | 4.07 ± 0.09 b |
| Glycosides | 3.50 ± 0.52 a | 0.43 ± 0.02 b | 0.34 ± 0.11 b |
| Total | 20.04 ± 0.59 a | 10.39 ± 0.63 b | 9.67 ± 0.74 b |
| Sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) | |||
| Free | tr** | tr | tr |
| Esters | 0.24 ± 0.04 a | 0.17 ± 0.03 a | 0.09 ± 0.02 a |
| Glycosides | tr | tr | tr |
| Total | 0.24 ± 0.04 a | 0.17 ± 0.03 a | 0.09 ± 0.02 a |
| p-Coumaric acid (trans-4-hydroxycinnamic acid) | |||
| Free | 0.36 ± 0.01 a | 0.13 ± 0.02 b | 0.11 ± 0.02 b |
| Esters | 1.75 ± 0.04 a | 0.92 ± 0.04 b | 0.92 ± 0.01 b |
| Glycosides | 0.38 ± 0.01 a | 0.03 ± 0.01 b | 0.03 ± 0.01 b |
| Total | 2.49 ± 0.05 a | 1.08 ± 0.07 b | 1.07 ± 0.03 b |
| Protocatechuic acid (3,4-dihydroxybenzoic acid) | |||
| Free | 0.09 ± 0.05 a | 0.04 ± 0.03 a | 0.08 ± 0.01 a |
| Esters | 0.04 ± 0.01a | 0.03 ± 0.00 a | 0.03 ± 0.00 a |
| Glycosides | 0.03 ± 0.00 a | 0.04 ± 0.01 a | 0.03 ± 0.00 a |
| Total | 0.16 ± 0.05 a | 0.11 ± 0.03 a | 0.14 ± 0.01a |
| Ellagic acid | |||
| Free | 1.16 ± 0.17 a | 1.00 ± 0.35 a | 0.76 ± 0.19 a |
| Esters | 0.26 ± 0.02 a | 0.17 ± 0.01 a | 0.15 ± 0.01 a |
| Glycosides | 0.59 ± 0.07 a | 0.61 ± 0.12 a | 0.48 ± 0.06 a |
| Total | 2.01 ± 0.27 a | 1.77 ± 0.47 a | 1.39 ± 0.25 a |
| Caffeic acid | |||
| Free | 38.85 ± 1.39 c | 54.41 ± 0.22 a | 49.50 ± 1.06 b |
| Esters | 3.56 ± 0.03 a | 2.74 ± 0.13 b | 2.04 ± 0.23 b |
| Glycosides | 6.72 ± 1.00 a | 1.03 ± 0.22 b | 1.29 ± 0.13 b |
| Total | 49.12 ± 2.42 b | 58.19 ± 0.87 a | 52.83 ± 1.41b |
| Caftaric acid (2-caffeoyl-L-tartaric acid) | |||
| Free | 42.17 ± 1.14 a | 30.32 ± 1.61 b | 37.48 ± 4.83 ab |
| Esters | 3.53 ± 0.91 a | 2.58 ± 0.25 a | 2.73 ± 0.11 a |
| Glycosides | tr | tr | tr |
| Total | 45.69 ± 2.06 a | 32.90 ± 1.86 b | 40.21 ± 4.94 ab |
| Chicoric acid (2,3-dicaffeoyl-L-tartaric acid) | |||
| Free | 0.09 ± 0.02 a | 0.03 ± 0.01 a | 0.17 ± 0.02 a |
| Esters | tr | tr | tr |
| Glycosides | 0.01 ± 0.00 b | 0.12 ± 0.01 a | 0.04 ± 0.02 b |
| Total | 0.10 ± 0.02 a | 0.15 ± 0.01 a | 0.20 ± 0.04 a |
| Flavonoid Forms | Control, Water | NPA, 5 mg/L | NPA, 10 mg/L |
|---|---|---|---|
| Quercetin | |||
| Free | tr | 0.10±0.01 | tr |
| Esters | tr** | tr | tr |
| Glycosides | 0.05 ± 0.01 a* | 0.03 ± 0.02 a | 0.02 ± 0.02 a |
| Total | 0.05 ± 0.01a | 0.13 ± 0.03 a | 0.02 ± 0.02 a |
| Apigenin | |||
| Free | 1.60 ± 0.08 a | 0.67 ± 0.06 b | 0.41 ± 0.01 c |
| Esters | 0.07 ± 0.01 | tr | tr |
| Glycosides | 2.01 ± 0.04 a | 0.14 ± 0.02 b | 0.08 ± 0.01 b |
| Total | 3.67 ± 0.13 a | 0.81 ± 0.06 b | 0.49 ± 0.01 c |
| Kaempferol | |||
| Free | tr | tr | tr |
| Esters | tr | tr | tr |
| Glycosides | tr | tr | tr |
| Total | tr | tr | tr |
| Luteolin | |||
| Free | 0.03 ± 0.01 | tr | tr |
| Esters | tr | tr | tr |
| Glycosides | 0.16 ± 0.02 a | 0.06 ± 0.01 b | 0.05 ± 0.01 b |
| Total | 0.19 ± 0.03 a | 0.06 ± 0.01 b | 0.05 ± 0.01 b |
| Naringenin | |||
| Free | 0.06 ± 0.02 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a |
| Esters | tr | tr | tr |
| Glycosides | 0.19 ± 0.05 | tr | tr |
| Total | 0.25 ± 0.06 a | 0.03 ± 0.01 b | 0.02 ± 0.01 b |
| Catechin | |||
| Free | 42.85 ± 5.56 a | 28.05 ± 4.58 a | 32.54 ± 0.07 a |
| Esters | 21.85 ± 3.06 a | 12.23 ± 1.64 a | 13.69 ± 2.93 a |
| Glycosides | 2.58 ± 1.11 b | 5.88 ± 0.31 a | 6.92 ± 0.33 a |
| Total | 67.28 ± 9.73 a | 46.16 ± 6.53 a | 53.16 ± 3.32 a |
| Anthocyanin | Control, Water | NPA, 5 mg/L | NPA, 10 mg/L |
|---|---|---|---|
| Cyanidin | tr** | tr | tr |
| Cyanidin 3-glucoside | 0.10 ± 0.02 a* | 0.11 ± 0.08 a | 0.15 ± 0.07 a |
| Cyanidin diglucoside | 0.16 ± 0.01 a | 0.13 ± 0.01 a | 0.14 ± 0.01 a |
| Peonidin diglucoside | tr | tr | tr |
| Peonidin 3-glucoside | 0.40 ± 0.03 a | 0.18 ± 0.01 b | 0.13 ± 0.01 b |
| Delphinidin diglucoside | 0.38 ± 0.02 a | 0.22 ± 0.01 b | 0.19 ± 0.02 b |
| Delphinidin 3-glucoside | 0.05 ± 0.01 a | 0.04 ± 0.01 a | 0.03 ± 0.01 a |
| Pelargonidin | tr | tr | tr |
| Pelargonidin 3-glucoside | 1.43 ± 0.03 a | 0.61 ± 0.04 b | 0.61 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasek-Ciołakowska, A.; Machlańska, A.; Wiczkowski, W.; Szawara-Nowak, D.; Lahuta, L.B.; Góraj-Koniarska, J.; Miyamoto, K.; Ueda, J.; Saniewski, M.; Horbowicz, M. Effects of 1-N-Naphthylphthalamic Acid on Root and Leaf Development of Muscari armeniacum and the Related Metabolic and Physiological Features. Int. J. Mol. Sci. 2025, 26, 6431. https://doi.org/10.3390/ijms26136431
Marasek-Ciołakowska A, Machlańska A, Wiczkowski W, Szawara-Nowak D, Lahuta LB, Góraj-Koniarska J, Miyamoto K, Ueda J, Saniewski M, Horbowicz M. Effects of 1-N-Naphthylphthalamic Acid on Root and Leaf Development of Muscari armeniacum and the Related Metabolic and Physiological Features. International Journal of Molecular Sciences. 2025; 26(13):6431. https://doi.org/10.3390/ijms26136431
Chicago/Turabian StyleMarasek-Ciołakowska, Agnieszka, Aleksandra Machlańska, Wiesław Wiczkowski, Dorota Szawara-Nowak, Lesław B. Lahuta, Justyna Góraj-Koniarska, Kensuke Miyamoto, Junichi Ueda, Marian Saniewski, and Marcin Horbowicz. 2025. "Effects of 1-N-Naphthylphthalamic Acid on Root and Leaf Development of Muscari armeniacum and the Related Metabolic and Physiological Features" International Journal of Molecular Sciences 26, no. 13: 6431. https://doi.org/10.3390/ijms26136431
APA StyleMarasek-Ciołakowska, A., Machlańska, A., Wiczkowski, W., Szawara-Nowak, D., Lahuta, L. B., Góraj-Koniarska, J., Miyamoto, K., Ueda, J., Saniewski, M., & Horbowicz, M. (2025). Effects of 1-N-Naphthylphthalamic Acid on Root and Leaf Development of Muscari armeniacum and the Related Metabolic and Physiological Features. International Journal of Molecular Sciences, 26(13), 6431. https://doi.org/10.3390/ijms26136431








