Low-Molecular-Weight Collagen Peptide Improves Skin Dehydration and Barrier Dysfunction in Human Dermal Fibrosis Cells and UVB-Exposed SKH-1 Hairless Mice
Abstract
1. Introduction
2. Results
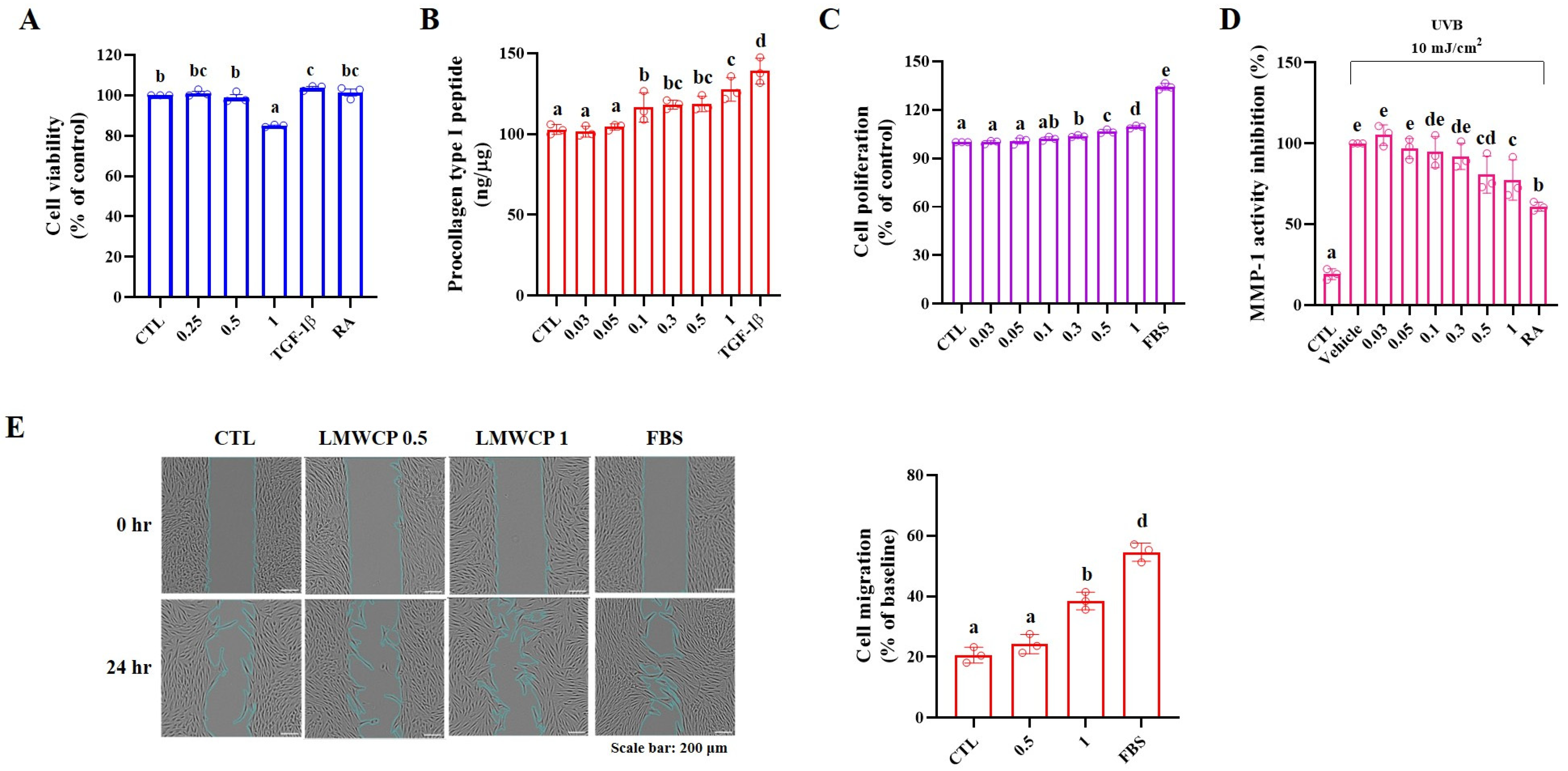
2.1. Cell Viability, Procollagen Type I Peptide Level and MMP-1 Activity in HDFs
2.2. Cell Migration in HDFs
2.3. LMWCP Improved the Condition of Skin on UVB-Exposed SKH-1 Hairless Mice
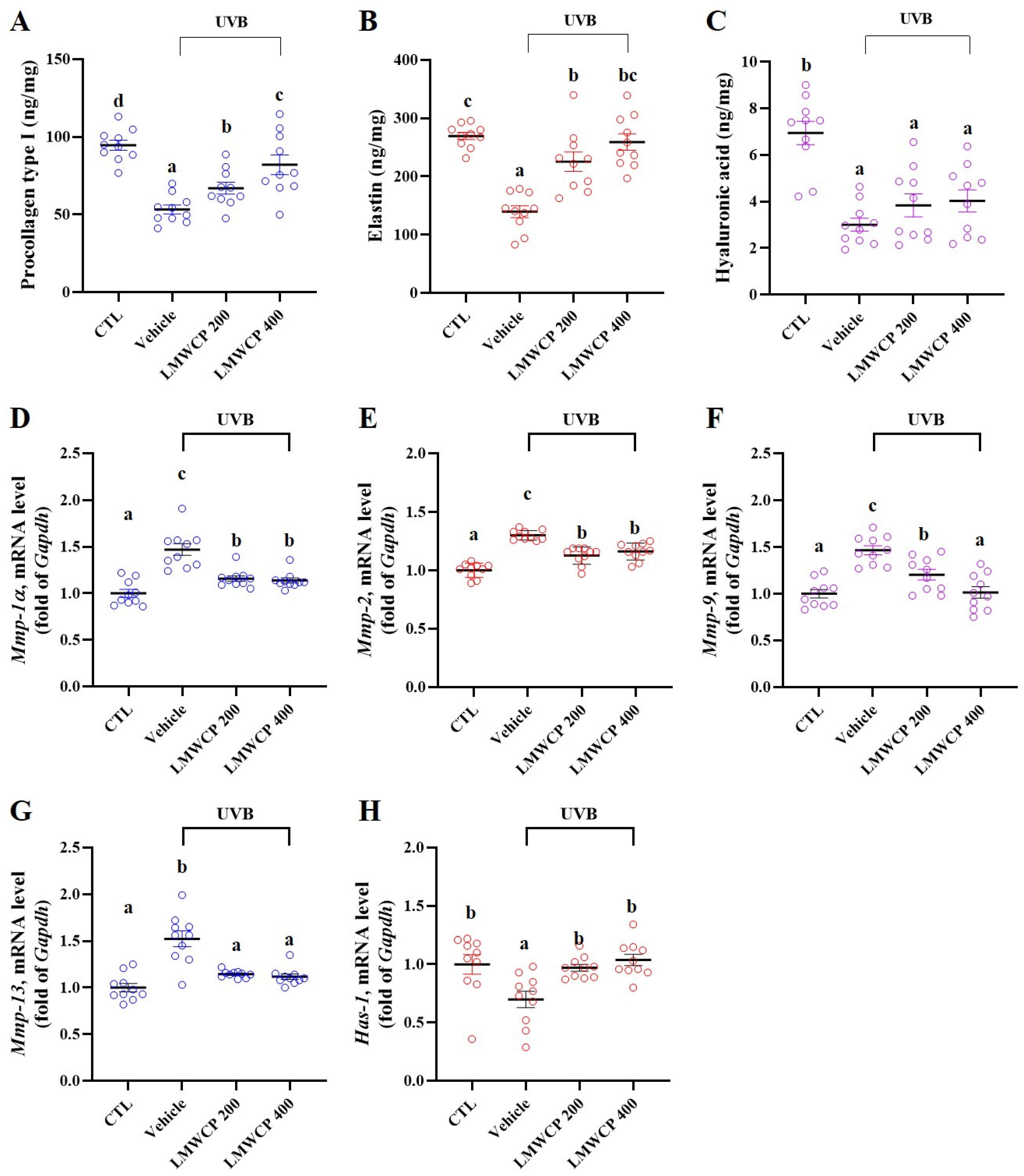
2.4. LMWCP Recovered Skin Barrier Function and Structure in SKH-1 Hairless Mice
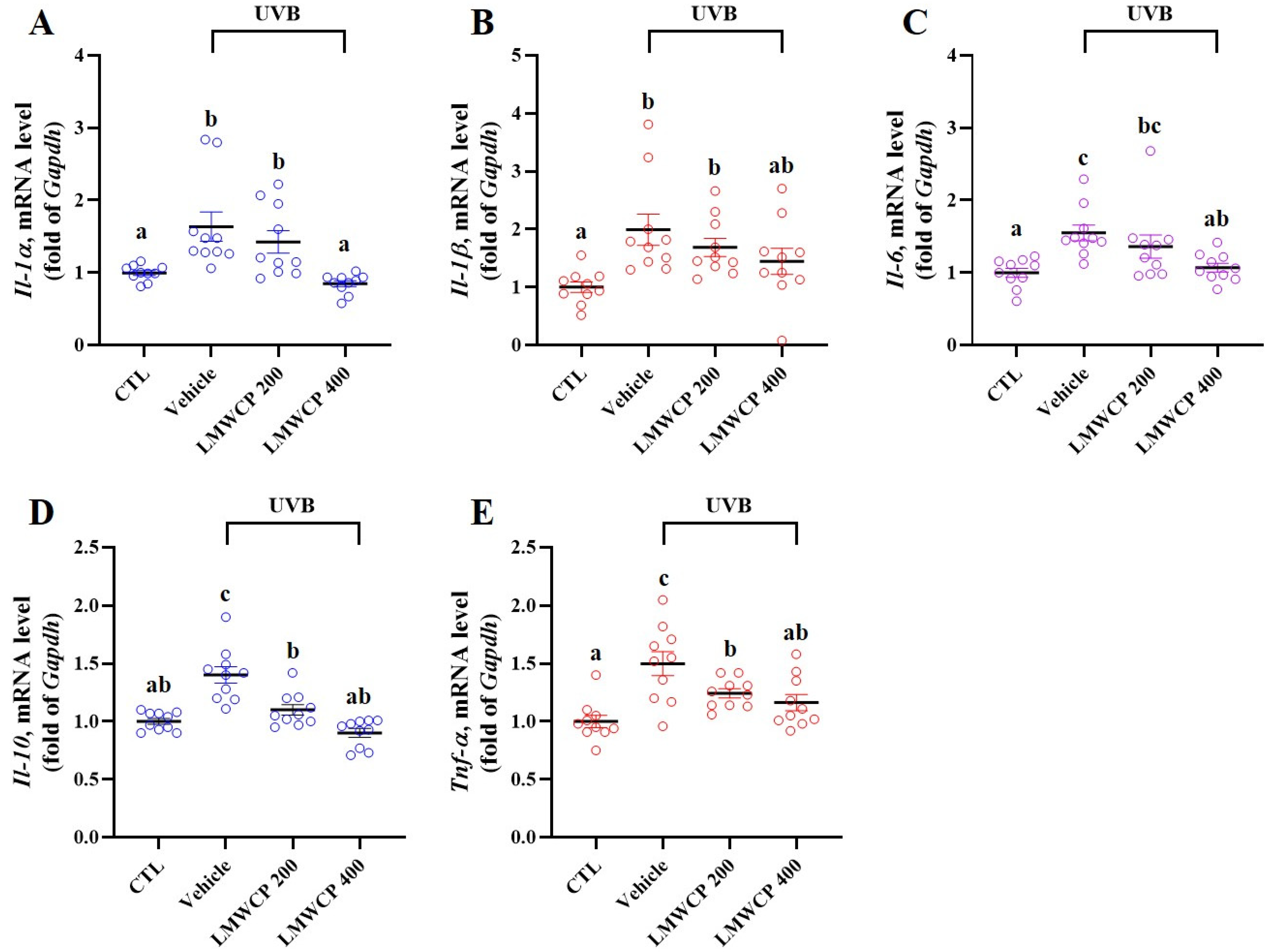
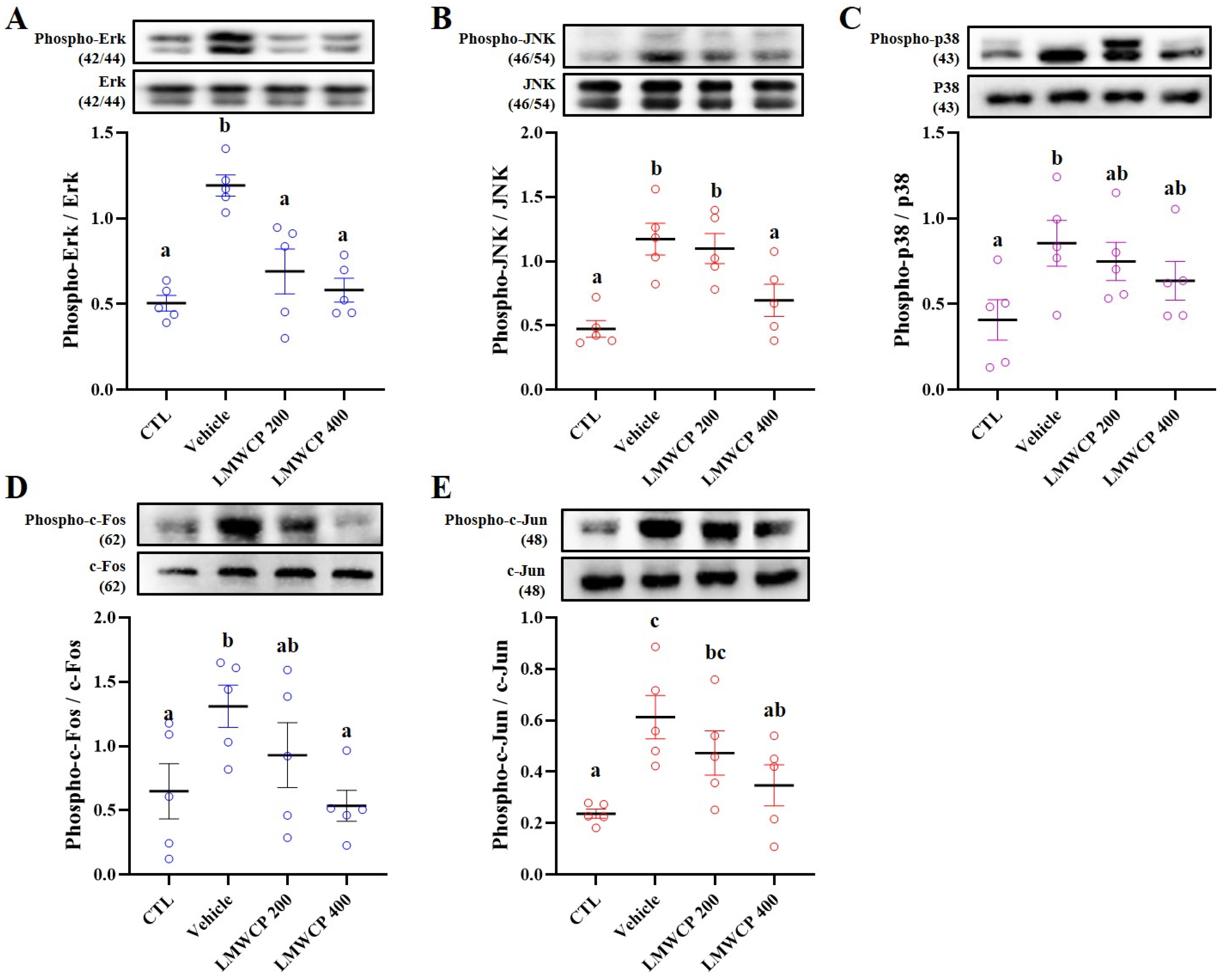
2.5. LMWCP Suppresses UVB-Induced Inflammation in SKH-1 Hairless Mice
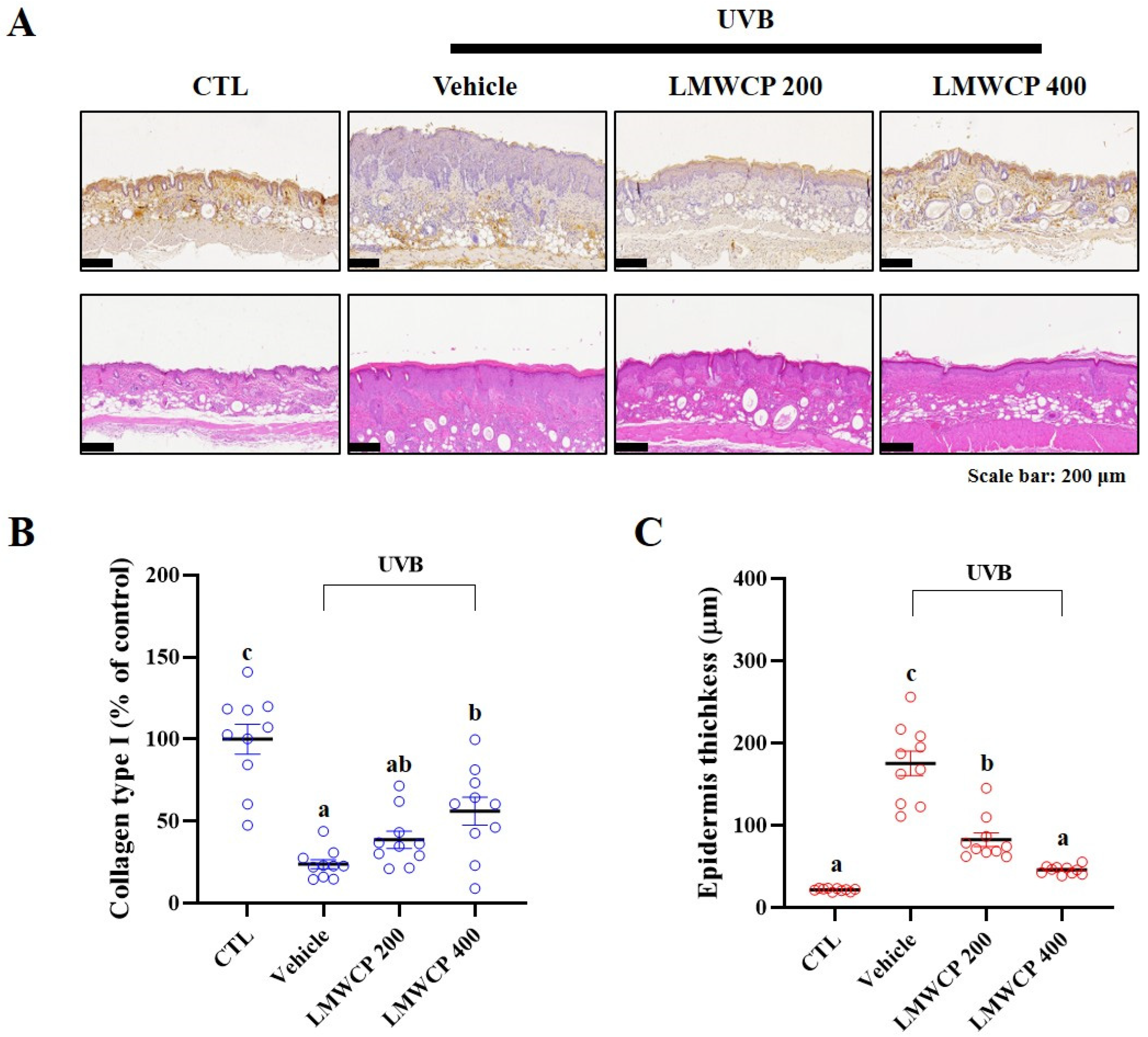
2.6. LMWCPs Improve UVB-Induced Epidermal Structural Changes and Collagen Enhancement in SKH-1 Hairless Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Low-Molecular-Weight Collagen Peptides (LMWCPs)
4.2. Cell Culture and Cell Viability on Human Derma Fibroblasts Cells (HDFs)
4.3. Collagen Production and MMP-1 Activity Inhibition in HDFs
4.4. Proliferation and Migration of HDFs
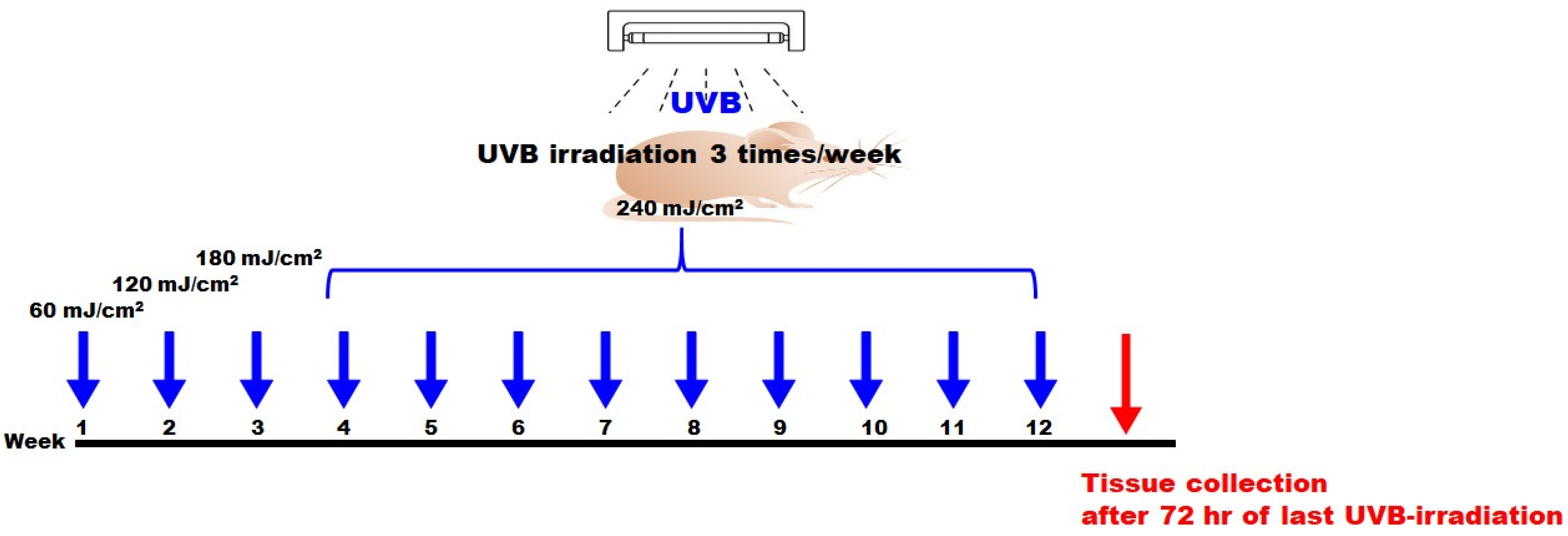
4.5. UVB-Induced Photodamage and Skin Monitoring
4.6. Skin Monitoring on SKH-1 Hairless Mice
4.7. ELISA and Western Blot
4.8. mRNA Expression
4.9. H&E Stain and Immunohistochemistry
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Archer, C.B. Functions of the Skin. In Rook’s Textbook of Dermatology, 7th ed.; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Blackwell Science: Malden, MA, USA, 2004; Volume 1, pp. 4.1–4.12. [Google Scholar] [CrossRef]
- Geerligs, M. Skin Layer Mechanics; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2010. [Google Scholar]
- Wysocki, A.B. Skin anatomy, physiology, and pathophysiology. Nurs. Clin. N. Am. 1999, 34, 777–797. [Google Scholar] [CrossRef]
- Lloyd, D.H.; Patel, A. Structure and function of the skin. In BSAVA Manual of Canine and Feline Dermatology; BSAVA Library: Gloucester, UK, 2012; pp. 1–11. [Google Scholar]
- Kligman, A.M.J.E.D. What is the ‘true’ function of skin? Exp. Dermatol. 2002, 11, 159. [Google Scholar]
- Yadav, N.; Parveen, S.; Chakravarty, S.; Banerjee, M.J.S.A.; Exposure, C.A.U.-R. Skin Anatomy and Morphology; Springer: Singapore, 2019; pp. 1–10. [Google Scholar]
- Vilela, F.M.; Oliveira, F.M.; Vicentini, F.T.; Casagrande, R.; Verri, W.A., Jr.; Cunha, T.M.; Fonseca, M.J. Commercial sunscreen formulations: UVB irradiation stability and effect on UVB irradiation-induced skin oxidative stress and inflammation. J. Photochem. Photobiol. B Biol. 2016, 163, 413–420. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chander, R.; Jetten, A.M.; Slominski, A.T. Neuro–immuno–endocrinology of the skin: How environment regulates body homeostasis. Nat. Rev. Endocrinol. 2025, 1–15. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Prabhakaran, M.P.; Sireesha, M.; Ramakrishna, S. Collagen in human tissues: Structure, function, and biomedical implications from a tissue engineering perspective. In Polymer Composites–Polyolefin Fractionation–Polymeric Peptidomimetics–Collagens; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–206. [Google Scholar]
- Geng, R.; Kang, S.-G.; Huang, K.; Tong, T.J.N. Boosting the photoaged skin: The potential role of dietary components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.Q.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.M.; Zhang, H.; Liu, Z. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. Int. J. Clin. Dermatol. Res. 2019, 7, 209–240. [Google Scholar]
- Yang, D.; Liu, Q.; Xu, Q.; Zheng, L.; Zhang, S.; Lu, S.; Xiao, G.; Zhao, M. Effects of collagen hydrolysates on UV-induced photoaging mice: Gly-Pro-Hyp as a potent anti-photoaging peptide. Food Funct. 2024, 15, 3008–3022. [Google Scholar] [CrossRef]
- Song, B.; Liu, D.; Liu, T.C.; Li, K.; Wang, S.; Liu, J.; Regenstein, J.M.; Wu, Y.; Zhou, P. The combined effect of commercial tilapia collagen peptides and antioxidants against UV-induced skin photoaging in mice. Food Funct. 2023, 14, 5936–5948. [Google Scholar] [CrossRef]
- Woo, H.; Jeong, G.A.; Choi, H.; Lee, C.J. Characterization of low-molecular-weight collagen from Korean native chicken feet hydrolyzed using alcalase. J. Microbiol. Biotechnol. 2023, 33, 656. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Lee, Y.I.; Lee, J.; Choi, S.; Kim, I.A.; Suk, J.; Jung, I.; Baeg, C.; Kim, J.; Oh, D. Low-molecular-weight collagen peptides supplement promotes a healthy skin: A randomized, double-blinded, placebo-controlled study. J. Cosmet. Dermatol. 2024, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Naughton, R.J.; Clifford, T.; Harper, L.D.; Corr, L. The effects of collagen peptide supplementation on body composition, collagen synthesis, and recovery from joint injury and exercise: A systematic review. Amino Acids 2021, 53, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Cha, A.; Kim, G.J.; Jang, Y.; Lee, J.-Y.; Apostolidis, E.; Kim, T.Y.; Kwon, Y.-I. Comparing Molecular Weight Dependent Absorption Rates of Collagen in Oral Mucosal and Epidermis/dermis Tissue Models. J. Food Hyg. Saf. 2024, 39, 299–303. [Google Scholar] [CrossRef]
- Chae, M.; Bae, I.-H.; Lim, S.; Jung, K.; Roh, J.; Kim, W. AP collagen peptides prevent cortisol-induced decrease of collagen type I in human dermal fibroblasts. Int. J. Mol. Sci. 2021, 22, 4788. [Google Scholar] [CrossRef]
- Zhao, T.; Su, G.; Zhang, L.; Chen, J.; Zhang, Y.; Liu, W.; Zhao, M.; Zhang, J.; Huang, Q. A comprehensive review of specific activity and intrinsic connections of food-derived bioactive peptides for human health. Food Front. 2024, 5, 1145–1165. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Liu, S.-X. Pathogenesis of photoaging in human dermal fibroblasts. Int. J. Dermatol. Venereol. 2020, 3, 37–42. [Google Scholar] [CrossRef]
- Varani, J.; Schuger, L.; Dame, M.K.; Leonard, C.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J. Investig. Dermatol. 2004, 122, 1471–1479. [Google Scholar] [CrossRef]
- Greenberg, J.H.; Seppä, S.; Seppä, H.; Hewitt, A.T. Role of collagen and fibronectin in neural crest cell adhesion and migration. Dev. Biol. 1981, 87, 259–266. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, W.; Zheng, Y.; Liu, C.; Gong, Z.; Lu, C.; Lai, W.; Maibach, H.I. Ultraviolet A-induced cathepsin K expression is mediated via MAPK/AP-1 pathway in human dermal fibroblasts. PLoS ONE 2014, 9, e102732. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.K.; Bae, S.; Kim, H.; Park, Y.; Chu, N.K.; Kim, S.G.; Kim, H.-R.; Hwang, Y.-i.; Kang, J.S. The anti-inflammatory effect of alloferon on UVB-induced skin inflammation through the down-regulation of pro-inflammatory cytokines. Immunol. Lett. 2013, 149, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.-H.; Kim, S.Y. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxidative Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Alhasaniah, A.; Sherratt, M.J.; O’Neill, C.A. The impact of ultraviolet radiation on barrier function in human skin: Molecular mechanisms and topical therapeutics. Curr. Med. Chem. 2018, 25, 5503–5511. [Google Scholar] [CrossRef]
- Lee, H.R.; Hong, S.-M.; Cho, K.; Kim, S.H.; Ko, E.; Lee, E.; Kim, H.J.; Jeon, S.Y.; Do, S.G.; Kim, S.Y. Potential Role of Dietary Salmon Nasal Cartilage Proteoglycan on UVB-Induced Photoaged Skin. Biomol. Ther. 2024, 32, 249. [Google Scholar] [CrossRef]
- Choi, F.D.; Sung, C.T.; Juhasz, M.; Mesinkovsk, N.A. Oral collagen supplementation: A systematic review of dermatological applications. J. Drugs Dermatol. 2019, 18, 9–16. [Google Scholar]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A scoping review on the effects of carotenoids and flavonoids on skin damage due to ultraviolet radiation. Nutrients 2022, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gui, M.; Tian, X.; Xin, X.; Wang, T.; Li, J. Effects of UV-B on vitamin C, phenolics, flavonoids and their related enzyme activities in mung bean sprouts (Vigna radiata). Int. J. Food Sci. Technol. 2017, 52, 827–833. [Google Scholar] [CrossRef]
- Watson, R.E.; Gibbs, N.K.; Griffiths, C.E.; Sherratt, M.J. Damage to skin extracellular matrix induced by UV exposure. Antioxid. Redox Signal. 2014, 21, 1063–1077. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on skin and intrinsic aging: Biological, environmental, and therapeutic insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef] [PubMed]
- Holleran, W.; Uchida, Y.; Halkier-Sorensen, L.; Haratake, A.; Hara, M.; Epstein, J.; Elias, P. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol. Photoimmunol. Photomed. 1997, 13, 117–128. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.G.; Jang, Y.A.; Bayazid, A.B.; Ou Lim, B. Fermented black rice and blueberry with Lactobacillus plantarum MG4221 improve UVB-induced skin injury. Food Agric. Immunol. 2021, 32, 499–515. [Google Scholar] [CrossRef]
- Muthusamy, V.; Piva, T.J. The UV response of the skin: A review of the MAPK, NFκB and TNFα signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef]
- Zhang, M.; Hwang, E.; Lin, P.; Gao, W.; Ngo, H.T.; Yi, T.-H. Prunella vulgaris L. exerts a protective effect against extrinsic aging through NF-κB, MAPKs, AP-1, and TGF-β/Smad signaling pathways in UVB-aged normal human dermal fibroblasts. Rejuvenation Res. 2018, 21, 313–322. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Zouboulis, C.C. The human skin as a hormone target and an endocrine gland. HORMONES-ATHENS- 2004, 3, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Shuster, E. Hormonal influences in multiple sclerosis. Adv. Mult. Scler. Exp. Demyelinating Dis. 2008, 267–311. [Google Scholar] [CrossRef]
- Choi, M.S.; Yoo, M.S.; Son, D.J.; Jung, H.Y.; Lee, S.H.; Jung, J.K.; Lee, B.C.; Yun, Y.P.; Pyo, H.B.; Hong, J.T. Increase of collagen synthesis by obovatol through stimulation of the TGF-β signaling and inhibition of matrix metalloproteinase in UVB-irradiated human fibroblast. J. Dermatol. Sci. 2007, 46, 127–137. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef]
- Shastri, M.; Sharma, M.; Sharma, K.; Sharma, A.; Minz, R.W.; Dogra, S.; Chhabra, S. Cutaneous-immuno-neuro-endocrine (CINE) system: A complex enterprise transforming skin into a super organ. Exp. Dermatol. 2024, 33, e15029. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Plonka, P.M.; Szaflarski, J.P.; Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 2018, 159, 1992–2007. [Google Scholar] [CrossRef]
- Owczarzy, A.; Kurasiński, R.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Collagen-structure, properties and application. Eng. Biomater. 2020, 23, 17–23. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Puizina-Ivic, N. Skin aging. Acta Dermatovenerol. Alp. Panon. Adriat. 2008, 17, 47. [Google Scholar]
- Schoenfeld, P. The Collagen Diet: Rejuvenate Skin, Strengthen Joints and Feel Younger by Boosting Collagen Intake and Production; Simon and Schuster: New York, NY, USA, 2018. [Google Scholar]
- Yakaew, S.; Itsarasook, K.; Ngoenkam, J.; Jessadayannamaetha, A.; Viyoch, J.; Ungsurungsie, M. Ethanol extract of Terminalia chebula fruit protects against UVB-induced skin damage. Pharm. Biol. 2016, 54, 2701–2707. [Google Scholar] [CrossRef]
- Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat extract oil (WEO) attenuates UVB-induced photoaging via collagen synthesis in human keratinocytes and hairless mice. Nutrients 2020, 12, 300. [Google Scholar] [CrossRef]
- Imokawa, G. Mechanism of UVB-Induced Wrinkling of the Skin: Paracrine Cytokine Linkage Between Keratinocytes and Fibroblasts Leading to the Stimulation of Elastase; Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2009; pp. 36–43. [Google Scholar]
- Horváth, S.; Komlódi, R.; Perkecz, A.; Pintér, E.; Gyulai, R.; Kemény, Á. Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Sci. Rep. 2019, 9, 3685. [Google Scholar] [CrossRef]
- Neu, S.D.; Strzepa, A.; Martin, D.; Sorci-Thomas, M.G.; Pritchard, K.A., Jr.; Dittel, B.N. Myeloperoxidase inhibition ameliorates plaque psoriasis in mice. Antioxidants 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]

| Group | 0 | 4 | 8 | 12 | |
|---|---|---|---|---|---|
| Skin hydration (A.U.) | CTL | 38.60 ± 0.83 a | 29.53 ± 1.30 b | 31.85 ± 1.57 c | 34.60 ± 1.09 c |
| Vehicle | 37.50 ± 2.11 a | 16.68 ± 1.62 a | 10.35 ± 1.21 a | 10.00 ± 1.07 a | |
| UVB LMWCP 200 | 39.60 ± 1.14 a | 16.33 ± 1.83 a | 14.90 ± 1.75 b | 15.90 ± 0.86 b | |
| UVB LMWCP 400 | 41.20 ± 0.77 a | 19.85 ± 1.60 a | 16.40 ± 1.49 b | 15.80 ± 0.53 b | |
| TEWL (g/m2/h) | CTL | 3.78 ± 0.29 a | 6.84 ± 0.35 a | 6.25 ± 0.41 a | 3.25 ± 0.33 a |
| Vehicle | 3.80 ± 0.46 a | 26.03 ± 1.11 b | 30.60 ± 2.74 c | 31.40 ± 1.93 d | |
| UVB LMWCP 200 | 4.05 ± 0.22 a | 24.07 ± 1.16 b | 14.50 ± 0.92 b | 13.55 ± 0.61 c | |
| UVB LMWCP 400 | 3.30 ± 0.33 a | 23.79 ± 1.83 b | 10.60 ± 0.82 b | 9.80 ± 0.65 b |
| Group | 0 | 4 | 8 | 12 | |
|---|---|---|---|---|---|
| Elasticity (mm) | CTL | 12.23 ± 0.29 b | 12.51 ± 0.18 c | 13.11 ± 0.23 c | 13.29 ± 0.29 d |
| Vehicle | 11.04 ± 0.13 a | 9.65 ± 0.07 a | 8.89 ± 0.13 a | 7.58 ± 0.23 a | |
| UVB LMWCP 200 | 10.99 ± 0.26 a | 10.05 ± 0.14 a | 9.88 ± 0.13 b | 9.83 ± 0.27 b | |
| UVB LMWCP 400 | 11.77 ± 0.20 b | 10.90 ± 0.24 b | 10.31 ± 0.12 b | 10.95 ± 0.36 c | |
| Erythema Score | CTL | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Vehicle | 0.00 ± 0.00 a | 1.70 ± 0.12 c | 2.38 ± 0.14 c | 2.40 ± 0.14 d | |
| UVB LMWCP 200 | 0.00 ± 0.00 a | 1.73 ± 0.12 c | 1.18 ± 0.12 b | 1.65 ± 0.12 c | |
| UVB LMWCP 400 | 0.00 ± 0.00 a | 1.37 ± 0.12 b | 1.08 ± 0.05 b | 1.18 ± 0.09 b | |
| Scaling score | CTL | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Vehicle | 0.00 ± 0.00 a | 1.10 ± 0.07 b | 2.15 ± 0.20 c | 1.43 ± 0.12 c | |
| UVB LMWCP 200 | 0.00 ± 0.00 a | 1.17 ± 0.07 b | 1.45 ± 0.12 b | 1.08 ± 0.04 b | |
| UVB LMWCP 400 | 0.00 ± 0.00 a | 1.03 ± 0.03 b | 1.35 ± 0.15 b | 1.00 ± 0.00 b |
| Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Erythema | Fleshy pink | Minor reddening Across surface | Medium red Across surface | Medium red across surface with dark red patches | Dark red |
| Scaling | No scaling | Slight scaling | Moderate scaling | Marked scaling | Maximal scaling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.; Joo, H.; Kim, M.; Kim, D.-U.; Chung, H.-C.; Kim, J.G. Low-Molecular-Weight Collagen Peptide Improves Skin Dehydration and Barrier Dysfunction in Human Dermal Fibrosis Cells and UVB-Exposed SKH-1 Hairless Mice. Int. J. Mol. Sci. 2025, 26, 6427. https://doi.org/10.3390/ijms26136427
Choi E, Joo H, Kim M, Kim D-U, Chung H-C, Kim JG. Low-Molecular-Weight Collagen Peptide Improves Skin Dehydration and Barrier Dysfunction in Human Dermal Fibrosis Cells and UVB-Exposed SKH-1 Hairless Mice. International Journal of Molecular Sciences. 2025; 26(13):6427. https://doi.org/10.3390/ijms26136427
Chicago/Turabian StyleChoi, Eunjung, Heeyeon Joo, Myunghee Kim, Do-Un Kim, Hee-Chul Chung, and Jae Gon Kim. 2025. "Low-Molecular-Weight Collagen Peptide Improves Skin Dehydration and Barrier Dysfunction in Human Dermal Fibrosis Cells and UVB-Exposed SKH-1 Hairless Mice" International Journal of Molecular Sciences 26, no. 13: 6427. https://doi.org/10.3390/ijms26136427
APA StyleChoi, E., Joo, H., Kim, M., Kim, D.-U., Chung, H.-C., & Kim, J. G. (2025). Low-Molecular-Weight Collagen Peptide Improves Skin Dehydration and Barrier Dysfunction in Human Dermal Fibrosis Cells and UVB-Exposed SKH-1 Hairless Mice. International Journal of Molecular Sciences, 26(13), 6427. https://doi.org/10.3390/ijms26136427








