Abstract
Parkinson’s disease (PD) is considered the second most common neurodegenerative disease worldwide; treating this disease remains quite challenging. Environmental and genetic factors may play a role in the pathophysiology of PD. α-synuclein aggregation, oxidative stress, ferroptosis, mitochondrial failure, neuroinflammation, and gut dysbiosis are among the known risk factors of PD. The pathophysiology of Parkinson’s disease is complicated by the interconnections between these molecular pathways, which also present significant obstacles to treatment development. However, due to its complex mechanism and long latency, PD is difficult to diagnose and detect, which presents a barrier to treatment. In addition, the need to develop new treatments for PD is increased by the fact that the majority of traditional therapeutic methods have major side effects and limited effects. Therefore, a deeper understanding of the fundamental mechanisms underlying PD is required. This review provides a comprehensive analysis of the current landscape of PD pathophysiology, paying particular attention to the molecular processes of PD, as well as the traditional research models, clinical diagnostic standards, documented medication therapeutic approaches, and recently disclosed drug candidates in clinical trials. We also highlighted the herbal-derived components that have recently been identified for their effects in the treatment of PD to provide a review and perspectives for the development of the next generation of drugs and preparations for the treatment of PD.
1. Introduction
Parkinson’s disease (PD) is the second most prevalent neurological illness worldwide, with its prevalence increasing by 74.3% between 1990 and 2016. James Parkinson was the first to describe Parkinson’s disease (PD) as a neurological condition in his 1817 monograph, “an Essay on the Shaking Palsy”. A succession of researchers, starting with Jean-Martin Charcot, has contributed to a thorough explanation of the clinical spectrum and anatomical foundation of PD, including neuropathological alterations of the substantia nigra (SN), Lewy bodies, motor and non-motor symptoms, and dopamine (DA) function. Following these discoveries (Figure 1), extremely effective treatments emerged to successfully manage the symptoms, such as deep brain stimulation and pharmaceutical DA substitution (levodopa therapy). However, none of these therapies can reverse the progression of PD, especially the worsening of treatment-resistant motor and non-motor symptoms that continue to plague the difficult lives of Parkinson’s patients. To provide a summary and outlook for the development of the next class of drugs and therapies for the treatment of PD, we summarized the molecular principles underlying PD and the research models, characterized the clinical features and diagnostic criteria of PD, listed drugs used in clinical practice and on the market, and discussed the available treatments for PD like such as compounds [1,2,3,4].
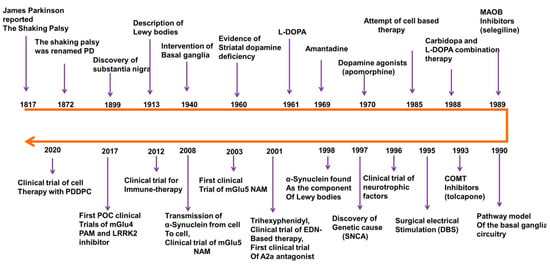
Figure 1.
Basic research and drug development history for PD disease and therapy, modified from Charvin et al., 2018 [5]. A2a adenosine receptor subtype 2a, mGlu metabotropic glutamate receptor, NAM negative allosteric modulator, PAM positive allosteric modulator, EDN embryonic dopamine neuron, PDDPC personalized iPSC-derived dopamine progenitor cell, iPSC induced pluripotent stem cell, DBS deep brain stimulation. This figure was generated by using Microsoft PowerPoint.
2. Epidemiology, Clinical Features, and Diagnostic Criteria
After age 60, the prevalence of PD increases sharply to over 3% among people aged 80. Men are more likely than women to have Parkinson’s disease in most populations. The disparity in frequency between locations and races is probably explained by differences in living conditions and environment. Although dietary practices can modify the prevalence of the disease, environmental pollutants can cause symptoms of PD. Notable examples include reduced risks among smokers and frequent caffeine users. The majority of clinical features of PD are composed of both motor and non-motor symptoms. A range of motor symptoms, including bradykinesia, muscle stiffness, rest tremor, and posture and walking problems, are present in patients with PD. Based on clinical data, PD can be classified into two basic types: tremor-dominant PD and non-tremor-dominant PD. Tremor-dominant PD is often associated with less functional impairment and a slower rate of development than non-tremor-dominant PD [6,7,8,9,10,11,12,13,14,15,16,17]. Constipation, melancholia, olfactory dysfunction, cognitive impairment, and sleep problems include excessive daytime sleepiness and fast eye movement. Non-motor features include pain, fatigue, autonomic dysfunction, and sleep behavior disorders. In the early stages of PD, non-motor symptoms often occur before motor symptoms. Some problems, including psychosis, motor and non-motor fluctuations, dyskinesia, decline in motor functions, and long-term symptomatic medication, will occur as PD progresses. Up to 50% of patients with PD report choking, and up to 80% of patients experience gait freezing and falls after about 17 years of the disease. It is also thought that people who have had PD for at least 20 years are more likely to develop dementia. One of the main pathogenic features of PD is the progressive loss of only a portion of neurons in specific brain regions, such as the SN. In early stages, dopaminergic neurons are lost only in the ventrolateral SN, but, in later stages, this damage spreads.
Additionally, abnormally high amounts of α-synuclein collect in the cytoplasm of some neurons in various parts of the brain. Lewy bodies, a common sign of neuropathology, are produced by aggregated α-synuclein in olfactory neurons, brainstem cholinergic and monoaminergic neurons, and other neurons. As PD progresses, Lewy bodies proliferate and affect not only the limbic and neocortical areas of the brain but also non-dopaminergic neurons in other parts of the brain [18,19,20,21,22,23,24,25,26,27]. Finally, PD causes the degeneration of neurons outside the central nervous system (CNS), such as those in the mesenteric or olfactory bulb. Due to the complexity of disease presentation and the risk of early symptoms of PD, 10% of diagnosed patients were initially classified as having other diseases. The International Parkinson and Movement Disorder Society has developed criteria to improve diagnostic accuracy. These guidelines state that Parkinson’s syndrome is defined as the presence of bradykinesia plus at least one additional cardinal motor sign (4–6-Hz rest tremor or limb rigidity). “Red flags” for alternative diagnoses and excluded clinical features were also mentioned. Clinical diagnosis of PD often relies on genetic testing, diffusion-weighted magnetic resonance imaging (MR-DWI), structural magnetic resonance imaging (MRI), and DA transporter–single-photon emission computed tomography (DATSPECT). Because approximately 90% of people with PD experience hyposmia or anosmia, testing of olfactory function using the UPSIT or Sniffin Stick tests is sometimes part of the initial clinical examination. Advanced magnetic resonance imaging techniques have revealed MRI features that are highly specific for atypical Parkinsonism. These include neuromelanin imaging (NMI), which utilizes the paramagnetic properties of neuromelanin, and quantitative susceptibility mapping (QSM), which determines iron accumulation in the SN. Interestingly, NMI may indicate changes in prodromal PD. Ioflupane I-123 single-photon emission computed tomography (SPECT) is another method used to differentiate PD from clinical symptoms that are not associated with presynaptic nigrostriatal terminal dysfunction [28,29,30,31,32,33,34,35,36]. Despite these advances, there is still room for improvement in one area of clinical diagnosis: the application of genetic testing, which is now only used when a specific hereditary etiology is suspected. But as PD progresses, we also see an increase in the number of genes associated with complex symptoms, including Parkinson’s symptoms. Regular genetic testing may be beneficial in certain situations.
3. Etiology and Pathogenesis of PD
3.1. Genetic and Environmental Factors and Pathogenesis of PD
Both hereditary and environmental variables play a role in the complex etiology of PD. Environmental factors include things like stress, brain damage, physical inactivity, and pesticide exposure. The symptoms of 1-methyl-4-phenyl-1,2,3,6-tetrahydrodropyridine (MPTP) poisoning are almost the same as those of PD, and the main metabolite of MPTP, 1-methyl-4-phenylpyridinium (MPP+), shares structural similarities with paraquat. According to human epidemiological research, living in a rural area and being exposed to pesticides and herbicides are linked to an increased risk of PD [37,38,39,40,41]. However, convincing evidence linking certain chemicals to PD is still required.
3.2. Autosomal-Dominant Genes and PD Pathogenesis
The PARK1/PARK4 gene for α-synuclein expression has been shown to be linked to aberrant pathological aggregation of insoluble α-synuclein fibril in patients with SNCA mutations whose brains exhibited α-synuclein aggregation, manifesting as DA neuron loss and the appearance of Lewy bodies. A mutation in the leucine-rich repeat kinase 2 (LRRK2) gene, also known as PARK8, is the most common genetic cause of Parkinson’s disease. The majority of people with late-onset LRRK2 mutations are over 50 years old. G2019S, R1441C, R1441G, and R1441H are the most prevalent variants of this mutation, which can disrupt a number of physiological functions, such as vesicle transport, cytoskeletal function, protein synthesis, and the lysosomal system, leading to the death and degeneration of DA neurons. PARK13, the HTRA2 serine peptidase 2 (HTRA2) gene, has been shown to be essential for preservation of regular mitochondrial function and is released into the cytoplasm from damaged mitochondria. Under stressful circumstances, it has also been shown to have a neuroprotective effect; PARK13 mutant animals exhibit increased reactive oxygen species (ROS), mitochondrial dysfunction, and PD symptoms [42,43,44,45,46,47]. PINK1-mediated phosphorylation regulates its activity, which is essential for preserving mitochondrial integrity under stressful situations. Degradation of misfolded SNCA is central to PARK13. Endosome proteins from the pre-lysosomal compartment network must be retro-transferred to the trans-Golgi network via the PARK17 gene, which is a component of the reverse transcriptome complex (VPS35). In an endosomal compartment, the cation-independent mannose 6-phosphate receptor (CI-MPR) may attach to VPS35 and become trapped in recycling tubules, blocking its transfer to lysosomes or vacuoles. The PARK18 gene, which encodes the eukaryotic translation initiation factor 4 gamma 1 (EIF4G1), regulates the start of the translation of mRNAs encoding growth, survival, and mitochondrial proteins in response to various stimuli. Two mutations, EIF4G1 p.A502V and EIF4G1 p.R1205H, have been shown to alter the eIF4G1-eIF4E or eIF4G1-eIF3e binding, which is thought to act as the molecular bridge between the mRNA cap-binding complex and the 40S subunit and cause mitochondria-related imbalance [48,49,50,51,52,53,54,55,56].
3.3. Autosomal-Recessive Genes and PD Pathogenesis
The parkin RBR E3 ubiquitin protein ligase gene (PRKN) encodes PARK2, the most common cause of autosomal-recessive early-onset Parkinson’s syndrome. Studies show that up to 50% of PD cases above the age of 25 and up to 7% of patients between the ages of 30 and 35 had the PARK2 mutation. Parkin is an E3 ubiquitin ligase that functions as an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugating enzyme to break down specific proteins in the ubiquitin–proteasome system, which is believed to be a multifunctional neuroprotective agent against a range of toxic injuries, including mitochondrial poisons, and is crucial for the survival of DA neurons. The PTEN-induced putative kinase 1 gene (PINK1) encodes the serine/threonine protein kinase PARK6, which has been shown to interact with parkin to promote selective autophagy in depolarized mitochondria and preserve mitochondrial integrity. After being phosphorylated by PINK1 to activate its E3 ligase activity, parkin is normally recruited to depolarized mitochondria to initiate autophagy and remove the damaged or defective mitochondria. Because it cannot be sent to the inner mitochondrial membrane for breakdown, PINK1 accumulates in the outer mitochondrial membrane of inefficient mitochondria to initiate the removal of damaged mitochondria from the cell. The parkinsonism-associated deglycase gene PARK7, often referred to as DJ-1, protects DA neurons in the model system against damage caused by mutant synuclein, rotenone, 6-hydroxydopamine (6-OHDA), and hydrogen peroxide. PARK9, the ATPase 13A2 gene (ATP13A2), encodes transmembrane endo-/lysosomal related proteins, and the lysosomal signaling lipids phosphatidylic acid and phosphatidylinositol (3,5) biphosphate interact with the N-terminus of ATP13A2, which acts as the catalyst for ATP13A2 action and regulates endo/lysosomal cargo sorting. Most ATP13A2 mutations affect its functional domains, specifically, its transmembrane and E1-E2 adenosine triphosphatase domains. If ATP13A2 is functionally lost, this may result in Zn2+ dysregulation and abnormal cell metabolism, including dysfunctional energy production and decreased lysosomal proteolysis [57,58,59,60,61,62,63,64,65,66,67,68]. Furthermore, it has been shown that ATP13A2 lessens α-synuclein’s neurotoxicity. The F-box protein 7 gene (FBXO7) encodes the PARK15 protein, which is a subunit of the F-box protein that acts as an adapter protein in the SKP1/cullin-1/F-box protein E3 ubiquitin ligase complex. It recognizes and mediates the non-degrading ubiquitination of the translocase of outer mitochondrial membrane 20 and glycogen synthase kinase (GSK-3β). This process regulates mitophagy, mitochondrial motility, mitochondrial membrane potential, mitochondrial bioenergetics, biogenesis, and apoptosis linked to the mitochondria. A malfunction may also reduce complex-I’s activity in the electron transport chain, which would lower ATP levels and mitochondrial membrane potential while increasing cytoplasmic ROS because FBXO7 is stress-responsive [69,70,71,72,73,74,75].
4. Molecular Mechanisms of PD
4.1. Role of α-Synuclein Aggregation in PD Pathology
Numerous molecular and cellular alterations, such as α-synuclein aggregation, abnormal protein handling, excitotoxicity, oxidative stress, apoptosis, and mitochondrial dysfunction, have been connected to neural degeneration. One of the most significant theories explaining the loss of nigrostriatal neurones in Parkinson’s disease is abnormal α-synuclein aggregation. A putative chaperone, α-synuclein, is found in the cytosol, mitochondria, and nucleus and is involved in intracellular trafficking, synaptic vesicle dynamics, and mitochondrial activity. According to some data, the protein may be involved in the brain’s lipid metabolism, which has a role in the pathophysiology of Parkinson’s disease. When soluble α-synuclein monomers combine to form oligomers, which then form small protofibrils and finally giant, insoluble fibrils, α-synuclein itself can become neurotoxic. The accumulation of α-synuclein may be significantly influenced by age-related declines in the brain’s proteolytic defense mechanisms [76,77,78,79,80,81,82,83,84]. In particular, the lysosomal autophagy and ubiquitin–proteasome mechanisms preserve intracellular α-synuclein homeostasis. α-synuclein cleavage is sometimes attributed to extracellular proteases that are not a component of either system. Thus, α-synuclein buildup may be facilitated by disruption of these degradation processes.
4.2. Role of Oxidative Stress (OS) in PD Pathology
One of the main aging processes that directly damage the central nervous system is oxidative stress (OS). Free radicals, or ROS, play a crucial role in apoptosis, gene transcription, host defense, and the regulation of neural plasticity under physiological settings. On the other hand, OS happens when ROS outweighs antioxidant action in cells. In different neurons, including DA-neuronal tissue, cytotoxic chemicals then build up to induce cell death, lipid breakdown, protein collapse, and enzyme failure (Figure 2).
These dysfunctions may be the cause of Alzheimer’s disease (AD), as well as contribute to the pathophysiology of Parkinson’s disease (PD). At the moment, NADPH oxidase (NOX) is thought to be the most significant ROS generator, and it is essential for initiating OS and neurotoxicity. ROS is also produced in large quantities by mitochondria. The majority of ROS is believed to be generated in mitochondria by complexes I and III of the electron transport chain. One electron moving from one oxygen molecule to another oxygen molecule produces the superoxide radical, the primary ROS produced in mitochondria. The superoxide radical may be converted to hydrogen peroxide by superoxide dismutase 2 (MnSOD), which can then be detoxified by the catalase enzyme. However, when metal ions like Fe2+ are present, hydrogen peroxide can change into a highly reactive hydroxyl radical due to the Fenton reaction, which severely oxidizes DNA or lipids. Iron ion homeostasis imbalance is linked to the mechanism of ferroptosis, suggesting a link between ferroptosis and OS. The hydroxyl radicals from the Fenton reaction can oxidize lipids to create lipid peroxides, which can lead to ferroptotic cell death [85,86,87,88,89,90,91,92,93,94,95,96,97,98]. Another biochemical indicator of ferroptosis is glutathione depletion, which exacerbates intracellular OS by encouraging the accumulation of lipid peroxides that cause ferroptosis. Moreover, elevated OS can damage the lysosomal autophagy mechanism and reduce lysosomes, linking OS to the accumulation of α-synuclein. According to a different theory, DA-quinones can be produced by simply oxidizing excess cytosolic DA. Then, α-synuclein may self-assemble as a result of the DA quinine-modified α-synuclein partially inhibiting chaperone-mediated autophagy. In the meantime, intracellular α-synuclein aggregation development raised mitochondrial OS [99,100,101,102].
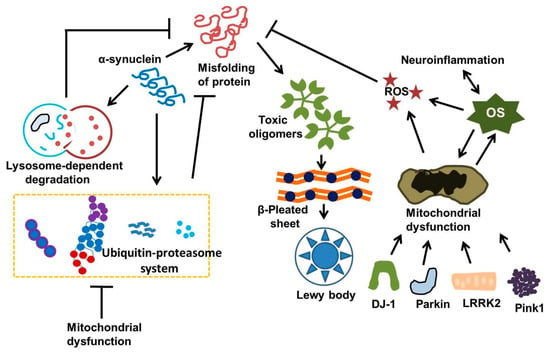
Figure 2.
Intracellular α-synuclein balance is preserved through the ubiquitin–proteasome and lysosomal autophagy pathways. Dysfunction of these degradation systems due to oxidative stress, mitochondrial issues, or neuroinflammation may lead to the buildup of α-synuclein. Additionally, gene mutations such as LRRK2, DJ-1, Parkin, and Pink1 lead to mitochondrial impairment and enhance cell mortality. Ultimately, OS and neuroinflammation seem to be linked. This figure was generated by using Microsoft PowerPoint. This figure was modified from Xu Dong-Chen et al., 2023 [103].
4.3. Role of Ferroptosis in PD Pathology
Ferroptosis, an iron-dependent form of regulated cell death that leads to OS and cell death, is brought on by an abnormal iron metabolism and extreme lipid peroxidation (Figure 3).
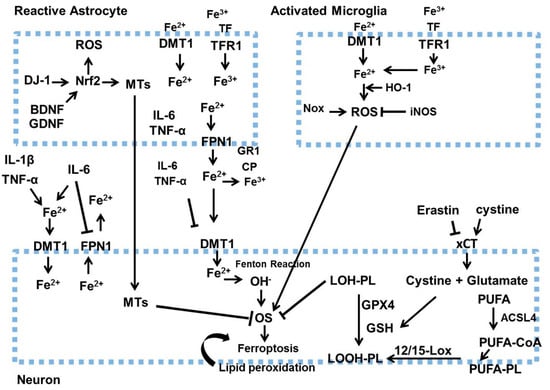
Figure 3.
Initially, inflammatory cytokines (IL-1β, TNF-α, IL-6) secreted by activated microglia and astrocytes enhance iron build-up in neurons by increasing DMT1 expression and decreasing FPN1 expression. Activated astrocytes release BDNF and GDNF, which decrease iron buildup in neurons by lowering DMT1 levels. Secondly, ROS generated by activated microglia enhances neuronal oxidative stress. The increase in Nrf2 and the secretion of metallothioneins in astrocytes enhance neuronal resistance to oxidative stress. BDNF stands for brain-derived neurotrophic factor, GDNF refers to glial cell line-derived neurotrophic factor, HO-1 is heme oxygenase-1, IL-1β denotes interleukin-1β, IL-6 signifies interleukin 6, iNOS indicates inducible nitric oxide synthase, NOX represents NADPH oxidase, Nrf2 is nuclear factor-erythroid factor-2, Tf stands for transferrin, TNF-α is tumor necrosis factor α, 12/15-LOX refers to lipoxygenases 12/15, LOOH-PL means lipid hydroperoxide–phospholipid, and LOH-PL signifies lipid alcohol–phospholipid. This figure was generated by using Microsoft PowerPoint. This figure was modified from Xu Dong-Chen et al., 2023 [103].
It was also linked to the loss of DA neurons in Parkinson’s disease. In the cytosol, coenzyme A (CoA) is changed into free polyunsaturated fatty acids (PUFAs) by the enzyme acyl-CoA synthetase long-chain family member 4 (ACSL4). Lipid peroxidation can then result from the incorporation of PUFA-CoA into phospholipids, which are oxidized by lipoxygenases 12/15. Glutathione (GSH), an antioxidant produced by the body from glutamate and cysteine, can decrease ferroptosis by stopping lipid peroxidation. Cysteine, the rate-limiting substrate, can be absorbed by the xCT antiporter as an oxidized cysteine dimer or generated from methionine by the transsulfuration pathway. DJ-1, another cellular antioxidant enzyme, acts as a ferroptosis inhibitor and protects the synthesis of cysteine and GSH by preventing the destruction of the transsulfuration pathway [104,105,106,107,108]. Glutathione peroxidase 4 (GPX4) is the sole member of the glutathione peroxidase family that can reduce lipid hydroperoxides in physiological conditions. GPX4 needs reduced GSH to convert lipid hydroperoxides to lipid alcohols, and direct inactivation of GPX4 by RAS-selective lethal is one of the most widely used experimental techniques to cause ferroptosis. Ferroptosis genes themselves can be linked to Parkinson’s disease (PD), and the characteristics of ferroptosis induction are extremely comparable with the pathogenic changes observed in PD patients. Iron and α-synuclein coexist in Lewy bodies in the midbrain of Parkinson’s disease patients. As a metal-binding protein, α-synuclein will alter its conformation when it binds iron, which will cause it to aggregate [109,110,111,112,113,114,115]. Astrocytes and microglia control iron homeostasis in brain networks. Iron deposition in the central nervous system may increase as a result of iron buildup in activated microglia and the consequent generation of proinflammatory cytokines. Neuronal iron deposition was exacerbated by ferroportin 1 (FPN1), which drastically downregulated expression, while divalent metal transporter 1 (DMT1), iron regulatory protein 1, and transferrin receptor 1 (TfR1) significantly increased expression. NOX in active microglia not only produced ROS but also resulted in OS, which triggered ferroptosis in DA neurons. Additionally, microglia responded to inflammatory signals by significantly increasing inducible nitric oxide synthase (iNOS), which enabled it to suppress 15-lipoxygenase activity and prevent ferroptosis. Astrocytes primarily use protein interaction cascades, especially ceruloplasmin (CP), to transport different types of iron. Iron efflux from cells may be facilitated by the effective oxidation of Fe2+ into Fe3+ by CP’s ferroxidase activity, but nearly 80% of this activity was diminished in PD patients’ SN, indicating that lower CP expression and the resulting iron accumulation contribute to neuronal death in PD. This overexpression of heme oxygenase-1 by microglia and astrocytes will also contribute to the neurotoxic accumulation of iron in the brain. Reactive astrocytes may also generate other antioxidant molecules, like GSH and metallothioneins, to eliminate ROS from DA neurons. In order to shield the DA neurons from OS, Nrf2 activation in astrocytes can also increase antioxidant enzymes, such as MTs and GSH synthesis enzymes [112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127].
4.4. Role of Mitochondrial Dysfunction in PD Pathology
The significance of mitochondrial dysfunction in the pathophysiology of Parkinson’s disease is increasingly acknowledged. Several studies have found that ongoing ROS generation and dopaminergic neurotoxicity result from mitochondrial dysfunction. Observations after the targeted inhibition of mitochondrial complex I through MPTP infusions offered the initial proof of this connection. Additional inhibitors of this complex, including rotenone, pyridaben, fenpyroximate, and trichloroethylene, exhibited similar negative effects. Additionally, animals with elevated levels of α-synuclein were more susceptible to toxins compared to mice without it, suggesting that mitochondrial α-synuclein enhances toxicity. Key contributors to mitochondrial dysfunction are believed to be the dysregulation of transcription factors and the subsequent changes in mitochondrial biogenesis. A key regulator of mitochondrial biogenesis is the transcription-factor coactivator peroxisome proliferator-activated receptor gamma coactivator-1a (PGC-1a). Although PGC-1a overexpression protects against neurotoxicity, dopaminergic cells in PGC-1a-deficient mice exhibit increased susceptibility to MPTP. Furthermore, harmful mutations in certain genes, such as Parkin, DJ-1, LRRK2, and PINK1, may lead to mitochondrial dysfunction. E3, a ligase of ubiquitin proteases, is produced by Parkin. Animals lacking Parkin are especially susceptible to the mitochondrial complex-I inhibitor known as rotenone. Mutations in PINK1 lead to an autosomal-recessive form of Parkinson’s disease (PD), probably through enhancing α-synuclein aggregation and reducing mitochondrial respiration and ATP production [128,129,130,131,132,133,134,135,136,137,138,139,140]. Additionally, it seems that PINK1 failure affects mitophagy and results in abnormalities in mitochondrial localization. In Drosophila, studies on combined Parkin and PINK1 knockouts revealed that they are part of the same pathway, with PINK1 upstream of Parkin. Parkin is recruited by the cytosol to mediate selective autophagic clearance of damaged and depolarized mitochondria. Dysfunctional mitochondria must collect PINK1 and activate kinases in order for Parkin to translocate. Given that SHP2 knockdown prevents the process, research indicates that Src homology 2 domain-containing tyrosine phosphatase-2 (SHP2) is crucial for mitochondrial translocation and ubiquitination of Parkin. One possible method by which SHP2 regulates Parkin activity is tyrosine dephosphorylation. Lovastatin is a medication that may be used to treat Parkinson’s disease because it increases SHP2 activity. In addition to increasing vulnerability to OS-induced cell death, loss-of-function mutations in the DJ-1 gene also result in a rare autosomal-recessive type of Parkinson’s disease. Humans with DJ-1 mutations and DJ-1 knockout animals both have mitochondria with compromised respiration [141,142,143,144,145,146,147,148,149,150]. On the other hand, LRRK2 mutations are linked to autosomal-dominant Parkinson’s disease. In Caenorhabditis elegans with G2019SLRRK2 mutations, the DA neurons showed mitochondrial abnormalities, as did the striatum of older homozygous LRRK2G2019S knock-in mice. Generally speaking, LRRK2 mutations mediated by dynamin-like proteins are linked to mitochondrial fission [151,152,153].
4.5. Role of Neuroinflammation in PD Pathology
Preclinical and clinical research has shown that neuroinflammation may influence the pathophysiology of PD, linked to its initiation and advancement. Analysis of the brains of Parkinson’s disease (PD) patients after death has shown signs of damage associated with neuroinflammation through both cellular and molecular investigations. The progression of PD is shaped by both inherent and adaptive immune responses. Activated microglia, innate immune cells residing in the brain, elevate nuclear factor kappa-B (NF-κB) and NLR family pyrin domain-containing 3 (NLRP3), leading to an increase in cytokines such as TNF-α and IL-1β. Patients with early-stage Parkinson’s disease exhibit greater densities of activated microglia in the midbrain and putamen, correlated with reduced DA transporter ligand activity. While chronic inflammation in Parkinson’s disease is well-recognized, the precise mechanism of neuroinflammation remains unclear. When α-synuclein accesses cells via the Toll-like receptor (TLR)-2, it can trigger a proinflammatory response as a damage-associated molecular pattern (DAMP) [154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169]. DAMPs, IL-1α, or mitochondrial ROS are also released by dying or injured cells, and when they engage with pattern recognition receptors (PRRs), they set off an innate immune response. Subsequent NLRP3 activation then increases the manufacturing of IL1β, which starts more innate immune reactions. Consequently, PRR-mediated reactions to DAMPs may be the cause of microglial activation in PD. Microglia progressively repolarized from an anti-inflammatory M2 to a proinflammatory M1 phenotype in animal models of neurodegeneration caused by 6-OHDA. Following repolarization, NF-κB triggers the synthesis of cytokines in M1 cells, which results in the transcription of procaspase-1 and interleukin. Together with caspase-1, these mechanisms generate the inflammasome NLRP3, which triggers the release of proinflammatory IL-1β; other proinflammatory proteins, like as TNFα and iNOS, that are generated by M1 cells also have a role in neurodegeneration in Parkinson’s disease. Lastly, TLR-4 contributes to neuroinflammation, as Noelker et al. found that TLR-4 deletion reduced the quantity of activated microglial cells and protected against SN dopaminergic degeneration [170,171,172,173,174,175,176,177,178,179,180]. Neuroinflammation with Parkinson’s disease is also influenced by adaptive immune responses. Numerous studies suggest that T-cell subpopulations play a role in the pathogenesis of Parkinson’s disease. For instance, patients with PD had considerable infiltration of both CD4 and CD8 T cells into their SN, with CD8 T-cell numbers being especially high. CD8 T cells appear to be crucial at the onset of the disease because this infiltration happens in early-stage Parkinson’s disease and decreases as the condition worsens. The population and activity changes of CD4 T-cells in PD patients, as well as an increase in human leukocyte antigen-DR positive antigen-presenting microglia, provide additional evidence of their role in neurodegeneration. This theory has been supported by research on Th17 cells’ function in Parkinson’s disease. Neurons eventually undergo NF-κB-dependent cell death and seem to be more vulnerable to IL-17 or autologous Th17 cells. Furthermore, pharmacological inhibition or CD4 T cell deletion reduces the expression of major histocompatibility complex (MHC) II in CNS myeloid cells and guards against the loss of tyrosine hydroxylase (TH) neurons in the ipsilateral SN pars compacta (SNpc) [177,178,179,180,181].
4.6. Role of Gut Dysbiosis in PD Pathology
There is much interest in the connection between gut microbiota and neurological disorders. The central neural system, enteric nervous system, autonomic nervous system, and hypothalamic–pituitary–adrenal axis are all included in gut–brain microbiota signaling. Metabolites, hormones, the immune system, and afferent nerves are all involved in the signaling pathways that connect the central nervous system to the enteric nervous system. Enteric nerve system inflammation can be mediated by microbiota (Figure 4).
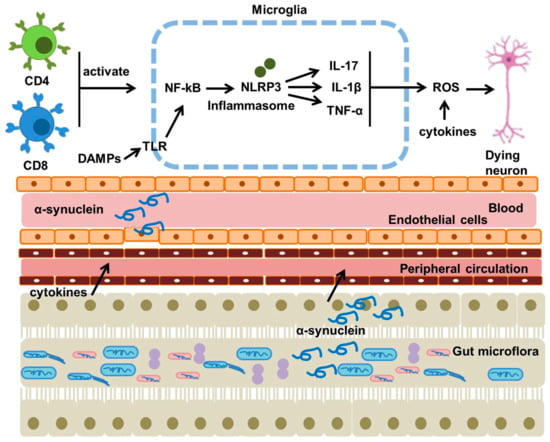
Figure 4.
DAMPs (such as α-synuclein) initiate an innate immune response when they interact with pattern recognition receptors found in microglial cells. Activation of microglia subsequently raises levels of NF-κB and NLRP3, resulting in the upregulation of cytokines. Gut dysbiosis communicates with the CNS and enteric nervous system through metabolites, hormones, and the immune system, thereby facilitating neuroinflammation. This figure was generated by using Microsoft PowerPoint. This figure was modified from Xu Dong-Chen et al., 2023 [103].
Given that patients have elevated levels of calprotectin, a marker of intestinal inflammation, as well as zonulin and alpha-1-antitrypsin, indicators of intestinal barrier disruption, intestinal inflammation plays a role in the pathophysiology of Parkinson’s disease [182,183,184]. Systemic inflammatory reactions have been intimately linked to certain microbial species. For example, the abundance of Bacteroides was linked to plasma TNF levels, whereas the presence of Verrucomicrobiaceae was linked to plasma interferon (IFN)-γ levels. Roseburia promoted immunological homeostasis by negatively regulating the NF-κB pathway and upregulating innate immunity genes. The etiology of Parkinson’s disease may be influenced by gut bacteria and their metabolites through these effects on intestinal inflammation. In this context, lipopolysaccharide (LPS) is a noteworthy metabolite that increases intestinal permeability and α-synuclein accumulation in the enteric nervous system. Additionally, LPS treatment dramatically reduced intestinal epithelial cells’ levels of two tight junction proteins (zona occludens 1 and E-cadherin) in Thy1-α-synuclein animals, suggesting a connection between gut microbiota and Parkinson’s disease etiology [185,186,187,188]. The discovery that exposure to bacteria that produce curli enhances the deposition and aggregation of α-synuclein in intestinal ganglion cells and the brain, causing inflammation, is one example of evidence that microbiota plays a role in Parkinson’s disease. Injecting α-synuclein into the intestinal wall results in pathological alterations in the central nervous system, and numerous animal studies have also shown that α-synuclein disease can travel along the gut–brain axis from the intestine to the brain. Nevertheless, pathogenic processes may not always follow the gut–brain or brain–gut axes. As a disease progresses, pathologies may arise independently in the central nervous system and the enteric nervous system. Given this scenario, Arotcarena et al. suggested a mechanism in which endogenous α-synuclein is transmitted long-distance and in both directions via the general circulation between the intestinal tract and CNS [188,189,190,191,192,193,194]. Table 1 has shown the main molecular mechanisms involved in the pathophysiology of Parkinson disease.

Table 1.
Major molecular and cellular mechanisms involved in Parkinson’s disease pathology.
5. Research Models of PD
Many models have enhanced our comprehension of the pathophysiology, etiology, and molecular mechanisms underlying Parkinson’s disease. The SNpc relies on SNpc dopaminergic neurons that can quickly deteriorate due to toxins such as 6-OHDA, MPP+, and MPTP, resulting in significant and unique motor impairments. The traditional models used in PD research are toxin-based models. Animal models for PD utilize both vertebrate and invertebrate species. C. elegans that overexpress α-synuclein exhibit damage to dopaminergic neurons, despite having few α-synuclein inclusions, and this degeneration is not progressive. Drosophila that overexpressed wild-type (WT), A53T, and A30P α-synuclein exhibited various Parkinson’s disease (PD) characteristics, including Lewy body-like filamentous inclusions and age-related, selective loss of dopaminergic neurons [195,196,197,198,199,200]. However, they lack the intricacy of vertebrates in expressing α-synuclein, and these models are unable to display important clinical characteristics such as stiffness, bradykinesia, and resting tremors. Although transgenic PD mice’s nigrostriatal system shows functional problems, their dopaminergic neurons do not show overt degenerative degeneration. The mouse prion promoter A53T α-synuclein transgenic mice (MitoPark) were the only animal models that replicated the entire spectrum of α-synuclein pathology seen in humans. As PD models, these MitoPark mice are therefore especially promising. They are produced when dopaminergic neurons’ gene encoding mitochondrial transcription-factor A (Tfam) is selectively disrupted. The mutation reduces the number of copies of mitochondrial DNA, which is comparable to traits seen in Parkinson’s disease in humans [201,202,203,204,205,206]. Furthermore, a respiratory chain shortage brought on by Tfam alteration leads to a gradual degenerative phenotype. Human Parkinson’s disease has both of these mitochondrial dysfunctional characteristics, highlighting the model’s value once more. A new model that highlights the aggregated, misfolded forms of α-synuclein found in Lewy bodies has recently become an important tool in the study of Parkinson’s disease. Researchers created preformed fibrils (PFFs) by incubating recombinant α-synuclein monomeric proteins under specific conditions. These fibrils mirror the structural elements of Lewy bodies and Lewy neurites. Synaptic dysfunction, alterations in cell excitability, and cell death can be caused by PFFs in primary neuronal cultures from WT mice and cell lines that overexpress disease-related proteins. Intracerebral injection of PFFs into the dorsal striatum results in behavioral deficits, neurodegeneration in the SNpc, and dysregulation of striatal DA release in mice overexpressing disease-related proteins or non-transgenic animals [207,208,209,210,211,212]. With early α-synuclein pathology in PD-relevant brain regions and the onset of DA dysfunction, nigral degeneration, and motor deficits months after induction, this type of model shows a longer time course of degeneration than other models, indicating a progression that is comparable to that of the human condition. The model’s failure to consistently produce pathogenic PFFs has limited the repeatability of the results and the investment in a model with a disease time course that takes several months to create.
6. Therapeutic Strategies for PD
6.1. Commercially Available Drugs for PD
Numerous medications have become effective therapies; we distinguish between them based on their pharmacological goals and list them in Table 2.

Table 2.
List of commercially available drugs for PD treatment.
6.1.1. Levodopa
Levodopa (L-DOPA), a traditional treatment for Parkinson’s disease, includes a number of unfavourable side effects, such as drug-induced dyskinesias and motor-response oscillations. These motor problems finally result from non-physiological pulsatile striatal DA receptor stimulation and are mediated by both presynaptic and postsynaptic pathways. Due to the short half-life of L-DOPA, as well as variations in gastrointestinal absorption and blood-brain barrier transit, discontinuous drug delivery is the primary source of maladaptive neuronal responses. Novel sustained-release formulations of L-DOPA and continuous delivery methods are constantly being developed to solve these issues. These include subcutaneous distribution using mini pumps and intestine delivery using percutaneous endoscopic gastro jejunostomy tubes [213,214,215,216,217].
6.1.2. DA Agonists
Two kinds of DA receptors are found in spiny neurons in the striatal medium. Dopaminomimetics, like the ergot alkaloid bromocriptine, are receptor agonists that specifically target the D2 receptor family. In addition to activating 5-hydroxytryptamine (5-HT) receptors, including the 5-HT2B subtype, ergot alkaloids are ergoline derivatives. They have, however, been linked to pleuropulmonary fibrosis and cardiac valvular fibrosis, which raises serious safety concerns. Non-ergoline medications, on the other hand, are favored for treating Parkinson’s disease because they do not have this problem. DA agonists are excellent options for adjunct therapy in individuals with motor fluctuations since they have a longer half-life than L-DOPA. They are less effective overall than L-DOPA, though, and they are more likely to impair impulse control and induce drowsiness. Among DA agonists, apomorphine is distinct in that it acts on both D1 and D2 receptors simultaneously and has an identical affinity for L-DOPA. Limiting motor-response variability and reducing pre-existing L-DOPA-induced dyskinesias have been associated with continuous subcutaneous apomorphine infusions. New apomorphine formulations for sublingual usage are now being developed in clinical settings [217,218,219,220,221,222,223,224,225].
6.1.3. Catechol-O-Methyltransferase and Monoamine Oxidase Type B Inhibitors
Catechol-O-methyltransferase (COMT) ortho-methylates L-DOPA through a secondary metabolic pathway during peripheral metabolism. The bioavailability and half-life of L-DOPA are enhanced when COMT is suppressed. Because of this impact, L-DOPA and COMT inhibitors are now used as part of the first-line treatment for PD patients. Entacapone and opicapone are two of the three COMT inhibitor formulations that are currently approved for clinical usage. In glial cells, monoamine oxidase type B (MAO-B) is the main mechanism for clearing synaptically produced dopamine. The action of DA is prolonged and its synaptic concentrations are raised when MAO-B is inhibited (for example, by the selective inhibitor selegiline). But while selegiline is irreversible, safinamide is a reversible MAO-B inhibitor that can be used to treat Parkinson’s disease [225,226,227,228,229,230].
6.1.4. Non-Dopaminergic Targets
Even if dopaminergic medication has a great effect on PD symptoms, other target therapies are still required. First, L-DOPA-resistant (“non-dopaminergic”) motor features (e.g., treatment-resistant tremors, postural instability, frozen gait, swallowing difficulties, and speech disturbances), as well as L-DOPA-induced dyskinesia and motor fluctuations, require new treatments. Amantadine is now the only readily available and efficient pharmaceutical treatment for L-DOPA-induced dyskinesia; it is thought to be an antagonist of the N-methyl-D-aspartate receptor. Second, new therapies need to target PD’s non-motor symptoms, namely, autonomic failure, cognitive impairment, and depression. The fact that many non-motor symptoms do not improve with DA replacement therapy, and that some are even triggered or made worse by it, is a significant issue. Cholinesterase inhibitors help people with dementia and Parkinson’s disease who have cognitive impairments. This favourable result may be linked to dementia’s substantial loss of cholinergic projections from the nucleus basalis of Meynert [218,231,232]. The best treatment for psychotic symptoms in PD is clozapine. Lastly, autonomic dysfunction is rather prevalent in Parkinson’s disease, particularly in its latter stages. There are several pharmacological treatments that primarily target the autonomic nerve system. Mineralocorticoid fludro cortisone, adrenergic agents (like midodrine and etilefrine), anti-muscarinics (like tolterodine, oxybutynin, or trospium chloride) for urinary urgency or incontinence, noradrenaline precursor (droxidopa) for orthostatic hypotension, and prokinetic medications (like macrogol or lubiprostone) for constipation are some of these [218,219,220,221,232,233].
6.2. Drugs for PD Treatment Under Clinical Trials
The newly discovered medications are being tested in a number of clinical trials (Table 3). A few of them have displayed signs of becoming PD treatment candidates. Tavapadon is a strong, very selective partial agonist for the DA D1/D5 receptor that is taken orally. In a clinical experiment (NCT02847650), tavapadon demonstrated a greater improved impact than a placebo, which served as the foundation for the present clinical phase III trial (NCT04760769). IRL790 was created as an experimental treatment for PD’s L-DOPA-induced dyskinesia, impulse control problem, and psychosis because it may interact with the DA D3 receptor. In the study, patients with severe Parkinson’s disease on IRL790 reported fewer motor symptoms and no significant side effects. Phase II clinical trials are continuing in the early stages of follow-up stages (NCT03368170). Deferiprone decreased SN iron deposition and the advancement of motor impairment in PD patients in Phase II randomized double-blind placebo-controlled clinical studies (NCT00943748, NCT01539837). In a Phase I dose-escalation study in early PD patients (NCT03204929), Cu (II) ATSM had a beneficial effect by avoiding lipid peroxidation, which raises the possibility of PD treatment. A monoclonal antibody called prasinezumab targets α-synuclein. Its discovery has drawn much attention as a potential treatment approach against a crucial target in Parkinson’s disease (PD), but in a recent phase II clinical research (NCT03100149), it had no therapeutic benefit when compared to a placebo and raised safety concerns [234,235,236,237,238,239,240]. However, additional research may be necessary to confirm its effectiveness, and fresh clinical trials are currently being conducted (NCT04777331). In the double-blind randomized clinical phase I trial (NCT04056689) conducted by Denali Therapeutics, DNL151, an LRRK2 inhibitor, showed a fairly clear therapeutic benefit on Parkinson’s disease. Since then, it has started clinical phase II (NCT05348785) and III phase studies (NCT05418673). If the outcomes are positive, it could demonstrate how effective LRRK2 is at treating Parkinson’s disease.

Table 3.
List of drugs for PD treatment under clinical trials.
6.3. Phytochemical-Based Therapeutic Strategy for PD Treatment
New remedies ought to be created in light of the negative impacts of Western medicine and the intrusiveness of external physical interventions. The usefulness of several natural compounds derived from medicinal plants and their formulations in the treatment of Parkinson’s disease has been the subject of numerous studies in recent years. Several natural items have been shown to regulate Parkinson’s disease at the molecular level. The list of compounds suggested to have therapeutic potential for PD treatment are summarized in Table 4.

Table 4.
List of natural compounds with therapeutic potential for PD treatment.
6.3.1. Phenol
Yang et al. showed the preventive benefits of curcumin on the damaged hippocampus using a 6-OHDA-induced PD model. Significant improvements in mental status, elevations of dopamine and norepinephrine, hippocampus tissue regeneration, and activation of proteins involved in cell survival-related pathways, including BDNF, tropomyosin receptor kinase (Trk) B, and phosphoinositide 3-kinase (PI3K), were among these advantages. This finding is supported by additional research using 6-OHDA PD models, which demonstrate that curcumin promotes neural regeneration via activating Trk/PI3K signalling, which reduces TNF-α and caspase activity while raising BDNF levels. Additional investigation into curcumin’s mode of action suggests that it requires at least some interaction with a nicotinic acetylcholine receptor (α7).
Curcumin reduces aberrant turning behavior and increases the survival of striatal TH fibers and neurons in the SNpc by this mechanism. Additionally, curcumin inhibits a variety of inflammatory substances, such as glial fibrillary acidic protein, cytokines, ILs, chemokines, inflammatory enzymes, cycloxygenase-2, and cyclin D1. Moreover, curcumin inhibits many players in apoptotic pathways, including c-Jun Nterminal kinase (JNK), iNOS, LPS-induced TNF-α, IL-1β, and IL-6 [240,241,242,243,244,245,246]. Data demonstrating that curcumin affects the impact of numerous inflammatory mediators further supports these anti-inflammatory qualities. Resveratrol has been demonstrated to have neuroprotective benefits in PD models derived from 6-OHDA, MPP+, and rotenone in both in vitro and in vivo experiments. Resveratrol blocks apoptosis by activating the pro-survival PI3K/protein kinase B (Akt) pathway, increasing the ratio of B-cell lymphoma (Bcl-2) to Bcl-2-associated X (Bax), and decreasing cytochrome C release, which keeps caspase-3 dormant. Additionally, following exposure to MPTP/MPP+ and rotenone, resveratrol boosts antioxidant defenses and reduces the generation of ROS. Resveratrol prevents mitochondrial dysfunction in experimentally produced Parkinson’s disease (PD) by boosting complex-I activity and mitochondrial biogenesis while reversing alterations in mitochondrial shape and membrane potential. Resveratrol reduces α-synuclein expression in the striatum and promotes autophagic clearance of α-synuclein following sirtuin (SIRT) 1 activation in a number of animal models. Similarly to gastrodin activity, resveratrol also increases autophagic flow by activating the HO-1 and mitogen-activated protein kinase (MAPK) pathways. Neuroprotection also involves the control of astroglial activity. When taken with L-DOPA, resveratrol exhibits synergistic effects, which is encouraging for therapeutic use [247,248,249,250,251,252,253,254,255,256,257,258,259].
6.3.2. Alkaloids
Berberine treatment dramatically reduced dopaminergic neuronal degeneration in the SN compacta and improved motor impairment in mice treated with MPTP. Additionally, berberine increased autophagy linked to microtubule-associated protein light chain 3 (LC3-II) and lowered α-synuclein levels. Additionally, berberine activated adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK). One noteworthy advantage of AMPK is that it protects cells against rotenone and reduces α-synuclein-induced toxicity. Another study examining berberine’s effects in animal models revealed that mice given berberine had dramatically reduced levels of NLRP3 inflammasome and NLRP3-associated neuroinflammation [260,261,262,263]. The phenylalanine–tyrosine–DA pathway’s rate-limiting enzyme, TH, may be the target of berberine’s particular mode of action. This route uses tetrahydrobiopterin as a coenzyme to produce L-DOPA and supply DA to the brain. It has been demonstrated that bacterial nitroreductase converts berberine to dihydroberberine in the intestines. Tetrahydrobiopterin concentrations rise as a result of this process, which also provides and boosts TH activity. In the end, the creation of L-DOPA by gut bacteria is accelerated. Isorhynchophylline (IRN) facilitates the removal of WT, A53T, and A30P α-synuclein aggregates from neuronal cells via the autophagy–lysosome route. IRN-induced autophagy depends on Beclin 1 function but is not mediated by the mTOR pathway. IRN treatment significantly decreased endoplasmic-reticulum stress responses mediated by MPP+. Furthermore, it appears that IRN suppression of the apoptotic signal-regulating kinase 1 (ASK1)/JNK pathway inhibits mitochondria-dependent apoptosis, indicating neuronal protection [258,259,260,261,262,263,264,265,266,267,268,269,270,271].
6.3.3. Flavonoids
Nrf2 is necessary for the anti-parkinsonian actions of the flavonoid puerarin. Puerarin controlled the phosphorylation of Fyn and GSK-3β in the ventral midbrain of mice given MPTP. De novo glutathione synthesis is facilitated by Nrf2 increase in the nucleus, which is facilitated by the Fyn/GSK-3β pathway. The information that is now available suggests that puerarin uses progesterone receptors to enhance dopaminergic neuron survival, proliferation, and differentiation. Additionally, puerarin inhibits GSK-3 activity in neurons and reduces inflammatory responses by acting on the PI3K/Akt pathway, which limits the generation of caspase-3 and the apoptosis that goes along with it. The way puerarin prevents MPP+-induced neuroblastoma SH-SY5Y cell death is explained by these interactions, as well as the regulation of nuclear p53 accumulation. The injection of baicalein restored the proinflammatory cytokine increase, dopaminergic neuronal loss, and motor impairment caused by MPTP. Furthermore, baicalein prevented disease-associated proinflammatory microglia from activating and proliferating. This protective effect’s fundamental mechanism is most likely the suppression of the NLRP3/caspase-1/gasdermin D pathway. Pyroptosis, a form of programmed cell death linked to this system, contributes to the degeneration of dopaminergic neurons. NLRP3 inflammasome activation initiates pyroptosis, which, in turn, stimulates caspase-1 maturation [271,272,273,274,275,276,277,278,279,280,281,282,283]. The pyroptosis executive protein gasdermin D is then oligomerized by caspase-1, which promotes the release of proinflammatory IL-1β and IL-18. Additionally, studies have shown that baicalein protects synaptic plasticity and decreases α-synuclein aggregation via acting on the BDNF/TrkB/Cyclic AMP response-element binding protein (CREB) pathway. Baicalein’s medicinal benefits have been further demonstrated by numerous studies employing it in various settings. For example, patients’ gait function was greatly restored when baicalein and low-dose L-DOPA were combined, reaching a level similar to that of high-dose L-DOPA treatment, despite some L-DOPA side effects. Lastly, baicalein decreased Bcl-2, phosphorylated extracellular signal-regulated kinases, increased Bax and cleaved caspase-3, and inhibited rotenone-induced ROS overproduction (ERK) [284,285,286,287].
6.3.4. Terpenoids
Celastrol alleviates nigrostriatal dopaminergic degeneration and motor impairments by inhibiting the NLRP3 inflammasome via the Nrf2-NLRP3-caspase-1 pathway. Celastrol stimulates mitophagy, autophagy, and autophagosome biogenesis in neurons, most likely in connection with MAPK pathways. Celastrol prevents ATP loss, mitochondrial membrane depolarization, and dopaminergic neuronal death in MPP+-induced PD cell models. Additionally, studies employing these models indicate that celastrol preserves mitochondrial quality by enclosing faulty mitochondria in autophagosomes for eventual destruction. Following fibril-induced microglial activation mediated by α-synuclein, triptolide therapy inhibited microglial activation and reduced the release of proinflammatory cytokines. The medication specifically inhibited NF-κB’s activity in the inositol polyphosphate 5-phosphatase (SHIP) 1 pathway by targeting the miR155-5p/Src homology 2 (SH2) domain. Research indicates that the overexpression of miR155-5p triggers NF-κB activity by suppressing SHIP1. Triptolide reduces the inflammatory response via interfering with the regulation of SHIP1 by miR155-5p. Triptolide’s anti-inflammatory effects were shown to be lessened when metabotropic glutamate receptor subtype 5 (mGlu5) was blocked in an LPS-induced Parkinson’s disease model. Furthermore, the impact of triptolide on microglia-induced astrocyte activation in vitro and in vivo seems to be mediated by mGlu5. Additionally, triptolide has been reported to be a strong inducer of autophagy in neuronal cells, assisting in the removal of different types of α-synuclein through the autophagic pathway [288,289,290,291,292,293].
6.3.5. Saponins
Promoting hippocampus CA3 α-synuclein expression, restoring glutamate in the CA3-schaffer collateral-CA1 route, and gradually raising postsynaptic density-95 expression are the neuroprotective mechanisms behind ginsenoside Rb1-improvements to synaptic plasticity. Ginsenoside Rb1 therapy significantly reduced apomorphine-induced rotations, SN inflammation, and DA (plus metabolites) depletion in the striatum of rats given LPS. These effects could be linked to the NF-κB signaling pathway being inhibited. The ginsenoside Rg3 decreased ROS levels in the SN while increasing the number of TH-positive neurons in the SN, the mean density of TH-positive nerve fibers, and the DA concentration in the striatum [294,295,296,297].
7. Discussion and Perspectives
The key pathological characteristics of Parkinson’s disease (PD) involve the degeneration of dopaminergic neurons in the SN and the accumulation of α-synuclein. PD results from various complex processes, such as ferroptosis, neuroinflammation, mitochondrial dysfunction, gut dysbiosis, oxidative stress, α-synuclein aggregation, and additional factors. Interactions play a crucial part in the progression and development of Parkinson’s disease. Besides the toxin, transgenic, and PFFs models previously discussed, additional models are essential for experimental studies to propel PD research forward, as the complex mechanisms driving the effects of these strategies on PD still need exploration. The main drug employed in managing Parkinson’s disease (PD) remains L-DOPA, while additional medications like COMT inhibitors and MAO-B inhibitors are often utilized alongside L-DOPA. Additionally, individuals with advanced Parkinson’s disease often do not adequately respond to existing medications, which greatly diminishes their quality of life. Consequently, additional drugs are still necessary for therapy. Researching new drugs that focus on ferroptosis, oxidative stress, α-synuclein aggregation, and other related factors can be founded on the mechanisms underlying Parkinson’s disease. The associated medicines currently under development have not shown favorable outcomes and might still require further investigation. Alongside well-known chemical, biological, and various other treatments for Parkinson’s disease, several studies have verified the efficacy of traditional Chinese medicines, which show significant potential for managing the condition. Traditional Chinese medicines contain substances that have complex pharmacological properties and cure the degenerative pathways of Parkinson’s disease (PD), including OS, neuroinflammation, and α-synuclein aggregation. Many natural medications, including tanshinone and andrographolide, which have been documented to have anti-inflammatory properties and may be useful in the treatment of Parkinson’s disease, have been suggested for additional research in the Zhu et al. study [298]. Furthermore, given the intricate mechanisms, multi-drug combinations, such as a mix of chemical and biological medications or natural small molecules, may provide a fresh viewpoint on the treatment of Parkinson’s disease. Some surgical treatment approaches additionally contain patients’ new ideal strategies in addition to medication therapies. High-frequency (100–200 Hz) electrodes can create deep brain interference, which mimics the effects of a lesion without causing damage to brain tissue [220]. Utilizing this method, a clinical experiment has improved gait-function recovery in PD patients by combining treadmill gait training with transcranial direct current stimulation (NCT04591236). Recent research has also shown that transcutaneous magnetic stimulation of the spinal cord, which can non-invasively stimulate neural components, may be a viable treatment option for gait abnormalities (NCT05008289). The application of stem cells is another exciting field of study. Autologous iPSCs were used in a clinical trial to differentiate patient-derived midbrain dopaminergic progenitor cells. The putamen was subsequently transplanted with these cells. Following treatment, patients’ PD symptoms subsided, enabling a 6% reduction in the daily dose of L-DOPA equivalent [297]. These imply that scientists might find a new therapeutic strategy in the field of stem cell transplantation.
The review presented in this work covers a wide range of current data on the pathophysiology of PD. We have particularly focused on describing key molecular processes in PD, as well as the traditional research models, clinical diagnostic standards, documented medication therapeutic approaches, and recently disclosed drug candidates in clinical trials. By reviewing recent advances and highlighting future research directions, we aim to shed light on the promising role of drugs in addressing unmet therapeutic needs in Parkinson disease.
Author Contributions
M.S.K. conceived and designed the study. M.S.K. has written the manuscript. S.N. edited the manuscript. J.C.B. reviewed the manuscript. J.C.B. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge T32 HL007446 (J.C.B.).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J. An essay on the shaking palsy. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef]
- Twelves, D.; Perkins, K.S.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. 2003, 18, 19–31. [Google Scholar] [CrossRef]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Ahlskog, J.E.; Rocca, W.A. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013, 70, 859–866. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Baldereschi, M.; Di Carlo, A.; Rocca, W.A.; Vanni, P.; Maggi, S.; Perissinotto, E.; Grigoletto, F.; Amaducci, L.; Inzitari, D. Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. Neurology 2000, 55, 1358–1363. [Google Scholar] [CrossRef]
- Kusumi, M.; Nakashima, K.; Harada, H.; Nakayama, H.; Takahashi, K. Epidemiology of Parkinson’s disease in Yonago City, Japan: Comparison with a study carried out 12 years ago. Neuroepidemiology 1996, 15, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.H.; Mehal, J.M.; Holman, R.C.; Rowland, A.S.; Cheek, J.E. Parkinson’s disease among American Indians and Alaska natives: A nationwide prevalence study. Mov. Disord. 2012, 27, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Davis, J.W.; Grandinetti, A.; Ross, G.W.; Popper, J.S.; White, L.R. Epidemiologic observations on Parkinson’s disease: Incidence and mortality in a prospective study of middle-aged men. Neurology 1996, 46, 1044–1050. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef]
- Marras, C.; Lang, A. Parkinson’s disease subtypes: Lost in translation? J. Neurol. Neurosurg. Psychiatry 2013, 84, 409–415. [Google Scholar] [CrossRef]
- Jankovic, J.; McDermott, M.; Carter, J.; Gauthier, S.; Goetz, C.; Golbe, L.; Huber, S.; Koller, W.; Olanow, C.; Shoulson, I.; et al. Variable expression of Parkinson’s disease: A base-line analysis of the DATATOP cohort. Neurology 1990, 40, 1529–1534. [Google Scholar] [CrossRef]
- Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Coleman, S.; O’Brien, J.T.; Brooks, D.J.; Barker, R.A.; Burn, D.J. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013, 80, 276–281. [Google Scholar] [CrossRef]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying prodromal Parkinson’s disease: Pre-motor disorders in Parkinson’s disease. Mov. Disord. 2012, 27, 617–626. [Google Scholar] [CrossRef]
- Hely, M.A.; Morris, J.G.; Reid, W.G.; Trafficante, R. Sydney Multicenter Study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord. 2005, 20, 190–199. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122 Pt 8, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.A.; Voorn, P.; Berendse, H.W.; Groenewegen, H.J.; Netherlands Brain Bank; Rozemuller, A.J.; van de Berg, W.D. Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov. Disord. 2014, 29, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Iacono, D.; Geraci-Erck, M.; Rabin, M.L.; Adler, C.H.; Serrano, G.; Beach, T.G.; Kurlan, R. Parkinson disease and incidental Lewy body disease: Just a question of time? Neurology 2015, 85, 1670–1679. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Hirsch, E.; Graybiel, A.M.; Agid, Y.A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 1988, 334, 345–348. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Orieux, G.; Muriel, M.P.; Francois, C.; Feger, J. Nondopaminergic neurons in Parkinson’s disease. Adv. Neurol. 2003, 91, 29–37. [Google Scholar]
- Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A.; Logroscino, G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology 2016, 86, 566–576. [Google Scholar] [CrossRef]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef]
- Krismer, F.; Pinter, B.; Mueller, C.; Mahlknecht, P.; Nocker, M.; Reiter, E.; Djamshidian-Tehrani, A.; Boesch, S.M.; Wenning, G.K.; Scherfler, C.; et al. Sniffing the diagnosis: Olfactory testing in neurodegenerative parkinsonism. Park. Relat. Disord. 2017, 35, 36–41. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Lees, A.J. Olfaction and Parkinson’s syndromes: Its role in differential diagnosis. Curr. Opin. Neurol. 2004, 17, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, C.; Sasaki, M.; Konno, K.; Koide, M.; Kato, K.; Takahashi, J.; Takahashi, S.; Kudo, K.; Yamashita, F.; Terayama, Y. Changes in substantia nigra and locus coeruleus in patients with early-stage Parkinson’s disease using neuromelanin-sensitive MR imaging. Neurosci. Lett. 2013, 541, 93–98. [Google Scholar] [CrossRef]
- Langkammer, C.; Schweser, F.; Krebs, N.; Deistung, A.; Goessler, W.; Scheurer, E.; Sommer, K.; Reishofer, G.; Yen, K.; Fazekas, F.; et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 2012, 62, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Ehrminger, M.; Latimier, A.; Pyatigorskaya, N.; Garcia-Lorenzo, D.; Leu-Semenescu, S.; Vidailhet, M.; Lehericy, S.; Arnulf, I. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain 2016, 139 Pt 4, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Stoessl, A.J.; Lehericy, S.; Strafella, A.P. Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet 2014, 384, 532–544. [Google Scholar] [CrossRef]
- Politis, M. Neuroimaging in Parkinson disease: From research setting to clinical practice. Nat. Rev. Neurol. 2014, 10, 708–722. [Google Scholar] [CrossRef]
- Kalinderi, K.; Bostantjopoulou, S.; Fidani, L. The genetic background of Parkinson’s disease: Current progress and future prospects. Acta Neurol. Scand. 2016, 134, 314–326. [Google Scholar] [CrossRef]
- Tambasco, N.; Nigro, P.; Romoli, M.; Prontera, P.; Simoni, S.; Calabresi, P. A53T in a parkinsonian family: A clinical update of the SNCA phenotypes. J. Neural Transm. 2016, 123, 1301–1307. [Google Scholar] [CrossRef]
- Marras, C.; Canning, C.G.; Goldman, S.M. Environment, lifestyle, and Parkinson’s disease: Implications for prevention in the next decade. Mov. Disord. 2019, 34, 801–811. [Google Scholar] [CrossRef]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef]
- Tanner, C.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1992, 10, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, T.; Kakita, A.; Shiga, A.; Kasuga, K.; Kaneko, H.; Tan, C.F.; Idezuka, J.; Wakabayashi, K.; Onodera, O.; Iwatsubo, T.; et al. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch. Neurol. 2008, 65, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, L.; Pan, H.; Liu, Z.; Jiang, L.; He, Y.; Zeng, Q.; Zhou, X.; Zhou, X.; Zhou, Y.; et al. The role of genetics in Parkinson’s disease: A large cohort study in Chinese mainland population. Brain 2020, 143, 2220–2234. [Google Scholar] [CrossRef]
- Monfrini, E.; Di Fonzo, A. Leucine-Rich Repeat Kinase (LRRK2) Genetics and Parkinson’s Disease. Adv. Neurobiol. 2017, 14, 3–30. [Google Scholar]
- Deng, H.; Wang, P.; Jankovic, J. The genetics of Parkinson disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Hedrich, K.; Winkler, S.; Hagenah, J.; Kabakci, K.; Kasten, M.; Schwinger, E.; Volkmann, J.; Pramstaller, P.P.; Kostic, V.; Vieregge, P.; et al. Recurrent LRRK2 (Park8) mutations in early-onset Parkinson’s disease. Mov. Disord. 2006, 21, 1506–1510. [Google Scholar] [CrossRef]
- Martins, L.M.; Morrison, A.; Klupsch, K.; Fedele, V.; Moisoi, N.; Teismann, P.; Abuin, A.; Grau, E.; Geppert, M.; Livi, G.P.; et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol. Cell. Biol. 2004, 24, 9848–9862. [Google Scholar] [CrossRef]
- Plun-Favreau, H.; Klupsch, K.; Moisoi, N.; Gandhi, S.; Kjaer, S.; Frith, D.; Harvey, K.; Deas, E.; Harvey, R.J.; McDonald, N.; et al. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat. Cell Biol. 2007, 9, 1243–1252. [Google Scholar] [CrossRef]
- Moisoi, N.; Klupsch, K.; Fedele, V.; East, P.; Sharma, S.; Renton, A.; Plun-Favreau, H.; Edwards, R.E.; Teismann, P.; Esposti, M.D.; et al. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009, 16, 449–464. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Camprubi, M.D.; Dunn, L.; Wu, H.C.; Ip, N.Y.; Kruger, R.; Martins, L.M.; Wood, N.W.; Plun-Favreau, H. Phosphorylation of HtrA2 by cyclin-dependent kinase-5 is important for mitochondrial function. Cell Death Differ. 2012, 19, 257–266. [Google Scholar] [CrossRef]
- Basak, I.; Pal, R.; Patil, K.S.; Dunne, A.; Ho, H.P.; Lee, S.; Peiris, D.; Maple-Grodem, J.; Odell, M.; Chang, E.J.; et al. Arabidopsis AtPARK13, which confers thermotolerance, targets misfolded proteins. J. Biol. Chem. 2014, 289, 14458–14469. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Hurley, J.H. Retromer. Curr. Opin. Cell Biol. 2008, 20, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Arighi, C.N.; Hartnell, L.M.; Aguilar, R.C.; Haft, C.R.; Bonifacino, J.S. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 2004, 165, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chartier-Harlin, M.C.; Dachsel, J.C.; Vilarino-Guell, C.; Lincoln, S.J.; Lepretre, F.; Hulihan, M.M.; Kachergus, J.; Milnerwood, A.J.; Tapia, L.; Song, M.S.; et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am. J. Hum. Genet. 2011, 89, 398–406. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.; Jankovic, J. The EIF4G1 gene and Parkinson’s disease. Acta Neurol. Scand. 2015, 132, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Hattori, N.; Mizuno, Y. Twenty years since the discovery of the parkin gene. J. Neural Transm. 2017, 124, 1037–1054. [Google Scholar] [CrossRef]
- Klein, C.; Lohmann-Hedrich, K.; Rogaeva, E.; Schlossmacher, M.G.; Lang, A.E. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 2007, 6, 652–662. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Seirafi, M.; Kozlov, G.; Gehring, K. Parkin structure and function. FEBS J. 2015, 282, 2076–2088. [Google Scholar] [CrossRef]
- van der Merwe, C.; Jalali Sefid Dashti, Z.; Christoffels, A.; Loos, B.; Bardien, S. Evidence for a common biological pathway linking three Parkinson’s disease-causing genes: Parkin, PINK1 and DJ-1. Eur. J. Neurosci. 2015, 41, 1113–1125. [Google Scholar] [CrossRef]
- Wilhelmus, M.M.; Nijland, P.G.; Drukarch, B.; de Vries, H.E.; van Horssen, J. Involvement and interplay of Parkin, PINK1, and DJ1 in neurodegenerative and neuroinflammatory disorders. Free. Radic. Biol. Med. 2012, 53, 983–992. [Google Scholar] [CrossRef]
- Zondler, L.; Miller-Fleming, L.; Repici, M.; Goncalves, S.; Tenreiro, S.; Rosado-Ramos, R.; Betzer, C.; Straatman, K.R.; Jensen, P.H.; Giorgini, F.; et al. DJ-1 interactions with alpha-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell Death Dis. 2014, 5, e1350. [Google Scholar] [CrossRef]
- Demirsoy, S.; Martin, S.; Motamedi, S.; van Veen, S.; Holemans, T.; Van den Haute, C.; Jordanova, A.; Baekelandt, V.; Vangheluwe, P.; Agostinis, P. ATP13A2/PARK9 regulates endo-/lysosomal cargo sorting and proteostasis through a novel PI(3, 5)P2-mediated scaffolding function. Hum. Mol. Genet. 2017, 26, 1656–1669. [Google Scholar] [CrossRef]
- Holemans, T.; Sorensen, D.M.; van Veen, S.; Martin, S.; Hermans, D.; Kemmer, G.C.; Van den Haute, C.; Baekelandt, V.; Gunther Pomorski, T.; Agostinis, P.; et al. A lipid switch unlocks Parkinson’s disease-associated ATP13A2. Proc. Natl. Acad. Sci. USA 2015, 112, 9040–9045. [Google Scholar] [CrossRef]
- Park, J.S.; Koentjoro, B.; Veivers, D.; Mackay-Sim, A.; Sue, C.M. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum. Mol. Genet. 2014, 23, 2802–2815. [Google Scholar] [CrossRef]
- Tsunemi, T.; Krainc, D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum. Mol. Genet. 2014, 23, 2791–2801. [Google Scholar] [CrossRef]
- Park, J.S.; Blair, N.F.; Sue, C.M. The role of ATP13A2 in Parkinson’s disease: Clinical phenotypes and molecular mechanisms. Mov. Disord. 2015, 30, 770–779. [Google Scholar] [CrossRef]
- Kong, S.M.; Chan, B.K.; Park, J.S.; Hill, K.J.; Aitken, J.B.; Cottle, L.; Farghaian, H.; Cole, A.R.; Lay, P.A.; Sue, C.M.; et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum. Mol. Genet. 2014, 23, 2816–2833. [Google Scholar] [CrossRef]
- Teixeira, F.R.; Randle, S.J.; Patel, S.P.; Mevissen, T.E.; Zenkeviciute, G.; Koide, T.; Komander, D.; Laman, H. Gsk3beta and Tomm20 are substrates of the SCFFbxo7/PARK15 ubiquitin ligase associated with Parkinson’s disease. Biochem. J. 2016, 473, 3563–3580. [Google Scholar] [CrossRef]
- Ordureau, A.; Heo, J.M.; Duda, D.M.; Paulo, J.A.; Olszewski, J.L.; Yanishevski, D.; Rinehart, J.; Schulman, B.A.; Harper, J.W. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc. Natl. Acad. Sci. USA 2015, 112, 6637–6642. [Google Scholar] [CrossRef]
- Duarte, F.V.; Amorim, J.A.; Varela, A.T.; Teodoro, J.S.; Gomes, A.P.; Cunha, R.A.; Palmeira, C.M.; Rolo, A.P. Adenosine receptors: Regulatory players in the preservation of mitochondrial function induced by ischemic preconditioning of rat liver. Purinergic Signal. 2017, 13, 179–190. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Lee, J.C.T.; Tan, E.K. Pathophysiological mechanisms linking F-box only protein 7 (FBXO7) and Parkinson’s disease (PD). Mutat. Res. Rev. Mutat. Res. 2018, 778, 72–78. [Google Scholar] [CrossRef]
- Delgado-Camprubi, M.; Esteras, N.; Soutar, M.P.; Plun-Favreau, H.; Abramov, A.Y. Deficiency of Parkinson’s disease-related gene Fbxo7 is associated with impaired mitochondrial metabolism by PARP activation. Cell Death Differ. 2017, 24, 2210. [Google Scholar] [CrossRef]
- Maries, E.; Dass, B.; Collier, T.J.; Kordower, J.H.; Steece-Collier, K. The role of alpha-synuclein in Parkinson’s disease: Insights from animal models. Nat. Rev. Neurosci. 2003, 4, 727–738. [Google Scholar] [CrossRef]
- Vekrellis, K.; Xilouri, M.; Emmanouilidou, E.; Rideout, H.J.; Stefanis, L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011, 10, 1015–1025. [Google Scholar] [CrossRef]
- Wales, P.; Pinho, R.; Lazaro, D.F.; Outeiro, T.F. Limelight on alpha-synuclein: Pathological and mechanistic implications in neurodegeneration. J. Park. Dis. 2013, 3, 415–459. [Google Scholar] [CrossRef]
- Burre, J. The Synaptic Function of alpha-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar]
- Ruiperez, V.; Darios, F.; Davletov, B. Alpha-synuclein, lipids and Parkinson’s disease. Prog. Lipid Res. 2010, 49, 420–428. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.J. Controlling the mass action of alpha-synuclein in Parkinson’s disease. J. Neurochem. 2008, 107, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Melki, R. Role of Different Alpha-Synuclein Strains in Synucleinopathies, Similarities with other Neurodegenerative Diseases. J. Park. Dis. 2015, 5, 217–227. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef]
- Xilouri, M.; Brekk, O.R.; Stefanis, L. Alpha-Synuclein and protein degradation systems: A reciprocal relationship. Mol. Neurobiol. 2013, 47, 537–551. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Lee, D.H.; Gold, R.; Linker, R.A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: Therapeutic modulation via fumaric acid esters. Int. J. Mol. Sci. 2012, 13, 11783–11803. [Google Scholar] [CrossRef]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef]
- Kemppainen, S.; Lindholm, P.; Galli, E.; Lahtinen, H.M.; Koivisto, H.; Hamalainen, E.; Saarma, M.; Tanila, H. Cerebral dopamine neurotrophic factor improves long-term memory in APP/PS1 transgenic mice modeling Alzheimer’s disease as well as in wild-type mice. Behav. Brain Res. 2015, 291, 1–11. [Google Scholar] [CrossRef]
- Melief, E.J.; Cudaback, E.; Jorstad, N.L.; Sherfield, E.; Postupna, N.; Wilson, A.; Darvas, M.; Montine, K.S.; Keene, C.D.; Montine, T.J. Partial depletion of striatal dopamine enhances penetrance of cognitive deficits in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. Res. 2015, 93, 1413–1422. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Kovac, S.; Angelova, P.R.; Holmstrom, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 2015, 1850, 794–801. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Guiney, S.J.; Adlard, P.A.; Bush, A.I.; Finkelstein, D.I.; Ayton, S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem. Int. 2017, 104, 34–48. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Gallagher, E.; Hou, C.; Zhu, Y.; Hsieh, C.J.; Lee, H.; Li, S.; Xu, K.; Henderson, P.; Chroneos, R.; Sheldon, M.; et al. Positron Emission Tomography with [18F]ROStrace Reveals Progressive Elevations in Oxidative Stress in a Mouse Model of Alpha-Synucleinopathy. Int. J. Mol. Sci. 2024, 25, 4943. [Google Scholar] [CrossRef]
- Dehay, B.; Bove, J.; Rodriguez-Muela, N.; Perier, C.; Recasens, A.; Boya, P.; Vila, M. Pathogenic lysosomal depletion in Parkinson’s disease. J. Neurosci. 2010, 30, 12535–12544. [Google Scholar] [CrossRef]
- Hernandes, M.S.; Cafe-Mendes, C.C.; Britto, L.R. NADPH oxidase and the degeneration of dopaminergic neurons in parkinsonian mice. Oxidative Med. Cell. Longev. 2013, 2013, 157857. [Google Scholar] [CrossRef]
- Dryanovski, D.I.; Guzman, J.N.; Xie, Z.; Galteri, D.J.; Volpicelli-Daley, L.A.; Lee, V.M.; Miller, R.J.; Schumacker, P.T.; Surmeier, D.J. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 2013, 33, 10154–10164. [Google Scholar] [CrossRef]
- Zhu, Y.; Kohli, N.; Young, A.; Sheldon, M.; Coni, J.; Rajasekaran, M.; Robinson, L.; Chroneos, R.; Riley, S.; Guarnieri, J.W.; et al. PET Imaging with [18F]ROStrace Detects Oxidative Stress and Predicts Parkinson’s Disease Progression in Mice. Antioxidants 2024, 13, 1226. [Google Scholar] [CrossRef]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Do Van, B.; Gouel, F.; Jonneaux, A.; Timmerman, K.; Gele, P.; Petrault, M.; Bastide, M.; Laloux, C.; Moreau, C.; Bordet, R.; et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 2016, 94, 169–178. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014, 136, 4551–4556. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Jiang, L.; Lu, B.; Yuan, M.; Zhu, D.; Zhu, H.; He, Q.; Yang, B.; Ying, M. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat. Commun. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef]
- Cozza, G.; Rossetto, M.; Bosello-Travain, V.; Maiorino, M.; Roveri, A.; Toppo, S.; Zaccarin, M.; Zennaro, L.; Ursini, F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: The polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic. Biol. Med. 2017, 112, 1–11. [Google Scholar] [CrossRef]
- Castellani, R.J.; Siedlak, S.L.; Perry, G.; Smith, M.A. Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol. 2000, 100, 111–114. [Google Scholar] [CrossRef]
- Golts, N.; Snyder, H.; Frasier, M.; Theisler, C.; Choi, P.; Wolozin, B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J. Biol. Chem. 2002, 277, 16116–16123. [Google Scholar] [CrossRef]
- Guo, J.J.; Yue, F.; Song, D.Y.; Bousset, L.; Liang, X.; Tang, J.; Yuan, L.; Li, W.; Melki, R.; Tang, Y.; et al. Intranasal administration of alpha-synuclein preformed fibrils triggers microglial iron deposition in the substantia nigra of Macaca fascicularis. Cell Death Dis. 2021, 12, 81. [Google Scholar] [CrossRef]
- Kenkhuis, B.; Somarakis, A.; de Haan, L.; Dzyubachyk, O.; IJsselsteijn, M.E.; de Miranda, N.F.C.C.; Lelieveldt, B.P.F.; Dijkstra, J.; van Roon-Mom, W.M.C.; Hollt, T.; et al. Iron loading is a prominent feature of activated microglia in Alzheimer’s disease patients. Acta Neuropathol. Commun. 2021, 9, 27. [Google Scholar] [CrossRef]
- Friedrich, I.; Reimann, K.; Jankuhn, S.; Kirilina, E.; Stieler, J.; Sonntag, M.; Meijer, J.; Weiskopf, N.; Reinert, T.; Arendt, T.; et al. Cell specific quantitative iron mapping on brain slices by immuno-microPIXE in healthy elderly and Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9, 47. [Google Scholar] [CrossRef]
- Healy, S.; McMahon, J.; Owens, P.; FitzGerald, U. Significant glial alterations in response to iron loading in a novel organotypic hippocampal slice culture model. Sci. Rep. 2016, 6, 36410. [Google Scholar] [CrossRef]
- Wang, J.; Song, N.; Jiang, H.; Wang, J.; Xie, J. Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim. Biophys. Acta 2013, 1832, 618–625. [Google Scholar] [CrossRef]
- Urrutia, P.; Aguirre, P.; Esparza, A.; Tapia, V.; Mena, N.P.; Arredondo, M.; Gonzalez-Billault, C.; Nunez, M.T. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 2013, 126, 541–549. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Apocyanin, a Microglial NADPH Oxidase Inhibitor Prevents Dopaminergic Neuronal Degeneration in Lipopolysaccharide-Induced Parkinson’s Disease Model. Mol. Neurobiol. 2016, 53, 3326–3337. [Google Scholar] [CrossRef]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Song, N.; Wang, J.; Jiang, H.; Xie, J. New Progress on the Role of Glia in Iron Metabolism and Iron-Induced Degeneration of Dopamine Neurons in Parkinson’s Disease. Front. Mol. Neurosci. 2017, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; David, S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J. Biol. Chem. 2003, 278, 27144–27148. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Lei, P.; Duce, J.A.; Wong, B.X.; Sedjahtera, A.; Adlard, P.A.; Bush, A.I.; Finkelstein, D.I. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 2013, 73, 554–559. [Google Scholar] [CrossRef]
- Wang, J.; Bi, M.; Xie, J. Ceruloplasmin is Involved in the Nigral Iron Accumulation of 6-OHDA-Lesioned Rats. Cell. Mol. Neurobiol. 2015, 35, 661–668. [Google Scholar] [CrossRef]
- Fernandez-Mendivil, C.; Luengo, E.; Trigo-Alonso, P.; Garcia-Magro, N.; Negredo, P.; Lopez, M.G. Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism. Redox Biol. 2021, 38, 101789. [Google Scholar] [CrossRef]
- Song, W.; Cressatti, M.; Zukor, H.; Liberman, A.; Galindez, C.; Schipper, H.M. Parkinsonian features in aging GFAP.HMOX1 transgenic mice overexpressing human HO-1 in the astroglial compartment. Neurobiol. Aging 2017, 58, 163–179. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yuan, L.; Li, W.; Li, J.Y. Ferroptosis in Parkinson’s disease: Glia-neuron crosstalk. Trends Mol. Med. 2022, 28, 258–269. [Google Scholar] [CrossRef]
- Burns, R.S.; LeWitt, P.A.; Ebert, M.H.; Pakkenberg, H.; Kopin, I.J. The clinical syndrome of striatal dopamine deficiency. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N. Engl. J. Med. 1985, 312, 1418–1421. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Beal, M.F. Mitochondrial approaches for neuroprotection. Ann. N. Y. Acad. Sci. 2008, 1147, 395–412. [Google Scholar] [CrossRef]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef]
- Borland, M.K.; Trimmer, P.A.; Rubinstein, J.D.; Keeney, P.M.; Mohanakumar, K.; Liu, L.; Bennett, J.P., Jr. Chronic, low-dose rotenone reproduces Lewy neurites found in early stages of Parkinson’s disease, reduces mitochondrial movement and slowly kills differentiated SH-SY5Y neural cells. Mol. Neurodegener. 2008, 3, 21. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Klivenyi, P.; Siwek, D.; Gardian, G.; Yang, L.; Starkov, A.; Cleren, C.; Ferrante, R.J.; Kowall, N.W.; Abeliovich, A.; Beal, M.F. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 2006, 21, 541–548. [Google Scholar] [CrossRef]
- Thomas, B.; Beal, M.F. Parkinson’s disease. Hum. Mol. Genet. 2007, 16, R183–R194. [Google Scholar] [CrossRef]
- Mudo, G.; Makela, J.; Di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Malkia, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Casarejos, M.J.; Menendez, J.; Solano, R.M.; Rodriguez-Navarro, J.A.; Garcia de Yebenes, J.; Mena, M.A. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J. Neurochem. 2006, 97, 934–946. [Google Scholar] [CrossRef]
- Palacino, J.J.; Sagi, D.; Goldberg, M.S.; Krauss, S.; Motz, C.; Wacker, M.; Klose, J.; Shen, J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004, 279, 18614–18622. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, C.; Yin, J.; Li, X.; Cheng, F.; Li, Y.; Yang, H.; Ueda, K.; Chan, P.; Yu, S. α-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci. Lett. 2009, 454, 187–192. [Google Scholar] [CrossRef]
- Bertolin, G.; Ferrando-Miguel, R.; Jacoupy, M.; Traver, S.; Grenier, K.; Greene, A.W.; Dauphin, A.; Waharte, F.; Bayot, A.; Salamero, J.; et al. The TOMM machinery is a molecular switch in PINK1 and PARK2/PARKIN-dependent mitochondrial clearance. Autophagy 2013, 9, 1801–1817. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; de Vries, R.L.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 2010, 107, 378–383. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Shen, L.; Zhu, Y.; Ma, H.; Liu, B.; Ouyang, L.; Guo, W.; Xu, Q.; Sun, Y. SHP2-mediated mitophagy boosted by lovastatin in neuronal cells alleviates parkinsonism in mice. Signal Transduct. Target. Ther. 2021, 6, 34. [Google Scholar] [CrossRef]
- Lavara-Culebras, E.; Paricio, N. Drosophila DJ-1 mutants are sensitive to oxidative stress and show reduced lifespan and motor deficits. Gene 2007, 400, 158–165. [Google Scholar] [CrossRef]
- Irrcher, I.; Aleyasin, H.; Seifert, E.L.; Hewitt, S.J.; Chhabra, S.; Phillips, M.; Lutz, A.K.; Rousseaux, M.W.; Bevilacqua, L.; Jahani-Asl, A.; et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef]
- Krebiehl, G.; Ruckerbauer, S.; Burbulla, L.F.; Kieper, N.; Maurer, B.; Waak, J.; Wolburg, H.; Gizatullina, Z.; Gellerich, F.N.; Woitalla, D.; et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS ONE 2010, 5, e9367. [Google Scholar] [CrossRef]
- Saha, S.; Guillily, M.D.; Ferree, A.; Lanceta, J.; Chan, D.; Ghosh, J.; Hsu, C.H.; Segal, L.; Raghavan, K.; Matsumoto, K.; et al. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J. Neurosci. 2009, 29, 9210–9218. [Google Scholar] [CrossRef]
- Yue, M.; Hinkle, K.M.; Davies, P.; Trushina, E.; Fiesel, F.C.; Christenson, T.A.; Schroeder, A.S.; Zhang, L.; Bowles, E.; Behrouz, B.; et al. Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis. 2015, 78, 172–195. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.H.; Fujioka, H.; Liu, J.; Wilson-Delfosse, A.; Chen, S.G.; Perry, G.; Casadesus, G.; Zhu, X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 2012, 21, 1931–1944. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Riederer, P.; Narabayashi, H.; Fujita, K.; Nagatsu, T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994, 165, 208–210. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Narabayashi, H.; Riederer, P.; Nagatsu, T. Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci. Lett. 1995, 193, 129–132. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Nagatsu, T. Interleukin-2 but not basic fibroblast growth factor is elevated in parkinsonian brain. J. Neural Transm. 1996, 103, 1077–1081. [Google Scholar] [CrossRef]
- Sommer, A.; Fadler, T.; Dorfmeister, E.; Hoffmann, A.C.; Xiang, W.; Winner, B.; Prots, I. Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model. J. Neuroinflamm. 2016, 13, 174. [Google Scholar] [CrossRef]
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef]
- Stone, D.K.; Reynolds, A.D.; Mosley, R.L.; Gendelman, H.E. Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid. Redox Signal. 2009, 11, 2151–2166. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Comi, C.; Magistrelli, L.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Minafra, B.; Riboldazzi, G.; et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J. Neuroinflamm. 2018, 15, 205. [Google Scholar] [CrossRef]
- Panicker, N.; Sarkar, S.; Harischandra, D.S.; Neal, M.; Kam, T.I.; Jin, H.; Saminathan, H.; Langley, M.; Charli, A.; Samidurai, M.; et al. Fyn kinase regulates misfolded alpha-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 2019, 216, 1411–1430. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yagi, S.; Yokokura, M.; Sakamoto, M. Neuroinflammation in the living brain of Parkinson’s disease. Park. Relat. Disord. 2009, 15 (Suppl. 3), S200–S204. [Google Scholar] [CrossRef]
- Haque, M.E.; Akther, M.; Jakaria, M.; Kim, I.S.; Azam, S.; Choi, D.K. Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov. Disord. 2020, 35, 20–33. [Google Scholar] [CrossRef]
- Labzin, L.I.; Heneka, M.T.; Latz, E. Innate Immunity and Neurodegeneration. Annu. Rev. Med. 2018, 69, 437–449. [Google Scholar] [CrossRef]
- Earls, R.H.; Menees, K.B.; Chung, J.; Barber, J.; Gutekunst, C.A.; Hazim, M.G.; Lee, J.K. Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J. Neuroinflamm. 2019, 16, 250. [Google Scholar] [CrossRef]
- Kuhbandner, K.; Hoffmann, A.; Gonzalez Alvarado, M.N.; Seyler, L.; Bauerle, T.; Winkler, J.; Linker, R.A. alpha-Synuclein: A Modulator During Inflammatory CNS Demyelination. J. Mol. Neurosci. 2020, 70, 1038–1049. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammation and the degenerative diseases of aging. Ann. N. Y. Acad. Sci. 2004, 1035, 104–116. [Google Scholar] [CrossRef]
- Ambrosi, G.; Kustrimovic, N.; Siani, F.; Rasini, E.; Cerri, S.; Ghezzi, C.; Dicorato, G.; Caputo, S.; Marino, F.; Cosentino, M.; et al. Complex Changes in the Innate and Adaptive Immunity Accompany Progressive Degeneration of the Nigrostriatal Pathway Induced by Intrastriatal Injection of 6-Hydroxydopamine in the Rat. Neurotox. Res. 2017, 32, 71–81. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-kappaB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Hunot, S.; Boissiere, F.; Faucheux, B.; Brugg, B.; Mouatt-Prigent, A.; Agid, Y.; Hirsch, E.C. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 1996, 72, 355–363. [Google Scholar] [CrossRef]
- Noelker, C.; Morel, L.; Lescot, T.; Osterloh, A.; Alvarez-Fischer, D.; Breloer, M.; Henze, C.; Depboylu, C.; Skrzydelski, D.; Michel, P.P.; et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci. Rep. 2013, 3, 1393. [Google Scholar] [CrossRef]
- Sommer, A.; Marxreiter, F.; Krach, F.; Fadler, T.; Grosch, J.; Maroni, M.; Graef, D.; Eberhardt, E.; Riemenschneider, M.J.; Yeo, G.W.; et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell 2018, 23, 123–131.e6. [Google Scholar] [CrossRef]
- Galiano-Landeira, J.; Torra, A.; Vila, M.; Bove, J. CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 2020, 143, 3717–3733. [Google Scholar] [CrossRef]
- Karikari, A.A.; McFleder, R.L.; Ribechini, E.; Blum, R.; Bruttel, V.; Knorr, S.; Gehmeyr, M.; Volkmann, J.; Brotchie, J.M.; Ahsan, F.; et al. Neurodegeneration by alpha-synuclein-specific T cells in AAV-A53T-alpha-synuclein Parkinson’s disease mice. Brain Behav. Immun. 2022, 101, 194–210. [Google Scholar] [CrossRef]
- Williams, G.P.; Schonhoff, A.M.; Jurkuvenaite, A.; Gallups, N.J.; Standaert, D.G.; Harms, A.S. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 2021, 144, 2047–2059. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Schwiertz, A.; Spiegel, J.; Dillmann, U.; Grundmann, D.; Burmann, J.; Fassbender, K.; Schafer, K.H.; Unger, M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Park. Relat. Disord. 2018, 50, 104–107. [Google Scholar] [CrossRef]
- Patterson, A.M.; Mulder, I.E.; Travis, A.J.; Lan, A.; Cerf-Bensussan, N.; Gaboriau-Routhiau, V.; Garden, K.; Logan, E.; Delday, M.I.; Coutts, A.G.P.; et al. Human Gut Symbiont Roseburia hominis Promotes and Regulates Innate Immunity. Front. Immunol. 2017, 8, 1166. [Google Scholar] [CrossRef]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.J.; Zhu, L.; et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1−/− mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef]
- Kelly, L.P.; Carvey, P.M.; Keshavarzian, A.; Shannon, K.M.; Shaikh, M.; Bakay, R.A.; Kordower, J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. 2014, 29, 999–1009. [Google Scholar] [CrossRef]
- Gorecki, A.M.; Preskey, L.; Bakeberg, M.C.; Kenna, J.E.; Gildenhuys, C.; MacDougall, G.; Dunlop, S.A.; Mastaglia, F.L.; Akkari, P.A.; Koengten, F.; et al. Altered Gut Microbiome in Parkinson’s Disease and the Influence of Lipopolysaccharide in a Human alpha-Synuclein Over-Expressing Mouse Model. Front. Neurosci. 2019, 13, 839. [Google Scholar] [CrossRef]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded alpha-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e7. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Yagi, H.; Uemura, M.T.; Hatanaka, Y.; Yamakado, H.; Takahashi, R. Inoculation of alpha-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 2018, 13, 21. [Google Scholar] [CrossRef]
- Van Den Berge, N.; Ferreira, N.; Gram, H.; Mikkelsen, T.W.; Alstrup, A.K.O.; Casadei, N.; Tsung-Pin, P.; Riess, O.; Nyengaard, J.R.; Tamguney, G.; et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019, 138, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Arotcarena, M.L.; Dovero, S.; Prigent, A.; Bourdenx, M.; Camus, S.; Porras, G.; Thiolat, M.L.; Tasselli, M.; Aubert, P.; Kruse, N.; et al. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 2020, 143, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Neuropathology of Parkinson disease. Park. Relat. Disord. 2018, 46 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef]
- Ding, F.; Luan, L.; Ai, Y.; Walton, A.; Gerhardt, G.A.; Gash, D.M.; Grondin, R.; Zhang, Z. Development of a stable, early stage unilateral model of Parkinson’s disease in middle-aged rhesus monkeys. Exp. Neurol. 2008, 212, 431–439. [Google Scholar] [CrossRef]
- Kuwahara, T.; Koyama, A.; Gengyo-Ando, K.; Masuda, M.; Kowa, H.; Tsunoda, M.; Mitani, S.; Iwatsubo, T. Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J. Biol. Chem. 2006, 281, 334–340. [Google Scholar] [CrossRef]
- Lakso, M.; Vartiainen, S.; Moilanen, A.M.; Sirvio, J.; Thomas, J.H.; Nass, R.; Blakely, R.D.; Wong, G. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J. Neurochem. 2003, 86, 165–172. [Google Scholar] [CrossRef]
- Chesselet, M.F. In vivo alpha-synuclein overexpression in rodents: A useful model of Parkinson’s disease? Exp. Neurol. 2008, 209, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.; Mandir, A.; Lee, M. Animal models of PD: Pieces of the same puzzle? Neuron 2002, 35, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Duda, J.E.; Quinn, S.M.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 2002, 34, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Stirling, W.; Xu, Y.; Xu, X.; Qui, D.; Mandir, A.S.; Dawson, T.M.; Copeland, N.G.; Jenkins, N.A.; Price, D.L. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8968–8973. [Google Scholar] [CrossRef]
- Ekstrand, M.I.; Falkenberg, M.; Rantanen, A.; Park, C.B.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.M.; Larsson, N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef]
- Grunewald, A.; Kumar, K.R.; Sue, C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Luk, K.C.; Song, C.; O’Brien, P.; Stieber, A.; Branch, J.R.; Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20051–20056. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.M.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J. Exp. Med. 2012, 209, 975–986. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Paumier, K.L.; Luk, K.C.; Manfredsson, F.P.; Kanaan, N.M.; Lipton, J.W.; Collier, T.J.; Steece-Collier, K.; Kemp, C.J.; Celano, S.; Schulz, E.; et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 2015, 82, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Abdelmotilib, H.; Maltbie, T.; Delic, V.; Liu, Z.; Hu, X.; Fraser, K.B.; Moehle, M.S.; Stoyka, L.; Anabtawi, N.; Krendelchtchikova, V.; et al. α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration. Neurobiol. Dis. 2017, 105, 84–98. [Google Scholar] [CrossRef] [PubMed]
- PD MED Collaborative Group. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): A large, open-label, pragmatic randomised trial. Lancet 2014, 384, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Fahn, S. Levodopa therapy for Parkinson disease: A look backward and forward. Neurology 2016, 86 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef]
- Olanow, C.W.; Obeso, J.A.; Stocchi, F. Continuous dopamine-receptor treatment of Parkinson’s disease: Scientific rationale and clinical implications. Lancet Neurol. 2006, 5, 677–687. [Google Scholar] [CrossRef]
- Cenci, M.A. Presynaptic Mechanisms of l-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications. Front. Neurol. 2014, 5, 242. [Google Scholar] [CrossRef]
- Poewe, W.; Antonini, A. Novel formulations and modes of delivery of levodopa. Mov. Disord. 2015, 30, 114–120. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; de Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C.; Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26 (Suppl. 3), S42–S80. [Google Scholar] [CrossRef]
- Jankovic, J.; Poewe, W. Therapies in Parkinson’s disease. Curr. Opin. Neurol. 2012, 25, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; Mehta, A.R.; Hallett, M. Impulse control disorders in Parkinson’s disease: Recent advances. Curr. Opin. Neurol. 2011, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Frankel, J.P.; Lees, A.J.; Kempster, P.A.; Stern, G.M. Subcutaneous apomorphine in the treatment of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1990, 53, 96–101. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Hughes, A.; Evans, A.; Manson, A.J.; Hoffman, M.; Swinn, L.; Watt, H.; Bhatia, K.; Quinn, N.; Lees, A.J. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: A prospective study using single-dose challenges. Mov. Disord. 2005, 20, 151–157. [Google Scholar] [CrossRef]
- Hauser, R.A.; Olanow, C.W.; Dzyngel, B.; Bilbault, T.; Shill, H.; Isaacson, S.; Dubow, J.; Agro, A. Sublingual apomorphine (APL-130277) for the acute conversion of OFF to ON in Parkinson’s disease. Mov. Disord. 2016, 31, 1366–1372. [Google Scholar] [CrossRef]
- Muller, T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs 2015, 75, 157–174. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Lees, A.; Rocha, J.F.; Poewe, W.; Rascol, O.; Soares-da-Silva, P.; Bi-Park 1 Investigators. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: A randomised, double-blind, controlled trial. Lancet Neurol. 2016, 15, 154–165. [Google Scholar] [CrossRef]
- Schapira, A.H. Monoamine oxidase B inhibitors for the treatment of Parkinson’s disease: A review of symptomatic and potential disease-modifying effects. CNS Drugs 2011, 25, 1061–1071. [Google Scholar] [CrossRef]
- Birkmayer, W.; Riederer, P.; Ambrozi, L.; Youdim, M.B. Implications of combined treatment with ‘Madopar’ and L-deprenil in Parkinson’s disease. A long-term study. Lancet 1977, 1, 439–443. [Google Scholar] [CrossRef]
- Schapira, A.H.; Fox, S.H.; Hauser, R.A.; Jankovic, J.; Jost, W.H.; Kenney, C.; Kulisevsky, J.; Pahwa, R.; Poewe, W.; Anand, R. Assessment of Safety and Efficacy of Safinamide as a Levodopa Adjunct in Patients with Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 216–224. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.; Fox, S.H. Treatment of cognitive, psychiatric, and affective disorders associated with Parkinson’s disease. Neurotherapeutics 2014, 11, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Rey, M.V.; Pavy-Le Traon, A.; Rascol, O. Emerging drugs for autonomic dysfunction in Parkinson’s disease. Expert Opin. Emerg. Drugs 2013, 18, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, R.; Werth, J.; Zhang, Y.; Duvvuri, S.; Gray, D. PF-06649751 efficacy and safety in early Parkinson’s disease: A randomized, placebo-controlled trial. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420911296. [Google Scholar] [CrossRef]
- Svenningsson, P.; Johansson, A.; Nyholm, D.; Tsitsi, P.; Hansson, F.; Sonesson, C.; Tedroff, J. Safety and tolerability of IRL790 in Parkinson’s disease with levodopa-induced dyskinesia-a phase 1b trial. npj Park. Dis. 2018, 4, 35. [Google Scholar] [CrossRef]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garcon, G.; Rouaix, N.; et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Ward, R.J.; Newbould, R.; Piccini, P.; Sharp, D.; Kabba, C.; Patel, M.C.; Spino, M.; Connelly, J.; Tricta, F.; et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017, 7, 1398. [Google Scholar] [CrossRef]
- Hung, L.W.; Villemagne, V.L.; Cheng, L.; Sherratt, N.A.; Ayton, S.; White, A.R.; Crouch, P.J.; Lim, S.; Leong, S.L.; Wilkins, S.; et al. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J. Exp. Med. 2012, 209, 837–854. [Google Scholar] [CrossRef]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef]
- Yang, J.; Song, S.; Li, J.; Liang, T. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson’s disease rat. Pathol. Res. Pract. 2014, 210, 357–362. [Google Scholar] [CrossRef]
- Baj, T.; Seth, R. Role of Curcumin in Regulation of TNF-alpha Mediated Brain Inflammatory Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 69–77. [Google Scholar] [CrossRef] [PubMed]
- El Nebrisi, E.; Javed, H.; Ojha, S.K.; Oz, M.; Shehab, S. Neuroprotective Effect of Curcumin on the Nigrostriatal Pathway in a 6-Hydroxydopmine-Induced Rat Model of Parkinson’s Disease is Mediated by alpha7-Nicotinic Receptors. Int. J. Mol. Sci. 2020, 21, 7329. [Google Scholar] [CrossRef] [PubMed]
- Cretu, E.; Trifan, A.; Vasincu, A.; Miron, A. Plant-derived anticancer agents—Curcumin in cancer prevention and treatment. Rev. Med. Chir. Soc. Med. Nat. Iasi 2012, 116, 1223–1229. [Google Scholar] [PubMed]
- Ghasemi, F.; Bagheri, H.; Barreto, G.E.; Read, M.I.; Sahebkar, A. Effects of Curcumin on Microglial Cells. Neurotox. Res. 2019, 36, 12–26. [Google Scholar] [CrossRef]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1beta-Induced Neuronal Apoptosis and Depression-like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2018, 12, 516. [Google Scholar]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Albani, D.; Polito, L.; Batelli, S.; De Mauro, S.; Fracasso, C.; Martelli, G.; Colombo, L.; Manzoni, C.; Salmona, M.; Caccia, S.; et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009, 110, 1445–1456. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, W.; Wang, H.; Bao, L.; Li, G.; Li, T.; Song, S.; Li, H.; Hao, J.; Sun, J. Resveratrol Protects PC12 Cell against 6-OHDA Damage via CXCR4 Signaling Pathway. Evid.-Based Complement. Altern. Med. 2015, 2015, 730121. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, W.; Lu, F.; Gao, L.; Gao, G. Resveratrol attenuates MPP+-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3beta pathway in SN4741 cells. Neurosci. Lett. 2017, 637, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y.; et al. Resveratrol Suppresses Rotenone-induced Neurotoxicity Through Activation of SIRT1/Akt1 Signaling Pathway. Anat. Rec. 2018, 301, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Zhang, Y.; Chen, M.; Jin, H.; Nie, J.; Luo, Y.; Zhou, S.; Shi, J.; Jin, F. Resveratrol delays 6-hydroxydopamine-induced apoptosis by activating the PI3K/Akt signaling pathway. Exp. Gerontol. 2019, 124, 110653. [Google Scholar] [CrossRef] [PubMed]
- Abolaji, A.O.; Adedara, A.O.; Adie, M.A.; Vicente-Crespo, M.; Farombi, E.O. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2018, 503, 1042–1048. [Google Scholar] [CrossRef]
- Lin, K.L.; Lin, K.J.; Wang, P.W.; Chuang, J.H.; Lin, H.Y.; Chen, S.D.; Chuang, Y.C.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol provides neuroprotective effects through modulation of mitochondrial dynamics and ERK1/2 regulated autophagy. Free Radic. Res. 2018, 52, 1371–1386. [Google Scholar] [CrossRef]
- Palle, S.; Neerati, P. Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 445–453. [Google Scholar] [CrossRef]
- Peng, K.; Tao, Y.; Zhang, J.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Zhao, J.; Wu, Q.; et al. Resveratrol Regulates Mitochondrial Biogenesis and Fission/Fusion to Attenuate Rotenone-Induced Neurotoxicity. Oxidative Med. Cell. Longev. 2016, 2016, 6705621. [Google Scholar] [CrossRef]
- Guo, Y.J.; Dong, S.Y.; Cui, X.X.; Feng, Y.; Liu, T.; Yin, M.; Kuo, S.H.; Tan, E.K.; Zhao, W.J.; Wu, Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of alpha-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016, 60, 2161–2175. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, D.; Jiang, P.; Tang, X.; Lang, Q.; Yu, Q.; Zhang, S.; Che, Y.; Feng, X. Resveratrol synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson disease in mice. Behav. Brain Res. 2019, 367, 10–18. [Google Scholar] [CrossRef]
- Lin, T.K.; Chen, S.D.; Chuang, Y.C.; Lin, H.Y.; Huang, C.R.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014, 15, 1625–1646. [Google Scholar] [CrossRef]
- Deng, H.; Ma, Z. Protective effects of berberine against MPTP-induced dopaminergic neuron injury through promoting autophagy in mice. Food Funct. 2021, 12, 8366–8375. [Google Scholar] [CrossRef] [PubMed]
- Dulovic, M.; Jovanovic, M.; Xilouri, M.; Stefanis, L.; Harhaji-Trajkovic, L.; Kravic-Stevovic, T.; Paunovic, V.; Ardah, M.T.; El-Agnaf, O.M.; Kostic, V.; et al. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol. Dis. 2014, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Lin, Y.; Liu, M.; Li, Y.; Mao, H.; Zhang, Z.; Zhang, Y.; Ye, P.; Ding, L.; et al. Berberine Protects Against NLRP3 Inflammasome via Ameliorating Autophagic Impairment in MPTP-Induced Parkinson’s Disease Model. Front. Pharmacol. 2020, 11, 618787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Hou, Y.S.; Guan, J.J.; Xu, H.D.; Wu, F.; Sheng, R.; Qin, Z.H. Sestrin2 Protects Dopaminergic Cells against Rotenone Toxicity through AMPK-Dependent Autophagy Activation. Mol. Cell. Biol. 2015, 35, 2740–2751. [Google Scholar] [CrossRef]
- Lu, J.H.; Tan, J.Q.; Durairajan, S.S.; Liu, L.F.; Zhang, Z.H.; Ma, L.; Shen, H.M.; Chan, H.Y.; Li, M. Isorhynchophylline, a natural alkaloid, promotes the degradation of alpha-synuclein in neuronal cells via inducing autophagy. Autophagy 2012, 8, 98–108. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, X.J.; Dong, M.X. Isorhynchophylline Attenuates MPP+-Induced Apoptosis Through Endoplasmic Reticulum Stress- and Mitochondria-Dependent Pathways in PC12 Cells: Involvement of Antioxidant Activity. Neuromol. Med. 2017, 19, 480–492. [Google Scholar] [CrossRef]
- Hu, X.; Weng, Z.; Chu, C.T.; Zhang, L.; Cao, G.; Gao, Y.; Signore, A.; Zhu, J.; Hastings, T.; Greenamyre, J.T.; et al. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J. Neurosci. 2011, 31, 247–261. [Google Scholar] [CrossRef]
- Kim, S.M.; Chung, M.J.; Ha, T.J.; Choi, H.N.; Jang, S.J.; Kim, S.O.; Chun, M.H.; Do, S.I.; Choo, Y.K.; Park, Y.I. Neuroprotective effects of black soybean anthocyanins via inactivation of ASK1-JNK/p38 pathways and mobilization of cellular sialic acids. Life Sci. 2012, 90, 874–882. [Google Scholar] [CrossRef]
- Lee, K.W.; Zhao, X.; Im, J.Y.; Grosso, H.; Jang, W.H.; Chan, T.W.; Sonsalla, P.K.; German, D.C.; Ichijo, H.; Junn, E.; et al. Apoptosis signal-regulating kinase 1 mediates MPTP toxicity and regulates glial activation. PLoS ONE 2012, 7, e29935. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.; Zhuang, J.; Xu, C.; Zhang, F. Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic stretch-induced pyroptosis and release of IL-1beta in human periodontal ligament cells. Oncotarget 2016, 7, 68292–68302. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhang, X.; Dong, M. Puerarin suppresses MPP+/MPTP-induced oxidative stress through an Nrf2-dependent mechanism. Food Chem. Toxicol. 2020, 144, 111644. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qu, X.; Jin, G.; Nie, F.; Liu, F.; Chen, S.; Hu, F. Puerarin promoted proliferation and differentiation of dopamine-producing cells in Parkinson’s animal models. Biomed. Pharmacother. 2018, 106, 1236–1242. [Google Scholar]
- Zhao, Y.; Zhao, J.; Zhang, X.; Cheng, Y.; Luo, D.; Lee, S.M.; Lao, L.; Rong, J. Botanical Drug Puerarin Promotes Neuronal Survival and Neurite Outgrowth against MPTP/MPP+-Induced Toxicity via Progesterone Receptor Signaling. Oxidative Med. Cell. Longev. 2020, 2020, 7635291. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, D.; Zhang, B.; Lu, H. Bergenin Ameliorates MPTP-Induced Parkinson’s Disease by Activating PI3K/Akt Signaling Pathway. J. Alzheimer’s Dis. 2019, 72, 823–833. [Google Scholar] [CrossRef]
- Luo, S.; Kang, S.S.; Wang, Z.H.; Liu, X.; Day, J.X.; Wu, Z.; Peng, J.; Xiang, D.; Springer, W.; Ye, K. Akt Phosphorylates NQO1 and Triggers its Degradation, Abolishing Its Antioxidative Activities in Parkinson’s Disease. J. Neurosci. 2019, 39, 7291–7305. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Liu, L.; Xie, A. The Role of Insulin/IGF-1/PI3K/Akt/GSK3beta Signaling in Parkinson’s Disease Dementia. Front. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Wu, S.; Li, Q. Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem. Int. 2012, 60, 400–408. [Google Scholar] [CrossRef]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/caspase-1/GSDMD Pathway in MPTP Induced Mice Model of Parkinson’s Disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, eaah4066. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, W.T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Zhao, X.; Kong, D.; Zhou, Q.; Wei, G.; Song, J.; Liang, Y.; Du, G. Baicalein alleviates depression-like behavior in rotenone- induced Parkinson’s disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother. 2021, 140, 111556. [Google Scholar] [CrossRef]
- Zheng, Z.V.; Cheung, C.Y.; Lyu, H.; Chan, H.Y.; Li, Y.; Bian, Z.X.; Wang, K.K.W.; Poon, W.S. Baicalein enhances the effect of low dose Levodopa on the gait deficits and protects dopaminergic neurons in experimental Parkinsonism. J. Clin. Neurosci. 2019, 64, 242–251. [Google Scholar] [CrossRef]
- Song, J.X.; Choi, M.Y.; Wong, K.C.; Chung, W.W.; Sze, S.C.; Ng, T.B.; Zhang, K.Y. Baicalein antagonizes rotenone-induced apoptosis in dopaminergic SH-SY5Y cells related to Parkinsonism. Chin. Med. 2012, 7, 1. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Wang, B.; Su, Z.; Guo, B.; Qin, L.; Zhang, W.; Zheng, R. The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson’s disease. Redox Biol. 2021, 47, 102134. [Google Scholar] [CrossRef]
- Lin, M.W.; Lin, C.C.; Chen, Y.H.; Yang, H.B.; Hung, S.Y. Celastrol Inhibits Dopaminergic Neuronal Death of Parkinson’s Disease through Activating Mitophagy. Antioxidants 2019, 9, 37. [Google Scholar] [CrossRef]
- Feng, Y.; Zheng, C.; Zhang, Y.; Xing, C.; Cai, W.; Li, R.; Chen, J.; Duan, Y. Triptolide Inhibits Preformed Fibril-Induced Microglial Activation by Targeting the MicroRNA155-5p/SHIP1 Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 6527638. [Google Scholar] [CrossRef]
- Lu, S.; Liao, Q.S.; Tang, L. MiR-155 affects osteosarcoma cell proliferation and invasion through regulating NF-kappaB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7633–7639. [Google Scholar] [PubMed]
- Huang, Y.Y.; Zhang, Q.; Zhang, J.N.; Zhang, Y.N.; Gu, L.; Yang, H.M.; Xia, N.; Wang, X.M.; Zhang, H. Triptolide up-regulates metabotropic glutamate receptor 5 to inhibit microglia activation in the lipopolysaccharide-induced model of Parkinson’s disease. Brain Behav. Immun. 2018, 71, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gong, X.; Wang, L.; Liu, M.; Liu, Y.; Fu, X.; Wang, W.; Zhang, T.; Wang, X. Triptolide Promotes the Clearance of alpha-Synuclein by Enhancing Autophagy in Neuronal Cells. Mol. Neurobiol. 2017, 54, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Meng, X.; Liu, Y.; Zhang, X.; Zhang, Y. Ginsenoside Rb1 prevents MPTP-induced changes in hippocampal memory via regulation of the alpha-synuclein/PSD-95 pathway. Aging 2019, 11, 1934–1964. [Google Scholar] [CrossRef]
- Li, D.W.; Zhou, F.Z.; Sun, X.C.; Li, S.C.; Yang, J.B.; Sun, H.H.; Wang, A.H. Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen. Res. 2019, 14, 1814–1822. [Google Scholar]
- Han, Y.; Wang, T.; Li, C.; Wang, Z.; Zhao, Y.; He, J.; Fu, L.; Han, B. Ginsenoside Rg3 exerts a neuroprotective effect in rotenone-induced Parkinson’s disease mice via its anti-oxidative properties. Eur. J. Pharmacol. 2021, 909, 174413. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New opportunities and challenges of natural products research: When target identification meets single-cell multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).