Polymorphism’s MBOAT7 as Risk and MTARC1 as Protection for Liver Fibrosis in MASLD
Abstract
1. Introduction
2. Results
Impact of the rs641738 Variant of the MBOAT7 Gene on Significant Fibrosis in Patients with MASLD
3. Discussion
- Europeans: CC (46%), CG (32%), and GG (22%);
- Asians: CC (18%), CG (42%), and GG (40%);
- Africans: CC (96%), CG (3.9%), and GG (0.1%);
- Latin Americans (Brazil): CC (46.8%), CG (40.5%), and GG (12.6%);
- Chinese: CC (39.2%), CG (47.6%), and GG (13.2%).
- Europeans: CC (35%), CT (45%), and TT (20%);
- Latin Americans (Brazil): CC (44%), CT (46%), and TT (10%);
- Africans: CC (46%), CT (44%), and TT (10%);
- East Asia: CC (32%), CT (49%), and TT (19%);
- South Asia: CC (22%), CT (50%), and TT (28%);
- South Europe: CC (32%), CT (50%), and TT (19%);
- North Europe: CC (33%), CT (47%), and TT (20%).
- Europeans: AA (53%), AG (39%), and GG (8%);
- African-Americans: AA (90%), AG (9%), and GG (1%);
- Asians: AA (95%), AG (5%), and GG (0%).
4. Materials and Methods
4.1. Study Population
4.2. Inclusion Criteria
4.3. Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP | Alpha-Fetoprotein |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Transaminase |
| BMI | Body Mass |
| DLP | Dyslipidemia |
| GCKR | Glucokinase Regulator |
| GGT | Gamma-Glutamyl Transpeptidase |
| HCC | Hepatocellular Carcinoma |
| HCFMUSP | Hospital Das Clínicas Da Faculdade De Medicina Da Universidade De São Paulo |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HSD17B13 | Hydroxysteroid 17-Beta Dehydrogenase 13 |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| MBOAT7 | Membrane Bound O-Acyltransferase Domain Containing 7 |
| MTARC1 | Mitochondrial Amidoxime Reducing Component 1 |
| NAS | NAFLD Activity Score |
| PNPLA3 | Patatin Like Phospholipase Domain Containing 3 |
| SNPs | Single Nucleotide Polymorphisms |
| TC | Total Cholesterol |
| T2DM | Type 2 Diabetes |
| TG | Triacyl Glyceride |
| TM6SF2 | Transmembrane 6 Superfamily Member 2 |
| USP | Universidade de São Paulo |
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. Reply: A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K. New concept in fatty liver diseases. Hepatology Research: The official journal of the japan society of hepatology. Hepatol. Res. 2024, 54, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Allen, A.M.; Jarvis, H.; Zelber-Sagi, S.; Cusi, K.; Dillon, J.F.; Caussy, C.; Francque, S.M.; Younossi, Z.; Alkhouri, N.; et al. A multistakeholder approach to innovations in NAFLD care. Commun. Med. 2023, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Lewis, L.C.; Chen, L.; Hameed, L.S.; Kitchen, R.R.; Maroteau, C.; Nagarajan, S.R.; Norlin, J.; Daly, C.E.; Szczerbinska, I.; Hjuler, S.T.; et al. Hepatocyte mARC1 promotes fatty liver disease. JHEP Rep. 2023, 5, 100693. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Hashimoto, E.; Taniai, M.; Tokushige, K. Characteristics and diagnosis of NAFLD/NASH. J. Gastroenterol. Hepatol. 2013, 28, 64–70. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Wang, Y.; Fleishman, J.S.; Li, T.; Li, Y.; Ren, Z.; Chen, J.; Ding, M. Pharmacological therapy of metabolic dysfunction-associated steatotic liver disease-driven hepatocellular carcinoma. Front. Pharmacol. 2024, 14, 1336216. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Juuti, A.; Sammalkorpi, H.; Penttilä, A.K.; Orešič, M.; Hyötyläinen, T.; Arola, J.; Orho-Melander, M.; Yki-Järvinen, H. MARC1 variant rs2642438 increases hepatic phosphatidylcholines and decreases severity of non-alcoholic fatty liver disease in humans. J. Hepatol. 2020, 73, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Jamialahmadi, O.; Pelusi, S.; Baselli, G.; Dongiovanni, P.; Zanoni, I.; Santoro, L.; Maier, S.; Liguori, A.; Meroni, M.; et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J. Hepatol. 2021, 74, 775–782. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatol. Baltim. Md. 2011, 53, 1883–1894. [Google Scholar] [CrossRef]
- Hotta, K.; Yoneda, M.; Hyogo, H.; Ochi, H.; Mizusawa, S.; Ueno, T.; Chayama, K.; Nakajima, A.; Nakao, K.; Sekine, A. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med. Genet. 2010, 11, 172. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Manchiero, C.; Nunes, A.K.d.S.; Magri, M.C.; Dantas, B.P.; Mazza, C.C.; Barone, A.A.; Tengan, F.M. The rs738409 polymorphism of the PNPLA3 gene is associated with hepatic steatosis and fibrosis in Brazilian patients with chronic hepatitis C. BMC Infect. Dis. 2017, 17, 780. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Peng, Z.; Liu, W. The TM6SF2 rs58542926 T allele is significantly associated with non-alcoholic fatty liver disease in Chinese. J. Hepatol. 2015, 62, 1438–1439. [Google Scholar] [CrossRef]
- Petta, S.; Armandi, A.; Bugianesi, E. Impact of PNPLA3 I148M on Clinical Outcomes in Patients With MASLD. Liver Int. 2024, 45, e16133. [Google Scholar] [CrossRef]
- Kim, H.-S.; Xiao, X.; Byun, J.; Jun, G.; DeSantis, S.M.; Chen, H.; Thrift, A.P.; El-Serag, H.B.; Kanwal, F.; Amos, C.I. Synergistic Associations of PNPLA3 I148M Variant, Alcohol Intake, and Obesity with Risk of Cirrhosis, Hepatocellular Carcinoma, and Mortality. JAMA Netw. Open 2022, 5, e2234221. [Google Scholar] [CrossRef]
- Goble, S.; Akambase, J.; Prieto, J.; Balderramo, D.; Ferrer, J.D.; Mattos, A.Z.; Arrese, M.; Carrera, E.; Groothuismink, Z.M.A.; Oliveira, J.; et al. MBOAT7 rs641738 Variant Is Not Associated with an Increased Risk of Hepatocellular Carcinoma in a Latin American Cohort. Dig. Dis. Sci. 2023, 68, 4212–4220. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Wu, C.-C.; Ni, Y.-H. New Perspectives on Genetic Prediction for Pediatric Metabolic Associated Fatty Liver Disease. Front. Pediatr. 2020, 8, 603654. [Google Scholar] [CrossRef] [PubMed]
- Astarini, F.D.; Ratnasari, N.; Wasityastuti, W. Update on Non-Alcoholic Fatty Liver Disease-Associated Single Nucleotide Polymorphisms and Their Involvement in Liver Steatosis, Inflammation, and Fibrosis: A Narrative Review. Iran. Biomed. J. 2022, 26, 252–268. [Google Scholar] [CrossRef]
- Jones, A.K.; Bajrami, B.; Campbell, M.K.; Erzurumluoglu, A.M.; Guo, Q.; Chen, H.; Zhang, X.; Zeveleva, S.; Kvaskoff, D.; Brunner, A.D.; et al. mARC1 in MASLD: Modulation of lipid accumulation in human hepatocytes and adipocytes. Hepatol. Commun. 2024, 8, e0365. [Google Scholar] [CrossRef]
- dbSNP Short Genetic Variations. 2024. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2642438 (accessed on 25 April 2025).
- Guo, Y.; Gao, Z.; LaGory, E.L.; Kristin, L.W.; Gupte, J.; Gong, Y.; Rardin, M.J.; Liu, T.; Nguyen, T.T.; Long, J.; et al. Liver-specific mitochondrial amidoxime–reducing component 1 (Mtarc1) knockdown protects the liver from diet-induced MASH in multiple mouse models. Hepatol. Commun. 2024, 8, e0419. [Google Scholar] [CrossRef]
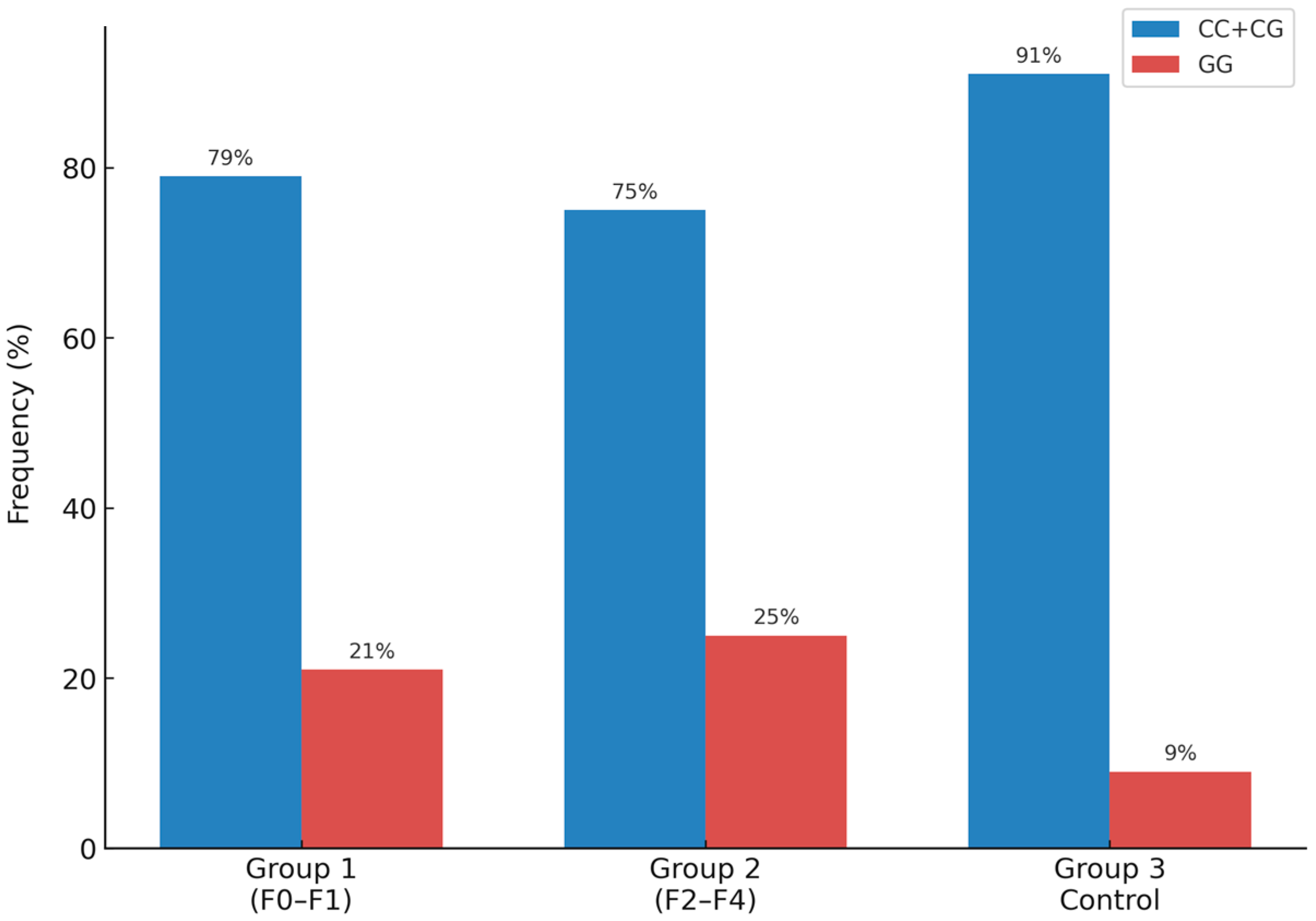

| Characteristic | Group 1 n = 110 (F0–F1) | Group 2 n = 102 (F2–F4) | p |
|---|---|---|---|
| Age, years | 52.8 ± 10.91 | 55.7 ± 11.46 | <0.05 |
| Sex (F/M) % | 68.14/31.86 | 69.70/30.30 | 0.19 |
| BMI, kg/m2 | 32.29 ± 6.49 | 30.8 ± 5.98 | <0.05 |
| DLP (Yes/No) | 56/42 | 38/52 | 0.06 |
| T2D (Yes/No) | 59/39 | 62/28 | 0.28 |
| Hypertension (Yes/No) | 70/28 | 64/26 | 1.00 |
| Glucose, mg/dL | 114.7 ± 37.52 | 129.30 ± 54.85 | <0.05 |
| Insulin, µU/mL | 21.36 ± 16.30 | 20.72 ± 12.33 | 0.83 |
| AST (U/L) | 31.95 ± 26.90 | 49.81 ± 40.24 | <0.05 |
| ALT (U/L) | 44.34 ± 35.72 | 58.16 ± 42.26 | <0.05 |
| GGT (U/L) | 74.58 ± 82.16 | 128.09 ± 157.49 | <0.05 |
| TC (mg/dL) | 197.77 ± 40.85 | 180.27 ± 44.77 | <0.05 |
| HDL-C (mg/dL) | 48.16 ± 15.32 | 44.34 ± 11.74 | 0.06 |
| LDL-C (mg/dL) | 115.26 ± 37.37 | 106.63 ± 37.60 | 0.12 |
| TG (mg/dL) | 184.13 ± 198.36 | 187.58 ± 180.5 | 0.90 |
| AFP (ng/mL) | 2.40 ± 1.13 | 3.81 ± 2.58 | <0.05 |
| MASH | 74 | 84 | - |
| NAS (0–8) | 4.07 ± 1.50 | 5.27 ± 1.70 | <0.05 |
| Steatosis (0–3) | 0: 7/1: 29/2: 45/3: 29 | 0: 5/1: 28/2: 37/3: 32 | - |
| Inflammation (0–3) | 0: 20/1: 58/2: 30/3: 2 | 0: 9/1: 32/2: 39/3: 22 | - |
| Balloonization (0–2) | 0: 23/1: 55/2: 32 | 0: 9/1: 27/2: 66 | - |
| Fibrosis (0–4) | 0: 52/1: 58 | 2: 35/3: 48/4: 19 | - |
| Gene Polymorphism | Genotype | Group 1 (F0–F1) | Group 2 (F2–F4) | Group 3 (Control Group) | p |
|---|---|---|---|---|---|
| PNPLA3 rs738409 | CC/CG (%) | 87 (79%) | 77 (75%) | 82 (91%) | <0.05 |
| GG (%) | 23 (21%) | 25 (25%) | 8 (9%) | ||
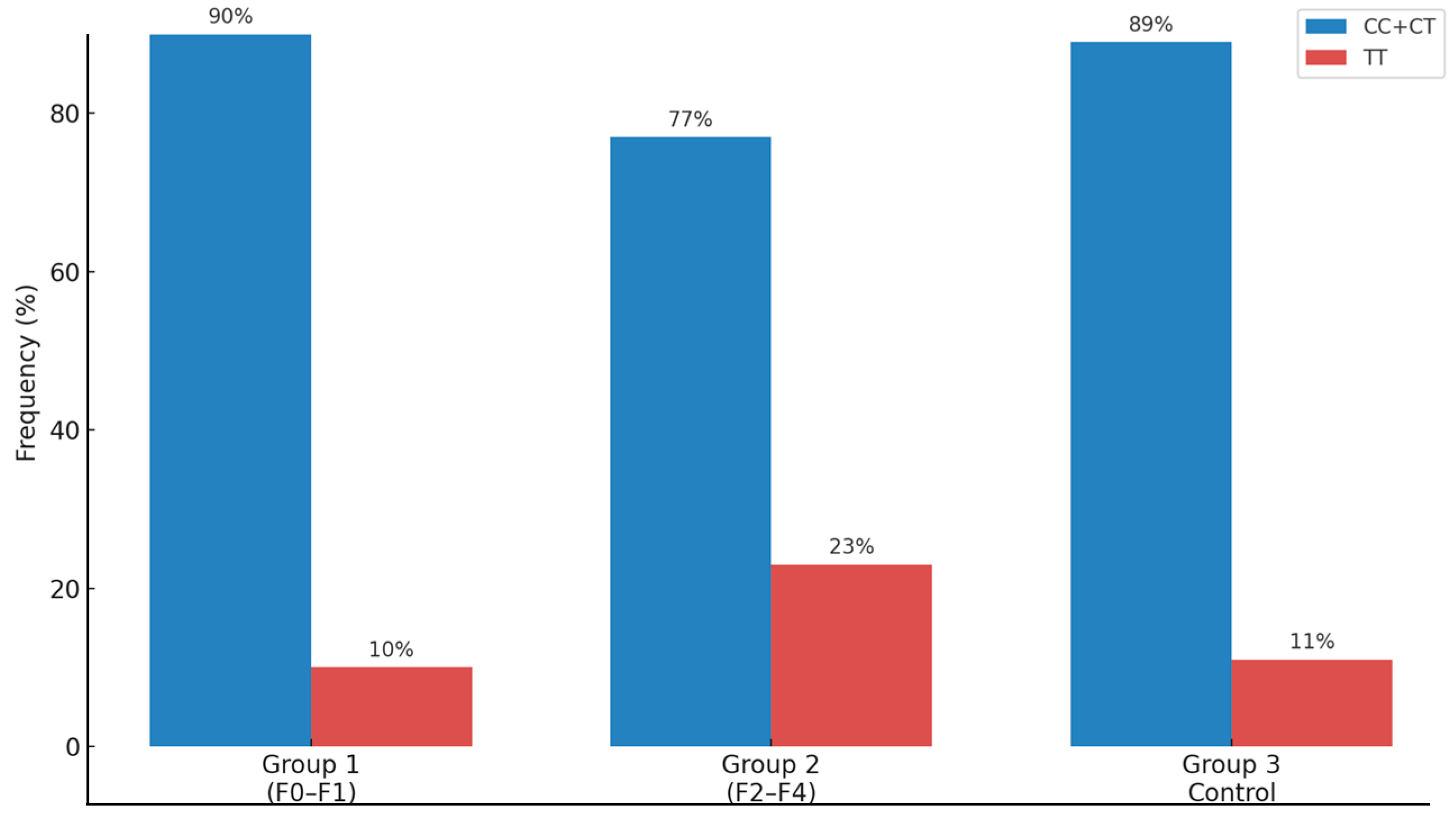
| MBOAT7 rs641738 | CC/CT (%) | 99 (90%) | 79 (77%) | 80 (89%) | <0.05 |
| TT (%) | 11 (10%) | 23 (23%) | 10 (11%) | ||
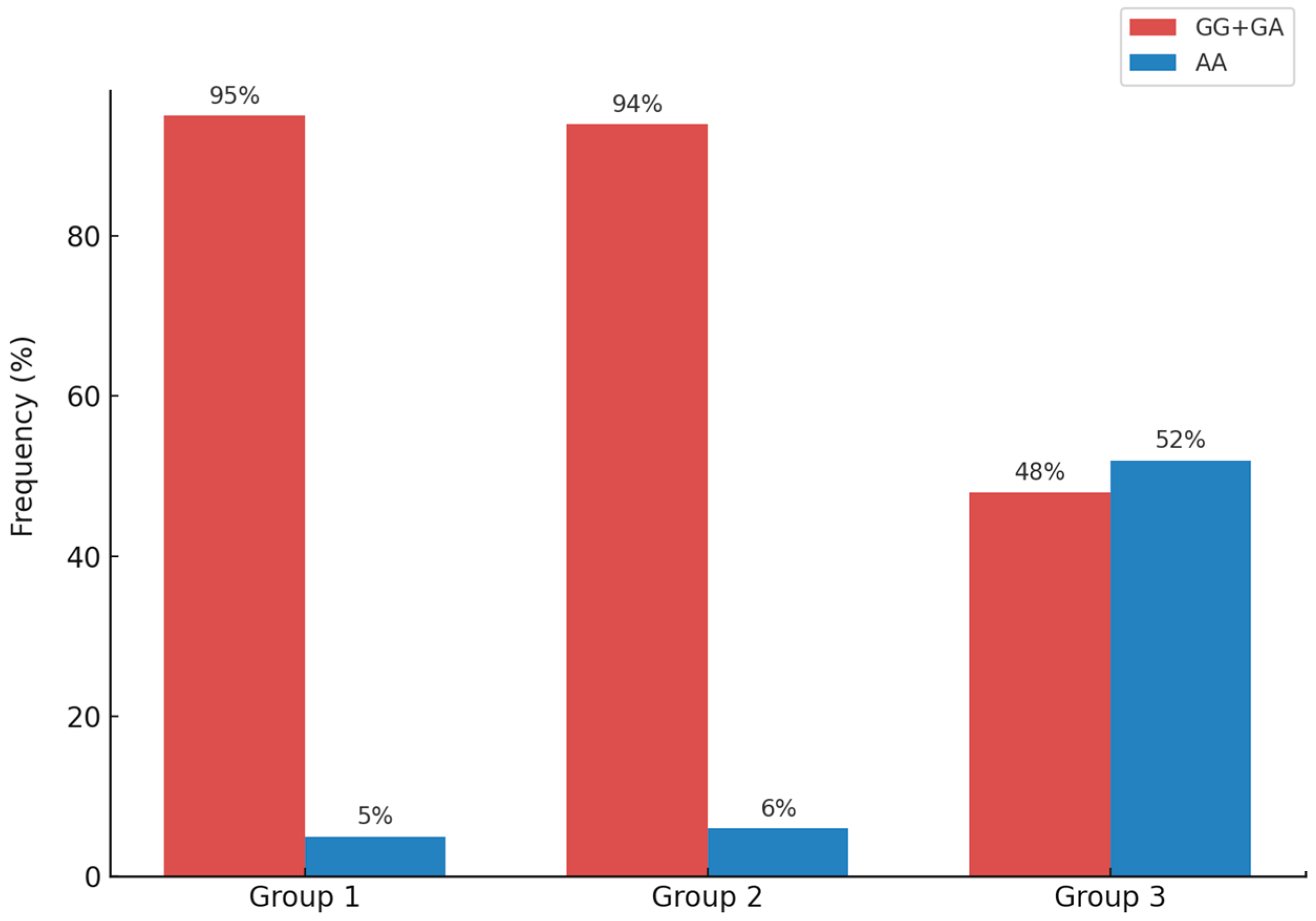
| MTARC1 rs2642438 | GG/GA (%) | 105 (95%) | 96 (94%) | 43 (48%) | <0.05 |
| AA (%) | 5 (5%) | 6 (6%) | 47 (52%) | ||
| TM6SF2 rs58542926 | CC/CT (%) | 108 (98%) | 101 (99%) | 90 (100%) | >0.05 |
| TT (%) | 2 (2%) | 1 (1%) | 0 (0%) | ||
| GCKR rs1260326 | CC/CT (%) | 92 (83%) | 78 (76%) | 75 (83%) | >0.05 |
| TT (%) | 18 (17%) | 24 (24%) | 15 (17%) | ||
| GCKR rs780094 | CC/CT (%) | 963 (84%) | 80 (78%) | 74 (82%) | >0.05 |
| TT (%) | 17 (16%) | 22 (22%) | 16 (18%) | ||
| HSD17B13 rs72613567 | TT (%) | 82 (74%) | 78 (76%) | 58 (65%) | >0.05 |
| TTA/TATA (%) | 28 (26%) | 24 (24%) | 32 (35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, S.; Oliveira, C.P.; Stefano, J.T.; Yokogawa, R.P.; Gomes-Gouvea, M.; Zitelli, P.M.Y.; Silva-Etto, J.M.K.; Martins, E.D.; Pessoa, M.G.; Alcantara, F.F.; et al. Polymorphism’s MBOAT7 as Risk and MTARC1 as Protection for Liver Fibrosis in MASLD. Int. J. Mol. Sci. 2025, 26, 6406. https://doi.org/10.3390/ijms26136406
Rocha S, Oliveira CP, Stefano JT, Yokogawa RP, Gomes-Gouvea M, Zitelli PMY, Silva-Etto JMK, Martins ED, Pessoa MG, Alcantara FF, et al. Polymorphism’s MBOAT7 as Risk and MTARC1 as Protection for Liver Fibrosis in MASLD. International Journal of Molecular Sciences. 2025; 26(13):6406. https://doi.org/10.3390/ijms26136406
Chicago/Turabian StyleRocha, Sofia, Claudia P. Oliveira, José Tadeu Stefano, Roberta P. Yokogawa, Michele Gomes-Gouvea, Patricia Momoyo Youshimura Zitelli, Joyce Matie Kinoshita Silva-Etto, Eduarda Donegá Martins, Mario G. Pessoa, Flavio F. Alcantara, and et al. 2025. "Polymorphism’s MBOAT7 as Risk and MTARC1 as Protection for Liver Fibrosis in MASLD" International Journal of Molecular Sciences 26, no. 13: 6406. https://doi.org/10.3390/ijms26136406
APA StyleRocha, S., Oliveira, C. P., Stefano, J. T., Yokogawa, R. P., Gomes-Gouvea, M., Zitelli, P. M. Y., Silva-Etto, J. M. K., Martins, E. D., Pessoa, M. G., Alcantara, F. F., Azevedo, R. S., & Rebello Pinho, J. R. (2025). Polymorphism’s MBOAT7 as Risk and MTARC1 as Protection for Liver Fibrosis in MASLD. International Journal of Molecular Sciences, 26(13), 6406. https://doi.org/10.3390/ijms26136406








