Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation
Abstract
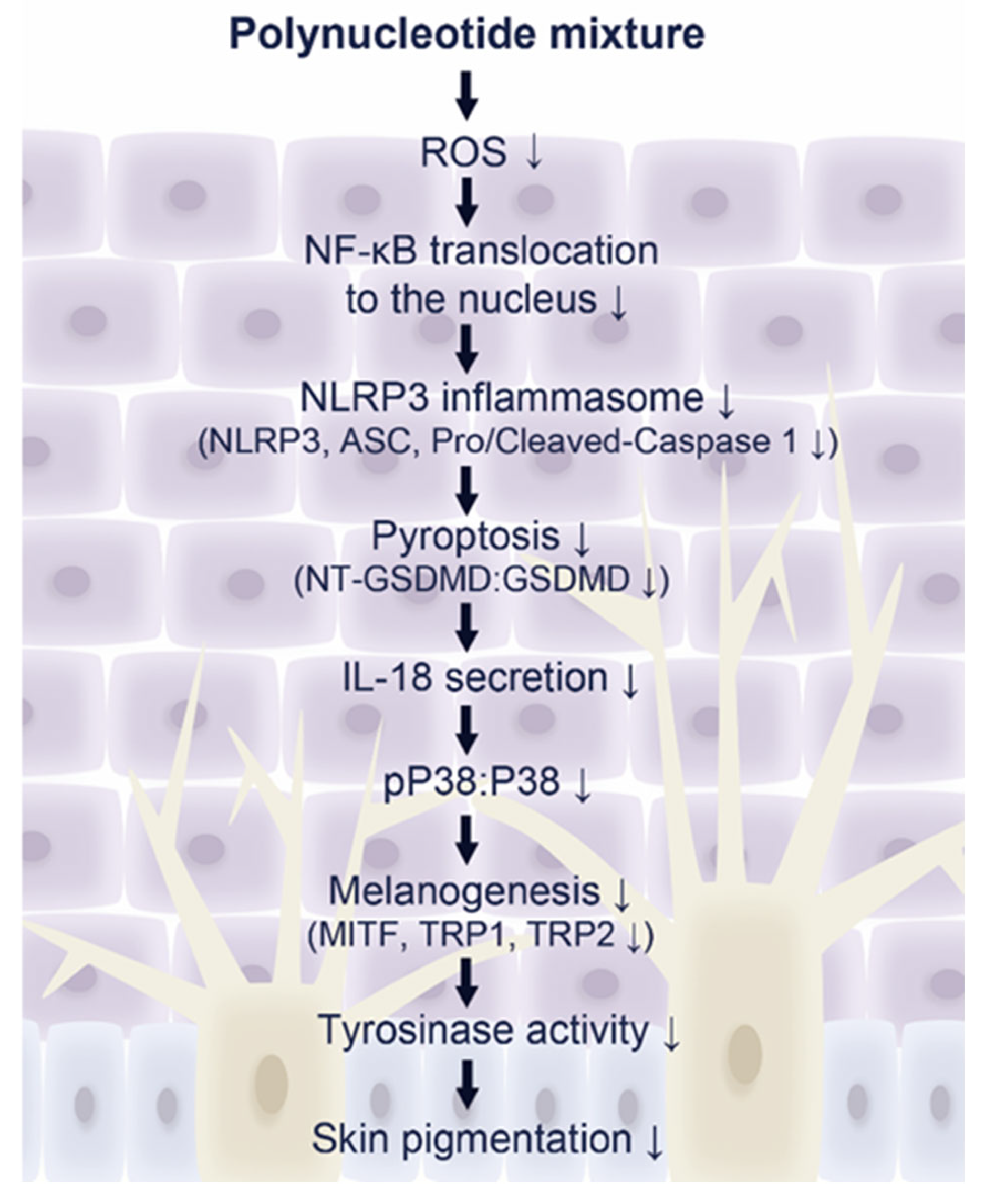
1. Introduction
2. Results
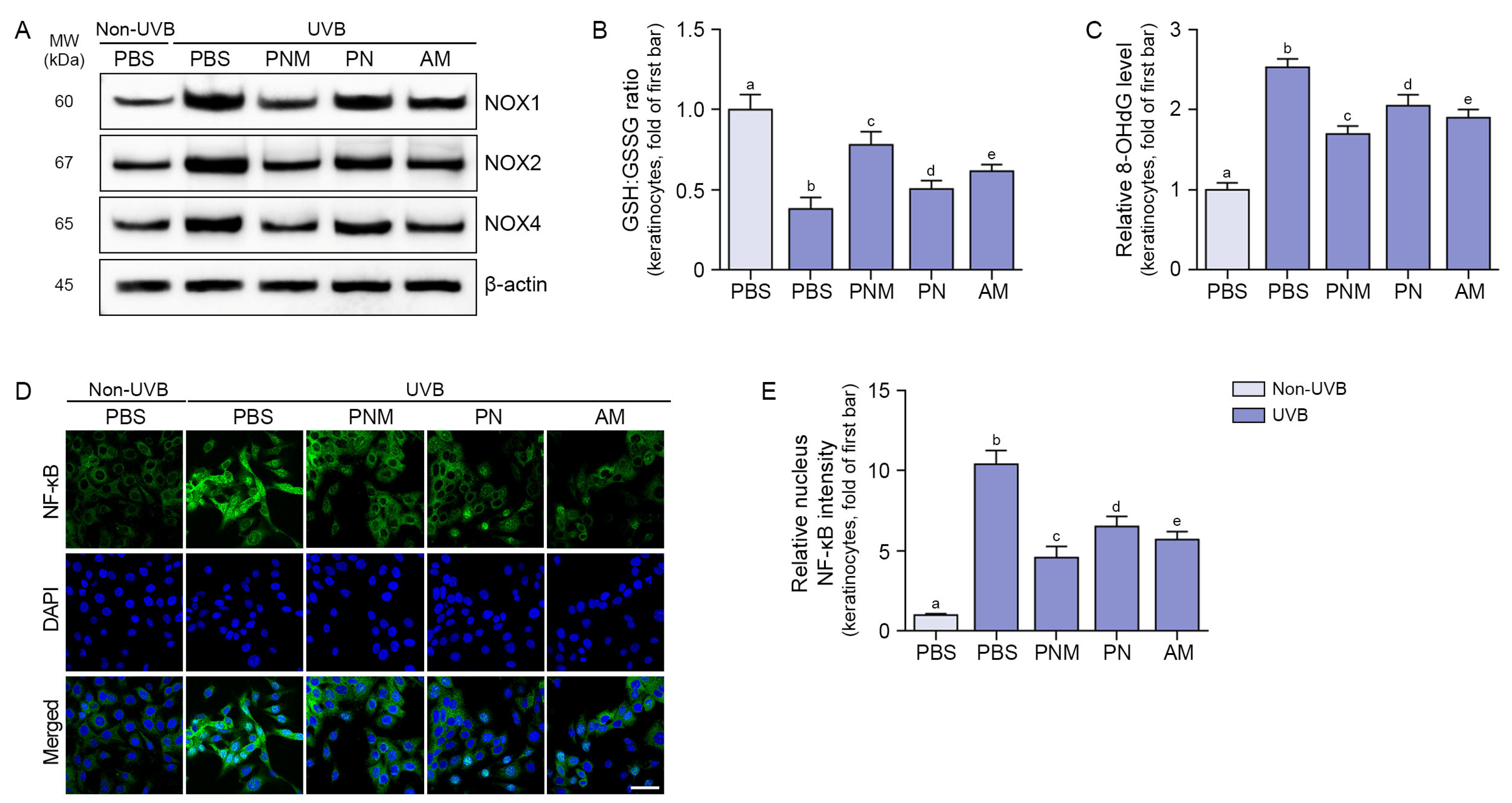
2.1. PNM, PN, and AM Decreased Oxidative Stress and NF-κB in UVB-Irradiated Keratinocytes
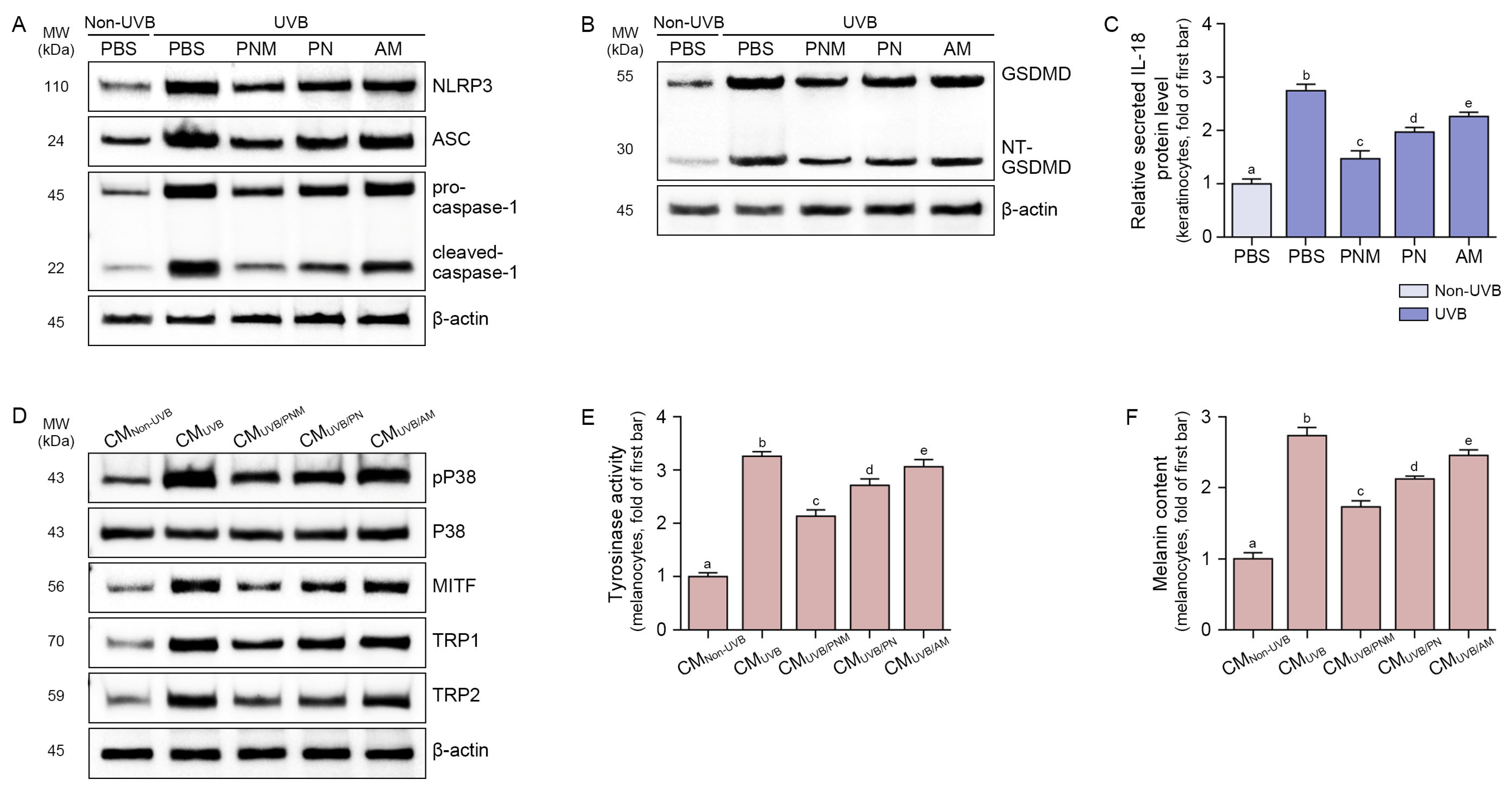
2.2. PNM, PN, and AM Decreased NLRP3 Inflammasomes and IL-18 in UVB-Irradiated Keratinocytes
2.3. PNM, PN, and AM Decreased Melanogenesis-Related Signals in the Melanocyte
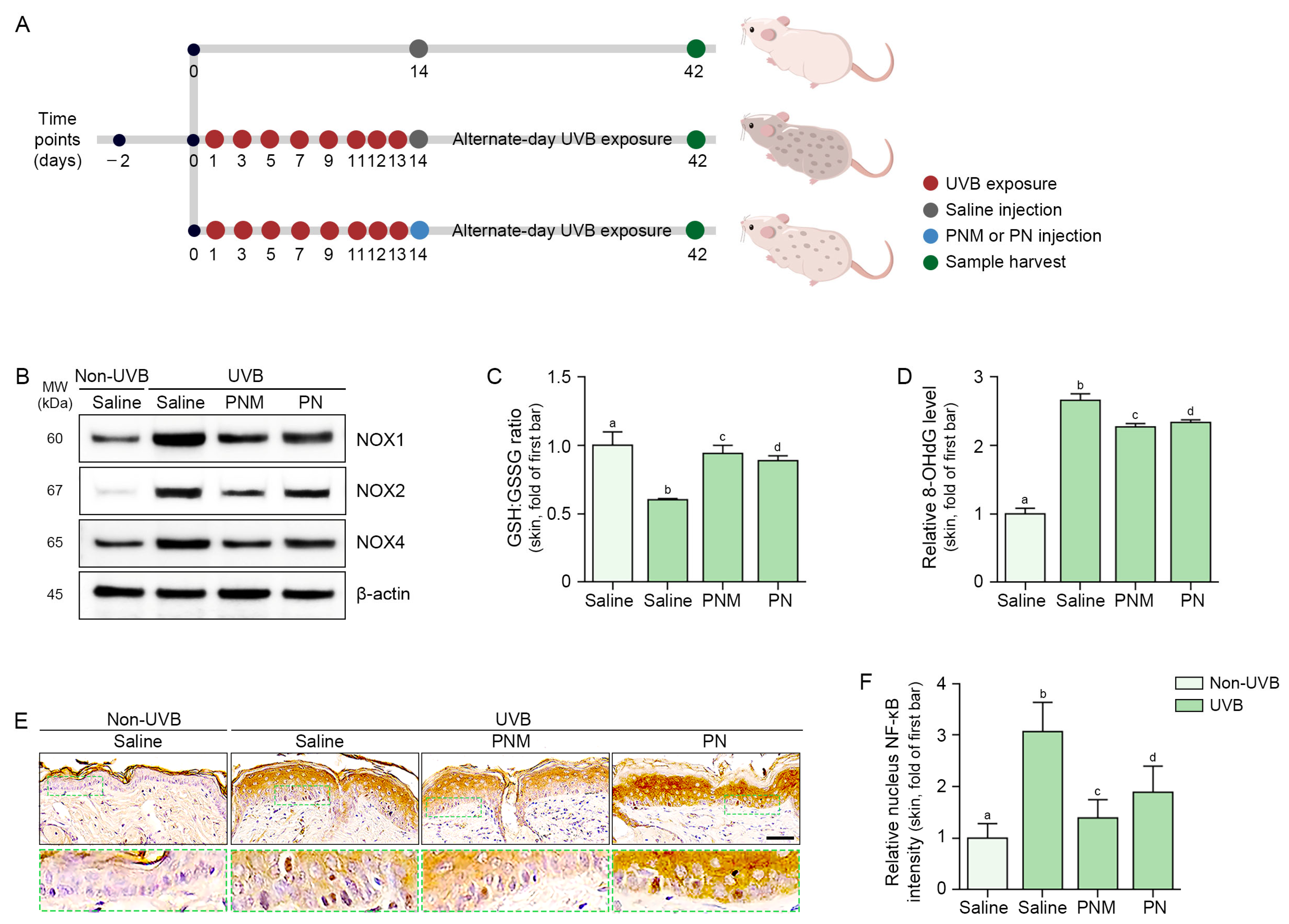
2.4. PN and PNM Decreased Oxidative Stress and NLRP3 Inflammasome Activation in UVB-Irradiated Skin
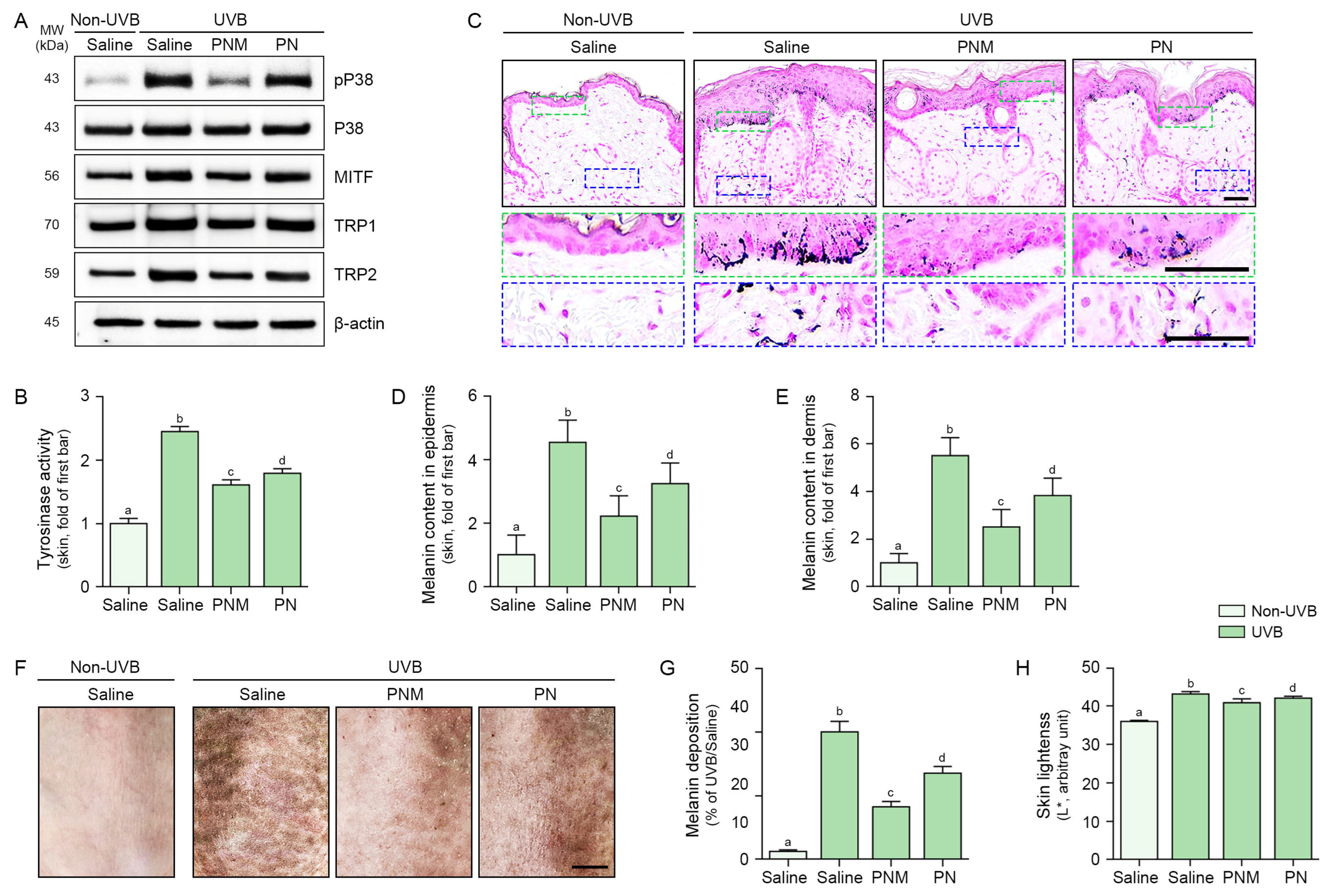
2.5. PN and PNM Decreased Melanogenesis Signals and Melanin Accumulation in UVB-Irradiated Skin
3. Discussion
4. Materials and Methods
4.1. AM, PN, and PNM Preparation
4.2. Cell Culture
4.3. Experimental Design for AM, PN, or PNM Treatment In Vitro
4.3.1. Establishment of a UVB Exposure Model for Keratinocytes
4.3.2. Establishment of CM-Treated Model for Melanoma
4.4. Animal Studies
4.4.1. Mouse Model and Maintenance
4.4.2. Experimental Design for PNM or PN Injections in UVB-Induced Pigmentation Animal Model
4.4.3. Skin Brightness Assessment
4.5. Sample Preparation
4.5.1. Protein Isolation and Concentration Quantification
4.5.2. Preparation of Paraffin-Embedded Skin Tissue Blocks
4.6. Cell Viability Assessment
4.7. Measurement of GSH:GSSG Ratio and Tyrosinase Activity
4.8. Western Blot Analysis
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Staining
4.10.1. Immunocytochemistry
4.10.2. Immunohistochemistry
4.10.3. Fontana–Masson Staining
4.11. Melanin Amount
4.12. Synergy Analysis Using the Bliss Independence Model
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Dang, Y.; Gao, W.; Zhang, Y.; Xu, P.; Gu, J.; Ye, X. P38 and JNK signal pathways are involved in the regulation of phlorizin against UVB-induced skin damage. Exp. Dermatol. 2015, 24, 275–279. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakashima, M.; Suzuki, Y. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem. Biophys. Res. Commun. 2016, 477, 329–335. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 540, 50. [Google Scholar] [CrossRef]
- Zhou, J.; Shang, J.; Song, J.; Ping, F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-κB pathway. Int. J. Biochem. Cell Biol. 2013, 45, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ling, J.; Wang, Y.; Shang, J.; Ping, F. Cross-talk between interferon-gamma and interleukin-18 in melanogenesis. J. Photochem. Photobiol. B 2016, 163, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Noh, T.K.; Chung, B.Y.; Kim, S.Y.; Lee, M.H.; Kim, M.J.; Youn, C.S.; Lee, M.W.; Chang, S.E. Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster. Int. J. Mol. Sci. 2016, 17, 1448. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A promising skin anti-aging agent. Chin. J. Plast. Reconstr. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M.; et al. PDRN, a Bioactive Natural Compound, Ameliorates Imiquimod-Induced Psoriasis through NF-κB Pathway Inhibition and Wnt/β-Catenin Signaling Modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.J.; Kweon, D.K.; Lim, S.T.; Lee, S.J. Polydeoxyribonucleotide Activates Mitochondrial Biogenesis but Reduces MMP-1 Activity and Melanin Biosynthesis in Cultured Skin Cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef]
- Kim, T.H.; Heo, S.Y.; Oh, G.; Heo, S.J.; Jung, W.K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Mar. Drugs 2021, 19, 296. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.T.; Lee, Y.J.; Paik, S.H.; Moon, Y.S.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Jung, J.M.; et al. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: A randomized, double-blind, split-face trial. J. Dermatol. Treat. 2022, 33, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, J.J.; Lee, Y.I.; Lee, W.J.; Lee, C.; Chung, W.Y.; Nam, K.H.; Lee, J.H. Preventive effect of polynucleotide on post-thyroidectomy scars: A randomized, double-blinded, controlled trial. Lasers Surg. Med. 2018, 50, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Di Stefano, V.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 313–321. [Google Scholar] [CrossRef]
- Yi, K.H.; Winayanuwattikun, W.; Kim, S.Y.; Wan, J.; Vachatimanont, V.; Putri, A.I.; Hidajat, I.J.; Yogya, Y.; Pamela, R. Skin boosters: Definitions and varied classifications. Ski. Res. Technol. 2024, 30, e13827. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Hossain, M.R.; Ansary, T.M.; Komine, M.; Ohtsuki, M. Diversified Stimuli-Induced Inflammatory Pathways Cause Skin Pigmentation. Int. J. Mol. Sci. 2021, 22, 3970. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of Melanogenesis Cascade for Identifying Pathophysiology and Therapeutic Approach of Pigmentary Disorders and Melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef]
- Fang, J.; Ouyang, M.; Qu, Y.; Wang, M.; Huang, X.; Lan, J.; Lai, W.; Xu, Q. Advanced Glycation End Products Promote Melanogenesis by Activating NLRP3 Inflammasome in Human Dermal Fibroblasts. J. Investig. Dermatol. 2022, 142, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Antonuccio, P.; Micali, A.G.; Romeo, C.; Freni, J.; Vermiglio, G.; Puzzolo, D.; Squadrito, F.; Irrera, N.; Marini, H.R.; Rana, R.A.; et al. NLRP3 Inflammasome: A New Pharmacological Target for Reducing Testicular Damage Associated with Varicocele. Int. J. Mol. Sci. 2021, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Siquier-Dameto, G.; Boisnic, S.; Boadas-Vaello, P.; Verdú, E. Anti-Aging and Depigmentation Effect of a Hyaluronic Acid Mechanically Stabilized Complex on Human Skin Explants. Polymers 2023, 15, 2438. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio 2015, 5, 476–484. [Google Scholar] [CrossRef]
- Sonthalia, S.; Daulatabad, D.; Sarkar, R. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian. J. Dermatol. Venereol. Leprol. 2016, 82, 262–272. [Google Scholar] [CrossRef]
- Villarama, C.D.; Maibach, H.I. Glutathione as a depigmenting agent: An overview. Int. J. Cosmet. Sci. 2005, 27, 147–153. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Shivhare, S.C.; Malviya, K.G.; Shivhare Malviya, K.K.; Jain, V. A review: Natural skin lighting and nourishing agents. Res. J. Top. Cosmet. Sci. 2013, 4, 21–25. [Google Scholar]
- Fisher, A.A. Current contact news. Hydroquinone uses and abnormal reactions. Cutis 1983, 31, 240–250. [Google Scholar]
- Zhou, H.; Kepa, J.K.; Siegel, D.; Miura, S.; Hiraki, Y.; Ross, D. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol. Pharmacol. 2009, 76, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Onodera, H.; Mitsumori, K.; Tamura, T.; Maruyama, S.; Ito, A. Changes in thyroid function during development of thyroid hyperplasia induced by kojic acid in F344 rats. Carcinogenesis 1999, 20, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs iodometric titration as analytical methods. LWT-Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair. Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Altavilla, D.; Arcoraci, V.; De Caridi, G.; De Feo, M.E.; Corrao, S.; Pallio, G.; Sterrantino, C.; Minutoli, L.; et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: Results of a clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, E746–E753. [Google Scholar]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Massirone, A.; Pia Palmieri, I.; Papagni, M.; Priori, M.; Trocchi, G. Consensus report on the use of PN-HPT™ (polynucleotides highly purified technology) in aesthetic medicine. J. Cosmet. Dermatol. 2021, 20, 922–928. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, T.R.; Lee, S.E.; Jang, Y.N.; Han, H.S.; Mun, S.K.; Kim, B.J. Comparative Evaluation of the Effectiveness of Novel Hyaluronic Acid-Polynucleotide Complex Dermal Filler. Sci. Rep. 2020, 10, 5127. [Google Scholar] [CrossRef]
- Krutmann, J.; Gilchrest, B.A. Photoaging of skin. In Skin Aging; Gilchrest, B.A., Krutmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–43. [Google Scholar]
- Zhao, W.; Sachsenmeier, K.; Zhang, L.; Sult, E.; Hollingsworth, R.E.; Yang, H. A New Bliss Independence Model to Analyze Drug Combination Data. J. Biomol. Screen. 2014, 19, 817–821. [Google Scholar] [CrossRef]
- Chen, H.W.; Chou, Y.S.; Young, T.H.; Cheng, N.C. Inhibition of melanin synthesis and melanosome transfer by chitosan biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1239–1250. [Google Scholar] [CrossRef]
- Yoon, Y.; Bae, S.; Kim, T.J.; An, S.; Lee, J.H. Nodakenin Inhibits Melanogenesis via the ERK/MSK1 Signaling Pathway. Pharmazie 2023, 78, 6–12. [Google Scholar]
- Byun, K.A.; Lee, S.Y.; Oh, S.; Batsukh, S.; Jang, J.W.; Lee, B.J.; Rheu, K.M.; Li, S.; Jeong, M.S.; Son, K.H.; et al. Fermented Fish Collagen Attenuates Melanogenesis via Decreasing UV-Induced Oxidative Stress. Mar. Drugs 2024, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.A.; Park, Y.; Oh, S.; Batsukh, S.; Son, K.H.; Byun, K. Co-Treatment with Phlorotannin and Extracellular Vesicles from Ecklonia cava Inhibits UV-Induced Melanogenesis. Antioxidants 2024, 13, 408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Jeon, H.-D.; Rho, N.-K.; Son, K.H.; Byun, K. Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation. Int. J. Mol. Sci. 2025, 26, 6399. https://doi.org/10.3390/ijms26136399
Oh S, Jeon H-D, Rho N-K, Son KH, Byun K. Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation. International Journal of Molecular Sciences. 2025; 26(13):6399. https://doi.org/10.3390/ijms26136399
Chicago/Turabian StyleOh, Seyeon, Hee-Dae Jeon, Nark-Kyoung Rho, Kuk Hui Son, and Kyunghee Byun. 2025. "Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation" International Journal of Molecular Sciences 26, no. 13: 6399. https://doi.org/10.3390/ijms26136399
APA StyleOh, S., Jeon, H.-D., Rho, N.-K., Son, K. H., & Byun, K. (2025). Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation. International Journal of Molecular Sciences, 26(13), 6399. https://doi.org/10.3390/ijms26136399





