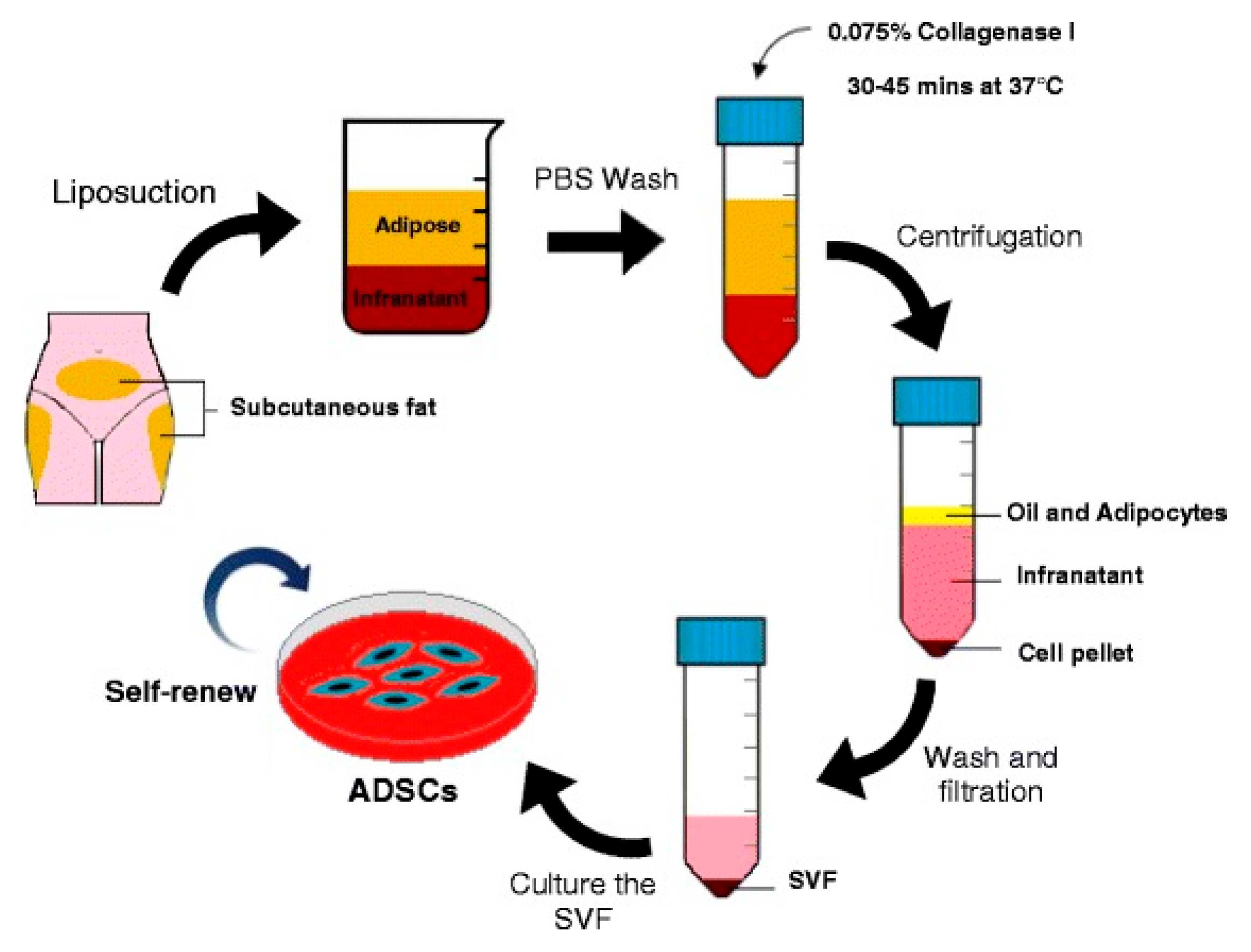
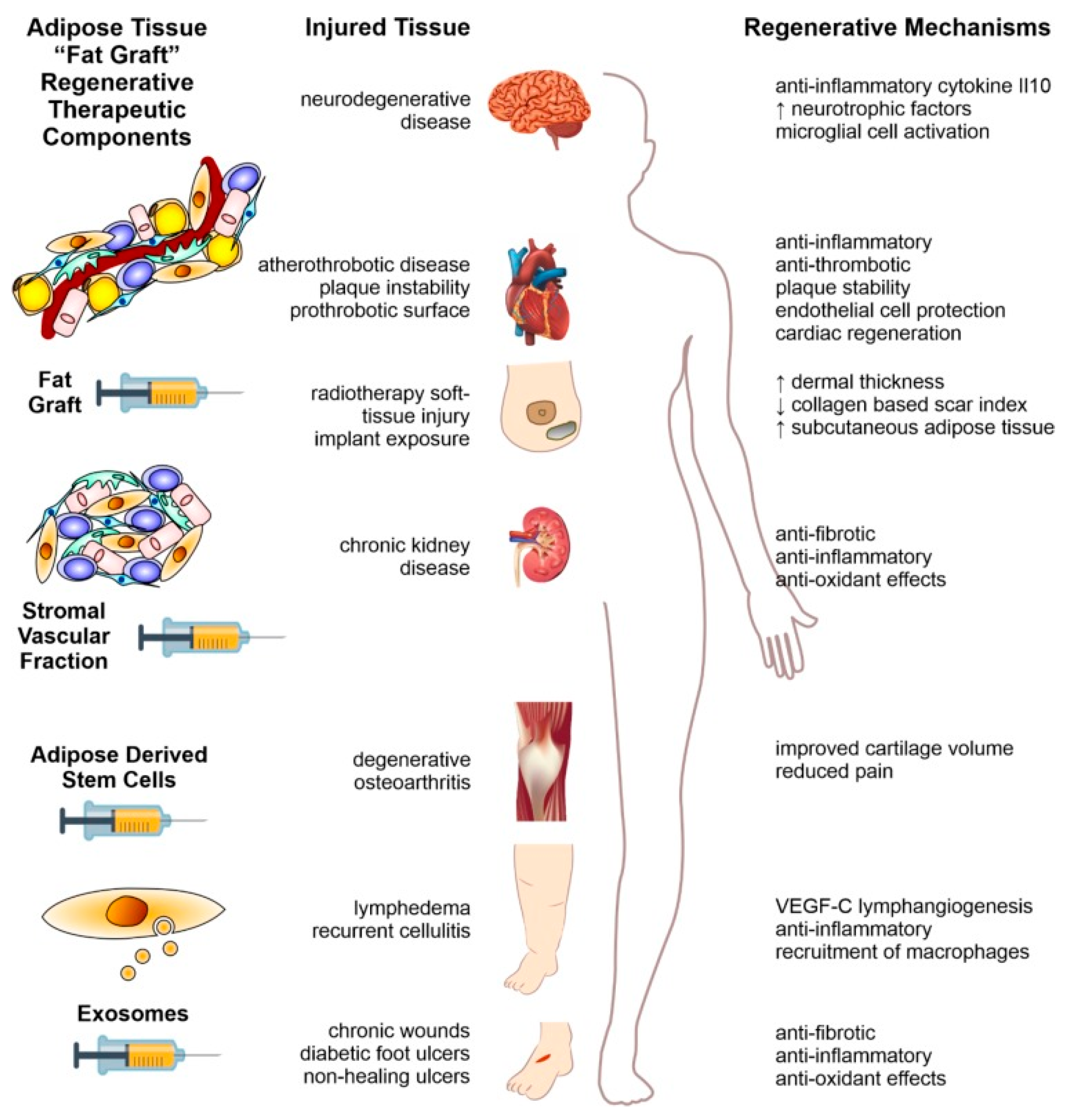
Following this screening, a total of 22 studies were included in the final review, covering a total of 953 patients.
Methodological quality was appraised with the Cochrane RoB 2 framework for randomized trials (nine studies) and ROBINS-I for non-randomized designs (13 studies). Agreement between assessors was excellent. Seven studies (32%) satisfied all critical standards and were judged at low risk of bias. Nine (41%) were rated moderate, most often because allocation concealment was insufficiently described or secondary outcomes were selectively reported. The remaining six (27%) were deemed high risk, typically owing to open-label conduct without blinded outcome assessment or attrition exceeding 20%.
This structured and transparent risk-of-bias assessment ensured a robust appraisal of methodological quality across study designs, thereby strengthening the interpretability and credibility of the review’s findings.
3.1. Overview of the Efficacy of AASC Treatment
Skin Lesions: Across four studies involving 190 patients, 103 (54.2%) received AASCs. Among those treated, 93 patients (90.3%) benefited from the treatment, while only 10 patients (5.3% of the total population studied) showed no improvement.
Crohn’s Disease: Three studies included 271 patients, with 151 (55.72%) receiving AASCs. Of these, 88 patients (58.28% of those treated) benefited from the treatment, whereas 63 patients (23.25% of the total population) experienced no improvement.
Glandular Dysfunction: Four studies involved 197 patients, of whom 102 (50%) received AASCs. Half of these treated patients (51, or 50%) showed improvements, while the other 51 patients (25.89% of the total population) received no benefit.
Lung Diseases: In three studies involving 172 patients, 90 (52.33%) received AASCs, with 62 patients (68.89% of those treated) benefiting from the treatment. Conversely, 28 patients (16.28% of the total population) did not experience any improvement.
Strokes: A single study included 13 patients, four of whom (30.77%) received AASCs. Notably, all four patients (100% of those treated) showed significant benefits from the treatment.
Renal Dysfunction: In a single study of 12 patients, all received AASCs. Among them, six patients (50%) benefited from the treatment.
Neurodegenerative Diseases: A single study treated 10 patients, including five (50% of the total population) who received AASCs. Remarkably, all five patients (100% of those treated) benefited from the treatment.
Osteoarthritis: Four studies involving 76 patients found that 56 patients (73.68%) received AASCs. Among those treated, 50 patients (89.29%) benefited from the treatment, while six patients (7.89% of the total population) showed no improvement.
Acute Graft-Versus-Host Disease (GvHD): A study of 12 patients, all of whom received AASCs, reported that nine patients (75%) benefited from the treatment.
Overall Results: Across 953 patients analyzed in these studies, 535 patients (56.14%) received AASCs. Among those treated, 368 patients (68.79%) experienced significant benefits, while 167 patients (17.52% of the total population) showed no improvement (
Table 2).
3.2. Skin Lesions
3.2.1. A Scar Treatment
According to Gentil [
14], 50 patients with scars and soft tissue deformities with hyperpigmentation treated with fat grafting, commonly known as “lipofilling”, enhanced with adipose tissue-derived mesenchymal stem cells (lipofilling-AASCs) and 50 patients treated with unenhanced lipofilling (lipofilling-NE) were prospectively evaluated. Preoperative evaluation included clinical assessment, photographic assessment, magnetic resonance imaging (MRI) and ultrasound. Postoperative follow-up was performed at weeks 1, 3, 7, 12, 24 and 48, then annually. Improvement in volume contours and pigmentation was clinically evaluated.
All those who underwent treatment (lipofilling-AASCs and lipofilling-NE) were satisfied with the improvement in pigmentation, texture and volume contours, with some differences. In the first subpopulation, one year after lipofilling-AASCs treatment, 25 patients were very satisfied (50%), 19 were satisfied (38%), and six patients were dissatisfied (12%). In the second subpopulation, 1 year after lipofilling-NE, 10 patients were very satisfied (20%),18 patients were satisfied (36%), and 22 patients were dissatisfied (44%). In conclusion, lipofilling-AASCs was the preferred option for improving contour deformities associated with increased scar pigmentation.
3.2.2. Treatment of Ulcers
Moon et al. [
15] conducted a clinical trial to evaluate the efficacy of a treatment based on adipose tissue-derived stem cells in 39 patients with diabetic foot ulcers. Inclusion criteria included age 18–80 years, type 1 or 2 diabetes, ulcer history of more than 4 weeks, wound size 1–25 cm
2, and a Wagner wound depth grade 1 or 2. Additional criteria included detectable blood flow, an ankle brachial index between >0.7 and <1.3, and transcutaneous oxygen pressure > 30 mmHg. Exclusion criteria included wound size changes of >30% in one week, wound infection, HIV status, HbA1c > 15% and postprandial glucose > 450 mg/dL. Patients were randomized into two treatment groups.
At week 8, 73% of subjects in the treatment group (16 of 22) achieved complete wound closure, compared with 47% in the control group (8 of 17). For secondary endpoints, at week 12, 82% of subjects in the treatment group achieved complete closure (18 of 22) versus 53% in the control group (9 of 17), with a trend towards significance (p = 0.053). Mean time to complete healing was 40.8 ± 5.3 days in the treatment group and 51.2 ± 3.9 days in the control group. According to the Kaplan–Meier median, the treatment group had a significantly shorter time to complete healing (28.5 days) than the control group (63.0 days) (p = 0.033).
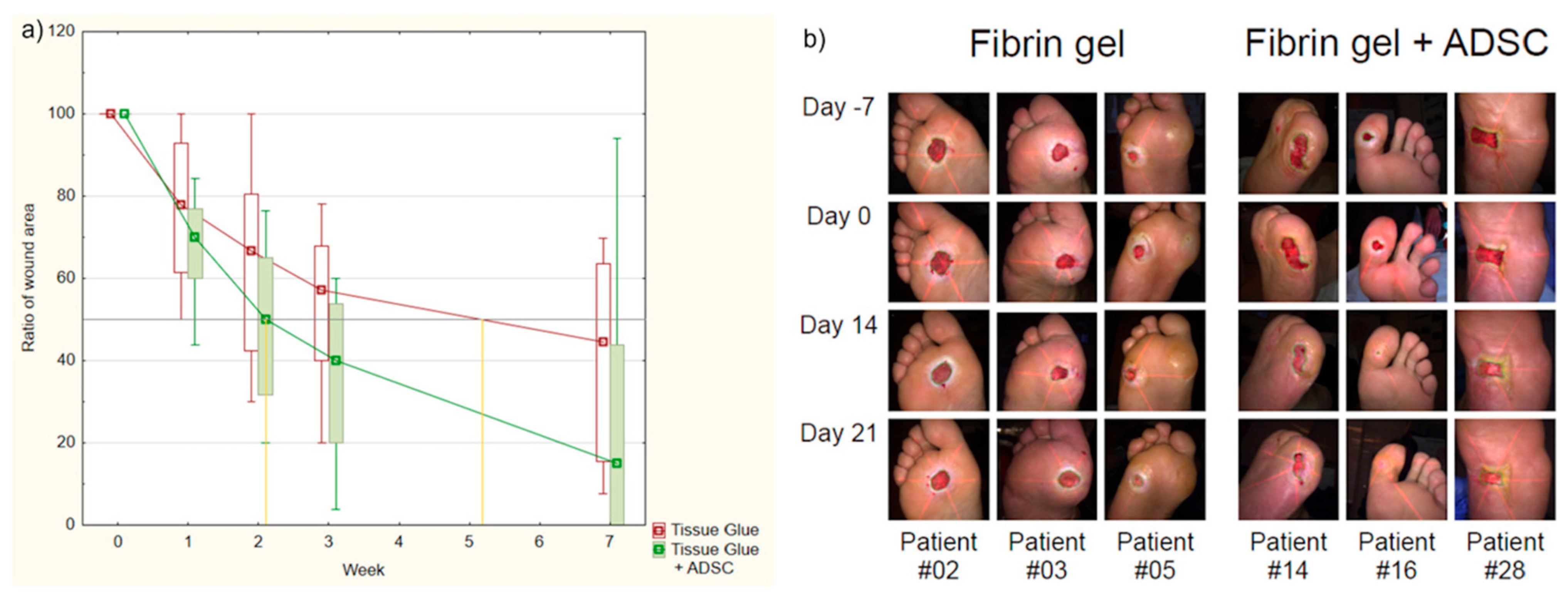
A study published by Mrozikiewicz-Rakowska et al. [
16] on this topic involved two equivalent groups of 23 participants receiving either fibrin gel with AASCs or fibrin gel alone. Clinical assessment was performed at four different time points: days 7, 14, 21 and 49. Wound size reduction was significantly greater on days 21 and 49 in patients receiving AASCs. Time to a 50% reduction in wound size in the fibrin gel group was 25.5 ± 4.2 days and 17.6 ± 1.5 days in the AASCs group (
p = 0.029).
Seven patients treated with AASCs achieved complete healing at the end of the study compared with one patient in the non-AASCs group. One week after the application of AASCs, 34 proteins differed significantly between groups, seven of which were positively correlated with the healing rate, including GAPDH, CAT, ACTN1, KRT1, KRT9, SCL4A1 and TPI. These results confirmed the improved wound healing associated with AASCs, thus offering molecular insights and contributing to the understanding of the role of AASCs in wound healing (
Figure 4).
3.2.3. C Psoriasis Treatment
A study on the topic of psoriasis treatment by Bajouri et al. [
17] involved five patients (three men and two women with a mean age of 32.8 ± 8.18 years. Three patients received a total of 3.10
6 cells/cm
2 of AASCs, while two patients received only 10
6 of these cells. The latter were injected into the subcutaneous tissue of each plaque in a single dose, and changes in clinical and histological indices, the number of B and T lymphocytes in local and peripheral blood, and serum levels of inflammatory cytokines were assessed.
No major adverse effects, such as burning, pain, itching or systemic side effects, were observed after the AASC injection, and lesions showed mild-to-considerable improvement after injection. The mRNA expression levels of pro-inflammatory factors were reduced in the dermis of patients after injection. Increased expression of the transcription factor Foxp3 in patients’ blood samples suggests modulation of inflammation after AASC administration.
Six months later, skin thickness, erythema and plaque desquamation, as well as the PASI score, decreased in most patients, according to clinical analysis by the physicians in charge. This study therefore suggests that ASSC injection can be considered as a safe and effective therapeutic approach for psoriatic plaques.
3.2.4. Crohn’s Disease
A study by Panés et al. [
18] on Crohn’s disease involved a total of 212 patients, 107 of whom were treated with AASCs. The results revealed that 53 patients derived significant benefit from this intervention, underlining the potential efficacy of AASCs in the management of their medical condition. This was a randomized, double-blind, parallel-group, placebo-controlled study conducted from 6 July 2012 to 27 July 2015, in 49 hospitals in seven European countries and Israel.
Adult patients (≥18 years of age) with Crohn’s disease and treatment-refractory draining complex perianal fistulas were randomly assigned using a pre-established randomization list to a single intralesional injection of 120 million AASCs (Cx601) to 24 mL saline (placebo). Treatment was administered by an unmasked surgeon, a gastroenterologist and a masked radiologist assessing the therapeutic effect. The primary endpoint was a combined remission at week 24 (i.e., clinical assessment of closure of all treated external orifices draining at baseline and the absence of >2 cm collections from treated perianal fistulae, confirmed by masked central magnetic resonance [MRI] imaging).
A total of 212 patients were randomized: 107 to Cx601 and 105 to placebo. A significantly higher proportion of patients treated with Cx601 versus placebo achieved combined remission in the intention-to-treat (ITT) populations (53 out of 107 [50%] vs. 36 out of 105 [34%]; a difference of 15.2% (97.5% confidence interval [CI] 0.2–30.3; p = 0.024)). A total of 18 of 103 patients in the Cx601 group (17%) vs. 30 (29%) of 103 patients in the placebo group experienced treatment-related adverse events, the most frequent being anal abscess (six in the Cx601 group versus nine in the placebo group) and proctalgia (five vs. nine). In conclusion, Cx601 is an effective and safe treatment for complex perianal fistulas in Crohn’s disease patients who have failed to respond to conventional and/or biologic therapies.
An ADMIRE-CD study conducted by Garcia-Olmo et al. [
19] evaluated the efficacy and safety of darvadstrocel (AASCs) for the treatment of complex perianal fistulas in Crohn’s disease over a 104-week period. Of the 131 patients who completed the initial 52-week follow-up, 40 participated in the extended follow-up, with 25 receiving darvadstrocel and 15 receiving a placebo. Patients in the darvadstrocel group had a mean age of 38.6 years compared to 42.7 years in the placebo group. The proportion of men was similar between the groups (56% for darvadstrocel vs. 53% for placebo). However, 60% of patients in the darvadstrocel group presented with more complex fistulas (more than one internal or external opening) compared to 33% in the placebo group.
During the 104-week period, four serious adverse events (SAEs) were reported: three in the darvadstrocel group (12%) and one in the placebo group (6.7%). None were attributed to the treatment. Events reported in the darvadstrocel group included anal abscess, anal fistula and fistula discharge, while fistula discharge was reported in the placebo group. These results showed a reduction in treatment-emergent serious adverse events (TESAEs) in the darvadstrocel group, decreasing from 24% at 52 weeks to 12% at 104 weeks.
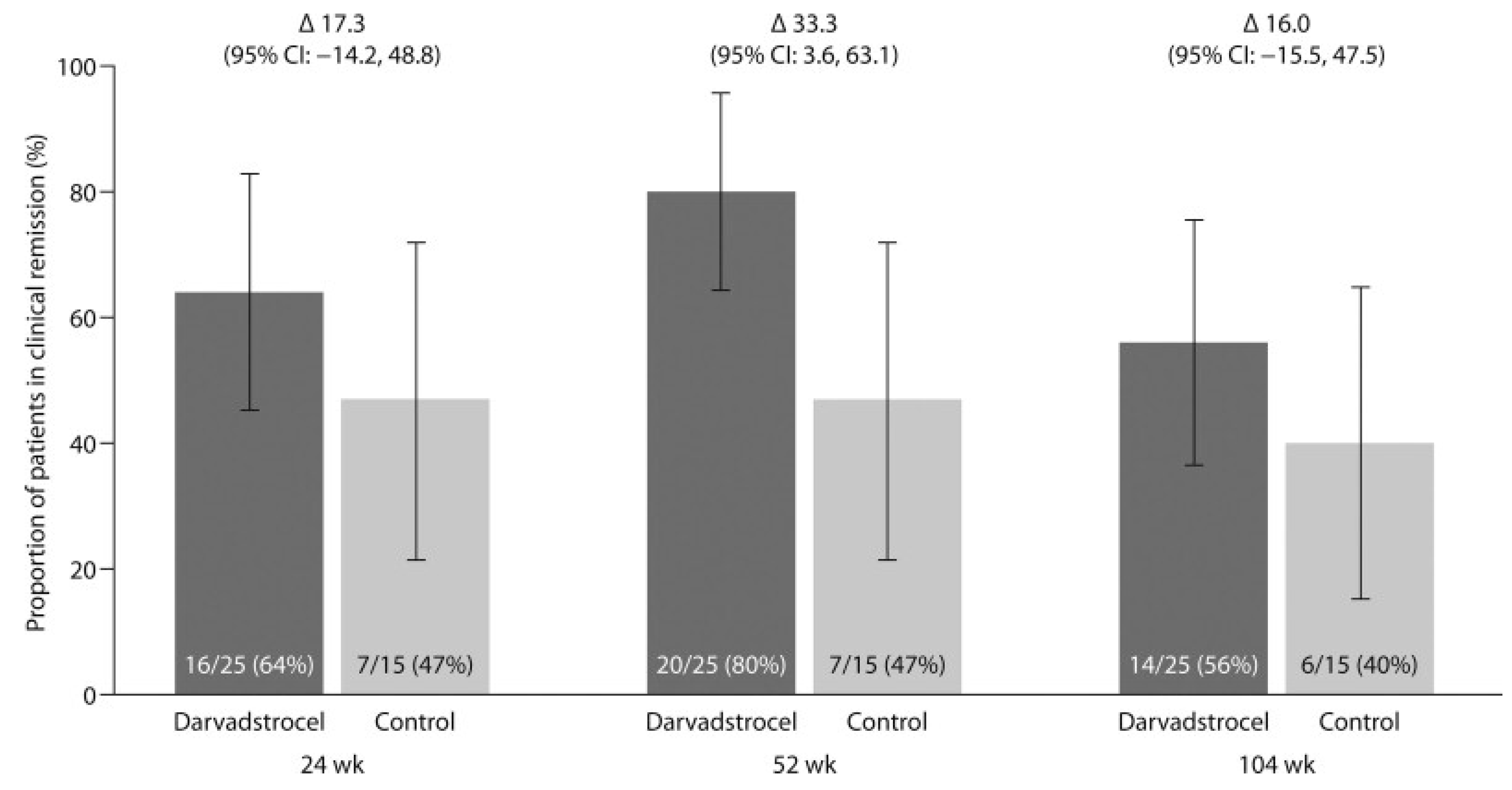
Clinical remission, defined as the closure of all treated external openings without drainage, was achieved by 64% of patients in the darvadstrocel group compared to 47% in the placebo group at 24 weeks. At 52 weeks, clinical remission reached 80% in the darvadstrocel group versus 47% in the placebo group, representing a difference of 33% (95% CI, 3.6 to 63.1). At 104 weeks, clinical remission remained superior in the darvadstrocel group at 56%, compared to 40% for the placebo. Among patients on concomitant anti-TNF therapy, 59% of those in the darvadstrocel group were in remission at 104 weeks, compared to 30% for the placebo. For patients not receiving anti-TNF therapy, remission was 50% with darvadstrocel and 60% with the placebo (
Figure 5).
In summary, darvadstrocel achieved significant clinical remission rates, reaching 80% at 52 weeks and remaining high at 56% at 104 weeks. The safety profile was comparable to the placebo, with no treatment-related serious adverse events. These results highlight the potential of darvadstrocel to provide durable remission of complex perianal fistulas in patients with Crohn’s disease, representing a significant advance in managing this challenging condition.
A study by Maciel et al. [
20] aimed to evaluate the safety of allogeneic mesenchymal stem cells (MSCs) in the treatment of complex anal fistulas in patients without Crohn’s disease.
Conducted as a prospective, non-randomized phase I clinical trial in a secondary hospital, it included 20 consecutive patients diagnosed with complex anal fistulas. Each patient received a total of 40 × 106 allogeneic MSCs, with 20 × 106 cells applied per fistula tract in cases with two tracts.
Patients were hospitalized for 24 h post-procedure and evaluated at weeks 1, 2, 4, 8, 16 and 24, with long-term follow-up conducted one year after treatment. The intervention was performed on 20 patients between 1 October 2016 and 31 October 2017, though one patient was excluded from the final analysis.
No adverse effects were reported within the first 24 h, and all patients were discharged without symptoms. Three patients (15%) developed perianal abscesses—one at week 4 and two at week 8. Complete fistula closure was achieved in 13 patients (69%).
Although this study had the limitation of being non-randomized, it demonstrated that the use of allogeneic MSCs is a safe option for treating complex anal fistulas not associated with Crohn’s disease.
3.2.5. Conclusions
Taken together, dermatologic trials show high clinical response, yet they rely on small sample sizes, inconsistent comparators and mostly non-blinded designs. The promising safety–efficacy profile therefore warrants larger, rigorously blinded studies before routine clinical adoption.
3.3. Glandular Dysfunctions
3.3.1. Treatment of Sjogren’s Disease
The study by Moller-Hansen et al. [
21] included 54 participants with severe dry eye disease secondary to Sjögren’s disease and divided them into three groups, i.e., those using AASCs (n = 20), an active comparator (n = 20), or a non-randomized observation group (n = 14). The intervention groups received a single injection of AASCs or an active comparator into the lacrimal gland of one eye, while the observation group received lubricating eye drops only (
Figure 6). The primary endpoint was change in the Ocular Surface Disease Index (OSDI) score, and the secondary endpoints were non-invasive tear break-up time, tear meniscus height, the Schirmer test and the Oxford score during a 12-month follow-up.
At baseline, the 54 study participants had a median OSDI score of 46.7 (interquartile range [IQR] 36.1–56.8), with no significant difference between the three groups. In the AASCs group, the OSDI score decreased significantly from the median of 39.8 at baseline by a mean of 16.6 points (−41.6%; p < 0.000) at 1-week follow-up, which was maintained at the 12-month follow-up (−16.1 points, −40.4%; p < 0.000). In the vehicle-treated group, the median OSDI score was 49.0 at baseline and decreased by a mean of 21.2 (−43.2%; p < 0.000) at 1-week post-treatment, which was also maintained at 12-months post-treatment (−20.8, −42.4%; p < 0.000).
In the observation group, the OSDI score did not change significantly during the 12-month follow-up. During the follow-up period, no significant difference was observed in the decrease of the OSDI score between the AASCs group and the vehicle group, while both interventions showed a significant decrease compared to the observation group (p = 0.03 and p = 0.004 for AASCs and vehicle, respectively).
The median Schirmer test score in the study eye was 3 mm (IQR 1–5 mm), with no difference between groups (p = 0.23). After 4 months’ follow-up, there was a significant mean increase in the Schirmer test score of 3.55 mm (125%; p = 0.01) in the AASCs group and 3.8 mm (115%; p = 0.008) in the vehicle group, which was maintained after the 12-month follow-up. Median tear osmolarity in the study eye at baseline was 314 mosm/L, with no difference between groups. In the AASCs group, a significant mean decrease in tear osmolarity was observed after the 12-month follow-up of 12.38 mosm/L (−3.9%; p < 0.05), with a trend towards a significant difference from the vehicle group after 4 months (p = 0.07) and at the 12-month follow-up (p = 0.098). An improvement in the subjective and objective signs and symptoms of this pathology was observed in both intervention groups after injection into the lacrimal gland, compared with the observation group.
3.3.2. Treatment of Xerostomia
Lynggaard et al. [
22] attempted to provide proof of concept of the efficacy and safety of AASCs injected into the major salivary glands of irradiated patients. Eligible patients with objective and subjective evidence of radiation-induced salivary gland damage after treatment of stage I-II oropharyngeal squamous cell carcinoma (UICC 8) were enrolled. In total, 25 million cryopreserved AASCs were injected into each submandibular gland and 50 million AASCs into each parotid gland. Data were analyzed using repeated measures linear mixed models.
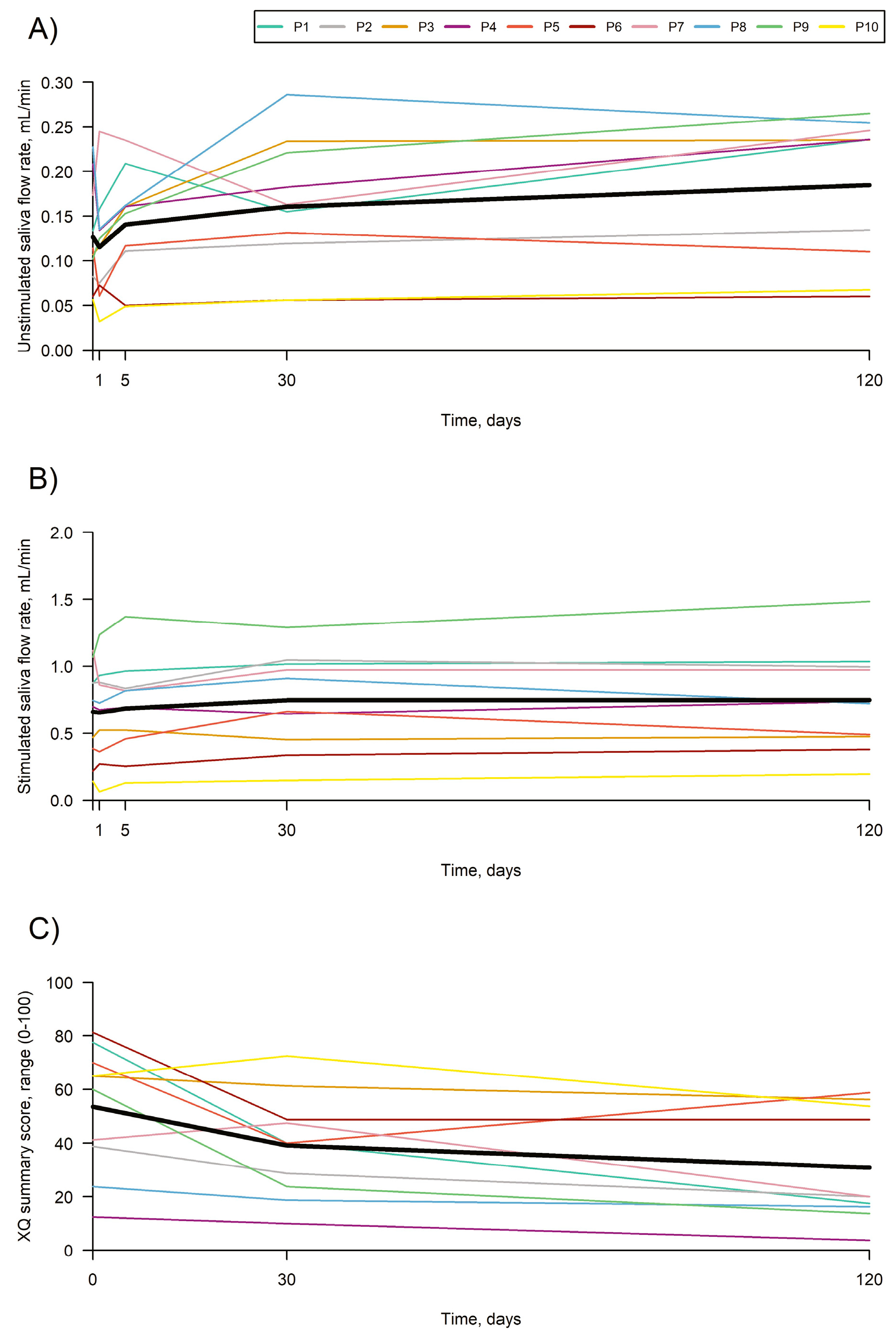
A total of 10 patients (seven men, three women; 59.5 years [range: 45–70]) were treated in four glands. No treatment-related serious adverse events occurred. Over 4 months, unstimulated saliva flow increased from 0.13 mL/mi at baseline to 0.18 mL/min, a change of 0.06 (p = 0.0009) mL/min. Stimulated saliva flow increased from 0.66 mL/min at baseline to 0.75 mL/min, a change of 0.09 (p = 0.017) mL/min.
The xerostomia questionnaire summary score decreased by 22.6 units (
p = 0.0004), the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck Module (EORTC QLQ-H&N35) (dry mouth domains decreased by 26.7 (
p = 0.0013), sticky saliva by 23.3 (
p = 0.0015) and swallowing by 10.0 (
p = 0.0016). This suggests that treatment of the major salivary glands with AASCs could be a safe option (
Figure 7).
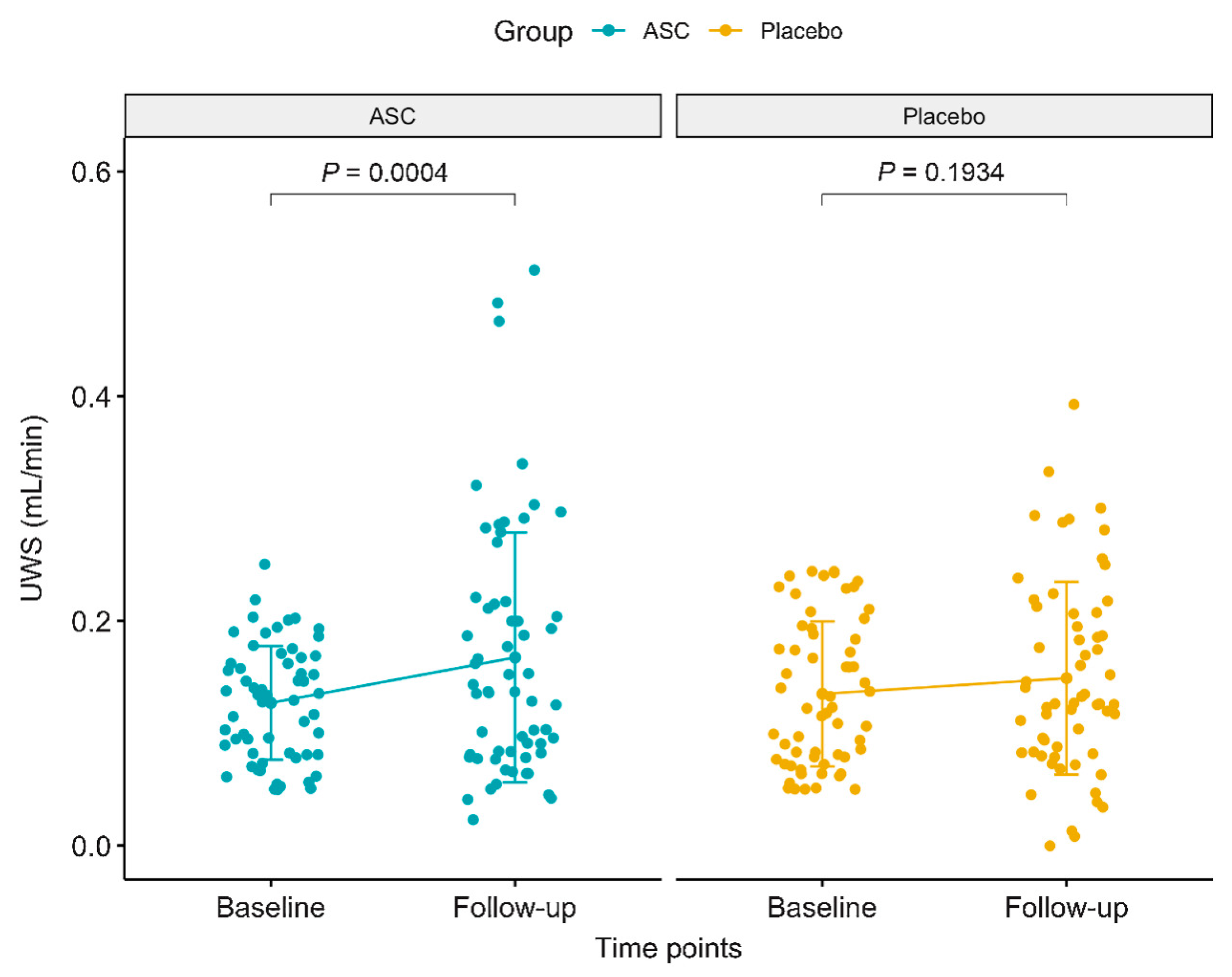
Jakobsen et al. [
23] enrolled 120 patients treated with AASCs an increase in unstimulated whole salivary flow rate (UWS) by 0.04 mL/min (38%) over four months, while the placebo group exhibited a smaller, non-significant increase of 0.01 mL/min (21%). In total, 13 patients (21.7%) in the AASCs group reached normal salivary flow rates (≥0.26 mL/min), compared to only eight patients (13.3%) in the placebo group. Despite these improvements, the difference in salivary function between the two groups was not statistically significant (
p = 0.11).
For the secondary endpoints, no significant improvement in stimulated salivary flow rate (SWS) was observed in either group. In terms of patient-reported outcomes, both the AASCs and placebo groups showed symptom reductions, particularly in dry mouth and sticky saliva. In the AASCs group, dry mouth symptoms decreased by 13.6 units, while sticky saliva reduced by 14.8 units. In comparison, the placebo group had reductions of 7.7 units for dry mouth and 9.3 units for sticky saliva. However, these reductions did not lead to a statistically significant difference between the groups in terms of patient-reported symptoms.
Regarding safety, there were no treatment-related serious adverse events (SAEs), but nine patients in the AASCs group experienced temporary swelling of the submandibular glands, which resolved within 3 weeks. Additionally, 31% of patients in the AASCs group developed de novo human leukocyte antigen (HLA) antibodies, which may have influenced the overall efficacy, as those with new HLA antibodies tended to have a lesser increase in salivary flow rates (
Figure 8).
In conclusion, ASC therapy improved salivary function and reduced xerostomia symptoms, though the improvements were not significantly greater than those observed in the placebo group.
3.3.3. Treatment of Type 1 Diabetes Mellitus
A study by Araujo et al. [
24] evaluated the safety and potential efficacy of adipose tissue-derived allogeneic stem cells (AASCs) combined with vitamin D in patients recently diagnosed with type 1 diabetes (T1D). A total of 13 patients participated: eight received a single dose of AASCs (1 × 10
6 cells/kg) along with vitamin D (2000 IU/day for three months), while five followed standard insulin therapy.
The results showed that adverse events were generally mild and transient. Among the eight patients who received AASCs, 100% reported transient headaches, 88% reported mild local reactions, 50% experienced tachycardia, and 13% reported abdominal cramps. Four patients (50%) developed superficial local thrombophlebitis, and two (25%) experienced transient “floaters”. One case of central retinal vein occlusion was reported at three months, with complete resolution.
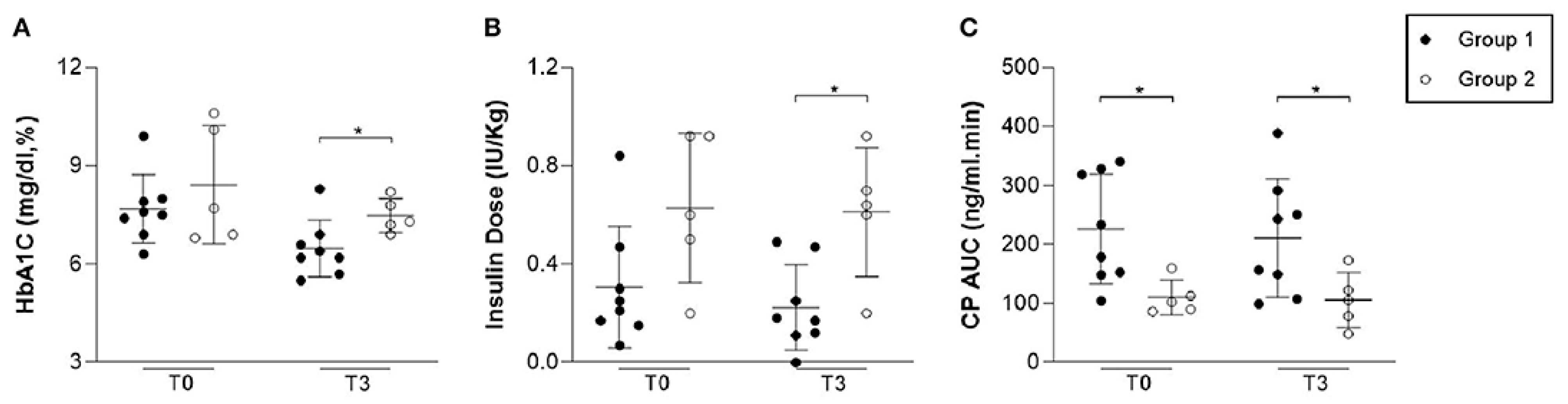
In terms of pancreatic function, C-peptide (CP) levels were significantly higher in the AASC group at the beginning of the study (225.90 ± 92.91 ng/mL) compared to the control group (110.55 ± 29.72 ng/mL, p = 0.02). One month after the intervention, CP levels were 250.22 ± 129.49 ng/mL in the AASC group versus 127.35 ± 18.31 ng/mL in the control group (p = 0.03). At three months, CP levels were 211.20 ± 100.42 ng/mL in the AASC group versus 106.05 ± 47.25 ng/mL in the control group, although the difference was no longer statistically significant (p = 0.06).
Glycemic control improved in the AASC group. Insulin requirements decreased significantly, from 0.31 ± 0.24 IU/kg at baseline to 0.22 ± 0.17 IU/kg at three months, compared to an increase in the control group (from 0.62 ± 0.30 IU/kg to 0.61 ± 0.26 IU/kg,
p = 0.01). HbA1c levels also reduced to 6.47 ± 0.86% in the AASC group compared to 7.48 ± 0.52% in the control group at three months (
p = 0.03). Two patients in the AASC group became temporarily insulin-independent (for 4 and 8 weeks), and 100% of the patients in this group were in the “honeymoon phase” at three months, compared to 0% in the control group (
p = 0.01). (
Figure 9).
This study demonstrates that the use of allogeneic stem cells derived from adipose tissue, combined with vitamin D, is associated with transient side effects, improved glycemic control, and a significant reduction in insulin requirements in newly diagnosed T1D patients. These results suggest promising therapeutic potential, although larger-scale studies with extended follow-up are needed to confirm these findings.
3.3.4. Conclusions
While short-term symptomatic relief (tear and salivary flow, OSDI scores) appears reproducible, variations in dosing, delivery route and concurrent therapies introduce substantial heterogeneity. Longer-term data and head-to-head comparisons are needed to clarify durability and optimal protocols.
3.5. Acute Ischemic Stroke
A phase IIa clinical trial published by De Celis-Ruiz et al. [
28] included 19 patients to evaluate the safety of intravenous administration of adipose-derived mesenchymal stem cells (AASCs) in patients with acute ischemic stroke. Of these 19 patients, six were excluded: two due to technical issues related to cell manufacturing and four for meeting exclusion criteria after randomization. The final sample consisted of 13 patients, divided into two groups: four received AASCs treatment, and nine received a placebo.
The median time from symptom onset to treatment administration was slightly longer in the AASCs group at 13 days (IQR: 13–13.75) compared to 12 days (IQR: 10–13) in the placebo group (p = 0.028). Baseline demographic and clinical characteristics were comparable between the two groups, with a median age of 78 years in the AASCs group and 76 years in the placebo group.
Over the 24-month follow-up period, 124 adverse events (AEs) were reported, with 50 in the AASCs group and 74 in the placebo group (p = 0.074). None of these events were attributed to the AASCs treatment. Additionally, 11 serious adverse events (SAEs) were documented: two in the AASCs group and nine in the placebo group, including one death in the placebo group during the first week, attributed to multiorgan failure.
In terms of efficacy parameters, no statistically significant differences were observed between the groups regarding functional scores (modified Rankin Scale, mRS) or infarct volume. However, a positive trend was noted in NIHSS scores, with a median score of 3 (IQR: 3–6.75) in the AASCs group compared to 7 (IQR: 0–12.5) in the placebo group at 24 months.
These findings indicate that the intravenous administration of AASCs is safe and well-tolerated, while also highlighting a potential clinical benefit, particularly in terms of long-term neurological score improvements (
Figure 12).
The trial proves feasibility and safety, but its tiny sample and late-treatment window mean efficacy remains speculative. A larger, earlier-intervention RCT is now indispensable before AASCs can be advocated for stroke recovery.
3.8. Osteoarthritis
The injection of mesenchymal stromal cells has been proposed as an innovative treatment for osteoarthritis of the knee. These cells, particularly those derived from adipose tissue, are considered a preferable choice in regenerative medicine due to their availability as ready-to-use products.
Sadri et al. [
31] studied three patients with osteoarthritis of the knee. Each received an intraarticular injection of one hundred million AASCs into each affected knee. Follow-up lasted 6 months, during which time clinical outcomes, MRI and serum inflammatory biomarkers were assessed. The primary objective was to verify the safety and feasibility of injecting AASCs during 6 months of follow-up. No serious adverse events were reported. Analysis of secondary outcomes, such as the Visual Analogue Scale, the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index and the Knee Osteoarthritis Outcome Score, showed improvement in all patients. The decrease in the WOMAC score, considered as an indicator of improvement, initially occurred with a steep slope and fell from 54, 43 and 53 to 16, 19 and 8 for patient 1, patient 2 and patient 3, respectively, after 3 months, and to decrease until the end of the 6-month follow-up.
Changes in cartilage volume and thickness, joint effusion and subchondral edema were identified by comparing MRI results before and 6 months after AASCs treatment. AASCs injection not only increased cartilage volume, but also reduced subchondral edema and joint effusion. In addition, the decrease in serum levels of cartilage oligomeric matrix protein and hyaluronic acid suggests a reduction in cartilage degeneration. Quantification of interleukin-10 and interleukin-6 levels showed immunomodulation after cell injection (
Figure 14).
In a randomized, triple-blind, phase II study, Sadri et al. [
32] included 40 patients with knee osteoarthritis. Of these, 20 received an intra-articular injection of 100 × 10
6 AASCs, while 20 received a placebo (saline solution). Four patients were excluded during the study, leaving 36 participants for the final analysis (18 in each group). The mean age was 52.8 ± 7.5 years in the AASCs group and 56.1 ± 7.2 years in the placebo group.
No serious adverse events (SAEs) were observed. Two patients in the AASCs group reported swelling and local pain at the injection site, which resolved within 2 to 3 days without intervention. Laboratory parameters, such as CRP and erythrocyte sedimentation rate, remained normal.
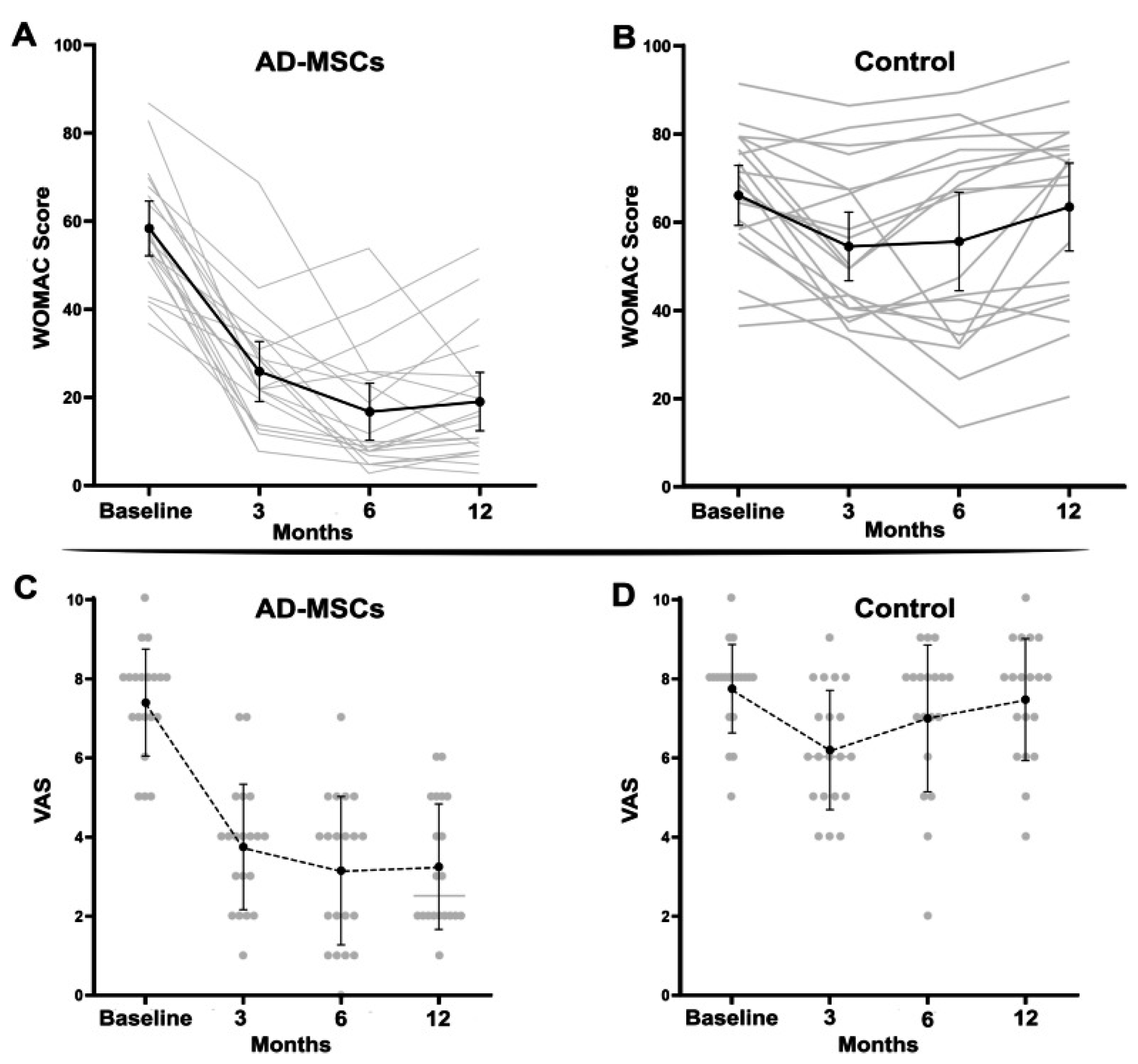
The clinical outcomes following treatment with allogeneic adipose-derived mesenchymal stem cells (AASCs) demonstrated significant and sustained improvements across multiple validated patient questionnaires. Specifically, results from the WOMAC questionnaire indicated a substantial improvement in the AASCs group, with mean scores decreasing notably from 58.35 ± 13.25 at baseline to 16.75 ± 13.81 after 6 months and maintaining at 19.05 ± 14.12 at 12 months, reflecting a reduction of more than 70% at 6 months. Conversely, patients in the placebo group showed minimal variation, with scores marginally declining from 65.42 ± 14.63 at baseline to 63.47 ± 20.68 after 12 months.
Pain assessments via the Visual Analog Scale (VAS) corroborated these findings, revealing a marked decrease in reported pain intensity in the AASCs-treated patients, from an initial score of 7.40 ± 1.35 down to 3.15 ± 1.87 at 6 months, and stabilizing at 3.25 ± 1.58 by the 12-month mark. The placebo group, however, experienced negligible change, maintaining pain scores around baseline levels (7.73 ± 1.14 initially and 7.47 ± 1.54 after 12 months).
Evaluations using the KOOS score, indicative of knee functionality, further highlighted the effectiveness of the AASCs intervention. In the treatment group, the mean KOOS score markedly improved from 28.30 ± 14.90 at baseline to 69.15 ± 12.22 at 6 months, with a slight decline to 63.75 ± 15.40 at 12 months. In stark contrast, scores in the placebo group remained essentially unchanged, evolving minimally from 22.05 ± 9.57 at baseline to 23.84 ± 15.09 at the end of the study period.
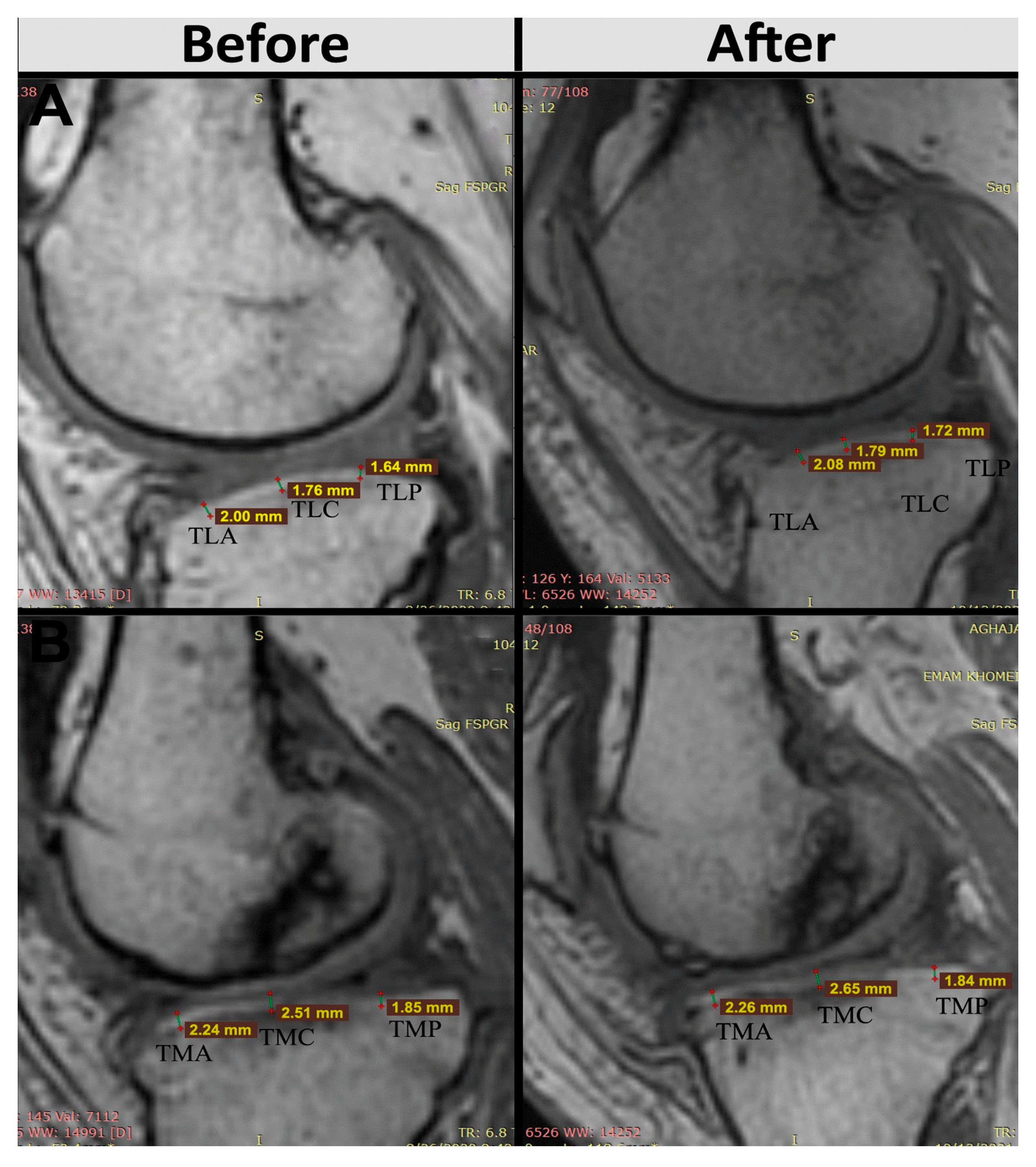
In addition to these clinical improvements, structural changes in articular cartilage thickness were objectively assessed at predefined anatomical landmarks on the tibia and femur. Patients treated with AASCs exhibited increased cartilage thickness in the medial anterior tibial region (TMA), rising from 1.86 ± 0.53 mm to 1.98 ± 0.56 mm at 12 months, and in the medial posterior tibial region (TMP), which increased from 2.01 ± 0.29 mm to 2.07 ± 0.26 mm. Conversely, placebo recipients experienced a reduction in TMA cartilage thickness, dropping from 1.49 ± 0.88 mm to 1.37 ± 0.81 mm, with TMP thickness remaining essentially unchanged (1.81 ± 0.25 mm to 1.78 ± 0.18 mm) (
Figure 15).
These combined clinical and imaging findings clearly illustrate the therapeutic potential of AASCs for improving both symptomatic and structural outcomes in patients.
Chen et al. [
33] studied 11 patients with knee osteoarthritis received intra-articular injections of AASCs at two different doses: a low dose of 6.7 × 10
6 cells (n = 5) and a high dose of 4 × 10
7 cells (n = 6). Over a follow-up period of 12 weeks, significant improvements were observed, particularly in the high-dose group.
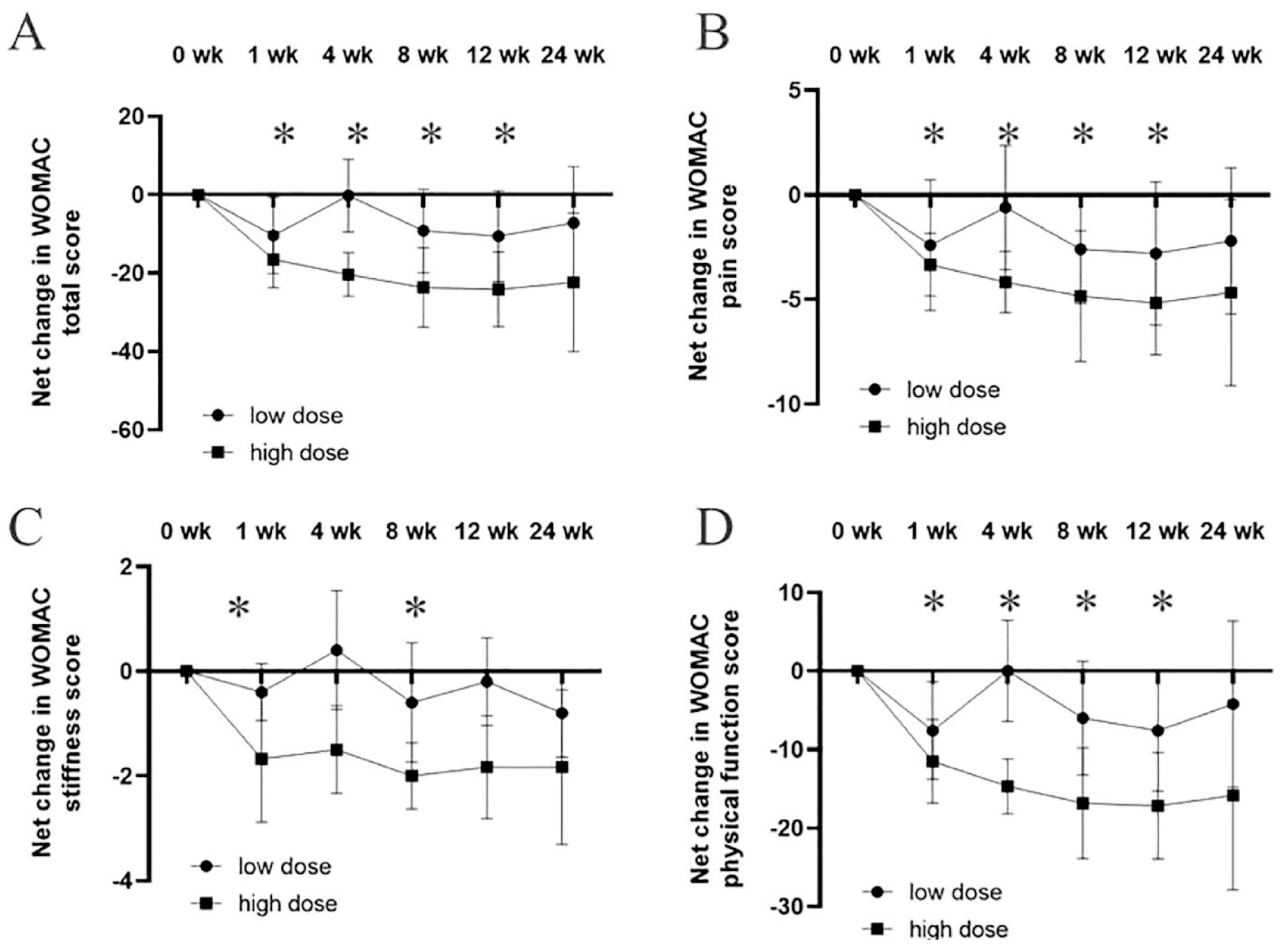
The results showed that the WOMAC total score, which evaluates pain, stiffness and physical function, dropped substantially in the high-dose group compared to baseline. At week 12, the WOMAC pain scores decreased by an average of 7.7 points, while stiffness scores improved by 3.1 points, and physical function scores saw an improvement of 14.3 points. In contrast, the low-dose group showed only moderate improvements, with smaller reductions in WOMAC scores across all categories.
VAS scores for pain also reflected similar trends. Patients in the high-dose group reported a significant reduction in pain, with an average decrease of 4.5 points on the VAS scale by week 12. The low-dose group, while also showing improvement, had a less pronounced reduction in pain, averaging around 2.1 points of improvement.
Additionally, MRI imaging conducted at 12 weeks post-injection demonstrated structural changes in the high-dose group. These patients showed increased cartilage volume, along with reduced subchondral edema and joint effusion, suggesting not only symptomatic relief but potential structural improvements in the knee joint. The low-dose group did not exhibit significant changes in MRI results.
Overall, 73% of patients in the high-dose group reported a marked improvement in their symptoms, while only 40% of patients in the low-dose group experienced comparable benefits. These results indicate that higher doses of AASCs lead to greater clinical improvements in pain relief, joint function, and even cartilage regeneration in patients with knee osteoarthritis (
Figure 16).
Lu et al. [
34] conducted a study involving 22 patients with bilateral knee osteoarthritis, divided into three groups based on the administered dose of AASCs: low dose (1 × 10
7 cells, seven patients), medium dose (2 × 10
7 cells, eight patients) and high dose (5 × 10
7 cells, seven patients). Among these participants, 19 were women, with a mean age of 57.9 years and a mean BMI of 26.3 kg/m
2. Three patients withdrew from the study for reasons unrelated to the treatment. Regarding safety, 19 patients (86.4%) reported at least one adverse event, the most common being localized pain (81.8%) and knee swelling (27.3%). These adverse effects were transient, resolving within three days, and no serious adverse events attributable to the treatment were observed.
Clinically, significant improvements were observed at 48 weeks. The total WOMAC score, assessing pain, stiffness and joint function, decreased by 23.71 points in the low-dose group, 16.50 points in the medium-dose group, and 10.71 points in the high-dose group, reflecting an improved quality of life for patients. Additionally, pain scores measured by the Visual Analog Scale (VAS) decreased by 2.19 points for the left knee in the low-dose group, 2.25 points in the medium-dose group, and 1.36 points in the high-dose group. Overall quality of life, measured by the SF-36 questionnaire, improved by 22.71 points in the low-dose group, 12.63 points in the medium-dose group, and 10.57 points in the high-dose group.
MRI analyses revealed an increase in cartilage volume in the low-dose group, with an average gain of 54.58 mm3 in total cartilage volume and 39.69 mm3 in tibial cartilage. Conversely, reductions in cartilage volume were observed in the medium- and high-dose groups. The WORMS score, assessing changes in joint structures, showed a decrease of 0.36 points in the left knee and 0.86 points in the right knee in the low-dose group, while the other groups exhibited minimal significant changes.
Pain, function and cartilage signals are encouraging, yet placebo and imaging-bias cannot be ruled out because two studies lacked sham controls. A multicenter, sham-controlled phase-III RCT is therefore essential to confirm clinical value.