Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives
Abstract
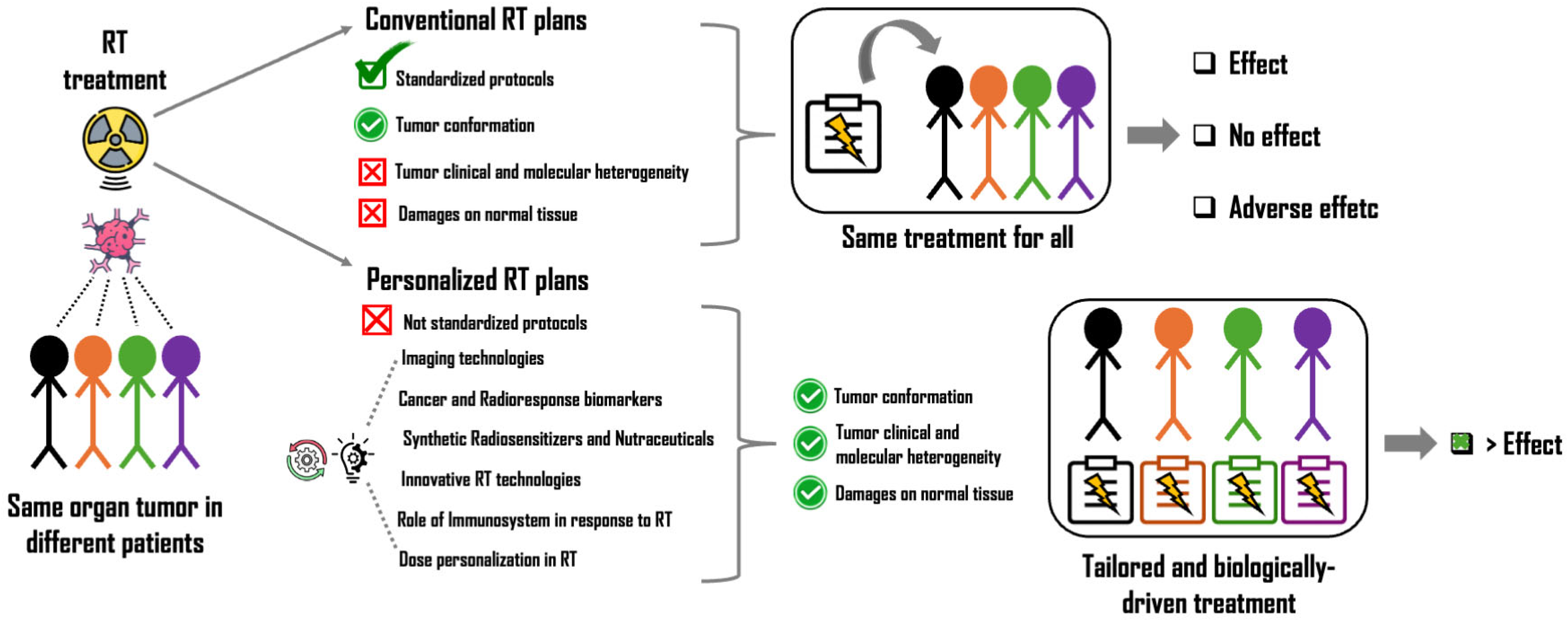
1. Introduction
2. Radioresponse Biomarkers
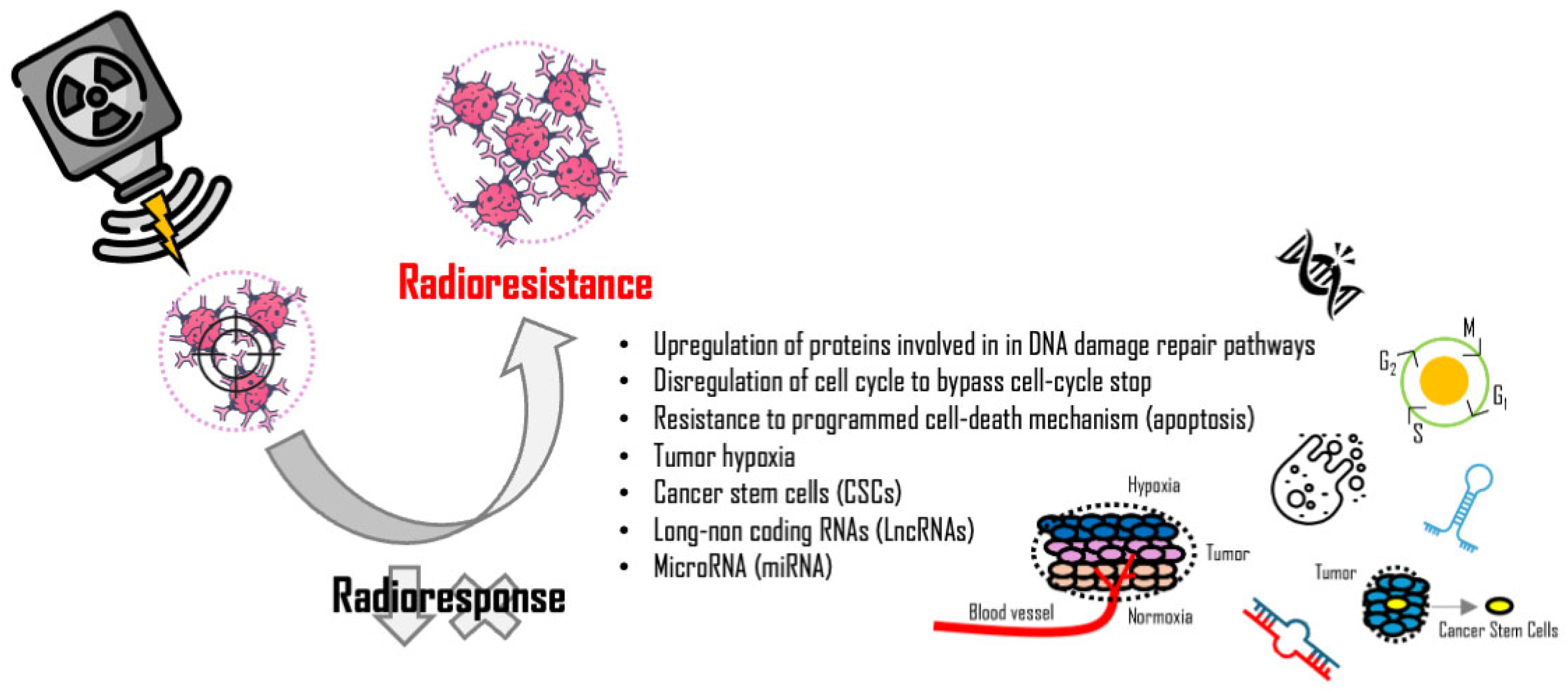
3. Mechanisms of Cancer Cell Radioresistance
4. Synthetic Radiosensitizers and Nutraceuticals
5. Role of the Immune System in Response to Radiotherapy
6. Clinical Trials Investigating the Impact of Hypofractionated RT Strategies
7. Innovative Radiotherapy Technologies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manem, V.S.; Taghizadeh-Hesary, F. Advances in personalized radiotherapy. BMC Cancer 2024, 24, 556. [Google Scholar] [CrossRef]
- Van Leeuwen, C.M.; Oei, A.L.; Crezee, J.; Bel, A.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat. Oncol. 2018, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Minafra, L.; Cammarata, F.P.; Calvaruso, M. The Role of Radiation in Cancer Treatment: New Insights towards Personalized Therapies. J. Pers. Med. 2022, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Luo, H.; Zhang, J.J.; Ge, H.; Ge, L. Clinical translation of ultra-high dose rate flash radiotherapy: Opportunities, challenges, and prospects. World J. Radiol. 2025, 17, 105722. [Google Scholar] [CrossRef]
- Dagar, G.; Gupta, A.; Shankar, A.; Chauhan, R.; Macha, M.A.; Bhat, A.A.; Das, D.; Goyal, R.; Bhoriwal, S.; Pandita, R.K.; et al. The future of cancer treatment: Combining radiotherapy with immunotherapy. Front. Mol. Biosci. 2024, 11, 1409300. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef]
- Meehan, J.; Gray, M.; Martínez-Pérez, C.; Kay, C.; Wills, J.C.; Kunkler, I.H.; Dixon, J.M.; Turnbull, A.K. A Novel Approach for the Discovery of Biomarkers of Radiotherapy Response in Breast Cancer. J. Pers. Med. 2021, 11, 796. [Google Scholar] [CrossRef]
- Jeggo, P.A.; Pearl, L.H.; Carr, A.M. DNA repair, genome stability and cancer: A historical perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef]
- Bouwman, P.; Jonkers, J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat. Rev. Cancer 2012, 12, 587–598. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Rivera, A.L.; Pelloski, C.E.; Gilbert, M.R.; Colman, H.; De La Cruz, C.; Sulman, E.P.; Bekele, B.N.; Aldape, K.D. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010, 12, 116–121. [Google Scholar] [CrossRef]
- Sherwani, Z.K.; Damast, S.; Fields, E.C.; Beriwal, S.; Horne, Z.D.; Kidd, E.A.; Leung, E.W.; Taunk, N.K.; Chino, J.; Russo, A.L.; et al. The Prognostic Impact of MLH1 Promoter Hypermethylation in Stage I-II Endometrial Cancer Treated with Adjuvant Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2025, 120, S32. [Google Scholar] [CrossRef]
- Dai, Y.H.; Wang, Y.F.; Shen, P.C.; Lo, C.H.; Yang, J.F.; Lin, C.S.; Chao, H.L.; Huang, W.Y. Radiosensitivity index emerges as a potential biomarker for combined radiotherapy and immunotherapy. NPJ Genom. Med. 2021, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): A cohort-based pooled analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef]
- Minafra, L.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Gilardi, M.C.; Forte, G.I. Radiation Gene-expression Signatures in Primary Breast Cancer Cells. Anticancer Res. 2018, 38, 2707–2715. [Google Scholar]
- Cammarata, F.P.; Forte, G.I.; Broggi, G.; Bravatà, V.; Minafra, L.; Pisciotta, P.; Calvaruso, M.; Tringali, R.; Tomasello, B.; Torrisi, F.; et al. Molecular Investigation on a Triple Negative Breast Cancer Xenograft Model Exposed to Proton Beams. Int. J. Mol. Sci. 2020, 21, 6337. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, F.P.; Torrisi, F.; Forte, G.I.; Minafra, L.; Bravatà, V.; Pisciotta, P.; Savoca, G.; Calvaruso, M.; Petringa, G.; Cirrone, G.A.P.; et al. Proton Therapy and Src Family Kinase Inhibitor Combined Treatments on U87 Human Glioblastoma Multiforme Cell Line. Int. J. Mol. Sci. 2019, 20, 4745. [Google Scholar] [CrossRef]
- Torrisi, F.; Minafra, L.; Cammarata, F.P.; Savoca, G.; Calvaruso, M.; Vicario, N.; Maccari, L.; Pérès, E.A.; Özçelik, H.; Bernaudin, M.; et al. SRC Tyrosine Kinase Inhibitor and X-rays Combined Effect on Glioblastoma Cell Lines. Int. J. Mol. Sci. 2020, 21, 3917. [Google Scholar] [CrossRef]
- Rajendran, J.G.; Krohn, K.A. F-18 fluoromisonidazole for imaging tumor hypoxia: Imaging the microenvironment for personalized cancer therapy. Semin. Nucl. Med. 2015, 45, 151–162. [Google Scholar] [CrossRef]
- Yazaki, S.; Salgado, R.; Shimoi, T.; Yoshida, M.; Shiino, S.; Kaneda, T.; Kojima, Y.; Sumiyoshi-Okuma, H.; Nishikawa, T.; Sudo, K.; et al. Impact of adjuvant chemotherapy and radiotherapy on tumour-infiltrating lymphocytes and PD-L1 expression in metastatic breast cancer. Br. J. Cancer 2023, 128, 568–575. [Google Scholar] [CrossRef]
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015, 25, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Moura, T.; Laranjeira, P.; Caramelo, O.; Gil, A.M.; Paiva, A. Breast Cancer and Tumor Microenvironment: The Crucial Role of Immune Cells. Curr. Oncol. 2025, 32, 143. [Google Scholar] [CrossRef] [PubMed]
- Bravatà, V.; Tinganelli, W.; Cammarata, F.P.; Minafra, L.; Calvaruso, M.; Sokol, O.; Petringa, G.; Cirrone, G.A.P.; Scifoni, E.; Forte, G.I.; et al. Hypoxia Transcriptomic Modifications Induced by Proton Irradiation in U87 Glioblastoma Multiforme Cell Line. J. Pers. Med. 2021, 11, 308. [Google Scholar] [CrossRef]
- Pucci, G.; Minafra, L.; Bravatà, V.; Calvaruso, M.; Turturici, G.; Cammarata, F.P.; Savoca, G.; Abbate, B.; Russo, G.; Cavalieri, V.; et al. Glut-3 Gene Knockdown as a Potential Strategy to Overcome Glioblastoma Radioresistance. Int. J. Mol. Sci. 2024, 25, 2079. [Google Scholar] [CrossRef]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A.; et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Nobrega, M.; Ferrier, T.; Dickinson, K.; Kaorey, N.; Nadeau, A.; Castillo, A.; Burnier, J.V. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. NPJ Precis. Oncol. 2025, 9, 84. [Google Scholar] [CrossRef]
- Ni, J.; Bucci, J.; Malouf, D.; Knox, M.; Graham, P.; Li, Y. Exosomes in Cancer Radioresistance. Front. Oncol. 2019, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jung, K.O.; Oh, S.; Kim, Y.H.; Lee, S.Y.; Hong, S.; Cho, S.H.; Kim, H.; Rhee, S.; Cheon, G.J.; et al. Radiation-induced exosomal miR-21 enhances tumor proliferation and invasiveness in breast cancer: Implications for poor prognosis in radiotherapy patients. Exp. Hematol. Oncol. 2024, 13, 120. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, X. Association of Exosomal miR-210 with Signaling Pathways Implicated in Lung Cancer. Genes 2021, 12, 1248. [Google Scholar] [CrossRef]
- Zebene, E.D.; Lombardi, R.; Pucci, B.; Medhin, H.T.; Seife, E.; Di Gennaro, E.; Budillon, A.; Woldemichael, G.B. Proteomic Analysis of Biomarkers Predicting Treatment Response in Patients with Head and Neck Cancers. Int. J. Mol. Sci. 2024, 25, 12513. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef]
- Liu, K.X.; Everdell, E.; Pal, S.; Haas-Kogan, D.A.; Milligan, M.G. Harnessing Lactate Metabolism for Radiosensitization. Front. Oncol. 2021, 11, 672339. [Google Scholar] [CrossRef]
- Busato, F.; Khouzai, B.E.; Mognato, M. Biological Mechanisms to Reduce Radioresistance and Increase the Efficacy of Radiotherapy: State of the Art. Int. J. Mol. Sci. 2022, 23, 10211. [Google Scholar] [CrossRef] [PubMed]
- Her, J.; Bunting, S.F. How cells ensure correct repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10502–10511. [Google Scholar] [CrossRef] [PubMed]
- Bright, S.J.; Flint, D.B.; Martinus, D.K.J.; Turner, B.X.; Manandhar, M.; Ben Kacem, M.; McFadden, C.H.; Yap, T.A.; Shaitelman, S.F.; Sawakuchi, G.O. Targeted Inhibition of DNA-PKcs, ATM, ATR, PARP, and Rad51 Modulate Response to X Rays and Protons. Radiat. Res. 2022, 198, 336–346. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef]
- Melia, E.; Fisch, A.S.; Tinhofer, I.; Parsons, J.L. Targeting Chk1 and Wee1 kinases enhances radiosensitivity of 2D and 3D head and neck cancer models to X-rays and low/high-LET protons. Cell Death Dis. 2025, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Omura-Minamisawa, M.; Chao, C.; Nakagami, Y.; Ito, M.; Inoue, T. Bcl-2 inhibitors potentiate the cytotoxic effects of radiation in Bcl-2 overexpressing radioresistant tumor cells. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 517–528. [Google Scholar] [CrossRef]
- Beckers, C.; Pruschy, M.; Vetrugno, I. Tumor hypoxia and radiotherapy: A major driver of resistance even for novel radiotherapy modalities. Semin. Cancer Biol. 2024, 98, 19–30. [Google Scholar] [CrossRef]
- Harada, H.; Kizaka-Kondoh, S.; Li, G.; Itasaka, S.; Shibuya, K.; Inoue, M.; Hiraoka, M. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene 2007, 26, 7508–7516. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Prabhakar, N.; Kumar, L.; Bhattacharjee, A.; Kar, S.; Malik, S.; Kumar, D.; Ruokolainen, J.; Negi, A.; Jha, N.K.; et al. Crosstalk between long noncoding RNA and microRNA in Cancer. Cell Oncol. 2023, 46, 885–908. [Google Scholar] [CrossRef] [PubMed]
- Yazarlou, F.; Martinez, I.; Lipovich, L. Radiotherapy and breast cancer: Finally, an lncRNA perspective on radiosensitivity and radioresistance. Front. Oncol. 2024, 14, 1437542. [Google Scholar]
- Lei, C.; Li, S.; Fan, Y.; Hua, L.; Pan, Q.; Li, Y.; Long, Z.; Yang, R. LncRNA DUXAP8 induces breast cancer radioresistance by modulating the PI3K/AKT/mTOR pathway and the EZH2-E-cadherin/RHOB pathway. Cancer Biol. Ther. 2022, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cang, H.; Xiao, J.; Wu, H.; Wang, B.; Shao, Q. LncRNA DNAH17-AS1 promotes gastric cancer proliferation and radioresistance by sponging miR-202-3p to upregulate ONECUT2. Discov. Oncol. 2024, 15, 432. [Google Scholar] [CrossRef]
- Xu, K.; Guo, H.; Xia, A.; Wang, Z.; Wang, S.; Wang, Q. Non-coding RNAs in radiotherapy resistance: Roles and therapeutic implications in gastrointestinal cancer. Biomed. Pharmacother. 2023, 161, 114485. [Google Scholar] [CrossRef]
- Hu, H.; Yang, H.; Fan, S.; Jia, X.; Zhao, Y.; Li, H. LncRNA HOTAIR promotes DNA damage repair and radioresistance by targeting ATR in colorectal cancer. Oncol. Res. 2024, 32, 1335–1346. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Q.; Zheng, W.; Zhou, Y.; Bai, Y.; Pan, Y.; Zhang, J.; Shao, C. LncRNA CASC19 Enhances the Radioresistance of Nasopharyngeal Carcinoma by Regulating the miR-340-3p/FKBP5 Axis. Int. J. Mol. Sci. 2023, 24, 3047. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Y.; Wan, M.; Zeng, X.; Wu, J. miR-340: A multifunctional role in human malignant diseases. Int. J. Biol. Sci. 2021, 17, 236–246. [Google Scholar] [CrossRef]
- Sun, B.; Liu, C.; Li, H.; Zhang, L.; Luo, G.; Liang, S.; Lü, M. Research progress on the interactions between long non-coding RNAs and microRNAs in human cancer. Oncol. Lett. 2020, 19, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Montaño, S.; Peralta-Zaragoza, O.; Vélez-Uriza, D.E.; Arriaga-Canon, C.; Guajardo-Barreto, X.; Bautista-Sánchez, D.; Sosa-León, R.; Hernández-González, O.; et al. Radio-miRs: A comprehensive view of radioresistance-related microRNAs. Genetics 2024, 227, iyae097. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S. miR-214 promotes radioresistance in human ovarian cancer cells by targeting PETN. Biosci. Rep. 2017, 37, BSR20170327. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, Y.F.; Tan, D.M.; Xu, Y.; Chen, H.X.; Wang, D. miR-183-5p enhances the radioresistance of colorectal cancer by directly targeting ATG5. J. Biosci. 2019, 44, 92. [Google Scholar] [CrossRef]
- Liu, J.; Yan, S.; Hu, J.; Ding, D.; Liu, Y.; Li, X.; Pan, H.S.; Liu, G.; Wu, B.; Liu, Y. MiRNA-4537 functions as a tumor suppressor in gastric cancer and increases the radiosensitivity of gastric cancer cells. Bioengineered 2021, 12, 8457–8467. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, M.; Cui, M.; Feng, G.; Dong, J.; Li, Y.; Xiao, H.; Fan, S. MiR-365 enhances the radiosensitivity of non-small cell lung cancer cells through targeting CDC25A. Biochem. Biophys. Res. Commun. 2019, 512, 392–398. [Google Scholar] [CrossRef]
- Al-Hawary, S.I.S.; Abdalkareem Jasim, S.; Altalbawy, F.M.A.; Kumar, A.; Kaur, H.; Pramanik, A.; Jawad, M.A.; Alsaad, S.B.; Mohmmed, K.H.; Zwamel, A.H. miRNAs in radiotherapy resistance of cancer; a comprehensive review. Cell Biochem. Biophys. 2024, 82, 1665–1679. [Google Scholar] [CrossRef]
- Zdrowowicz, M.; Spisz, P.; Hać, A.; Herman-Antosiewicz, A.; Rak, J. Influence of Hypoxia on Radiosensitization of Cancer Cells by 5-Bromo-2’-deoxyuridine. Int. J. Mol. Sci. 2022, 23, 1429. [Google Scholar] [CrossRef]
- Wardman, P. Nitroimidazoles as hypoxic cell radiosensitizers and hypoxia probes: Misonidazole, myths and mistakes. Br. J. Radiol. 2019, 92, 20170915. [Google Scholar] [CrossRef]
- Lesueur, P.; Chevalier, F.; Austry, J.B.; Waissi, W.; Burckel, H.; Noël, G.; Habrand, J.L.; Saintigny, Y.; Joly, F. Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: A systematic review of pre-clinical and clinical human studies. Oncotarget 2017, 8, 69105–69124. [Google Scholar] [CrossRef]
- Valdes, G.; Iwamoto, K.S. Re-evaluation of cellular radiosensitization by 5-fluorouracil: High-dose, pulsed administration is effective and preferable to conventional low-dose, chronic administration. Int. J. Radiat. Biol. 2013, 89, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005, 3, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Tahara, M.; Mizusawa, J.; Kodaira, T.; Fujii, H.; Yamazaki, T.; Mitani, H.; Iwae, S.; Fujimoto, Y.; Onozawa, Y.; et al. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 1980–1990. [Google Scholar] [CrossRef]
- Candelaria, M.; Garcia-Arias, A.; Cetina, L.; Dueñas-Gonzalez, A. Radiosensitizers in cervical cancer. Cisplatin and beyond. Radiat. Oncol. 2006, 1, 15. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef]
- Bennett, M.; Feldmeier, J.; Smee, R.; Milross, C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst. Rev. 2018, 2018, CD005007. [Google Scholar] [CrossRef]
- Chen, A. PARP inhibitors: Its role in treatment of cancer. Chin. J. Cancer 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Scheper, J.; Hildebrand, L.S.; Faulhaber, E.M.; Deloch, L.; Gaipl, U.S.; Symank, J.; Fietkau, R.; Distel, L.V.; Hecht, M.; Jost, T. Tumor-specific radiosensitizing effect of the ATM inhibitor AZD0156 in melanoma cells with low toxicity to healthy fibroblasts. Strahlenther. Onkol. 2023, 199, 1128–1139. [Google Scholar] [CrossRef]
- Samuels, M.; Falkenius, J.; Bar-Ad, V.; Dunst, J.; van Triest, B.; Yachnin, J.; Rodriguez-Gutierrez, A.; Kuipers, M.; You, X.; Sarholz, B.; et al. A Phase 1 Study of the DNA-PK Inhibitor Peposertib in Combination With Radiation Therapy With or Without Cisplatin in Patients With Advanced Head and Neck Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Antrobus, J.; Parsons, J.L. Histone Deacetylases and Their Potential as Targets to Enhance Tumour Radiosensitisation. Radiation 2022, 2, 149–167. [Google Scholar] [CrossRef]
- Thangaretnam, K.; Islam, M.O.; Lv, J.; El-Rifai, A.; Perloff, A.; Soutto, H.L.; Peng, D.; Chen, Z. WEE1 inhibition in cancer therapy: Mechanisms, synergies, preclinical insights, and clinical trials. Crit. Rev. Oncol. Hematol. 2025, 211, 104710. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Chen, X.; Yang, Z.; Mei, X.; Ma, J.; Zhang, Z.; Guo, X.; Yu, X. CDK4/6 inhibitors: A novel strategy for tumor radiosensitization. J. Exp. Clin. Cancer Res. 2020, 39, 188. [Google Scholar] [CrossRef]
- Kriegs, M.; Gurtner, K.; Can, Y.; Brammer, I.; Rieckmann, T.; Oertel, R.; Wysocki, M.; Dorniok, F.; Gal, A.; Grob, T.J.; et al. Radiosensitization of NSCLC cells by EGFR inhibition is the result of an enhanced p53-dependent G1 arrest. Radiother. Oncol. 2015, 115, 120–127. [Google Scholar] [CrossRef]
- Bossi, P.; Platini, F. Radiotherapy plus EGFR inhibitors: Synergistic modalities. Cancers Head Neck 2017, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lei, L.; Zhang, Z.; Du, M.; Chen, Z. The potential of vascular normalization for sensitization to radiotherapy. Heliyon 2024, 10, e32598. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Vázquez, L.A.; Méndez-García, A.; Rodríguez, A.L.; Sahare, P.; Pathak, S.; Banerjee, A.; Duttaroy, A.K.; Paul, S. Applications of nanotechnologies for miRNA-based cancer therapeutics: Current advances and future perspectives. Front. Bioeng. Biotechnol. 2023, 11, 1208547. [Google Scholar] [CrossRef]
- Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int. J. Mol. Sci. 2019, 20, 5267. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Cotino-Nájera, S.; Herrera, L.A.; Domínguez-Gómez, G.; Díaz-Chávez, J. Molecular mechanisms of resveratrol as chemo and radiosensitizer in cancer. Front. Pharmacol. 2023, 14, 1287505. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, T.; Bao, Y.; Li, P.; Zhao, T.; Liu, Y.; Wang, H.; Sun, C. Genistein Implications in Radiotherapy: Kill Two Birds with One Stone. Molecules 2025, 30, 188. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yu, Y.; Zhao, H.G.; Yang, A.; Yan, H.; Cui, Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012, 104, 395–400. [Google Scholar] [CrossRef]
- Montoro, A.; Obrador, E.; Mistry, D.; Forte, G.I.; Bravatà, V.; Minafra, L.; Calvaruso, M.; Cammarata, F.P.; Falk, M.; Schettino, G.; et al. Radioprotectors, Radiomitigators, and Radiosensitizers. In Radiobiology Textbook; Baatout, S., Ed.; Springer: Cham, Switzerland, 2023; pp. 571–628. [Google Scholar]
- Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.B.; Sridhar, K.; Sharma, M. Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering 2022, 9, 478. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front. Oncol. 2012, 2, 153. [Google Scholar] [CrossRef]
- Troitskaya, O.S.; Novak, D.D.; Richter, V.A.; Koval, O.A. Immunogenic Cell Death in Cancer Therapy. Acta Naturae 2022, 14, 40–53. [Google Scholar] [CrossRef]
- Lind, H.T.; Hall, S.C.; Strait, A.A.; Goon, J.B.; Aleman, J.D.; Chen, S.M.Y.; Karam, S.D.; Young, C.D.; Wang, J.H.; Wang, X.J. MHC class I upregulation contributes to the therapeutic response to radiotherapy in combination with anti-PD-L1/anti-TGF-β in squamous cell carcinomas with enhanced CD8 T cell memory-driven response. Cancer Lett. 2025, 608, 217347. [Google Scholar] [CrossRef]
- Nabrinsky, E.; Macklis, J.; Bitran, J. A Review of the Abscopal Effect in the Era of Immunotherapy. Cureus 2022, 14, e29620. [Google Scholar] [CrossRef]
- Karimi, S.; Bakhshali, R.; Bolandi, S.; Zahed, Z.; Mojtaba Zadeh, S.S.; Kaveh Zenjanab, M.; Jahanban Esfahlan, R. For and against tumor microenvironment: Nanoparticle-based strategies for active cancer therapy. Mater. Today Bio 2025, 31, 101626. [Google Scholar] [CrossRef]
- Mohamad, O.; Diaz de Leon, A.; Schroeder, S.; Leiker, A.; Christie, A.; Zhang-Velten, E.; Trivedi, L.; Khan, S.; Desai, N.B.; Laine, A.; et al. Safety and efficacy of concurrent immune checkpoint inhibitors and hypofractionated body radiotherapy. Oncoimmunology 2018, 7, e1440168. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.H.; Lei, Z.; Yang, H.N.; Tang, Z.; Yang, M.Q.; Wang, Y.; Sui, J.D.; Wu, Y.Z. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: A narrative review. Ann. Transl. Med. 2022, 10, 1406. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Guha, C.; Schoenfeld, J.; Morris, Z.; Monjazeb, A.; Sikora, A.; Crittenden, M.; Shiao, S.; Khleif, S.; Gupta, S.; et al. Radiation dose and fraction in immunotherapy: One-size regimen does not fit all settings, so how does one choose? J. Immunother. Cancer 2021, 9, e002038. [Google Scholar] [CrossRef] [PubMed]
- Antelo, G.; Comas, S.; Casas, F.; Valduvieco, I.; Barreto, T.; Laplana, M.; Mases, J.; Oses, G.; Mollà, M. Clinical outcomes and timing on the combination of focal radiation therapy and immunotherapy for the treatment of brain metastases. Front. Immunol. 2023, 14, 1236398. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 391, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, Y.; Umezawa, R.; Nakagawa, T.; Anbai, A.; Makiguchi, T.; Tanaka, H.; Horii, Y.; Suzuki, A.; Morita, R.; Nogawa, H.; et al. Phase 2 Trial of Combination Radiotherapy and Pembrolizumab Plus Chemotherapy in Patients With Previously Untreated Metastatic NSCLC: NJLCG 1902. JTO Clin. Res. Rep. 2025, 6, 100817. [Google Scholar] [CrossRef]
- Brenner, D.J. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin. Radiat. Oncol. 2008, 18, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Savoca, G.; Calvaruso, M.; Minafra, L.; Bravatà, V.; Cammarata, F.P.; Iacoviello, G.; Abbate, B.; Evangelista, G.; Spada, M.; Forte, G.I.; et al. Local Disease-Free Survival Rate (LSR) Application to Personalize Radiation Therapy Treatments in Breast Cancer Models. J. Pers. Med. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. CHHiP Investigators. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Gillespie, E.F.; Khan, A.J.; Cahlon, O.; Braunstein, L.Z. Are 5-Year Randomized Clinical Trial Results Sufficient for Implementation of Short-Course Whole Breast Radiation Therapy? Pract. Radiat. Oncol. 2021, 11, 301–304. [Google Scholar] [CrossRef]
- Berk, L.B. Radiation therapy as primary and adjuvant treatment for local and regional melanoma. Cancer Control 2008, 15, 233–238. [Google Scholar] [CrossRef]
- Kao, Y.S. Preoperative ultra-hypofractionation radiotherapy in extremity/trunk wall soft tissue sarcoma—A meta-analysis of prospective studies. Cancer Radiother. 2023, 27, 96–102. [Google Scholar] [CrossRef]
- Brand, D.H.; Yarnold, J.R. The Linear-Quadratic Model and Implications for Fractionation. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 673–677. [Google Scholar] [CrossRef]
- Pedicini, P.; Fiorentino, A.; Simeon, V.; Tini, P.; Chiumento, C.; Pirtoli, L.; Salvatore, M.; Storto, G. Clinical radiobiology of glioblastoma multiforme: Estimation of tumor control probability from various radiotherapy fractionation schemes. Strahlenther. Onkol. 2014, 190, 925–932. [Google Scholar] [CrossRef]
- Wright, C.M.; Dreyfuss, A.D.; Baron, J.A.; Maxwell, R.; Mendes, A.; Barsky, A.R.; Doucette, A.; Svoboda, J.; Chong, E.A.; Jones, J.A.; et al. Radiation Therapy for Relapsed or Refractory Diffuse Large B-Cell Lymphoma: What Is the Right Regimen for Palliation? Adv. Radiat. Oncol. 2022, 7, 101016. [Google Scholar] [CrossRef]
- Catton, C.N.; Lukka, H.; Gu, C.S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.P.; Ahmed, S.; Cheung, P.; et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M.; Agrawal, R.K.; Aird, E.G.; Barrett, J.M.; Barrett-Lee, P.J.; Bliss, J.M.; Brown, J.; Dewar, J.A.; Dobbs, H.J.; Haviland, J.S.; et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008, 9, 331–341. [Google Scholar]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. FAST-Forward Trial Management Group. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.M.; Le-Rademacher, J.; Haffty, B.G., Jr.; Hansen, E.K.; Agarwal, J.; Wagner, J.; Kong, I.; Armer, J.; Arthur, D.W.; Whelan, T.J.; et al. Randomized Trial of Hypofractionated Post-Mastectomy Radiation Therapy (PMRT) in Women with Breast Reconstruction (RT CHARM, Alliance A221505). Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S11. [Google Scholar] [CrossRef]
- Fransson, P.; Nilsson, P.; Gunnlaugsson, A.; Beckman, L.; Tavelin, B.; Norman, D.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): Patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol. 2021, 22, 235–245. [Google Scholar] [CrossRef]
- van As, N.; Griffin, C.; Tree, A.; Patel, J.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; et al. Phase 3 Trial of Stereotactic Body Radiotherapy in Localized Prostate Cancer. N. Engl. J. Med. 2024, 391, 1413–1425. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, S.; Xu, X.; Du, S.; Zhu, G.; Jiang, C.; Xia, S.A.; Li, Q.; Wang, Q.; Qian, D.; et al. Efficacy and Toxicity of Moderately Hypofractionated Radiation Therapy with Helical TomoTherapy Versus Conventional Radiation Therapy in Patients with Unresectable Stage III Non-Small Cell Lung Cancer Receiving Concurrent Chemotherapy: A Multicenter, Randomized Phase 3 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 422–431. [Google Scholar]
- Rehman, S.; Roach, M.C.; Bradley, J.D.; Robinson, C.G. Lung Stereotactic Body Radiation Therapy. Mo. Med. 2015, 112, 361–365. [Google Scholar]
- Matsuo, Y. Stereotactic Body Radiotherapy for Hepatocellular Carcinoma: A Brief Overview. Curr. Oncol. 2023, 30, 2493–2500. [Google Scholar] [CrossRef]
- Guckenberger, M.; Dahele, M.; Ong, W.L.; Sahgal, A. Stereotactic Body Radiation Therapy for Spinal Metastases: Benefits and Limitations. Semin. Radiat. Oncol. 2023, 33, 159–171. [Google Scholar] [CrossRef]
- Shintani, T.; Anami, S.; Sano, K.; Okada, W.; Tanooka, M. Stereotactic Body Radiation Therapy for Prostate Cancer Using Tomotherapy With Synchrony Fiducial Tracking. Cureus 2023, 15, e40778. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Arcangeli, S.; Filippi, A.R.; Ricardi, U.; Scorsetti, M. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012, 17, 1100–1107. [Google Scholar] [CrossRef]
- Rosenzweig, K. Stereotactic Body Radiation Therapy as an Alternative to Surgery in Early-Stage Non-Small-Cell Lung Cancer. Oncology 2017, 31, 492–498. [Google Scholar] [PubMed]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Rana, S. Intensity modulated radiation therapy versus volumetric intensity modulated arc therapy. J. Med. Radiat. Sci. 2013, 60, 81–83. [Google Scholar] [CrossRef]
- Studenski, M.T.; Bar-Ad, V.; Siglin, J.; Cognetti, D.; Curry, J.; Tuluc, M.; Harrison, A.S. Clinical experience transitioning from IMRT to VMAT for head and neck cancer. Med. Dosim. 2013, 38, 171–175. [Google Scholar] [CrossRef]
- Samir, F.; Meaz, T.M.; Hussiny, F.A.; Ahmed, A.A.; Mahmoud, A.A.; Refaat, T.; Gawish, A.; Abouegylah, M. Analytical dosimetric study of intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) for prostate cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 6239–6246. [Google Scholar] [CrossRef]
- White, K.L.; Varrassi, E.; Routledge, J.A.; Barraclough, L.H.; Livsey, J.E.; McLaughlin, J.; Davidson, S.E. Does the Use of Volumetric Modulated Arc Therapy Reduce Gastrointestinal Symptoms after Pelvic Radiotherapy? Clin. Oncol. (R. Coll. Radiol.) 2018, 30, e22–e28. [Google Scholar] [CrossRef] [PubMed]
- Bravatà, V.; Cammarata, F.P.; Minafra, L.; Pisciotta, P.; Scazzone, C.; Manti, L.; Savoca, G.; Petringa, G.; Cirrone, G.A.P.; Cuttone, G.; et al. Proton-irradiated breast cells: Molecular points of view. J. Radiat. Res. 2019, 60, 451–465. [Google Scholar] [CrossRef]
- Petringa, G.; Calvaruso, M.; Conte, V.; Bláha, P.; Bravatà, V.; Cammarata, F.P.; Cuttone, G.; Forte, G.I.; Keta, O.; Manti, L.; et al. Radiobiological Outcomes, Microdosimetric Evaluations and Monte Carlo Predictions in Eye Proton Therapy. Appl. Sci. 2021, 11, 8822. [Google Scholar] [CrossRef]
- Sokol, O.; Durante, M. Carbon Ions for Hypoxic Tumors: Are We Making the Most of Them? Cancers 2023, 15, 4494. [Google Scholar] [CrossRef] [PubMed]
- Matuszak, N.; Suchorska, W.M.; Milecki, P.; Kruszyna-Mochalska, M.; Misiarz, A.; Pracz, J.; Malicki, J. FLASH radiotherapy: An emerging approach in radiation therapy. Rep. Pract. Oncol. Radiother. 2022, 27, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Mazal, A.; Prezado, Y.; Ares, C.; de Marzi, L.; Patriarca, A.; Miralbell, R.; Favaudon, V. FLASH and minibeams in radiation therapy: The effect of microstructures on time and space and their potential application to protontherapy. Br. J. Radiol. 2020, 93, 20190807. [Google Scholar] [CrossRef]
- Landry, G.; Kurz, C.; Traverso, A. The role of artificial intelligence in radiotherapy clinical practice. BJR Open 2023, 5, 20230030. [Google Scholar] [CrossRef]

| Immunotherapy and RT Combinations: Clinical Trials | ||||
|---|---|---|---|---|
| Clinical Trial | Molecule (ICI) | Pathway Target | Cancer Type | Ref. |
| PACIFIC Trial | Durvalumab | PD L1 blockage | Non-Small Cell Lung Cancer | [100] |
| CheckMate 577 Trial | Nivolumab | PD 1 blockage | Esophagogastric Cancer | [101] |
| ADRIATIC Trial | Durvalumab Durvalumab plus Tremelimumab | PD L1 blockage PD-L1 and CTLA-4 blockage | Small Cell Lung Cancer | [102] |
| NJLCG 1902 Trial | Pembrolizumab | PD 1 blockage | Head and Neck Squamous Cell Cancer | [103] |
| Hypofractionated RT Strategies: Clinical Trials | |||
|---|---|---|---|
| Clinical Trial | RT Plans Specification | Cancer Type | Ref. |
| UK START Trial | 41.6 Gy or 39 Gy in 13 fractions vs. 50 Gy in 25 fractions, over 5 weeks | Breast Cancer | [114] |
| FAST FORWARD Trial | 26 Gy or 27 Gy in 5 fractions (over 1 week) vs. 40 Gy in 15 fractions (over 3 weeks) | Breast Cancer | [115] |
| CHARM Trial | 42.56 Gy in 16 fractions vs. 50 Gy in 25 fractions, 5 days per week | Breast Cancer | [116] |
| HYPO-RT-PC Trial | 42.7 Gy in 7 fractions (3 days per week for 2.5 weeks) vs. 78 Gy in 39 fractions (5 days per week for 8 weeks) | Prostate Cancer | [117] |
| PACE-B Trial | 36.25 Gy in 5 fractions (1 or 2 weeks) vs. 78 Gy in 39 fractions (7.5 weeks) or 62 Gy in 20 fractions (4 weeks) | Prostate Cancer | [118] |
| A Multicenter, Randomized Phase 3 Trial | 60 Gy in 20 fractions vs. 60 Gy in 30 fractions, 5 days per week | Non-Small Cell Lung Cancer | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvaruso, M.; Pucci, G.; Alberghina, C.; Minafra, L. Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives. Int. J. Mol. Sci. 2025, 26, 6375. https://doi.org/10.3390/ijms26136375
Calvaruso M, Pucci G, Alberghina C, Minafra L. Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives. International Journal of Molecular Sciences. 2025; 26(13):6375. https://doi.org/10.3390/ijms26136375
Chicago/Turabian StyleCalvaruso, Marco, Gaia Pucci, Cristiana Alberghina, and Luigi Minafra. 2025. "Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives" International Journal of Molecular Sciences 26, no. 13: 6375. https://doi.org/10.3390/ijms26136375
APA StyleCalvaruso, M., Pucci, G., Alberghina, C., & Minafra, L. (2025). Radiation Therapy Personalization in Cancer Treatment: Strategies and Perspectives. International Journal of Molecular Sciences, 26(13), 6375. https://doi.org/10.3390/ijms26136375









