Applications of Limonene in Neoplasms and Non-Neoplastic Diseases
Abstract
1. Introduction
2. Applications of Limonene in Neoplasms
2.1. Breast Cancer
2.2. Hepatocellular Carcinoma
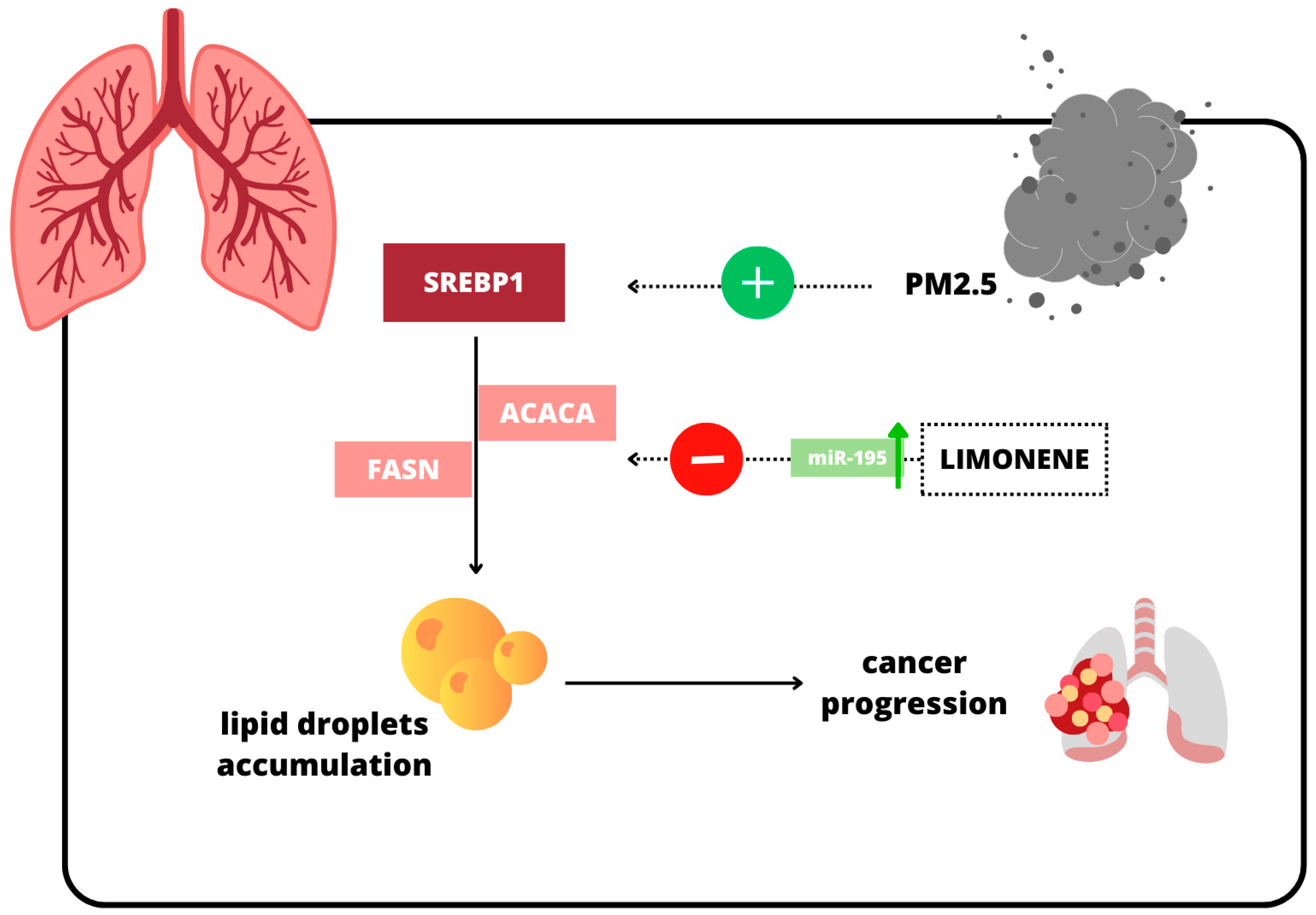
2.3. Lung Cancer
3. Applications of Limonene in Non-Neoplastic Diseases
3.1. Diabetes Mellitus and Other Metabolic Diseases
3.2. Gastrointestinal Diseases
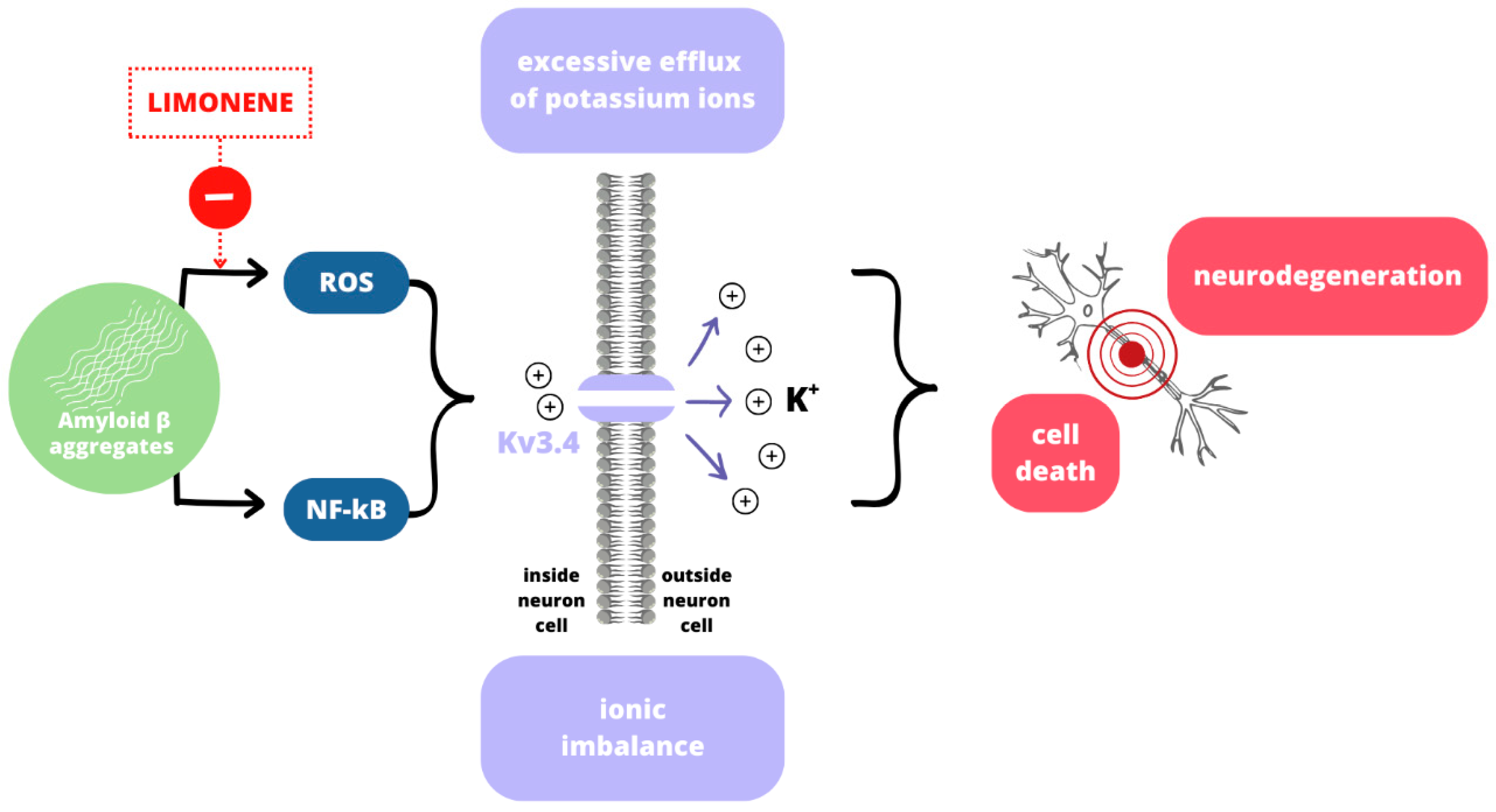
3.3. Neurodegenerative Diseases
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| 2-AAF | 2-acetylaminofluorene |
| ACACA | Acetyl-CoA Carboxylase |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AGE | Advanced glycation end product |
| ALP | Alkaline phosphatase |
| ALS | Amyotrophic lateral sclerosis |
| ALT | Alanine aminotransferase |
| AMG | Aminoguanidine |
| AR | Aldose reductase |
| AST | Aspartate aminotransferase |
| ATG5 | Autophagy-related gene 5 |
| Aβ | Amyloid beta |
| Bax | Bcl-2-associated X protein |
| BC | Breast cancer |
| CAT | Catalase |
| CB2 | Cannabinoid receptor type 2 |
| CD | Conjugated dienes |
| COX-2 | Cyclooxygenase-2 |
| DEN | Ciethylnitrosamine |
| DT-diaphorase | NAD(P)H:quinone oxidoreductase |
| EGFR | Epidermal growth factor receptor |
| FFA | Free fatty acids |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HR | Heart rate |
| HOMA-IR | Homeostasis model assessment for insulin resistance |
| IC50 | Half maximal inhibitory concentration |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| iNOS | Inducible nitric oxide synthase |
| lb-LDL | Large buoyant low-density lipoprotein |
| LC3-II | Microtubule-associated proteins 1A/1B light chain 3B |
| LDL | Low-density lipoprotein |
| LDL-c | LDL cholesterol |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| LOOH | Lipid hydroperoxides |
| LUAD | Lung adenocarcinoma |
| LXRβ | Liver X receptor beta |
| MDA | Malondialdehyde |
| MCF-7 | Human breast cancer cell line |
| mnSOD | Manganese superoxide dismutase, p-AMPK |
| MPO | Myeloperoxidase |
| MS | Multiple sclerosis |
| ND | Neurodegenerative disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| OGTT | Oral glucose tolerance test |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| p-AMPK | Phosphorylated adenosine monophosphate-activated protein kinase |
| PD | Parkinson’s disease |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | Phosphoinositide 3-kinase |
| PPAR | Peroxisome proliferator-activated receptor |
| PUFA | Polyunsaturated fatty acid |
| RBC | Red blood cells |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| sd-LDL | Small dense low-density lipoprotein |
| SIRT1 | Sirtuin 1 |
| STZ | Streptozotocin |
| TBARS | Thiobarbituric acid-reactive substances |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-alpha |
| TRPA1 | Transient receptor potential cation channel subfamily A member 1 |
| WAT | White adipocyte tissue |
| WBC | White blood cell |
References
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical composition, antioxidant and antimicrobial activity of essential oils from tangerine (Citrus reticulata L.), grapefruit (Citrus paradisi L.), lemon (Citrus lemon L.) and cinnamon (Cinnamomum zeylanicum Blume). Z. Naturforsch C J. Biosci. 2020, 76, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Chatterjee, J. Identification of proapoptopic, anti-inflammatory, anti- proliferative, anti-invasive and anti-angiogenic targets of essential oils in cardamom by dual reverse virtual screening and binding pose analysis. Asian Pac. J. Cancer Prev. 2013, 14, 3735–3742. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Manassero, C.A.; Girotti, J.R.; Mijailovsky, S.; De Bravo, M.G.; Polo, M. In vitro comparative analysis of antiproliferative activity of essential oil from mandarin peel and its principal component limonene. Nat. Prod. Res. 2013, 27, 1475–1478. [Google Scholar] [CrossRef]
- Panaskar, S.N.; Joglekar, M.M.; Taklikar, S.S.; Haldavnekar, V.S.; Arvindekar, A.U. Aegle marmelos Correa leaf extract prevents secondary complications in streptozotocin-induced diabetic rats and demonstration of limonene as a potent antiglycating agent. J. Pharm. Pharmacol. 2013, 65, 884–894. [Google Scholar] [CrossRef]
- Igimi, H.; Hisatsugu, T.; Nishimura, M. The use of d-limonene preparation as a dissolving agent of gallstones. Am. J. Dig. Dis. 1976, 21, 926–939. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. Available online: https://europepmc.org/article/med/18072821 (accessed on 30 November 2024).
- Hirota, R.; Roger, N.N.; Nakamura, H.; Song, H.S.; Sawamura, M.; Suganuma, N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J. Food Sci. 2010, 75, H87–H92. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.C.; Neder Morato, P.; Barbosa, M.d.S.; Croda, J.; Sampson, J.; Kong, X.; Konkiewitz, E.C.; Ziff, E.B.; Amaya-Farfan, J.; Kassuya, C.A.L. Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1 β and protein expression in spinal cord of mice. Life Sci. 2017, 174, 28–34. [Google Scholar] [CrossRef]
- Kaimoto, T.; Hatakeyama, Y.; Takahashi, K.; Imagawa, T.; Tominaga, M.; Ohta, T. Involvement of transient receptor potential A1 channel in algesic and analgesic actions of the organic compound limonene. Eur. J. Pain. 2016, 20, 1155–1165. [Google Scholar] [CrossRef]
- Chebet, J.J.; Ehiri, J.E.; McClelland, D.J.; Taren, D.; Hakim, I.A. Effect of d-limonene and its derivatives on breast cancer in human trials: A scoping review and narrative synthesis. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, K.; Szlasa, W.; Sauer, N.; Saczko, J.; Kulbacka, J. Molecular relation between biological stress and carcinogenesis. Mol. Biol. Rep. 2022, 49, 9929–9945. [Google Scholar] [CrossRef]
- Bacanli, M.; Başaran, A.A.; Başaran, N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Beg, Z.H. Mitigating role of thymoquinone rich fractions from Nigella sativa oil and its constituents, thymoquinone and limonene on lipidemic-oxidative injury in rats. Springerplus 2014, 3, 316. [Google Scholar] [CrossRef]
- Mandal, D.; Sahu, B.R.; Parija, T. Combination of tamoxifen and D-limonene enhances therapeutic efficacy in breast cancer cells. Med. Oncol. 2023, 40, 216. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Panaskar, S.N.; Chougale, A.D.; Kulkarni, M.J.; Arvindekar, A.U. A novel mechanism for antiglycative action of limonene through stabilization of protein conformation. Mol. Biosyst. 2013, 9, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Ayuningtyas, N.F.; Hendarti, H.T.; Soebadi, B.; Surboyo, M.D.C.; Hadi, P.; Ganesha, R.; Ernawati, D.S.; Marsetyo, R.I. Expression of VEGF and CD-31 in traumatic ulcer of diabetic Wistar rats after application of Citrus limon peel essential oil. J. Oral. Biol. Craniofac Res. 2023, 13, 380. [Google Scholar] [CrossRef]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Rocha, L.R.M.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Rathinasamy, B.; Lohanathan, B.P.; Thiyagarajan, V.; Weng, C.F. Neuroprotective Role of Phytochemicals. Molecules 2018, 23, 2485. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- de Araújo-Filho, H.G.; dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Salim, E.I.; Alabasy, M.M.; El Nashar, E.M.; Al-Zahrani, N.S.; Alzahrani, M.A.; Guo, Z.; Beltagy, D.M.; Shahen, M. Molecular interactions between metformin and D-limonene inhibit proliferation and promote apoptosis in breast and liver cancer cells. BMC Complement. Med. Ther. 2024, 24, 185. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: Potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement. Med. Ther. 2021, 21, 186. [Google Scholar] [CrossRef]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.-H.S. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev. Res. 2015, 8, 86–93. [Google Scholar] [CrossRef]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2017, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Hafidh, R.R.; Hussein, S.Z.; MalAllah, M.Q.; Abdulamir, A.S.; Bakar, F.A. A High-throughput Quantitative Expression Analysis of Cancer-related Genes in Human HepG2 Cells in Response to Limonene, a Potential Anticancer Agent. Curr. Cancer Drug Targets 2018, 18, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, C.; Li, L.; Zhang, H.; Zha, W.; Ma, L.; Chen, L.; Gan, J. The Role of Oxidative Stress in the Development and Therapeutic Intervention of Hepatocellular Carcinoma. Curr. Cancer Drug Targets 2023, 23, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, R.S.; Abdel-Moneim, A.; Zoheir, K.M.; Mohamed, E.E.; Abou-Seif, H.S.; Hefnawy, M.; Ahmed, O.M. Anti-carcinogenic effects and mechanisms of actions of Citrus limon fruit peel hydroethanolic extract and limonene in diethylnitrosmine/2-acetylaminofluorene-induced hepatocellular carcinoma in Wistar rats. Am. J. Cancer Res. 2024, 14, 5193. [Google Scholar] [CrossRef]
- Lung Cancer Awareness Month 2024—IARC. Available online: https://www.iarc.who.int/featured-news/lung-cancer-awareness-month-2024/ (accessed on 4 March 2025).
- Zhu, T.; Li, Y.; Feng, T.; Yang, Y.; Zhang, K.; Gao, J.; Quan, X.; Qian, Y.; Yu, H.; Qian, B. d-Limonene inhibits the occurrence and progression of LUAD through suppressing lipid droplet accumulation induced by PM2.5 exposure in vivo and in vitro. Respir. Res. 2022, 23, 338. [Google Scholar] [CrossRef]
- Chen, X.-F.; Ding, Y.-Y.; Guan, H.-R.; Zhou, C.-J.; He, X.; Shao, Y.-T.; Wang, Y.-B.; Wang, N.; Li, B.; Lv, G.-Y.; et al. The Pharmacological Effects and Potential Applications of Limonene From Citrus Plants: A Review. Nat. Prod. Commun. 2024, 19, 12. [Google Scholar] [CrossRef]
- Liu, S.Z.; Yao, S.J.; Yang, H.; Liu, S.J.; Wang, Y.J. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.J.; Zhang, H. Exploring the role of autophagy-related gene 5 (ATG5ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front. Immunol. 2018, 9, 377336. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020, 2, 27–59. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Su, Z.; Sun, Q.; Zhao, Y.; Feng, T.; Jiang, J.; Zhang, F.; Ma, H. Lipid metabolism gene-wide profile and survival signature of lung adenocarcinoma. Lipids Health Dis. 2020, 19, 222. [Google Scholar] [CrossRef]
- Kuhlmann-Hogan, A.; Cordes, T.; Xu, Z.; Kuna, R.S.; Traina, K.A.; Robles-Oteíza, C.; Ayeni, D.; Kwong, E.M.; Levy, S.; Globig, A.-M.; et al. EGFR-Driven Lung Adenocarcinomas Co-opt Alveolar Macrophage Metabolism and Function to Support EGFR Signaling and Growth. Cancer Discov. 2024, 14, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Study Details|Limonene for Pulmonary Nodule Chemoprevention|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05525260?intr=limonene&page=1&rank=2#more-information (accessed on 17 March 2025).
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J. Clin. 2024, 74, 50–81. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Joglekar, M.M.; Bavkar, L.N.; Sistla, S.; Arvindekar, A.U. Effective inhibition of protein glycation by combinatorial usage of limonene and aminoguanidine through differential and synergistic mechanisms. Int. J. Biol. Macromol. 2017, 99, 563–569. [Google Scholar] [CrossRef]
- Kumar, M.P.; Poornima; Mamidala, E.; Al-Ghanim, K.; Al-Misned, F.; Ahmed, Z.; Mahboob, S. Effects of D-Limonene on aldose reductase and protein glycation in diabetic rats. J. King Saud. Univ. Sci. 2020, 32, 1953–1958. [Google Scholar] [CrossRef]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Santiago, J.V.A.; Jayachitra, J.; Shenbagam, M.; Nalini, N. Dietary d-limonene alleviates insulin resistance and oxidative stress-induced liver injury in high-fat diet and L-NAME-treated rats. Eur. J. Nutr. 2012, 51, 57–68. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013, 715, 46–55. [Google Scholar] [CrossRef]
- More, T.A.; Kulkarni, B.R.; Nalawade, M.L.; Arvindekar, A.U. Antidiabetic Activity of Linalool and Limonene in Streptozotocin-Induced Diabetic Rat: A Combinatorial Therapy Approach. Int. J. Pharm. Phamrac. Sci. 2014, 8, 159–163. [Google Scholar]
- Bacanlı, M.; Anlar, H.G.; Aydın, S.; Çal, T.; Arı, N.; Bucurgat, Ü.Ü.; Başaran, A.A.; Başaran, N. D-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2017, 110, 434–442. [Google Scholar] [CrossRef]
- Bagheri, S.; Sarabi, M.M.; Gholami, M.; Assadollahi, V.; Khorramabadi, R.M.; Moradi, F.H.; Ahmadvand, H. D-limonene in diabetic rats. J. Ren. Inj. Prev. 2021, 10, 1–8. [Google Scholar] [CrossRef]
- Shakeel, S.; Tabassum, N. Effect of Some Naturally Occurring Monoterpenes viz d-Limonene, p-Cymene and Terpinolene on the Glycemic and Hepatic Function in a Rat Model of Type 2 Diabetes Mellitus. Pharmacogn. Res. 2022, 14, 446–453. [Google Scholar] [CrossRef]
- Lawal, R.; Aluwong, T.; Suleiman, M.; Adeniran, L.; Onakpa, M.; Ajagbonna, P. D-Limonene and Vitamin E Combinatory Therapy Stimulate Erythropoiesis and Repair of Β-Cells Atrophy in Alloxan–Induced Diabetic Rats. Alex. J. Vet. Sci. 2023, 79, 60. [Google Scholar] [CrossRef]
- Han, X.; Qi, H.; Niu, J. L-limonene reduces aortic artery atherosclerosis by inhibiting oxidative stress/inflammatory responses in diabetic rats fed high-fat diet. Chin. J. Physiol. 2023, 66, 129–136. [Google Scholar] [CrossRef]
- Benchoula, K.; Serpell, C.J.; Mediani, A.; Albogami, A.; Misnan, N.M.; Ismail, N.H.; Parhar, I.S.; Ogawa, S.; Hwa, W.E. 1H NMR metabolomics insights into comparative diabesity in male and female zebrafish and the antidiabetic activity of DL-limonene. Sci. Rep. 2024, 14, 3823. [Google Scholar] [CrossRef]
- Valerii, M.C.; Turroni, S.; Ferreri, C.; Zaro, M.; Sansone, A.; Dalpiaz, A.; Botti, G.; Ferraro, L.; Spigarelli, R.; Bellocchio, I.; et al. Effect of a fiber d-limonene-enriched food supplement on intestinal microbiota and metabolic parameters of mice on a high-fat diet. Pharmaceutics 2021, 13, 1753. [Google Scholar] [CrossRef]
- Murali, R.; Karthikeyan, A.; Saravanan, R. Protective Effects of d-Limonene on Lipid Peroxidation and Antioxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2013, 112, 175–181. [Google Scholar] [CrossRef]
- Sharma, S.; Bansal, N. D-Limonene Ameliorates Diabetic Neuropathic Pain in Rats. Int. J. Med. Health Res. 2016, 2, 34–39. [Google Scholar]
- Amirfakhryan, H.; New, K.J. The role of myeloperoxidase as a biomarker in atherosclerotic cardiovascular disease. Cardiol. Plus 2024, 9, 195–209. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R -Limonene Enhances Differentiation and 2-Deoxy-D-Glucose Uptake in 3T3-L1 Preadipocytes by Activating the Akt Signaling Pathway. Evid.-Based Complement. Altern. Med. 2018, 2018, 4573254. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Li, M.; Gao, Y.; Feng, Y.; Xu, Y.; Liu, W.; Zhang, Z.-F.; Ren, J.-N.; Yang, J.; Fan, G.; et al. Limonene alleviates hepatic lipid accumulation by regulating fatty acids metabolism: Insights from the transcriptome. Food Biosci. 2024, 62, 105391. [Google Scholar] [CrossRef]
- Lv, W.; Tan, X.; Chen, X.; Hu, T.; Jiang, J.; Li, Q.; Chen, X.; Tan, H.; Qian, B. D-Limonene for regulating metabolism-associated fatty liver disease (MAFLD) and analysis of the TCM constitution: A protocol for an exploratory, randomized, double-blind, placebo-controlled trial (DL-MAFLD-TCM). Food Front. 2022, 3, 550–559. [Google Scholar] [CrossRef]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, B.S.; Özbek, H. Investigation of the anti-inflammatory, hypoglycemic activity and median lethal dose (LD50) level of limonene in mice and rats. Acta Pharm. Sci. 2018, 56, 85–94. [Google Scholar] [CrossRef]
- Igimi, H.; Tamura, R.; Toraishi, K.; Yamamoto, F.; Kataoka, A.; Ikejiri, Y.; Hisatsugu, T.; Shimura, H. Medical Dissolution of Gallstones Clinical Experience of d-Limonene as a Simple, Safe, and Effective Solvent. Dig. Dis. Sci. 1991, 36, 200–208. [Google Scholar] [CrossRef]
- Arrout, A.; El Ghallab, Y.; Lefriyekh, M.R.; Said, A.A.H. Citrus essential oils and main terpenes: Chemical composition and good litholytic activity on gallstones. Vegetos 2021, 34, 600–605. [Google Scholar] [CrossRef]
- D’Alessio, P.A.; Ostan, R.; Bisson, J.F.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-Limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Kathem, S.H.; Nasrawi, Y.S.; Mutlag, S.H.; Nauli, S.M. Limonene Exerts Anti-Inflammatory Effect on LPS-Induced Jejunal Injury in Mice by Inhibiting NF-κB/AP-1 Pathway. Biomolecules 2024, 14, 334. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Dakpa, G.; Wang, S. Dietary limonene promotes gastrointestinal barrier function via upregulating tight/adherens junction proteins through cannabinoid receptor type-1 antagonistic mechanism and alters cellular metabolism in intestinal epithelial cells. BioFactors 2025, 51, e2106. [Google Scholar] [CrossRef]
- Moraes, T.M.; Rozza, A.L.; Kushima, H.; Pellizzon, C.H.; Rocha, L.R.M.H.; Hiruma-Lima, C.A. Healing actions of essential oils from citrus aurantium and d-limonene in the Gastric Mucosa: The roles of VEGF, PCNA, and COX-2 in cell proliferation. J. Med. Food 2013, 16, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef]
- Lu, X.-G.; Zhan, L.-B.; Feng, B.-A.; Qu, M.-Y.; Yu, L.-H.; Xie, J.-H. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J. Gastroenterol. 2004, 10, 2140–2144. [Google Scholar] [CrossRef]
- Yi, S.; Yue, W.; Shanshan, S.; Hongbin, L. The Effects of D-limonene on COX-2 Expression and Lymphangion Genesis in Mice with Gastric Cancer. Rev. Científica De La Fac. De Cienc. Vet. 2020, 30, 2317–2324. [Google Scholar]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef] [PubMed]
- Dhureja, M. Neuroprotective Potential of Limonene in LPS Induced Parkinson’s Disease in Rats. Alzheimer’s Dement. 2023, 19, e067741. [Google Scholar] [CrossRef]
- Yu, Z.; Luo, F. The Role of Reactive Oxygen Species in Alzheimer’s Disease: From Mechanism to Biomaterials Therapy. Adv. Heal. Mater. 2024, 13, 2304373. [Google Scholar] [CrossRef]
- Song, M.S.; Ryu, P.D.; Lee, S.Y. Kv3.4 is modulated by HIF-1α to protect SH-SY5Y cells against oxidative stress-induced neural cell death. Sci. Rep. 2017, 7, 2075. [Google Scholar] [CrossRef]
- Boz, B.; Diraman, E.; Sezgin, F.G. Pathogenesis and Molecular Mechanisms of Parkinson’s Disease. Am. J. Biomed. Sci. Res. 2024, 24, AJBSR.MS.ID.003203. [Google Scholar] [CrossRef]
- Eddin, L.B.; Azimullah, S.; Jha, N.K.; Meeran, M.F.N.; Beiram, R.; Ojha, S. Limonene, a Monoterpene, Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Modulating Neuroinflammation, Hippo Signaling and Apoptosis in Rats. Int. J. Mol. Sci. 2023, 24, 5222. [Google Scholar] [CrossRef]
- Kim, T.-K.; Bae, E.-J.; Jung, B.C.; Choi, M.; Shin, S.J.; Park, S.J.; Kim, J.T.; Jung, M.K.; Ulusoy, A.; Song, M.-Y.; et al. Inflammation promotes synucleinopathy propagation. Exp. Mol. Med. 2022, 54, 2148–2161. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G. Alpha Synuclein: Neurodegeneration and Inflammation. Int. J. Mol. Sci. 2023, 24, 5914. [Google Scholar] [CrossRef] [PubMed]
- Lorigooini, Z.; Boroujeni, S.N.; Sayyadi-Shahraki, M.; Rahimi-Madiseh, M.; Bijad, E.; Amini-Khoei, H. Limonene through Attenuation of Neuroinflammation and Nitrite Level Exerts Antidepressant-Like Effect on Mouse Model of Maternal Separation Stress. Behav. Neurol. 2021, 2021, 8817309. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishida, Y.; Tabata, M.; Isobayashi, A.; Tomizawa, H.; Miyahara, Y.; Sugizaki, Y. Molecular Interactions between an Enzyme and Its Inhibitor for Selective Detection of Limonene. Anal. Chem. 2022, 94, 7692–7702. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]


| Author, Year | Model | Dose | Effects |
|---|---|---|---|
| Santiago et al., 2012 [49] | HFD-fed, L-NAME-treated rats | 2% d- limonene diet | ↓ fasting blood glucose, plasma insulin, HOMA-IR, pancreatic B-cell mass and hyperplasia, and B-cell nucleus ↓ SBP and HR ↓ lipid peroxidation byproducts ↑ GST and DT-diaphorase ↑ GSH, vitamin C, and vitamin E ↓ AST, ALT, and ALP ↑ TC, TG, and FFA ↓ hepatic enzymes: cytochrome P450, cytochrome b5, cytochrome P4502E1, NADPH-cytochrome P450 reductase, and NADH-cytochrome b5 reductase ↓ hepatic fat deposition and hepatosteatosis |
| Murali et al., 2012 [48] | STZ-induced diabetic rats | 50 mg/kg, 100 mg/kg, 200 mg/kg | ↓ blood glucose (maximum effect at 100 mg/kg) and HbA1c ↑ body weight, hemoglobin, and plasma insulin ↑ enzyme activity in hepatic tissue ↑ glycolysis, glycogenesis, pentose oxidative pathway, and glycogen content in liver ↓ gluconeogenesis |
| Jing et al., 2013 [50] | HFD-induced obese mice | 0.6 g/kg | ↓ blood glucose ↑ improved impaired glucose tolerance at 60 and 90 min ↓ plasma LDL-c and serum TG ↑ plasma HDL-c TC, body weight unaffected ↑ PPARα transactivity ↓ LXRβ signaling ↑ PGC-1α gene expression in WAT ↓ hepatic lipid deposition ↓ size of white and brown adipocytes |
| Murali et al., 2013 [59] | STZ-induced diabetic rats | 100 mg/kg | ↓ plasma glucose ↑ plasma insulin ↑ SOD, CAT, GPx, and GST activities ↑ GSH, vitamin C, vitamin E ↓ TBARS, LOOH, and CD - normal liver and kidney architecture |
| Panaskar et al., 2013 [8] | STZ-induced diabetic rats | A. marmelos extract: 150 µg/kg, 300 µg/kg Limonene: 10 µM, 50 µM, 100 µM | - potent antiglycative properties similar to AMG at an almost 20-fold lower concentration ↓ blood glucose ↓ progression of nephropathy and cataract formation in vivo |
| Joglekar et al., 2013 [21] | Bovine serum albumin | 25 µM, 50 µM, 100 µM | - excellent protein glycation inhibitor - blocking transition of α-helix to β-sheet - stabilizing structure through hydrophobic interactions |
| Nalawade et al., 2014 [51] | STZ-induced diabetic rats Bovine serum albumin | 20 mg/kg 25 µM, 50 µM, 100 µM | ↓ fructosamine formation comparable to a tenfold greater AMG concentration ↑ SOD and CAT activity ↓ TBARS formation ↓ urine glucose, albumin, and creatinine - no blood glucose decrease in OGTT |
| Sharma et al., 2016 [60] | STZ-induced diabetic rats | 100 mg/kg, 200 mg/kg | - attenuated behavioral and biochemical alterations of neuropathy ↑ CAT, GSH, and total protein levels ↓ nitrite and TBARS level - no hypoglycemic effect on healthy rats |
| Joglekar et al., 2017 [46] | Bovine serum albumin | 25 µM, 50 µM, 100 µM | - reinforced mechanism of glycation inhibition - combination can reduce dosage of AMG by twenty times - combinatorial treatment of AMG and limonene inhibited AGE-related fluorescence and pentosidine formation |
| Bacanlı et al., 2017 [52] | STZ-induced diabetic rats | 50 mg/kg | ↓ plasma insulin levels ↓ GR, 8-OHdG, and MDA levels ↑ GSH, CAT, SOD, and GPx ↓ serum LDL, TC, and TG ↑ serum HDL ↓ AST and GGT ↓ DNA damage in blood, liver, and kidney cells |
| Soundharrajan et al., 2018 [62] | 3T3-L1 preadipocytes | 5 µM | - probable induction of differentiation and glucose uptake in 3T3-L1 preadipocytes - regulated adipogenesis and lipogenesis via induction of PPARγ, C/EBP- α, C/EBP-β |
| Yilmaz et al., 2018 [66] | Alloxan-induced diabetic mice | 0.15 mL/kg, 0.3 mL/kg, 0.6 mL/kg | - inflammatory effect (peak at 0.30 mL/kg) - no hypoglycemic effect |
| Kumar et al., 2020 [47] | STZ-induced diabetic rats, rat lenses | 1–100 µM/mL, 13.49 µM/mL for lens incubation | ↓ AR and AGE ↑ increased crystalline chaperone activity - delayed development of diabetic cataracts |
| Bagheri et al., 2021 [53] | Alloxan-induced diabetic rats | 100 mg/kg | ↓ serum glucose, creatinine, and urea ↑ GSH, mRNA of GPx, CAT, and SOD ↓ MDA, MPO, and NO |
| Valerii et al., 2021 [58] | HFD-fed mice | 30 mg/kg 60 mg/kg | ↓ fasting glycemia and TG ↓ weight gain ↓ HFD-associated liver steatosis ↑ liver PUFA levels |
| Shakeel et al., 2022 [54] | STZ-induced diabetic rats | 300 mg/kg | ↓ blood glucose, HbA1c ↑ serum insulin ↓ ALP, ALT, AST, and GGT ↑ albumin and total protein ↓ progression of liver degeneration |
| Han et al., 2023 [56] | HFD-fed, low-dose STZ diabetic atherosclerosis model in rats | 200 mg/kg | ↓ blood glucose ↓ cholesterol, TG, and LDL ↑ HDL/LDL ratio ↓ atherogenic index, morphological irregularities of the intima ↑ mnSOD and GSH ↓ 8-isoprostane ↓ TNF-α and IL-6 ↑ IL-10 ↑ expression of p-AMPK/ AMPK, SIRT1, and p-p65/p65 proteins |
| Lawal et al., 2023 [55] | Alloxan-induced diabetic rats | 10 mg/kg, 5 mg/kg with 25 mg/kg vitamin E | ↓ blood glucose level comparable to metformin ↑ body weight ↓ feed intake ↑ RBC and WBC levels ↑ hepatic glycogen levels ↓ MDA ↓ TC, TG, and LDH - ameliorative effect on β-cell of pancreas |
| Benchoula et al., 2024 [57] | HFD-induced type 2 diabetes-related obese zebrafish | 5 mg/L, 20 mL/L | ↓ fasting blood glucose and BMI - reverses changes in metabolites due to diabesity - reverses elevated expression of AKT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakoczy, K.; Szymańska, N.; Stecko, J.; Kisiel, M.; Maruszak, M.; Niedziela, M.; Kulbacka, J. Applications of Limonene in Neoplasms and Non-Neoplastic Diseases. Int. J. Mol. Sci. 2025, 26, 6359. https://doi.org/10.3390/ijms26136359
Rakoczy K, Szymańska N, Stecko J, Kisiel M, Maruszak M, Niedziela M, Kulbacka J. Applications of Limonene in Neoplasms and Non-Neoplastic Diseases. International Journal of Molecular Sciences. 2025; 26(13):6359. https://doi.org/10.3390/ijms26136359
Chicago/Turabian StyleRakoczy, Katarzyna, Natalia Szymańska, Jakub Stecko, Michał Kisiel, Monika Maruszak, Michał Niedziela, and Julita Kulbacka. 2025. "Applications of Limonene in Neoplasms and Non-Neoplastic Diseases" International Journal of Molecular Sciences 26, no. 13: 6359. https://doi.org/10.3390/ijms26136359
APA StyleRakoczy, K., Szymańska, N., Stecko, J., Kisiel, M., Maruszak, M., Niedziela, M., & Kulbacka, J. (2025). Applications of Limonene in Neoplasms and Non-Neoplastic Diseases. International Journal of Molecular Sciences, 26(13), 6359. https://doi.org/10.3390/ijms26136359








