NGF, BDNF, and NO in Myopic Subjects: Relationships Between Aqueous Levels and Lens Epithelial Cells’ Activation
Abstract
1. Introduction
2. Results
2.1. NGF and BDNF Proteins Humor Aqueous Decreased with Myopia Severity
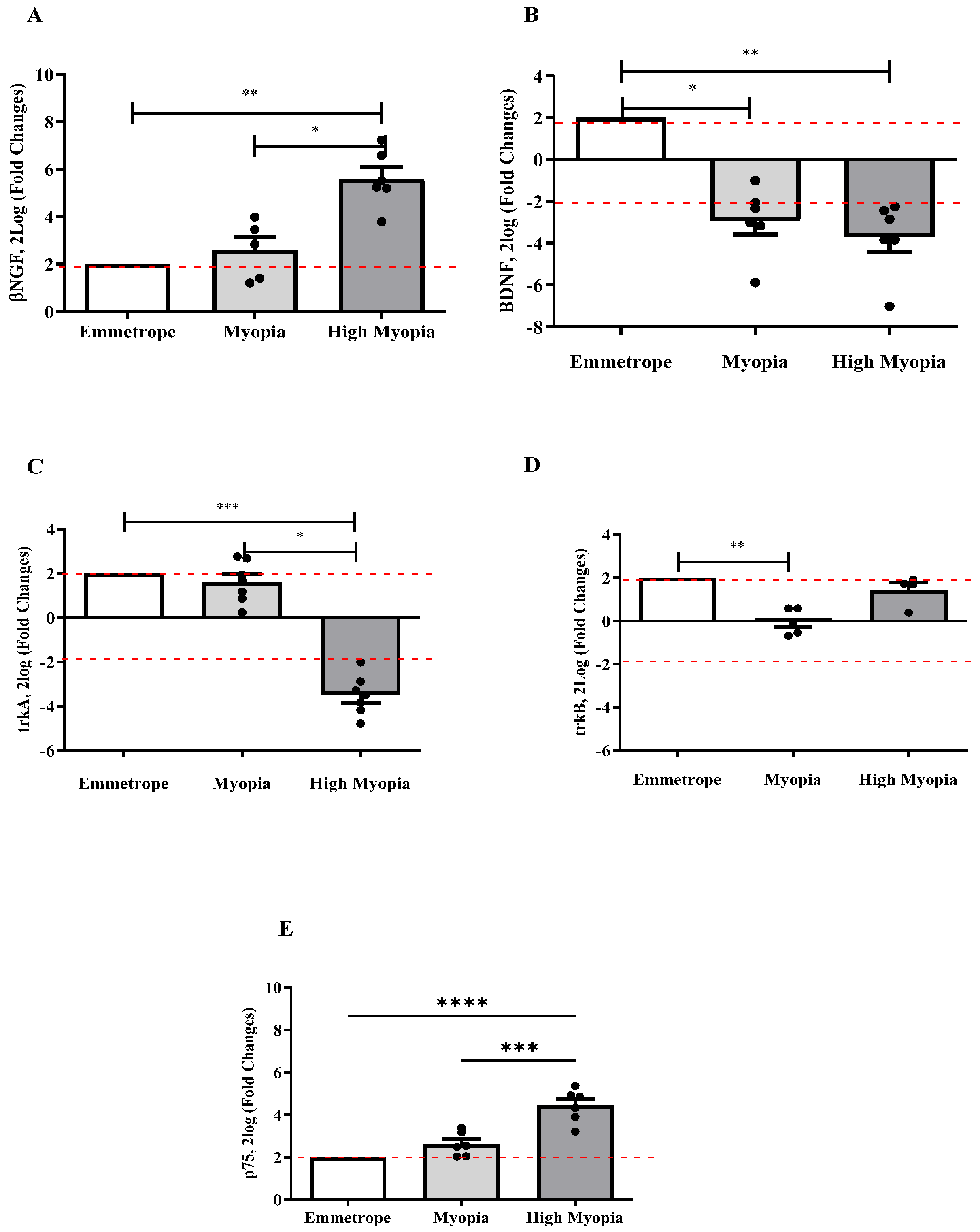
2.2. NGF and BDNF Transcripts Were Differentially Expressed in Anterior Capsule (LEC)
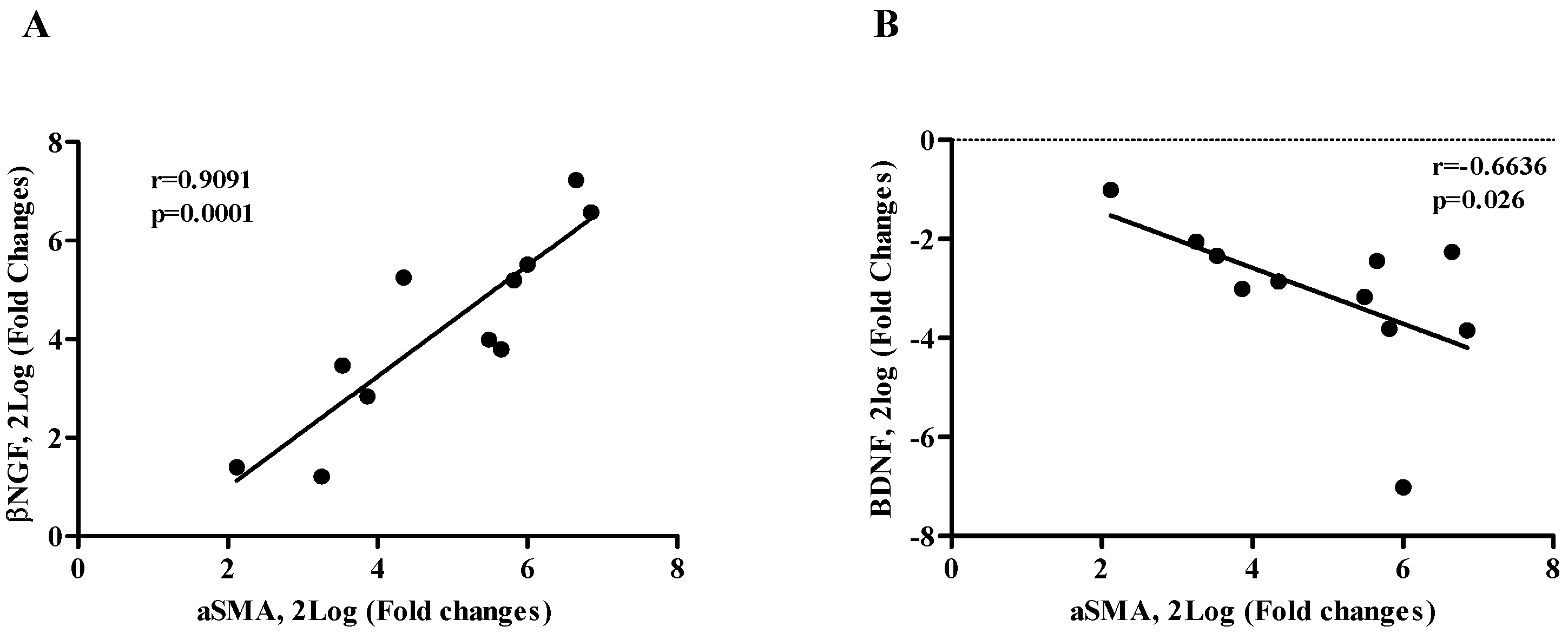
2.3. βNGF and BDNF Correlate with αSMA Expression (EMT Phenotype)
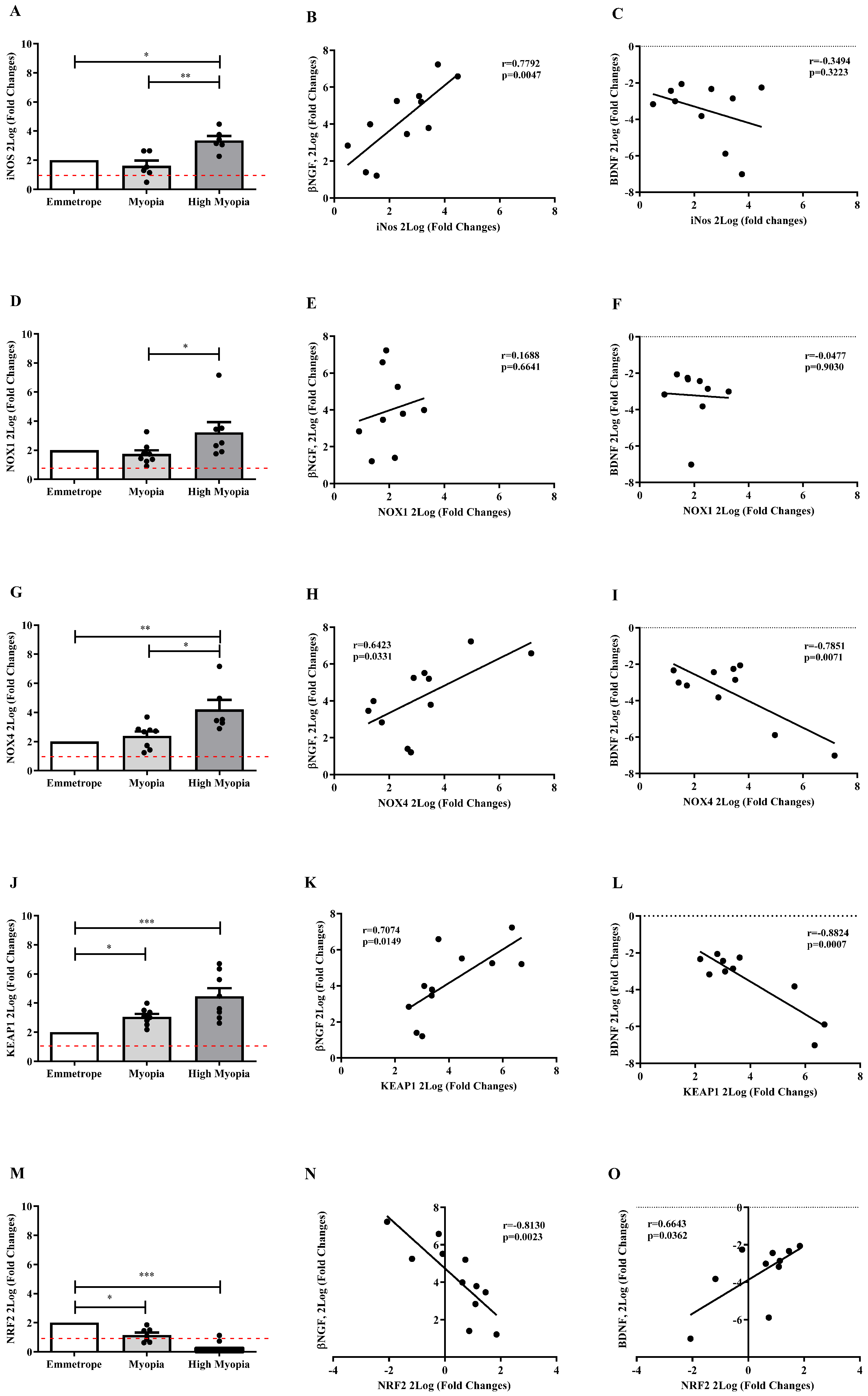
2.4. NO and Nitrites Are Released in Myopic Aqueous and Correlate with NGF and BDNF
2.5. Oxidative Stress Transcripts Expression in Anterior Capsule (LEC)
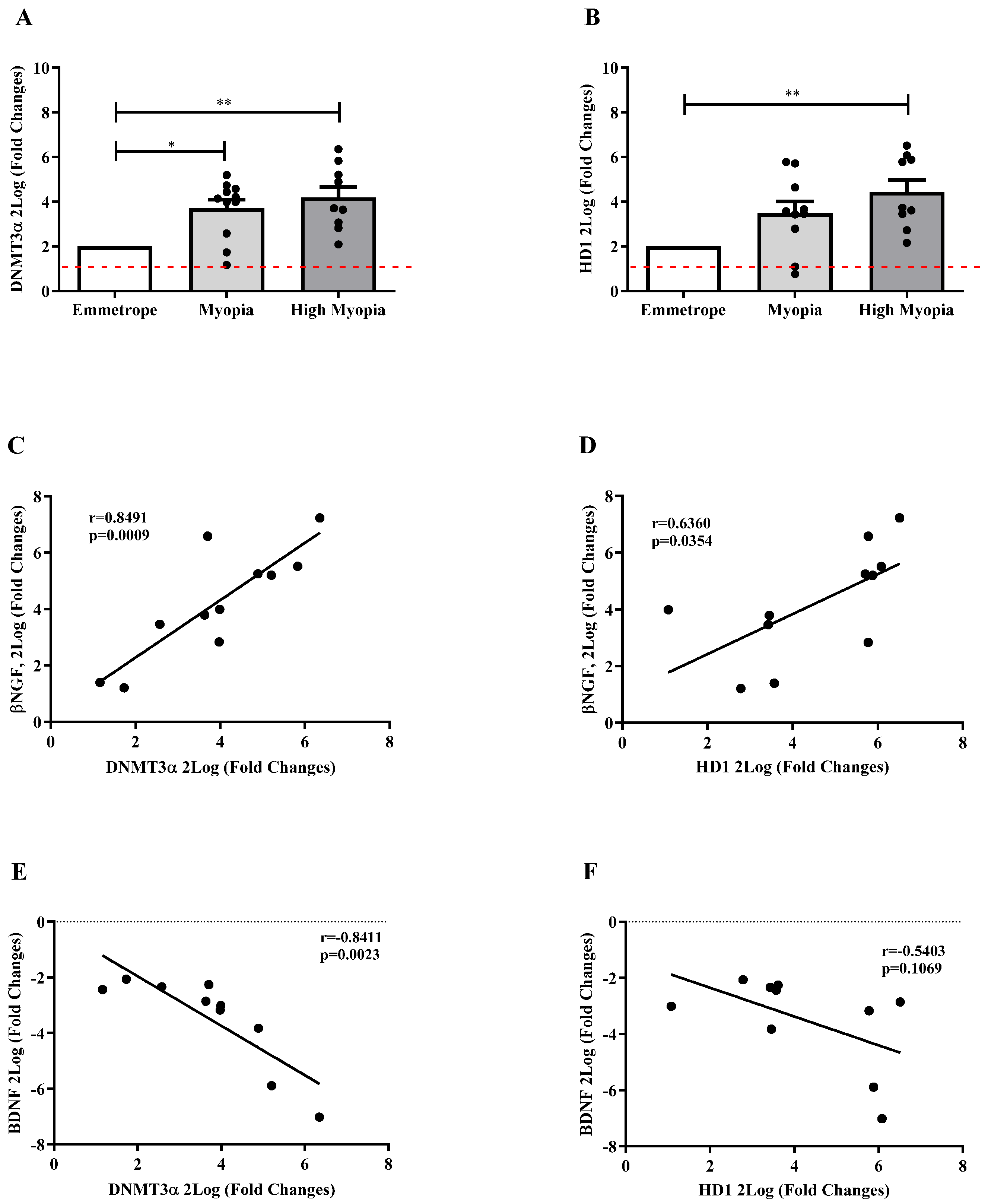
2.6. Epigenetic Mediators Are Activated in Anterior Capsule Myopic LEC
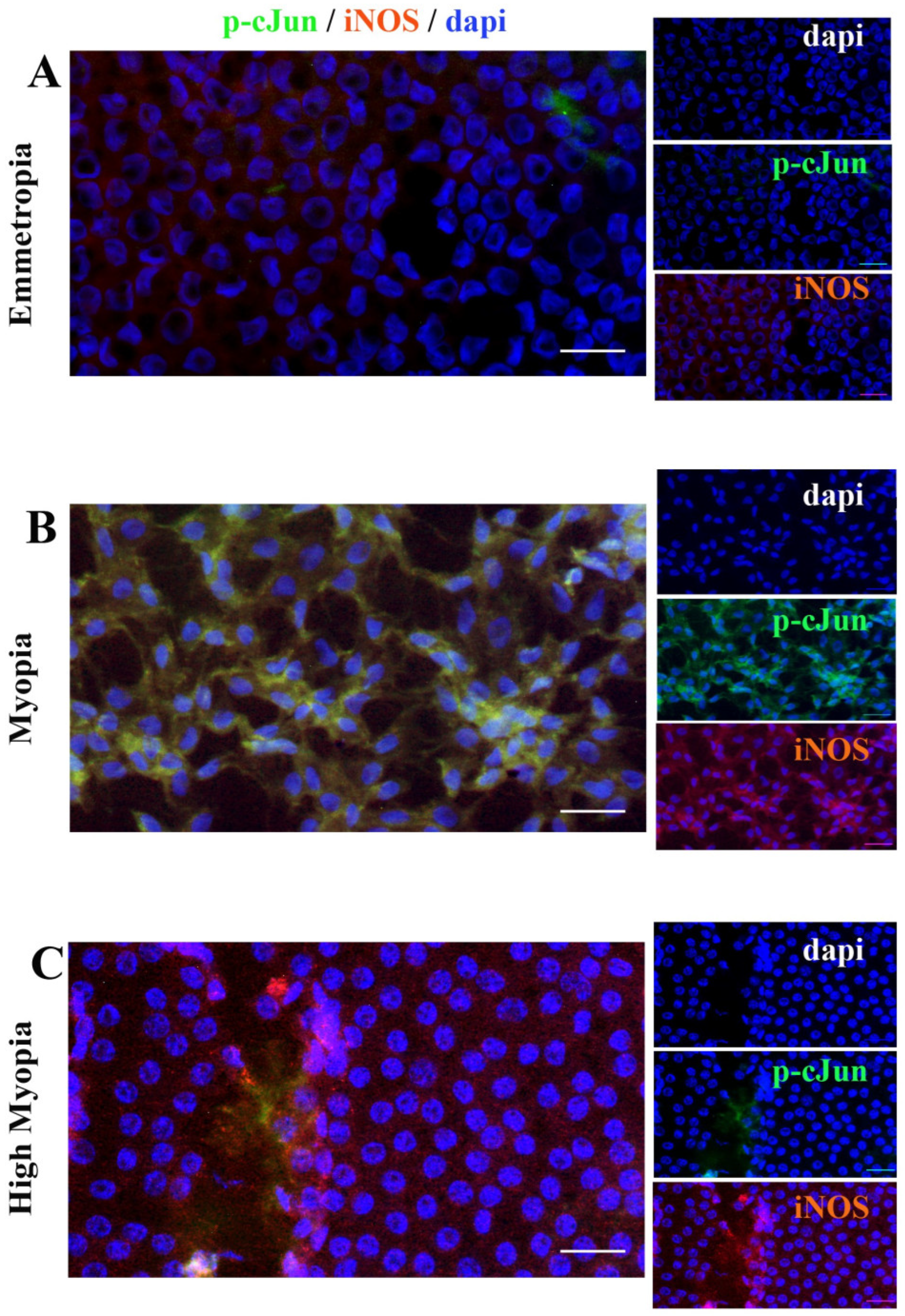
2.7. Anterior Capsule Myopic LEC Differentially Express iNOS and cJun
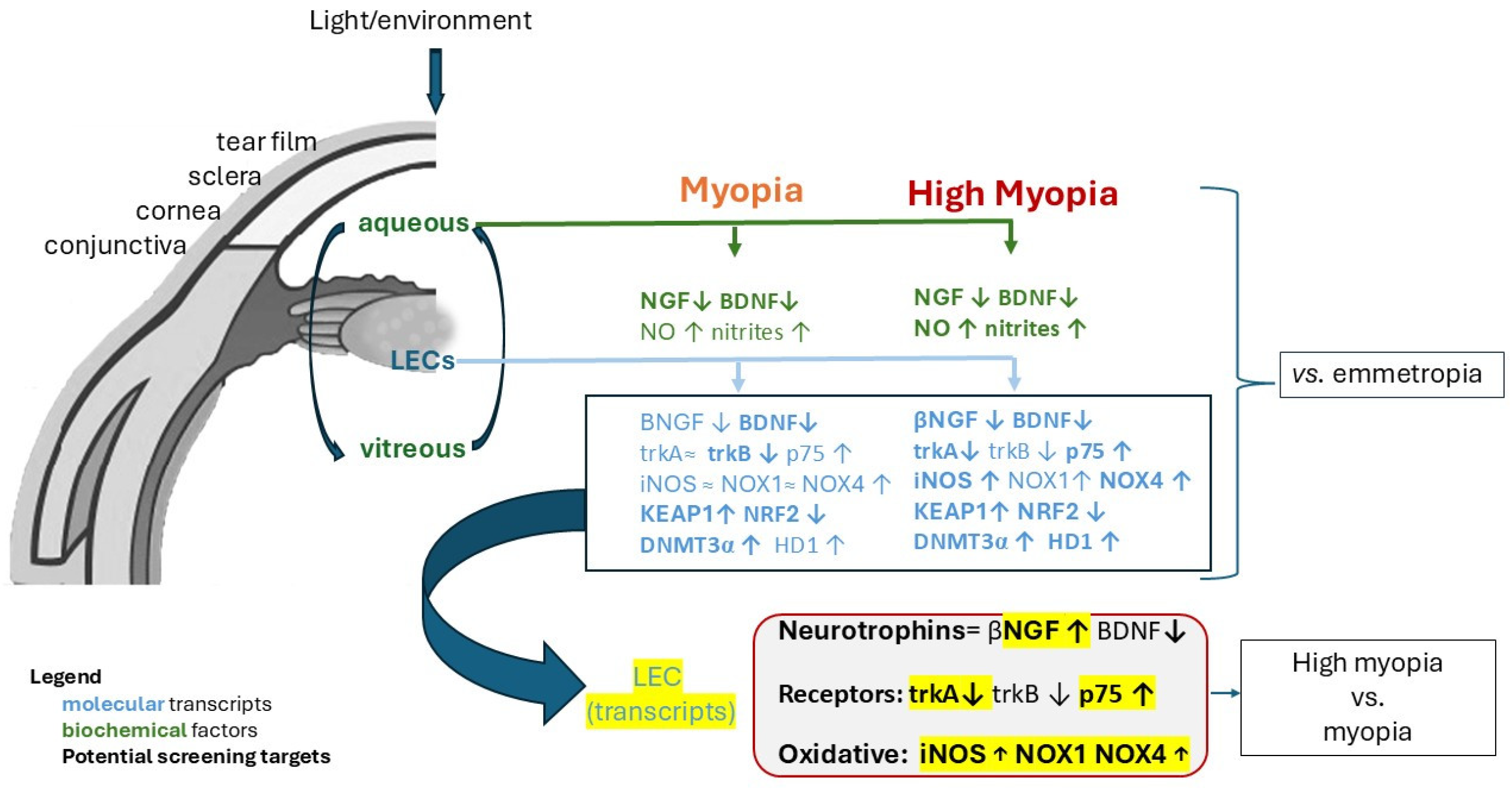
3. Discussion
4. Materials and Methods
4.1. Study Population: Clinical Assessment and Sampling
4.2. Pre-Analytical Procedures
4.3. Biochemical Analysis
4.4. Molecular Analysis: Two-Step Real Time PCR
4.5. Immunofluorescence and Epifluorescent Microscopy
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Walline, J.J.; Lindsley, K.B.; Vedula, S.S.; Cotter, S.A.; Mutti, D.O.; Ng, S.M.; Twelker, J.D. Interventions to slow progression of myopia in children. Cochrane Database Syst. Rev. 2020, 1, CD004916. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.M.; Gazzard, G.; Shin-Yen, E.C.; Chua, W.H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, R.; Sinha, S.; Gangadharaiah, D.; Gadde, S.G.K.; Mohan, A.; Shetty, R.; Yadav, N.K. Retinal structural-vascular-functional relationship using optical coherence tomography and optical coherence tomography—Angiography in myopia. Eye Vis. 2019, 6, 8. [Google Scholar] [CrossRef]
- Zeppieri, M.; Marsili, S.; Enaholo, E.S.; Shuaibu, A.O.; Uwagboe, N.; Salati, C.; Spadea, L.; Musa, M. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina 2023, 59, 2114. [Google Scholar] [CrossRef]
- Xu, R.; Zheng, J.; Liu, L.; Zhang, W. Effects of inflammation on myopia: Evidence and potential mechanisms. Front. Immunol. 2023, 14, 1260592. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Zheng, R.; Cui, T.; Qin, J.; Su, Z.; Shang, M.; Bao, Y. High irisin and low BDNF levels in aqueous humor of high myopia. Adv. Clin. Exp. Med. 2021, 30, 893–904. [Google Scholar] [CrossRef]
- Nubile, M.; Lambiase, A.; Sgrulletta, R.; Costantino, O.; Mastropasqua, L.; Bonini, S. Effectiveness of Nerve growth factor treatment on corneal innervation following photorefractive keratectomy. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4881. [Google Scholar]
- Fiore, M.; Chaldakov, G.N.; Aloe, L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009, 20, 133–145. [Google Scholar] [CrossRef]
- Micera, A.; Lambiase, A.; Aloe, L.; Bonini, S.; Levi-Schaffer, F.; Bonini, S. Nerve growth factor involvement in the visual system: Implications in allergic and neurodegenerative diseases. Cytokine Growth Factor Rev. 2004, 15, 411–417. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L. Brain-derived neurotrophic factor and Alzheimer’s disease: Physiopathology and beyond. Neuromolecular Med. 2011, 13, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chaldakov, G. The metabotrophic NGF and BDNF: An emerging concept. Arch. Ital. Biol. 2011, 149, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Ferraguti, G.; Tarani, L.; Fanfarillo, F.; Tirassa, P.; Ralli, M.; Iannella, G.; Polimeni, A.; Lucarelli, M.; Greco, A.; et al. Nerve Growth Factor and Autoimmune Diseases. Curr. Issues Mol. Biol. 2023, 45, 8950–8973. [Google Scholar] [CrossRef] [PubMed]
- De Piano, M.; Cacciamani, A.; Balzamino, B.O.; Scarinci, F.; Cosimi, P.; Cafiero, C.; Ripandelli, G.; Micera, A. Biomarker Signature in Aqueous Humor Mirrors Lens Epithelial Cell Activation: New Biomolecular Aspects from Cataractogenic Myopia. Biomolecules 2023, 13, 1328. [Google Scholar] [CrossRef]
- Esposito, G.; Balzamino, B.O.; Rocco, M.L.; Aloe, L.; Micera, A. Nerve Growth Factor (NGF) as Partaker in the Modulation of UV-Response in Cultured Human Conjunctival Fibroblasts. Int. J. Mol. Sci. 2022, 23, 6337. [Google Scholar] [CrossRef]
- Rocco, M.L.; Balzamino, B.O.; Aloe, L.; Micera, A. NGF protects corneal, retinal, and cutaneous tissues/cells from phototoxic effect of UV exposure. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 729–738. [Google Scholar] [CrossRef]
- Micera, A.; Lambiase, A.; Stampachiacchiere, B.; Bonini, S.; Bonini, S.; Levi-Schaffer, F. Nerve growth factor and tissue repair remodeling: trkA(NGFR) and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007, 18, 245–256. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Aloe, L.; Tirassa, P.; Lambiase, A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol. Res. 2008, 57, 253–258. [Google Scholar] [CrossRef]
- Lambiase, A.; Sacchetti, M.; Bonini, S. Nerve growth factor therapy for corneal disease. Curr. Opin. Ophthalmol. 2012, 23, 296–302. [Google Scholar] [CrossRef]
- Mérida, S.; Villar, V.M.; Navea, A.; Desco, C.; Sancho-Tello, M.; Peris, C.; Bosch-Morell, F. Imbalance Between Oxidative Stress and Growth Factors in Human High Myopia. Front. Physiol. 2020, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Becquet, F.; Courtois, Y.; Goureau, O. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv. Ophthalmol. 1997, 42, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Dorner, G.T.; Garhofer, G.; Kiss, B.; Polska, E.; Polak, K.; Riva, C.E.; Schmetterer, L. Nitric oxide regulates retinal vascular tone in humans. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H631–H636. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO generation from nitrite and its role in vascular control. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 915–922. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef]
- Mutti, D.O.; Cooper, M.E.; O’Brien, S.; Jones, L.A.; Marazita, M.L.; Murray, J.C.; Zadnik, K. Candidate gene and locus analysis of myopia. Mol. Vis. 2007, 13, 1012–1019. [Google Scholar] [PubMed]
- Chen, J.; Ikeda, S.I.; Negishi, K.; Tsubota, K.; Kurihara, T. Identification of Potential Therapeutic Targets for Myopic Choroidal Neovascularization via Discovery-Driven Data Mining. Curr. Eye Res. 2023, 48, 1160–1169. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Y.; Wang, Z.; Long, Q.; Li, Y.; Chen, D. Complement-mediated inflammation and mitochondrial energy metabolism in the proteomic profile of myopic human corneas. J. Proteomics 2023, 15, 104949. [Google Scholar] [CrossRef]
- Sayanagi, K.; Fujimoto, S.; Hara, C.; Fukushima, Y.; Kawasaki, R.; Sato, S.; Sakaguchi, H.; Nishida, K. Characteristics of choroidal neovascularization in elderly eyes with high myopia not meeting the pathologic myopia definition. Sci. Rep. 2022, 12, 13795. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Puxeddu, I.; Lambiase, A.; Antonelli, A.; Bonini, S.; Bonini, S.; Aloe, L.; Pe’er, J.; Levi-Schaffer, F. The pro-fibrogenic effect of nerve growth factor on conjunctival fibroblasts is mediated by transforming growth factor-beta. Clin. Exp. Allergy 2005, 35, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Buono, R.; Catanzaro, G.; De Palma, C.; Longhi, R.; Meneveri, R.; Bresolin, N.; Bassi, M.T.; Cossu, G.; Clementi, E.; et al. The low-affinity receptor for neurotrophins p75 NTR plays a key role for satellite cell function in muscle repair acting via RhoA. Mol. Biol. Cell 2009, 20, 3620–3627. [Google Scholar] [CrossRef]
- Sun, C.; Li, P.; Zhang, G.; Geng, W.; Wang, C.; Bao, S.; Liu, X.M.; Ji, M.; Guan, H. Investigation of Mitochondrial Homeostasis Changes in Lens Epithelium of High-Myopic Cataract. Curr. Eye Res. 2024, 49, 158–167. [Google Scholar] [CrossRef]
- Pierini, D.; Bryan, N.S. Nitric oxide availability as a marker of oxidative stress. Methods Mol. Biol. 2015, 1208, 63–71. [Google Scholar] [CrossRef]
- Li, Y.; Yip, M.; Ning, Y.; Chung, J.; Toh, A.; Leow, C.; Liu, N.; Ting, D.; Schmetterer, L.; Saw, S.M.; et al. Topical Atropine for Childhood Myopia Control: The Atropine Treatment Long-Term Assessment Study. JAMA Ophthalmol. 2024, 142, 15–23. [Google Scholar] [CrossRef]
- Knaus, K.R.; Hipsley, A.; Blemker, S.S. The action of ciliary muscle contraction on accommodation of the lens explored with a 3D model. Biomech. Model. Mechanobiol. 2021, 20, 879–894. [Google Scholar] [CrossRef]
- Francisco, B.M.; Salvador, M.; Amparo, N. Oxidative stress in myopia. Oxid. Med. Cell Longev. 2015, 2015, 750637. [Google Scholar] [CrossRef]
- Brewer, T.F.; Garcia, F.J.; Onak, C.S.; Carroll, K.S.; Chang, C.J. Chemical Approaches to Discovery and Study of Sources and Targets of Hydrogen Peroxide Redox Signaling Through NADPH Oxidase Proteins. Annu. Rev. Biochem. 2015, 84, 765–790. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ouyang, X.; Fu, H.; Hou, X.; Liu, Y.; Xie, Y.; Yu, H.; Wang, G. Advances in biomedical study of the myopia-related signaling pathways and mechanisms. Biomed. Pharmacother. 2022, 145, 112472. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A. Quantitative aspects of nitric oxide production from nitrate and nitrite. EXCLI J. 2022, 21, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, R.A.; Saavedra, G.M.; Franklin, J.L. Rapid activation of antioxidant defenses by nerve growth factor suppresses reactive oxygen species during neuronal apoptosis: Evidence for a role in cytochrome c redistribution. J. Neurosci. 2007, 27, 11315–11326. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: Oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Annunziato, A.T.; Hansen, J.C. Role of histone acetylation in the assembly and modulation of chromatin structures. Gene Expr. 2000, 9, 37–61. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, K.K.; Lu, Y.; Zhu, X.J. Myopia and Cataract. Nat. Cell Sci. 2023, 1, 24–31. [Google Scholar] [CrossRef]
- Bruscolini, A.; Iannitelli, A.; Segatto, M.; Rosso, P.; Fico, E.; Buonfiglio, M.; Lambiase, A.; Tirassa, P. Psycho-Cognitive Profile and NGF and BDNF Levels in Tears and Serum: A Pilot Study in Patients with Graves’ Disease. Int. J. Mol. Sci. 2023, 24, 8074. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal epithelium-derived Neurotrophic factors promote nerve regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve Growth Factor: A Focus on Neuroscience and Therapy. Curr. Neuropharmacol. 2015, 13, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Haarman, A.E.G.; Klaver, C.C.W.; Tedja, M.S.; Roosing, S.; Astuti, G.; Gilissen, C.; Hoefsloot, L.H.; van Tienhoven, M.; Brands, T.; Magielsen, F.J.; et al. Identification of Rare Variants Involved in High Myopia Unraveled by Whole Genome Sequencing. Ophthalmol. Sci. 2023, 3, 100303. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, Y.; Chen, Y.; Cai, J.; Chen, Y.; Li, N.; Xu, C.; Zhou, Q.; Wang, F.; Wang, J. Expression of cytokines in the aqueous humor of cataract patients with pathologic myopia and simple high myopia. Mol. Vis. 2024, 30, 369–377. [Google Scholar] [PubMed]
- Shao, Y.; Li, M.; Wang, W.; Shi, Y.; Sun, L.; Qian, Y.; Ma, X.; Zou, J. The impact of soft contact lenses on the corneal neuromediators and optical quality in high myopia. Cont. Lens Anterior Eye 2025, 10, 102425. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Nubile, M.; Acerra, G.; Detta, N.; Pelusi, L.; Lanzini, M.; Mattioli, S.; Santalucia, M.; Pietrangelo, L.; Allegretti, M.; et al. Bioengineered Human Stromal Lenticule for Recombinant Human Nerve Growth Factor Release: A Potential Biocompatible Ocular Drug Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 887414. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Yu, Y.; Li, T.; Guo, L.; Hu, G.; Wei, H.; Yang, Z.; Liu, J.; Hao, Y.; et al. Covariation of scleral remodeling and PI3K/Akt signaling pathway in experimental myopia. Sci. Rep. 2025, 15, 12476. [Google Scholar] [CrossRef]


| Population | Emmetropia | Myopia | High Myopia | p Value | |
|---|---|---|---|---|---|
| Patients | 65 | 23 | 24 | 18 | |
| Mean age ± SD | 76.25 ± 9.40 | 81.00 ± 6.70 | 75.96 ± 7.30 | 70.56 ± 11.68 | p > 0.05 |
| Sex F/M | 36/29 | 12/11 | 12/12 | 12/6 | p > 0.05 |
| Axial length | 25.52 ± 2.74 | 23.14 ± 0.45 | 24.83 ± 0.50 | 29.46 ± 1.68 | p < 0.05 |
| Genes | Primer Sequence | GeneBank | |
|---|---|---|---|
| Reference gene | |||
| 18S | 5′-GGAGAGGGAGCCTGAGAAAC-3′ | 5′-AGGGCCTCGAAAGAGTCCT–3′ | M10098 |
| H3 | 5′-GTCTGCAGGCTGGCATAGAAG-3′ | 5′-TCGCCTTCTGGGTTGAGTG-3′ | NM005324.4 |
| Target genes | |||
| βNGF | 5′-TGAAGCTGCAGACACTCAGG-3′ | 5′-CACCTCCTTGCCCTTGATGT-3′ | BC126150.1 |
| BDNF | 5′-CCT TT GAG CCT CCT CTT CTC-3′ | 5′-ACT GTC ACA GCT CAG CTC-3′ | M61176.1 |
| trkA | 5′-GCT CAG TCG CCT GAA TCT CT-3′ | 5′-GCA CAA GAA CAG TGC AGA GG-3′ | M23102 |
| trkB | 5′-TGG TGG TGA TTG CGT CTG-3′ | 5′-CCA CA CCC CTT TCT CTG T-3′ | AF400441 |
| p75 | 5′-CCT ACG GCT ACT ACC AGG ATG AG-3′ | 5′-TGG CCT CGT CGG AAT ACG-3′ | AF187064 |
| iNOS | 5′-CCCCTTCAATGGCTGGTACA-3′ | 5′-GTTTCCAGGCCCATTCTCCT-3′ | U31511.1 |
| NOX1 | 5′-CCAGGATTGAAGTGGATGGT-3′ | 5′-AGGTTGTGGTCTGCACACTG-3′ | BC075014.2 |
| NOX4 | 5′-CTCAGCGGAATCAATCAGCTGTG-3′ | 5′-AGAGGAACACGACAATCAGCCTTAG-3′ | BC040105.1 |
| KEAP1 | 5′-TTCAGCTACACCCTGGAGGA-3′ | 5′-CTTGAAGACAGGGCTGGATG-3′ | BC002417.2 |
| NRF2 | 5′-ACACGGTCCACAGCTCATC-3′ | 5′-TGCCTCCAAAGTATGTCAATCA-3′ | BC011558.1 |
| DNMT3α | 5′-GCA CTC AAG GGC AGC AGA TA-3′ | 5′-TTC CAG GCT TCC AGG GTT AG-3′ | C032392 |
| HD1 | 5′-GGG ATC GGT TAG GTT GCT TC-3′ | 5′-AGG GCC ACA GCT GTC CTC ATA-3′ | U50079 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Piano, M.; Cacciamani, A.; Scarinci, F.; Squitti, R.; Cosimi, P.; Bruno, M.; Ripandelli, G.; Palanza, P.; Micera, A. NGF, BDNF, and NO in Myopic Subjects: Relationships Between Aqueous Levels and Lens Epithelial Cells’ Activation. Int. J. Mol. Sci. 2025, 26, 6350. https://doi.org/10.3390/ijms26136350
De Piano M, Cacciamani A, Scarinci F, Squitti R, Cosimi P, Bruno M, Ripandelli G, Palanza P, Micera A. NGF, BDNF, and NO in Myopic Subjects: Relationships Between Aqueous Levels and Lens Epithelial Cells’ Activation. International Journal of Molecular Sciences. 2025; 26(13):6350. https://doi.org/10.3390/ijms26136350
Chicago/Turabian StyleDe Piano, Maria, Andrea Cacciamani, Fabio Scarinci, Rosanna Squitti, Pamela Cosimi, Marisa Bruno, Guido Ripandelli, Paola Palanza, and Alessandra Micera. 2025. "NGF, BDNF, and NO in Myopic Subjects: Relationships Between Aqueous Levels and Lens Epithelial Cells’ Activation" International Journal of Molecular Sciences 26, no. 13: 6350. https://doi.org/10.3390/ijms26136350
APA StyleDe Piano, M., Cacciamani, A., Scarinci, F., Squitti, R., Cosimi, P., Bruno, M., Ripandelli, G., Palanza, P., & Micera, A. (2025). NGF, BDNF, and NO in Myopic Subjects: Relationships Between Aqueous Levels and Lens Epithelial Cells’ Activation. International Journal of Molecular Sciences, 26(13), 6350. https://doi.org/10.3390/ijms26136350











