From Nuclear Receptor Regulation to Spleen Activating and Accumulation Resolving Therapy: A Review of Traditional Chinese Medicine Against Diabetes and Inflammation
Abstract
1. Introduction
2. Nuclear Receptors and Metabolic Disorders in Diabetes
2.1. Multifaceted Roles of FXR in Regulating Glucose and Lipid Metabolism Disorders in Diabetes: Focusing on Bile Acids, Glucose, and Lipid Regulation
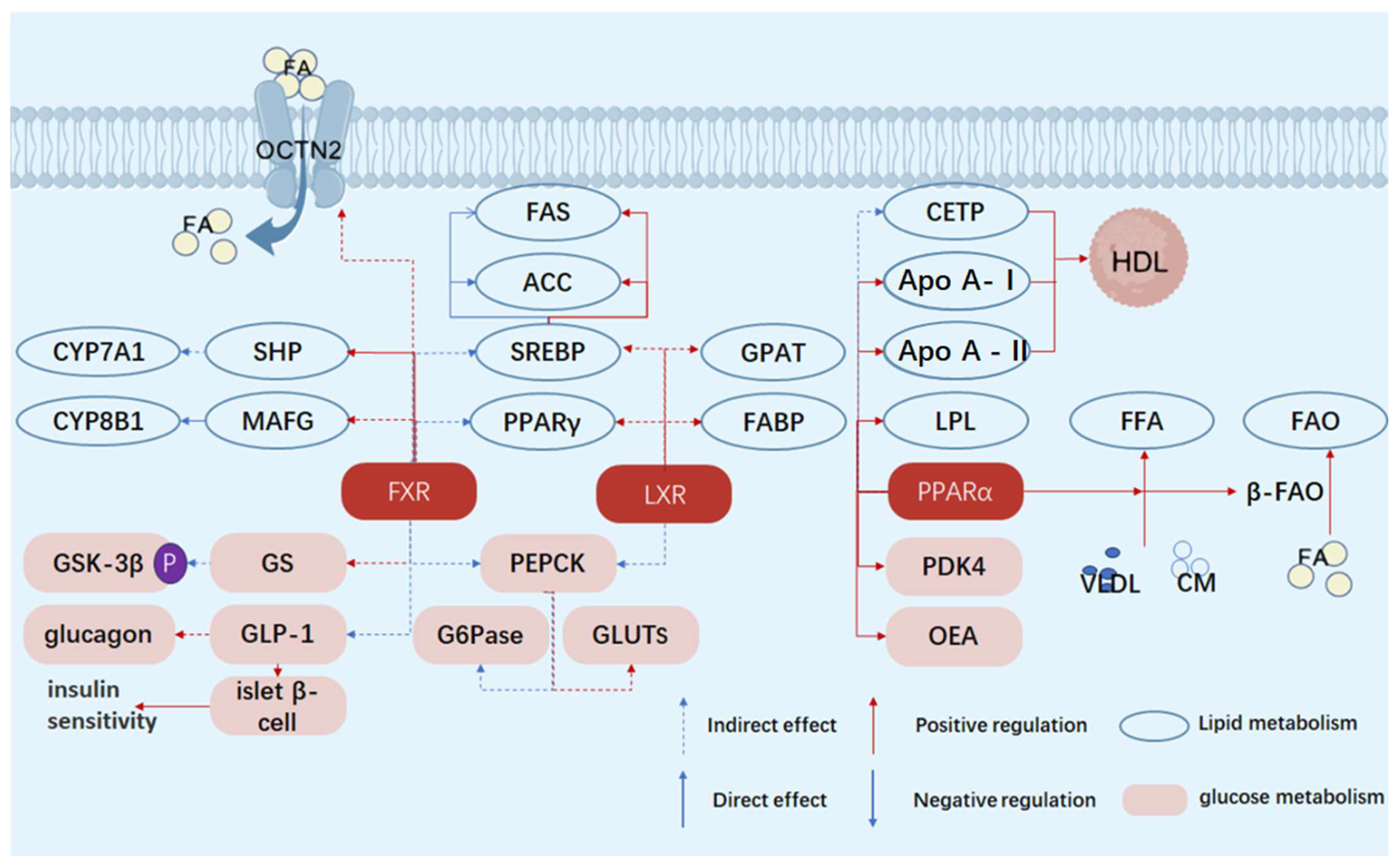
2.1.1. Regulation of Bile Acid Metabolism
2.1.2. Regulation of Glucose Metabolism
2.1.3. Regulation of Lipid Metabolism
2.1.4. Differences in the Effects of FXR on Type 2 Diabetes Mellitus (T1DM) and Type 2 Diabetes Mellitus (T2DM)
2.2. Dual Roles of LXR in Glucose and Lipid Metabolism in T2DM: Promoting Lipid Synthesis and Regulating Glucose
2.2.1. Regulation of Lipid Metabolism
2.2.2. Regulation of Glucose Metabolism
2.2.3. Differences in the Effects of LXR on T1DM and T2DM
2.3. Multifaceted Roles of PPARα in Metabolic Regulation in Diabetes: Lipids, Glucose, and Beyond
2.3.1. Regulation of Lipid Metabolism
2.3.2. Regulation of Glucose Metabolism and Other Aspects
| Characteristics | Receptors | Functions/Effects | References |
|---|---|---|---|
| Core metabolic pathways | PPARα | Fatty acid oxidation and energy metabolism | [39] |
| LXR | Cholesterol metabolism and lipogenesis | [40] | |
| FXR | Bile acid metabolism and gluconeogenesis inhibition | [41] | |
| Key points of blood glucose regulation | PPARα | Reduce hepatic glucose output and improve insulin sensitivity | [42] |
| LXR | Cholesterol balance and potential inhibition of gluconeogenesis | [43] | |
| FXR | Directly inhibit hepatic glucose output and enhance GLP-1 | [44] | |
| Impact on the liver | PPARα | Reduce lipotoxicity and improve insulin sensitivity | [45] |
| LXR | Promote lipogenesis (potential insulin resistance) | [16] | |
| FXR | Inhibit gluconeogenesis and improve insulin signaling | [46] | |
| Tissue specificity | PPARα | Liver muscle | [47] |
| LXR | Liver adipose tissue | [48] | |
| FXR | Liver and intestine | [49] | |
| Drug applications | PPARα | Fibrates (such as fenofibrate) | [50] |
| LXR | Limited (due to the risk of fatty liver) | [51] | |
| FXR | Obeticholic acid (in clinical trials) | [52] |
3. Nuclear Receptors and Diabetes Inflammation
3.1. Inflammation in Diabetes
3.2. Inflammation Exacerbates Diabetes
3.2.1. Islet Beta Cell Damage
3.2.2. Influence on Blood Glucose Metabolism
3.3. LXR’s Regulation of Inflammatory Response
3.4. ER’s Regulation of Inflammatory Response
3.5. FXR’s Regulation of Inflammatory Response
3.6. PPARs Regulating Inflammatory Response

| Characteristics | Receptors | Cell Types/Effects | References |
|---|---|---|---|
| Regulatory Cells | LXR | Macrophages | [79] |
| FXR | Monocytes, macrophages, dendritic cells, and intestinal cells | [80,81] | |
| PPARs | Kupffer cells (PPARγ) | [68,69] | |
| Regulatory Mechanisms | LXR | Regulates lipid metabolism to inhibit inflammation; Interferes with NF-κB pathway through conformational changes | [82,83] |
| ER | Interacts with NF-κB to inhibit its transcriptional activity | [84] | |
| FXR | Reduces pro-inflammatory cytokines; Regulates lipid metabolism to avoid inflammation | [85,86] | |
| PPARs | PPARγ promotes M2 polarization of Kupffer cells; PPARα inhibits NF-κB activation | [68,69,87] | |
| Impact on Diabetic Inflammation | LXR | Alleviates inflammation and improves insulin resistance | [88] |
| ER | Inhibits liver inflammation genes | [84] | |
| FXR | Reduces systemic inflammation and maintains intestinal inflammation homeostasis | [89] | |
| PPARs | Improves liver inflammation and regulates immune cell function | [90] |
4. New Nuclear Receptor Targets for the Treatment of Diabetes
4.1. FXR Improves Glycolipid Metabolism by Regulating ZBTB18
4.2. KLF16 Regulates PPARα to Improve Glycolipid Metabolism
4.3. Nur77 Affects AMPK Glucose Metabolism
5. Drugs Targeting Nuclear Receptors
5.1. PPARs Agonists
5.2. Metadichol
5.3. Retinoic Acid Receptor-Related Orphan Receptor Gamma (RORγ) and SR1001
5.4. Nuclear Receptor Drugs in Development
6. Traditional Chinese Medicine
6.1. Spleen Deficiency Compound
6.1.1. Nourishing Yin, Moistening Dryness—Yunv Decoction
6.1.2. Nourishing Yin and Tonifying Kidney—Liuwei Dihuang Pill
6.1.3. Tonifying Kidney and Yang—Shenqi Wan
6.2. Example of Spleen Deficiency Single Flavor Medicine
6.2.1. Qi-Tonifying Herb
6.2.2. Blood Tonic Herb
6.2.3. Yin-Tonifying Herb
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Van Zutphen, T.; Stroeve, J.H.; Yang, J.; Bloks, V.W.; Jurdzinski, A.; Roelofsen, H.; Huijkman, N.C.; Van Dijk, T.H.; Vonk, R.J.; Van Deursen, J.; et al. FXR overexpression alters adipose tissue architecture in mice and limits its storage capacity leading to metabolic derangements. J. Lipid Res. 2019, 60, 1547–1561. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, J.S.; Choi, H.-I.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. The role of the farnesoid X receptor in kidney health and disease: A potential therapeutic target in kidney diseases. Exp. Mol. Med. 2023, 55, 304–312. [Google Scholar] [CrossRef]
- Chiang, J.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell. Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Ahn, H.; Hagey, L.R.; Romanoski, C.E.; Lee, R.G.; Graham, M.J.; Motohashi, H.; Yamamoto, M.; Edwards, P.A. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 2015, 21, 298–311. [Google Scholar] [CrossRef]
- Popescu, I.R.; Helleboid-Chapman, A.; Lucas, A.; Vandewalle, B.; Dumont, J.; Bouchaert, E.; Derudas, B.; Kerr-Conte, J.; Caron, S.; Pattou, F.; et al. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010, 584, 2845–2851. [Google Scholar] [CrossRef]
- Cipriani, S.; Mencarelli, A.; Palladino, G.; Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010, 51, 771–784. [Google Scholar] [CrossRef]
- Kirk, R.K.; Pyke, C.; von Herrath, M.G.; Hasselby, J.P.; Pedersen, L.; Mortensen, P.G.; Knudsen, L.B.; Coppieters, K. Immunohistochemical assessment of glucagon-like peptide 1 receptor (GLP-1R) expression in the pancreas of patients with type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 705–712. [Google Scholar] [CrossRef]
- Heller, R.S.; Kieffer, T.J.; Habener, J.F. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes 1997, 46, 785–791. [Google Scholar] [CrossRef]
- Al Mahtab, M.; Akbar, S.M.F.; Roy, P.P.; Rahim, M.A.; Yesmin, S.S.; Bin Islam, S. Treatment of Nonalcoholic Steatohepatitis by Obeticholic Acid: Current Status. Euroasian J. Hepatogastroenterol. 2022, 12 (Suppl. S1), S46–S50. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, Y.; Ding, H.; Wang, X.; Chen, L.; Jiang, H.; Shen, X. Farnesoid X receptor induces GLUT4 expression through FXR response element in the GLUT4 promoter. Cell. Physiol. Biochem. 2008, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, X.X.; Scherzer, P.; Wilson, P.; Tallman, J.; Takahashi, H.; Li, J.; Iwahashi, M.; Sutherland, E.; Arend, L.; et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 2007, 56, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, M.; Caron, S.; Duhem, C.; Prawitt, J.; Dumont, J.; Lucas, A.; Bouchaert, E.; Briand, O.; Brozek, J.; Kuipers, F.; et al. The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways. J. Biol. Chem. 2010, 285, 36759–36767. [Google Scholar] [CrossRef]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.-M.A.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes. Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef]
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes. Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef]
- Wolfrum, C.; Borrmann, C.M.; Börchers, T.; Spener, F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha- and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 2323–2328. [Google Scholar] [CrossRef]
- Ogihara, T.; Chuang, J.-C.; Vestermark, G.L.; Garmey, J.C.; Ketchum, R.J.; Huang, X.; Brayman, K.L.; Thorner, M.O.; Repa, J.J.; Mirmira, R.G.; et al. Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling. J. Biol. Chem. 2010, 285, 5392–5404. [Google Scholar] [CrossRef]
- Su, F.; Koeberle, A. Regulation and targeting of SREBP-1 in hepatocellular carcinoma. Cancer Metastasis Rev. 2024, 43, 673–708. [Google Scholar] [CrossRef]
- Bilotta, M.T.; Petillo, S.; Santoni, A.; Cippitelli, M. Liver X Receptors: Regulators of Cholesterol Metabolism, Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2020, 11, 584303. [Google Scholar] [CrossRef]
- Laffitte, B.A.; Chao, L.C.; Li, J.; Walczak, R.; Hummasti, S.; Joseph, S.B.; Castrillo, A.; Wilpitz, D.C.; Mangelsdorf, D.J.; Collins, J.L.; et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl. Acad. Sci. USA 2003, 100, 5419–5424. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Liang, Y.; Broderick, C.L.; Oldham, B.A.; Schmidt, R.J.; Zhang, Y.; Stayrook, K.R.; Suen, C.; Otto, K.A.; Miller, A.R.; et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 2003, 278, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elheiga, L.; Matzuk, M.M.; Abo-Hashema, K.A.; Wakil, S.J. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 2001, 291, 2613–2616. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Ferré, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53 (Suppl. S1), S43–S50. [Google Scholar] [CrossRef]
- Montanez, J.E.; Peters, J.M.; Correll, J.B.; Gonzalez, F.J.; Patterson, A.D. Metabolomics: An essential tool to understand the function of peroxisome proliferator-activated receptor alpha. Toxicol. Pathol. 2013, 41, 410–418. [Google Scholar] [CrossRef]
- Barbier, O.; Torra, I.P.; Duguay, Y.; Blanquart, C.; Fruchart, J.-C.; Glineur, C.; Staels, B. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 717–726. [Google Scholar] [CrossRef]
- Chou, C.J.; Haluzik, M.; Gregory, C.; Dietz, K.R.; Vinson, C.; Gavrilova, O.; Reitman, M.L. WY14,643, a peroxisome proliferator-activated receptor alpha (PPARalpha) agonist, improves hepatic and muscle steatosis and reverses insulin resistance in lipoatrophic A-ZIP/F-1 mice. J. Biol. Chem. 2002, 277, 24484–24489. [Google Scholar] [CrossRef]
- Garelnabi, M.; Lor, K.; Jin, J.; Chai, F.; Santanam, N. The paradox of ApoA5 modulation of triglycerides: Evidence from clinical and basic research. Clin. Biochem. 2013, 46, 12–19. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M.; Van Rooyen, D.M.; Brooling, J.; Ghatora, K.; Farrell, G.C. Peroxisome proliferator-activated receptor-α agonist, Wy 14,643, improves metabolic indices, steatosis and ballooning in diabetic mice with non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2012, 27, 341–350. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Jetten, A.M. Prospero-related homeobox 1 (Prox1) functions as a novel modulator of retinoic acid-related orphan receptors α- and γ-mediated transactivation. Nucleic Acids Res. 2013, 41, 6992–7008. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Torra, I.P.; Fruchart, J.-C.; Staels, B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin. Chem. Lab. Med. 2000, 38, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Orsó, E.; Broccardo, C.; Kaminski, W.E.; Böttcher, A.; Liebisch, G.; Drobnik, W.; Götz, A.; Chambenoit, O.; Diederich, W.; Langmann, T.; et al. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 2000, 24, 192–196. [Google Scholar] [CrossRef]
- Duszka, K.; Wahli, W. Peroxisome Proliferator-Activated Receptors as Molecular Links between Caloric Restriction and Circadian Rhythm. Nutrients 2020, 12, 3476. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, P.F. PPARα: An emerging target of metabolic syndrome, neurodegenerative and cardiovascular diseases. Front. Endocrinol. 2022, 13, 1074911. [Google Scholar] [CrossRef]
- Kim, H.; Haluzik, M.; Asghar, Z.; Yau, D.; Joseph, J.W.; Fernandez, A.M.; Reitman, M.L.; Yakar, S.; Stannard, B.; Heron-Milhavet, L.; et al. Peroxisome proliferator-activated receptor-alpha agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes 2003, 52, 1770–1778. [Google Scholar] [CrossRef]
- Billon, C.; Sitaula, S.; Burris, T.P. Metabolic Characterization of a Novel RORα Knockout Mouse Model without Ataxia. Front. Endocrinol. 2017, 8, 141. [Google Scholar] [CrossRef]
- Kersten, S. Integrated physiology and systems biology of PPARalpha. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef]
- Calkin, A.C.; Tontonoz, P. Liver x receptor signaling pathways and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1513–1518. [Google Scholar] [CrossRef]
- Modica, S.; Gadaleta, R.M.; Moschetta, A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal 2010, 8, e005. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Olson, P.; Evans, R.M. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003, 144, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Mitro, N.; Mak, P.A.; Vargas, L.; Godio, C.; Hampton, E.; Molteni, V.; Kreusch, A.; Saez, E. The nuclear receptor LXR is a glucose sensor. Nature 2007, 445, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 2018, 68, 1574–1588. [Google Scholar] [CrossRef]
- Staels, B.; Fruchart, J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yi, Q.; Luo, L.; Xiong, Y. Regulation of bile acids and their receptor FXR in metabolic diseases. Front. Nutr. 2024, 11, 1447878. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Krone, W. Peroxisome proliferator-activated receptor alpha (PPARalpha) and athero-sclerosis. Curr. Drug. Targets Cardiovasc. Haematol. Disord. 2005, 5, 513–523. [Google Scholar] [CrossRef]
- Archer, A.; Stolarczyk, É.; Doria, M.L.; Helguero, L.; Domingues, R.; Howard, J.K.; Mode, A.; Korach-André, M.; Gustafsson, J. LXR activation by GW3965 alters fat tissue distribution and adipose tissue inflammation in ob/ob female mice. J. Lipid Res. 2013, 54, 1300–1311. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlström, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, I.-K.; Ahn, K.J.; Chung, H.Y.; Hwang, Y.-C. Fenofibrate, a PPARalpha agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism 2021, 120, 154798. [Google Scholar] [CrossRef]
- Jung, U.J.; Millman, P.N.; Tall, A.R.; Deckelbaum, R.J. n-3 fatty acids ameliorate hepatic steatosis and dysfunction after LXR agonist ingestion in mice. Biochim. Biophys. Acta 2011, 1811, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; She, P.; Mozzoli, M.; Cheung, P.; Gumireddy, K.; Reddy, P.; Xiang, X.; Luo, Z.; Ruderman, N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 2005, 54, 3458–3465. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Zhao, L.; Lei, W.; Deng, C.; Wu, Z.; Sun, M.; Jin, Z.; Song, Y.; Yang, Z.; Jiang, S.; Shen, M.; et al. The roles of liver X receptor α in inflammation and inflammation-associated diseases. J. Cell. Physiol. 2021, 236, 4807–4828. [Google Scholar] [CrossRef]
- Glaría, E.; Letelier, N.A.; Valledor, A.F. Integrating the roles of liver X receptors in inflammation and infection: Mechanisms and outcomes. Curr. Opin. Pharmacol. 2020, 53, 55–65. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Z.; Zhou, E.; Zhang, N.; Yang, Z. Cyanidin-3-O-β-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. J. Lipid Res. 2014, 55, 1111–1119. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef]
- Yu, S.-X.; Chen, W.; Hu, X.-Z.; Feng, S.-Y.; Li, K.-Y.; Qi, S.; Lei, Q.-Q.; Hu, G.-Q.; Li, N.; Zhou, F.-H.; et al. Liver X receptors agonists suppress NLRP3 inflammasome activation. Cytokine 2017, 91, 30–37. [Google Scholar] [CrossRef]
- Evans, M.J.; Eckert, A.; Lai, K.; Adelman, S.J.; Harnish, D.C. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ. Res. 2001, 89, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Cao, L.; Jiang, C.; Che, Y.; Zhang, S.; Takahashi, S.; Wang, G.; Gonzalez, F.J. Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis. Cell Metab. 2017, 25, 856–867.e5. [Google Scholar] [CrossRef]
- Guan, B.; Tong, J.; Hao, H.; Yang, Z.; Chen, K.; Xu, H.; Wang, A. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta Pharm. Sin. B 2022, 12, 2129–2149. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Wu, H.M.; Ni, X.X.; Xu, Q.Y.; Wang, Q.; Li, X.Y.; Hua, J. Regulation of lipid-induced macrophage polarization through modulating peroxisome proliferator-activated receptor-gamma activity affects hepatic lipid metabolism via a Toll-like receptor 4/NF-κB signaling pathway. J. Gastroenterol. Hepatol. 2020, 35, 1998–2008. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Crisafulli, C.; Cuzzocrea, S. The role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor alpha (PPAR-alpha) in the regulation of inflammation in macrophages. Shock 2009, 32, 62–73. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, Q.; Liu, H.; Guo, Y.; Xu, H. PPAR-α Agonist WY-14643 Inhibits LPS-Induced Inflammation in Synovial Fibroblasts via NF-kB Pathway. J. Mol. Neurosci. 2016, 59, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Ousman, S.S.; Sobel, R.A.; Zuniga, L.; Baranzini, S.E.; Youssef, S.; Crowell, A.; Loh, J.; Oksenberg, J.; Steinman, L. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J. Exp. Med. 2007, 204, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef]
- Tao, S.; Yang, Y.; Li, J.; Wang, H.; Ma, Y. Bixin Attenuates High-Fat Diet-Caused Liver Steatosis and Inflammatory Injury through Nrf2/PPARα Signals. Oxidative. Med. Cell Longev. 2021, 2021, 6610124. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Oishi, Y.; Spann, N.J.; Link, V.M.; Muse, E.D.; Strid, T.; Edillor, C.; Kolar, M.J.; Matsuzaka, T.; Hayakawa, S.; Tao, J.; et al. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell Metab. 2017, 25, 412–427. [Google Scholar] [CrossRef]
- Dong, X.; Qi, M.; Cai, C.; Zhu, Y.; Li, Y.; Coulter, S.; Sun, F.; Liddle, C.; Uboha, N.V.; Halberg, R.; et al. Farnesoid X receptor mediates macrophage-intrinsic responses to suppress colitis-induced colon cancer progression. JCI Insight 2024, 9, e170428. [Google Scholar] [CrossRef]
- Massafra, V.; Ijssennagger, N.; Plantinga, M.; Milona, A.; Pittol, J.M.R.; Boes, M.; van Mil, S.W. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochim. Biophys. Acta 2016, 1862, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Barish, G.D.; Evans, R.M. A nuclear strike against Listeria--the evolving life of LXR. Cell 2004, 119, 149–151. [Google Scholar] [CrossRef]
- Stein, B.; Yang, M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 1995, 15, 4971–4979. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.; Renooij, W.; Murzilli, S.; Klomp, L.W.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Tian, S.-Y.; Chen, S.-M.; Pan, C.-X.; Li, Y. FXR: Structures, biology, and drug development for NASH and fibrosis diseases. Acta Pharmacol. Sin. 2022, 43, 1120–1132. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Z.; Liu, S.; Shen, L.; Zheng, L.; Ding, L.; Liu, L.; Wu, L.; Li, L.; Hu, Z.; et al. Lupeol alleviates autoimmune myocarditis by suppressing macrophage pyroptosis and polarization via PPARalpha/LACC1/NF-kappaB signaling pathway. Phytomedicine 2024, 123, 155193. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, B.; Zhao, J.; Feng, Q.; Peng, J.; Hu, Y.; Zhao, Y. Biological mechanisms and related natural modulators of liver X receptor in nonalcoholic fatty liver disease. Biomed. Pharmacother. 2019, 113, 108778. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef]
- Lefere, S.; Puengel, T.; Hundertmark, J.; Penners, C.; Frank, A.K.; Guillot, A.; De Muynck, K.; Heymann, F.; Adarbes, V.; Defrêne, E.; et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages(☆). J. Hepatol. 2020, 73, 757–770. [Google Scholar] [CrossRef]
- Hemming, I.A.; Clément, O.; Gladwyn-Ng, I.E.; Cullen, H.D.; Ng, H.L.; See, H.B.; Ngo, L.; Ulgiati, D.; Pfleger, K.D.; Agostino, M.; et al. Disease-associated missense variants in ZBTB18 disrupt DNA binding and impair the development of neurons within the embryonic cerebral cortex. Hum. Mutat. 2019, 40, 1841–1855. [Google Scholar] [CrossRef] [PubMed]
- Fedele, V.; Dai, F.; Masilamani, A.P.; Heiland, D.H.; Kling, E.; Gätjens-Sanchez, A.M.; Ferrarese, R.; Platania, L.; Doostkam, S.; Kim, H.; et al. Epigenetic Regulation of ZBTB18 Promotes Glioblastoma Progression. Mol. Cancer Res. 2017, 15, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, R.; Izzo, A.; Andrieux, G.; Lagies, S.; Bartmuss, J.P.; Masilamani, A.P.; Wasilenko, A.; Osti, D.; Faletti, S.; Schulzki, R.; et al. ZBTB18 inhibits SREBP-dependent lipid synthesis by halting CTBPs and LSD1 activity in glioblastoma. Life Sci. Alliance 2023, 6, e202201400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Yang, X.; Shen, C.; Huang, J.; Zhang, D.; Liu, N.; Liu, C.; Zhong, Y.; Chen, Y.; et al. Hepatic Zbtb18 (Zinc Finger and BTB Domain Containing 18) alleviates hepatic steatohepatitis via FXR (Farnesoid X Receptor). Signal Transduct. Target. Ther. 2024, 9, 20. [Google Scholar] [CrossRef]
- Kumari, A.; Pal, P.D.; Asthana, S. Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of Lipid Metabolism. Int. J. Biol. Sci. 2020, 16, 2308–2322. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, J.; Han, S.; Zhong, L.; Liu, Z.; Li, B.; Zhang, R.; Zhou, L.; Zheng, X.; Liu, Z.; et al. The KLF16/MYC feedback loop is a therapeutic target in bladder cancer. J. Exp. Clin. Cancer Res. 2024, 43, 303. [Google Scholar] [CrossRef]
- Sun, N.; Shen, C.; Zhang, L.; Wu, X.; Yu, Y.; Yang, X.; Yang, C.; Zhong, C.; Gao, Z.; Miao, W.; et al. Hepatic Kruppel-like factor 16 (KLF16) targets PPARalpha to improve steatohepatitis and insulin resistance. Gut 2021, 70, 2183–2195. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef]
- Supale, S.; Li, N.; Brun, T.; Maechler, P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol. Metab. 2012, 23, 477–487. [Google Scholar] [CrossRef]
- Pirola, L.; Johnston, A.M.; Van Obberghen, E. Modulation of insulin action. Diabetologia 2004, 47, 170–184. [Google Scholar] [CrossRef]
- Hasanvand, A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: A new perspective for treatment and prevention of diseases. Inflammopharmacology 2022, 30, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.-Y.; Chen, Y.; Zhang, Q.; Zhuang, J.-J.; Tian, M.; Chen, H.-Z.; Zhang, L.-R.; Zhang, H.-K.; He, J.-P.; Wang, W.-J.; et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat. Chem. Biol. 2012, 8, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, J.; Kang, Q.; Yuan, H.; Liu, C.; Li, Z.; Liu, J.; Li, M. Knockout of Nur77 Leads to Amino Acid, Lipid, and Glucose Metabolism Disorders in Zebrafish. Front. Endocrinol. 2022, 13, 864631. [Google Scholar] [CrossRef] [PubMed]
- Eldor, R.; DeFronzo, R.A.; Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: Glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 2013, 36 (Suppl. S2), S162–S174. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Filipova, E.; Uzunova, K.; Kalinov, K.; Vekov, T. Effects of pioglitazone therapy on blood parameters, weight and BMI: A meta-analysis. Diabetol. Metab. Syndr. 2017, 9, 90. [Google Scholar] [CrossRef]
- Chen, R.; Yan, J.; Liu, P.; Wang, Z. Effects of thiazolidinedione therapy on inflammatory markers of type 2 diabetes: A meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0123703. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, X.; Ma, F.; Sun, W.; Wang, W.; Yu, J.; Shi, Y.; Cai, L.; Xu, Z. The Role of Akt2 in the Protective Effect of Fenofibrate against Diabetic Nephropathy. Int. J. Biol. Sci. 2020, 16, 553–567. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Guo, W.; Li, F.; Sun, W.; Chen, J.; Zhang, C.; Lu, X.; Tan, Y.; Feng, W.; et al. Up-regulation of Nrf2 is involved in FGF21-mediated fenofibrate protection against type 1 diabetic nephropathy. Free Radic. Biol. Med. 2016, 93, 94–109. [Google Scholar] [CrossRef]
- Raghavan, P. Metadichol® and Vitamin C Increase In Vivo, an Open-Label Study. Vitam. Miner. 2017, 6, 163. [Google Scholar]
- Solt, L.A.; Banerjee, S.; Campbell, S.; Kamenecka, T.M.; Burris, T.P. ROR inverse agonist suppresses insulitis and prevents hyperglycemia in a mouse model of type 1 diabetes. Endocrinology 2015, 156, 869–881. [Google Scholar] [CrossRef]
- Zapadka, T.E.; Lindstrom, S.I.; Taylor, B.E.; Lee, C.A.; Tang, J.; Taylor, Z.R.; Howell, S.J.; Taylor, P.R. RORgammat Inhibitor-SR1001 Halts Retinal Inflammation, Capillary Degeneration, and the Progression of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 3547. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.C.; Santos, R.D.; Aguilar-Salinas, C.; Aikawa, M.; Al Rasadi, K.; Amarenco, P.; Barter, P.J.; Ceska, R.; Corsini, A.; Després, J.P.; et al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha) paradigm: Conceptual framework and therapeutic potential: A consensus statement from the International Atherosclerosis Society (IAS) and the Residual Risk Reduction Initiative (R3i) Foundation. Cardiovasc. Diabetol. 2019, 18, 71. [Google Scholar]
- Arai, H.; Yamashita, S.; Araki, E.; Yokote, K.; Tanigawa, R.; Saito, A.; Yamasaki, S.; Suganami, H.; Ishibashi, S. Efficacy and Safety of Pemafibrate Extended-Release Tablet: A Phase 3, Multicenter, Randomized, Double-Blind, Active-Controlled, Parallel-Group Comparison Trial. J. Atheroscler. Thromb. 2024, 31, 1517–1538. [Google Scholar] [CrossRef]
- Osinski, V.; Bauknight, D.K.; Dasa, S.S.K.; Harms, M.J.; Kroon, T.; Marshall, M.A.; Garmey, J.C.; Nguyen, A.T.; Hartman, J.; Upadhye, A.; et al. In vivo liposomal delivery of PPARα/γ dual agonist tesaglitazar in a model of obesity enriches macrophage targeting and limits liver and kidney drug effects. Theranostics 2020, 10, 585–601. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Chen, B.; Fan, Z.; Li, N.; Yue, J.; Ye, Q. FXR activation alleviates tacrolimus-induced post-transplant diabetes mellitus by regulating renal gluconeogenesis and glucose uptake. J. Transl. Med. 2019, 17, 418. [Google Scholar] [CrossRef]
- Vitulo, M.; Gnodi, E.; Rosini, G.; Meneveri, R.; Giovannoni, R.; Barisani, D. Current Therapeutical Approaches Targeting Lipid Metabolism in NAFLD. Int. J. Mol. Sci. 2023, 24, 12748. [Google Scholar] [CrossRef]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef]
- Patel, K.; Harrison, S.A.; Elkhashab, M.; Trotter, J.F.; Herring, R.; Rojter, S.E.; Kayali, Z.; Wong, V.W.-S.; Greenbloom, S.; Jayakumar, S.; et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology 2020, 72, 58–71. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J.D.A. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Licong, Z. Clinical Experience of Professor Xu Jianyang in Using Yu’nyu Decoction. Chin. Med. Mod. Distance Educ. China 2024, 22, 84–86+100. [Google Scholar]
- Cui, Z.; Qin, P.; Min, Z.; Min, W. Wu Min’s Experience in Treating Diabetes with Yunu Jian. Hubei J. Tradit. Chin. Med. 2022, 44, 24–26. [Google Scholar]
- Yue, G.; Fang, H.; Weijian, Z. Clinical Study on Modified Yunv Jian for Type 2 Diabetes Mellitus with Intense Stomach Heat Syndrome. New Chin. Med. 2024, 56, 31–35. [Google Scholar]
- Liang, R.; Wenjing, G.E.; Pengtao, S.H.A.N.; Gengsheng, L.I.; Zheng, W.E.I.; Zhang, M. Effect of Yunvjian with or Without Achyranthis Bidentatae Radix on Glucose and Lipid Metabolism and Inflammatory Response in Diabetic Rats with Syndrome of Yin Deficiency and Internal Heat. Chin. J. Exp. Tradit. 2024, 30, 46–55. [Google Scholar]
- Xiaoping, R. Effect of Liuwei Dihuang Pill combined with metformin in the treatment of type 2 diabetes mellitus of Shen Yin Kui Xu Type and the influence of HbA1c,2 hPG and FPG levels. Chin. Community Dr. 2021, 37, 101–102. [Google Scholar]
- Junyu, N.; Juanhong, C.; Jianping, X. Clinical effect of Liuwei Dihuang pills combined with metformin for type 2 diabetes mellitus with Yin deficiency of liver and kidney. Chin. J. Clin. Ration. Drug Use 2023, 16, 28–31. [Google Scholar]
- Jun, X.; Xiaojun, Z.; Senli, L.; Lizhen, X. Cost-Effectiveness Analysis of Liuwei Dihuang Pill Combined with Empagliflozin in the Treatment of Type 2 Diabetes Mellitus. Jilin Med. J. 2024, 45, 2738–2740. [Google Scholar]
- Lina, W. Annotation of Jingui Yaolue (Synopsis of the Golden Chamber) by Doctors in the Ming and Qing Dynasties; Beijing University of Chinese Medicine: Beijing, China, 2005. [Google Scholar]
- Lianhe, H.; Wenjuan, L.; Lizhen, C. Clinical Effect of Integrated Traditional Chinese and Western Medicine on Patients with Early Diabetic Nephropathy. Diabetes New World 2021, 24, 36–39. [Google Scholar]
- Yuwen, G.; Feng, Q.; Changlong, Q. Protective Effect of Shenqi Pils on Rats with Diabetic Nephropathy Induced by Streptozotocin and Effect of Nrf2/HO-1 Pathway. Chin. Arch. Tradit. Chin. 2023, 41, 92–96+273. [Google Scholar]
- Jingzu, Z.; Jigong, Z.; Peixin, S.; Yu, Z. Clinical Observation on the Treatment of Yin and Yang Deficiency Type Diabetic Nephropathy with Modified Jinkui Shenqi Pills. China’s Naturopathy 2019, 27, 56–59. [Google Scholar]
- Qunju, Z.; Lin, Q.; Ziling, D.; Fanglin, H. Study on the Mechanism of Shenqi Pills in Treating Kidney-Yang Deficiency Syndrome of Type 2 Diabetes Based on Metabolomics. Lishizhen Med. Mater. Med. Res. 2019, 30, 53–56. [Google Scholar]
- Xiaocui, W.; Yawei, C.; Yongjie, Z.; Yi, D.; Lei, C.; Su, W.; Xiaotang, Q. Effects of Modified Jinkui Shenqi Pills on RBP4, GSK-3β, TGF-β1, NAG, and Renal Function in Patients with Diabetic Nephropathy (Spleen-Kidney Yang Deficiency Type). Chin. J. Gerontol. 2022, 42, 1676–1680. [Google Scholar]
- Xiao, M.; Jing, Y. Study on the hypoglycemic and hypolipidemic effects of Anemarrhena asphodeloides Bge-Panax notoginseng (Burk.) FH Chen powder on diabetic rats. J. Food Saf. Qual. 2022, 13, 4352–4360. [Google Scholar]
- Peng, J.; Li, Q.; Li, K.; Zhu, L.; Lin, X.; Lin, X.; Shen, Q.; Li, G.; Xie, X. Quercetin Improves Glucose and Lipid Metabolism of Diabetic Rats: Involvement of Akt Signaling and SIRT1. J. Diabetes Res. 2017, 2017, 3417306. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Tran, H.D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Andriana, Y.; Tuyen, P.T. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities and Potential Constituents of Canarium tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef]
- Congyu, L.; Shijie, C.; Feng, Q.; Xuejie, Q.; Ning, K. Research Progress on Anti-Diabetic Mechanism of Common Sweet-Taste Herbs. Chin. Tradit. Herb. Drugs 2022, 53, 3531–3537. [Google Scholar]
- Smits, M.M.; van Raalte, D.H.; Tonneijck, L.; A Muskiet, M.H.; Kramer, M.H.H.; Cahen, D.L. GLP-1 based therapies: Clinical implications for gastroenterologists. Gut 2016, 65, 702–711. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Liu, J.; Hong, J.; Deng, Y.; Yang, F.; Jin, X.; Gao, J.; Li, J.; Fang, H.; et al. Efficacy and safety of tangshen formula on patients with type 2 diabetic kidney disease: A multicenter double-blinded randomized placebo-controlled trial. PLoS ONE 2015, 10, e0126027. [Google Scholar] [CrossRef]
- Shen, Q.; Fang, J.; Guo, H.; Su, X.; Zhu, B.; Yao, X.; Wang, Y.; Cao, A.; Wang, H.; Wang, L. Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic. Biol. Med. 2023, 203, 45–57. [Google Scholar] [CrossRef]
- Luo, M.J.; Wang, Y.; Chen, S.Y.; Yang, Z.M. Astragalus Polysaccharides Alleviate Type 2 Diabetic Rats by Reversing the Expressions of Sweet Taste Receptors and Genes Related to Glycolipid Metabolism in Liver. Front. Pharmacol. 2022, 13, 916603. [Google Scholar] [CrossRef]
- Dai, Y.; Qiu, C.; Zhang, D.; Li, M.; Liu, W. Yam Gruel alone and in combination with metformin regulates hepatic lipid metabolism disorders in a diabetic rat model by activating the AMPK/ACC/CPT-1 pathway. Lipids Health Dis. 2024, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.S.; Chen, W.; Wu, H.T.; Cheng, K.C.; Wen, Y.J.; Lin, K.C.; Cheng, J.T. Decrease of plasma glucose by allantoin, an active principle of yam (Dioscorea spp.), in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 12031–12035. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, L.; Miao, Y.; Ha, W.; Li, Z.; Mi, D. Angelica polysaccharides relieve blood glucose levels in diabetic KKAy mice possibly by modulating gut microbiota: An integrated gut microbiota and metabolism analysis. BMC Microbiol. 2023, 23, 281. [Google Scholar] [CrossRef]
- Zou, Y.F.; Li, C.Y.; Fu, Y.P.; Jize, X.P.; Zhao, Y.Z.; Peng, X.; Wang, J.Y.; Yin, Z.Q.; Li, Y.P.; Song, X.; et al. Angelica sinensis aboveground part polysaccharide and its metabolite 5-MT ameliorate colitis via modulating gut microbiota and TLR4/MyD88/NF-kappaB pathway. Int. J. Biol. Macromol. 2023, 242, 124689. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Zhang, Y.; Liu, X.; Wu, Z.; Gilbert, R.G.; Deng, B.; Wang, K. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef]
- Mou, Z.; Zhao, Y.; Ye, F.; Shi, Y.; Kennelly, E.J.; Chen, S.; Zhao, D. Identification, Biological Activities and Biosynthetic Pathway of Dendrobium Alkaloids. Front. Pharmacol. 2021, 12, 605994. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Xu, L.; Liu, W.; Lin, C.; Tang, Y.; Shen, C.; Gao, Y. From Nuclear Receptor Regulation to Spleen Activating and Accumulation Resolving Therapy: A Review of Traditional Chinese Medicine Against Diabetes and Inflammation. Int. J. Mol. Sci. 2025, 26, 6345. https://doi.org/10.3390/ijms26136345
Huang J, Xu L, Liu W, Lin C, Tang Y, Shen C, Gao Y. From Nuclear Receptor Regulation to Spleen Activating and Accumulation Resolving Therapy: A Review of Traditional Chinese Medicine Against Diabetes and Inflammation. International Journal of Molecular Sciences. 2025; 26(13):6345. https://doi.org/10.3390/ijms26136345
Chicago/Turabian StyleHuang, Jiawen, Like Xu, Weiru Liu, Chuanquan Lin, Ying Tang, Chuangpeng Shen, and Yong Gao. 2025. "From Nuclear Receptor Regulation to Spleen Activating and Accumulation Resolving Therapy: A Review of Traditional Chinese Medicine Against Diabetes and Inflammation" International Journal of Molecular Sciences 26, no. 13: 6345. https://doi.org/10.3390/ijms26136345
APA StyleHuang, J., Xu, L., Liu, W., Lin, C., Tang, Y., Shen, C., & Gao, Y. (2025). From Nuclear Receptor Regulation to Spleen Activating and Accumulation Resolving Therapy: A Review of Traditional Chinese Medicine Against Diabetes and Inflammation. International Journal of Molecular Sciences, 26(13), 6345. https://doi.org/10.3390/ijms26136345





