Microwave Irradiation Pre-Treatment as a Sustainable Method to Obtain Bioactive Hydrolysates from Chicken Feathers
Abstract
1. Introduction
2. Results and Discussion
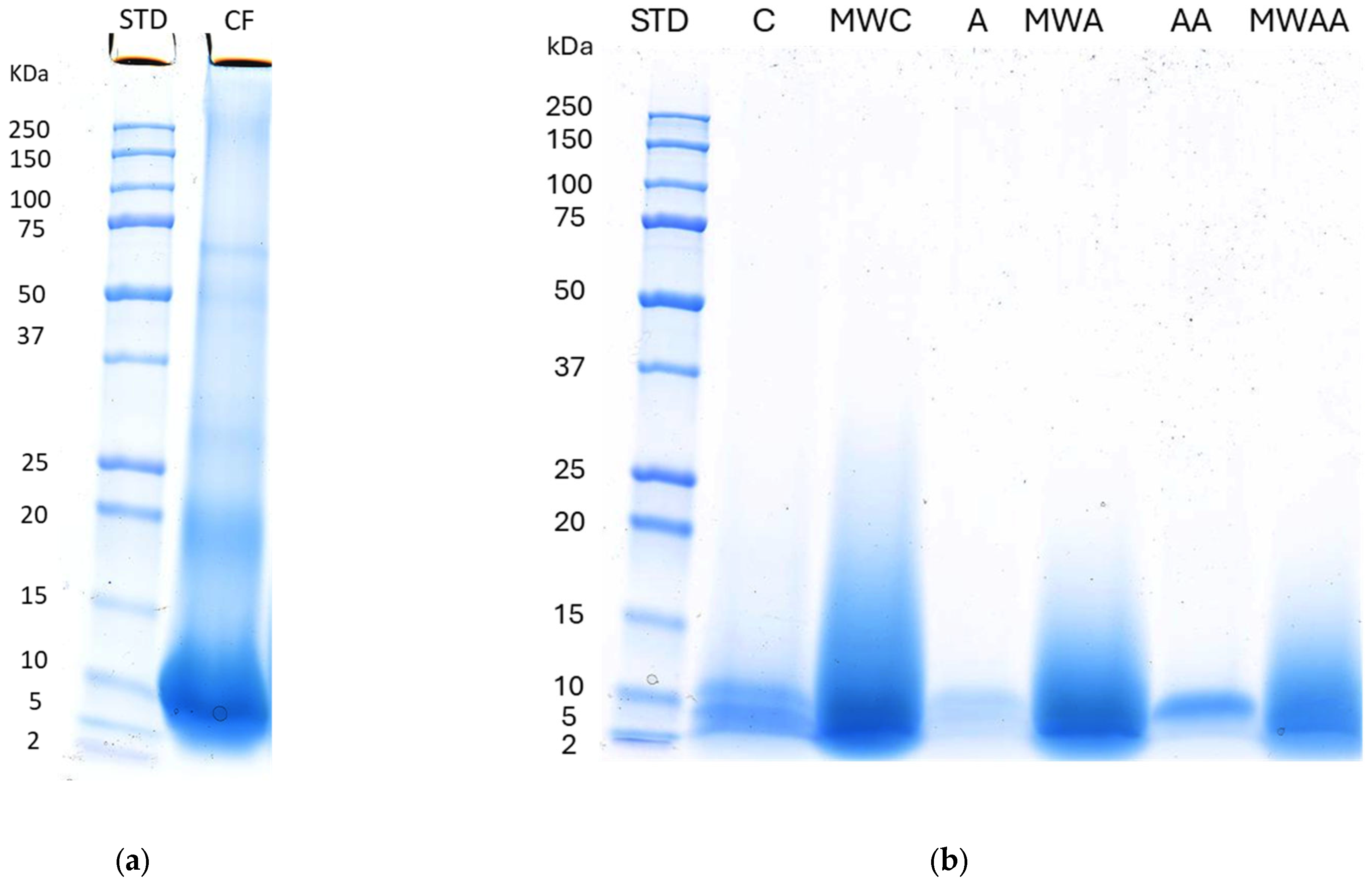
2.1. Chicken Feather Characterisation
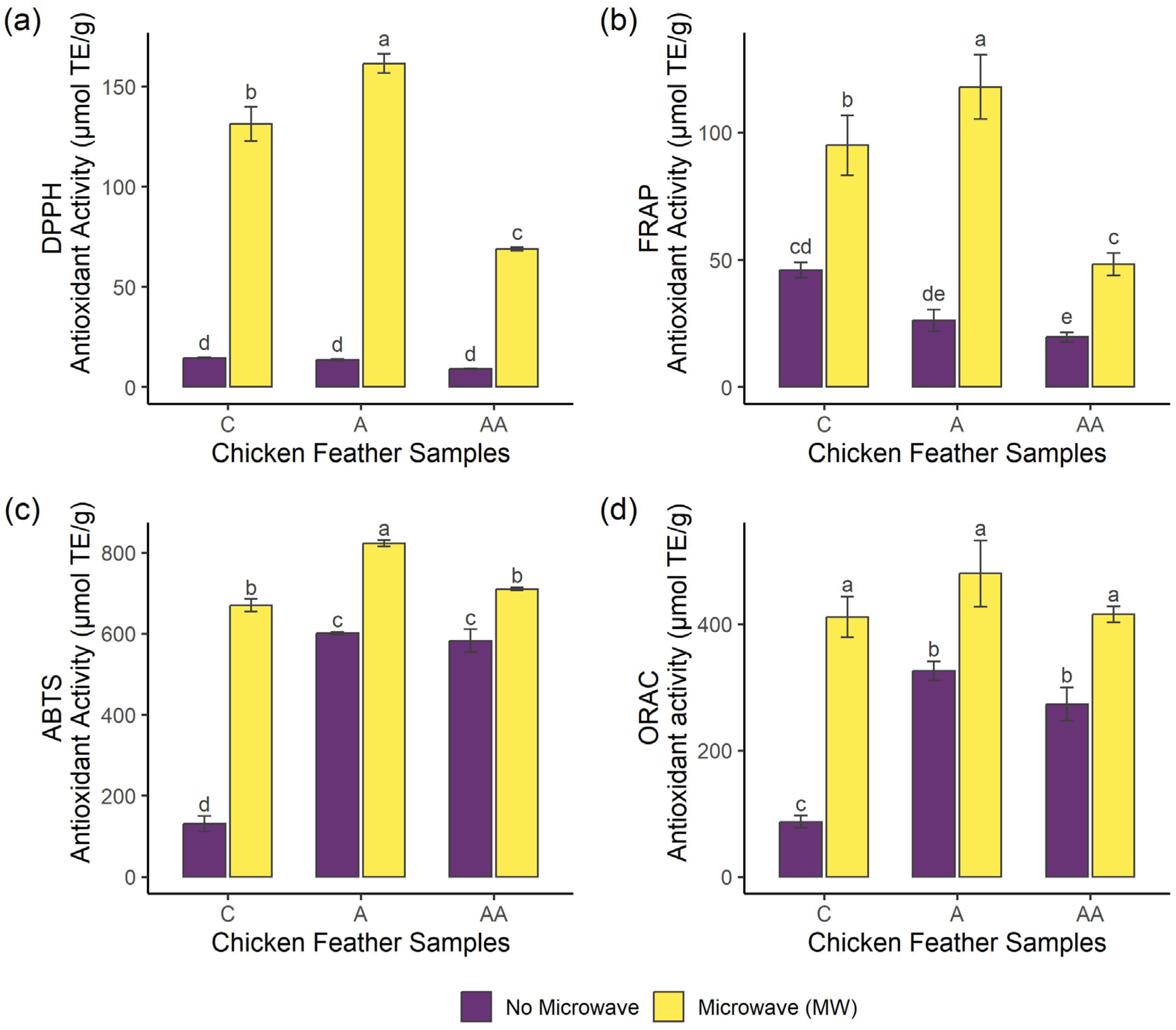
2.2. Effects of Microwave Irradiation Pre-Treatment and Enzymatic Hydrolysis on the Bioactivity of Samples
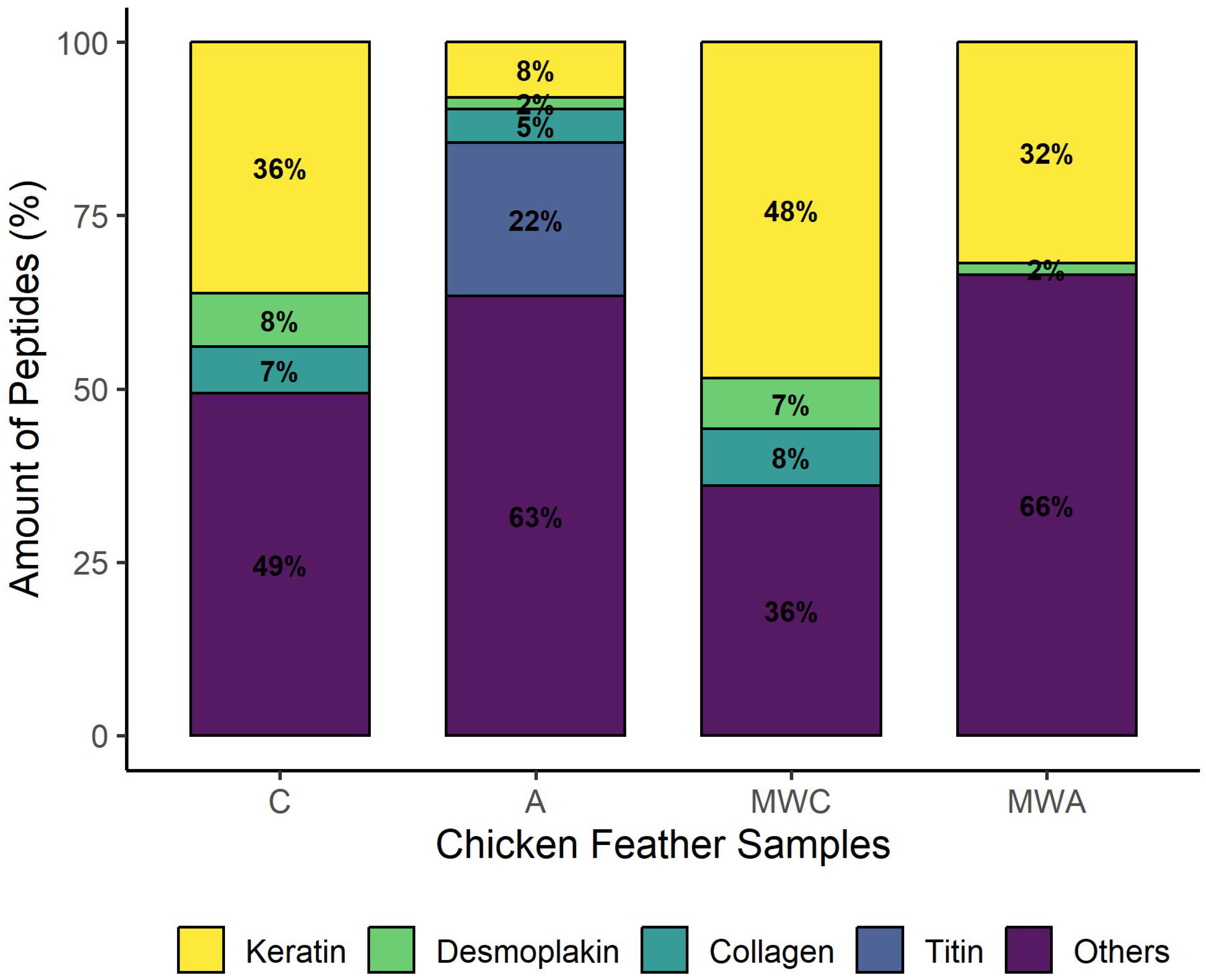
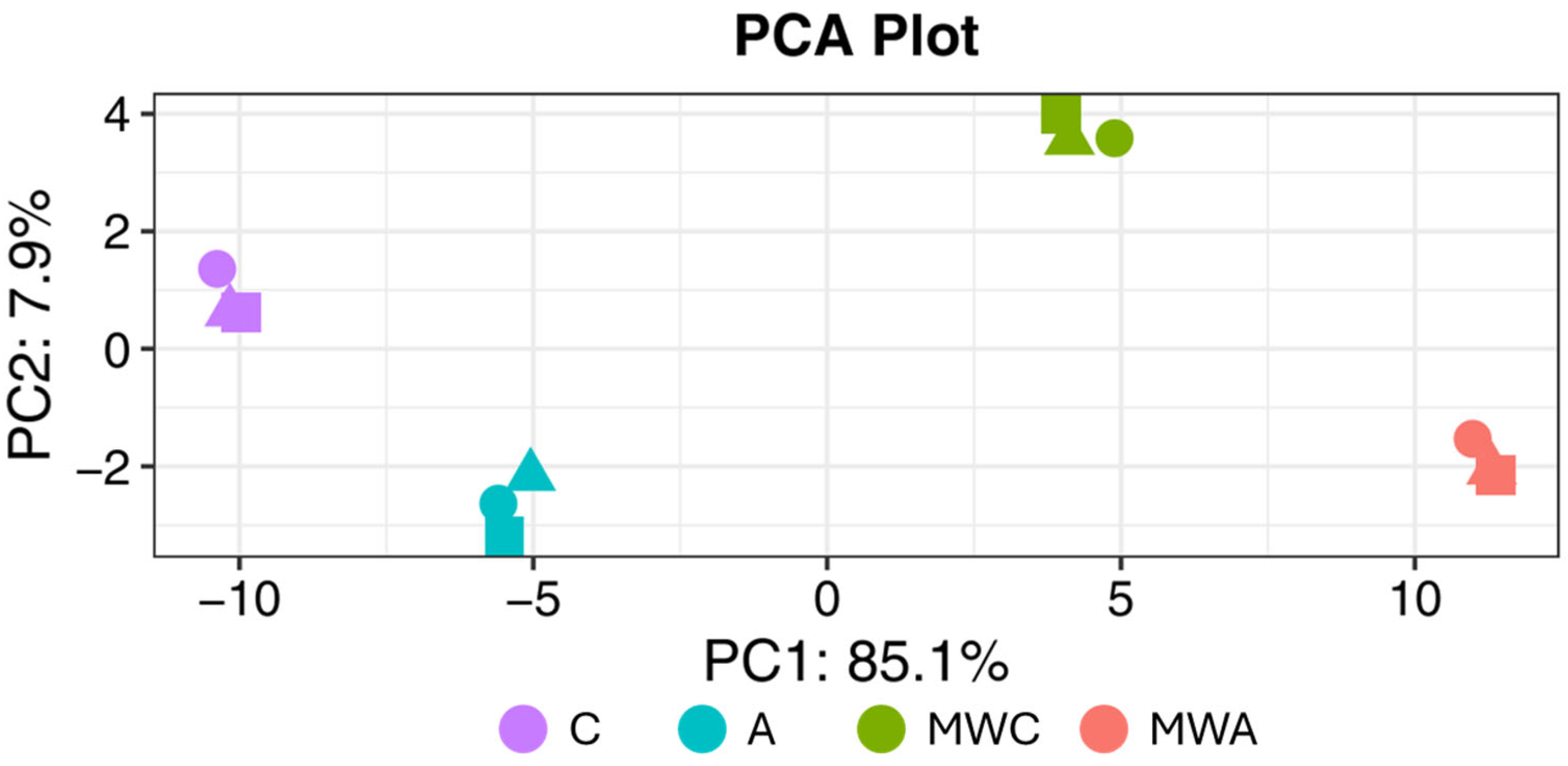
2.3. Peptidomics Characterisation of the Samples
3. Materials and Methods
3.1. Chemicals and Reagents
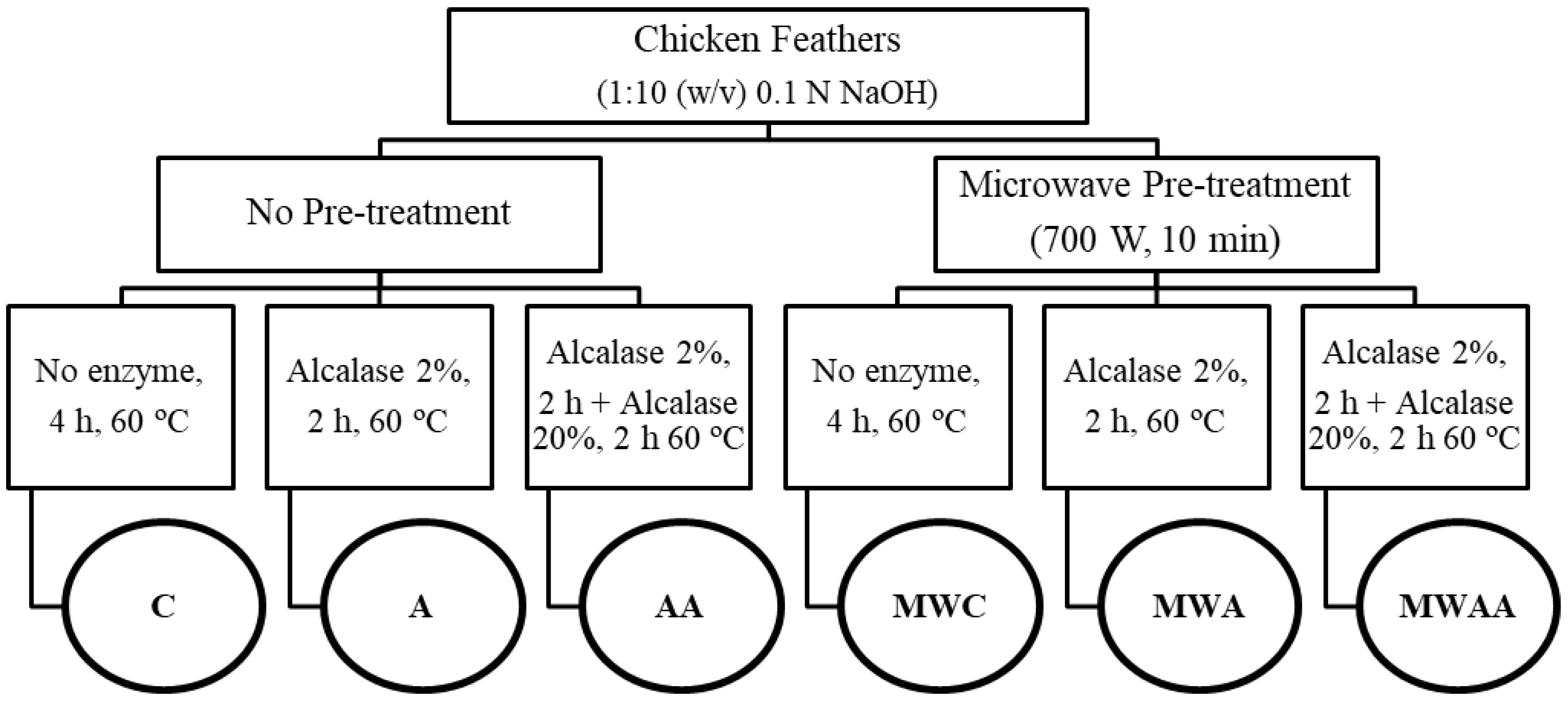
3.2. Obtention of Chicken Feather Samples
3.2.1. Preparation of Chicken Feather Meal
3.2.2. Chemical Composition of Chicken Feathers
3.2.3. Microwave Irradiation Pre-Treatment and Enzymatic Hydrolysis
3.3. SDS-PAGE Electrophoresis
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging Activity
3.4.2. Ferric-Reducing Antioxidant Power (FRAP)
3.4.3. ABTS Radical Scavenging Capacity
3.4.4. Oxygen Radical Absorbance Capacity Assay (ORAC)
3.5. DPP-IV-Inhibitory Activity
3.6. Neprilysin-Inhibitory Activity
3.7. ACE-I-Inhibitory Activity
3.8. Peptidomic Characterisation of the Samples
3.8.1. Peptide Analysis by LC-MS/MS
3.8.2. Identification and Label-Free Quantification Data Analysis
3.8.3. In Silico Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poultry Production. Available online: https://ourworldindata.org/grapher/poultry-production-tonnes (accessed on 17 May 2025).
- Qin, X.; Xu, X.; Guo, Y.; Shen, Q.; Liu, J.; Yang, C.; Scott, E.; Bitter, H.; Zhang, C. A Sustainable and Efficient Recycling Strategy of Feather Waste into Keratin Peptides with Antimicrobial Activity. Waste Manag. 2022, 144, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lu, L.; Yin, M.; Yang, R.; Zhang, Z.; Zhao, W. Valorization of Refractory Keratinous Waste Using a New and Sustainable Bio-Catalysis. Chem. Eng. J. 2020, 397, 125420. [Google Scholar] [CrossRef]
- Kshetri, P.; Singh, P.L.; Chanu, S.B.; Singh, T.S.; Rajiv, C.; Tamreihao, K.; Singh, H.N.; Chongtham, T.; Devi, A.K.; Sharma, S.K.; et al. Biological Activity of Peptides Isolated from Feather Keratin Waste through Microbial and Enzymatic Hydrolysis. Electron. J. Biotechnol. 2022, 60, 11–18. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, M.; Wu, L.; Yang, Y.; Sun, Y.; Wang, Q.; Gao, X. Bioconversion of Feather Waste into Bioactive Nutrients in Water by Bacillus Licheniformis WHU. Appl. Microbiol. Biotechnol. 2023, 107, 7055–7070. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D.; Chunilall, V. Valorisation of Chicken Feathers: Characterisation of Chemical Properties. Waste Manag. 2017, 68, 626–635. [Google Scholar] [CrossRef]
- Metin, Ö.; Yildiz, M.; Eldem, V.; Adabi, S.G. The Effects of Using Hydrolyzed Feather Meal, Amino Acids, and Probiotics in the Diet of Juvenile Rainbow Trout on Growth, Digestibility, and Expression of Growth-Related Genes. Aquac. Int. 2024, 32, 9671–9693. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, Mechanical Properties, Occurrence in Biological Organisms, and Efforts at Bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Martinez, J.P.D.O.; Cai, G.; Nachtschatt, M.; Navone, L.; Zhang, Z.; Robins, K.; Speight, R. Challenges and Opportunities in Identifying and Characterising Keratinases for Value-Added Peptide Production. Catalysts 2020, 10, 184. [Google Scholar] [CrossRef]
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond Plucking: Feathers Bioprocessing into Valuable Protein Hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel Technologies for the Production of Bioactive Peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Chen, M.; Ma, A.; Sun, Z.; Xie, B.; Shi, L.; Chen, S.; Chen, L.; Xiong, G.; Wang, L.; Wu, W. Enhancing Activity of Food Protein-Derived Peptides: An Overview of Pretreatment, Preparation, and Modification Methods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4698–4733. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Phang, L.Y.; Ahmad, S.A.; Ooi, P.T. Microwave-Alkali Treatment of Chicken Feathers for Protein Hydrolysate Production. Waste Biomass Valorization 2016, 7, 1147–1157. [Google Scholar] [CrossRef]
- Hall, F.; Liceaga, A. Effect of Microwave-Assisted Enzymatic Hydrolysis of Cricket (Gryllodes sigillatus) Protein on ACE and DPP-IV Inhibition and Tropomyosin-IgG Binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwal, S.; Huang, J.Y.; Liceaga, A.M. Selective Separation and Characterisation of Dual ACE and DPP-IV Inhibitory Peptides from Rainbow Trout (Oncorhynchus mykiss) Protein Hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 1062–1073. [Google Scholar] [CrossRef]
- Li, M.; Xia, S.; Zhang, Y.; Li, X. Optimization of ACE Inhibitory Peptides from Black Soybean by Microwave-Assisted Enzymatic Method and Study on Its Stability. LWT 2018, 98, 358–365. [Google Scholar] [CrossRef]
- Noman, A.; Qixing, J.; Xu, Y.; Abed, S.M.; Obadi, M.; Ali, A.H.; AL-Bukhaiti, W.Q.; Xia, W. Effects of Ultrasonic, Microwave, and Combined Ultrasonic-Microwave Pretreatments on the Enzymatic Hydrolysis Process and Protein Hydrolysate Properties Obtained from Chinese Sturgeon (Acipenser sinensis). J. Food Biochem. 2020, 44, e13292. [Google Scholar] [CrossRef]
- Santos, M.M.F.; Grisi, C.V.B.; de Souza, E.G.T.; de Morais Lima, J.; da Silva Ferreira, V.C.; Kurozawa, L.E.; Madruga, M.S.; da Silva, F.A.P. Biotransformation of Free-Range Chicken Feather into Functional Protein Hydrolysates Using Microwave Alkaline Pretreatment. Food Biosci. 2024, 59, 103897. [Google Scholar] [CrossRef]
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “Sisters” α-Helices of Collagen, Elastin and Keratin Recovered from Animal by-Products: Functionality, Bioactivity and Trends of Application. Trends Food Sci. Technol. 2016, 51, 65–75. [Google Scholar] [CrossRef]
- Chen, J.; Ding, S.; Ji, Y.; Ding, J.; Yang, X.; Zou, M.; Li, Z. Microwave-Enhanced Hydrolysis of Poultry Feather to Produce Amino Acid. Chem. Eng. Process. Process Intensif. 2015, 87, 104–109. [Google Scholar] [CrossRef]
- Škerget, M.; Čolnik, M.; Zemljič, L.F.; Gradišnik, L.; Semren, T.Ž.; Lovaković, B.T.; Maver, U. Efficient and Green Isolation of Keratin from Poultry Feathers by Subcritical Water. Polymers 2023, 15, 2658. [Google Scholar] [CrossRef]
- Khumalo, M.; Sithole, B.; Tesfaye, T. Valorisation of Waste Chicken Feathers: Optimisation of Keratin Extraction from Waste Chicken Feathers by Sodium Bisulphite, Sodium Dodecyl Sulphate and Urea. J. Environ. Manag. 2020, 262, 110329. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, Q.; Guan, X.; Song, H.; Zhang, Y.; Xie, J.; Fang, Y. Effect of Conventional and Microwave Heating Treatment on Antioxidant Activity of Quinoa Protein after Simulated Gastrointestinal Digestion. Food Chem. 2023, 415, 135763. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Huang, K.; Shi, J.; Li, M.; Sun, R.; Guan, W.; Cao, H.; Guan, X.; Zhang, Y. Intervention of Microwave Irradiation on Structure and Quality Characteristics of Quinoa Protein Aggregates. Food Hydrocoll. 2022, 130, 107677. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Y.; Yan, B.; Zhang, N.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Microwave Treatment on Structure and Digestibility Characteristics of Spirulina Platensis Protein. Curr. Res. Food Sci. 2023, 7, 100581. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the Production of Bioactive Peptides: A Review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- Groleau, P.E.; Morin, P.; Gauthier, S.F.; Pouliot, Y. Effect of Physicochemical Conditions on Peptide−Peptide Interactions in a Tryptic Hydrolysate of β-Lactoglobulin and Identification of Aggregating Peptides. J. Agric. Food Chem. 2003, 51, 4370–4375. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Ullah, A. Effects of Ultrasound-Assisted Alkaline Extraction on Antioxidant Activity and Functional Characteristics of Chicken Feather Keratin Peptides. ChemistrySelect 2020, 5, 13788–13794. [Google Scholar] [CrossRef]
- Cai, G.; Moffitt, K.; Navone, L.; Zhang, Z.; Robins, K.; Speight, R. Valorisation of Keratin Waste: Controlled Pretreatment Enhances Enzymatic Production of Antioxidant Peptides. J. Environ. Manag. 2022, 301, 113945. [Google Scholar] [CrossRef]
- Hong, C.; Zhu, J.Q.; Zhao, Y.M.; Ma, H. Effects of Dual-Frequency Slit Ultrasound on the Enzymolysis of High-Concentration Hydrolyzed Feather Meal: Biological Activities and Structural Characteristics of Hydrolysates. Ultrason. Sonochemistry 2022, 89, 106135. [Google Scholar] [CrossRef] [PubMed]
- Jeampakdee, P.; Puthong, S.; Srimongkol, P.; Sangtanoo, P.; Saisavoey, T.; Karnchanatat, A. The Apoptotic and Free Radical–Scavenging Abilities of the Protein Hydrolysate Obtained from Chicken Feather Meal. Poult. Sci. 2020, 99, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, N.D.A.; de Andrade, C.J.; Petrus, J.C.C.; Monteiro, A.R. Sequential Hydrolysis of Chicken Feathers Composed of Ultrasound and Enzymatic Steps: An Enhanced Protein Source with Bioactive Peptides. Biomass 2022, 2, 237–249. [Google Scholar] [CrossRef]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Effects of Different Pretreatments and Proteases on Recovery, Umami Taste Compound Contents and Antioxidant Potentials of Labeo Rohita Head Protein Hydrolysates. J. Food Sci. Technol. 2019, 56, 1966–1977. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Li, J.; Yang, M.; Yao, S. Swelling and Microwave-Assisted Hydrolysis of Animal Keratin in Ionic Liquids. J. Mol. Liq. 2021, 341, 117306. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Taraszkiewicz, A.; Sinkiewicz, I.; Sommer, A.; Dąbrowska, M.; Staroszczyk, H. Prediction of Bioactive Peptides from Chicken Feather and Pig Hair Keratins Using In Silico Analysis Based on Fragmentomic Approach. Curr. Pharm. Des. 2022, 28, 841–851. [Google Scholar] [CrossRef]
- Callegaro, K.; Welter, N.; Daroit, D.J. Feathers as Bioresource: Microbial Conversion into Bioactive Protein Hydrolysates. Process Biochem. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Carrera-Alvarado, G.; Toldrá, F.; Mora, L. DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. Int. J. Mol. Sci. 2022, 23, 14140. [Google Scholar] [CrossRef]
- Esser, N.; Zraika, S. Neprilysin Inhibition: A New Therapeutic Option for Type 2 Diabetes? Diabetologia 2019, 62, 1113–1122. [Google Scholar] [CrossRef]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin Inhibition in Heart Failure: Mechanisms and Substrates beyond Modulating Natriuretic Peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mariscal, C.; Carrera-Alvarado, G.; Mora, L.; Toldrá, F. Neprilysin (NEP) and Angiotensin Converting Enzyme-I (ACE-I) Inhibitory Dipeptides from Chicken Carcass Hydrolysates. LWT 2025, 221, 117591. [Google Scholar] [CrossRef]
- Carrera-Alvarado, G.; Toldrá, F.; Mora, L. Effect of Thermal Pretreatment and Gastrointestinal Digestion on the Bioactivity of Dry-Cured Ham Bone Enzymatic Hydrolyzates. Food Res. Int. 2024, 188, 114513. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldrá, F. Effect of Ultrasound and Enzymatic Pre-Treatments on the Profile of Bioactive Peptides of Beef Liver Hydrolysates. Food Res. Int. 2024, 197, 115240. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Health Relevance of Antihypertensive Peptides in Foods. Curr. Opin. Food Sci. 2018, 19, 8–14. [Google Scholar] [CrossRef]
- Berraquero-García, C.; Rivero-Pino, F.; Ospina, J.L.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Guadix, A.; García-Moreno, P.J.; Guadix, E.M. Activity, Structural Features and in Silico Digestion of Antidiabetic Peptides. Food Biosci. 2023, 55, 102954. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive Peptides from Germinated Soybean with Anti-Diabetic Potential by Inhibition of Dipeptidyl Peptidase-IV, α-Amylase, and α-Glucosidase Enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Ojeda-Montes, M.J.; Gimeno, A.; Tomas-Hernández, S.; Cereto-Massagué, A.; Beltrán-Debón, R.; Valls, C.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. Activity and Selectivity Cliffs for DPP-IV Inhibitors: Lessons We Can Learn from SAR Studies and Their Application to Virtual Screening. Med. Res. Rev. 2018, 38, 1874–1915. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-Originating ACE Inhibitors, Including Antihypertensive Peptides, as Preventive Food Components in Blood Pressure Reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Angiotensin-Converting Enzyme–Lisinopril Complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a Dipeptide Library to Identify Human Dipeptidyl Peptidase IV Inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.W.; Lee, Y.S.; Ahmad, S.A.; Ooi, P.T.; Phang, L.Y. Chicken Feather Valorization by Thermal Alkaline Pretreatment Followed by Enzymatic Hydrolysis for Protein-Rich Hydrolysate Production. Waste Manag. 2018, 79, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, P. (Ed.) AOAC International Official Method 992.15. In Official Methods of Analysis of the AOAC International; AOAC International: Gaithersburg, MD, USA, 1992. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Quero-Jiménez, P.C.; Montenegro, O.N.; Sosa, R.; De La Torre, J.B.; Valero Acosta, J.; López Pérez, D.; Santana Rodríguez, A.; Ramos Méndez, R.; Alonso, A.C.; Corrales, A.J.; et al. Total Carbohydrates Concentration Evaluation in Products of Microbial Origin. Afinidad 2019, 76, 587. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Effect of Cooking and in Vitro Digestion on the Antioxidant Activity of Dry-Cured Ham by-Products. Food Res. Int. 2017, 97, 296–306. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Toldrá, F. A Fluorescence-Based Protocol for Quantifying Angiotensin-Converting Enzyme Activity. Nat. Protoc. 2006, 1, 2423–2427. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]

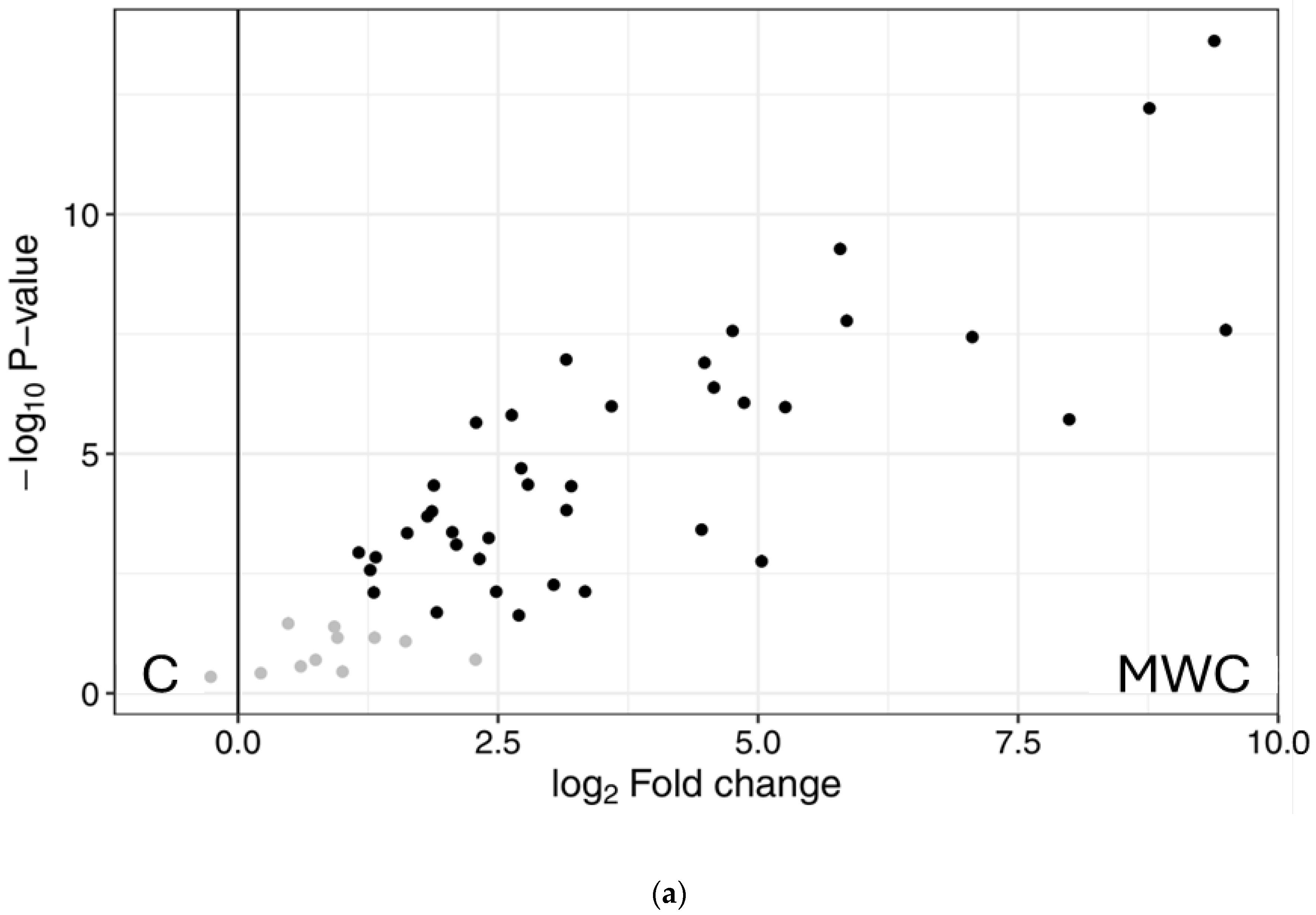
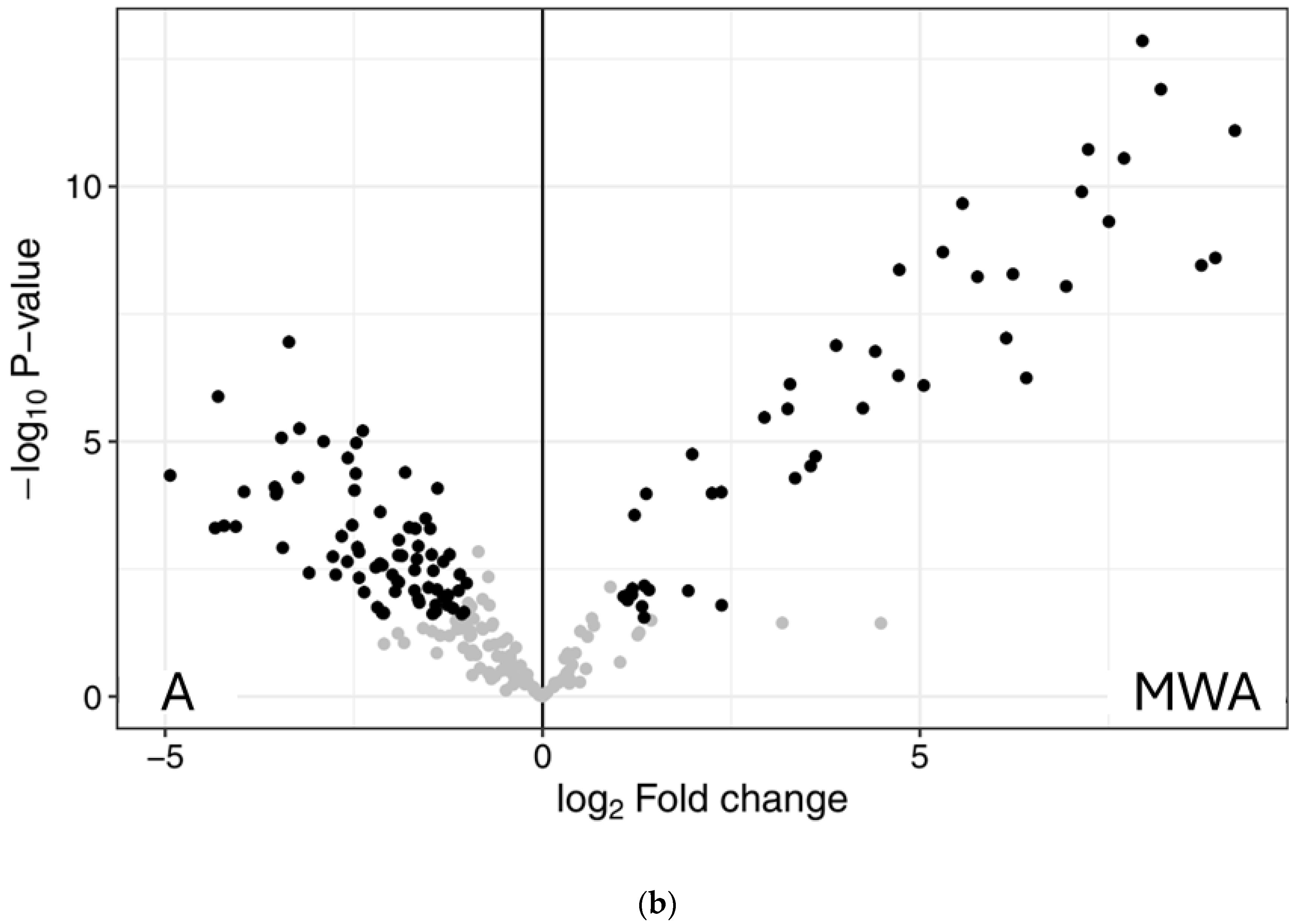
| Sample d | Peptide Sequence | Parent Protein | PRR a | Hydrophobicity | A b | Bioactivity c | Potential Bioactive Peptide Fragments c | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Antioxidant Peptides | ACE-I-Inhibitory Peptides | DPP-IV-Inhibitory Peptides | Neprilysin-Inhibitory Peptides | |||||||
| C | GLGGGLGGGLGGGLAGGSSGS | Keratin, type II cytoskeletal 5 (Positions 511–531) | 0.9778 | 0.17 | 0.9048 | ACE inhibitor | - | LA, GL, AG, GS, GG, SG, LG | LA, GL, AG, GG | GG |
| 0.6190 | DPP-IV inhibitor | |||||||||
| 0.3333 | NEP inhibitor | |||||||||
| GGGGFGGGGIGGGGFGGF | Keratin, type II cytoskeletal 1 (Positions 105–122) | 0.9777 | 0.27 | 1.0556 | ACE inhibitor | - | GF, IG, GI, FG, GG, FGG | GF, GG, GI | FG, GG | |
| 0.7778 | DPP-IV inhibitor | |||||||||
| 0.6667 | NEP inhibitor | |||||||||
| GGGGFGGGGFGGGGIGGGGF | Keratin, type II cytoskeletal 1 (Positions 100–119) | 0.9758 | 0.26 | 1.0500 | ACE inhibitor | - | GF, IG, GI, FG, GG | GF, GG, GI | FG, GG | |
| 0.8000 | DPP-IV inhibitor | |||||||||
| 0.7000 | NEP inhibitor | |||||||||
| GLGGGLGGGLGGSLAGGGSGS | Keratin, type II cytoskeletal 5 (Positions 513–533) | 0.9711 | 0.17 | 0.9048 | ACE inhibitor | - | LA, GL, AG, GS, GG, SG, LG | LA, SL, GL, AG, GG | GG | |
| 0.6190 | DPP-IV inhibitor | |||||||||
| 0.3333 | NEP inhibitor | |||||||||
| GGGYGGGGFGGGGFGGGGF | Keratin, type II cytoskeletal 1 (Positions 91–109) | 0.9711 | 0.22 | 1.1579 | ACE inhibitor | - | GGY, GY, YG, GF, FG, GG, YGG, FGG | GF, GG, GY, YG | FG, GG | |
| 0.8421 | DPP-IV inhibitor | |||||||||
| 0.6842 | NEP inhibitor | |||||||||
| A | PFWPGLFA | 60S acidic ribosomal protein P1 (Positions 41–48) | 0.9826 | 0.3 | 0.6250 | ACE inhibitor | - | LF, PGL, GL, PG, PW | FA, WP, GL, PF, PG | FW |
| 0.6250 | DPP-IV inhibitor | |||||||||
| 0.1250 | NEP inhibitor | |||||||||
| PPGPPGPPGPPSGGF | Collagen alpha-1(I) chain (Positions 1171–1185) | 0.9749 | 0.04 | 0.4000 | Antioxidative | GPP, GP | GP, GF, GG, SG, PG, GPP, PP | GP, PP, PPG, GF, GG, PG, PS | GG, GP | |
| 1.0667 | ACE inhibitor | |||||||||
| 1.0667 | DPP-IV inhibitor | |||||||||
| 0.2667 | NEP inhibitor | |||||||||
| GAAFMLGF | Vitellogenin-2 (Positions 1494–1501) | 0.9736 | 0.35 | 0.3750 | Antioxidative | GAA, LGF, GA | AF, AA, GF, GA, LG, LGF | GA, AA, AF, GF, ML | - | |
| 0.7500 | ACE inhibitor | |||||||||
| 0.6250 | DPP-IV inhibitor | |||||||||
| GGGFGGGFGGGF | Keratin, type I cytoskeletal 9 (Positions 111–122) | 0.9706 | 0.27 | 1.0833 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.7500 | DPP-IV inhibitor | |||||||||
| 0.6667 | NEP inhibitor | |||||||||
| MWC | QFGFFLPL | Ephrin type-A receptor 6 (Positions 12–19) | 0.9845 | 0.29 | 0.2500 | Antioxidative | LPL, FGF | PL, FFL, GF, FG, LPL, FF, LP, FGF | LP, FL, LPL, PL, GF, QF | FG |
| 1.0000 | ACE inhibitor | |||||||||
| 0.8750 | DPP-IV inhibitor | |||||||||
| 0.1250 | NEP inhibitor | |||||||||
| FGGGGFGGGGFGGGFGG | Keratin, type I cytoskeletal 10 (Positions 117–133) | 0.9726 | 0.27 | 1.1765 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.7059 | DPP-IV inhibitor | |||||||||
| 0.7647 | NEP inhibitor | |||||||||
| MWA | GGLGGGLGGGLGGGLGGGLGGG | Keratin, type II cytoskeletal 5 (Positions 511–532) | 0.9947 | 0.24 | 0.9545 | ACE inhibitor | - | GL, GG, LG | GL, GG | GG |
| 0.7273 | DPP-IV inhibitor | |||||||||
| 0.5000 | NEP inhibitor | |||||||||
| GSGSGYGGGLGGGLGGGLGGG | Keratin, type II cytoskeletal 5 (Positions 503–523) | 0.9863 | 0.17 | 1.0000 | ACE inhibitor | - | GY, YG, GL, GS, GG, SG, LG, YGG | GL, GG, GY, YG | GG | |
| 0.6190 | DPP-IV inhibitor | |||||||||
| 0.3810 | NEP inhibitor | |||||||||
| GGGSFGGGGFGGGGFGGGF | Keratin, type I cytoskeletal 10 (Positions 113–131) | 0.9813 | 0.23 | 1.1053 | ACE inhibitor | - | GF, FG, GS, GG, SF, FGG | GF, GG, SF | FG, GG | |
| 0.7368 | DPP-IV inhibitor | |||||||||
| 0.6842 | NEP inhibitor | |||||||||
| GRCCLEGPFWHFL | Spatacsin (Positions 76–88) | 0.9793 | 0.02 | 0.3077 | ACE inhibitor | - | GP, GR, EG, FW | GP, FL, EG, HF, PF, WH, GPF | FW, GP | |
| 0.5385 | DPP-IV inhibitor | |||||||||
| 0.1538 | NEP inhibitor | |||||||||
| FFYPLDF | Peroxiredoxin-2 (Positions 41–47) | 0.9768 | 0.23 | 0.1429 | Antioxidative | FY | FY, YP, PL, DF, FF | YP, PL, FF | - | |
| 0.7143 | ACE inhibitor | |||||||||
| 0.4286 | DPP-IV inhibitor | |||||||||
| GGFGGGGFGGGGFGGGGF | Keratin, type II cytoskeletal 1 (Positions 117–134/122–139) | 0.9762 | 0.26 | 1.1111 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.7778 | DPP-IV inhibitor | |||||||||
| 0.7222 | NEP inhibitor | |||||||||
| GGGGFGGGGFGGGGIGGGGF | Keratin, type II cytoskeletal 1 (Positions 100–119) | 0.9758 | 0.26 | 1.0500 | ACE inhibitor | - | GF, IG, GI, FG, GG, FGG | GF, GG, GI | FG, GG | |
| 0.8000 | DPP-IV inhibitor | |||||||||
| 0.7000 | NEP inhibitor | |||||||||
| NFDMPFIF | Protein-glutamine gamma-glutamyltransferase E (Positions 383–390) | 0.9755 | 0.17 | 0.3750 | ACE inhibitor | - | IF, NF, DM | MP, NF, PF, MPF | - | |
| 0.5000 | DPP-IV inhibitor | |||||||||
| GSGFGGFGGF | Keratin, type I cytoskeletal 9 (Positions 128–137) | 0.9743 | 0.25 | 1.1000 | ACE inhibitor | - | GF, FG, GS, GG, SG, FGG | GF, GG | FG, GG | |
| 0.5000 | DPP-IV inhibitor | |||||||||
| 0.4000 | NEP inhibitor | |||||||||
| GGGGFGGGGFGGGF | Keratin, type I cytoskeletal 10 (Positions 118–131) | 0.9741 | 0.26 | 1.0714 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.7857 | DPP-IV inhibitor | |||||||||
| 0.7143 | NEP inhibitor | |||||||||
| GGGGFGGGGFGGGGF | Keratin, type II cytoskeletal 1 (Positions 95–109) | 0.9729 | 0.25 | 1.0667 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.8000 | DPP-IV inhibitor | |||||||||
| 0.7333 | NEP inhibitor | |||||||||
| GGLGSGLGQSLGQVGGSLASLTGQIS | Thyroid receptor-interacting protein 11 (Positions 6–31) | 0.9720 | 0.08 | 0.0385 | Antioxidative | LT | LA, VG, GL, GS, GQ, GG, SG, LG, TG, ASL | LA, SL, GL, AS, GG, LT, QI, QS, QV, TG, VG | GG | |
| 0.6538 | ACE inhibitor | |||||||||
| 0.5769 | DPP-IV inhibitor | |||||||||
| 0.0769 | NEP inhibitor | |||||||||
| GGGSFGGGSFGGGGFGGGGF | Keratin, type I cytoskeletal 10 (Positions 108–127) | 0.9719 | 0.21 | 1.1000 | ACE inhibitor | - | GF, FG, GS, GG, SF, FGG | GF, GG, SF | FG, GG | |
| 0.7000 | DPP-IV inhibitor | |||||||||
| 0.6500 | NEP inhibitor | |||||||||
| GSGFGGGSGFGGGGFGGGGF | Keratin, type II cytoskeletal 2 epidermal (Positions 97–116) | 0.9719 | 0.21 | 1.1000 | ACE inhibitor | - | GF, FG, GS, GG, SG, FGG | GF, GG | FG, GG | |
| 0.6000 | DPP-IV inhibitor | |||||||||
| 0.5500 | NEP inhibitor | |||||||||
| GGSGFGGGGFGGGGF | Keratin, type II cytoskeletal 2 epidermal (Positions 102–116/118–132) | 0.9718 | 0.22 | 1.0667 | ACE inhibitor | - | GF, FG, GS, GG, SG, FGG | GF, GG | FG, GG | |
| 0.6667 | DPP-IV inhibitor | |||||||||
| 0.6000 | NEP inhibitor | |||||||||
| GGGYGGGGFGGGGFGGGGF | Keratin, type II cytoskeletal 1 (Positions 91–109) | 0.9711 | 0.22 | 1.1579 | ACE inhibitor | - | GGY, GY, YG, GF, FG, GG, YGG, FGG | GF, GG, GY, YG | FG, GG | |
| 0.8421 | DPP-IV inhibitor | |||||||||
| 0.6842 | NEP inhibitor | |||||||||
| GGGFGGGGFGGGGFG | Keratin, type II cytoskeletal 1 (Positions 114–128) | 0.971 | 0.25 | 1.0667 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.7333 | DPP-IV inhibitor | |||||||||
| 0.7333 | NEP inhibitor | |||||||||
| SPGGFGPGGF | Keratin, type II cytoskeletal 3 (Positions157–166) | 0.9707 | 0.16 | 0.1000 | Antioxidative | GP | GP, GF, FG, GG, PG | GP, SP, GF, GG, PG | FG, GG, GP | |
| 0.8000 | ACE inhibitor | |||||||||
| 0.8000 | DPP-IV inhibitor | |||||||||
| 0.4000 | NEP inhibitor | |||||||||
| GGGGFGGGGFGGGFG | Keratin, type I cytoskeletal 10 (Positions 118–132) | 0.9703 | 0.25 | 1.0667 | ACE inhibitor | - | GF, FG, GG, FGG | GF, GG | FG, GG | |
| 0.7333 | DPP-IV inhibitor | |||||||||
| 0.7333 | NEP inhibitor | |||||||||
| GGGSFGGGGFGGGGF | Keratin, type I cytoskeletal 10 (Positions 113–127) | 0.97 | 0.22 | 1.0667 | ACE inhibitor | - | GF, FG, GS, GG, SF, FGG | GF, GG, SF | FG, GG | |
| 0.7333 | DPP-IV inhibitor | |||||||||
| 0.6667 | NEP inhibitor | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torices-Hernández, Á.; Gallego, M.; Mora, L.; Toldrá, F. Microwave Irradiation Pre-Treatment as a Sustainable Method to Obtain Bioactive Hydrolysates from Chicken Feathers. Int. J. Mol. Sci. 2025, 26, 6344. https://doi.org/10.3390/ijms26136344
Torices-Hernández Á, Gallego M, Mora L, Toldrá F. Microwave Irradiation Pre-Treatment as a Sustainable Method to Obtain Bioactive Hydrolysates from Chicken Feathers. International Journal of Molecular Sciences. 2025; 26(13):6344. https://doi.org/10.3390/ijms26136344
Chicago/Turabian StyleTorices-Hernández, Álvaro, Marta Gallego, Leticia Mora, and Fidel Toldrá. 2025. "Microwave Irradiation Pre-Treatment as a Sustainable Method to Obtain Bioactive Hydrolysates from Chicken Feathers" International Journal of Molecular Sciences 26, no. 13: 6344. https://doi.org/10.3390/ijms26136344
APA StyleTorices-Hernández, Á., Gallego, M., Mora, L., & Toldrá, F. (2025). Microwave Irradiation Pre-Treatment as a Sustainable Method to Obtain Bioactive Hydrolysates from Chicken Feathers. International Journal of Molecular Sciences, 26(13), 6344. https://doi.org/10.3390/ijms26136344










