Oxidative Stress and Low-Grade Endotoxemia in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Insights from a Cross-Sectional Study
Abstract
1. Introduction
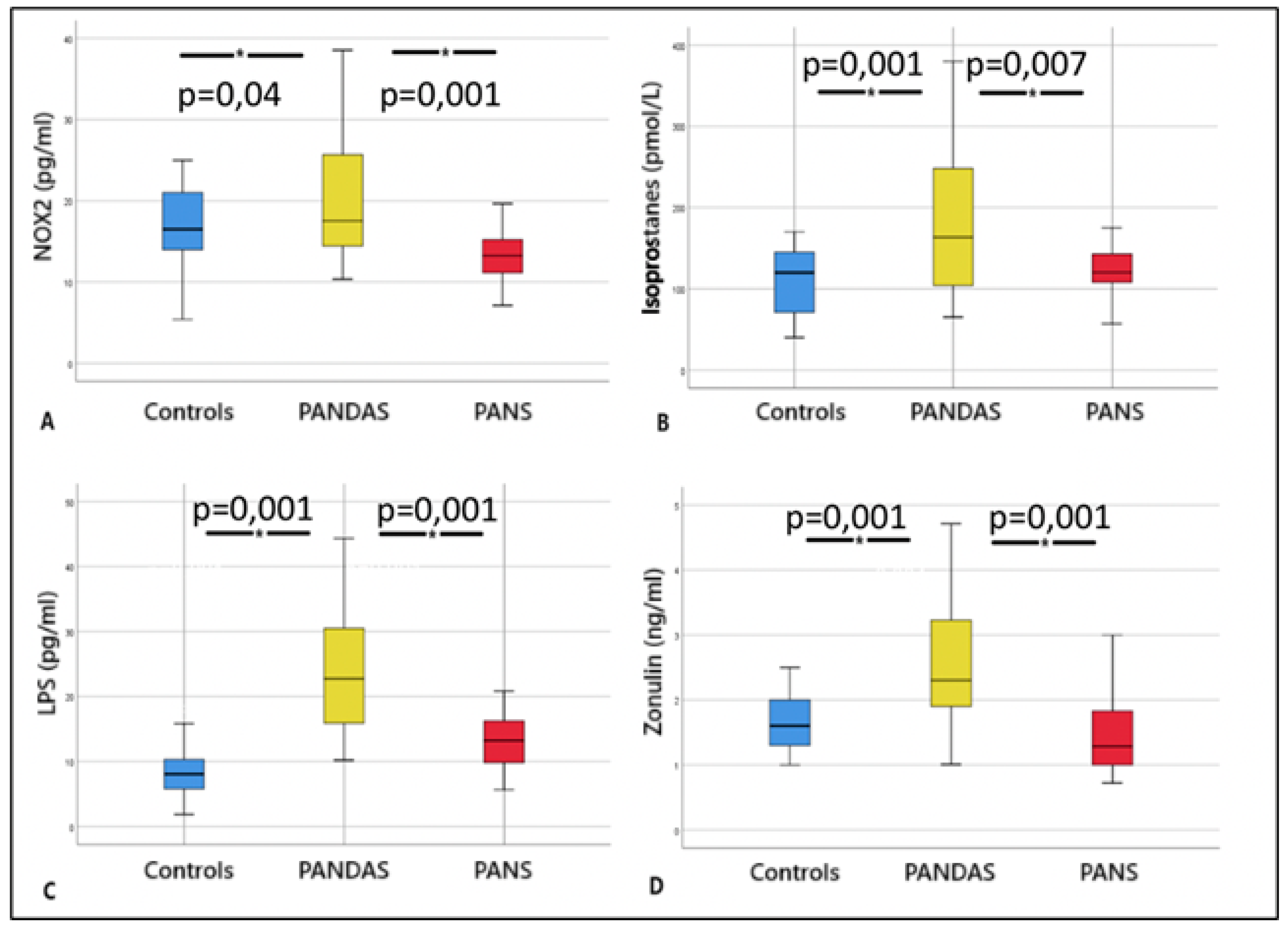
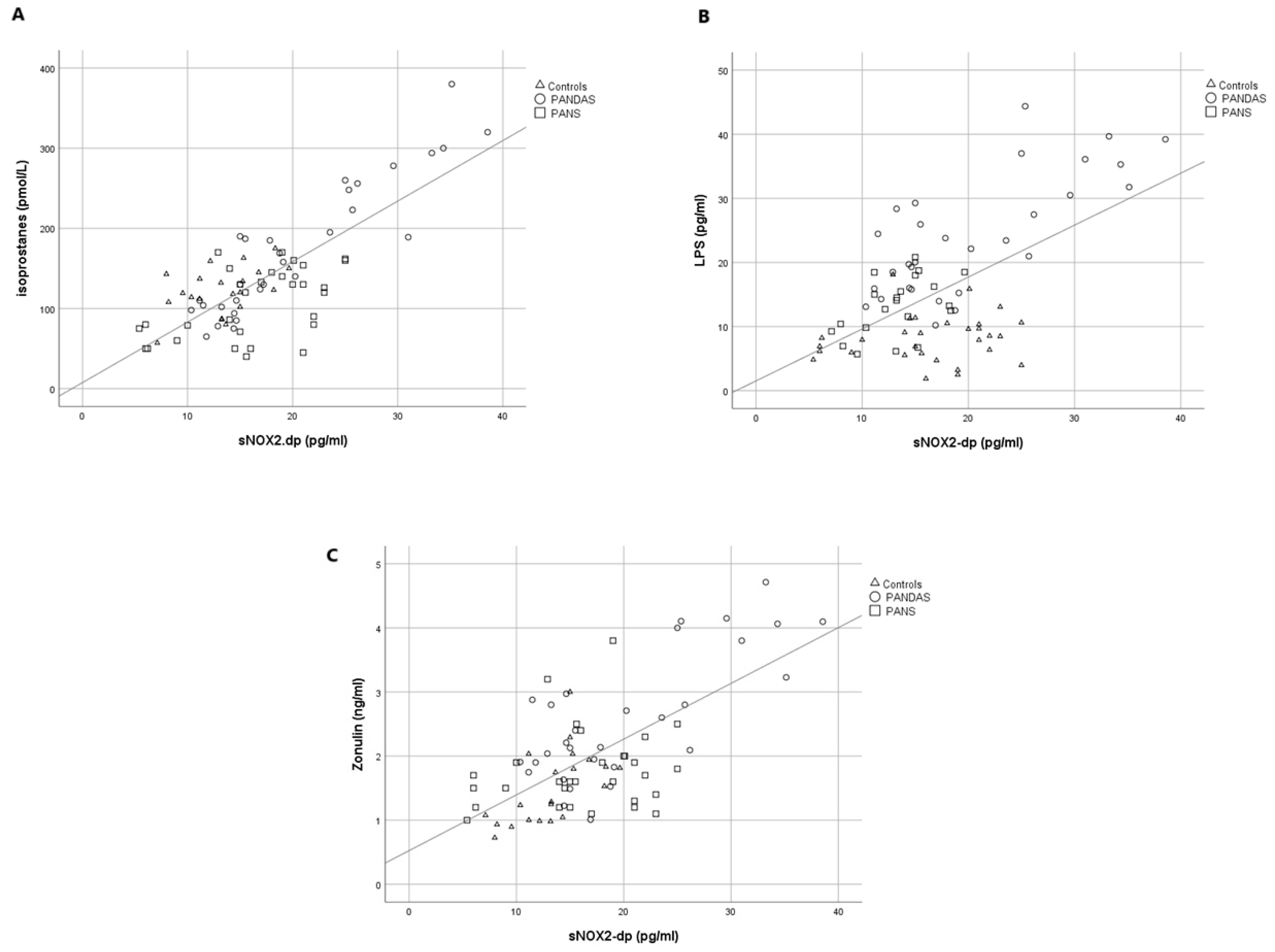
2. Results
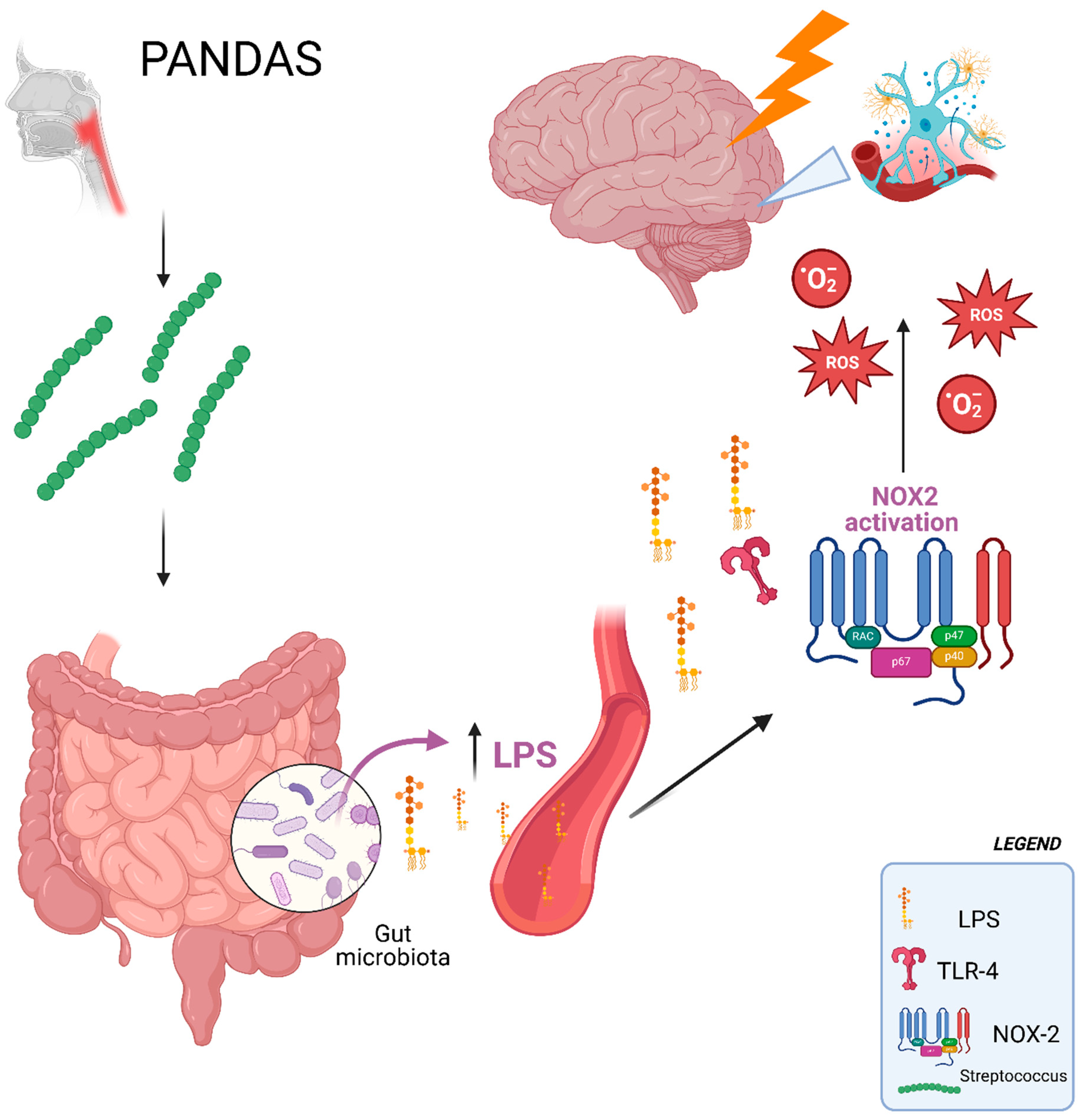
3. Discussion
3.1. Limitations
3.2. Clinical Implications
4. Methods
- -
- Clinical diagnosis of Obsessive–Compulsive Disorder (OCD) and/or tic disorders.
- -
- Symptom onset occurring between the age of 3 and the onset of puberty.
- -
- Disease presenting in a relapsing–remitting pattern.
- -
- Symptom exacerbations temporally related to group A streptococcal (GAS) infections.
- -
- Presence of neurological features such as choreiform movements or motor hyperactivity during flare-ups.
- -
- Sydenham’s chorea
- -
- Tourette syndrome
- -
- Autoimmune encephalitis
- -
- Systemic autoimmune diseases
- -
- Wilson’s disease
- -
- Congenital cardiac anomalies
- -
- Chronic kidney disease
- -
- Malignancies
- -
- Hepatic failure
- -
- Acute illnesses
- -
- Any prior treatment with immunosuppressants
- -
- Diabetes
- -
- Dyslipidemia
- -
- Hypertension
- -
- Use of antibiotics, probiotics, or antioxidant therapies within the 4 weeks prior to enrollment in the study.
4.1. Blood Collection
4.2. Measurement of sNOX2-Dp
4.3. Quantification of 8-Iso-Prostaglandin F2α
4.4. Zonulin Detection
4.5. Lipopolysaccharide (LPS) Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandya, D.; Johnson, T.P. Chronic and delayed neurological manifestations of persistent infections. Curr. Opin. Neurol. 2023, 36, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Snider, L.A.; Swedo, S.E. Post-streptococcal autoimmune disorders of the central nervous system. Curr. Opin. Neurol. 2003, 16, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, Y.; Miyata, R.; Tanuma, N.; Hongou, K.; Tanaka, K.; Shimoda, K.; Kanda, S.; Hoshino, A.; Hanafusa, Y.; Kumada, S.; et al. Autoimmune neurological disorders associated with group-A beta-hemolytic streptococcal infection. Brain Dev. 2013, 35, 670–674. [Google Scholar] [CrossRef]
- Leonardi, L.; Lorenzetti, G.; Carsetti, R.; Piano Mortari, E.; Guido, C.A.; Zicari, A.M.; Forster-Waldl, E.; Loffredo, L.; Duse, M.; Spalice, A. Immunological characterization of an Italian PANDAS cohort. Front. Pediatr. 2023, 11, 1216282. [Google Scholar] [CrossRef]
- Dale, R.C. Post-streptococcal autoimmune disorders of the central nervous system. Dev. Med. Child Neurol. 2005, 47, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, L.; Perna, C.; Bernabei, I.; Fiore, M.; Ma, M.; Frankovich, J.; Tarani, L.; Spalice, A. Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities. Children 2024, 11, 1043. [Google Scholar] [CrossRef]
- Loffredo, L.; Ivanov, V.; Ciobanu, N.; Deseatnicova, E.; Gutu, E.; Mudrea, L.; Ivanov, M.; Nocella, C.; Cammisotto, V.; Orlando, F.; et al. Is There an Association Between Atherosclerotic Burden, Oxidative Stress, and Gut-Derived Lipopolysaccharides? Antioxid. Redox Signal. 2020, 33, 761–766. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Loffredo, L.; Ettorre, E.; Zicari, A.M.; Inghilleri, M.; Nocella, C.; Perri, L.; Spalice, A.; Fossati, C.; De Lucia, M.C.; Pigozzi, F.; et al. Oxidative Stress and Gut-Derived Lipopolysaccharides in Neurodegenerative Disease: Role of NOX2. Oxid. Med. Cell. Longev. 2020, 2020, 8630275. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Tu, D.; Velagapudi, R.; Gao, Y.; Hong, J.S.; Zhou, H.; Gao, H.M. Activation of neuronal NADPH oxidase NOX2 promotes inflammatory neurodegeneration. Free Radic. Biol. Med. 2023, 200, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, C.; Magna, A.; Mereu, E.; Bernardini, S.; Bartimoccia, S.; Marti, R.; Lazzerini, P.E.; D’Amico, A.; Ettorre, E.; Desideri, G.; et al. Impact of Hospitalization on Sarcopenia, NADPH-Oxidase 2, Oxidative Stress, and Low-Grade Endotoxemia in Elderly Patients. Antioxidants 2025, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, A.; Del Chierico, F.; Russo, A.; Reddel, S.; Conte, G.; Lopetuso, L.R.; Ianiro, G.; Dallapiccola, B.; Cardona, F.; Gasbarrini, A.; et al. Gut Microbiota Profiling and Gut-Brain Crosstalk in Children Affected by Pediatric Acute-Onset Neuropsychiatric Syndrome and Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections. Front. Microbiol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Loffredo, L.; Spalice, A.; Salvatori, F.; De Castro, G.; Guido, C.A.; Zicari, A.M.; Ciacci, P.; Battaglia, S.; Brindisi, G.; Ettorre, E.; et al. Oxidative stress and gut-derived lipopolysaccharides in children affected by paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. BMC Pediatr. 2020, 20, 127. [Google Scholar] [CrossRef]
- Sarb, O.F.; Sarb, A.D.; Iacobescu, M.; Vlad, I.M.; Milaciu, M.V.; Ciurmarnean, L.; Vacaras, V.; Tantau, A.I. From Gut to Brain: Uncovering Potential Serum Biomarkers Connecting Inflammatory Bowel Diseases to Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 5676. [Google Scholar] [CrossRef] [PubMed]
- Mittli, D.; Tukacs, V.; Ravasz, L.; Csosz, E.; Kozma, T.; Kardos, J.; Juhasz, G.; Kekesi, K.A. LPS-induced acute neuroinflammation, involving interleukin-1 beta signaling, leads to proteomic, cellular, and network-level changes in the prefrontal cortex of mice. Brain Behav. Immun. Health 2023, 28, 100594. [Google Scholar] [CrossRef]
- Zarimeidani, F.; Rahmati, R.; Mostafavi, M.; Darvishi, M.; Khodadadi, S.; Mohammadi, M.; Shamlou, F.; Bakhtiyari, S.; Alipourfard, I. Gut Microbiota and Autism Spectrum Disorder: A Neuroinflammatory Mediated Mechanism of Pathogenesis? Inflammation 2024, 48, 501–519. [Google Scholar] [CrossRef]
- D’Eufemia, P.; Celli, M.; Finocchiaro, R.; Pacifico, L.; Viozzi, L.; Zaccagnini, M.; Cardi, E.; Giardini, O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996, 85, 1076–1079. [Google Scholar] [CrossRef]
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tildon, J.T. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 1999, 135, 559–563. [Google Scholar] [CrossRef]
- de Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Li, F.; Ke, H.; Wang, S.; Mao, W.; Fu, C.; Chen, X.; Fu, Q.; Qin, X.; Huang, Y.; Li, B.; et al. Leaky Gut Plays a Critical Role in the Pathophysiology of Autism in Mice by Activating the Lipopolysaccharide-Mediated Toll-Like Receptor 4-Myeloid Differentiation Factor 88-Nuclear Factor Kappa B Signaling Pathway. Neurosci. Bull. 2023, 39, 911–928. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Hager-Mair, F.F.; Andrukhov, O.; Schaffer, C. Oral streptococci: Modulators of health and disease. Front. Cell. Infect. Microbiol. 2024, 14, 1357631. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.H.; Randis, T.M.; Desai, P.V.; Sapra, K.J.; Ma, B.; Gajer, P.; Humphrys, M.S.; Ravel, J.; Gelber, S.E.; Ratner, A.J. Group B Streptococcus and the Vaginal Microbiota. J. Infect. Dis. 2017, 216, 744–751. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Scheffler, L.; Crane, A.; Heyne, H.; Tonjes, A.; Schleinitz, D.; Ihling, C.H.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A.; et al. Widely Used Commercial ELISA Does Not Detect Precursor of Haptoglobin2, but Recognizes Properdin as a Potential Second Member of the Zonulin Family. Front. Endocrinol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Loffredo, L.; Alfano, A.R.; Ettorre, E.; Desideri, G.; Carnevale, R.; Forte, M.; Maglione, V.; Bartimoccia, S.; Castellani, V.; Tote, C.M.; et al. Effect of the probiotic Escherichia coli Nissle 1917 on serum levels of NADPH oxidase-2 and lipopolysaccharide in patients with Alzheimer’s disease. J. Alzheimers Dis. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, A.; Baratta, F.; Maggio, E.; Palumbo, I.M.; Magna, A.; Trivigno, C.; Carnevale, R.; Simona, B.; Cammisotto, V.; Vidili, G.; et al. Dark chocolate’s impact on low-grade endotoxemia in metabolic dysfunction-associated steatohepatitis. Nutrition 2024, 131, 112643. [Google Scholar] [CrossRef]
- Swedo, S.E.; Leonard, H.L.; Garvey, M.; Mittleman, B.; Allen, A.J.; Perlmutter, S.; Lougee, L.; Dow, S.; Zamkoff, J.; Dubbert, B.K. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am. J. Psychiatry 1998, 155, 264–271. [Google Scholar] [CrossRef]
- Swedo, S.E.; Leckman, J.F.; Rose, N.R. From Research Subgroup to Clinical Syndrome: Modifying the PANDAS Criteria to Describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr. Ther. 2012, 2, 113. [Google Scholar] [CrossRef]
- Swedo, S.E.; Frankovich, J.; Murphy, T.K. Overview of Treatment of Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Child Adolesc. Psychopharmacol. 2017, 27, 562–565. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Green, S.B. How Many Subjects Does It Take To Do A Regression Analysis. Multivar. Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
| PANDAS (n = 30) | PANS (n = 21) | Controls (n = 30) | |
|---|---|---|---|
| Age | 9 ± 3 | 9 ± 3 | 9 ± 3 |
| Gender (male/female) | 24/6 | 15/6 | 24/6 |
| Glycaemia (mg/dL) | 83 ± 3.75 | 85 ± 3.6 | 87 ± 3 |
| Systolic blood pressure (mmHg) | 110 ± 3 | 109 ± 3 | 112 ± 3 |
| Diastolic blood pressure (mmHg) | 67 ± 2.55 | 68 ± 2 | 70 ± 2 |
| BMI | 18 ± 2 | 18 ± 2 | 17 ± 1 |
| Tic disorders (presence/absence) | 25/5 | 19/2 | 0 |
| OCD (presence/absence) | 10/20 | 11/10 | 0 |
| Anti-streptolysinic titer (UI/mL) | 409 ± 262 | 264 ± 21° | 0 |
| LPS (pg/mL) | 24.1 ± 9.2 | 13.1 ± 4.5° | 8.1 ± 3.6° |
| NOX2 (pg/mL) | 20.4 ± 8.1 | 13.2 ± 4.8° | 16.3 ± 5.7° |
| Zonulin (ng/mL) | 2.6 ± 1 | 1.5 ± 0.6° | 1.7 ± 0.6° |
| Isoprostanes (pmol/L) | 175 ± 84 | 122 ± 30° | 106 ± 43° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Totè, C.M.; Capponi, M.; Salvatori, F.; Zicari, A.M.; Guido, C.A.; Brindisi, G.; Iantorno, L.; Bartimoccia, S.; Baratta, F.; Forte, M.; et al. Oxidative Stress and Low-Grade Endotoxemia in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Insights from a Cross-Sectional Study. Int. J. Mol. Sci. 2025, 26, 6336. https://doi.org/10.3390/ijms26136336
Totè CM, Capponi M, Salvatori F, Zicari AM, Guido CA, Brindisi G, Iantorno L, Bartimoccia S, Baratta F, Forte M, et al. Oxidative Stress and Low-Grade Endotoxemia in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Insights from a Cross-Sectional Study. International Journal of Molecular Sciences. 2025; 26(13):6336. https://doi.org/10.3390/ijms26136336
Chicago/Turabian StyleTotè, Chiara Maria, Martina Capponi, Francesca Salvatori, Anna Maria Zicari, Cristiana Alessia Guido, Giulia Brindisi, Laura Iantorno, Simona Bartimoccia, Francesco Baratta, Maurizio Forte, and et al. 2025. "Oxidative Stress and Low-Grade Endotoxemia in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Insights from a Cross-Sectional Study" International Journal of Molecular Sciences 26, no. 13: 6336. https://doi.org/10.3390/ijms26136336
APA StyleTotè, C. M., Capponi, M., Salvatori, F., Zicari, A. M., Guido, C. A., Brindisi, G., Iantorno, L., Bartimoccia, S., Baratta, F., Forte, M., Picchio, V., Malvasi, M., Aloisio, S., Pacella, E., Pignatelli, P., Violi, F., Carnevale, R., Spalice, A., & Loffredo, L. (2025). Oxidative Stress and Low-Grade Endotoxemia in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Insights from a Cross-Sectional Study. International Journal of Molecular Sciences, 26(13), 6336. https://doi.org/10.3390/ijms26136336










