Diammonium Glycyrrhizinate Exerts Broad-Spectrum Antiviral Activity Against Human Coronaviruses by Interrupting Spike-Mediated Cellular Entry
Abstract
1. Introduction
2. Results
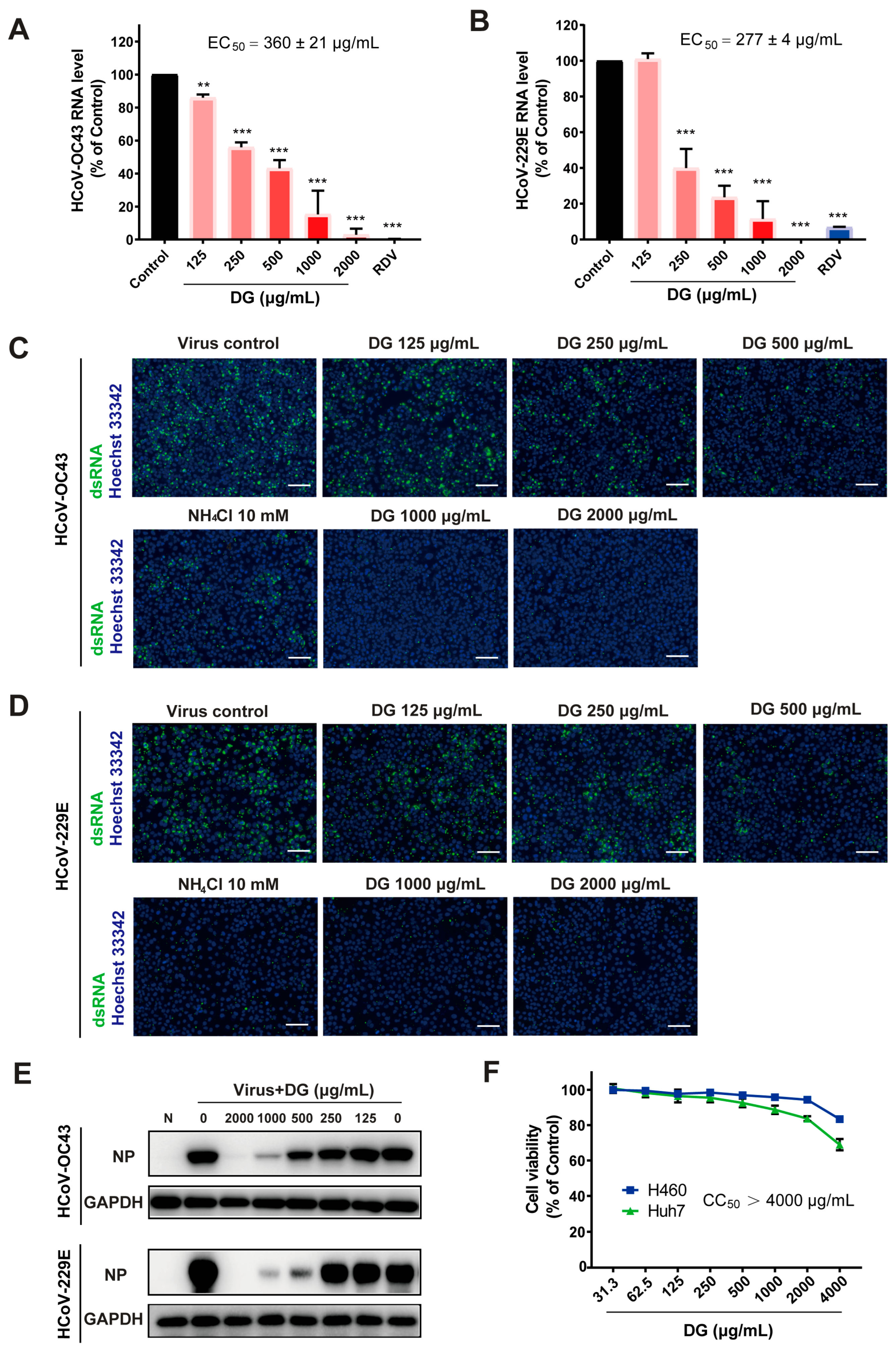
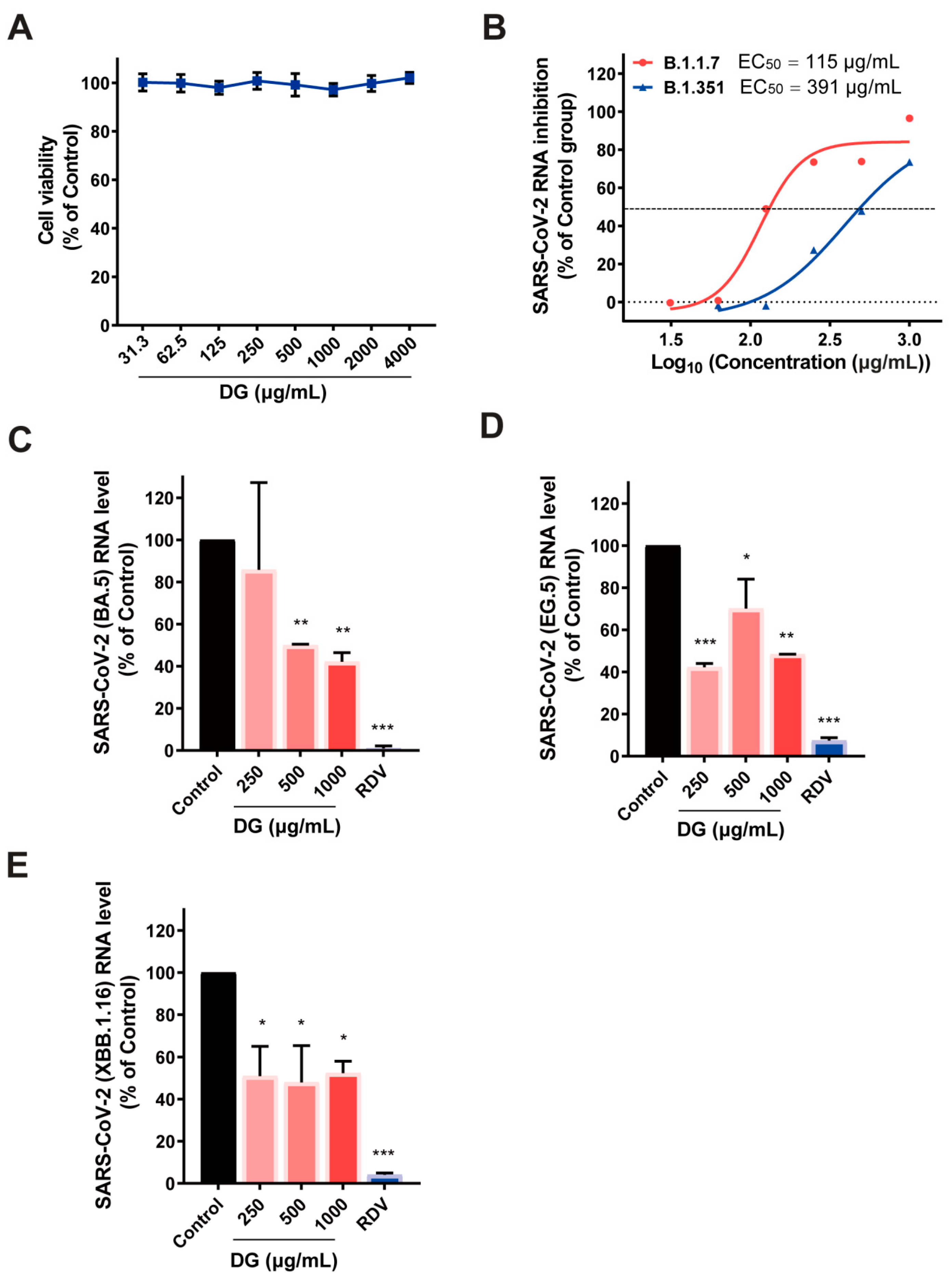
2.1. Diammonium Glycyrrhizinate Potently Inhibits Replication of Multiple Coronaviruses
2.2. Diammonium Glycyrrhizinate Exhibits Broad-Spectrum Interrupting Activity on Spike-Mediated Cellular Entry of HCoVs
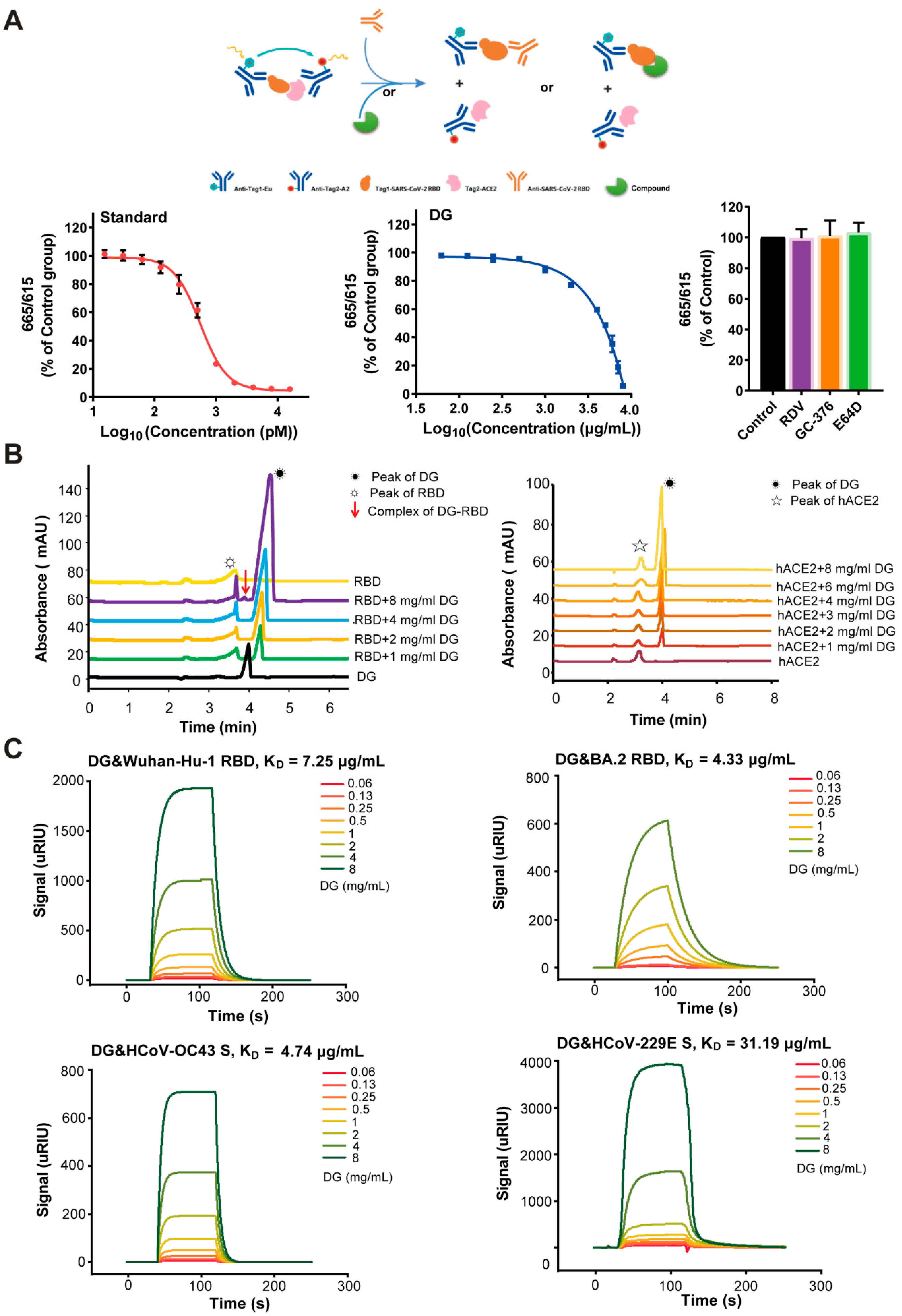
2.3. Diammonium Glycyrrhizinate Specifically Binds to the RBD Fragment to Block Interaction Between the SARS-CoV-2 Spike and Human ACE2
2.4. Molecular Docking Analysis Determines the Binding Sites of DG and HCoV RBD Complexes
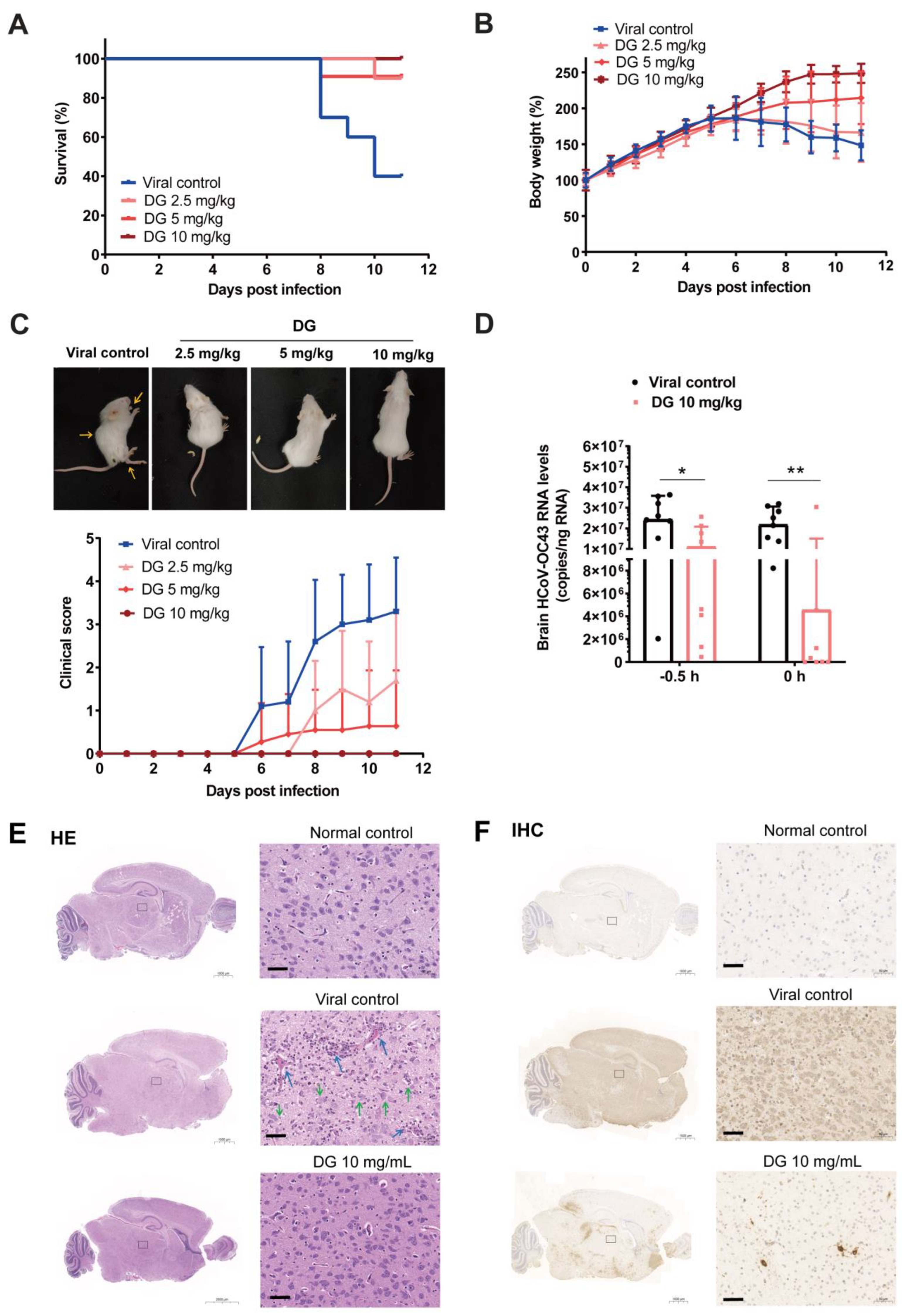
2.5. Intranasal Administration of Diammonium Glycyrrhizinate Significantly Protects Mice from HCoV-OC43 Infection
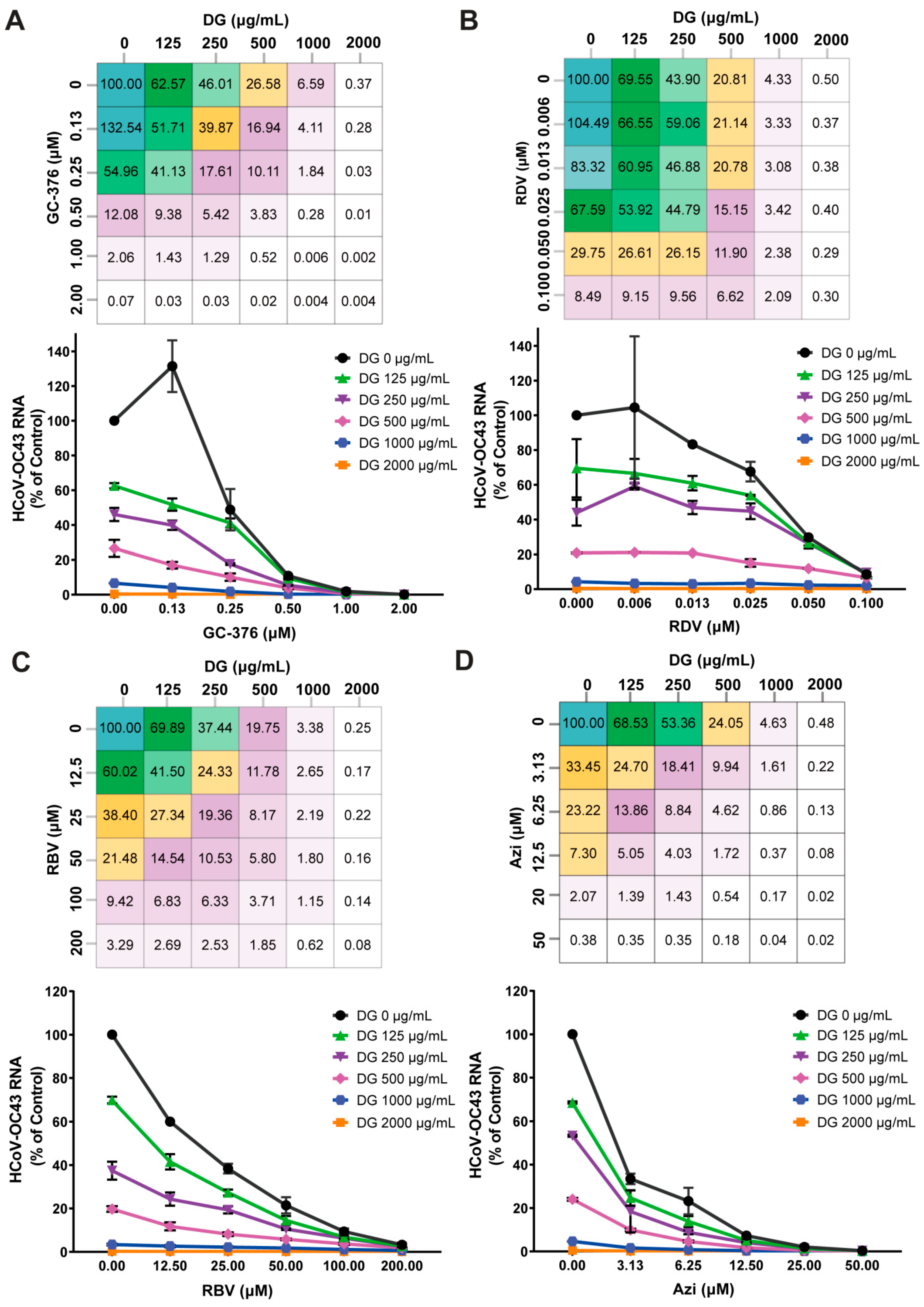
2.6. Combinations of Diammonium Glycyrrhizinate and Other HCoV Inhibitors Produce Synergistic Antiviral Effects
3. Discussion
4. Materials and Methods
4.1. Cells and Viruses
4.2. Compounds and Plasmids
4.3. Cell Cytotoxicity Assay
4.4. Antiviral Activity Assay
4.5. RNA Extraction and qRT-PCR Assay
4.6. Immunofluorescence Assay
4.7. Western Blot Assays
4.8. Time-of-Addition Assay
4.9. Attachment and Internalization Assays
4.10. Pseudovirus Infection and Luciferase Assay
4.11. Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET)-Based SARS-CoV-2 S-RBD-ACE2 Interaction Assay
4.12. Capillary Electrophoresis (CE)
4.13. Surface Plasmon Resonance (SPR) Assay
4.14. Molecular Docking
4.15. Animal Experiments
4.16. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brian, D.A.; Baric, R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005, 287, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Gunther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Q.; Zhang, R.; Aw, Z.Q.; Chen, P.; Wong, Y.H.; Hong, J.; Ju, B.; Shi, X.; Ding, Q.; et al. SARS-CoV-2 Omicron Variants Reduce Antibody Neutralization and Acquire Usage of Mouse ACE2. Front. Immunol. 2022, 13, 854952. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2022, 23, 189–199. [Google Scholar] [CrossRef]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef]
- Ao, D.; Lan, T.; He, X.; Liu, J.; Chen, L.; Baptista-Hon, D.T.; Zhang, K.; Wei, X. SARS-CoV-2 Omicron variant: Immune escape and vaccine development. MedComm 2022, 3, e126. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Li, J.Y.; Cao, H.Y.; Liu, P.; Cheng, G.H.; Sun, M.Y. Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomed. Res. Int. 2014, 2014, 872139. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural products in licorice for the therapy of liver diseases: Progress and future opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Arase, Y.; Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Saitoh, S.; Kobayashi, M.; Kumada, H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79, 1494–1500. [Google Scholar] [CrossRef]
- Sun, Z.G.; Zhao, T.T.; Lu, N.; Yang, Y.A.; Zhu, H.L. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mini. Rev. Med. Chem. 2019, 19, 826–832. [Google Scholar] [CrossRef]
- Garcia-Salazar, G.; Urban-Morlan, Z.; Mendoza-Elvira, S.; Quintanar-Guerrero, D.; Mendoza, S. Broad Antiviral Spectrum of Glycyrrhizic Acid for Human and Veterinary Medicine: Reality or Fiction? Intervirology 2023, 66, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Meng, T.; Wang, Y.; Tang, W. A Review of the Antiviral Activities of Glycyrrhizic Acid, Glycyrrhetinic Acid and Glycyrrhetinic Acid Monoglucuronide. Pharmaceuticals 2023, 16, 641. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef]
- Li, J.; Xu, D.; Wang, L.; Zhang, M.; Zhang, G.; Li, E.; He, S. Glycyrrhizic Acid Inhibits SARS-CoV-2 Infection by Blocking Spike Protein-Mediated Cell Attachment. Molecules 2021, 26, 6090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, W.; Li, M. Diammonium Glycyrrhizinate Preparation for Liver Function Recovery in Chronic Hepatitis B in China: A Meta-analysis with Trial Sequential Analysis. Curr. Pharm. Des. 2022, 28, 2089–2112. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Yeo, K.L.; Chen, Y.L.; Xu, H.Y.; Dong, H.; Wang, Q.Y.; Yokokawa, F.; Shi, P.Y. Synergistic suppression of dengue virus replication using a combination of nucleoside analogs and nucleoside synthesis inhibitors. Antimicrob. Agents Chemother. 2015, 59, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lin, B.; Chu, V.; Hu, Z.; Hu, X.; Xiao, J.; Wang, A.Q.; Schweitzer, C.J.; Li, Q.; Imamura, M.; et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 2015, 7, 282ra249. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O.; et al. Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 2021, 13, 609. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Jittapiromsak, P.; Wu, A.; Deshmukh, P.; Feiz-Erfan, I.; Nakaji, P.; Spetzler, R.F.; Preul, M.C. Comparative analysis of extensions of transbasal approaches: Effect on access to midline and paramedian structures. Skull Base 2009, 19, 387–399. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, S.; Zhu, M.; He, W.; Pan, Z.; Su, S. Antiviral Effect of Lithium Chloride and Diammonium Glycyrrhizinate on Porcine Deltacoronavirus In Vitro. Pathogens 2019, 8, 144. [Google Scholar] [CrossRef]
- Xiang, R.; Yu, Z.; Wang, Y.; Wang, L.; Huo, S.; Li, Y.; Liang, R.; Hao, Q.; Ying, T.; Gao, Y.; et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm. Sin. B 2022, 12, 1591–1623. [Google Scholar] [CrossRef]
- Kalhor, H.; Sadeghi, S.; Abolhasani, H.; Kalhor, R.; Rahimi, H. Repurposing of the approved small molecule drugs in order to inhibit SARS-CoV-2 S protein and human ACE2 interaction through virtual screening approaches. J. Biomol. Struct. Dyn. 2022, 40, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Prasad, S.K.; Islam, M.A.; Gurav, S.S.; Patil, R.B.; AlFaris, N.A.; Aldayel, T.S.; AlKehayez, N.M.; Wabaidur, S.M.; Shakya, A. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: A pharmacoinformatics study. J. Biomol. Struct. Dyn. 2021, 39, 4686–4700. [Google Scholar] [CrossRef]
- Luo, P.; Liu, D.; Li, J. Pharmacological perspective: Glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105995. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Cai, X.; Cong, S.; Sun, J.; Du, W.; Cui, H.; Luo, L.; Ma, X.; Wang, L. Diammonium Glycyrrhizinate Inhibited Inflammatory Response and Modulated Serum Metabolism in Poly(I:C)-Induced Pneumonia Model Mice. Shock 2024, 61, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, L.; Li, S.; Liu, J.; Wang, H.; Lin, Y.; Cai, D. Diammonium glycyrrhizinate ameliorates portal hypertension by regulating portal macrophage oxidation and superoxide dismutase 3. Eur. J. Pharmacol. 2022, 929, 175115. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients 2020, 12, 1193. [Google Scholar] [CrossRef]
- Tan, R.; Xiang, X.; Chen, W.; Yang, Z.; Hu, W.; Qu, H.; Liu, J. Efficacy of diammonium glycyrrhizinate combined with vitamin C for treating hospitalized COVID-19 patients: A retrospective, observational study. QJM 2022, 115, 77–83. [Google Scholar] [CrossRef]
- Liao, F.L.; Peng, D.H.; Chen, W.; Hu, H.N.; Tang, P.; Liu, Y.Y.; Luo, Y.; Yao, T. Evaluation of serum hepatic enzyme activities in different COVID-19 phenotypes. J. Med. Virol. 2021, 93, 2365–2373. [Google Scholar] [CrossRef]
- Yan, H.; Sun, J.; Wang, K.; Wang, H.; Wu, S.; Bao, L.; He, W.; Wang, D.; Zhu, A.; Zhang, T.; et al. Repurposing carrimycin as an antiviral agent against human coronaviruses, including the currently pandemic SARS-CoV-2. Acta Pharm. Sin. B 2021, 11, 2850–2858. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Yang, G.; Wang, K.; Yan, H.; Wang, H.; Li, X.; Qiao, L.; Wu, M.; Wang, Y.; Jiang, J.-D.; et al. Diammonium Glycyrrhizinate Exerts Broad-Spectrum Antiviral Activity Against Human Coronaviruses by Interrupting Spike-Mediated Cellular Entry. Int. J. Mol. Sci. 2025, 26, 6334. https://doi.org/10.3390/ijms26136334
Wu S, Yang G, Wang K, Yan H, Wang H, Li X, Qiao L, Wu M, Wang Y, Jiang J-D, et al. Diammonium Glycyrrhizinate Exerts Broad-Spectrum Antiviral Activity Against Human Coronaviruses by Interrupting Spike-Mediated Cellular Entry. International Journal of Molecular Sciences. 2025; 26(13):6334. https://doi.org/10.3390/ijms26136334
Chicago/Turabian StyleWu, Shuo, Ge Yang, Kun Wang, Haiyan Yan, Huiqiang Wang, Xingqiong Li, Lijun Qiao, Mengyuan Wu, Ya Wang, Jian-Dong Jiang, and et al. 2025. "Diammonium Glycyrrhizinate Exerts Broad-Spectrum Antiviral Activity Against Human Coronaviruses by Interrupting Spike-Mediated Cellular Entry" International Journal of Molecular Sciences 26, no. 13: 6334. https://doi.org/10.3390/ijms26136334
APA StyleWu, S., Yang, G., Wang, K., Yan, H., Wang, H., Li, X., Qiao, L., Wu, M., Wang, Y., Jiang, J.-D., & Li, Y. (2025). Diammonium Glycyrrhizinate Exerts Broad-Spectrum Antiviral Activity Against Human Coronaviruses by Interrupting Spike-Mediated Cellular Entry. International Journal of Molecular Sciences, 26(13), 6334. https://doi.org/10.3390/ijms26136334




