Abstract
Hepatobiliary diseases, which include disorders of the liver, gallbladder, and bile ducts, remain a major global health concern. A significant proportion of deaths worldwide are attributed to hepatic diseases, accounting for 4% of the total global mortality in 2023. Among benign hepatobiliary diseases, metabolic dysfunction-associated steatotic liver disease is the most prevalent liver pathology, with a concerning rise in incidence, while it is recognized as the leading cause of liver transplantation in the United States. However, there is a notable rise over time in cases of autoimmune hepatobiliary disorders, including autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis. Meanwhile, hepatocellular carcinoma still remains the most frequently diagnosed hepatobiliary malignancy, constituting the third leading cause of malignancy-related mortality globally. Meanwhile, cholangiocarcinoma and gallbladder cancer are the second and third most common hepatobiliary malignancies, respectively, both exhibiting highly aggressive malignant behavior. Despite the notable advances in biomarkers and the development of therapeutic tools, early diagnosis and monitoring are considered pivotal for the management of the aforementioned pathologies. The development of new non-invasive biomarkers that can effectively identify, monitor these pathologies, and guide their management is considered a necessity. Extracellular vesicles (EVs) constitute nanoparticles with several embedded cargoes, with a significant role in intercellular communication, which are considered promising biomarkers in several diseases, including viral, metabolic, autoimmune, and malignant diseases. In this review, we will shed light on the role of EVs as novel frontiers in hepatobiliary diseases.
1. Introduction
Hepatic disorders and cholangiopathies comprise a wide range of diseases affecting hepatocytes, the biliary tree, and liver vasculature, posing a significant global health burden. Hepatic disorders involve metabolic, structural, and functional impairments and are classified accordingly. Some of the most common metabolic hepatic disorders are metabolic dysfunction-associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and their combination, the so-called metabolic-alcohol-associated liver disease, while viral hepatitis B and C constitute hepatic diseases that have significantly altered global mortality and morbidity for decades [1]. Meanwhile, some of the most commonly diagnosed autoimmune hepatobiliary disorders are autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) [1]. All the above disorders may eventually lead to several liver-related outcomes such as fibrogenesis, cirrhosis, as well as hepatobiliary oncogenesis, including gallbladder cancer (GC), hepatocellular carcinoma (HCC), and cholangiocarcinoma (CCA). HCC constitutes the most frequently diagnosed primary liver malignancy and the third cause of cancer-related death [2], while CCA is the second most diagnosed malignancy, followed by GC, which both constitute highly malignant diseases [2,3].
Despite advancements in diagnostic and therapeutic modalities, these conditions continue to impact many individuals globally, and the management of the aforementioned hepatobiliary diseases remains challenging. Extracellular vesicles (EVs) have been in the spotlight of biomarker research. These nanoparticles have a key role in intercellular communication, as they carry several types of cargo, which can alter the functional state of the recipient cells. The type of cargo can influence disease development and progression, and it can potentially serve as a target for the identification of EVs related to the disease [4]. In this review, we will shed light on the current knowledge regarding the emerging role of EVs as novel frontiers in the most commonly diagnosed benign and malignant hepatobiliary diseases, and we will provide a concise overview of their interplay.
2. Review of EV Biogenesis
EVs constitute quite heterogeneous, nanosized particles that are distinctly classified into 3 subclasses, which are closely related to their biogenesis pathway (Figure 1). More particularly, the 3 subclasses are (i) exosomes, (ii) microvesicles, and (iii) apoptotic bodies, starting with the smallest diameter (40–150 nm), towards the intermediate (150–1000 nm), and the largest (>1000 nm), respectively. There are three discrete pathogenetic pathways, including (a) inward membrane budding for exosome biogenesis, (b) outward membrane blebbing for microvesicles’ production, and (c) cellular apoptotic mechanism, for the biogenesis of apoptotic vesicles and eventually of apoptotic bodies [4,5]. However, their heterogeneity is also identified in the wide variety of cargoes that they can encompass. There is a multitude of cargoes, including coding and non-coding RNAs, autophagosomes, DNA molecules, several receptors, and other protein and lipid molecules, which can significantly alter the function of several other cells via intercellular communication. More specifically, the parental cell that produces several types of EVs, regarding their size and cargoes, can significantly alter the functional state of the recipient cells, which can receive these particles through several pathways. These nanoparticles are delivered either via receptor–ligand interaction or through a wide variety of endocytic processes, including receptor, lipid, or caveolin-mediated endocytosis, as well as micropinocytosis. Nevertheless, the delivery of the cargoes and their interactions with the recipient cells constitute mechanisms that are still under study [4,5,6].
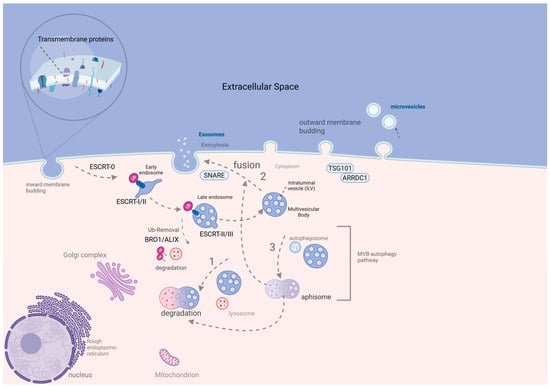
Figure 1.
Microvesicle and ESCRT-dependent exosome biogenesis [7]. The exosome biogenesis starts with the internalization of transmembrane proteins under a close dependence on ESCRT 0-III for cargo recruitment. ESCRT-0 (Vps27, Hse1) identifies the internalized ubiquitinated transmembrane proteins. The binding of Vps27 on the endosomal membrane permits the binding of ESCRT machinery on the endosome. ESCRT-0 binds to ESCRT-I (Vps23, Vps28) and the latter on ESCRT-II (Vps25, Vps36). Then, ESCRT-II binds and recruits ESCRT-III (Vps20), followed by the formation of ILVs (budding of late endosomal membrane), which are integrated into the lumen of MVB. On that point, MVB final formation requires the removal of Ubiquitin (Up) tags by Bro1/ALIX proteins (part of the ESCRT complex). Once MVB is formed, it can either be (1) fused with lysosome for degradation, (2) fused with plasma membrane for exosome exocytosis under SNARE proteins contribution, or (3) fused with autophagosome, forming amphisome, which is either degraded or fused with the cell membrane for exosome release. Microvesicles result from the outward blebbing of the plasma membrane under the TSG101-ARRDC1 interaction [7], created in BioRender. Trifylli, E. (2025) https://BioRender.com/e99v625 (accessed on 4 May 2025) (Agreement number EM28FVFH0Z). ALIX, ALG-2-interacting protein X; ARRDC1, arrestin domain-containing protein 1; BR01/BRO1, Bro1 domain protein; ESCRT, endosomal sorting complex required for transport; EV, extracellular vesicle; ILV, intraluminal vesicle; MVB, multivesicular body; SNARE, soluble NSF attachment protein receptor; TSG101, tumor susceptibility gene 101; Ub, ubiquitin; Vps, vacuolar protein sorting protein.
Exosomes are formed through inward budding of the plasma membrane, involving endosomal pathways. Early endosomes mature into late endosomes, which form intraluminal vesicles (ILVs) through inward invagination. These are then packaged into multivesicular bodies (MVBs). The Endosomal Sorting Complex Required for Transport (ESCRT) 0-III complex guides this process. ESCRT 0 and I initiate vesicle formation, and ESCRT-II recruits ESCRT-III to finalize ILV detachment. MVBs may fuse with lysosomes for degradation or with the plasma membrane to release exosomes [8]. Exosome formation may also occur via ESCRT-independent routes involving the membrane or cytoplasm. Fusion with autophagosomes creates amphisomes, which may also release exosomes. SNARE proteins (like Ykt6, VAMP7, and syntaxin 1A) mediate exosome exocytosis [8]. Microvesicles (150–1000 nm) bud directly from the cell membrane. Under hypoxic conditions, SNAREs and Rab-GTPases are involved in cargo recruitment. Under normoxia, ARRDC1 and TSG101 regulate this process. Additionally, ARF6 facilitates the packaging of MHC-I, RNAs, DNAs, and integrins [9]. Moreover, apoptotic bodies, the largest EVs, are released during programmed cell death. Apoptosis leads to nuclear fragmentation, chromatin condensation, and disintegration of organelles. Membrane protrusions like apoptopodia and microtubule spikes eventually form apoptotic vesicles, which segment into apoptotic bodies [10].
3. An Overview of the Interplay Between EV and Hepatobiliary Diseases and Their Potential Role as Biomarkers
3.1. Chronic Viral Hepatitis B and C
EVs are key in cell-to-cell communication between viral hepatitis B or C (HBV or HCV) and host cells, facilitating disease progression and chronicity. After six months, this leads to liver tissue destruction, fibrotic injury, cirrhosis, and hepatocellular carcinoma (HCC) development [7]. Infected host cells secrete EVs that contain viral elements, while these EVs are quite similar to viruses, as they share biogenesis, uptake mechanisms, and secretory pathways. Notably, viral nanoparticles (30–1000 nm) and EVs resemble each other in size, morphological heterogeneity, and transported cargoes. HBV and HCV are common chronic hepatitis viruses that are about 42 and 50 nm, respectively. Like EVs, viral particles are double-membrane vesicles carrying DNA (HBV) or RNA (HCV). Both utilize ESCRT-dependent and independent biogenesis pathways. Viruses can manipulate EV biogenesis to enhance transmissibility and contagiousness (e.g., HBV and HCV) [11,12,13,14].
EVs containing HBV DNA isolated from chronic HBV patient serum/plasma can infect non-infected HepG2 hepatocytes, confirmed by positive HBcAg, HBsAg, and DNA levels. Similarly, in mice, EVs from infected hepatocytes mediate HBV transmission to healthy ones [11,12]. Chronic HBV patients have elevated circulating EVs with viral genetic material and proteins that impair immune responses, notably reducing NK cell cytotoxicity and interferon gamma (INF-γ) production [11,12,13,14]. HBV-induced immune suppression is also mediated via EV-contained microRNAs (e.g., miR-21), which inhibit interleukins like interleukin (IL)-12, weakening the immune response and promoting HCC development [15]. Infected hepatocytes secrete exosomes and virions that overregulate programmed death-ligand 1 (PD-L1) and downregulate CD69, suppressing leukocyte activity, promoting viral replication, and hindering HBV eradication in mouse models [11,12,13,16]. Additionally, exosomes that are derived from HBV-infected LO2 cells and contain miR-222 promote LX-2 cell activation in animal models (mice), promoting fibrogenesis [17]. Similarly, HCV-infected hepatocyte-derived EVs containing viral RNA infect non-infected hepatocytes, promoting replication. In chronic HCV, high EV-contained RNA levels may bypass oral antiviral treatment [18]. EV-contained miR-19a, abundant in chronic infection, activates HSCs and promotes fibrosis [19], while platelet-derived EVs are more abundant in chronic HCV than HBV, correlating with fibrotic markers (procollagen III N-terminal propeptide, hyaluronate), though further in-depth research is needed [20]. HCV-derived EVs also engage in cross-talk with monocytes (immune escape) and HSCs (fibrosis) [13]. In Table 1, we demonstrate some of the promising EV-based diagnostic biomarkers for chronic hepatitis B and C.

Table 1.
EV-based diagnostic biomarkers for chronic hepatitis B and C.
3.2. Metabolic Diseases
3.2.1. MASLD
MASLD constitutes the major cause of chronic hepatopathies, with a big portion (20–30%) of the global population being affected, especially in the West. It includes a spectrum of pathological manifestations: steatosis (MASL), steatohepatitis (MASH), and cirrhosis. Among MASL patients, 3 of 10 will develop steatohepatitis, with or without fibrosis [23]. HCC can develop in cirrhosis and MASH alone, while MASLD is the leading cause of liver transplantation in the US. The scientific community is striving to develop non-invasive biomarkers, especially for early MASLD diagnosis [24]. Although liver biopsy is the gold standard for MASH and fibrosis diagnosis, it has limitations: invasiveness, complications, cost, sampling errors, inter-observer variability, inadequate sampling, and misinterpretation due to disease heterogeneity [25].
EVs are promising biomarkers for MASLD, playing a key role in its development and progression. They can be produced by hepatocytes or non-parenchymal liver cells like liver endothelial sinusoidal cells (LSECs), hepatic stellate cells (HSCs), portal fibroblasts, pre-adipocytes, and monocytes/macrophages [26]. EV alterations in quantity and quality are linked to MASLD pathogenesis and progression, especially in advanced cirrhosis (F3–F4) versus F1–F2 and healthy donors [27]. Patients with advanced fibrosis or cirrhosis show fewer circulating EVs, mainly from white blood cells and endothelial cells [28]. Similarly, in animal models of MASH and advanced fibrosis/cirrhosis, circulating EV levels are altered [29]. In mice, EVs from a rich fat diet injected into chow-fed mice increased inflammation, underscoring their role in MASH [30]. These vesicles can also identify MASLD among other chronic liver disorders, supporting their diagnostic and prognostic potential. Understanding MASLD progression, which is deeply rooted in cell-to-cell crosstalk, is essential for targeting EV cargos.
Lipotoxic hepatocytes, as parental EV producers, induce changes in parenchymal and non-parenchymal liver cells, such as hepatocytes, HSCs, macrophages, and LSECs, respectively, leading to inflammation, fibrogenesis, immune cell recruitment, and angiogenesis [31]. Lipid overload in the parenchyma triggers inflammation, which is amplified by monocytes/macrophages releasing IL-1b and IL-6. T-helper 17 cells, neutrophils, and Kupffer cells further activate HSCs via IL-17, IL-22, and TGF-β, respectively [32]. Quantitative and qualitative abnormalities in hepatocyte-derived EVs are reported in MASLD patients [33]. Moreover, HSC-derived EVs are also related to fibrogenesis and increased angiogenesis in the parenchyma. More particularly, it has been demonstrated that HSC-derived-EVs interact with LSECs, promoting angiogenesis and fibrosis via containing several protein molecules that promote fibrogenesis [34]. Meanwhile, it has to be underlined that angiogenesis is also promoted via Portal fibroblast-derived EVs that contain VEGF, a growth factor for endothelial cells [35]. Additionally, LSECs interact with HSCs via the release of LSEC-derived EV-sphingosine kinase 1 and/or EV-sphingosine-phosphate (S1P), a phenomenon that leads to HSCs activation, promoting fibrosis and MASLD progression [36]. These changes highlight the potential of EVs as a “liquid biopsy” for identifying disease and possibly stratifying severity [37]. Monocytes, macrophages, and platelets as parental cells also have a key role in EV-related MASLD development and disease progression [38]. On top of that, it has been demonstrated that there is a notable increase in circulating microvesicles that originate from monocytes, platelets, as well as endothelial cells in type 2 diabetes mellitus patients, which is one of the major aggravating factors for MASLD development and fibrosis progression [39]. Table 2a demonstrates several types of hepatocyte-derived EVs and their implication in MASLD progression (Table 2b).

Table 2.
(a). Effects of EV-cargoes in MASLD pathogenesis. (b). EV-based biomarkers in MASLD/MASH.
3.2.2. Alcohol-Associated Liver Disease (ALD)
Excessive alcohol consumption is closely associated with liver toxicity, including steatosis, steatohepatitis, and cirrhosis [64]. ALD shows aberrations in EV features, including increased circulating EV levels compared to healthy controls, as shown in animal ALD (mice) models [65]. Most circulating EVs originate from hepatocytes and HSCs, with levels proportionally increasing with disease severity, such as in alcoholic hepatitis (AH) compared to alcohol-abusing patients without AH [66]. These EVs significantly alter recipient cells like HSCs, macrophages, and endothelial cells [66,67]. In AH, hepatocytes release EVs that increase macrophage IL-17 and IL-1β expression, promoting fibrosis. HSCs are also activated by hepatocyte-derived EVs containing collagen a-SMA, demonstrated in AH models (mice) [66,67,68]. Additionally, hepatocyte-derived EV-CD40 ligand stimulates macrophages, while mitochondrial double-stranded RNA EVs induce neutrophilic infiltration, interacting with Kupffer cells and triggering IL-1b release [69].
ALD-induced EV miRs also promote inflammation and fibrotic injury. HSC-derived EVs-miR-19b play a key role in HSC activation and fibrogenesis [66,67]. AH-injured hepatocytes release EV-miR-181 and EV-miR-27a, promoting fibrosis via HSC activation in mice [69]. Monocyte-derived EV-miR-27a promotes M2 phenotype polarization of monocytes [70]. Macrophage activation (M2 phenotype) is also induced by hepatocyte-derived EVs containing heat shock HSP90 protein, shown in ALD animal models, while hepatocyte-EV CYP2E1 induces alcohol-related monocyte toxicity [67]. There is an interplay between EV biogenesis and the autophagy pathway. EVs suppress autophagy via miR-155 overexpression, decreasing LAMP1 and LAMP2 levels [71]. Thus, EVs with miR-155 inhibit autophagy in hepatocytes, a key toxic molecule-recycling process [71]. Hepatocyte-originated EVs containing miR-122 play a critical role in inflammation, inducing lipopolysaccharide and pro-inflammatory cytokine stimulation in monocytes [34]. Table 3 demonstrates EV-based biomarkers specifically associated with ALD and AH.

Table 3.
EV-based biomarkers in ALD and AH.
3.2.3. Hereditary Hemochromatosis (HH)
HH is a homeostatic disorder of iron, caused by a high iron Fe gene mutation (C282Y SNP), leading to decreased hepcidin despite increased iron, as well as increased intestinal absorption of it [74]. The iron overload in hepatic parenchyma leads to liver fibrosis, cirrhosis, and an increased risk of HCC [75]. EVs have a key role in iron homeostasis, as they carry ferritin and transferrin and can modulate hepcidin function and ferroptosis [76]. Under oxidative stress and iron overload, cells release more EVs containing antioxidant protein molecules and iron metabolism proteins, which help minimize injury and modulate ferroptosis [76]. Iron-embedded EVs can prevent injury in parental cells by redistributing iron but may damage recipient cells, contributing to disease progression in hepatic disorders like MASLD and HH [77,78]. Hepatocyte- and macrophage-derived EVs under iron-rich conditions show altered ferritin and iron-handling enzymes, serving as sensitive biomarkers of intracellular iron status [79]. Given their presence in fluids like blood, urine, and bile, EVs offer a non-invasive method to monitor iron accumulation and liver damage in HH. In Table 4, we summarize the key points of iron uptake regulation and EVs’ role in hemochromatosis diagnosis.

Table 4.
EVs in iron homeostasis and HH diagnosis [76,77,78,79].
3.3. Autoimmune Hepatobiliary Diseases
3.3.1. PBC and PSC
The emerging role of EVs in biliary tract pathophysiology is highlighted in current studies. EVs and their embedded cargoes are involved in the physiological function of cholangiocytes and cholangiopathies such as PBC and PSC [80]. Released from the apical part of polarized cholangiocytes, EVs contribute to biliary tract homeostasis by mediating cross-talk between parental cells and recipient cells like endothelial cells and hepatocytes, while hepatocyte-originated EVs carrying epidermal growth factor receptor (EGFR) and integrin beta 4 (ITGB4) are implicated in biliary tract oncogenesis [81,82]. PBC and PSC are characterized by cholestasis, inflammation, and fibrogenesis, with ductular reaction and cholangiocyte activation. EVs are closely related to cholangiocyte proliferation, where bile-originated EVs interact with primary cilia to suppress ERK signaling through miR-15a overexpression [83]. EVs from biliary tract cells also promote fibrotic injury, particularly those carrying long non-coding RNA (lncRNA) H19. These vesicles mediate interaction between cholangiocytes and hepatocytes, contributing to fibrosis progression in PSC patients. EV-H19 increases high mobility group AT-hook 2 levels, stimulating macrophage and HSC activation and chemotactic pathways [84].
3.3.2. Autoimmune Hepatitis (AIH)
AIH is an immune-mediated liver disease that can occur at any age and may lead to cirrhosis and transplantation [83]. Though the pathogenetic mechanism remains unclear, AIH is linked to auto-antibody production (antinuclear antibody, liver kidney microsomal type 1 and 3 antibody; anti-smooth muscle antibody, anti-liver-cytosol-1), cytokine overproduction (TNF-α, IL-1, -6, -17, -12, INF-γ), and T-cell imbalances [85]. Concanavalin A and hepatic S100 proteins induce AIH and serve as targets for mesenchymal stem cell (MSC)-derived EVs [86]. These EVs alter the AIH microenvironment, reducing inflammation and fibrosis. MSC-exosomes-miR-223 show liver-protective, anti-inflammatory, and anti-apoptotic effects by stimulating signal transducer and activator of transcription 3 (STAT3) in macrophages and hepatocytes [2]. EVs with miR-21 and miR-16 promote pro-inflammatory macrophages. T cells show functional aberrations in AIH. Regulatory T cell, T helper (Th) cell type-17, -1, -2 imbalances are addressed by MSC-EV-sphingosine 1-phosphate, which downregulates Th-17. MSC-exosomes promote Th-1 to Th-2 transition and inhibit T-cell immune function via PD-L1 overexpression [85]. Table 5 presents the role of EVs as biomarkers for autoimmune hepatobiliary diseases.

Table 5.
The role of EVs as biomarkers for autoimmune hepatobiliary diseases.
3.4. Complications of Chronic Hepatobiliary Diseases
Chronic hepatobiliary diseases may gradually lead to the development of liver-related outcomes and hepato- or cholangiocarcinogenesis. In this section, we focus on fibrogenesis, cirrhosis, biliary tract stenosis, as well as portal hypertension, ascites, hepatopulmonary and porto-pulmonary syndromes, coagulation disorders, and hepatobiliary malignancies. Meanwhile, chronic cholangiopathies might lead to biliary tract stenosis, chronic cholecystitis, and lithiasis, as well as GC [2].
3.5. Fibrotic Injury and Cirrhosis
The development of fibrotic injury and cirrhosis encompasses conditions that are characterized by increased fibrogenesis in the extracellular matrix, with the latter presenting distortion of hepatic tissue architecture and angiogenesis. Pro-fibrotic EVs have a key role in the activation of quiescent HSCs, such as EV-PDGF receptor α (PDGFRα), with the degradation of PDGFRα and MVBs being suppressed [50]. It has been demonstrated that patients with established cirrhosis have an increased amount of these EVs in their circulation compared with healthy individuals. Meanwhile, some other aberrations in EV levels that are identified under these fibrogenic conditions include the notably decreased amount of HSC-derived EVs that contain the Twist family basic helix-loop-helix transcription factor 1 (Twist1), as well as miR-214, leading to the paracrine activation of other HSCs [87]. Similarly, LSECs release EVs that contain SK1 in mice fibrosis models [88]. In addition, hepatocyte-originated EVs-miR-128-3p that are released under lipotoxic conditions interact with HSCs and subsequently activate them, promoting fibrogenesis through suppressing peroxisome proliferator-activated receptor (PPAR) γ, which has a pivotal role in quiescent HSC regulation and fibrosis inhibition in liver parenchyma, while the hepatocyte-derived EVs that carry miR-192 act in the same manner for HSC activation and fibrogenesis progression [89].
3.6. Portal Hypertension and Hepatopulmonary and Porto-Pulmonary Syndromes
Parenchymal structural modifications, due to fibrosis, lead to the establishment of portal hypertension (PT), hyperdynamic circulation, and portosystemic collaterals. Large-diameter EVs of unknown origin are implicated in systemic vasodilation, which has been found in patients with cirrhosis with a B or C grade of Child–Pugh (CP) score in comparison to healthy controls and CP A grade [90]. On the other hand, small EVs have been correlated with elevated intrahepatic resistance via over-activation of signaling pathways such as JAK2 and ROCK ones [7]. These EVs are received by endothelial cells, resulting in vasoconstriction suppression. EV-miR-194 levels are increased in hepatopulmonary syndrome in animal models, as well as portal myofibroblasts-EV-VEGF that is received by endothelial cells, leading to disease aggravation [91]. Meanwhile, EVs’ implication in porto-pulmonary hypertension has also been studied in animal models (mice), with its development via injection of EVs from diseased to healthy mice [92].
3.7. Coagulation Disorders
Coagulation disorders are closely associated with EVs-phospholipids and platelet-derived EVs-annexin V, with the latter being related to increased severity and mortality for cirrhotic patients [93]. Similarly, EV-Tissue factor is also implicated in coagulation and disease progression in animal and human cirrhotic models. Furthermore, increased angiogenesis is attributed to activated HSCs, cholangiocytes, and hepatocyte-originated EVs that are interacting with LSECs and lead to increased angiogenesis. Furthermore, increased angiogenesis is attributed to activated HSCs, cholangiocytes, and hepatocyte-originated EVs (e.g., EV-vanin-1 in mice models) that are interacting with LSECs and lead to increased angiogenesis, as well as endothelial cell-derived EVs (e.g., VEGF) that contain VEGF [19,26,49].
3.8. Ascites and Hepatic Encephalopathy
There are differences in the protein cargoes of circulating EVs between healthy controls and animal models of hepatic encephalopathy, while patients who present ascites have a notable increase in the amount of hepatocyte and endothelial-derived EVs. The former population of EVs is significantly associated with mortality, while small-sized vesicles inside the ascitic fluid have been correlated with patient proneness to inflammation [7,94].
3.9. Biliary Tract Stenosis and Chronic Cholecystitis
The cells from these stenotic areas produce a noticeable amount of EVs that enter the bile. The non-invasive identification of malignant common bile duct (CBD) stenosis, which can be present in chronic liver diseases such as PSC, is in the spotlight of the scientific community. Based on the study by Severino V et al., the concentration levels of bile-EVs could accurately (100% accuracy) identify the malignant CBD stenosis from the benign ones [95]. Dysbiosis constitutes a pivotal aggravating factor for cholecystitis development, with EVs playing a key role as they carry several bacteria, which are produced by microbial-infected cells. There are several qualitative aberrations in the serum exosomes between the chronic versus acute cases. Exosomes are significantly released under the condition of cholecystitis, a phenomenon that is closely related to exosomal function as regulators of gene expression and signaling pathways, which are implicated in cholecystitis. Interestingly, the bacterial and viral composition of the microbiome in the biliary tract can significantly affect the origin (e.g., host-infected cells) and cargoes of the released exosomes [96]. In Table 6, we demonstrate the complications of chronic hepatobiliary diseases.

Table 6.
The complications of chronic hepatobiliary diseases [26,87,88,89,90,91,92,93,94,95,96,97].
In the next paragraphs, we will shed light on the implication of EVs in hepatobiliary malignancies and their potential role as biomarkers [97].
3.10. Gallbladder Cancer (GC)
Although a wide variety of studies exist regarding the development of novel biomarkers for several malignancies, there is a noticeable lack of studies specifically regarding GC [98]. It has been recently demonstrated by M. Kong et al. that there are aberrations in exosomes between GC patients and patients with other gallbladder pathologies (cholecystitis, gastric polyps) and healthy controls [99]. They demonstrated that exosomal membrane integrity was altered (lost) due to a noticeable decrease in several unsaturated phosphatidylcholines/phosphatidylethanolamines in GC patients, compared to other groups [100]. Meanwhile, Ueta, E. et al. demonstrated that circulating serum EV-miRNAs constitute novel GC biomarkers, as there are several alterations between EV-miRNAs in GC and non-GC patients [101]. Some of the EV-miRNAs that they demonstrated are miR-451a- and miR-1246-based in silico. The former was noticeably decreased, whereas the latter increased [101]. Additionally, the first one is implicated in the apoptosis pathway and GC suppression of proliferation via suppression of cyclin-dependent kinase inhibitor 2D (CDKN2D), MIF, and PSMB8, having a prognostic value, whereas the second is in tumor progression, proliferation, as well as invasion [99,100,101]. The second one, if it is combined with the neoplastic biomarkers carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen CEA, provides high diagnostic accuracy (AUC of 0.816), which can serve as a diagnostic GC biomarker [99,100,101]. In Table 7, we demonstrate some of the EV-based biomarkers for GC.

Table 7.
EV-based biomarkers for GC, CCA, and HCC.
3.11. Hepatocellular Cancer
The emerging role of EVs in HCC pathogenesis is in the spotlight of scientific research, as it is the leading cause of cancer-related mortality globally. EVs have a key role in the intercellular communication, which eventually leads to hepatocarcinogenesis and tumor progression via altering the anti-cancer immune responses and cell survival [102,103]. EV-cargoes such as miRNAs and other non-coding RNAs (lncRNAs, circular RNAs (circRNAs), etc.) have a crucial role in HCC proliferation, progression, invasion, migration, and lastly metastatic dissemination via stimulating several signaling pathways such as PI3K/AKT and MAPK [104,105]. Moreover, EV-oncogenic protein and RNA molecules are significantly implicated in neoangiogenesis, promoting HCC typical hypervascularity, increased vascular permeability, and eventually migration and distant dissemination [104]. HCC-derived EV-miR-103 and miR-210 interact with endothelial cells, leading to their altered integrity and signal transducer and activator of transcription 6 (STAT6) and SMAD family member 4 (SMAD4) overexpression, respectively [106]. HCC-derived EVs interact with several other types of recipient cells in the tumor microenvironment (TME), altering their functional state through a paracrine and/or autocrine mode [104,105]. More particularly, HCC-derived EVs can promote EMT in TME via their embedded miR-3129, which has as a target the thioredoxin-interacting protein (TXNIP) [107]. At the same time, the interaction between HCC-EVs and other recipient cells leads to pro-inflammatory cytokine and metalloproteinase (MMP-2 and 9) overproduction that promotes HCC cell proliferation and migration [105]. Additionally, tumor macrophages in TME receive large-sized EVs let-7b, leading to overproduction of IL-6, while MMP-2 is also overproduced via the HCC-derived-EV-CD147 [102]. EMT and distant dissemination are also promoted via EVs-miR92a-3p [108]. Moreover, TME-fibroblasts are activated (cancer-associated fibroblasts (CAFs)) in highly malignant HCC, with an increased metastatic behavior via HCC-derived miR-1247-3p that alters the expression (decrease) of Beta-1,4-Galactosyltransferase 3 (B4GALT3) [109]. Interestingly, these EVs promote CAF-related pro-inflammatory cytokine production (IL-6 and 8), stimulating the formation of lung pre-metastatic niche fibroblasts, via the EMT phenomenon [109]. Meanwhile, small EVs-circ-PTGR1 modify the homeostasis of TME, facilitating HCC cell invasion in the surrounding tissues and migration to blood circulation [110]. HSCs are also receiving EVs such as EV-miR-21, a phenomenon that leads to their transformation into CAFs and the oversecretion of several growth factors, such as MMP-2 and 9, VEGF, as well as TGF-b [111]. Furthermore, the interaction between HCC-derived EVs-miR-23a and adipocytes and vice versa has been related to HCC progression, proliferation, and migration [112]. Meanwhile, adipocytes as EV-parental cells promote HCC growth by stimulating HCC deubiquitination and inhibiting miR-34a [105,112,113]. HSCs also receive HCC-derived EV-miR-21, which leads to their transformation into cancer-supporting cells [105,112,113]. The same is also reported in macrophages through HCC-derived-miR-23a-3p, while macrophages are polarized (M2-phenotype) via EVs-lncRNA TUC339, resulting in cytokine overproduction, tumor progression, and “defective” phagocytosis [105,112,114]. The aforementioned EVs are considered one of the most abundant, released by HCC. In addition, when macrophages receive HCC-derived EVs-miR-23a-3p, they increase PD-L1 expression, which impairs T-cell anti-cancer immune response, leading to HCC immune escape [115]. Some other tumor-promoting EVs’ non-coding RNAS are EV-miR-221, EV-miR-429, EV-CircFBLIM1, as well as EV-lncRNA FAL1 and EV-miR-25. The former has as a target p27/Kip1 in HCC, promoting its overexpression, the second targets Rb-binding protein 4 (RBBP4), promoting the expression of POU class 5 homeobox 1 (POU5F1), while the third is implicated in miR-338 and low-density lipoprotein receptor-related protein 6 (LRP6) axis, enhancing HCC progression [112,113,114,116]. The fourth is closely implicated in the gene expression of ZEB1 and alpha-fetoprotein (AFP), via targeting and competing miR-1236, whereas the fifth is implicated in HCC-resistance in sorafenib. Meanwhile, focusing on the EV-protein cargoes, HCC-derived-EV-CD147 and EV-complement factor H constitute tumor-promoting EVs, with the former interacting with fibroblast activation, while the latter with enhancing of inflammatory reaction and HCC progression via the overproduction of complement 5a and 3a [112,113,114,116].
Nevertheless, some of the tumor-suppressing EV cargoes are Huh7-derived EV-miR-122, which, when received by HepG2 cells in vitro, leads to the overproduction of IGF-1 and has a tumor-suppressive effect [112,113,114,117]. Likewise, EVs-EVs-circ-0051443 exhibit a HCC growth suppressive role via apoptosis promotion, as they act as sponges for miR-331-3p [112,113,114,117]. Additionally, HCC cells-derived EVs’ VEGF-suppressing proteins limit neoangiogenesis via AMPK signaling pathway activation, while EV-CLEC3B also suppresses EGF that is also implicated in angiogenesis [118]. Moreover, CAF-derived EV-miR-320a suppresses HCC proliferation and growth via targeting and inhibiting PBX3/ERK1/2/CDK2 signaling pathway in malignant hepatocytes [119]. Neoangiogenesis is also promoted by CD90-positive HCC-derived EV-H19 (lncRNA) via stimulating VEGF and VEGF receptor (VEGFR) overexpression in human umbilical vein endothelial cells (HUVECs) [120]. PI3K-Akt pathway, which has a key role in tumor progression and distant metastasis, is suppressed via HCC-derived EV-vacuolar protein sorting-associated protein 4A (Vps4A) [121]. In addition, it has been demonstrated that normal liver cells release SUMO-specific protease 3–eukaryotic initiation factor 4A1 complex (SENP3-EIF4A1) embedded in small-sized EVs. These act as tumor suppressors via decreasing the expression of miR-9-5p in the malignant hepatocytes [122]. In Table 8, we present a concise overview of EV-related HCC pathogenesis, while in Table 7, we demonstrate some of the EV-based biomarkers for HCC.

Table 8.
A concise overview of EV-related HCC pathogenesis.
3.12. Cholangiocarcinoma (CCA)
CCA constitutes a highly aggressive malignancy, which arises in the biliary tract, encompassing a group of malignancies based on the anatomical site of the malignant lesion: intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA). Despite the current diagnostic and therapeutic advances, there are limited options regarding the therapeutic modalities, while CCA diagnosis is usually in already established advanced stages, leading to a poor prognosis [123]. EVs have a key role in the intercellular communication between CCA cells and the TME cells in their vicinity, leading to CCA cell proliferation, growth, and metastatic dissemination. As it is widely known, the implication of TME components is pivotal in CCA progression. Desmoplasia is a key characteristic of CCA, which includes the presence of a strongly fibrotic stroma around the tumor, where ECM modifications take place, as well as overproduction of pro-inflammatory molecules, cytokines, and tumor-promoting molecules (periostin, metalloproteinases, osteopontin, tenascin-C, etc.). CCA-derived EVs are implicated in stromal modification, as well as in the impaired anti-neoplastic immune responses and tumor escape phenomenon. Tumor escape is also attributed to CCA-derived EVs, which alter cytokine-induced killer cells (CIKs) expression in vitro, a phenomenon that alters their secretion of TNF-a and perforin [84]. Tumor migration and invasive behavior are facilitated by several CCA-derived EVs, such as EV-integrin-a or -b, EV-frizzled class receptor 10 (FZD10), EV-vitronectin, and EV-lactadherin, which alter several signaling pathways in the recipient cells. More particularly, the β-catenin pathway (Wnt signaling pathway), which regulates cell proliferation, and differentiation and can potentially lead to carcinogenesis, is significantly modified by vitronectin, lactadherin, and integrin-a/b that are embedded in CCA-derived EVs, leading to the overexpression of β-catenin. This phenomenon leads not only to increased CCA migration and invasion but also to proliferation and distant metastasis [124]. Moreover, it has been demonstrated that iCCA-derived EV-ceramide and/or dihydroceramide are highly found in CCA patients, while they are implicated in blood dissemination of tumor cells in cases of the latter and monocyte cytokine overproduction in the case of the former [125]. Additionally, both types of EVs are derived from iCCA, with poor differentiation EVs that contain circ-CCAC1 are highly detected in bile and malignant tissue specimens, being deeply involved in neoangiogenesis as they interact with endothelial cells, as well as in Yin Yang 1 (YY1) overregulation via binding and sponging of miR-514a-5p. The overexpression of YY1 has been closely related to the migratory behavior of CCA cells, as well as to distant metastasis [83,84,126]. In addition, CCA-derived-EV-miR-183-5p induces overexpression of VEGF by mast cells, promoting neoangiogenesis, as well as protumorigenic prostaglandin E2 (PGE2) and prostaglandin E receptor 1 (PTGER1) [81,82,97,124,125].
Similarly, CCA-derived Circ-0000284 sponges miR-637, leading to LY6E upregulation, which eventually results in the malignant transformation of physiological cholangiocytes [126,127]. It is observed that HuCCT1-derived EVs, which are a population of vesicles that are derived from the HuCCT1 CCA line, highly contain CXCL-1, alpha-smooth muscle actin (α-SMA), vimentin, fibroblast activation protein, CCL2, as well as IL-6, with the latter acting as a growth factor for CCA. Meanwhile, the intercellular interactions between HUCCT1-derived EVs and MSCs in vitro lead to CCA progression [128]. MSCs are transformed into CAFs after interacting with CCA-derived EVs, leading to desmoplasia. Moreover, TAM-derived EVs-circ-0020256 enhance CCA proliferation, migration, and dissemination. T-CD8-positive cells’ chemotaxis is also modulated in CCA, via EV-B-cell-specific Moloney murine leukemia virus integration site 1 (BMI1), which is significantly elevated in the malignant tissue, leading to CCA growth, progression, migration, and metastasis, constituting a possible druggable target [81,128,129]. Furthermore, it has been demonstrated that there are several other CCA-derived-EV-miRNAs that are highly expressed in CCA, such as EV-miR-34c, miR-183-5p, as well as miR-200c-3p and miR-200b-3p [124]. The last two are significantly higher in CCA, proportionally to the tumor stage; the second is implicated in iCCA chemoresistance, as it induces PD-L1 overexpression on the surface of macrophages, while the former is related to increased CCA progression and growth. On the other hand, there are some tumor-suppressive vesicles, such as EV-miR-30e, which suppress EMT in CCA, impeding CCA cell dissemination and their invasive behavior. HSC-derived EV-miR-195 also acts as a tumor suppressor, inhibiting CCA progression, while EV-miR-195 also suppresses CCA cell growth in vitro [130]. In Table 9, we present EV-related CCA pathogenesis, while in Table 7, we demonstrate some of the EV-based biomarkers for CCA.

Table 9.
EV-related CCA pathogenesis.
4. Limitations of EVs Utilization as Biomarkers
Exosomes, a subtype of EVs, are promising noninvasive biomarkers for chronic hepatobiliary diseases [82,97]. These vesicles are released into various body fluids—such as blood, bile, and urine—and carry biomolecules like proteins, lipids, and nucleic acids, reflecting the pathological state of the cells or tissues from which they originate. A major advantage of EVs lies in their potential for early diagnosis [114]. Their cargo often undergoes molecular changes before symptoms appear or conventional biomarkers are altered. Their dynamic content makes them useful for monitoring disease progression and treatment response. Furthermore, their lipid bilayer offers protection from enzymatic degradation, which enhances their stability in circulation [131]. Despite their promise, several limitations remain. However, a major challenge is the lack of standardized methods for EV isolation and characterization (Table 10). Techniques like ultracentrifugation, immunoaffinity capture, filtration, and size-exclusion chromatography produce variable results in purity and subtype, affecting study reproducibility [114,132]. EVs are also heterogeneous in size, origin, and molecular cargo, which complicates functional analysis and data interpretation. Additionally, overlapping molecular signatures between different diseases can reduce biomarker specificity [81,114,132,133]. Analytical tools used to analyze EVs are often complex and not widely available in clinical settings. Storage conditions are also critical—freezing at −80 °C is recommended to maintain EV structure and molecular content, as repeated freeze–thaw cycles degrade particles and RNA. Stabilizers like trehalose may help reduce damage [131]. From a regulatory standpoint, there is no unified framework for classifying EV-based products, whether as diagnostics, therapeutics, or biologics. This ambiguity complicates clinical approvals. Ethical concerns also arise, particularly with engineered or donor-derived EVs, regarding donor safety, consent, and traceability [134]. Contaminants like protein aggregates and lipoproteins can co-purify with EVs, potentially skewing results from omics-based analyses [135]. Another major limitation is the incomplete understanding of EV bio-distribution, uptake, and clearance in vivo—critical factors for designing therapeutic delivery systems. On the production side, large-scale manufacturing of EVs faces issues like low yield, batch variability, and lack of GMP standards [136]. Moreover, current isolation methods are related to resources, implying several limitations such as the cost and infrastructure, with the standardization of these being necessary for clinical laboratories. Integration of EV analysis in combination with existing diagnostic modalities, such as biopsy, serological lab tests, and imaging modalities, that will provide a multi-modal interpretation, is considered a challenge as it requires an algorithmic approach and harmonization. In addition, the common EV profiling in several diseases constitutes another challenge for the interpretation of EV findings in the presence of multiple diseases in each patient (e.g., chronic hepatitis B and MASLD), so the development of disease-specific panels may overcome this issue. Finally, inconsistencies in EV definitions, characterization, and reporting across studies hinder reproducibility and consensus. In conclusion, while EVs show great potential as early, dynamic, and tissue-specific biomarkers for chronic liver diseases, several biological, technical, and regulatory challenges must be resolved before they can be widely adopted in clinical practice. Standardization, mechanistic understanding, ethical regulation, and clinical validation are all key steps needed for successful translation [114,131,132,133,134,135].

Table 10.
Limitations in EV research [114,131,132,133,134,135].
5. Conclusions and Future Directions
EVs can potentially serve as non-invasive biomarkers in hepatobiliary disease, especially due to their wide variety of cargoes that can be potentially targeted for multi-parametric analysis. The standardization of EV isolation, characterization, and storage protocols may increase the performance of EV-based diagnostics, while the combination of multi-omics findings may open new horizons in the development of novel biomarkers and may shed light on the complexity of hepatic and biliary pathologies. Finally, in addition to EVs’ role as diagnostic and prognostic biomarkers, they have an emerging role as promising tools for targeted therapies, due to their ability to transfer several cargoes. This ability opens new horizons for future perspectives in EV research and engineering for drug delivery and personalized hepatobiliary disease management.
Author Contributions
All authors participated in the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| A | |
| AFP | Alpha-fetoprotein |
| AIH | Autoimmune hepatitis |
| ALD | Alcohol-associated liver disease |
| AMPK | AMP-activated protein kinase |
| ANA | Anti-nuclear antibodies |
| ApoEVs | Apoptotic extracellular vesicles |
| ARF6 | ADP-ribosylation factor 6 |
| ARRDC1 | Arrestin domain-containing protein 1 |
| ASGPR1 | Asialoglycoprotein receptor 1 |
| ASMA | Anti-smooth muscle antibody |
| B | |
| B4GALT3 | Beta-1,4-galactosyltransferase 3 |
| BMI1 | B lymphoma Mo-MLV insertion region 1 homolog |
| BM-EVs | Bone marrow-derived extracellular vesicles (context inferred) |
| C | |
| CAFs | Cancer-associated fibroblasts |
| CA19-9 | Carbohydrate antigen 19-9 |
| CCL | Chemokine (C-C motif) ligand |
| CCA | Cholangiocarcinoma |
| CDKN2D | Cyclin-dependent kinase inhibitor 2D |
| CEA | Carcinoembryonic antigen |
| circRNA | Circular RNA |
| CK18 | Cytokeratin 18 |
| CP | Child |
| Pugh | |
| CXCL | C-X-C motif chemokine ligand |
| D | |
| DM | Diabetes mellitus |
| DMT1 | Divalent metal transporter 1 |
| dCCA | Distal cholangiocarcinoma |
| E | |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinases |
| ESCRT | Endosomal sorting complex required for transport |
| EV | Extracellular vesicle |
| F | |
| FAP | Fibroblast activation protein |
| FZD10 | Frizzled class receptor 10 |
| F1–F4 | Fibrosis/cirrhosis staging |
| G | |
| GC | Gallbladder cancer |
| GI | Gastrointestinal |
| GMP | Good manufacturing practice |
| (γ-GT) | Gamma-glutamyl transferase (inferred) |
| H | |
| HBcAg | Hepatitis B core antigen |
| HBsAg | Hepatitis B surface antigen |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HE | Hepatic encephalopathy |
| HH | Hereditary hemochromatosis |
| HMGA2 | High-mobility group AT-Hook 2 |
| HSCs | Hepatic stellate cells |
| HSP | Heat shock protein |
| HUVECs | Human umbilical vein endothelial cells |
| HuCCT1 | HuCCT1 cholangiocarcinoma cell line |
| I | |
| IL | Interleukin |
| ILVs | Intraluminal vesicles |
| INF-γ | Interferon gamma |
| iCCA | Intrahepatic cholangiocarcinoma |
| ITGβ1 | Integrin beta 1 |
| ITGB4 | Integrin beta 4 |
| J | |
| JAK2 | Janus kinase 2 |
| K | |
| Kupffer cells | liver macrophages; no specific abbreviation |
| L | |
| LSECs | Liver sinusoidal endothelial cells |
| lncRNA | Long non-coding RNA |
| LRP6 | Low-density lipoprotein receptor-related protein 6 |
| LY6E | Lymphocyte antigen 6 family member E |
| M | |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MIF | Macrophage migration inhibitory factor |
| miRNA | MicroRNA |
| MLK3 | Mixed lineage kinase 3 |
| MMP | Matrix metalloproteinases |
| MSCs | Mesenchymal stem cells |
| mTOR | Mammalian target of rapamycin |
| MVBs | Multivesicular bodies |
| N | |
| NAFLD | Nonalcoholic fatty liver disease (inferred) |
| NASH | Nonalcoholic steatohepatitis (inferred) |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer |
| P | |
| PBC | Primary biliary cholangitis |
| PC | Phosphatidylcholine |
| PDGFRα | Platelet-derived growth factor receptor alpha |
| PD-L1 | Programmed death-ligand 1 |
| PE | Phosphatidylethanolamine |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| POU5F1 | POU class 5 homeobox 1 |
| R | |
| ROS | Reactive oxygen species |
| RBBP4 | Rb-binding protein 4 |
| Rb | Retinoblastoma protein |
| S | |
| STAT | Signal transducer and activator of transcription |
| siRNA | Small interfering RNA (inferred) |
| snRNA | Small nuclear RNA (possible) |
| snoRNA | Small nucleolar RNA (possible) |
| S1P | Sphingosine 1-phosphate |
| SK1 | Sphingosine kinase 1 |
| SLC27A5 | Solute carrier family 27 member 5 |
| SENP3–EIF4A1 | SUMO-specific protease 3–eukaryotic initiation factor 4A1 complex |
| T | |
| TAM | Tumor-associated macrophage |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| Twist1 | Twist family basic helix-loop-helix transcription factor 1 |
| U | |
| Ub | Ubiquitin (inferred) |
| V | |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| VAMP | Vesicle-associated membrane protein |
| Vps4A | Vacuolar protein sorting-associated protein 4A |
| VWF | von Willebrand factor |
| Y | |
| YY1 | Yin yang 1 |
References
- Gan, C.; Yuan, Y.; Shen, H.; Gao, J.; Kong, X.; Che, Z.; Guo, Y.; Wang, H.; Dong, E.; Xiao, J. Liver diseases: Epidemiology, causes, trends and predictions. Signal Transduct. Target. Ther. 2025, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagalli, S. Chronic Liver Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jiang, Y.; Jiang, L.; Li, F.; Li, Q.; Yuan, S.; Huang, S.; Fu, Y.; Yan, X.; Chen, J.; Li, H.; et al. The epidemiological trends of biliary tract cancers in the United States of America. BMC Gastroenterol. 2022, 22, 546. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sane, S.; Kim, J.E.; Yun, S.; Kim, H.J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and delivery of extracellular vesicles: Harnessing the power of EVs for diagnostics and therapeutics. Front. Mol. Biosci. 2023, 10, 1330400. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Kostallari, E.; Valainathan, S.; Biquard, L.; Shah, V.H.; Rautou, P.E. Role of extracellular vesicles in liver diseases and their therapeutic potential. Adv. Drug Deliv. Rev. 2021, 175, 113816. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Rey-Cadilhac, F.; Rachenne, F.; Missé, D.; Pompon, J. Viral Components Trafficking with(in) Extracellular Vesicles. Viruses 2023, 15, 2333. [Google Scholar] [CrossRef]
- Kakizaki, M.; Yamamoto, Y.; Otsuka, M.; Kitamura, K.; Ito, M.; Kawai, H.D.; Muramatsu, M.; Kagawa, T.; Kotani, A. Extracellular vesicles secreted by HBV-infected cells modulate HBV persistence in hydrodynamic HBV transfection mouse model. J. Biol. Chem. 2020, 295, 12449–12460. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Jeffrey, G.P.; Ramm, G.A.; Soekmadji, C. Pathogenesis of Viral Hepatitis-Induced Chronic Liver Disease: Role of Extracellular Vesicles. Front. Cell. Infect. Microbiol. 2020, 10, 587628. [Google Scholar] [CrossRef] [PubMed]
- Karamichali, E.; Foka, P.; Papadopoulou, G.; Loukaki-Gkountara, D.; Andresaki, K.; Koskinas, I.; Georgopoulou, U. Hepatitis Viruses Control Host Immune Responses by Modifying the Exosomal Biogenesis Pathway and Cargo. Int. J. Mol. Sci. 2022, 23, 10862. [Google Scholar] [CrossRef] [PubMed]
- Tahir, F.; Farooq, M.; Malik, M.A.; Manzoor, S. Extracellular Vesicles Contribute to Viral-Induced Hepatocellular Carcinoma: Understanding Their Involvement in Viral Hepatitis and Their Potential as Biomarkers for Early Hepatocellular Carcinoma Detection. Viral Immunol. 2024, 37, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Bi, Y.; Huang, Z.; Liao, C.; Li, X.; Hu, H.; Xie, H.; Huang, Y. CD69 Expression is Negatively Associated With T-Cell Immunity and Predicts Antiviral Therapy Response in Chronic Hepatitis B. Ann. Lab. Med. 2025, 45, 185–198. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, Y.; Zhang, Q.; Li, F.; Li, B.; Li, Z.; Dong, Y.; Lu, L.; Cai, X. Exosomes derived from hepatitis B virus-infected hepatocytes promote liver fibrosis via miR-222/TFRC axis. Cell Biol. Toxicol. 2023, 39, 467–481. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, Y.; Chen, Q.; Chen, Y.; Mao, L. Dual roles and potential applications of exosomes in HCV infections. Front. Microbiol. 2022, 13, 1044832. [Google Scholar] [CrossRef]
- Lee, C.; Han, J.; Jung, Y. Pathological Contribution of Extracellular Vesicles and Their MicroRNAs to Progression of Chronic Liver Disease. Biology 2022, 11, 637. [Google Scholar] [CrossRef]
- Grossini, E.; Smirne, C.; Venkatesan, S.; Tonello, S.; D’Onghia, D.; Minisini, R.; Cantaluppi, V.; Sainaghi, P.P.; Comi, C.; Tanzi, A.; et al. Plasma Pattern of Extracellular Vesicles Isolated from Hepatitis C Virus Patients and Their Effects on Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 10197. [Google Scholar] [CrossRef]
- Ye, B.; Shen, Y.; Chen, H.; Lin, S.; Mao, W.; Dong, Y.; Li, X. Differential proteomic analysis of plasma-derived exosomes as diagnostic biomarkers for chronic HBV-related liver disease. Sci. Rep. 2022, 12, 14428. [Google Scholar] [CrossRef]
- Cairoli, V.; Valle-Millares, D.; Terrón-Orellano, M.C.; Luque, D.; Ryan, P.; Dominguez, L.; Martín-Carbonero, L.; De Los Santos, I.; De Matteo, E.; Ameigeiras, B.; et al. MicroRNA signature from extracellular vesicles of HCV/HIV co-infected individuals differs from HCV mono-infected. J. Mol. Med. 2023, 101, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Rodriguez, L.A.; Schmittdiel, J.A.; Liu, L.; Macdonald, B.A.; Balasubramanian, S.; Chai, K.P.; Seo, S.I.; Mukhtar, N.; Levin, T.R.; Saxena, V. Hepatocellular Carcinoma in Metabolic Dysfunction-Associated Steatotic Liver Disease. JAMA Netw. Open 2024, 7, e2421019. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Jha, P.; Kleiner, D.E. Digital pathology for nonalcoholic steatohepatitis assessment. Nat. reviews. Gastroenterol. Hepatol. 2024, 21, 57–69. [Google Scholar] [CrossRef]
- Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Deutsch, M.; Aloizos, G.; Fortis, S.P.; Papageorgiou, E.G.; Tsagarakis, A.; Manolakopoulos, S. The Emerging Role of Extracellular Vesicles and Autophagy Machinery in NASH-Future Horizons in NASH Management. Int. J. Mol. Sci. 2022, 23, 12185. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, C.; Liu, M. The Role of Extracellular Vesicles in Liver Fibrosis: Friends or Foes? Biomedicines 2024, 12, 2665. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Muller, K.; Rowland, A. Circulating cell-specific extracellular vesicles as biomarkers for the diagnosis and monitoring of chronic liver diseases. Cell. Mol. Life Sci. CMLS 2022, 79, 232. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Eguchi, A.; Li, H.; Johnson, C.D.; Papouchado, B.G.; Wree, A.; Messer, K.; Feldstein, A.E. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE 2014, 9, e113651. [Google Scholar] [CrossRef]
- Wang, J.; Bao, S.; An, Q.; Li, C.; Feng, J. Roles of extracellular vesicles from different origins in metabolic-associated fatty liver disease: Progress and perspectives. Front. Immunol. 2025, 16, 1544012. [Google Scholar] [CrossRef]
- Iturbe-Rey, S.; Maccali, C.; Arrese, M.; Aspichueta, P.; Oliveira, C.P.; Castro, R.E.; Lapitz, A.; Izquierdo-Sanchez, L.; Bujanda, L.; Perugorria, M.J.; et al. Lipotoxicity-driven metabolic dysfunction-associated steatotic liver disease (MASLD). Atherosclerosis 2025, 400, 119053. [Google Scholar] [CrossRef]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef]
- Boccatonda, A.; Del Cane, L.; Marola, L.; D’Ardes, D.; Lessiani, G.; di Gregorio, N.; Ferri, C.; Cipollone, F.; Serra, C.; Santilli, F.; et al. Platelet, Antiplatelet Therapy and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Narrative Review. Life 2024, 14, 473. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Y.; Fu, Q.; Chen, M.; Liang, H.; Chen, X.; Long, X.; Zhang, B.; Zhao, J.; Chen, Q. Novel insights into the role of immunomodulatory extracellular vesicles in the pathogenesis of liver fibrosis. Biomark. Res. 2024, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, A.; Hamilton, T.; Carter, N.; Brown, M.; McPheat, W.; Dobrian, A. Endothelial Extracellular Vesicles: From Keepers of Health to Messengers of Disease. Int. J. Mol. Sci. 2021, 22, 4640. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Buelna, M.; Ramirez-Lopez, I.G.; San Juan-Garcia, C.A.; Garcia-Regalado, J.J.; Millan-Sanchez, M.S.; de la Cruz-Mosso, U.; Haramati, J.; Pereira-Suarez, A.L.; Macias-Barragan, J. Contribution of extracellular vesicles to steatosis-related liver disease and their therapeutic potential. World J. Hepatol. 2024, 16, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Saldaña, H.A.; Fernández-Garza, L.E.; Barrera-Barrera, S.A. Liquid biopsy in chronic liver disease. Ann. Hepatol. 2021, 20, 100197. [Google Scholar] [CrossRef]
- Balaphas, A.; Meyer, J.; Sadoul, K.; Fontana, P.; Morel, P.; Gonelle-Gispert, C.; Bühler, L.H. Platelets and Platelet-Derived Extracellular Vesicles in Liver Physiology and Disease. Hepatol. Commun. 2019, 3, 855–866. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Tveden-Nyborg, P. Extracellular Vesicles as Drivers of Non-Alcoholic Fatty Liver Disease: Small Particles with Big Impact. Biomedicines 2021, 9, 93. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.F.; Jiang, S.; Wu, J.; Liu, J.; Yuan, X.W.; Shen, D.; Zhang, J.Z.; Zhou, N.; He, J.; et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 2020, 11, 719. [Google Scholar] [CrossRef]
- Tomita, K.; Freeman, B.L.; Bronk, S.F.; LeBrasseur, N.K.; White, T.A.; Hirsova, P.; Ibrahim, S.H. CXCL10-Mediates Macrophage, but not Other Innate Immune Cells-Associated Inflammation in Murine Nonalcoholic Steatohepatitis. Sci. Rep. 2016, 6, 28786. [Google Scholar] [CrossRef]
- Srinivas, A.N.; Suresh, D.; Santhekadur, P.K.; Suvarna, D.; Kumar, D.P. Extracellular Vesicles as Inflammatory Drivers in NAFLD. Front. Immunol. 2020, 11, 627424. [Google Scholar] [CrossRef]
- Svobodová, G.; Horní, M.; Velecká, E.; Boušová, I. Metabolic dysfunction-associated steatotic liver disease-induced changes in the antioxidant system: A review. Arch. Toxicol. 2025, 99, 1–22. [Google Scholar] [CrossRef]
- Zhu, C.; Huai, Q.; Zhang, X.; Dai, H.; Li, X.; Wang, H. Insights into the roles and pathomechanisms of ceramide and sphigosine-1-phosphate in nonalcoholic fatty liver disease. Int. J. Biol. Sci. 2023, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Cao, H.X.; Wang, B.C.; Xin, F.Z.; Zhang, R.N.; Zhou, D.; Yang, R.X.; Zhao, Z.H.; Pan, Q.; Fan, J.G. miR-192-5p regulates lipid synthesis in non-alcoholic fatty liver disease through SCD-1. World J. Gastroenterol. 2017, 23, 8140–8151. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Furuta, K.; Lucien, F.; Gutierrez Sanchez, L.H.; Hirsova, P.; Krishnan, A.; Kabashima, A.; Pavelko, K.D.; Madden, B.; Alhuwaish, H.; et al. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J. Hepatol. 2019, 71, 1193–1205. [Google Scholar] [CrossRef]
- Hochreuter, M.Y.; Dall, M.; Treebak, J.T.; Barrès, R. MicroRNAs in non-alcoholic fatty liver disease: Progress and perspectives. Mol. Metab. 2022, 65, 101581. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Kawai, H.; Nakashima, K.; Nakaseko, Y.; Suto, D.; Yanagida, K.; Hashidate-Yoshida, T.; Mori, T.; Yoshio, S.; Ohtake, T.; et al. Sphingosine-1-phosphate promotes liver fibrosis in metabolic dysfunction-associated steatohepatitis. PLoS ONE 2024, 19, e0303296. [Google Scholar] [CrossRef]
- Grønbæk, H.; Mellemkjær, A.; Nielsen, S.; Magkos, F. The vascular endothelial growth factor system-a new player in the pathogenesis and development of metabolic dysfunction-associated steatotic liver disease. Hepatobiliary Surg. Nutr. 2023, 12, 963–965. [Google Scholar] [CrossRef]
- Dai, Q.; Ain, Q.; Seth, N.; Rooney, M.; Zipprich, A. Liver sinusoidal endothelial cells: Friend or foe in metabolic dysfunction- associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis. Dig. Liver Dis. 2025, 57, 493–503. [Google Scholar] [CrossRef]
- Povero, D.; Panera, N.; Eguchi, A.; Johnson, C.D.; Papouchado, B.G.; de Araujo Horcel, L.; Pinatel, E.M.; Alisi, A.; Nobili, V.; Feldstein, A.E. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 646–663.e644. [Google Scholar] [CrossRef]
- Luo, X.; Luo, S.Z.; Xu, Z.X.; Zhou, C.; Li, Z.H.; Zhou, X.Y.; Xu, M.Y. Lipotoxic hepatocyte-derived exosomal miR-1297 promotes hepatic stellate cell activation through the PTEN signaling pathway in metabolic-associated fatty liver disease. World J. Gastroenterol. 2021, 27, 1419–1434. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J. Targeting extracellular vesicles-mediated hepatic inflammation as a therapeutic strategy in liver diseases. Liver Int. 2020, 40, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, F.; Cheng, F.; Zhu, L.; Yan, Y. Lipotoxic hepatocyte derived LIMA1 enriched small extracellular vesicles promote hepatic stellate cells activation via inhibiting mitophagy. Cell. Mol. Biol. Lett. 2024, 29, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Xia, M.; Salas, S.S.; Ospina, J.A.; Buist-Homan, M.; Harmsen, M.C.; Moshage, H. Extracellular vesicles derived from liver sinusoidal endothelial cells inhibit the activation of hepatic stellate cells and Kupffer cells in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167020. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Hernández, R.; Rojas, Á.; Gato, S.; Gallego, J.; Gil-Gómez, A.; Castro, M.J.; Ampuero, J.; Romero-Gómez, M. Extracellular Vesicles as Biomarkers in Liver Disease. Int. J. Mol. Sci. 2022, 23, 16217. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, M.; Jiang, X.; Yu, H.; Qiu, Y.; Yu, J.; Yang, F.; Bao, Z. Identifying SLC27A5 as a potential prognostic marker of hepatocellular carcinoma by weighted gene co-expression network analysis and in vitro assays. Cancer Cell Int. 2021, 21, 174. [Google Scholar] [CrossRef]
- Ferro, A.; Saccu, G.; Mattivi, S.; Gaido, A.; Herrera Sanchez, M.B.; Haque, S.; Silengo, L.; Altruda, F.; Durazzo, M.; Fagoonee, S. Extracellular Vesicles as Delivery Vehicles for Non-Coding RNAs: Potential Biomarkers for Chronic Liver Diseases. Biomolecules 2024, 14, 277. [Google Scholar] [CrossRef]
- Hu, M.; Huang, H.; Jia, M.; Xu, M.; Chen, M.; Wu, J.; Gu, S.; Liang, H.; Zhou, H.; Gong, Y. A panel of miRNAs in the serum extracellular vesicles serve as novel diagnostic biomarkers for MASLD. Biomed. J. 2025, 100838. [Google Scholar] [CrossRef]
- Erceg, S.; Munjas, J.; Sopić, M.; Tomašević, R.; Mitrović, M.; Kotur-Stevuljević, J.; Mamić, M.; Vujčić, S.; Klisic, A.; Ninić, A. Expression Analysis of Circulating miR-21, miR-34a and miR-122 and Redox Status Markers in Metabolic Dysfunction-Associated Steatotic Liver Disease Patients with and Without Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 2392. [Google Scholar] [CrossRef]
- Nie, Z.; Xiao, C.; Wang, Y.; Li, R.; Zhao, F. Heat shock proteins (HSPs) in non-alcoholic fatty liver disease (NAFLD): From molecular mechanisms to therapeutic avenues. Biomark. Res. 2024, 12, 120. [Google Scholar] [CrossRef]
- Welsh, J.A.; Scorletti, E.; Clough, G.F.; Englyst, N.A.; Byrne, C.D. Leukocyte extracellular vesicle concentration is inversely associated with liver fibrosis severity in NAFLD. J. Leukoc. Biol. 2018, 104, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Amrollahi, P.; Parthasarathy, G.; Mauer, A.S.; Sehrawat, T.S.; Vanderboom, P.; Nair, K.S.; Nakao, K.; Allen, A.M.; Hu, T.Y.; et al. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomed. Nanotechnol. Biol. Med. 2021, 36, 102430. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Massaro, J.M.; Hoffmann, U.; Benjamin, E.J.; Naimi, T.S. Alcohol Use Is Associated With Hepatic Steatosis Among Persons with Presumed Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 1831–1841.e1835. [Google Scholar] [CrossRef]
- Saha, B.; Momen-Heravi, F.; Furi, I.; Kodys, K.; Catalano, D.; Gangopadhyay, A.; Haraszti, R.; Satishchandran, A.; Iracheta-Vellve, A.; Adejumo, A.; et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology 2018, 67, 1986–2000. [Google Scholar] [CrossRef]
- Cho, Y.E.; Song, B.J.; Akbar, M.; Baek, M.C. Extracellular vesicles as potential biomarkers for alcohol- and drug-induced liver injury and their therapeutic applications. Pharmacol. Ther. 2018, 187, 180–194. [Google Scholar] [CrossRef]
- Rahman, M.A.; Patters, B.J.; Kodidela, S.; Kumar, S. Extracellular Vesicles: Intercellular Mediators in Alcohol-Induced Pathologies. J. Neuroimmune Pharmacol. 2020, 15, 409–421. [Google Scholar] [CrossRef]
- Eguchi, A.; Yan, R.; Pan, S.Q.; Wu, R.; Kim, J.; Chen, Y.; Ansong, C.; Smith, R.D.; Tempaku, M.; Ohno-Machado, L.; et al. Comprehensive characterization of hepatocyte-derived extracellular vesicles identifies direct miRNA-based regulation of hepatic stellate cells and DAMP-based hepatic macrophage IL-1β and IL-17 upregulation in alcoholic hepatitis mice. J. Mol. Med. 2020, 98, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Li, H.; Wang, R.; Hirsova, P.; Mushref, M.; Liu, Y.; Cao, S.; Contreras, P.C.; Malhi, H.; Kamath, P.S.; et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J. Hepatol. 2016, 64, 651–660. [Google Scholar] [CrossRef]
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease outcomes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424. [Google Scholar] [CrossRef]
- Babuta, M.; Furi, I.; Bala, S.; Bukong, T.N.; Lowe, P.; Catalano, D.; Calenda, C.; Kodys, K.; Szabo, G. Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease. Hepatology 2019, 70, 2123–2141. [Google Scholar] [CrossRef]
- Sehrawat, T.S.; Arab, J.P.; Liu, M.; Amrollahi, P.; Wan, M.; Fan, J.; Nakao, Y.; Pose, E.; Navarro-Corcuera, A.; Dasgupta, D.; et al. Circulating Extracellular Vesicles Carrying Sphingolipid Cargo for the Diagnosis and Dynamic Risk Profiling of Alcoholic Hepatitis. Hepatology 2021, 73, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Schnabl, B. Acute alcohol-associated hepatitis: Latest findings in non-invasive biomarkers and treatment. Liver Int. 2025, 45, e15608. [Google Scholar] [CrossRef]
- Fleming, R.E.; Britton, R.S.; Waheed, A.; Sly, W.S.; Bacon, B.R. Pathophysiology of hereditary hemochromatosis. Semin. Liver Dis. 2005, 25, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Tantiworawit, A.; Leerapun, A.; Soontornpun, A.; Thongsawat, S. Secondary Iron Overload and the Liver: A Comprehensive Review. J. Clin. Transl. Hepatol. 2023, 11, 932–941. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Wu, Y.; Cui, N. The crosstalk between exosomes and ferroptosis: A review. Cell Death Discov. 2024, 10, 170. [Google Scholar] [CrossRef]
- Daou, Y.; Falabrègue, M.; Pourzand, C.; Peyssonnaux, C.; Edeas, M. Host and microbiota derived extracellular vesicles: Crucial players in iron homeostasis. Front. Med. 2022, 9, 985141. [Google Scholar] [CrossRef]
- Qu, H.; Zhou, L.; Wang, J.; Tang, D.; Zhang, Q.; Shi, J. Iron overload is closely associated with metabolic dysfunction-associated fatty liver disease in type 2 diabetes. Obesity 2025, 33, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Kong, Y.; Zheng, H.; Mi, D.; Katabuchi, M.; Motooka, Y.; Ito, F. Double-edged Sword Role of Iron-loaded Ferritin in Extracellular Vesicles. J. Cancer Prev. 2021, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, J.H.; Kim, S.E.; Jung, J.H.; Jang, M.K.; Park, S.H.; Lee, M.S.; Kim, H.S.; Suk, K.T.; Kim, D.J. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics. Biomedicines 2022, 10, 1288. [Google Scholar] [CrossRef]
- Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Vasileiadi, S.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Papageorgiou, E.G.; et al. The Arising Role of Extracellular Vesicles in Cholangiocarcinoma: A Rundown of the Current Knowledge Regarding Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 15563. [Google Scholar] [CrossRef]
- Olaizola, P.; Lee-Law, P.Y.; Arbelaiz, A.; Lapitz, A.; Perugorria, M.J.; Bujanda, L.; Banales, J.M. MicroRNAs and extracellular vesicles in cholangiopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Wang, Y.; Zhu, W.; Zhao, D.; Wang, X.; Yang, H.; Gurley, E.C.; Chen, W.; Hylemon, P.B.; et al. Cholangiocyte-Derived Exosomal lncRNA H19 Promotes Macrophage Activation and Hepatic Inflammation under Cholestatic Conditions. Cells 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Sucher, E.; Sucher, R.; Gradistanac, T.; Brandacher, G.; Schneeberger, S.; Berg, T. Autoimmune Hepatitis-Immunologically Triggered Liver Pathogenesis-Diagnostic and Therapeutic Strategies. J. Immunol. Res. 2019, 2019, 9437043. [Google Scholar] [CrossRef]
- Yang, F.; Ni, B.; Liang, X.; He, Y.; Yuan, C.; Chu, J.; Huang, Y.; Zhong, H.; Yang, L.; Lu, J.; et al. Mesenchymal stromal cell-derived extracellular vesicles as nanotherapeutics for concanavalin a-induced hepatitis: Modulating the gut-liver axis. Stem Cell Res. Ther. 2025, 16, 4. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Huang, M.; Wu, Y.; Jin, S.; Zhang, Y.; Gan, X.; Yu, T.; Yu, G.; Zhang, J.; et al. Mesenchymal Stem Cell-Derived Exosomes: Emerging as a Promising Cell-Free Therapeutic Strategy for Autoimmune Hepatitis. Biomolecules 2024, 14, 1353. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Piscaglia, F. Predictive role of microvesicles in cirrhotic patients: A promised land or a land of confusion? A narrative review. Ann. Hepatol. 2024, 30, 101563. [Google Scholar] [CrossRef]
- McConnell, M.J.; Kostallari, E.; Ibrahim, S.H.; Iwakiri, Y. The evolving role of liver sinusoidal endothelial cells in liver health and disease. Hepatology 2023, 78, 649–669. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.H. The emerging roles of extracellular vesicles as intercellular messengers in liver physiology and pathology. Clin. Mol. Hepatol. 2022, 28, 706–724. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Trebicka, J. Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy. JHEP Rep. Innov. Hepatol. 2021, 3, 100316. [Google Scholar] [CrossRef]
- Baweja, S.; Kumari, A.; Negi, P.; Thangariyal, S.; Subudhi, P.D.; Gautam, S.; Mittal, A.; Bihari, C. Vascular Extracellular Vesicles Indicate Severe Hepatopulmonary Syndrome in Cirrhosis. Diagnostics 2023, 13, 1272. [Google Scholar] [CrossRef]
- Conti, M.; Minniti, M.; Tiné, M.; De Francesco, M.; Gaeta, R.; Nieri, D.; Semenzato, U.; Biondini, D.; Camera, M.; Cosio, M.G.; et al. Extracellular Vesicles in Pulmonary Hypertension: A Dangerous Liaison? Biology 2023, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.; Bozbas, E.; Tannetta, S.D.; Alroqaiba, N.; Zhou, R.; Crawley, J.T.B.; Gibbins, J.M.; Jones, C.I.; Ahnström, J.; Yaqoob, P. Mode of induction of platelet-derived extracellular vesicles is a critical determinant of their phenotype and function. Sci. Rep. 2020, 10, 18061. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, A.; Stoess, C.; Sung, H.; Povero, D.; Eguchi, A.; Feldstein, A. Extracellular Vesicles as Therapeutic and Diagnostic Tools for Chronic Liver Diseases. Biomedicines 2023, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Severino, V.; Dumonceau, J.M.; Delhaye, M.; Moll, S.; Annessi-Ramseyer, I.; Robin, X.; Frossard, J.L.; Farina, A. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology 2017, 153, 495–504.e498. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, M.X.; Yu, M.C.; Ma, Q.W.; Huang, M.J.; Lu, C.W.; Chen, C.B.; Chung, W.H.; Chang, C.J. Altered microbiome of serum exosomes in patients with acute and chronic cholecystitis. BMC Microbiol. 2024, 24, 133. [Google Scholar] [CrossRef] [PubMed]
- Lapitz, A.; Arbelaiz, A.; Olaizola, P.; Aranburu, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Extracellular Vesicles in Hepatobiliary Malignancies. Front. Immunol. 2018, 9, 2270. [Google Scholar] [CrossRef]
- Lin, H.Z.; Zhang, T.; Chen, M.Y.; Shen, J.L. Novel biomarkers for the diagnosis and prognosis of gallbladder cancer. J. Dig. Dis. 2021, 22, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Hong, D.H.; Paudel, S.; Yoon, N.E.; Jung, B.H.; Kim, M.; Kim, T.H.; Jeong, J.; Choi, D.; Lee, H. Metabolomics and miRNA profiling reveals feature of gallbladder cancer-derived biliary extracellular vesicles. Biochem. Biophys. Res. Commun. 2024, 705, 149724. [Google Scholar] [CrossRef]
- Ueta, E.; Tsutsumi, K.; Kato, H.; Matsushita, H.; Shiraha, H.; Fujii, M.; Matsumoto, K.; Horiguchi, S.; Okada, H. Extracellular vesicle-shuttled miRNAs as a diagnostic and prognostic biomarker and their potential roles in gallbladder cancer patients. Sci. Rep. 2021, 11, 12298. [Google Scholar] [CrossRef]
- Priya, R.; Jain, V.; Akhtar, J.; Saklani, N.; Sakhuja, P.; Agarwal, A.K.; Polisetty, R.V.; Sirdeshmukh, R.; Kar, S.; Gautam, P. Proteomic profiling of cell line-derived extracellular vesicles to identify candidate circulatory markers for detection of gallbladder cancer. Front. Oncol. 2022, 12, 1027914. [Google Scholar] [CrossRef]
- Huang, D.-F.; Zhang, W.-J.; Chen, J.; Jiao, Z.-G.; Wang, X.-L.; Rao, D.-Y.; Li, W.-S.; Hu, D.; Xie, F.-F.; Wang, X.-X.; et al. Hepatocellular carcinoma cell-derived small extracellular vesicle-associated CD147 serves as a diagnostic marker and promotes endothelial cell angiogenesis via the PI3K/Akt pathway. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, L.; Wang, X.; Wang, Y.; Yu, J.; Li, M.; Ma, Z.; Chi-Lui Ho, P.; Chen, X.; Wang, L.; et al. Extracellular vesicles in the HCC microenvironment: Implications for therapy and biomarkers. Pharmacol. Res. 2024, 209, 107419. [Google Scholar] [CrossRef] [PubMed]
- Koustas, E.; Trifylli, E.M.; Sarantis, P.; Papadopoulos, N.; Papanikolopoulos, K.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Matthaios, D.; et al. An Insight into the Arising Role of MicroRNAs in Hepatocellular Carcinoma: Future Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 7168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Zhang, X.; Shao, T.; Luo, Y.; Wang, W.; Han, Y. Extracellular Vesicles and Hepatocellular Carcinoma: Opportunities and Challenges. Front. Oncol. 2022, 12, 884369. [Google Scholar] [CrossRef]
- Li, J.; Bao, H.; Huang, Z.; Liang, Z.; Wang, M.; Lin, N.; Ni, C.; Xu, Y. Little things with significant impact: miRNAs in hepatocellular carcinoma. Front. Oncol. 2023, 13, 1191070. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, F.; Guo, L.; Shi, J.; Wu, M.; Cheng, S.; Guo, W. Tumor cells derived-extracellular vesicles transfer miR-3129 to promote hepatocellular carcinoma metastasis by targeting TXNIP. Dig. Liver Dis. 2021, 53, 474–485. [Google Scholar] [CrossRef]
- Yamada, N.O.; Heishima, K.; Akao, Y.; Senda, T. Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4406. [Google Scholar] [CrossRef]
- Guo, J.; Wang, K.; Sun, Q.; Liu, J.; Zheng, J. Targeting B4GALT3 in BMSCs-EVs for Therapeutic Control of HCC via NF-κB pathway inhibition. Cell Biol. Toxicol. 2025, 41, 67. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Zhao, X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J. Hematol. Oncol. 2019, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Shoucair, I.; Weber Mello, F.; Jabalee, J.; Maleki, S.; Garnis, C. The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 6837. [Google Scholar] [CrossRef]
- Hu, W.; Liu, C.; Bi, Z.Y.; Zhou, Q.; Zhang, H.; Li, L.L.; Zhang, J.; Zhu, W.; Song, Y.Y.; Zhang, F.; et al. Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Mol. Cancer 2020, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Gondaliya, P.; Sayyed, A.A.; Driscoll, J.; Patel, K.; Patel, T. Extracellular vesicle RNA signaling in the liver tumor microenvironment. Cancer Lett. 2023, 558, 216089. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fan, L.; Yu, H.; Zhang, J.; He, Y.; Feng, D.; Wang, F.; Li, X.; Liu, Q.; Li, Y.; et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology 2019, 70, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Augello, G.; Cusimano, A.; Cervello, M.; Cusimano, A. Extracellular Vesicle-Related Non-Coding RNAs in Hepatocellular Carcinoma: An Overview. Cancers 2024, 16, 1415. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, Q.; Cao, K.; Sun, Y. Exosomes: Small vesicles with big roles in hepatocellular carcinoma. Oncotarget 2016, 7, 60687–60697. [Google Scholar] [CrossRef]
- Dai, W.; Wang, Y.; Yang, T.; Wang, J.; Wu, W.; Gu, J. Downregulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals. Cell Commun. Signal. CCS 2019, 17, 113. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sun, W.; Yue, S.; Yang, J.; Li, J.; Ma, B.; Wang, J.; Yang, X.; Pu, M.; et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017, 397, 33–42. [Google Scholar] [CrossRef]
- Conigliaro, A.; Costa, V.; Lo Dico, A.; Saieva, L.; Buccheri, S.; Dieli, F.; Manno, M.; Raccosta, S.; Mancone, C.; Tripodi, M.; et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 2015, 14, 155. [Google Scholar] [CrossRef]
- Wei, J.X.; Lv, L.H.; Wan, Y.L.; Cao, Y.; Li, G.L.; Lin, H.M.; Zhou, R.; Shang, C.Z.; Cao, J.; He, H.; et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 2015, 61, 1284–1294. [Google Scholar] [CrossRef]
- Wang, J.; Pu, J.; Zhang, Y.; Yao, T.; Luo, Z.; Li, W.; Xu, G.; Liu, J.; Wei, W.; Deng, Y. Exosome-transmitted long non-coding RNA SENP3-EIF4A1 suppresses the progression of hepatocellular carcinoma. Aging 2020, 12, 11550–11567. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.; Shi, Y.; Lu, Y.; Qiu, P.; Deng, Z.; Yao, W.; Wang, J. Exosomes in the tumor microenvironment of cholangiocarcinoma: Current status and future perspectives. J. Transl. Med. 2022, 20, 117. [Google Scholar] [CrossRef]
- Oliviero, B.; Dei Cas, M.; Zulueta, A.; Maiello, R.; Villa, A.; Martinelli, C.; Del Favero, E.; Falleni, M.; Montavoci, L.; Varchetta, S.; et al. Ceramide present in cholangiocarcinoma-derived extracellular vesicle induces a pro-inflammatory state in monocytes. Sci. Rep. 2023, 13, 7766. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Liu, C.G.; Xiang, X.; Le, M.T.N.; Sethi, G.; Wang, L.; Goh, B.C.; Ma, Z. The potential role of exosomal circRNAs in the tumor microenvironment: Insights into cancer diagnosis and therapy. Theranostics 2022, 12, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Trifylli, E.M. Role of exosomal circular RNAs as microRNA sponges and potential targeting for suppressing hepatocellular carcinoma growth and progression. World J. Gastroenterol. 2024, 30, 994–998. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Takahashi, K.; Wood, J.; Zubair, A.; Patel, T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J. Extracell. Vesicles 2015, 4, 24900. [Google Scholar] [CrossRef]
- Hadad, S.; Khalaji, A.; Sarmadian, A.J.; Sarmadian, P.J.; Janagard, E.M.; Baradaran, B. Tumor-associated macrophages derived exosomes; from pathogenesis to therapeutic opportunities. Int. Immunopharmacol. 2024, 136, 112406. [Google Scholar] [CrossRef]
- Li, L.; Piontek, K.; Ishida, M.; Fausther, M.; Dranoff, J.A.; Fu, R.; Mezey, E.; Gould, S.J.; Fordjour, F.K.; Meltzer, S.J.; et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017, 65, 501–514. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Allelein, S.; Medina-Perez, P.; Lopes, A.L.H.; Rau, S.; Hause, G.; Kölsch, A.; Kuhlmeier, D. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci. Rep. 2021, 11, 11585. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, S.; Jafari, N.; Tamadon, A.; Ghaffarzadeh, A.; Rahbarghazi, R.; Mahdipour, M. Different storage and freezing protocols for extracellular vesicles: A systematic review. Stem Cell Res. Ther. 2024, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- Stawarska, A.; Bamburowicz-Klimkowska, M.; Runden-Pran, E.; Dusinska, M.; Cimpan, M.R.; Rios-Mondragon, I.; Grudzinski, I.P. Extracellular Vesicles as Next-Generation Diagnostics and Advanced Therapy Medicinal Products. Int. J. Mol. Sci. 2024, 25, 6533. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanomed. 2025, 20, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).