A Mathematical Model of Metformin Action on COVID-19 Risk Infection in Cardiovascular Diabetic Patients Studied by FTIR Spectroscopy
Abstract
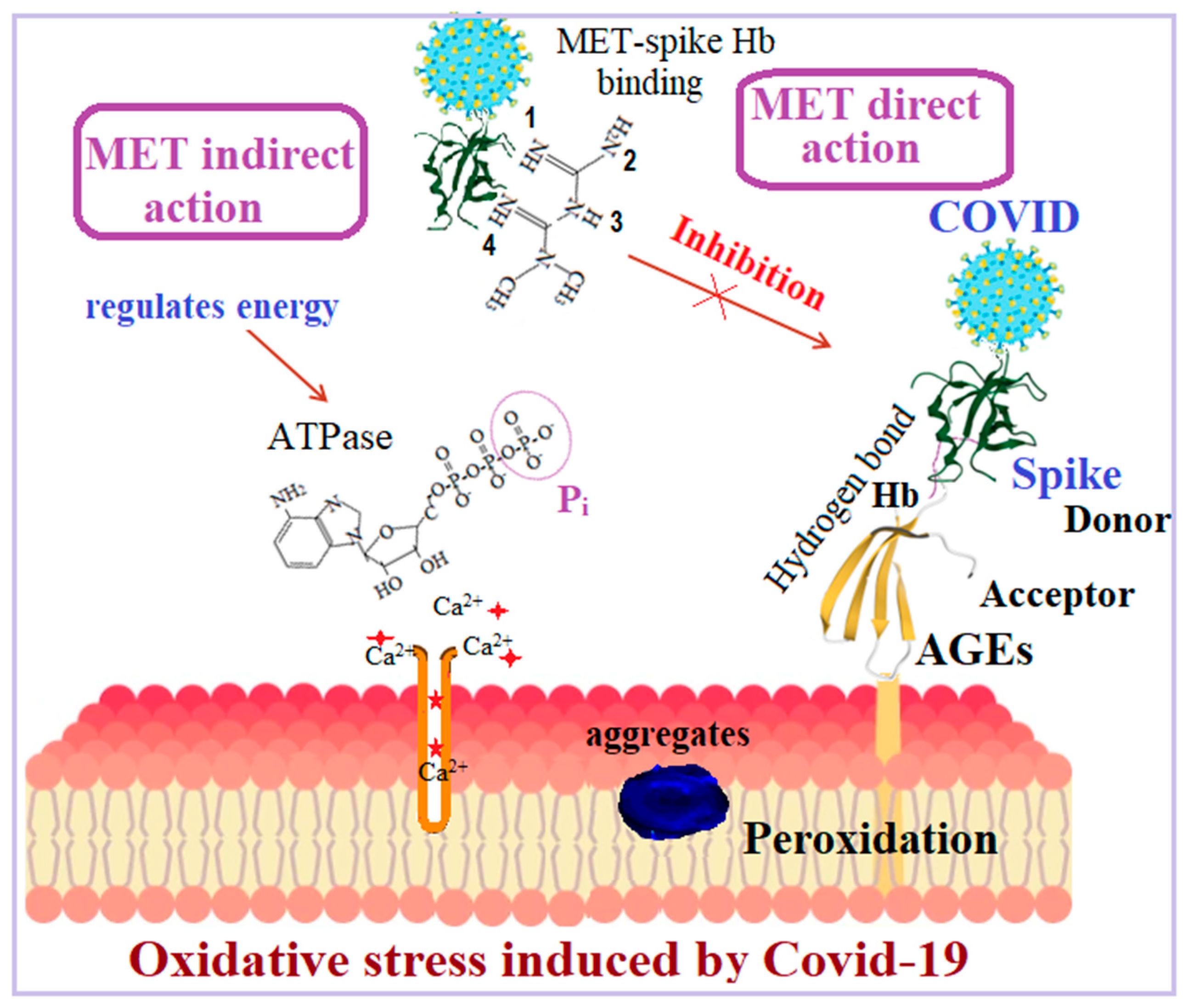
1. Introduction
2. Results
2.1. FTIR Spectra
2.2. Mathematical Model
3. Discussion
4. Materials and Methods
4.1. Mathematical Model
4.2. FTIR Spectroscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-Converting Enzyme 2 |
| AGEs | Advanced Glycation End Products |
| Arg | Arginine |
| ATPase | Adenosine Triphosphate kinase (enzyme) |
| FOC | Fractional-Order Calculus |
| FTIR | Fourier-Transform Infrared |
| GNPHO | Greek National Public Health Organization |
| H-bond | Hydrogen Bond |
| IgG | Immunoglobulin |
| MET | Metformin |
| NMR | Nuclear Magnetic Resonance |
| ODE | Ordinary Differential Equations |
| PECE | Predictor Corrector |
| SIR | Susceptible–Infected–Recovered |
| Tyr | Tyrosine |
| TZD | Thiazolidinedione |
References
- Kotoulas, C.; Mamarelis, I.; Koutoulakis, E.; Kyriakidou, M.; Mamareli, V.; Tanis, O.; Malesiou, E.; Theophanides, T.; Anastassopoulou, J. Evidence from the influence of diabetes on atherosclerosis and amyloid fibril formation of coronary arteries. An FT-IR spectroscopic study. Hell. J. Atheroscler. 2017, 8, 15–29. [Google Scholar]
- Know, C.S.; Ramachandram, D.S.; Hasan, S.S. Metformin therapy in COVID-19: Inhibition of NETosis. J. Thromb. Thrombolysis 2022, 54, 217–218. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Gonikman, D.; Kustovs, D. Antidiabetic Drug Efficacy in Reduction of Mortality during the COVID-19 Pandemic. Medicina 2023, 59, 1810. [Google Scholar] [CrossRef] [PubMed]
- Somasundarem, M.; Mathew, S.K.; Paul, S.; Kurian, S.J.; Kunhikatta, V.; Karanth, S.; Shetty, S.; Kudru, C.U.; Manu, M.K.; Saravu, K.; et al. Metformin use and its association with various outcomes in COVID-19 patients with diabetes mellitus: A retrospective cohort study in a tertiary care facility. Ann. Med. 2024, 56, 2425829. [Google Scholar] [CrossRef]
- Marín-Machuca, O.; Chacón, R.D.; Alvarez-Lovera, N.; Pesantes-Grados, P.; Pérez-Timaná, L.; Marín-Sánchez, O. Mathematical Modeling of COVID-19 Cases and Deaths and the Impact of Vaccinations during Three Years of the Pandemic in Peru. Vaccines 2023, 11, 1648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manabe, H.; Manabe, T.; Honda, Y.; Kawade, Y.; Kambayashi, D.; Manabe, Y.; Kudo, K. Simple mathematical model for predicting COVID-19 outbreaks in Japan based on epidemic waves with a cyclical trend. BMC Infect. Dis. 2024, 24, 465. [Google Scholar] [CrossRef]
- Jayatilaka, R.; Patela, R.; Brara, M.; Tanga, Y.; Jisrawia, N.M.; Chishtiec, F.; Drozdd, J.; Valluria, S.R. A mathematical model of COVID-19 transmission. Mater. Today Proc. 2021, 54, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Nave, O.; Hartuv, I.; Shemesh, U. Θ-SEIHRD mathematical model of COVID-19 stability analysis using fast-slow decomposition. PeerJ 2020, 8, e10019. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Mylonas, E.; Michali, E.; Lazopoulos, K.; Kotoulas, C.; Theophanides, T.; Mamarelis, I.; Markouizou, A.; Spiliopoulos, K. FT-IR spectroscopic study of coronary artery in cardiovascular patients and Mathematical simulation model of COVID-19 binding. Ann. Forensic Sci. Res. 2022, 1, 1006. [Google Scholar]
- Mylonas, E.; Filippakis, M.; Mamareli, C.; Anastassopoulou, J.; Mamarelis, I.; Theophanides, T. Mathematical Simulation of COVID-19 infection risk factor in cardiovascular diabetic patients. The effect of metformin and the act of viral volume. Atherosclerosis 2024, 395, 117627. [Google Scholar] [CrossRef]
- Mylonas, E.; Mamareli, C.; Filippakis, M.; Mamarelis, I.; Anastassopoulou, J.; Theophanides, T. A Mathematical Model of Statin Anti-Hyperlipidemic Drug Reactivity and Diverse Concentrations of Risk Toxicity. J. Clin. Med. 2025, 14, 2331. [Google Scholar] [CrossRef]
- Glöckler, J.; Mizaikoff, B.; Díaz de León-Martínez, L. SARS CoV-2 infection screening via the exhaled breath fingerprint obtained by FTIR spectroscopic gas-phase analysis. A proof of concept. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123066. [Google Scholar] [CrossRef]
- Markouizou, A.; Anastassopoulou, J.; Kolovou, P.; Theophanides, T.; Tsekeris, P. Fourier Transform Infrared spectroscopy in the study of discrimination of lobular breast cancers. Cancer Diagn. Progn. 2022, 2, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Theophanides, T.; Anastassopoulou, J.; Mavrogenis, A. Infrared and Raman spectroscopy of human diseases. Mol. Biophys. IJMS 2023. [Google Scholar]
- Barth, A.; Zscherp, C. What vibrations tell us about proteins. Q. Rev. Biophys. 2002, 35, 343–369. [Google Scholar] [CrossRef]
- Santos, M.C.D.; Morais, C.M.L.; Lima, K.M.G. ATR-FTIR Spectroscopy for Virus Identification: A Powerful Alternative. Biomed. Spectrosc. Imaging 2020, 9, 103–118. [Google Scholar] [CrossRef]
- Calvo-Gomez, O.; Calvo, H.; Cedillo-Barrón, L.; Vivanco-Cid, H.; Alvarado-Orozco, J.M.; Fernandez-Benavides, D.A.; Arriaga-Pizano, L.; Ferat-Osorio, E.; Anda-Garay, J.C.; López-Macias, C.; et al. Potential of ATR-FTIR-Chemometrics in COVID-19: Disease Recognition. ACS Omega 2022, 7, 30756–30767. [Google Scholar] [CrossRef]
- Bandeira, C.C.S.; Madureira, K.C.R.; Rossi, M.B.; Gallo, J.F.; Aguirra da Silva, A.P.R.; Torres, V.L.; Alves de Lima, V.; Júnior, N.K.; Almeida, J.D.; Zerbinati, R.M.; et al. Micro-Fourier-transform infrared reflectance spectroscopy as tool for probing IgG glycosylation in COVID-19 patients. Sci. Rep. 2022, 12, 4269. [Google Scholar] [CrossRef]
- Jizzini, M.; Shah, M.; Zhou, K. SARS-CoV-2 and Anti-Cardiolipin Antibodies. Clin. Med. Insights Case Rep. 2020, 13, 1179547620980381. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. Ser. A 1927, 115, 700–721. [Google Scholar]
- Almeida, R. A Caputo fractional derivative of a function with respect to another function. Commun. Nonlinear Sci. Numer. Simul. 2017, 44, 460–481. [Google Scholar] [CrossRef]
- Choi, H.W.; Choi, Y.J.; Chung, S.K. A predictor-corrector method for fractional evolution equations. Bull. Korean Math. Soc. 2016, 53, 1725–1739. [Google Scholar] [CrossRef]
- Garrappa, R. Predictor-Corrector PECE Method for Fractional Differential Equations. MATLAB Central File Exchange. Available online: https://www.mathworks.com/matlabcentral/fileexchange/32918-predictor-corrector-pece-method-for-fractional-dif-ferential-equations (accessed on 21 December 2024).
- Greek National Public Health Organization. Responsible for the Surveillance and Control of Diseases in Greece. Available online: https://eody.gov.gr/ (accessed on 21 December 2024).
- Akinosoglou, K.; Schinas, K.; Bletsa, E.; Bristianou, M.; Lanaras, L.; Michailides, C.; Katsikas, T.; Barkas, F.; Liberopoulos, E.; Kotsis, V.; et al. COVID-19 Outcomes and Diabetes Mellitus: A Comprehensive Multicenter Prospective Cohort Study. Microorganisms 2023, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Al-Salameh, A.; Lanoix, J.-P.; Bennis, Y.; Andrejak, C.; Brochot, E.; Deschasse, G.; Dupont, H.; Goeb, V.; Jaureguy, M.; Lion, S.; et al. Characteristics and outcomes of COVID-19 in hospitalized patients with and without diabetes. Diabetes Metab. Res. Rev. 2021, 37, e3388. [Google Scholar] [CrossRef]
- Wiernsperger, N.; Al-Salameh, A.; Cariou, B.; Lalau, J.D. Protection by metformin against severe COVID-19: An in-depth mechanistic analysis. Diabetes Metab. 2022, 48, 101359. [Google Scholar] [CrossRef]
- Yu, B.; Li, C.; Sun, Y.; Wang, D.W. Insulin treatment is associated with increased mortality in patients with COVID-19 and Type 2 diabetes. Cell Metab. 2021, 33, 65–77. [Google Scholar] [CrossRef]
- Sellegounder, D.; Zafari, P.; Rajabinejad, M.; Taghadosi, M.; Pankaj-Kapahi, P. Advanced glycation end products (AGEs) and its receptor, RAGE, modulate age-dependent COVID-19 morbidity and mortality. A review and hypothesis. Int. Immunopharmacol. 2021, 98, 107806. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Navarro, C.; Ortega, A.; Nava, M.; Morillo, D.; Torres, W.; Hernández, M.; Cabrera, M.; Angarita, M.; Ortiz, R.; et al. Advanced glycation end products: New clinical and molecular perspectives. Int. J. Environ. Res. Public Health 2021, 18, 7236. [Google Scholar] [CrossRef]
- Lenza, M.P.; Oyenarte, I.; Diercks, T.; Quintana, J.I.; Gimeno, A.; Coelho, H.; Diniz, A.; Peccati, F.; Delgado, S.; Bosch, A.; et al. Structural Characterization of N-Linked Glycans in the Receptor Binding Domain of the SARS-CoV-2 Spike Protein and their Interactions with Human Lectins. Angew. Chem. Int. Ed. 2020, 59, 23763–23771. [Google Scholar] [CrossRef]
- Raza, M.A.; Farwa, U.; Ain, N.Q.U.; Ishaque, F.; Yaseen, M.; Naveed, M.; Shabbir, M.A. Designing of Thiazolidinones for COVID-19 and its Allied Diseases: An In silic Evaluation. ChemistrySelect 2022, 7, e202201793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, S.; Jha, S.; Rani, P.; Kumar, S. Medical perspective of 2,4-thiazolidinediones derivatives: An insight into recent advancements. ChemistryOpen 2024, 13, e202400147. [Google Scholar] [CrossRef] [PubMed]
- Proto, M.C.; Fiore, D.; Piscopo, C.; Pagano, C.; Galgani, M.; Bruzzaniti, S.; Laezza, C.; Gazzerro, P.; Bifulco, M. Lipid homeostasis and mevalonate pathway in COVID-19: Basic concepts and potential therapeutic targets. Prog. Lipid Res. 2021, 82, 101099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jafary, F.; Jafari, S.; Ganjalikhany, M.R. In silico investigation of critical binding pattern in SARS-CoV-2 spike protein with angiotensin-converting enzyme 2. Sci. Rep. 2021, 11, 6927. [Google Scholar] [CrossRef]
- Cheng, X.; Xin, S.; Chen, Y.; Li, L.; Chen, W.; Li, W.; Zhou, B.; Li, C.; Gong, Y.; Li, F.; et al. Effects of metformin, insulin on COVID-19 patients with pre-existed type 2 diabetes: A multicentral retrospective study. Life Sci. 2021, 15, 275. [Google Scholar] [CrossRef]
- Wang, G.; Lin, F.; Wan, Q.; Wu, J.; Luo, M. Mechanisms of action of metformin and its regulatory effect on microRNAs related to angiogenesis. Pharm. Res. 2021, 164, 105390. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Mamarelis, I.; Theophanides, T. Study of the development of carotid artery atherosclerosis upon oxidative stress using infrared spectroscopy and scanning electron microscopy. OBM Geriatr. 2021, 5, 180. [Google Scholar] [CrossRef] [PubMed Central]



| Total | Hospitalized | Deaths |
|---|---|---|
| 339 | 276 | 63 |
| Metformin | 144 | 21 |
| Insulin | 72 | 14 |
| TZD | 60 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylonas, E.; Mamareli, C.; Filippakis, M.; Mamarelis, I.; Anastassopoulou, J.; Theophanides, T. A Mathematical Model of Metformin Action on COVID-19 Risk Infection in Cardiovascular Diabetic Patients Studied by FTIR Spectroscopy. Int. J. Mol. Sci. 2025, 26, 6332. https://doi.org/10.3390/ijms26136332
Mylonas E, Mamareli C, Filippakis M, Mamarelis I, Anastassopoulou J, Theophanides T. A Mathematical Model of Metformin Action on COVID-19 Risk Infection in Cardiovascular Diabetic Patients Studied by FTIR Spectroscopy. International Journal of Molecular Sciences. 2025; 26(13):6332. https://doi.org/10.3390/ijms26136332
Chicago/Turabian StyleMylonas, Evangelos, Christina Mamareli, Michael Filippakis, Ioannis Mamarelis, Jane Anastassopoulou, and Theophile Theophanides. 2025. "A Mathematical Model of Metformin Action on COVID-19 Risk Infection in Cardiovascular Diabetic Patients Studied by FTIR Spectroscopy" International Journal of Molecular Sciences 26, no. 13: 6332. https://doi.org/10.3390/ijms26136332
APA StyleMylonas, E., Mamareli, C., Filippakis, M., Mamarelis, I., Anastassopoulou, J., & Theophanides, T. (2025). A Mathematical Model of Metformin Action on COVID-19 Risk Infection in Cardiovascular Diabetic Patients Studied by FTIR Spectroscopy. International Journal of Molecular Sciences, 26(13), 6332. https://doi.org/10.3390/ijms26136332





