Right Ventricular Dynamics in Tricuspid Regurgitation: Insights into Reverse Remodeling and Outcome Prediction Post Transcatheter Valve Intervention
Abstract
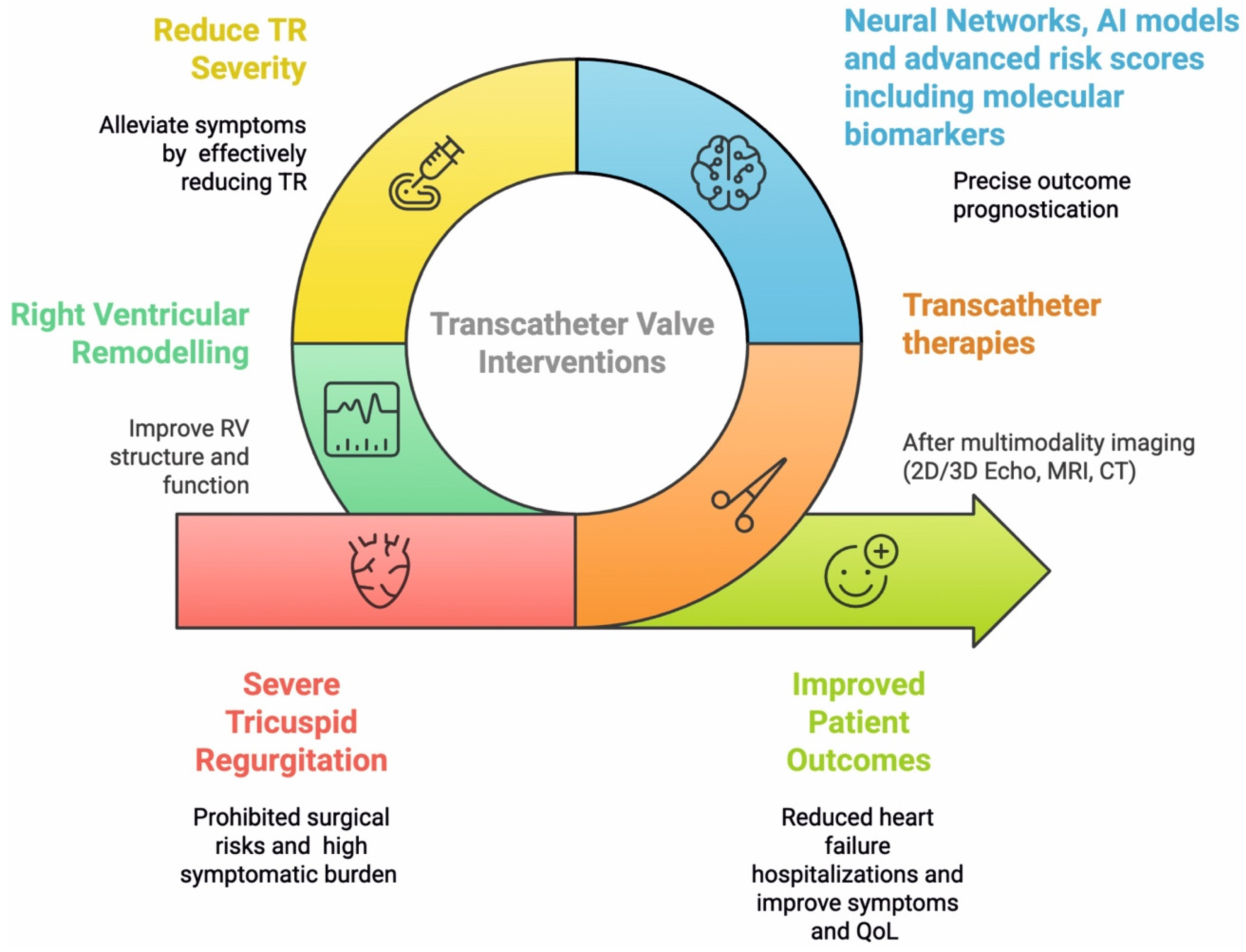
1. Introduction
2. Pathophysiological Landscape of Right Ventricular Remodeling in Tricuspid Regurgitation
2.1. Biomechanical Adaptations and Maladaptive Changes
2.2. Molecular Mechanisms of RV Maladaptive Remodeling
3. Assessing Right Ventricular Function and Evidence of Reverse Remodeling Post Transcatheter Interventions
3.1. Advanced Imaging for RV Function Assessment
3.2. Mechanisms and Manifestations of RVRR After TTVI
4. Predicting Outcomes and the Role of Artificial Intelligence
4.1. Clinical Importance of Outcome Prediction in TTVI
4.2. Biomechanical and Molecular Markers for RVRR Prediction
4.3. Leveraging Neural Networks and AI for Prognostication
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CMR | Cardiac magnetic resonance imaging |
| CT | Computed tomography |
| eGFR | Estimated glomerular filtration rate |
| IGFBP-2 | Insulin-like growth factor binding protein 2 |
| LV | Left ventricular/left ventricle |
| miRNAs | MicroRNAs |
| mPAP | Mean pulmonary arterial pressure |
| NT-proBNP | N-terminal pro–B-type natriuretic peptide |
| PASP | Pulmonary artery systolic pressure |
| PDK | Pyruvate dehydrogenase kinase |
| RA | Right atrial/right atrium |
| ROS | Reactive oxygen species |
| RV | Right ventricular/right ventricle |
| RVEDV | Right ventricular end-diastolic volume |
| RVEF | Right ventricular ejection fraction |
| RV GLS | Right ventricular global longitudinal strain |
| RV-PA | Right ventricular-pulmonary arterial |
| RVESV | Right ventricular end-systolic volume |
| RVRR | Right ventricular reverse remodeling |
| T-TEER | Transcatheter tricuspid valve edge-to-edge repair |
| TAPSE | Tricuspid annular plane systolic excursion |
| TR | Tricuspid regurgitation |
| TTVI | Transcatheter tricuspid valve interventions |
| TTVR | Transcatheter tricuspid valve replacement |
References
- Tagliari, A.P.; Taramasso, M. Transcatheter tricuspid interventions: Time to re-think guidelines? Aging 2020, 12, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, E.R.; Marinescu, D.; Florescu, C.; Donoiu, I.; Istrătoaie, O. Transcatheter Tricuspid Regurgitation Repair—An Overview of Techniques and Eligible Patient Selection. J. Clin. Med. 2024, 13, 6876. [Google Scholar] [CrossRef]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N. Engl. J. Med. 2023, 388, 1833–1842. [Google Scholar] [CrossRef]
- Weckbach, L.T.; Stolz, L.; Chatfield, A.G.; Fam, N.P.; Stephan von Bardeleben, R.; Davidson, C.J.; Hahn, R.T.; Hausleiter, J. Right Ventricular Reverse Remodeling After Transcatheter Tricuspid Valve Replacement in Patients with Heart Failure. J. Am. Coll. Cardiol. 2023, 81, 708–710. [Google Scholar] [CrossRef]
- Sharifi Kia, D.; Kim, K.; Simon, M.A. Current Understanding of the Right Ventricle Structure and Function in Pulmonary Arterial Hypertension. Front. Physiol. 2021, 12, 641310. [Google Scholar] [CrossRef]
- Rako, Z.A.; Kremer, N.; Yogeswaran, A.; Richter, M.J.; Tello, K. Adaptive versus maladaptive right ventricular remodelling. ESC Heart Fail. 2023, 10, 762–775. [Google Scholar] [CrossRef]
- Sharifi Kia, D.; Shen, Y.; Bachman, T.N.; Goncharova, E.A.; Kim, K.; Simon, M.A. The Effects of Healthy Aging on Right Ventricular Structure and Biomechanical Properties: A Pilot Study. Front. Med. 2021, 8, 751338. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, S.; Vang, A.; Mehdi, R.R.; Phelan, H.; Nicely, P.; Imran, T.; Zhang, P.; Choudhary, G.; Avazmohammadi, R. Right Ventricular Stiffening and Anisotropy Alterations in Pulmonary Hypertension: Mechanisms and Relations to Right Heart Failure. J. Am. Heart Assoc. 2025, 14, e037126. [Google Scholar] [CrossRef] [PubMed]
- Rain, S.; Handoko, M.L.; Trip, P.; Gan, C.T.; Westerhof, N.; Stienen, G.J.; Paulus, W.J.; Ottenheijm, C.A.; Marcus, J.T.; Dorfmüller, P.; et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013, 128, 2016–2025. [Google Scholar] [CrossRef]
- Wang, Z.; Patel, J.R.; Schreier, D.A.; Hacker, T.A.; Moss, R.L.; Chesler, N.C. Organ-level right ventricular dysfunction with preserved Frank-Starling mechanism in a mouse model of pulmonary arterial hypertension. J. Appl. Physiol. 2018, 124, 1244–1253. [Google Scholar] [CrossRef]
- Sommer, G.; Schriefl, A.J.; Andrä, M.; Sacherer, M.; Viertler, C.; Wolinski, H.; Holzapfel, G.A. Biomechanical properties and microstructure of human ventricular myocardium. Acta Biomater. 2015, 24, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.; Smerup, M.; Agger, P.; Frandsen, J.; Ringgard, S.; Pedersen, M.; Vestergaard, P.; Nyengaard, J.R.; Andersen, J.B.; Lunkenheimer, P.P.; et al. Normal right ventricular three-dimensional architecture, as assessed with diffusion tensor magnetic resonance imaging, is preserved during experimentally induced right ventricular hypertrophy. Anat. Rec. 2009, 292, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Badano, L.P.; Hahn, R.T.; Lang, R.M.; Delgado, V.; Wunderlich, N.C.; Donal, E.; Taramasso, M.; Duncan, A.; Lurz, P.; et al. Atrial secondary tricuspid regurgitation: Pathophysiology, definition, diagnosis, and treatment. Eur. Heart J. 2024, 45, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Lahm, T.; Hansmann, G.; Hemnes, A.R. Molecular mechanisms of right ventricular dysfunction in pulmonary arterial hypertension: Focus on the coronary vasculature, sex hormones, and glucose/lipid metabolism. Cardiovasc. Diagn. Ther. 2020, 10, 1522–1540. [Google Scholar] [CrossRef]
- Reddy, S.; Bernstein, D. Molecular Mechanisms of Right Ventricular Failure. Circulation 2015, 132, 1734–1742. [Google Scholar] [CrossRef]
- Schüttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. [Google Scholar] [CrossRef]
- Prihadi, E.A.; van der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients with Significant Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Kossaify, A. Echocardiographic Assessment of the Right Ventricle, from the Conventional Approach to Speckle Tracking and Three-Dimensional Imaging, and Insights into the “Right Way” to Explore the Forgotten Chamber. Clin. Med. Insights Cardiol. 2015, 9, 65–75. [Google Scholar] [CrossRef]
- Furlani, A.C.; Garcia, M.J. Right Ventricular Strain. Circ. Cardiovasc. Imaging 2019, 12, e008862. [Google Scholar] [CrossRef]
- Longobardo, L.; Suma, V.; Jain, R.; Carerj, S.; Zito, C.; Zwicke, D.L.; Khandheria, B.K. Role of Two-Dimensional Speckle-Tracking Echocardiography Strain in the Assessment of Right Ventricular Systolic Function and Comparison with Conventional Parameters. J. Am. Soc. Echocardiogr. 2017, 30, 937–946.e6. [Google Scholar] [CrossRef] [PubMed]
- Stolz, L.; Doldi, P.M.; Weckbach, L.T.; Stocker, T.J.; Braun, D.; Orban, M.; Wild, M.G.; Hagl, C.; Massberg, S.; Näbauer, M.; et al. Right ventricular function in transcatheter mitral and tricuspid valve edge-to-edge repair. Front. Cardiovasc. Med. 2022, 9, 993618. [Google Scholar] [CrossRef]
- Doldi, P.M.; Weckbach, L.T.; Fink, N.; Stolz, L.; Ennin, C.; Dinkel, J.; Lurz, P.; Thiele, H.; Hahn, R.T.; Cavalcante, J.L.; et al. 3D Echocardiographic and CMR Imaging for the Assessment of Right Ventricular Function and Tricuspid Regurgitation Severity. Circ. Cardiovasc. Imaging 2025, 18, e017638. [Google Scholar] [CrossRef]
- Ahn, Y.; Koo, H.J.; Kang, J.W.; Yang, D.H. Tricuspid Valve Imaging and Right Ventricular Function Analysis Using Cardiac CT and MRI. Korean J. Radiol. 2021, 22, 1946–1963. [Google Scholar] [CrossRef]
- Stolz, L.; Weckbach, L.T.; Glaser, H.; Doldi, P.M.; Schmid, S.; Stocker, T.J.; Hagl, C.; Näbauer, M.; Massberg, S.; Hausleiter, J. Biphasic Right Ventricular Reverse Remodeling Following Tricuspid Valve Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2024, 17, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Orban, M.; Braun, D.; Deseive, S.; Stolz, L.; Stocker, T.J.; Stark, K.; Stremmel, C.; Orban, M.; Hagl, C.; Massberg, S.; et al. Transcatheter Edge-to-Edge Repair for Tricuspid Regurgitation Is Associated with Right Ventricular Reverse Remodeling in Patients with Right-Sided Heart Failure. JACC Cardiovasc. Imaging 2019, 12, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Margonato, D. Unveiling Right Ventricle Remodeling Following Tricuspid Valve Intervention: New Light in the Dark. JACC Cardiovasc. Interv. 2024, 17, 2823–2825. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, L.T.; Stolz, L.; Doldi, P.M.; Glaser, H.; Ennin, C.; Kothieringer, M.; Stocker, T.J.; Näbauer, M.; Kassar, M.; Bombace, S.; et al. Relevance of residual tricuspid regurgitation for right ventricular reverse remodelling after tricuspid valve intervention in patients with severe tricuspid regurgitation and right-sided heart failure. Eur. J. Heart Fail. 2024. [Google Scholar] [CrossRef]
- Brener, M.I.; Lurz, P.; Hausleiter, J.; Rodés-Cabau, J.; Fam, N.; Kodali, S.K.; Rommel, K.P.; Muntané-Carol, G.; Gavazzoni, M.; Nazif, T.M.; et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J. Am. Coll. Cardiol. 2022, 79, 448–461. [Google Scholar] [CrossRef]
- Hahn, R.T.; Meduri, C.U.; Davidson, C.J.; Lim, S.; Nazif, T.M.; Ricciardi, M.J.; Rajagopal, V.; Ailawadi, G.; Vannan, M.A.; Thomas, J.D.; et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty: SCOUT Trial 30-Day Results. J. Am. Coll. Cardiol. 2017, 69, 1795–1806. [Google Scholar] [CrossRef]
- Rommel, K.P.; Besler, C.; Noack, T.; Blazek, S.; von Roeder, M.; Fengler, K.; Ender, J.; Gutberlet, M.; Desch, S.; Borger, M.A.; et al. Physiological and Clinical Consequences of Right Ventricular Volume Overload Reduction After Transcatheter Treatment for Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2019, 12, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Fortmeier, V.; Lachmann, M.; Stolz, L.; von Stein, J.; Rommel, K.P.; Kassar, M.; Gerçek, M.; Schöber, A.R.; Stocker, T.J.; Omran, H.; et al. Simplified Outcome Prediction in Patients Undergoing Transcatheter Tricuspid Valve Intervention by Survival Tree-Based Modelling. JACC Adv. 2025, 4, 101575. [Google Scholar] [CrossRef]
- Schlotter, F.; Miura, M.; Kresoja, K.P.; Alushi, B.; Alessandrini, H.; Attinger-Toller, A.; Besler, C.; Biasco, L.; Braun, D.; Brochet, E.; et al. Outcomes of transcatheter tricuspid valve intervention by right ventricular function: A multicentre propensity-matched analysis. EuroIntervention 2021, 17, e343–e352. [Google Scholar] [CrossRef]
- Lal, S. AIding, Not Replacing, Clinicians: A Machine-Based Learning Approach to Predict Successful Outcomes in Transcatheter Tricuspid Valve Interventions. JACC Adv. 2025, 4, 101574. [Google Scholar] [CrossRef]
- Cai, H.; Yu, C.; Li, X.; Wang, X.; Yang, Y.; Lan, C. Circulating miRNA-486 as a novel diagnostic biomarker for right ventricular remodeling. Front. Cardiovasc. Med. 2025, 12, 1518022. [Google Scholar] [CrossRef] [PubMed]
- Gröger, M.; Felbel, D.; Paukovitsch, M.; Schneider, L.M.; Markovic, S.; Rottbauer, W.; Keßler, M. Insulin-Like Growth Factor Binding Protein 2 Predicts Right Ventricular Reverse Remodeling and Improvement of Concomitant Tricuspid Regurgitation After Transcatheter Edge-to-Edge Mitral Valve Repair. Clin. Cardiol. 2024, 47, e70048. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Abu-Amero, K.K. Utility of circulating microRNAs as clinical biomarkers for cardiovascular diseases. Biomed. Res. Int. 2015, 2015, 821823. [Google Scholar] [CrossRef]
- Wang, W.; Yu, K.; Zhao, S.Y.; Mo, D.G.; Liu, J.H.; Han, L.J.; Li, T.; Yao, H.C. The impact of circulating IGF-1 and IGFBP-2 on cardiovascular prognosis in patients with acute coronary syndrome. Front. Cardiovasc. Med. 2023, 10, 1126093. [Google Scholar] [CrossRef]
- Goretti, E.; Vausort, M.; Wagner, D.R.; Devaux, Y. Association between circulating microRNAs, cardiovascular risk factors and outcome in patients with acute myocardial infarction. Int. J. Cardiol. 2013, 168, 4548–4550. [Google Scholar] [CrossRef]
- Kirchner, J.; Gerçek, M.; Omran, H.; Friedrichs, K.P.; Rudolph, F.; Rossnagel, T.; Piran, M.; Goncharov, A.; Ivannikova, M.; Rudolph, V.; et al. Predictive value of CT-based and AI-reconstructed 3D-TAPSE in patients undergoing transcatheter tricuspid valve repair. Front. Cardiovasc. Med. 2024, 11, 1463978. [Google Scholar] [CrossRef]
- Stolz, L.; Weckbach, L.T.; Doldi, P.M.; Stocker, T.J.; Trimborn, F.; Orban, M.; Näbauer, M.; Massberg, S.; Grayburn, P.; Hausleiter, J. Right Ventricular Reverse Remodeling Following Mitral Valve Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Imaging 2023, 16, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Rizk, R.; Chiu, C.; Zhang, J.J.; Scholl, J.L.; Bosch, T.J.; Singh, A.; Baugh, L.A.; McGough, J.S.; Santosh, K.C.; et al. The Heart of Transformation: Exploring Artificial Intelligence in Cardiovascular Disease. Biomedicines 2025, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Maturi, B.; Dulal, S.; Sayana, S.B.; Ibrahim, A.; Ramakrishna, M.; Chinta, V.; Sharma, A.; Ravipati, H. Revolutionizing Cardiology: The Role of Artificial Intelligence in Echocardiography. J. Clin. Med. 2025, 14, 625. [Google Scholar] [CrossRef]
- Lin, A.; Kolossváry, M.; Motwani, M.; Išgum, I.; Maurovich-Horvat, P.; Slomka, P.J.; Dey, D. Artificial Intelligence in Cardiovascular Imaging for Risk Stratification in Coronary Artery Disease. Radiol. Cardiothorac. Imaging 2021, 3, e200512. [Google Scholar] [CrossRef]
- Deb, B.; Scott, C.; Pislaru, S.V.; Nkomo, V.T.; Kane, G.C.; Alkhouli, M.; Crestanello, J.A.; Arruda-Olson, A.; Pellikka, P.A.; Anand, V. Machine learning facilitates the prediction of long-term mortality in patients with tricuspid regurgitation. Open Heart 2023, 10, e002417. [Google Scholar] [CrossRef]
- Vrudhula, A.; Vukadinovic, M.; Haeffele, C.; Kwan, A.C.; Berman, D.; Liang, D.; Siegel, R.; Cheng, S.; Ouyang, D. Automated Deep Learning Phenotyping of Tricuspid Regurgitation in Echocardiography. JAMA Cardiol. 2025, 10, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Tolu-Akinnawo, O.Z.; Ezekwueme, F.; Omolayo, O.; Batheja, S.; Awoyemi, T. Advancements in Artificial Intelligence in Noninvasive Cardiac Imaging: A Comprehensive Review. Clin. Cardiol. 2025, 48, e70087. [Google Scholar] [CrossRef]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef]
| Category | Molecular Factor | Role in Maladaptive Remodeling | Potential for RVRR |
|---|---|---|---|
| Oxidative Stress and Metabolism | Reactive Oxygen Species (ROS) | Increased production; early failure of antioxidant defenses (SOD, GPX) in RV vs. LV; greater mitochondrial ROS generation. Leads to damage and apoptosis. | Reduction in oxidative stress (e.g., with antioxidants like EUK-134) improves RV systolic function. |
| PGC1α | Decreased expression, leading to impaired fatty acid oxidation, reduced mitochondrial mass/number, decreased oxidative capacity, increased ROS, mitochondrial DNA damage. | Upregulation could restore metabolic function. | |
| HIF-1α | Activation associated with complex II-mediated ROS production in RVH; impaired angiogenic response (decreased VEGF, unchanged capillarity). | Modulation could improve angiogenesis and reduce ROS. | |
| Pyruvate Dehydrogenase Kinase (PDK) | Increased expression mediates shift to aerobic glycolysis (Warburg effect), reducing ATP efficiency and RV contractility. | Pharmacologic inhibition improves RV contractility. | |
| Fatty Acid Metabolism (Dysfunctional) | Decreased fatty acid oxidation, increased lipid accumulation, production of toxic intermediates (ceramide, palmitate), lipotoxic cardiomyopathy. | Restoration of fatty acid oxidation. | |
| Angiogenesis and Epigenetics | MicroRNAs (miR-143/145, miR-34, miR-379, miR-503, miR-126, miR-486) | Dysregulation in RV failure (vascular tone, apoptosis, endothelial proliferation, VEGF pathway inhibition). | Targeted delivery (e.g., miR-126) shows increased RV vascularity/function. Circulating miR-486 is a diagnostic biomarker for maladaptive RV remodeling. |
| IGFBP-2 (Insulin-Like Growth Factor Binding Protein 2) | Elevated levels at baseline predict non-development of RVRR and persistent TR/RV dilation after M-TEER. | Lower levels associated with RVRR. | |
| Signaling Pathways | PI3K/Akt/mTOR pathway | Inactivated in pathological hypertrophy (pressure overload). | Activated in physiological hypertrophy (exercise-induced); enhances ventricular hypertrophy and function, suppresses apoptosis; gene therapy with constitutively active PI3K can improve function. |
| Modality | Strengths | Parameters Measured | Utility for RVRR | Limitations |
|---|---|---|---|---|
| Echocardiography (2D and 3D) | First-line, widely available, real-time, non-invasive. 2D strain (speckle tracking) is angle-independent, sensitive for early dysfunction, good reproducibility. 3D echo provides comprehensive anatomical and volumetric assessment. | TAPSE, RVFAC, RVMPI, RV-Sa (conventional). RV peak systolic strain (RVPSS), RV global longitudinal strain (RV GLS). RV volumes (RVEDV3D, RVESV3D), RVEF3D (3D echo). | Early detection of myocardial deformation impairment. Quantifies RV volume unloading and structural remodeling. Measures improvement in RV FW-GLS and RV-PA coupling (TAPSE/PASP). | 2D methods limited by suboptimal acoustic windows, geometric assumptions, and inability to capture complex 3D anatomy. 2D-TAPSE alone may fail to predict outcomes. |
| Cardiac Magnetic Resonance Imaging (CMR) | Gold standard for volumetric quantification of RV. Excellent endocardial definition, no ionizing radiation. Quantitative TR measurement via phase-contrast imaging. Can assess regional RV performance with tissue tagging. | RV volumes (end-diastolic, end-systolic), RVEF, RV mass. TR regurgitant volume, TR fraction. Regional shortening. | Precise quantification of RV volume reduction and changes in EF. Baseline RVESV (CMR-derived) is a strong predictor of RVRR. | Impractical for some patients (inability to stay supine, implanted devices). Less available than echo. |
| Cardiac Computed Tomography (CT) | Detailed anatomy of TV and RV. Full-cycle CT captures complex RV anatomy and annular plane dynamics. AI-augmented software automates post-processing and quantification. | RV volumes (RVEDV, RVESV), RVEF. CT-based 3D-TAPSE (anterior, posterior, septal, lateral), iTAPSE, iTAPSE volume. | Substantial reduction in RV-EDV after TTVR (35%). Posterior iTAPSE and iTAPSE volume are independent predictors of cardiovascular outcomes after TTVI. | Ionizing radiation. Contrast use. |
| Biomechanical Change/Marker | Observed Change | Impact on RV |
|---|---|---|
| Tricuspid Regurgitation (TR) Severity Reduction | Immediate and significant reduction in TR grade (e.g., to ≤2+ in 83% of patients at 6 months, 41% reduction in vena contracta, 50% in TR volume, 54% in EROA). Optimal procedural results (residual TR ≤ 1+) associated with more pronounced RVRR. | Reduces RV volume overload and wall stress, improving cardiac efficiency. |
| RV Volume Reduction (Reverse Remodeling) | Biphasic pattern: early RV volume unloading (RVEDV reduction, e.g., −9.7% at discharge, −35% after TTVR) and later structural remodeling (RVESV reduction, e.g., −5.4% at 6 months). Average RV and TV dimensions decrease significantly. | Improved RV geometry, reduced wall stress, and enhanced cardiac output. Associated with improved survival. |
| RV Ejection Fraction (RVEF) | May initially decline post-TTVI but gradually increases over time, returning to baseline values by 2 years. Effective RVEF improves immediately post-procedure. | Reflects improved pump function and overall RV efficiency. |
| RV Global Longitudinal Strain (RV GLS) | Initial decline followed by late recovery to pre-procedural baseline values. Improvement in RV FW-GLS is a definition of RVRR (>10% improvement). | Direct assessment of myocardial deformation, indicating improved contractility and less impairment. |
| RV-Pulmonary Artery (RV-PA) Coupling (TAPSE/PASP ratio) | Significant improvement (e.g., from 0.36 to 0.42). Improvement in RV-PA coupling is a definition of RVRR (>10% improvement). | Reflects improved RV efficiency in handling afterload, crucial for prognosis. |
| Biventricular Interaction | Reduction in RV volume overload improves biventricular interaction, alleviating leftward bowing of the septum and improving early LV filling. Increased LV forward stroke volume (e.g., 30% increase). | Enhanced overall cardiac output and systemic perfusion. |
| Model Type | Input Data Types | Key Input Parameters (Examples) | Risk Stratification (Example Cut-Offs) | Model Output | Utility/ Significance |
|---|---|---|---|---|---|
| Survival Tree-Based Model | Preprocedural clinical, laboratory, echocardiographic, and hemodynamic data. | Mean pulmonary artery pressure (mPAP), NT-proBNP levels, Right Atrial (RA) area, Estimated Glomerular Filtration Rate (eGFR). | Low-Risk: mPAP ≤ 28 mmHg AND NT-proBNP ≤ 2728 pg/mL (2-year survival: 85.5%) High-Risk: mPAP > 28 mmHg AND RA area > 32.5 cm2 AND eGFR ≤ 51 mL/min (2-year survival: 52.6%) | 2-year survival rate. | Effectively stratifies patients into distinct risk categories, comparable to TRI-Score and outperforms EuroScore II in identifying high-risk patients. Informs patient selection and personalized treatment. |
| Penalized Cox Proportional Hazard Regression, Random Survival Forest (RSF), Extreme Gradient Boosting | 27 clinical and echocardiographic features from echocardiography reports and electronic medical records. | Heart rate, right ventricular systolic pressure (RVSP), blood pressure, diuretic use, age, BMI, chronic kidney disease, prior cardiac surgery, signs of congestion/hypoperfusion (AST, creatinine, hyponatremia), LV ejection fraction, LV end-diastolic dimension. | Identifies top contributing features for mortality prediction. | 1-year and 3-year mortality prediction (C-index 0.74–0.75 for 1-year). | Good overall performance in predicting long-term mortality in TR patients. Conditional RSF often ranks highest. |
| Deep Learning for RVEF Prediction from 2D Echo | 2D apical 4-chamber view echocardiographic videos. | Video frames processed by convolutional networks. | Reduced RVEF < 45% (significantly worse 1-year survival: 80.3% vs. 92.1%). | Predicted RVEF. | Refines prognostication, superior to conventional 2D TAPSE in predicting 1-year mortality. Can screen for patients needing intensified follow-up. |
| AI-augmented CT analysis for 3D-TAPSE | Full cardiac cycle CT images (axial thin slices). | 3D tricuspid annulus dynamics (anterior, posterior, septal, lateral TAPSE measurements, iTAPSE volume). | Posterior iTAPSE > 4.5 mm/m2 (1-year combined endpoint: 17.2% vs. 63.6%). iTAPSE volume > 9 mL/m2 (1-year combined endpoint: 16.4% vs. 57.1%). | CT-based 3D-TAPSE values, prediction of hospitalization and mortality. | Automates complex measurements, provides incremental predictive value over 2D-TAPSE, refines risk stratification. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doldi, P.M.; Thienel, M.; Willy, K. Right Ventricular Dynamics in Tricuspid Regurgitation: Insights into Reverse Remodeling and Outcome Prediction Post Transcatheter Valve Intervention. Int. J. Mol. Sci. 2025, 26, 6322. https://doi.org/10.3390/ijms26136322
Doldi PM, Thienel M, Willy K. Right Ventricular Dynamics in Tricuspid Regurgitation: Insights into Reverse Remodeling and Outcome Prediction Post Transcatheter Valve Intervention. International Journal of Molecular Sciences. 2025; 26(13):6322. https://doi.org/10.3390/ijms26136322
Chicago/Turabian StyleDoldi, Philipp M., Manuela Thienel, and Kevin Willy. 2025. "Right Ventricular Dynamics in Tricuspid Regurgitation: Insights into Reverse Remodeling and Outcome Prediction Post Transcatheter Valve Intervention" International Journal of Molecular Sciences 26, no. 13: 6322. https://doi.org/10.3390/ijms26136322
APA StyleDoldi, P. M., Thienel, M., & Willy, K. (2025). Right Ventricular Dynamics in Tricuspid Regurgitation: Insights into Reverse Remodeling and Outcome Prediction Post Transcatheter Valve Intervention. International Journal of Molecular Sciences, 26(13), 6322. https://doi.org/10.3390/ijms26136322







