Designing a Multi-Epitope Vaccine Against MPXV and HIV Based on an Immunoinformatic Approach
Abstract
1. Introduction
2. Results
2.1. T-Cell Epitopes
2.2. Linear B-Cell Epitopes
2.3. Population Coverage
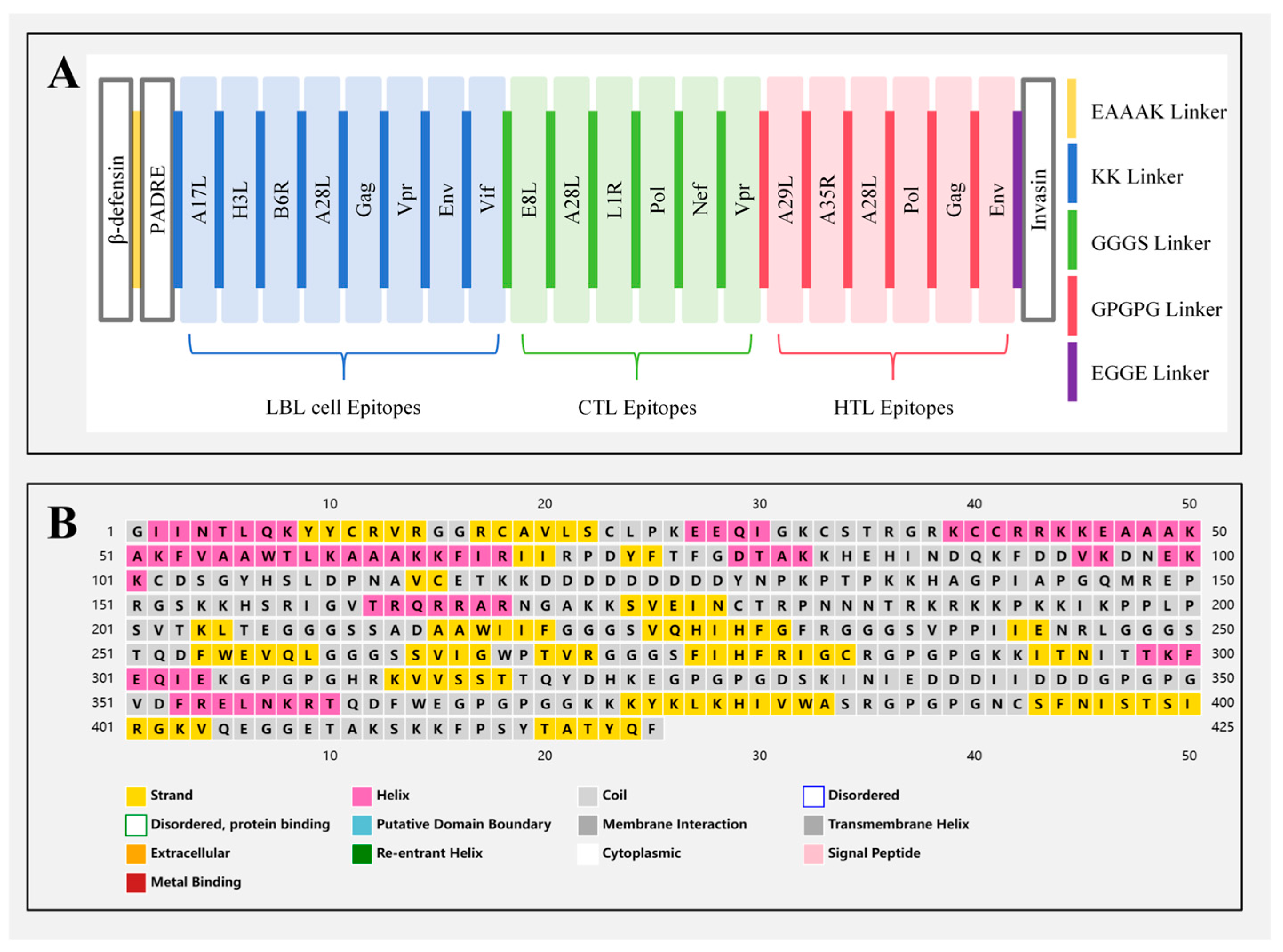
2.4. Multi-Epitope Vaccine Construction
2.5. Physicochemical Properties of Multi-Epitope Vaccine
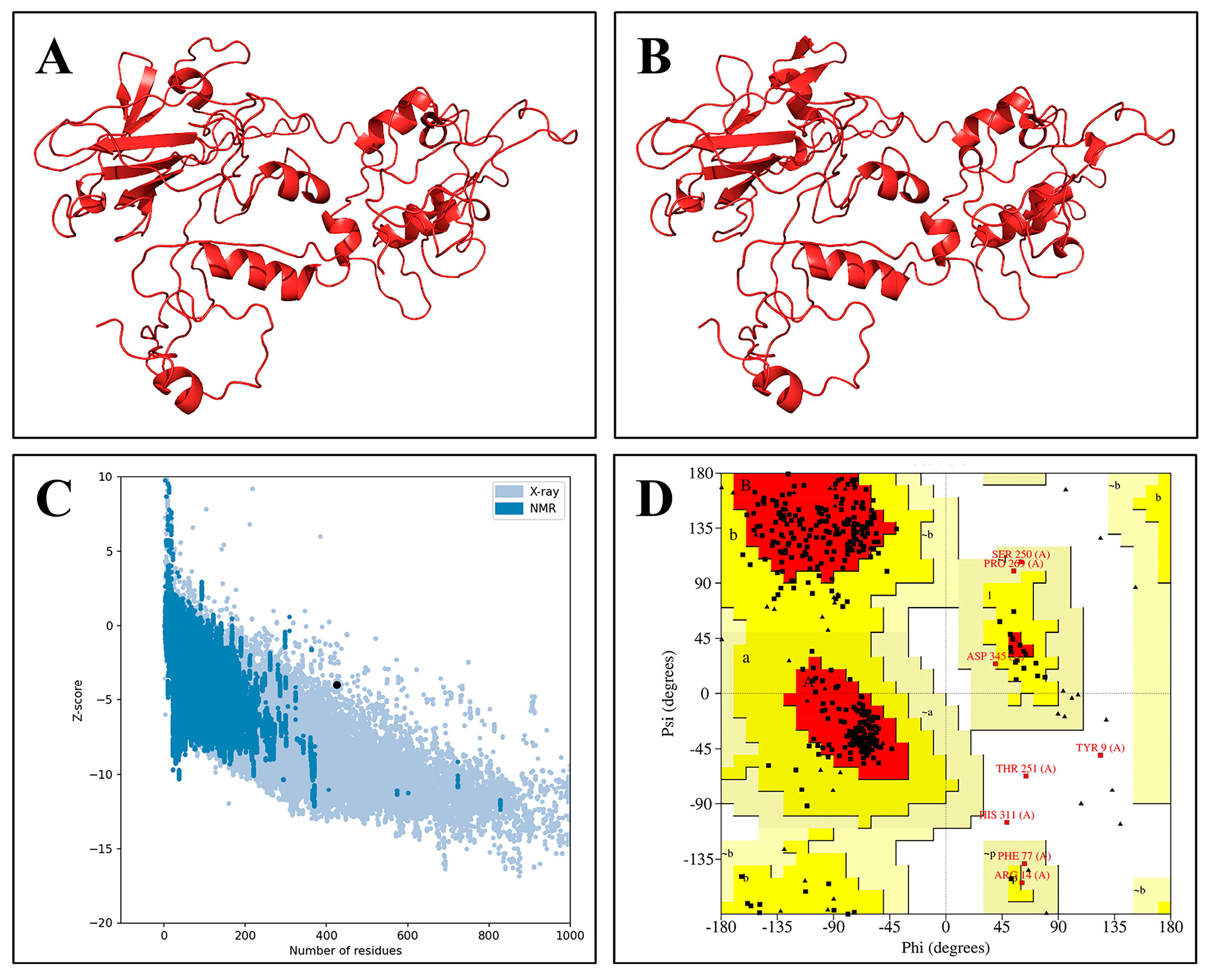
2.6. Secondary and 3D Structure Analysis of the Vaccine Construct
2.7. Prediction of Conformational B-Cell Epitopes
2.8. Molecular Docking
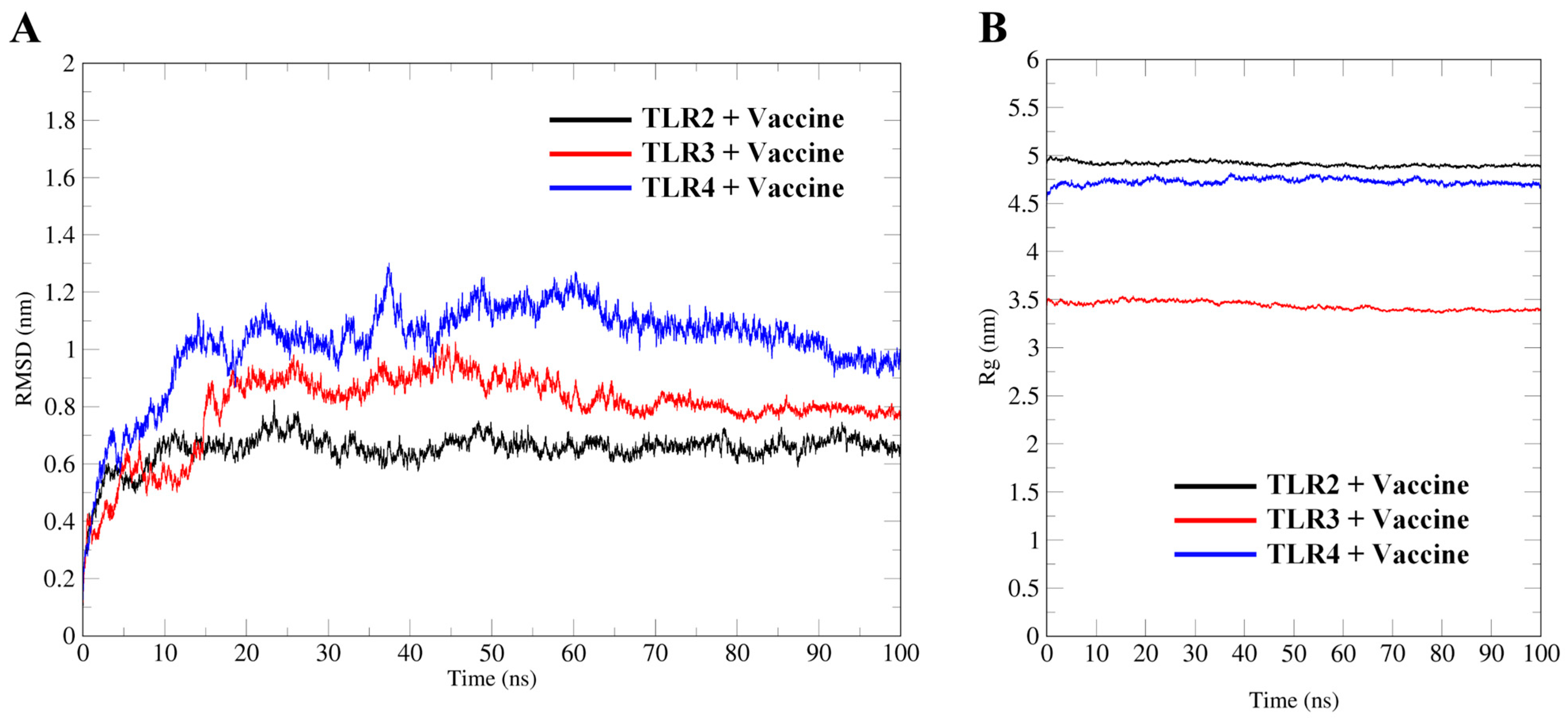
2.9. Molecular Dynamics Simulation
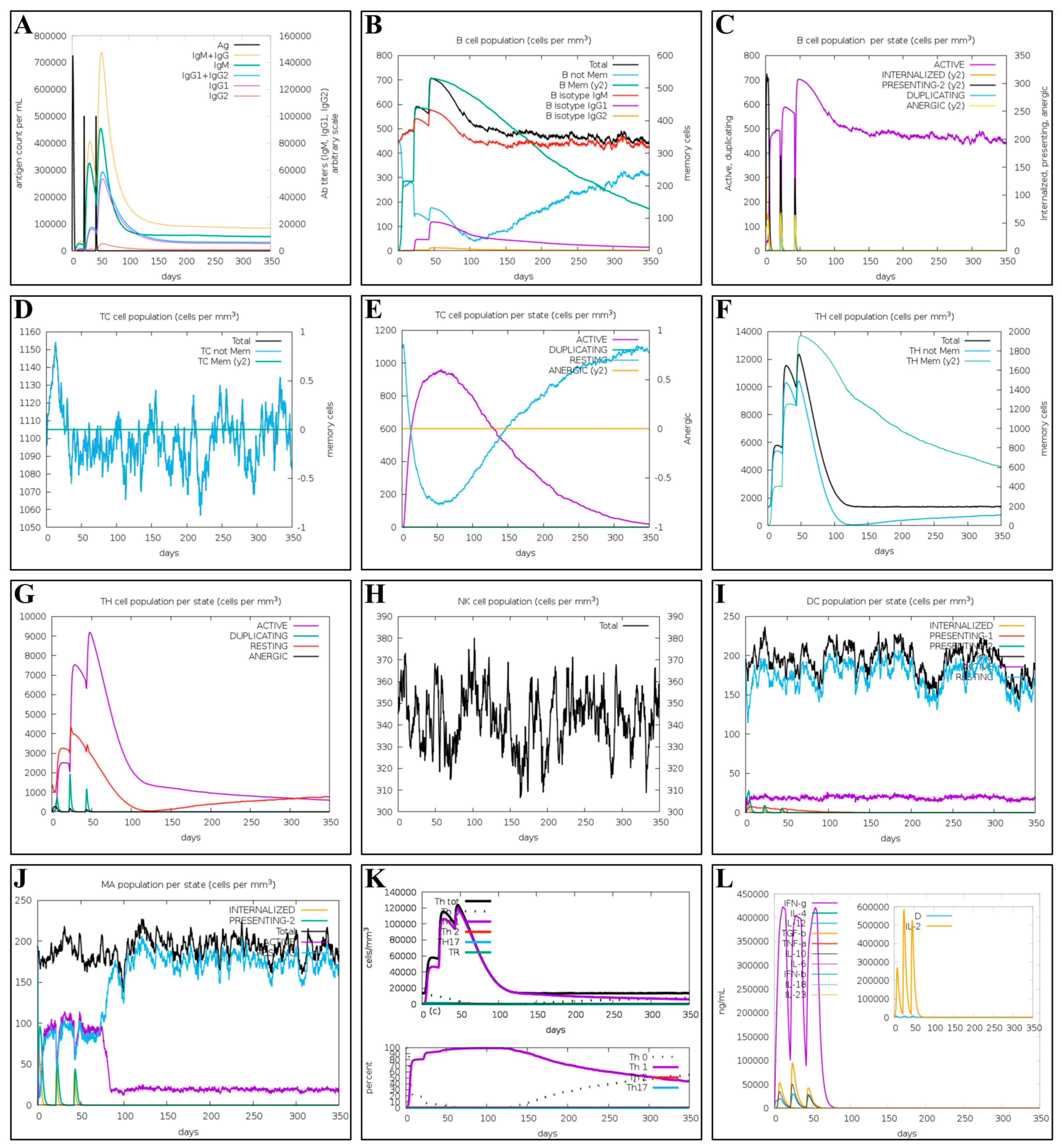
2.10. Immune Simulation of the Multi-Epitope Vaccine
2.11. Codon Optimization and In Silico Cloning
3. Discussion
4. Materials and Methods
4.1. Sequence Retrieval
4.2. T-Cell Epitope Prediction Analysis
4.3. Linear B Cell Epitope Prediction Analysis
4.4. Population Coverage Analysis of the Candidate Epitopes
4.5. Multi-Epitope Vaccine Construction
4.6. Evaluation of Physicochemical Properties of the Multi-Epitope Vaccine
4.7. Prediction and Optimisation of Multi-Epitope Vaccine Structures
4.8. Prediction of Conformational B-Cell Epitopes
4.9. Molecular Docking
4.10. Molecular Dynamic Simulations (MDs)
4.11. Immune Simulation of the Multi-Epitope Vaccine
4.12. Codon Optimisation and In Silico Cloning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. 2022–24 Mpox (Monkeypox) Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global (accessed on 8 January 2025).
- WHO. Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Upsurge of Mpox 2024. Available online: https://www.who.int/news/item/28-11-2024-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-upsurge-of-mpox-2024 (accessed on 8 January 2025).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Hsu, C.K.; Yen, M.Y.; Lee, P.I.; Ko, W.C.; Hsueh, P.R. Monkeypox: An emerging global threat during the COVID-19 pandemic. J. Microbiol. Immunol. Infect. 2022, 55, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Pastula, D.M.; Tyler, K.L. An Overview of Monkeypox Virus and Its Neuroinvasive Potential. Ann. Neurol. 2022, 92, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Samara, A.; O’Brien, P.; Morris, E.; Draycott, T.; Lees, C.; Ladhani, S. Monkeypox vaccines in pregnancy: Lessons must be learned from COVID-19. Lancet Glob. Health 2022, 10, e1230–e1231. [Google Scholar] [CrossRef]
- WHO. Vaccines and Immunization for Monkeypox: Interim Guidance, 16 November 2022. Available online: https://iris.who.int/handle/10665/364527 (accessed on 8 January 2025).
- Lane, J.M.; Goldstein, J. Adverse events occurring after smallpox vaccination. Semin. Pediatr. Infect. Dis. 2003, 14, 189–195. [Google Scholar] [CrossRef]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.M.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat. Commun. 2023, 14, 5925. [Google Scholar] [CrossRef]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Gotz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Liu, Q.; Ruan, L.; Ren, F.; Han, Y.; Zhang, Y.; Yang, L.; Li, S.; Sun, H.; et al. Vaccinia virus tiantan strain is inefficient in eliciting cross-reactive immunity against the emerging monkeypox virus strain. Emerg. Microbes Infect. 2024, 13, 2306967. [Google Scholar] [CrossRef]
- Kamali, P.; Zandi, M.; Ghasemzadeh-Moghaddam, H.; Fani, M. Comparison between various biosensor methods for human T-lymphotropic virus-1 (HTLV-1) detection. Mol. Biol. Rep. 2022, 49, 1513–1517. [Google Scholar] [CrossRef]
- Zandi, M.; Soltani, S.; Fani, M.; Abbasi, S.; Ebrahimi, S.; Ramezani, A. Severe acute respiratory syndrome coronavirus 2 and respiratory syncytial virus coinfection in children. Osong Public Health Res. Perspect. 2021, 12, 286–292. [Google Scholar] [CrossRef]
- UNAIDS Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 9 January 2025).
- Conway, J.M.; Meily, P.; Li, J.Z.; Perelson, A.S. Unified model of short- and long-term HIV viral rebound for clinical trial planning. J. R. Soc. Interface 2021, 18, 20201015. [Google Scholar] [CrossRef] [PubMed]
- Pavlakis, G.N.; Felber, B.K. A new step towards an HIV/AIDS vaccine. Lancet 2018, 392, 192–194. [Google Scholar] [CrossRef]
- Fomsgaard, A.; Karlsson, I.; Gram, G.; Schou, C.; Tang, S.; Bang, P.; Kromann, I.; Andersen, P.; Andreasen, L.V. Development and preclinical safety evaluation of a new therapeutic HIV-1 vaccine based on 18 T-cell minimal epitope peptides applying a novel cationic adjuvant CAF01. Vaccine 2011, 29, 7067–7074. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Manzardo, C.; Sanchez-Bernabeu, A.; Coll, P.; Moron-Lopez, S.; Puertas, M.C.; Rosas-Umbert, M.; Cobarsi, P.; Escrig, R.; Perez-Alvarez, N.; et al. Therapeutic Vaccination Refocuses T-cell Responses Towards Conserved Regions of HIV-1 in Early Treated Individuals (BCN 01 study). eClinicalMedicine 2019, 11, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.M.; Rakhmanina, N.Y.; Yang, Z.; Bukrinsky, M.I. Mpox (Monkeypox) Virus and Its Co-Infection with HIV, Sexually Transmitted Infections, or Bacterial Superinfections: Double Whammy or a New Prime Culprit? Viruses 2024, 16, 784. [Google Scholar] [CrossRef]
- Ortiz-Saavedra, B.; Montes-Madariaga, E.S.; Cabanillas-Ramirez, C.; Alva, N.; Ricardo-Martinez, A.; Leon-Figueroa, D.A.; Barboza, J.J.; Mohanty, A.; Padhi, B.K.; Sah, R. Epidemiologic Situation of HIV and Monkeypox Coinfection: A Systematic Review. Vaccines 2023, 11, 246. [Google Scholar] [CrossRef]
- Cao, K.; Wang, X.; Peng, H.; Ding, L.; Wang, X.; Hu, Y.; Dong, L.; Yang, T.; Hong, X.; Xing, M.; et al. A Single Vaccine Protects against SARS-CoV-2 and Influenza Virus in Mice. J. Virol. 2022, 96, e0157821. [Google Scholar] [CrossRef]
- Sher, H.; Sharif, H.; Zaheer, T.; Khan, S.A.; Ali, A.; Javed, H.; Javed, A. Employing computational tools to design a multi-epitope vaccine targeting human immunodeficiency virus-1 (HIV-1). BMC Genom. 2023, 24, 276. [Google Scholar] [CrossRef]
- Khairkhah, N.; Namvar, A.; Kardani, K.; Bolhassani, A. Prediction of cross-clade HIV-1 T-cell epitopes using immunoinformatics analysis. Proteins 2018, 86, 1284–1293. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A.; Namvar, A. An overview of in silico vaccine design against different pathogens and cancer. Expert Rev. Vaccines 2020, 19, 699–726. [Google Scholar] [CrossRef]
- Nain, Z.; Abdulla, F.; Rahman, M.M.; Karim, M.M.; Khan, M.S.A.; Sayed, S.B.; Mahmud, S.; Rahman, S.M.R.; Sheam, M.M.; Haque, Z.; et al. Proteome-wide screening for designing a multi-epitope vaccine against emerging pathogen Elizabethkingia anophelis using immunoinformatic approaches. J. Biomol. Struct. Dyn. 2020, 38, 4850–4867. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Maskey, U.; Thada, P.K.; Mustansir, M.; Sarfraz, A.; Sarfraz, Z. Re-Emergence of monkeypox amidst delta variant concerns: A point of contention for public health virology? J. Med. Virol. 2022, 94, 805–806. [Google Scholar] [CrossRef]
- Cortes-Ciriano, I.; Gulhan, D.C.; Lee, J.J.; Melloni, G.E.M.; Park, P.J. Computational analysis of cancer genome sequencing data. Nat. Rev. Genet. 2022, 23, 298–314. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Vartak, A.; Sucheck, S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, H.; Meng, J.; Wang, J.; Wu, J.; Liu, S.; Tong, J.; Nie, J.; Huang, W. Multi-epitope vaccine design for hepatitis E virus based on protein ORF2 and ORF3. Front. Microbiol. 2024, 15, 1372069. [Google Scholar] [CrossRef]
- Rcheulishvili, N.; Mao, J.; Papukashvili, D.; Feng, S.; Liu, C.; Wang, X.; He, Y.; Wang, P.G. Design, evaluation, and immune simulation of potentially universal multi-epitope mpox vaccine candidate: Focus on DNA vaccine. Front. Microbiol. 2023, 14, 1203355. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Ismail, S.; Ullah, A.; Bibi, S. Immuno-informatics profiling of monkeypox virus cell surface binding protein for designing a next generation multi-valent peptide-based vaccine. Front. Immunol. 2022, 13, 1035924. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, A.; Dawoud, T.M.; Nafidi, H.A.; Singh, N.; Bourhia, M. Immunoinformatics and reverse vaccinology approach in designing a novel highly immunogenic multivalent peptide-based vaccine against the human monkeypox virus. Front. Mol. Biosci. 2023, 10, 1295817. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, Y.; Xue, Y.; Cheng, P.; Wang, J.; Lian, J.; Gong, W. Developing a multiepitope vaccine for the prevention of SARS-CoV-2 and monkeypox virus co-infection: A reverse vaccinology analysis. Int. Immunopharmacol. 2023, 115, 109728. [Google Scholar] [CrossRef]
- Hashempour, A.; Khodadad, N.; Akbarinia, S.; Ghasabi, F.; Ghasemi, Y.; Nazar, M.; Falahi, S. Reverse vaccinology approaches to design a potent multiepitope vaccine against the HIV whole genome: Immunoinformatic, bioinformatics, and molecular dynamics approaches. BMC Infect. Dis. 2024, 24, 873. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Gomez-Flores, R.; De La Garza-Ramos, M.A.; Tamez-Guerra, P.; Lucio-Sauceda, D.G.; Rodríguez-Padilla, M.C. Immunoinformatics Approach to Design a Novel Epitope-Based Oral Vaccine Against Helicobacter pylori. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2019, 26, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Wlodawer, A. Stereochemistry and Validation of Macromolecular Structures. Methods Mol. Biol. 2017, 1607, 595–610. [Google Scholar] [PubMed]
- Iscla, I.; Wray, R.; Blount, P.; Larkins-Ford, J.; Conery, A.L.; Ausubel, F.M.; Ramu, S.; Kavanagh, A.; Huang, J.X.; Blaskovich, M.A.; et al. A new antibiotic with potent activity targets MscL. J. Antibiot. 2015, 68, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Fadaka, A.O.; Sibuyi, N.R.S.; Martin, D.R.; Goboza, M.; Klein, A.; Madiehe, A.M.; Meyer, M. Immunoinformatics design of a novel epitope-based vaccine candidate against dengue virus. Sci. Rep. 2021, 11, 19707. [Google Scholar] [CrossRef]
- Abdulla, F.; Adhikari, U.K.; Uddin, M.K. Exploring T & B-cell epitopes and designing multi-epitope subunit vaccine targeting integration step of HIV-1 lifecycle using immunoinformatics approach. Microb. Pathog. 2019, 137, 103791. [Google Scholar]
- Kato, K.; Nakayoshi, T.; Kurimoto, E.; Oda, A. Molecular dynamics simulations for the protein–ligand complex structures obtained by computational docking studies using implicit or explicit solvents. Chem. Phys. Lett. 2021, 781, 139022. [Google Scholar] [CrossRef]
- Yamamoto, E.; Akimoto, T.; Mitsutake, A.; Metzler, R. Universal Relation between Instantaneous Diffusivity and Radius of Gyration of Proteins in Aqueous Solution. Phys. Rev. Lett. 2021, 126, 128101. [Google Scholar] [CrossRef]
- Rapin, N.; Lund, O.; Bernaschi, M.; Castiglione, F. Computational immunology meets bioinformatics: The use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 2010, 5, e9862. [Google Scholar] [CrossRef]
- Mullane, K.; Lee, C.; Bressler, A.; Buitrago, M.; Weiss, K.; Dabovic, K.; Praestgaard, J.; Leeds, J.A.; Blais, J.; Pertel, P. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrob. Agents Chemother. 2015, 59, 1435–1440. [Google Scholar] [CrossRef]
- Devi, A.; Chaitanya, N.S.N. In silico designing of multi-epitope vaccine construct against human coronavirus infections. J. Biomol. Struct. Dyn. 2021, 39, 6903–6917. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Ahmad, S.; Azam, S.S. Vaccinomics to design a novel single chimeric subunit vaccine for broad-spectrum immunological applications targeting nosocomial Enterobacteriaceae pathogens. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 146, 105258. [Google Scholar] [CrossRef]
- Tahir Ul Qamar, M.; Shokat, Z.; Muneer, I.; Ashfaq, U.A.; Javed, H.; Anwar, F.; Bari, A.; Zahid, B.; Saari, N. Multiepitope-Based Subunit Vaccine Design and Evaluation against Respiratory Syncytial Virus Using Reverse Vaccinology Approach. Vaccines 2020, 8, 288. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chatterjee, S.; Nag, S.; Dhama, K.; Chakraborty, C. Designing, characterization, and immune stimulation of a novel multi-epitopic peptide-based potential vaccine candidate against monkeypox virus through screening its whole genome encoded proteins: An immunoinformatics approach. Travel. Med. Infect. Dis. 2022, 50, 102481. [Google Scholar] [CrossRef]
- Rahmani, A.; Baee, M.; Rostamtabar, M.; Karkhah, A.; Alizadeh, S.; Tourani, M.; Nouri, H.R. Development of a conserved chimeric vaccine based on helper T-cell and CTL epitopes for induction of strong immune response against Schistosoma mansoni using immunoinformatics approaches. Int. J. Biol. Macromol. 2019, 141, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, K.; Zhou, H. Immunogenic proteins and potential delivery platforms for mpox virus vaccine development: A rapid review. Int. J. Biol. Macromol. 2023, 245, 125515. [Google Scholar] [CrossRef] [PubMed]
- Papukashvili, D.; Rcheulishvili, N.; Liu, C.; Wang, X.; He, Y.; Wang, P.G. Strategy of developing nucleic acid-based universal monkeypox vaccine candidates. Front. Immunol. 2022, 13, 1050309. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Vir, P.; Raghava, G.P. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct 2013, 8, 30. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. Identifying candidate subunit vaccines using an alignment-independent method based on principal amino acid properties. Vaccine 2007, 25, 856–866. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Open Source Drug Discovery, C.; Raghava, G.P. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Raghava, G.P. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef]
- Bui, H.H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, J.; Zhang, Z.; Liang, X.; Liu, S.; Pan, Y.; Wei, J.; Huang, Y.; Huang, X.; Qin, Q. An improved oral vaccine with molecular adjuvant β-defensin protects grouper against nervous necrosis virus infection. Fish Shellfish Immunol. 2023, 136, 108709. [Google Scholar] [CrossRef]
- La Rosa, C.; Longmate, J.; Lacey, S.F.; Kaltcheva, T.; Sharan, R.; Marsano, D.; Kwon, P.; Drake, J.; Williams, B.; Denison, S.; et al. Clinical Evaluation of Safety and Immunogenicity of PADRE-Cytomegalovirus (CMV) and Tetanus-CMV Fusion Peptide Vaccines with or Without PF03512676 Adjuvant. J. Infect. Dis. 2012, 205, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Marillonnet, S.; Hause, G.; Klimyuk, V.; Gleba, Y. Immunoabsorbent nanoparticles based on a tobamovirus displaying protein, A. Proc. Natl. Acad. Sci. USA 2006, 103, 17678–17683. [Google Scholar] [CrossRef]
- Fadilah, F.; Paramita, R.I.; Erlina, L.; Istiadi, K.A.; Wuyung, P.E.; Tedjo, A. Linker Optimization in Breast Cancer Multiepitope Peptide Vaccine Design Based on Molecular Study. In Proceedings of the 4th International Conference on Life Sciences and Biotechnology (ICOLIB 2021), Jember, Indonesia, 15–16 November 2021; pp. 528–538. [Google Scholar]
- Hu, W.; Li, F.; Yang, X.; Li, Z.; Xia, H.; Li, G.; Wang, Y.; Zhang, Z. A flexible peptide linker enhances the immunoreactivity of two copies HBsAg preS1 (21–47) fusion protein. J. Biotechnol. 2004, 107, 83–90. [Google Scholar] [CrossRef]
- Livingston, B.; Crimi, C.; Newman, M.; Higashimoto, Y.; Appella, E.; Sidney, J.; Sette, A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J. Immunol. 2002, 168, 5499–5506. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Hebditch, M.; Carballo-Amador, M.A.; Charonis, S.; Curtis, R.; Warwicker, J.; Valencia, A. Protein–Sol: A web tool for predicting protein solubility from sequence. Bioinformatics 2017, 33, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999, 292, 195–202. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Heo, L.; Park, H.; Seok, C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013, 41, W384–W388. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Rullmannn, J.A.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef]
- Martin, J.; Frezza, E. A dynamical view of protein-protein complexes: Studies by molecular dynamics simulations. Front. Mol. Biosci. 2022, 9, 970109. [Google Scholar] [CrossRef] [PubMed]
- Grote, A.; Hiller, K.; Scheer, M.; Munch, R.; Nortemann, B.; Hempel, D.C.; Jahn, D. JCat: A novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005, 33, W526–W531. [Google Scholar] [CrossRef] [PubMed]



| Protein | Peptide Sequence | Percentile Rank | Immunogenicity Score | Antigenicity Score | Toxicity | Allergenicity |
|---|---|---|---|---|---|---|
| E8L | SADAAWIIF | 0.43 | 0.4833 | 0.8244 | Non-Toxin | Non-Allergen |
| A28L | VQHIHFGFR | 0.35 | 0.3831 | 3.4743 | Non-Toxin | Non-Allergen |
| L1R | VPPIIENRL | 0.16 | 0.3790 | 0.9944 | Non-Toxin | Non-Allergen |
| Pol | TQDFWEVQL | 0.22 | 0.4021 | 0.7160 | Non-Toxin | Non-Allergen |
| Nef | SVIGWPTVR | 0.03 | 0.3394 | 0.7639 | Non-Toxin | Non-Allergen |
| Vpr | FIHFRIGCR | 0.38 | 0.3011 | 3.1138 | Non-Toxin | Non-Allergen |
| Protein | Peptide Sequence | Percentile Rank | Antigenicity Score | IFN-γ Inducer Score | Toxicity | Allergenicity |
|---|---|---|---|---|---|---|
| A29L | KKITNITTKFEQIEK | 0.32 | 0.5631 | 0.8282 | Non-Toxin | Non-Allergen |
| A35R | HRKVVSSTTQYDHKE | 0.24 | 0.7656 | 0.5314 | Non-Toxin | Non-Allergen |
| A28L | DSKINIEDDDIIDDD | 0.14 | 0.5307 | 0.4342 | Non-Toxin | Non-Allergen |
| Pol | VDFRELNKRTQDFWE | 0.44 | 1.0134 | 0.6691 | Non-Toxin | Non-Allergen |
| Gag | GKKKYKLKHIVWASR | 0.47 | 1.5616 | 0.5550 | Non-Toxin | Non-Allergen |
| Env | NCSFNISTSIRGKVQ | 0.42 | 0.6658 | 0.3292 | Non-Toxin | Non-Allergen |
| Protein | Peptide Sequence | ABC Pred Score | Antigenicity Score | Toxicity | Allergenicity |
|---|---|---|---|---|---|
| A17L | FIRIIRPDYFTFGDTA | 0.97 | 0.5255 | Non-Toxin | Non-Allergen |
| H3L | HEHINDQKFDDVKDNE | 0.97 | 0.5947 | Non-Toxin | Non-Allergen |
| B6R | CDSGYHSLDPNAVCET | 0.94 | 0.7127 | Non-Toxin | Non-Allergen |
| A28L | DDDDDDDDDYNPKPTP | 0.91 | 0.9543 | Non-Toxin | Non-Allergen |
| Gag | HAGPIAPGQMREPRGS | 0.93 | 0.6008 | Non-Toxin | Non-Allergen |
| Vpr | HSRIGVTRQRRARNGA | 0.92 | 1.0919 | Non-Toxin | Non-Allergen |
| Env | SVEINCTRPNNNTRKR | 0.92 | 0.9122 | Non-Toxin | Non-Allergen |
| Vif | PKKIKPPLPSVTKLTE | 0.92 | 0.4677 | Non-Toxin | Non-Allergen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, D.; Wu, S.; Wang, Y.; Huang, W. Designing a Multi-Epitope Vaccine Against MPXV and HIV Based on an Immunoinformatic Approach. Int. J. Mol. Sci. 2025, 26, 6313. https://doi.org/10.3390/ijms26136313
Tang D, Wu S, Wang Y, Huang W. Designing a Multi-Epitope Vaccine Against MPXV and HIV Based on an Immunoinformatic Approach. International Journal of Molecular Sciences. 2025; 26(13):6313. https://doi.org/10.3390/ijms26136313
Chicago/Turabian StyleTang, Ding, Siwen Wu, Youchun Wang, and Weijin Huang. 2025. "Designing a Multi-Epitope Vaccine Against MPXV and HIV Based on an Immunoinformatic Approach" International Journal of Molecular Sciences 26, no. 13: 6313. https://doi.org/10.3390/ijms26136313
APA StyleTang, D., Wu, S., Wang, Y., & Huang, W. (2025). Designing a Multi-Epitope Vaccine Against MPXV and HIV Based on an Immunoinformatic Approach. International Journal of Molecular Sciences, 26(13), 6313. https://doi.org/10.3390/ijms26136313






