Abstract
To further extend the structure–activity relationships on previously identified anti-proliferative imidazo-pyrazoles, a novel series of compounds was designed and synthesized. In the obtained derivatives (1), the imidazo-pyrazole scaffold was formally condensed with a substituted triazole moiety, known for its biological properties. All derivatives were tested for anti-proliferative activity on a panel of 60 different cancer cell lines and compound 1h was identified as the most promising derivative, being highly effective against melanoma cells. Additional investigations demonstrated a cytotoxic and pro-oxidant action of the compound 1h on human metastatic melanoma cell lines (MeOV and MeTA) but not on healthy keratinocytes (HaCAT), confirming the selective activity of the compound. In silico calculations predicted favorable drug-like and pharmacokinetic properties and pre-formulation studies evaluated the effect of Tween 80 on 1h solubility. Overall, the collected data confirmed the pharmacological potential of the imidazo-pyrazole scaffold and indicated 1h as an interesting lead structure for the development of novel anti-melanoma agents.
1. Introduction
The 1,2,4-triazole nucleus, unfused or linked to other nitrogenous heterocycles, represents a pivotal pharmacophore, being embedded in different biologically active compounds including anticonvulsants [1], fungicides [2], herbicides [3], antibacterials [4], antigastrics [5], anti-oxidants [6], modulators or antagonists of ion channels [7], agents effective on metabolic syndrome (e.g., GPR131 and 11β hydroxysteroid dehydrogenase inhibitors) [8,9], and D3 antagonists [10,11,12]. Moreover, 1,2,4-triazole-containing compounds showed relevant anti-cancer activity [13,14,15,16,17]; interestingly, the presence of a sulfur-containing substituent at position 3 and a nitrogen heterocycle at position 5 of the triazole ring were correlated with the most potent anti-proliferative activity [18].
In previous studies, we synthesized different imidazo-pyrazoles I (Figure 1) with interesting anti-cancer, anti-angiogenic, and anti-inflammatory activities, showing anti-proliferative activity with IC50 values in the low micromolar range against selected tumor cell lines [19,20]. Furthermore, derivative Ia proved to be effective against patient-isolated melanoma (MEOV NT) and Vemurafenib (PLX4032, Figure 2)-resistant (MEOV PLX-R) cells, representing a valuable lead compound for the development of novel therapeutics for melanoma treatment [21]. Additionally, imidazo-pyrazole derivative Ib (Figure 1) showed low micromolar IC50 values against the skin melanoma SKMEL-28 cell line [22] and proteomics studies evidenced its ability to downregulate Ras-responsive element binding protein 1 (RREB1), a crucial molecular target in SKMEL-28 melanoma cells [23]. Collectively, these studies confirmed the imidazo-pyrazole moiety as an interesting chemical scaffold to develop novel anti-melanoma agents.
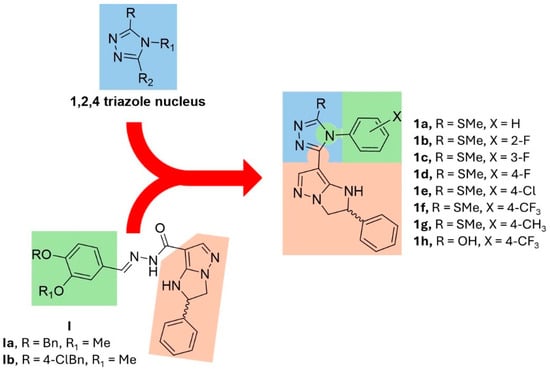
Figure 1.
Structures of 1,2,4 triazole nucleus, previous anti-cancer imidazo-pyrazoles I, and new designed compounds 1a–h.
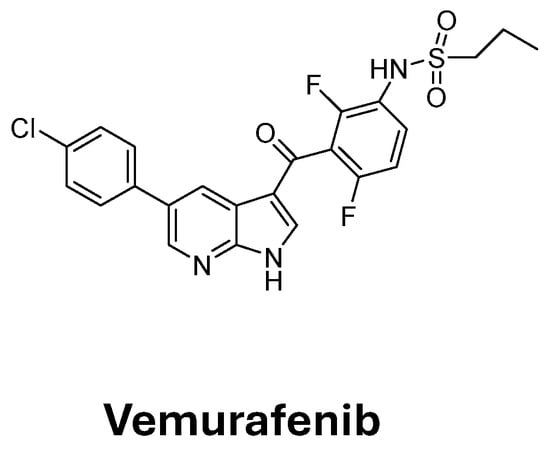
Figure 2.
Structure of Vemurafenib.
To further extend the structure–activity relationships (SARs) of imidazo-pyrazoles I and to obtain more active anti-proliferative agents, we designed and synthesized novel compounds 1a–g (Figure 1) in which the imidazo-pyrazole substructure was functionalized with a 1,2,4 triazole nucleus. In detail, (i) 1,2,4-triazole nucleus was inserted at position 7 of the imidazo-pyrazole scaffold, in analogy to the functionalization pattern of previous I; (ii) a substituted phenyl ring, structurally related to the catechol moiety of I, was inserted at position 4 of triazole; (iii) the position 3 of 1,2,4 triazole was substituted with a thiomethyl (compounds 1a–g) or a hydroxy group (1h).
To identify potential hit compounds, derivatives 1 were initially tested for anti-proliferative activity on a panel of 60 different cancer cell lines by the National Cancer Institute (Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis). Out of this initial screening, compound 1h was selected as the most promising analogue and submitted to further investigations. In detail, the cytotoxic and pro-oxidant effects of 1h were evaluated in two human metastatic melanoma cell lines, namely MeOV (characterized by the most common BRAFV600D mutation) and MeTA (characterized by BRAFV600E mutation). Furthermore, the same evaluations were carried out on healthy HaCAT keratinocytes to verify the cytotoxicity of 1h against non-cancerous cells. Moreover, in silico drug-like and pharmacokinetic properties were analyzed and the micellar solubilization of 1h was performed to provide new imidazo-pyrazole-based drug delivery systems with enhanced and anti-cancer activity.
2. Results
2.1. Chemistry
Derivatives 1 were synthesized according to a sequential strategy starting from the key carbohydrazide intermediate 2 (Scheme 1) previously prepared by us following the literature procedure [20]. In particular, 2 was reacted with the suitable substituted phenyl isothiocyanates in ethanol at reflux for 6–18 h to obtain intermediates 3a–f that were cyclized in a basic condition (2M NaOH) for 4–12 h to afford the 1,2,4 triazole derivatives 4 (Scheme 1). Compounds 1a–g were synthesized by the methylation of the corresponding thiol precursors with iodomethane in the presence of either sodium acetate in absolute ethanol (compounds 1a,b,d) or potassium carbonate in anhydrous DMF (compounds 1c,e–g). Compound 1h was prepared by a one-pot, two-step reaction by condensing 4f with iodomethane in the presence of potassium carbonate in anhydrous DMF and prolonging stirring for 24 h.
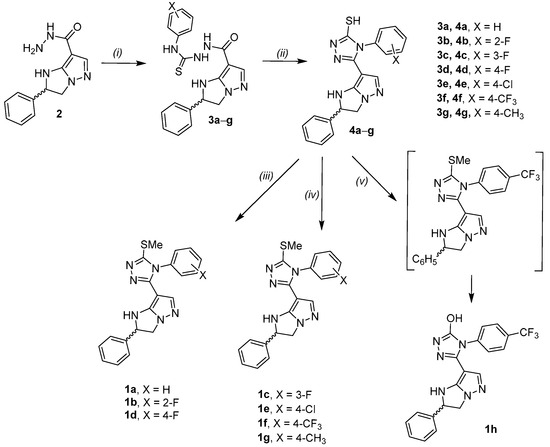
Scheme 1.
Reaction and conditions: (i) proper phenylisothiocyanate, EtOH ass., 6–18 h; (ii) 2M NaOH, 80 °C, 4–12 h; (iii) CH3I, AcONa, EtOH ass., reflux 3–4 h; (iv) CH3I, K2CO3. an. DMF, r.t., 1–18 h; (v) CH3I, K2CO3. an. DMF, r.t. 24 h.
2.2. Biological Results
2.2.1. Anti-Proliferative Activity
New synthesized compounds 1a–h were tested for anti-proliferative activity by the National Cancer Institute (Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis) at a fixed concentration of 10 µM [24]. Out of the eight compounds tested, only derivatives 1a, 1e and 1h evidenced percentage growth values below ≤60% for at least one of the cell lines considered (Table 1 and Supplementary Materials Figures S31–S33). In detail, two compounds (bearing a thiomethyl substituent on a triazole scaffold) were able to inhibit the growth of leukemia and non-small-cell lung cancer (NSCLC) cells (1a), and melanoma and renal cancer (1e), whereas 1h, characterized by a 3-hydroxy substituted triazole nucleus, strongly inhibited the growth of several cancer cell lines investigated, affecting particularly leukemia, colon cancer, and melanoma cell proliferation. Interestingly, the replacement of 1h hydroxy group with a thiomethyl substituent completely abolished anti-cancer activity (compare 1h and 1f).

Table 1.
Cell growth percent values of most active derivatives on different cancer cell lines at 10 μM concentration for 72 h. For each compound, only cell lines with a growth percent values ≤60% are indicated. * A negative mean value indicates lethality.
2.2.2. 1h Affects the Viability of MeOV and MeTA Cell Lines
Based on NCI screening results, human metastatic melanoma cells MeOV and MeTA, were treated with 1h (10 µM) for 24 h, 48 h, and 72 h. As shown in Figure 3, 24 h treatment decreased the viability of MeOV and MeTA by 60% and 46%, respectively. Incubation for 48 h and 72 h further increased the cytotoxic effect of 1h, reducing the viability of MeOV and MeTA cells by 77% and 85%, respectively, at 72 h (Figure 3).
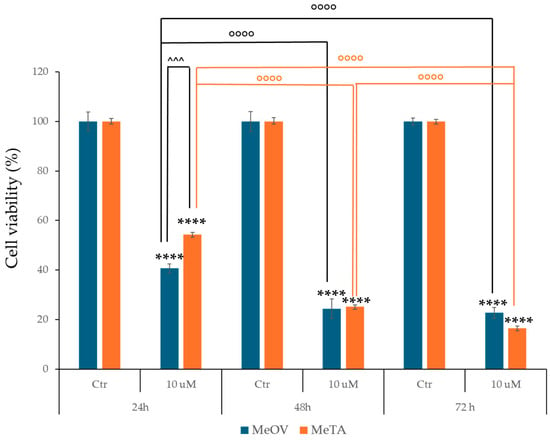
Figure 3.
1h exposure reduces cell viability of melanoma cells. Cell viability was evaluated by MTS assay in MeOV (blue bars) and in MeTA cells (orange bars) treated with 10 µM 1h for 24, 48, and 72 h. The graph reports the percentage viability of cells treated for indicated times in comparison with that of untreated ones (100%). Histograms summarize quantitative data of means ± S.E.Ms of at least four independent experiments. **** p < 0.0001 vs. untreated MeOV or MeTRAV cells. ◦◦◦◦ p < 0.0001 vs. respective treatments indicated by the bars.; ^^^ p < 0.001 vs. respective treatments indicated by the bars.
2.2.3. 1h Stimulates the Production of Reactive Oxygen Species (ROS) in MeOV and MeTA Cell Lines
Considering that the cytotoxic effect of imidazo-pyrazoles I is mediated by ROS production [21], the amount of H2O2, the most long-lived ROS [25,26], was monitored in both cell lines under each treatment condition. As shown in Figure 4, 1h exposure increased H2O2 levels in MeOV and MeTA cells in a time-dependent way, reaching, at 72 h, an increase of 3.5 fold and 4 fold, respectively.
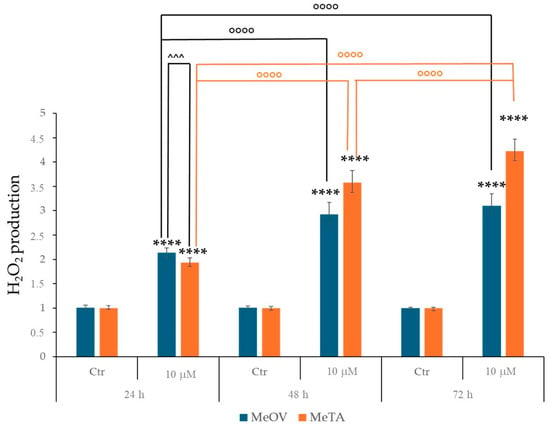
Figure 4.
1h exposure enhances H2O2 production in a time-dependent way. H2O2 production was analysed by DCFH-DA labelling in MeOV (blue bars) MeTA cells (orange bars) treated with 10 µM 1h for 24, 48, and 72 h. Results are expressed as variation of H2O2 production under treatment conditions in comparison with untreated ones (1). Histograms summarize quantitative data of means ± S.E.Ms of four independent experiments. **** p < 0.0001 vs. untreated MeOV or MeTRAV cells. ◦◦◦◦ p < 0.0001 vs. respective treatments indicated by the bars.; ^^^ p < 0.001 vs. respective treatments indicated by the bars.
2.2.4. 1h Is Not Cytotoxic for Healthy HaCAT Keratinocytes and Does Not Stimulate H2O2 Production in These Cells
In order to evaluate the impact of 1h on non-cancerous cells, the same treatment performed on melanoma cells was also performed on healthy HaCAT keratinocytes. As shown in Figure 5, 1h treatment, at any time of exposure, did not affect HaCAT viability (Figure 5a) and did not induce significant changes in H2O2 production (Figure 5b).
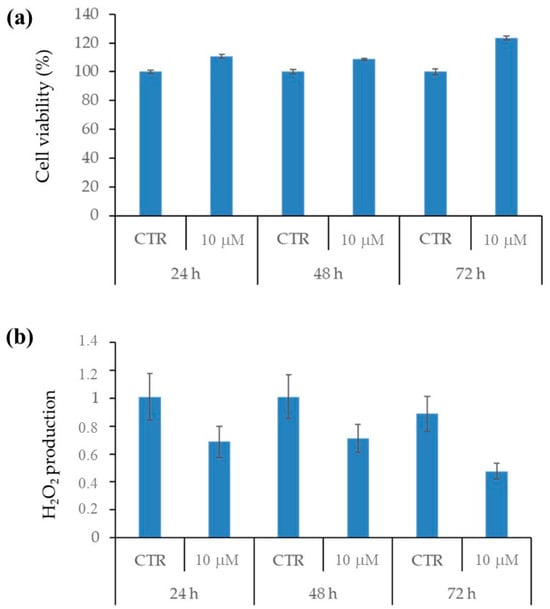
Figure 5.
1h exposure did not alter viability and did not induce H2O2 production in healthy HaCAT keratinocytes. Cell viability (a) and H2O2 production (b) were evaluated by MTS assay and DCFH-DA labelling, respectively, in HaCAT cells treated with 10 µM 1h for 24, 48, and 72 h. Histograms summarize quantitative data of means ± S.E.Ms of at least four independent experiments.
2.3. In Silico Prediction of ADMET Properties of 1h
In silico predictions of absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties are exploited as valuable tools in drug design [27,28,29]. To estimate the drug-like profile of compound 1h, the ADMET properties of 1h were calculated via the ACD/Lab Percepta platform [30]. Putative violations of Veber’s [31] and Lipinski’s rules [32] were considered and the following descriptors were calculated: the (i) logarithmic ratio of the octanol–water partitioning coefficient (cLogP), (ii) molecular weight (MW) values of the compounds, (iii) H-bonding acceptor number (HBA), H-bonding donor moieties (HBD), (iv) number of rotatable bonds (nRot_bond), and (v) topological polar surface area (TPSA) (see Supplementary Materials Table S2). In addition, further pharmacokinetic parameters were taken into account such as human intestinal absorption (HIA), the estimation of the plasmatic protein binding (% PPB), the volume of distribution (Vd), ligand affinity toward human serum albumin (LogKa HSA), and putative oral bioavailability, as a percentage (F %) (Supplementary Materials Table S2).
Regarding hepatic metabolism, the potential inhibitory activity of 1h against certain isoenzymes of cytochrome P450, particularly CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4, was evaluated; it is important to emphasize that the compound was not predicted to inhibit CYP3A4, an enzyme of fundamental importance in the metabolism of numerous drugs, especially in patients undergoing polypharmacy.
In general, the comparison of the chemical–physical properties of 1h with the thiomethyl derivatives 1a–g evidenced that all these new triazolo-imidazo-pyrazoles here reported had similar profiles in terms of pharmacokinetic properties, metabolism, and druglikeness characteristics. However, it should be noted that 1h, having an additional hydroxy group on the triazole nucleus, would exhibit better hydrophilicity and solubility (5.67 × 10−3 mg/mL), as predicted by the ESOL method [33,34], and a greater number of H-bond acceptors (seven for 1 h instead of six for 1a–g) and H-bond donors (two for 1h instead of one for 1a–g).
Finally, the in silico evaluation of toxicity properties in terms of cytochrome inhibition and of the lethal dose (LD50) via mouse oral administration was explored (Supplementary Materials Table S3).
2.4. Micellar Solubilization of 1h
To evaluate the behavior of 1h in solution, a pre-formulative study was carried out. Initially, the UV-visible spectra of 1h in water and DMSO (0.05 mM) were recorded to identify the maximum absorbance value (λ max = 290 nm; Figure 6A). Then, the absorption profile of 1h in the presence of different amount of the Tween 80 (TW-80) surfactant was measured (Figure 6B) and the critical micelle concentration (CMC) was determined (Figure 7). When the TW-80 concentration exceeds the CMC, a large number of micelles are formed, promoting compound encapsulation and enhancing its solubility and stability. As a result, this phenomenon leads to an increase in absorption efficiency [35].
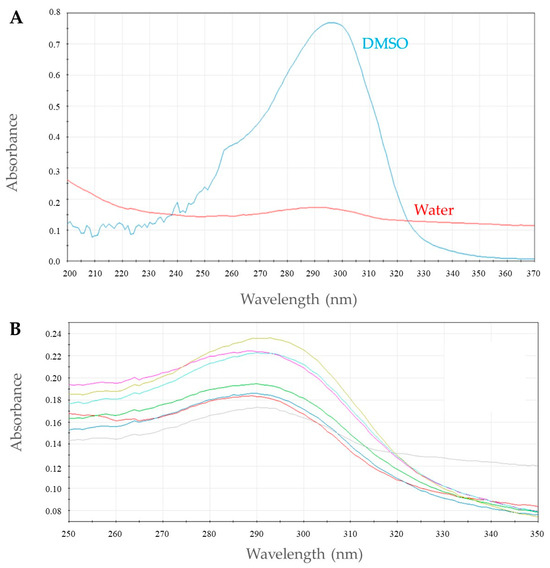
Figure 6.
Plot of UV absorption spectra of 1h in water and DMSO (A) and in micellar media of TW-80 at different concentrations. Each color represents a different concentration (B).
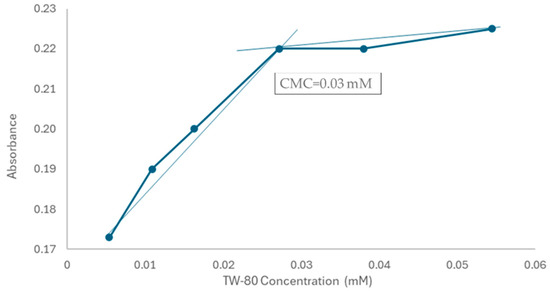
Figure 7.
Plot of absorbance of 1h against different concentrations of TW-80.
3. Discussion
The development of a sequential synthetic approach based on key intermediate 2 allowed the isolation of a series of eight unreported imidazo-pyrazole derivatives characterized by a 1,2,4 triazole nucleus. The nature and the position of the N-phenyl ring substituent (X group, Scheme 1) affected the reactivity of the thiol group of compounds 4 and required the setup of two different protocols for the methylation reaction. In particular, unsubstituted (4a) and ortho or para fluoro-substituted (4b and 4d) derivatives were successfully methylated in absolute ethanol using sodium acetate as a base. Conversely, compounds 4c,e–g proved to be unreactive under these conditions and required the use of potassium carbonate as a base and dry DMF as a solvent. Interestingly, the nucleophilic substitution of the thiomethyl group of 1f was observed by prolonging stirring in DMF for 24 h, leading to the isolation of the hydroxy derivative 1h. Under the same experimental conditions, the thiomethyl displacement did not occur for the other derivatives, thus indicating the involvement of the 4-trifluoromethyl substituent in the reaction.
The substituents of the triazolyl substructure affected the anti-proliferative profile of the derivatives 1). Thus, among the SMe series, compounds 1b (X = 2-F), 1c (X = 3-F), 1d (X = 4-F), 1f (X = 4-CF3), and 1g (X = 4-Me) did not show any relevant anti-cancer activity against NCI cell line panel. Conversely, unsubstituted (1a) and 4-chlorosubstituted derivatives (1e) were found to affect the proliferation of selected cell lines (Table 1). The replacement of the SMe group with a hydroxyl functionality dramatically improved activity (compare 1f and 1h), leading to the identification of the most promising compound of the series. Derivative 1h showed widespread anti-proliferative activity and proved to be lethal particularly for melanoma MDA-MB-435 cells.
The effectiveness of 1h was confirmed in the human MeOV and MeTA melanoma cell lines, bearing different BRAF mutations. In fact, incubation with 10 µM 1h markedly decreased the viability of both cell populations and this event was accompanied by a time-dependent increase in H2O2 levels. It is noteworthy that 1h exerts its anti-proliferative and pro-oxidant activities selectively on melanoma cells while it is ineffective on healthy keratinocytes (HaCAT).
Computational studies (ACD/Lab Percepta platform) supported the drug-like profile of 1h as no violations of drug-like property rules, such as Lipinski’s or Veber’s rules, were observed. Oral bioavailability was predicted to be 99%, with 1h being chemically stable in acidic conditions and predicted with a dose/solubility ratio of 0.83. Passive absorption across the intestinal barrier was calculated to be greater than 70%. In silico toxicology results pointed out no cytochrome 3A4 and 2D6 inhibition events (based on the reported reliability indices) and an estimated LD50 value of 570 mg/Kg for mouse acute oral toxicity.
Poorly water-soluble drugs often require specialized formulation strategies to enhance bioavailability [36,37]. One promising approach involves the use of surfactants—i.e., amphiphilic compounds capable of reducing surface tension between two immiscible phases. These molecules contain both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions within the same structure. Surfactants can improve drug solubility by forming colloidal-sized structures known as micelles in suitable solvents. When present at higher concentrations than the CMC, these amphiphilic molecules spontaneously self-assemble into micelles. Thanks to this micelle-forming ability, surfactants have recently gained significant attention as potential carriers in drug delivery systems [38,39]. On this basis, the behavior of the hydrophilic non-ionic surfactant TW-80 and its ability to form micelles and encapsulate 1h were studied. In the presence of different TW-80 concentrations, the compound absorbance raised sharply in the pre-micellar region until reaching the CMC, after which it gradually decreased as the drug became fully incorporated into the micelles (Figure 6 and Figure 7). The typical CMC value of TW-80 in water is 0.015 mM [40], but after compound incorporation, the CMC increases at surfactant concentration values of 0.03 mM (Figure 7). It has been hypothesized that this could be due to the drug disrupting micelle formation, making the environment unfavorable for micellization, with the CMC consequently increasing [41]. Collectively, this preliminary study suggested a potential increase in 1h solubility and will be helpful in selecting the most suitable drug delivery system for subsequent formulation studies.
4. Materials and Methods
4.1. General Information
All chemicals were purchased from Chiminord srl and Merk (formerly Aldrich Chemical) (Milan, Italy). Solvents were reagent-grade. All commercial reagents were used without further purification. Aluminum-backed silica gel plates (Merck DC-Alufolien Kieselgel 60 F254, Darmstad, Germany) were used in thin-layer chromatography (TLC) for routine of monitoring the reaction course. Merck silica gel, 230–400 mesh, was used for chromatography. Flash chromatography was performed using Isolera one instrument (Biotage, Uppsala, Sweden) using Silicagel column. Melting points were not “corrected” and were measured with a Buchi M-560 instrument (Buchi instruments, Flawil, Switzerland). 1H and 13C NMR spectra (Supplementary Materials Figures S1–S30) were recorded on a JEOL JNM ECZ-400S/L1 (400 MHz, Tokyo, Japan); chemical shifts are reported as δ (ppm) relative to tetramethylsilane (TMS) as internal standard; signals were characterized as s (singlet), d (doublet), t (triplet), n t (near triplet), q (quartet), m (multiplet), or br s (broad signal); J values were reported in Hz. IR spectra were recorded on Perkin-Elmer 398.HPLC/ESI-MS analyses were carried out with 1100 HPLC coupled with an Agilent 1100 series LC/MSD XCT Ion Trap (Agilent Technologies, Santa Clara, CA, USA).
Elemental analysis was determined with an elemental analyzer EA 1110 (Fison Instruments, Milan, Italy); products were considered pure when the difference between calculated and found values was ± 0.4 (see Supplementary Materials Table S1). Compound 2 was prepared as previously reported [20,42].
4.2. Chemistry
4.2.1. General Procedure for Synthesis of Intermediates 3a–g
To a solution of 2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbohydrazide 2 (1.22 g, 5 mmol) in absolute ethanol (40 mL), the proper phenyl isothiocyanate (6 mmol) solved in absolute ethanol (2 mL) was added and the mixture was heated at reflux for 6–18 h. After cooling to room temperature, the obtained solid was filtered and recrystallized by absolute ethanol or by a 1/1 mixture of absolute ethanol/methanol.
N-phenyl-2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)hydrazine-1-carbothioamide 3a. Yield: 92%. M.p.: 139–141 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.82 (t, J = 9.5 Hz, 1H, H3), 4.56 (t, J = 9.5 Hz, H, H3), 5.36–5.43 (m, 1H, H2), 7.06–7.12 (m, 1H, Ar), 7.19 (br s, 1H, NH exchangeable with D2O), 7.23–7.45 (m, 9H, Ar), 7.72 (s, 1H, H6), 9.51 (br s, 1H, NH exchangeable with D2O), 9.62 (br s, 1H, NH exchangeable with D2O), 9.68 (br s, 1H, NH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 183.98, 164.62, 148.24, 146.82, 142.46, 140.91, 139.31, 130.38, 128.56, 127.86, 126.64, 125.63, 93.90, 64.95, 53.10. IR (KBr): 3430 (NH), 1652 (CO) cm−1. Anal calcd. for C19H18N6OS.
N-(2-fluorophenyl)-2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)hydrazine-1-carbothioamide 3b. Yield: 66%. M.p.: 190–193 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.73 (t, J = 9.5 Hz, 1H, H3), 4.56 (t, J = 9.5 Hz, H, H3), 5.32–5.42 (m, 1H, H2), 7.09–7.41 (m, 10H, 9Ar + NH), 7.72 (s, 1H, H6), 9.49 (br s, 1H, NH exchangeable with D2O), 9.62–9.76 (m, 2H, 2xNH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 183.00, 166.30, 159.56 (d, J = 247.9 Hz), 141.71, 140.92, 133.54, 130.42, 128.56, 127.86, 127.39, 126.63, 126.04, 123.84, 115.62 (d, J = 19.8 Hz), 93.81, 64.95, 53.10. IR (KBr): 3385 (NH), 1674 (CO) cm−1. Anal calcd. for C19H17FN6OS.
N-(3-fluorophenyl)-2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)hydrazine-1-carbothioamide 3c. Yield: 73%. M.p.: 163–165 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.78 (t, J = 9.5 Hz, 1H, H3), 4.61 (t, J = 9.5 Hz, H, H3), 5.39–5.48 (m, 1H, H2), 6.97 (br s, 1H, NH exchangeable with D2O), 7.20–7.46 (m, 9H, Ar), 7.77 (s, 1H, H6), 9.63–9.84 (m, 3H, 3xNH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 184.14, 166.27, 155.70, 142.16, 141.31, 140.90, 129.88, 129.14, 128.57, 127.87, 126.64, 121.09, 112.95, 103.58, 93.84, 64.96, 53.10.IR (KBr): 3356 (NH), 1655 (CO) cm−1. Anal calcd. for C19H17FN6OS.
N-(4-fluorophenyl)-2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)hydrazine-1-carbothioamide 3d. Yield: 99%. M.p.: 150–153 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.77 (t, J = 9.5 Hz, 1H, H3), 4.59 (t, J = 9.5 Hz, H, H3), 5.38–5.48 (m, 1H, H2), 7.11–7.17 (m, 2H, Ar), 7.21 (br s, 1H, NH exchangeable with D2O), 7.30–7.47 (m, 7H, Ar), 7.75 (s, 1H, H6), 9.52–9.78 (m, 3H, 3xNH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 182.12, 167.68, 157.91, 142.94, 141.45, 136.18, 133.12, 129.10, 128.41, 127.17, 120.63, 115.18, 94.43, 65.48, 53.64. IR (KBr): 3431 (NH), 1653 (CO) cm−1. Anal calcd. for C19H17FN6OS.
N-(4-chlorophenyl)-2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)hydrazine-1-carbothioamide 3e. Yield: 63%. M.p.: 162–165 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.78 (t, J = 9.5 Hz, 1H, H3), 4.59 (t, J = 9.5 Hz, H, H3), 5.39–5.46 (m, 1H, H2), 7.22 (br s, 1H, NH exchangeable with D2O), 7.29–7.44 (m, 7H, Ar), 7.47–7.52 (m, 2H, Ar), 7.75 (s, 1H, H6), 9.61–9.70 (m, 2H, 2xNH exchangeable with D2O), 9.78 (br s, 1H, NH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 184.10, 164.74, 155.92, 142.96, 141.44, 135.09, 129.10, 128.41, 128.30, 127.79, 127.17, 124.13, 94.47, 65.49, 53.64.IR (KBr): 3419 (NH), 1652 (CO) cm−1. Anal calcd. for C19H17ClN6OS.
2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)-N-(4-(trifluoromethyl)phenyl)hydrazine-1-carbothioamide 3f. Yield: 55%. M.p.: 150–152 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.77 (t, J = 9.5 Hz, 1H, H3), 4.59 (t, J = 9.5 Hz, H, H3), 5.37–5.47 (m, 1H, H2), 7.10–7.18 (m, 2H, Ar), 7.22 (br s, 1H, NH exchangeable with D2O), 7.28–7.48 (m, 7H, Ar), 7.75 (s, 1H, H6), 9.59 (br s, 1H, NH exchangeable with D2O), 9.67 (m, 1H, NH exchangeable with D2O), 9.73 (br s, 1H, NH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 182.21, 160.62, 142.93, 141.44, 136.19, 129.62, 129.10, 128.41, 127.17, 126.18, 121.22, 114.98, 94.43, 65.48, 53.63. IR (KBr): 3335 (NH), 1657 (CO) cm−1. Anal calcd. for C20H17F3N6OS.
2-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carbonyl)-N-(p-tolyl)hydrazine-1-carbothioamide 3g. Yield: 73%. M.p.: 152–154 °C. 1H-NMR (400 MHz, DMSO-d6): δ 2.24 (s, 3H, CH3), 3.77 (t, J = 9.5 Hz, 1H, H3), 4.59 (t, J = 9.5 Hz, 1H, H3), 5.37–5.42 (m, 1H, H2), 7.07–7.41 (m, 9H Ar), 7.72 (s, 1H, H6), 9.45 (br s, 1H, NH exchangeable with D2O), 9.61 (br s, 1H, NH exchangeable with D2O). 13C-NMR (101 MHz, DMSO-d6): δ 182.26, 161,88, 154.55, 142.43, 140.93, 133.95, 136.74, 128.59, 128.39, 127.89, 126.66, 125.82, 93.94, 64.96, 53.12, 20.57. Anal calcd. for C21H20N6OS.
4.2.2. General Procedure for Synthesis of Intermediates 4a–g
The proper compound 3a–g (2 mmol) was suspended in 2M NaOH (16 mL) and the mixture was heated at 80 °C for 4–12 h (TLC monitoring). After cooling to room temperature, concentrated AcOH was added until a pH of 5 was reached. The obtained solid was filtered and crystallized by absolute ethanol or by a 1/1 mixture of absolute ethanol/methanol.
4-phenyl-5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 4a. Yield: 92%. M.p.: 264–267 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.71–3.76 (m, 1H, H3), 4.55–4.60 (m, 1H, H3), 5.38–5.43 (m, 1H, H2), 6.16 (br s, 1H, NH exchangeable), 6.90 (br s, 1H, NH exchangeable), 7.31–7.44 (m, 8H, 7Ar + H6), 7.59–7.63 (m, 3H, Ar). 13C-NMR (101 MHz, DMSO-d6): δ 167.09, 152.82, 146.13, 140.81, 139.42, 134.72, 129.88, 129.67, 128.97, 128.51, 127.83, 126.66, 85.64, 64.92, 53.23. IR (KBr): 3278 (NH) cm−1. Anal calcd. for C19H16N6S.
4-(2-fluorophenyl)-5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 4b. Yield: 55%. M.p.: 266–272 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.73–3.80 (m, 1H, H3), 4.57–4.62 (m, 1H, H3), 5.41–5.45 (m, 1H, H2), 6.22 (br s, 1H, NH exchangeable), 6.27 (br s, 1H, NH exchangeable), 7.03–7.07 (m, 1H, Ar), 7.31–7.72 (m, 9H, 9Ar + H6). 13C-NMR (101 MHz, DMSO-d6): δ 167.39, 158.71, 152.91, 146.11, 140.75, 138.60, 132.70, 131.66, 128.53, 127.86, 126.71, 125.65, 122.30, 116.98, 85.30, 65.03, 53.25. IR (KBr): 3282 (NH) cm−1. Anal calcd. for C19H15FN6S.
4-(3-fluorophenyl)-5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 4c. Yield: 66%. M.p.: 273–274 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.71–3.76 (m, 1H, H3), 4.56–4.61 (m, 1H, H3), 5.38–5.43 (m, 1H, H2), 6.34 (br s, 1H, NH exchangeable), 6.90 (br s, 1H, NH exchangeable), 7.26–7.49 (m, 9H, 8Ar + H6), 7.61–7.67 (m, 1H, Ar). 13C-NMR (101 MHz, DMSO-d6): δ 167.05, 163.35, 152.76, 145.89, 140.87, 139.62, 136.25, 131.27, 128.52, 127.84, 126.63, 125.45, 125.41, 116.80, 85.56, 64.89, 53.29. IR (KBr): 3287 (NH) cm−1. Anal calcd. for C19H15FN6S.
4-(4-fluorophenyl)-5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 4d. Yield: 66%. M.p.: 294–295 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.68–3.77 (m, 1H, H3), 4.54–4.63 (m, 1H, H3), 5.35–5.41 (m, 1H, H2), 6.33 (br s, 1H, NH exchangeable), 6.71 (br s, 1H, NH exchangeable), 7.11–7.48 (m, 10H, 9Ar + H6).13C-NMR (101 MHz, DMSO-d6): δ 173.93, 166.86, 163.17, 152.31, 145.63, 141.06, 139.47, 131.25, 128.50, 127.78, 126.63, 116.20, 87.11, 64.81, 53.29. IR (KBr): 3268 (NH) cm−1. Anal calcd. for C19H15FN6S.
4-(4-chlorophenyl)-5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 4e. Yield: 60%. M.p.: 287–291 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.69–3.74 (m, 1H, H3), 4.56–4.61 (m, 1H, H3), 5.37–5.42 (m, 1H, H2), 6.45 (br s, 1H, NH exchangeable), 6.84 (br s, 1H, NH exchangeable), 7.30–7.53 (m, 8H, 7Ar + H6), 7.65–7.70 (m, 2H, Ar). 13C-NMR (101 MHz, DMSO-d6): δ 167.12, 152.72, 146.01, 140.83, 139.91, 134.52, 133.50, 130.93, 129.68, 128.55, 127.85, 126.61, 85.39, 64.89, 53.32. IR (KBr): 3344 (NH) cm−1. Anal calcd. for C19H15ClN6S.
5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-4-(4-(trifluoromethyl)phenyl)-2,4-dihydro-3H-1,2,4-triazole-3-one 4f. Yield: 63%. M.p.: 163–166 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.79–3.73 (m, 1H, H3), 4.49–4.52 (m, 1H, H3), 5.48–5.51 (m, 1H, H2), 6.53 (br s, 1H, NH exchangeable), 7.23–7.37 (m, 8H, 7Ar + H6), 7.61–7.64 (m, 2H, Ar). 13C-NMR (101 MHz, DMSO-d6): δ 166.36, 155.18, 138.87, 135.58, 133.61, 128.43, 128.34, 127.77, 126.44, 125.05, 123.62, 122.97, 119.62, 86.24, 67.25, 58.03. IR (KBr): 3381 (NH) cm−1. Anal calcd. for C20H15F3N6.
5-(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-4-(p-tolyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione 4g. Yield: 52%. M.p.: 247–249 °C. 1H-NMR (400 MHz, DMSO-d6): δ 2.42 (s, 3H, CH3), 3.70–3.75 (m, 1H, H3), 4.57–4.59 (m, 1H, H3), 5.37–5.42 (m, 1H, H2), 6.25 (br s, 1H, NH exchangeable), 6.81 (br s, 1H, NH exchangeable), 7.25–7.40 (m, 11H, 9Ar + H6). 13C-NMR (101 MHz, DMSO-d6): δ 167.67, 153.23, 146.71, 141.37, 140.10, 132.85, 130.61, 129.19, 129.05, 128.36, 127.18, 125.70, 86.49, 65.46, 53.78, 21.39. Anal calcd. for C20H18N6S.
4.2.3. Synthesis of Compounds 1a,b,d
To an absolute ethanol solution (10 mL) of the proper compounds 4 (1 mmol), sodium acetate (0.500 g, 6 mmol) and iodomethane (0.150 g, 1 mmol) were added and the mixture was heated at reflux for 3–4 h, monitoring, by TLC, the product formation. After cooling to room temperature, the solvent was evaporated under reduced pressure and the crude was solved in dichloromethane (20 mL); the organic phase was washed with water (2 × 10 mL), then dried (MgSO4) and concentrated under reduced pressure. The obtained solid was filtered and recrystallized by absolute ethanol or diethyl ether.
7-(5-(methylthio)-4-phenyl-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1a. Yield: 53%. M.p.: 98–100 °C. 1H-NMR (400 MHz, CDCl3): δ 2.69 (s, 3H, SCH3), 3.92–3.97 (m, 1H, H3), 4.55–4.60 (m, 1H, H3), 5.66–5.70 (m, 1H, H2), 6.34 (br s, 1H, NH exchangeable), 7.32–7.48 (m, 8H, 7Ar + H6), 7.68–7.75 (m, 3H, Ar). 13C-NMR (101 MHz, CDCl3): δ 154.72, 154.11, 147.41, 140.46, 139.32, 132.34, 131.65, 131.23, 129.09, 128.63, 127.60, 126.60, 125.48, 65.96, 54.23, 14.48. IR (KBr): 3213 (NH) cm−1. Anal calcd. for C20H18N6S.
7-(4-(2-fluorophenyl)-5-(methylthio)-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1b. Yield: 52%. M.p.: 195–196 °C. 1H-NMR (400 MHz, CDCl3): δ 2.53 (s, 3H, SCH3), 3.80–3.85 (m, 1H, H3), 4.40–4.45 (m, 1H, H3), 5.42–5.47 (m, 1H, H2), 6.32 (br s, 1H, NH exchangeable), 7.19–7.34 (m, 9H, 8Ar + H6), 7.51–7.54 (m, 1H, Ar). 13C-NMR (101 MHz, CDCl3): δ 157.84 (d, J = 255.8 Hz), 153.86, 152.64, 149.28, 139.43 (m), 133.69 (m), 129.79 (d, J = 7.2 Hz), 129.40, 129.11, 128.71, 126.58, 126.00 (m), 125.47, 117.98 (m), 104.83, 66.14, 54.37, 14.94. IR (KBr): 3326 (NH) cm−1. Anal calcd. for C20H17FN6S.
7-(4-(4-fluorophenyl)-5-(methylthio)-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1d. Yield: 70%. M.p.: 195–197 °C. 1H-NMR (400 MHz, DMSO-d6): δ 2.61 (s, 3H, SCH3), 3.99–4.03 (m, 1H, H3), 4.61–4.65 (m, 1H, H3), 5.62–5.66 (m, 1H, H2), 6.50 (br s, 1H, NH exchangeable), 7.24–7.37 (m, 4H, Ar), 7.45–7.52 (m, 3H, 2Ar + H6), 7.56–7.59 (m, 3H, Ar). 13C-NMR (101 MHz, DMSO-d6): δ 161.56 (d, J = 246.5 Hz), 147.93, 147.10, 138.47, 138.23, 138.11, 133.76 (d, J = 3.0 Hz), 129.34 (d, J = 8.4 Hz), 127.50, 126.35, 126.01, 115.92 (d, J = 22.5 Hz), 89.62, 64.10, 56.53, 20.29. IR (KBr): 3361 (NH) cm−1. Anal calcd. for C20H17FN6S.
4.2.4. Synthesis of Compounds 1c,e–g
To an anhydrous DMF solution (2 mL) of the proper compound 4 (1 mmol), anhydrous potassium carbonate (0.171 g, 1.24 mmol) and iodomethane (0.300 g, 2.13 mmol) were added and the mixture was stirred at rt for 1 h (compounds 1c and 1f) or 18 h (compound 1e and 1g), monitoring, by TLC, the product. Then water was added (20 mL); the precipitated solid was filtered, washed 3 times with water, and recrystallized from absolute ethanol.
7-(4-(3-fluorophenyl)-5-(methylthio)-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1c. Yield: 62%. M.p.: 105–108 °C. 1H-NMR (400 MHz, CDCl3): δ 2.67 (s, 3H, SCH3), 3.95–4.00 (m, 1H, H3), 4.54–4.59 (m, 1H, H3), 5.53–5.57 (m, 1H, H2), 6.49 (br s, 1H, NH exchangeable), 7.12–7.14 (m, 1H, Ar), 7.32–7.43 (m, 7H, 6Ar + H6), 7.58–7.60 (m, 1H, Ar). 13C-NMR (101 MHz, CDCl3): δ 163.16 (d, J = 251.8 Hz), 153.54, 151.81, 149.58, 139.57, 139.59, 134.49 (d, J = 9.1 Hz), 131.92 (d, J = 8.7 Hz), 129.12, 128.74, 126.56, 123.76, 118.42 (d, J = 20.7 Hz), 115.56 (J = 23.7 Hz), 85.87, 66.17, 54.83, 14.87. IR (KBr): 3267 (NH) cm−1. Anal calcd. for C20H17FN6S.
7-(4-(4-chlorophenyl)-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1e. Yield: 63%. M.p.: 127–129 °C. 1H-NMR (400 MHz, CDCl3): δ 2.69 (s, 3H, SCH3), 3.94–3.98 (m, 1H, H3), 4.55–4.60 (m, 1H, H3), 5.62–5.65 (m, 1H, H2), 6.46 (br s, 1H, NH exchangeable), 7.32–7.48 (m, 7H, 6Ar + H6), 7.62–7.72 (m, 3H, Ar). 13C-NMR (101 MHz, CDCl3): δ 154.24, 153.19, 148.31, 140.14, 139.37, 138.22, 131.91, 131.30, 130.67, 129.12, 129.04, 128.70, 126.58, 66.06, 54.29, 14.68. IR (KBr): 3342 (NH) cm−1. Anal calcd. for C20H17ClN6S.
7-(5-(methylthio)-4-(4-(trifluoromethyl)phenyl)-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1f. Yield: 42%. M.p.: 147–149 °C. 1H-NMR (400 MHz, CDCl3): δ 2.67 (s, 3H, SCH3), 3.71–3.75 (m, 1H, H3), 4.40–4.44 (m, 1H, H3), 5.19–5.22 (m, 1H, H2), 6.47 (br s, 1H, NH exchangeable), 7.24–7.37 (m, 8H, 7Ar + H6), 7.81–7.85 (m, 2H, Ar). 13C-NMR (101 MHz, CDCl3): δ 153.65, 151.92, 149.44, 139.91, 139.59, 136.34, 129.33, 129.12 (d, J = 5.7 Hz), 128.75 (d, J = 4.3 Hz), 128.50, 127.82, 126.63 (d, J = 6.7 Hz), 119.84 (d, J = 169.5 Hz), 114.41, 66.19, 54.36, 14.93. Anal calcd. for C21H17F3N6S.
7-(5-(methylthio)-4-(p-tolyl)-4H-1,2,4-triazole-3-yl)-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole 1g. Yield: 52%. M.p.: 87–89 °C. 1H-NMR (400 MHz, CDCl3): δ 2.43 (s, 3H, CH3), 2.54 (s, 3H, CH3), 3.70–3.75 (m, 1H, H3), 4.56–4.61 (m, 1H, H3), 5.38–5.43 (m, 1H, H2), 6.40 (br s, 1H, NH exchangeable), 7.30–7.43 (m, 10H, 9Ar + H6). 13C-NMR (101 MHz, DMSO-d6): δ 153.90, 150.44, 141.55, 140.66, 131.74, 131.06, 129.95, 129.03, 128.21, 127.30, 125.72, 117.30, 86.98, 65.68, 53.83, 21.38, 15.31. Anal calcd. for C21H20N6S.
4.2.5. Synthesis of 5-(2-Phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)-4-(4-(trifluoromethyl)phenyl)-4H-1,2,4-triazole-3-ol 1h
To an anhydrous DMF (2 mL) solution of compound 4f (428 mg, 1 mmol), anhydrous potassium carbonate (0.171 g, 1.24 mmol) and iodomethane (300 mg, 2.13 mmol) were added and the mixture was stirred at rt for 24 h, monitoring, by TLC, the product. Then water was added (20 mL); a pale-rose solid was precipitated and filtered, washed 3 times with water, and crystallized from absolute ethanol.
Yield: 55%. M.p.: 223–225 °C. 1H-NMR (400 MHz, DMSO-d6): δ 3.86–3.91 (m, 1H, H3), 4.68–4.73 (m, 1H, H3), 5.48–5.53 (m, 1H, H2), 7.32–7.49 (m, 6H, 5Ar + NH exchangeable), 7.66–7.78 (m, 5H, 4Ar + H6), 10.92 (br s, 1H, NH exchangeable). 13C-NMR (101 MHz, DMSO-d6): δ 157.63, 154.02, 152.43, 142.48, 140.73, 140.69, 128.59, 127.94, 126.75, 126.59, 126.41, 121.23 (d, J = 207.9 Hz), 116.68, 84.27, 65.17, 53.39. IR (KBr): 3450 (NH) cm−1. Anal calcd. for C20H15F3N6O. HPLC/ESI-MS analysis [M + H]+ 413.3.
4.3. Biological Evaluation
4.3.1. NCI Anti-Proliferative Screening
All compounds, 1a–h, were tested for anti-proliferative activity by National Cancer Institute (Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis) at a fixed concentration of 10 µM [24].
4.3.2. Cell Culture Conditions
MeOV and MeTA cells were kindly provided by Prof. Gabriella Pietra (University of Genoa, Genoa, Italy). HaCAT keratinocytes were kindly provided by Dr. Marco Ponassi (Biologic Bank and Cell Factory, IRCCS Policlinico San Martino, Genoa, Italy). Cell lines were maintained in RPMI 1640 medium (Euroclone Spa, Pavia, Italy) supplemented with 10% Fetal Bovine Serum (FBS, Euroclone Spa, Pavia, Italy), 1% L-Glutamine (Euroclone Spa, Pavia, Italy), and 1% Penicillin/Streptomicin (Euroclone Spa, Pavia, Italy) and grown in standard conditions (37 °C humidified incubator with 5% CO2).
4.3.3. Treatments
MEOV and MeTA cells were treated for 24, 48, and 72 h with increasing concentrations (0–100 μM) of 1h. Cell cultures were carefully monitored before and during the experiments to ensure optimal cell density. Notably, samples were discarded if the cell confluence reached >90%.
4.3.4. Cell Viability Assay
Cell viability was determined by using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) and following the supplier’s instructions. Briefly, cells (10,000 cells/well) were seeded into 96-well plates (Corning Incorporated, Corning, NY, USA) and then treated. Next, cells were incubated with 20 mL of CellTiter and the absorbance at 490 nm was recorded using a microplate reader (EL-808, BIO-TEK Instruments Inc., Winooski, VT, USA).
4.3.5. Detection of Hydrogen Peroxide (H2O2) Production
The production of H2O2 was evaluated using 2′-7′-dichlorofluorescein-diacetate (DCFH-DA; Merk Life Science S.r.l., Milan, Italy) as previously reported [43].
4.3.6. Statistical Analysis
The results are expressed as means ± S.E.Ms of at least four independent experiments. The statistical significance of the parametric differences between the experimental data sets was assessed by one-way ANOVA followed by Dunnett’s test for multiple comparisons using GraphPad Prism 8.0.1 (GraphPad Software v8.0, San Diego, CA, USA). p < 0.05 was considered statistically significant.
4.4. In Silico Prediction of ADMET Properties
The prediction of ADMET parameters was carried out by means of the Advanced Chemistry Development (ACD) Percepta platform [30]. The software prediction is based on the software implemented training libraries, which include experimentally determined pharmacokinetic and safety properties for different series of compounds.
4.5. Preparation of Micellar Solutions
The stock solution of 1h, at the concentration of 0.05 mM, was prepared for spectroscopic measurement. The surfactant solutions were prepared in deionized water and diluted with compound’s solution. The TW-80 concentrations were set to range from 0.014 to 0.14 mM. The solutions were kept for 2 h before being used to take measurements. The UV absorption spectra were carried out using a Thermo Scientific UV/VIS spectrophotometer (Evolution 300, Fischer Scientific, GmbH, Schwerte, Germany) at λ = 290 nm.
5. Conclusions
The SAR extension study on imidazo-pyrazole compounds led to the identification of the novel derivative 1h, characterized by a substituted 1,2,4-triazolyl substructure. The compound showed selective anti-proliferative activity against different melanoma cell lines without affecting the viability of healthy keratinocytes. Furthermore, the 1h increased the H2O2 levels in BRAF-mutated MEOV and MeTA cells.
As indicated by in silico predictions, 1h would be endowed with promising ADME and toxicological profiles. Furthermore, the preliminary solubility of 1h Tween-80 has provided a solid foundation for the rational selection of the most suitable drug delivery system in subsequent formulation studies.
Overall, the collected data support the pharmacological value of derivative 1h as a novel lead compound for the development of new and effective anti-melanoma agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26136312/s1.
Author Contributions
Synthesis of compounds, M.L. and C.B.; conceptualization, C.B. and A.S. (Andrea Spallarossa); biological investigations, B.M. and C.D.; drug delivery study, E.R. and D.C.; data curation, A.S. (Andrea Spallarossa); in silico study, E.C.; HPLC analysis, A.S. (Annalisa Salis); writing—original draft preparation, C.B. and A.S. (Andrea Spallarossa). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank M. Anzaldi, F. Rapetti, and R. Raggio for recording elemental analysis and 1H and 13 C spectra. We thank the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (NCI, http://dtp.cancer.gov) for their anti-proliferative evaluation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ulloora, S.; Shabaraya, R.; Adhikari, A.V. New 6-bromoimidazo [1,2-a]pyridine-2-carbohydrazide derivatives: Synthesis and anticonvulsant studies. Med. Chem. Res. 2014, 23, 3019–3028. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Zhang, F.; Yang, Y.; Wang, X. Preparation of Triazole Derivatives as Fungicides. Chinese Patent CN103373987, 30 October 2013. [Google Scholar]
- Xinghai, L.; Zhai, Z.; Zhao, W.; Sun, Z.; Tan, C.; Weng, J.; Liu, X. Preparation of Triazoles as Agrochemical Herbicides. Chinese patent CN106220610, 14 December 2016. [Google Scholar]
- Al-Wahaibi, L.H.; Rabea, S.M.; Mahmoud, M.A.; Youssif, B.G.M.; Bräse, S.; Abdel-Aziz, S.A. Synthesis and Antimicrobial Evaluation of New 1,2,4-Triazolo[1,5-a]pyrimidine-Based Derivatives as Dual Inhibitors of Bacterial DNA Gyrase and DHFR. ACS Omega 2024, 9, 47261–47273. [Google Scholar] [CrossRef]
- Azam, J.; Noman, M.; Nadeem, H.; Ahmad, N.; Ul-Haq, Z.; Hilal, F.; Irshad, N. Pharmacological investigation of selected 1,2,4 triazole derivative against ethanol induced gastric ulcer. Bioorganic Chem. 2024, 154, 108040. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, F.; Xiao, Y.; Cen, S.Y.; Wan, B.W.; Li, X.; Zhao, Y.; He, Y.; Yuan, H.X.; Nie, S. Discovery and optimization of 1,2,4-triazole derivatives as novel ferroptosis inhibitors. Europ. J. Med. Chem. 2024, 284, 117192. [Google Scholar] [CrossRef]
- Finlay, H.J.; Jiang, J.; Caringal, Y.; Kover, A.; Conder, M.L.; Xing, D.; Levesque, P.; Harper, T.; Hsueh, M.M.; Atwal, K.; et al. Triazolo and imidazo dihydropyrazolopyrimidine potassium channel antagonists. Bioorg. Med. Chem. Lett. 2013, 23, 1743–1747. [Google Scholar] [CrossRef]
- Song, J.; Pham, P.; Ma, J.; Novack, A.R.; Nashashibi, I.; Wone, D.W.G.; Shi, D.F.; Chen, X. Preparation of Bicyclic Agonists of GPR131 and Uses Thereof. WO2013032939, 7 March 2013. [Google Scholar]
- Andersen, H.S.; Kampen, G.C.T.; Christensen, I.T.; Mogensen, J.P.; Larsen, A.R. Pharmaceutical Use of Substituted 1,2,4-Triazoles for Modulating the Activity of 11β-Hydroxy Steroid Dehydrogenase Type 1 (11βHSD1). WO2004089367, 21 October 2004. [Google Scholar]
- Micheli, F.; Bernardelli, A.; Bianchi, F.; Braggio, S.; Castelletti, L.; Cavallini, P.; Cavanni, P.; Cremonesi, S.; Dal Cin, M.; Feriani, A.; et al. 1,2,4-Triazolyl octahydropyrrolo[2,3-b]pyrroles: A new series of potent and selective dopamine D3 receptor antagonists. Bioorg. Med. Chem. 2016, 24, 1619–1636. [Google Scholar] [CrossRef]
- Micheli, F.; Bacchi, A.; Braggio, S.; Castelletti, L.; Cavallini, P.; Cavanni, P.; Cremonesi, S.; Dal Cin, M.; Feriani, A.; Gehanne, S.; et al. 1,2,4-Triazolyl 5-Azaspiro[2.4]heptanes: Lead Identification and Early Lead Optimization of a New Series of Potent and Selective Dopamine D3 Receptor Antagonists. J. Med. Chem. 2016, 59, 8549–8576. [Google Scholar] [CrossRef]
- Arista, L.; Bonanomi, G.; Capelli, A.M.; Damiani, F.; Di Fabio, R.; Gentile, G.; Hamprecht, D.; Micheli, F.; Tarsi, L.; Tedesco, G.; et al. Preparation of N-(4H-1,2,4-Triazol-3-ylthioalkyl)-3-azabicyclo[3.1.0]hexane Derivatives Useful as Modulators of Dopamine D3 Receptors. WO2005080382, 1 September 2005. [Google Scholar]
- Kaya-Sezginer, E.; Oz Bedir, B.E.; Terzi, E.; Ozdemir Sanci, T.; Maryam, Z.; Acar Çevik, U. Design, Synthesis, and Biological Evaluation of New Benzimidazole-1,2,4-Triazole Derivatives as Potential Anticancer Agents. Chem. Biol. Drug Des. 2024, 104, e70033. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ab-delhamid, D.; Youssif, B.G.M.; Gomaa, H.A.M.; Hayallah, A.M.; Abdel-Aziz, M. Design, synthesis, and antiproliferative activity of new indole/1,2,4-triazole/chalcone hybrids as EGFR and/or c-MET inhibitors. Arch. Pharm. 2024, 357, e2300562. [Google Scholar] [CrossRef]
- Deb, M.; Singh, H.; Manhas, D.; Nandi, U.; Guru, S.K.; Das, P. Development of di-arylated 1,2,4-triazole-based derivatives as therapeutic agents against breast cancer: Synthesis and biological evaluation. RSC Med. Chem. 2024, 15, 3097–3113. [Google Scholar] [CrossRef]
- Zhu, H.; Man, R.; Li, Z. Preparation of 3-[5-(benzylthio)-4-phenyl-4H-1,2,4-triazol-3-yl]-1-methyl-1H-indole Derivatives Useful as Anticancer Agents. Chinese Patent CN107082774, 22 August 2017. [Google Scholar]
- Mikhina, E.A.; Stepanycheva, D.V.; Maksimova, V.P.; Sineva, O.N.; Markelova, N.N.; Grebenkina, L.E.; Lesovaya, E.A.; Yakubovskaya, M.G.; Matveev, A.V.; Zhidkova, E.M. Synthesis of Alkyl/Aryloxymethyl Derivatives of 1,2,4-Triazole-3-Carboxamides and Their Biological Activities. Molecules 2024, 29, 4808. [Google Scholar] [CrossRef]
- Crider, A.M.; Hospital, A.; Sandoval, K.E.; Neumann, W.L.; Kukielski, S.; Garic, L.; Ingold, K.; Dunahoo, M.; Srabony, K.N.; Frare, R.; et al. 3-Thio-3,4,5-trisubstituted-1,2,4-triazoles: High affinity somatostatin receptor-4 agonist synthesis and structure-activity relationships. RSC Med. Chem. 2025, 16, 945–960. [Google Scholar] [CrossRef]
- Signorello, M.G.; Rapetti, F.; Meta, E.; Sidibè, A.; Bruno, O.; Brullo, C. New Series of Pyrazoles and Imidazo-pyrazoles Targeting Different Cancer and Inflammation Pathways. Molecules 2021, 26, 5735. [Google Scholar] [CrossRef]
- Brullo, C.; Rapetti, F.; Alfei, S.; Maric, I.; Rizzelli, F.; Mapelli, M.; Rosano, C.; Viale, M.; Bruno, O. Discovery of new antiproliferative imidazopyrazole acylhydrazone able to interact with microtubule system. ChemMedChem 2020, 15, 961–969. [Google Scholar] [CrossRef]
- Alfei, S.; Milanese, M.; Brullo, C.; Valenti, G.E.; Domenicotti, C.; Russo, E.; Marengo, B. Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics 2023, 15, 2425. [Google Scholar] [CrossRef]
- Spallarossa, A.; Rapetti, F.; Signorello, M.G.; Rosano, C.; Iervasi, E.; Ponassi, M.; Brullo, C. Insights into the Pharmacological Activity of the Imidazo-Pyrazole Scaffold. ChemMedChem 2023, 18, e202300252. [Google Scholar] [CrossRef]
- Iervasi, E.; Coronel Vargas, G.; Bachetti, T.; Tkachenko, K.; Spallarossa, A.; Brullo, C.; Rosano, C.; Carta, S.; Barboro, P.; Profumo, A.; et al. A Proteomics Approach Identifies RREB1 as a Crucial Molecular Target of Imidazo-Pyrazole Treatment in SKMEL-28 Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 6760. [Google Scholar] [CrossRef]
- Available online: http://dtp.cancer.gov (accessed on 30 April 2025).
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Smolyarova, D.D.; Podgorny, O.V.; Bilan, D.S.; Belousov, V.V. A guide to genetically encoded tools for the study of H2O2. FEBS J. 2022, 289, 5382–5395. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Parodi, A.; Righetti, G.; Pesce, E.; Salis, A.; Tasso, B.; Urbinati, C.; Tomati, V.; Damonte, G.; Rusnati, M.; Pedemonte, N.; et al. Discovery of novel VX-809 hybrid derivatives as F508del-CFTR correctors by molecular modeling, chemical synthesis and biological assays. Eur. J. Med. Chem. 2020, 208, 112833. [Google Scholar] [CrossRef]
- Abbotto, E.; Casini, B.; Piacente, F.; Scarano, N.; Cerri, E.; Tonelli, M.; Astigiano, C.; Millo, E.; Sturla, L.; Bruzzone, S.; et al. Novel Thiazole-Based SIRT2 Inhibitors Discovered via Molecular Modelling Studies and Enzymatic Assays. Pharmaceuticals 2023, 16, 1316. [Google Scholar] [CrossRef]
- ACD/Percepta Platform; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2015.
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Model. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Noor, S.; Taj, M.B. Mixed-micellar approach for enhanced dye entrapment: A spectroscopic study. J. Mol. Liq. 2021, 338, 116701. [Google Scholar] [CrossRef]
- Parodi, B.; Russo, E.; Caviglioli, G.; Baldassari, S.; Gaglianone, N.; Schito, A.M.; Cafaggi, S. A chitosan lactate/poloxamer 407-based matrix containing Eudragit RS microparticles for vaginal delivery of econazole: Design and in vitro evaluation. Drug Dev. Ind. Pharm. 2013, 39, 1911–1920. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology for Pediatric Retinoblastoma Therapy. Pharmaceuticals 2022, 15, 1087. [Google Scholar] [CrossRef]
- Shakeel, F.; Alshehri, S.; Ibrahim, M.A.; Altamimi, M.; Haq, N.; Elzayat, E.M.; Shazly, G.A. Solubilization and thermodynamic properties of simvastatin in various micellar solutions of different non-ionic surfactants: Computational modeling and solubilization capacity. PLoS ONE 2021, 16, e0249485. [Google Scholar] [CrossRef]
- Rana, A.A.; Yusaf, A.; Shahid, S.; Usman, M.; Ahmad, M.; Aslam, S.; Al-Hussain, S.A.; Zaki, M.E.A. Unveiling the Role of Nonionic Surfactants in Enhancing Cefotaxime Drug Solubility: A UV-Visible Spectroscopic Investigation in Single and Mixed Micellar Formulations. Pharmaceuticals 2023, 16, 1663. [Google Scholar] [CrossRef]
- Mahmood, M.E.; Al-Koofee, D.A. Effect of temperature changes on critical micelle concentration for tween series surfactant. Glob. J. Sci. Front. Res. Chem. 2013, 13, 1–7. [Google Scholar]
- Hanif, S.; Usman, M.; Hussain, A.; Rasool, N.; Zubair, M.; Rana, U.A. Solubilization of Benzothiazole (BNZ) by micellar media of Sodium dodecyl sulphate and Cetyl trimethylammonium bromide. J. Mol. Liq. 2015, 211, 7–14. [Google Scholar] [CrossRef]
- Meta, E.; Brullo, C.; Sidibe, A.; Imhof, B.A.; Bruno, O. Design, synthesis and biological evaluation of new pyrazolyl-ureas and imidazopyrazolecarboxamides able to interfere with MAPK and PI3K upstream signaling involved in the angiogenesis. Eur. J. Med. Chem. 2017, 133, 24–35. [Google Scholar] [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-Mediated Antioxidant Response and Aerobic Metabolism: Two Crucial Factors Involved in Determining the Multi-Drug Resistance of High-Risk Neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).