Plasma Metabolic and Inflammatory Protein Signatures in Psychiatric Disorders
Abstract
1. Introduction
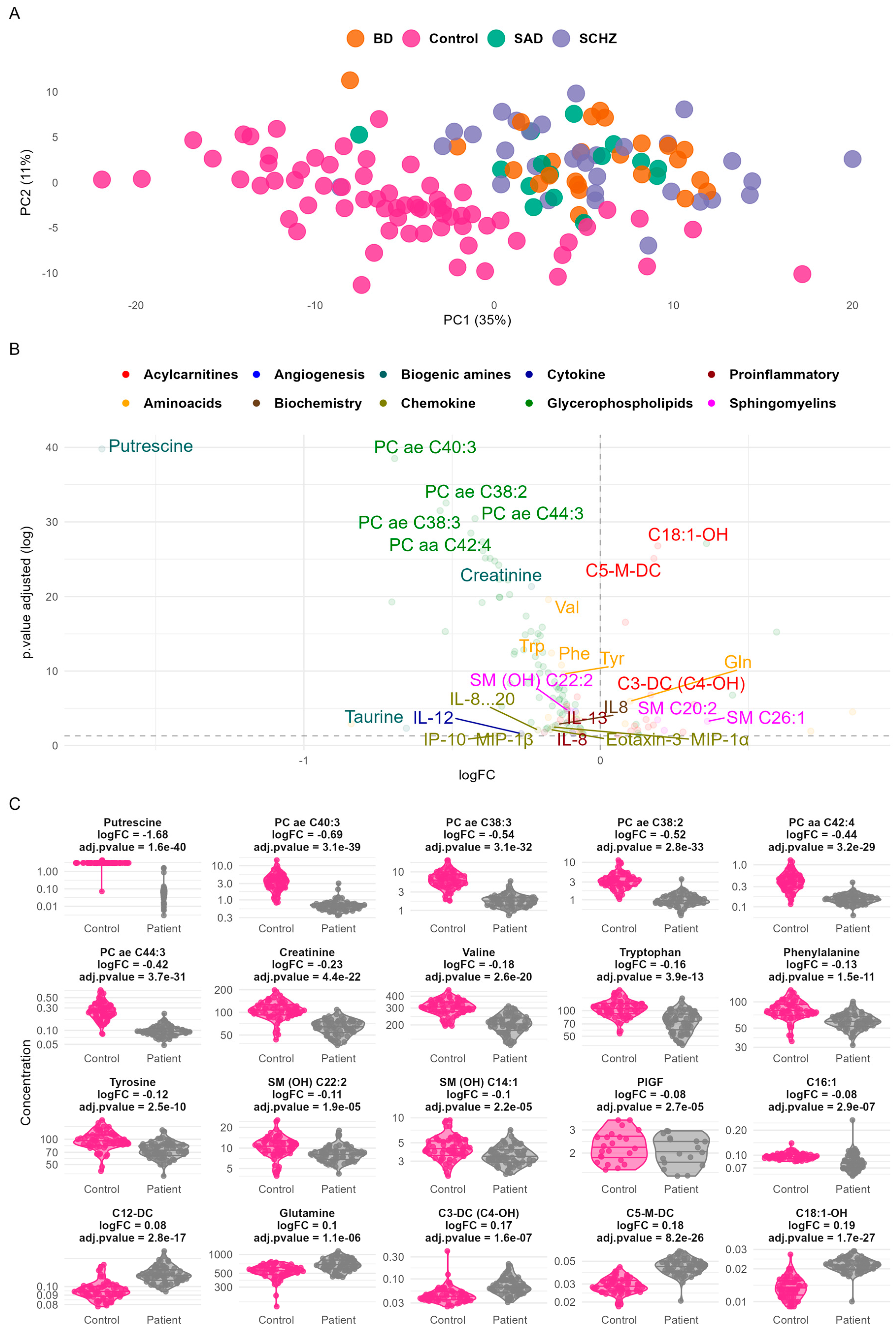
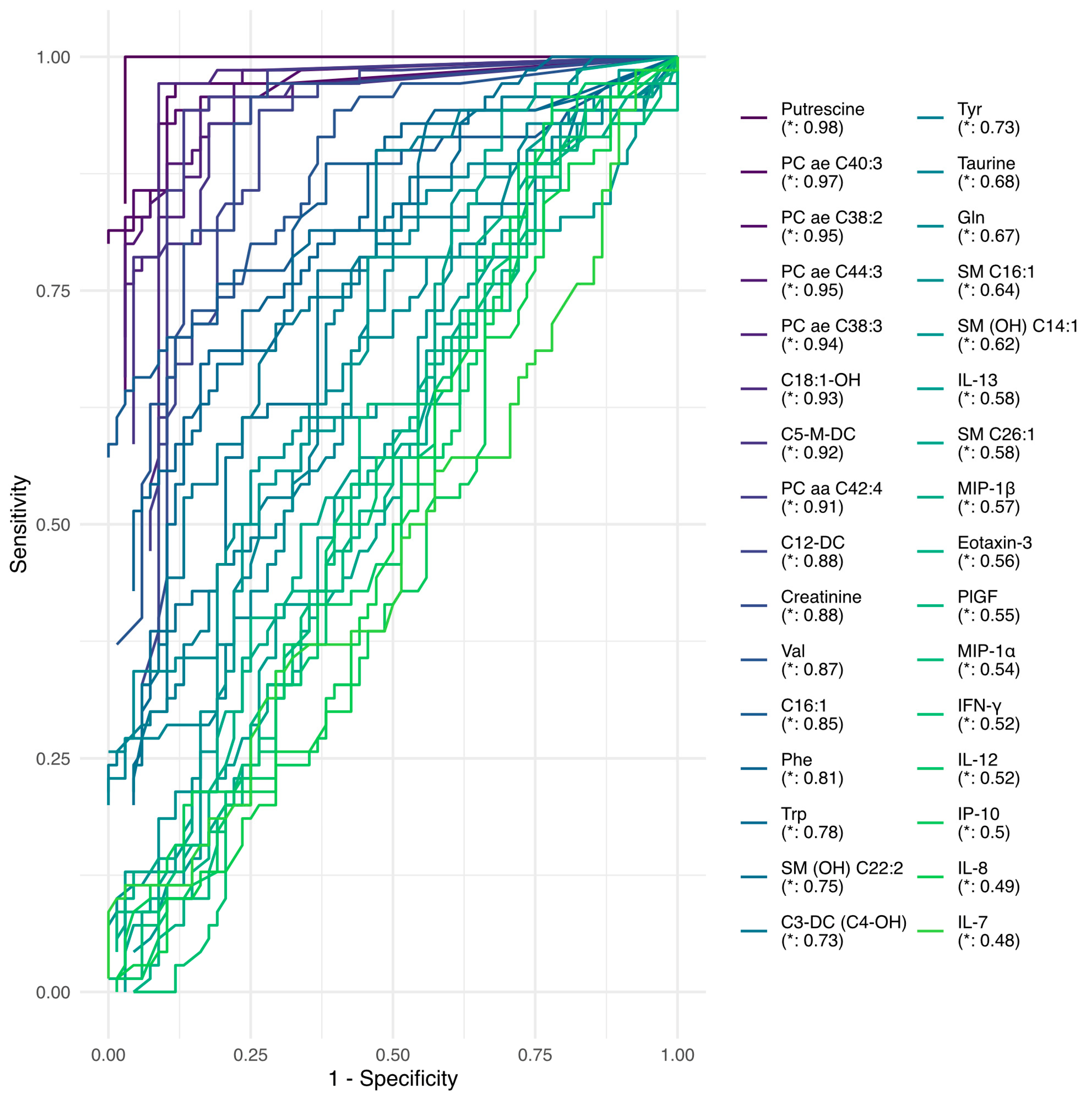
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Targeted Metabolomics Analysis
4.3. Targeted Proteomics Analysis
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BD | Bipolar Disorder |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th Edition |
| EDTA | Ethylenediaminetetraacetic Acid |
| FGF2 | Fibroblast Growth Factor 2 |
| FIA | Fluorescence Immunoassay |
| HPLC | High-Performance Liquid Chromatography |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| ICD-11 | International Classification of Diseases, 11th Revision |
| LDL | Low-Density Lipoprotein |
| LPC | Lysophosphatidylcholine |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MoCA | Montreal Cognitive Assessment |
| MRM | Multiple Reaction Monitoring |
| PANSS | The Positive and Negative Symptoms Scale |
| PC | Phosphatidylcholine |
| PLGF | Placental Growth Factor |
| RDoC | Research Domain Criteria |
| SAA | Serum Amyloid A |
| SAD | Schizoaffective Disorder |
| SCZ | Schizophrenia |
| SM | Sphingomyelin |
| TARC | Thymus- and Activation-Regulated Chemokine |
| TC | Total cholesterol |
| TG | Triglycerides |
| Tie-2 | Tyrosine Kinase with Immunoglobulin-Like and EGF-Like Domains 2 |
| TNF-β | Tumor necrosis factor beta |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
References
- Perälä, J.; Suvisaari, J.; Saarni, S.I.; Kuoppasalmi, K.; Isometsä, E.; Pirkola, S.; Partonen, T.; Tuulio-Henriksson, A.; Hintikka, J.; Kieseppä, T.; et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry 2007, 64, 19–28. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Y.; Su, X.; Wang, M.; Li, Q.; Shao, Z.; Sun, L. Global, regional and national burdens of bipolar disorders in adolescents and young adults: A trend analysis from 1990 to 2019. Gen. Psychiatry 2024, 37, e101255. [Google Scholar] [CrossRef]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef]
- Reed, G.M.; First, M.B.; Kogan, C.S.; Hyman, S.E.; Gureje, O.; Gaebel, W.; Maj, M.; Stein, D.J.; Maercker, A.; Tyrer, P.; et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 2019, 18, 3–19. [Google Scholar] [CrossRef]
- Wakefield, J.C. DSM-5, psychiatric epidemiology and the false positives problem. Epidemiol. Psychiatr. Sci. 2015, 24, 188–196. [Google Scholar] [CrossRef]
- Sharan, P.; Hans, G. Cultural Issues Related to ICD-11 Mental, Behavioural and Neurodevelopmental Disorders. Consort. Psychiatr. 2021, 2, 7–15. [Google Scholar] [CrossRef]
- Frances, A.J.; Nardo, J.M. ICD-11 should not repeat the mistakes made by DSM-5. Br. J. Psychiatry 2013, 203, 1–2. [Google Scholar] [CrossRef]
- Kamp-Becker, I. Autism spectrum disorder in ICD-11-a critical reflection of its possible impact on clinical practice and research. Mol. Psychiatry 2024, 29, 633–638. [Google Scholar] [CrossRef]
- Stein, D.J.; Lund, C.; Nesse, R.M. Classification systems in psychiatry: Diagnosis and global mental health in the era of DSM-5 and ICD-11. Curr. Opin. Psychiatry 2013, 26, 493–497. [Google Scholar] [CrossRef]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef]
- Musumeci, A.; Vinci, M.; Treccarichi, S.; Greco, D.; Rizzo, B.; Gloria, A.; Federico, C.; Saccone, S.; Musumeci, S.A.; Calì, F. Potential Association of the CSMD1 Gene with Moderate Intellectual Disability, Anxiety Disorder, and Obsessive–Compulsive Personality Traits. Int. J. Mol. Sci. 2025, 26, 4297. [Google Scholar] [CrossRef]
- Ursini, G.; Di Carlo, P.; Mukherjee, S.; Chen, Q.; Han, S.; Kim, J.; Deyssenroth, M.; Marsit, C.J.; Chen, J.; Hao, K.; et al. Prioritization of potential causative genes for schizophrenia in placenta. Nat. Commun. 2023, 14, 2613. [Google Scholar] [CrossRef]
- Hill, M.; Shannahan, K.; Jasinski, S.; Macklin, E.A.; Raeke, L.; Roffman, J.L.; Goff, D.C. Folate supplementation in schizophrenia: A possible role for MTHFR genotype. Schizophr. Res. 2011, 127, 41–45. [Google Scholar] [CrossRef]
- van Winkel, R.; Rutten, B.P.; Peerbooms, O.; Peuskens, J.; van Os, J.; De Hert, M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2010, 121, 193–198. [Google Scholar] [CrossRef]
- Tebani, A.; Afonso, C.; Marret, S.; Bekri, S. Omics-Based Strategies in Precision Medicine: Toward a Paradigm Shift in Inborn Errors of Metabolism Investigations. Int. J. Mol. Sci. 2016, 17, 1555. [Google Scholar] [CrossRef]
- Dalgleish, T.; Black, M.; Johnston, D.; Bevan, A. Transdiagnostic approaches to mental health problems: Current status and future directions. J. Consult. Clin. Psychol. 2020, 88, 179–195. [Google Scholar] [CrossRef]
- Wise, T.; Robinson, O.J.; Gillan, C.M. Identifying Transdiagnostic Mechanisms in Mental Health Using Computational Factor Modeling. Biol. Psychiatry 2023, 93, 690–703. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef]
- Myint, A.M.; Kim, Y.K.; Verkerk, R.; Scharpe, S.; Steinbusch, H.; Leonard, B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J. Affect. Disord. 2007, 98, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Ge, M.; Wang, J.; Kuang, D.; Wei, H.; Wang, Z.; Hu, Z.; Zhao, C.; Jin, Q.; Chen, M.; et al. Causal association between kynurenine and depression investigated using two-sample mendelian randomization. Sci. Rep. 2024, 14, 1821. [Google Scholar] [CrossRef]
- Bogielski, B.; Michalczyk, K.; Głodek, P.; Tempka, B.; Gębski, W.; Stygar, D. Association between small intestine bacterial overgrowth and psychiatric disorders. Front. Endocrinol. 2024, 15, 1438066. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- MacKay, M.; Yang, B.H.; Dursun, S.M.; Baker, G.B. The Gut-Brain Axis and the Microbiome in Anxiety Disorders, Post-Traumatic Stress Disorder and Obsessive-Compulsive Disorder. Curr. Neuropharmacol. 2024, 22, 866–883. [Google Scholar] [CrossRef]
- Mhanna, A.; Martini, N.; Hmaydoosh, G.; Hamwi, G.; Jarjanazi, M.; Zaifah, G.; Kazzazo, R.; Haji Mohamad, A.; Alshehabi, Z. The correlation between gut microbiota and both neurotransmitters and mental disorders: A narrative review. Medicine 2024, 103, e37114. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.; Jakobi, B.; Shi, Y.; Mulders, P.; Kist, J.D.; Collard, R.M.; Vrijsen, J.N.; van Eijndhoven, P.; Tendolkar, I.; Bloemendaal, M.; et al. Gut microbiota composition links to variation in functional domains across psychiatric disorders. Brain Behav. Immun. 2024, 120, 275–287. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, S.; Wu, Y.; Huang, Y.; Tao, S.; Liu, Y.; Tang, Y.; Xie, M.; Ma, Q.; Yin, Y.; et al. The interactions between host genome and gut microbiome increase the risk of psychiatric disorders: Mendelian randomization and biological annotation. Brain Behav. Immun. 2023, 113, 389–400. [Google Scholar] [CrossRef]
- Zhao, G.; Lu, Z.; Liao, Y.; Sun, Y.; Zhang, Y.; Kang, Z.; Feng, X.; Sun, J.; Yue, W. Association of intestinal anti-inflammatory drug target genes with psychiatric Disorders: A Mendelian randomization study. J. Adv. Res. 2025, 70, 545–553. [Google Scholar] [CrossRef]
- Roiser, J.P.; McLean, A.; Ogilvie, A.D.; Blackwell, A.D.; Bamber, D.J.; Goodyer, I.; Jones, P.B.; Sahakian, B.J. The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology 2005, 30, 775–785. [Google Scholar] [CrossRef]
- Yin, B.; Cai, Y.; Teng, T.; Wang, X.; Liu, X.; Li, X.; Wang, J.; Wu, H.; He, Y.; Ren, F.; et al. Identifying plasma metabolic characteristics of major depressive disorder, bipolar disorder, and schizophrenia in adolescents. Transl. Psychiatry 2024, 14, 163. [Google Scholar] [CrossRef]
- Gomez-Merino, D.; Bequet, F.; Berthelot, M.; Riverain, S.; Chennaoui, M.; Guezennec, C.Y. Evidence that the branched-chain amino acid L-valine prevents exercise-induced release of 5-HT in rat hippocampus. Int. J. Sports Med. 2001, 22, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Beneyto, M.; Kristiansen, L.V.; Oni-Orisan, A.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007, 32, 1888–1902. [Google Scholar] [CrossRef]
- Kruse, A.O.; Bustillo, J.R. Glutamatergic dysfunction in Schizophrenia. Transl. Psychiatry 2022, 12, 500. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C., Jr.; Machado-Vieira, R.; Henter, I.; Ibrahim, L.; Diazgranados, N.; Salvadore, G. Glutamatergic modulators: The future of treating mood disorders? Harv. Rev. Psychiatry 2010, 18, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Keilhoff, G.; Laube, G.; Dobrowolny, H.; Steiner, J. Polyamines and polyamine-metabolizing enzymes in schizophrenia: Current knowledge and concepts of therapy. World J. Psychiatry 2021, 11, 1177–1190. [Google Scholar] [CrossRef]
- Spathopoulou, A.; Sauerwein, G.A.; Marteau, V.; Podlesnic, M.; Lindlbauer, T.; Kipura, T.; Hotze, M.; Gabassi, E.; Kruszewski, K.; Koskuvi, M.; et al. Integrative metabolomics-genomics analysis identifies key networks in a stem cell-based model of schizophrenia. Mol. Psychiatry 2024, 29, 3128–3140. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, T.; Li, R.; Cui, Z.Q.; Du, J.; Yang, J.B.; Xue, F.; Chen, Y.H.; Tan, Q.R.; Peng, Z.W. Alterations in the Plasma Lipidome of Adult Women With Bipolar Disorder: A Mass Spectrometry-Based Lipidomics Research. Front. Psychiatry 2022, 13, 802710. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Wang, D.; Hu, X.; Jiang, J.; Qing, Y.; Yang, X.; Cui, G.; Wang, P.; Zhang, J.; et al. Impaired Membrane Lipid Homeostasis in Schizophrenia. Schizophr. Bull. 2022, 48, 1125–1135. [Google Scholar] [CrossRef]
- Rege, S.; Mackworth-Young, C. Antiphospholipid antibodies as biomarkers in psychiatry: Review of psychiatric manifestations in antiphospholipid syndrome. Transl. Dev. Psychiatry 2015, 3, 25452. [Google Scholar] [CrossRef]
- Matam, Y.; Ray, B.D.; Petrache, H.I. Direct affinity of dopamine to lipid membranes investigated by Nuclear Magnetic Resonance spectroscopy. Neurosci. Lett. 2016, 618, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Hou, W.; Tian, H.; Wang, L.; Li, R. Lipidomics of the brain, retina, and biofluids: From the biological landscape to potential clinical application in schizophrenia. Transl. Psychiatry 2020, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhong, Q.; Wu, W.-t.; Chen, J.-j. Multi-omics data reveals the important role of glycerophospholipid metabolism in the crosstalk between gut and brain in depression. J. Transl. Med. 2023, 21, 93. [Google Scholar] [CrossRef]
- Rezin, G.T.; Amboni, G.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 2009, 34, 1021–1029. [Google Scholar] [CrossRef]
- Kivimaki, M.; Shipley, M.J.; Batty, G.D.; Hamer, M.; Akbaraly, T.N.; Kumari, M.; Jokela, M.; Virtanen, M.; Lowe, G.D.; Ebmeier, K.P.; et al. Long-term inflammation increases risk of common mental disorder: A cohort study. Mol. Psychiatry 2014, 19, 149–150. [Google Scholar] [CrossRef]
- Giesbrecht, C.J.; O’ Rourke, N.; Leonova, O.; Strehlau, V.; Paquet, K.; Vila-Rodriguez, F.; Panenka, W.J.; MacEwan, G.W.; Smith, G.N.; Thornton, A.E.; et al. The Positive and Negative Syndrome Scale (PANSS): A Three-Factor Model of Psychopathology in Marginally Housed Persons with Substance Dependence and Psychiatric Illness. PLoS ONE 2016, 11, e0151648. [Google Scholar] [CrossRef][Green Version]
- Soron, T.R. Validation of Bangla Montgomery Asberg Depression Rating Scale (MADRSB). Asian J. Psychiatry 2017, 28, 41–46. [Google Scholar] [CrossRef]
- Hobson, J. The Montreal Cognitive Assessment (MoCA). Occup. Med. 2015, 65, 764–765. [Google Scholar] [CrossRef]
- Licht, R.W.; Jensen, J. Validation of the Bech-Rafaelsen Mania Scale using latent structure analysis. Acta Psychiatr. Scand. 1997, 96, 367–372. [Google Scholar] [CrossRef]
- Ducatez, F.; Mauhin, W.; Boullier, A.; Pilon, C.; Pereira, T.; Aubert, R.; Benveniste, O.; Marret, S.; Lidove, O.; Bekri, S.; et al. Parsing Fabry Disease Metabolic Plasticity Using Metabolomics. J. Pers. Med. 2021, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Tebani, A.; Mauhin, W.; Abily-Donval, L.; Lesueur, C.; Berger, M.G.; Nadjar, Y.; Berger, J.; Benveniste, O.; Lamari, F.; Laforêt, P.; et al. A Proteomics-Based Analysis Reveals Predictive Biological Patterns in Fabry Disease. J. Clin. Med. 2020, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Trygg, J.; Wold, S. A chemometrics toolbox based on projections and latent variables. J. Chemom. 2014, 28, 332–346. [Google Scholar] [CrossRef]
- Van Den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wright, M.N.; Ziegler, A. ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. J. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. 2020. Available online: https://CRAN.R-project.org/package=caret (accessed on 26 May 2025).

| Total Population Lipids | Controls | Patients | p-Value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| TC (mmol/L) | 68 | 4.41 | 0.87 | 70 | 3.64 | 0.77 | 1.57 × 10−7 |
| TG (mmol/L) | 68 | 1.17 | 0.50 | 70 | 1.01 | 0.43 | 3.85 × 10−2 |
| HDL-C (mmol/L) | 39 | 1.08 | 0.25 | 48 | 1.18 | 0.43 | 1.76 × 10−1 |
| LDL-C (mmol/L) | 39 | 2.72 | 0.75 | 48 | 2.08 | 0.71 | 1.11 × 10−4 |
| Tota PC (µmol/L) * | 68 | 1084.83 | 283.71 | 70 | 774.33 | 156.28 | 2.65 × 10−12 |
| Total LPC (µmol/L) * | 68 | 410.50 | 152.54 | 70 | 252.88 | 88.92 | 3.41 × 10−11 |
| Total SM (µmol/L) * | 67 | 255.81 | 75.75 | 70 | 228.15 | 52.16 | 1.46 × 10−2 |
| Total Acylcarnitines (µmol/L) * | 68 | 56.04 | 15.87 | 70 | 48.18 | 12.77 | 1.74 × 10−3 |
| Characteristic | Schizoaffective Disorder (SAD), N = 16 1 | Bipolar Disorder (BD), N = 26 1 | Schizophrenia (SCZ), N = 34 1 | p-Value 2 | q-Value 3 |
|---|---|---|---|---|---|
| Age | 30 (24, 33) | 36 (29, 47) | 36 (29, 44) | 0.022 | 0.070 |
| Unknown | 0 | 1 | 0 | ||
| Number of Tobacco Packs/Year | 0 (0, 4) | 2 (0, 12) | 0 (0, 15) | 0.7 | 0.7 |
| Unknown | 2 | 7 | 6 | ||
| Weight | 60 (57, 80) | 64 (60, 71) | 64 (57, 70) | 0.7 | 0.7 |
| Unknown | 3 | 8 | 7 | ||
| Height | 1.72 (1.70, 1.78) | 1.75 (1.66, 1.79) | 1.70 (1.66, 1.75) | 0.5 | 0.7 |
| Unknown | 4 | 11 | 9 | ||
| Body Mass Index (BMI) | 20.5 (18.8, 26.2) | 21.8 (20.9, 23.3) | 21.7 (19.3, 25.6) | 0.9 | 0.9 |
| Unknown | 4 | 11 | 9 | ||
| Marital Status | 0.002 | 0.016 | |||
| Divorced | 0 (0%) | 1 (4.0%) | 0 (0%) | ||
| Married | 0 (0%) | 9 (36%) | 3 (8.8%) | ||
| Unmarried | 16 (100%) | 15 (60%) | 31 (91%) | ||
| Unknown | 0 | 1 | 0 | ||
| Living Situation | 0.4 | 0.6 | |||
| Alone | 2 (13%) | 2 (8.0%) | 7 (21%) | ||
| With family | 13 (87%) | 23 (92%) | 27 (79%) | ||
| Unknown | 1 | 1 | 0 | ||
| Educational Level | 0.2 | 0.5 | |||
| Illiterate | 2 (12%) | 2 (8.0%) | 6 (18%) | ||
| Primary | 5 (31%) | 4 (16%) | 13 (38%) | ||
| Secondary | 6 (38%) | 11 (44%) | 12 (35%) | ||
| University-level | 3 (19%) | 8 (32%) | 3 (8.8%) | ||
| Unknown | 0 | 1 | 0 | ||
| Social Level | 0.017 | 0.070 | |||
| High | 0 (0%) | 2 (9.1%) | 0 (0%) | ||
| Low | 10 (67%) | 10 (45%) | 24 (86%) | ||
| Medium | 5 (33%) | 10 (45%) | 4 (14%) | ||
| Unknown | 1 | 4 | 6 | ||
| Profession | 0.003 | 0.016 | |||
| Active | 4 (27%) | 15 (60%) | 6 (18%) | ||
| Inactive | 11 (73%) | 10 (40%) | 28 (82%) | ||
| Unknown | 1 | 1 | 0 | ||
| Housing | 0.3 | 0.6 | |||
| Rural | 4 (29%) | 8 (33%) | 15 (48%) | ||
| Urban | 10 (71%) | 16 (67%) | 16 (52%) | ||
| Unknown | 2 | 2 | 3 | ||
| Psychoactive Substance | 8 (50%) | 20 (83%) | 19 (56%) | 0.045 | 0.12 |
| Unknown | 0 | 2 | 0 | ||
| Tobacco Use | 8 (50%) | 19 (76%) | 17 (50%) | 0.10 | 0.2 |
| Unknown | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naifar, M.; Ducatez, F.; Guidara, W.; Maalej, M.; Lesueur, C.; Pilon, C.; Plichet, T.; Maalej, M.; Ayadi, F.; Bekri, S. Plasma Metabolic and Inflammatory Protein Signatures in Psychiatric Disorders. Int. J. Mol. Sci. 2025, 26, 6260. https://doi.org/10.3390/ijms26136260
Naifar M, Ducatez F, Guidara W, Maalej M, Lesueur C, Pilon C, Plichet T, Maalej M, Ayadi F, Bekri S. Plasma Metabolic and Inflammatory Protein Signatures in Psychiatric Disorders. International Journal of Molecular Sciences. 2025; 26(13):6260. https://doi.org/10.3390/ijms26136260
Chicago/Turabian StyleNaifar, Manel, Franklin Ducatez, Wassim Guidara, Manel Maalej, Celine Lesueur, Carine Pilon, Thomas Plichet, Mohamed Maalej, Fatma Ayadi, and Soumeya Bekri. 2025. "Plasma Metabolic and Inflammatory Protein Signatures in Psychiatric Disorders" International Journal of Molecular Sciences 26, no. 13: 6260. https://doi.org/10.3390/ijms26136260
APA StyleNaifar, M., Ducatez, F., Guidara, W., Maalej, M., Lesueur, C., Pilon, C., Plichet, T., Maalej, M., Ayadi, F., & Bekri, S. (2025). Plasma Metabolic and Inflammatory Protein Signatures in Psychiatric Disorders. International Journal of Molecular Sciences, 26(13), 6260. https://doi.org/10.3390/ijms26136260







