ApoE Isoform-Dependent Effects on Extinction of Contextual Fear Memory and Passive Avoidance Memory
Abstract
1. Introduction
2. Results
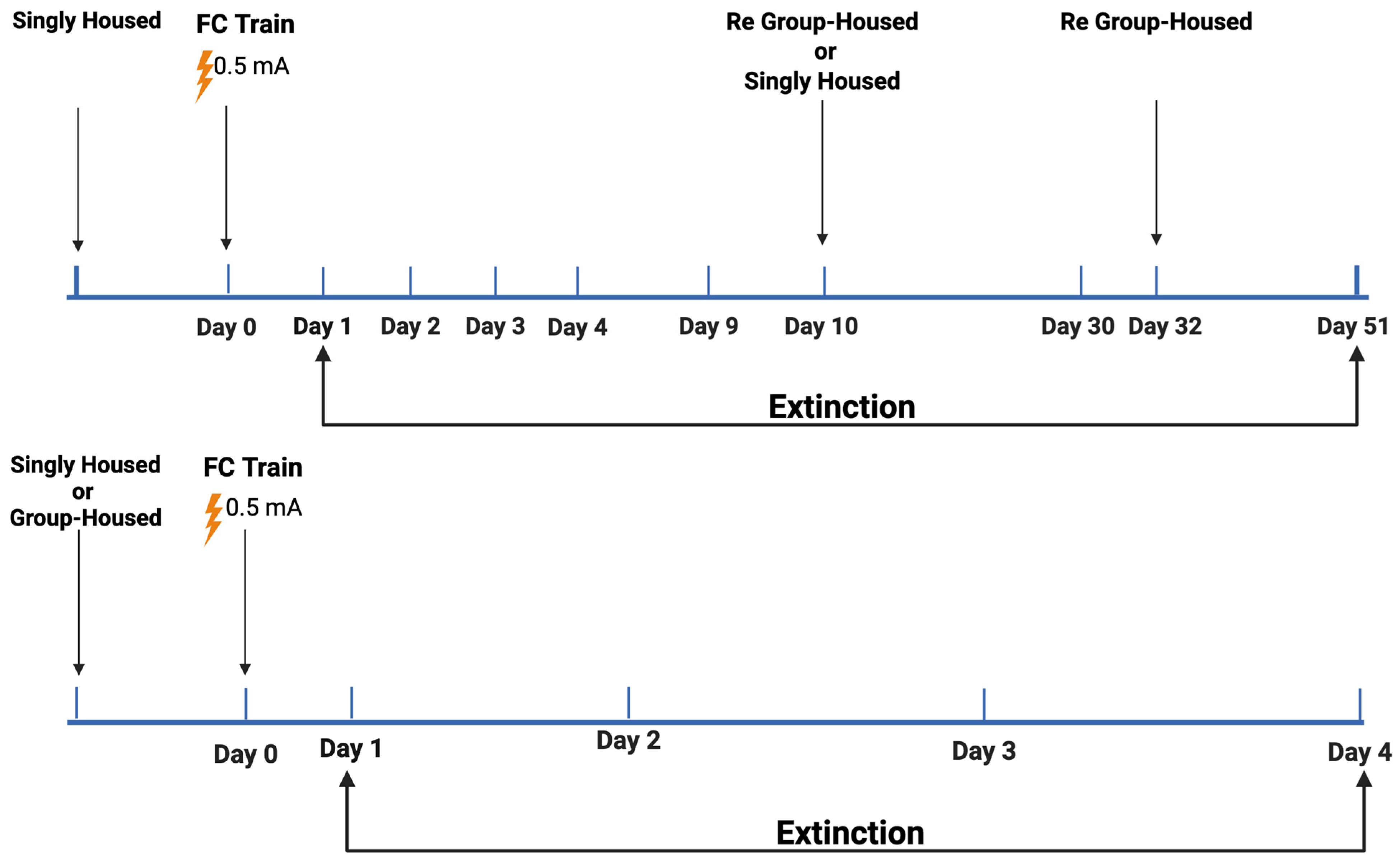
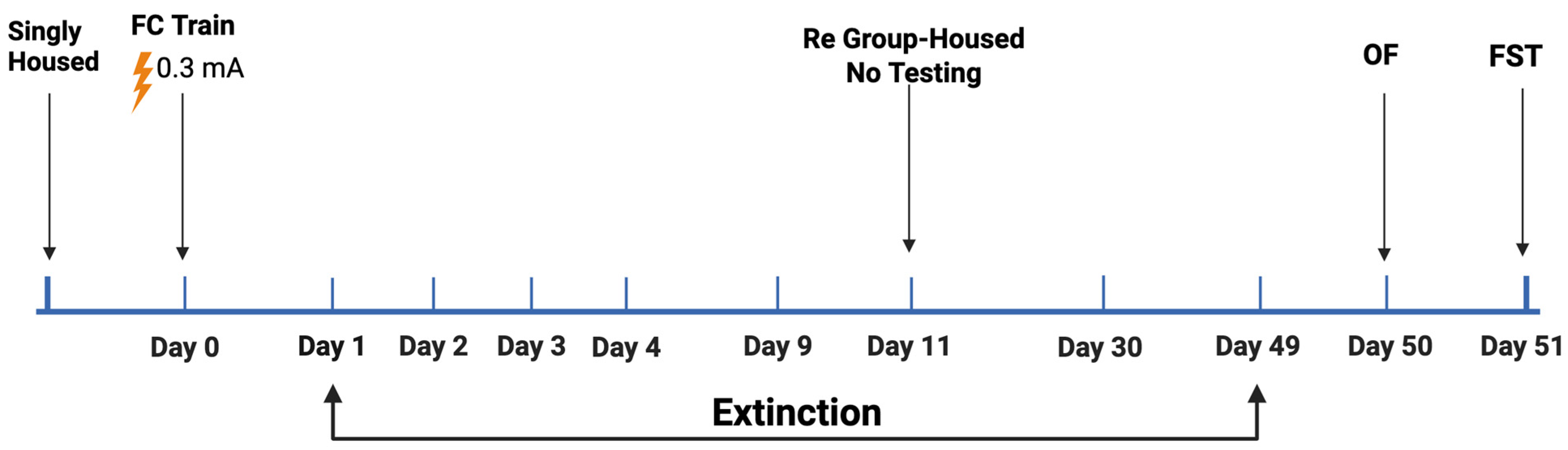
2.1. Study 1
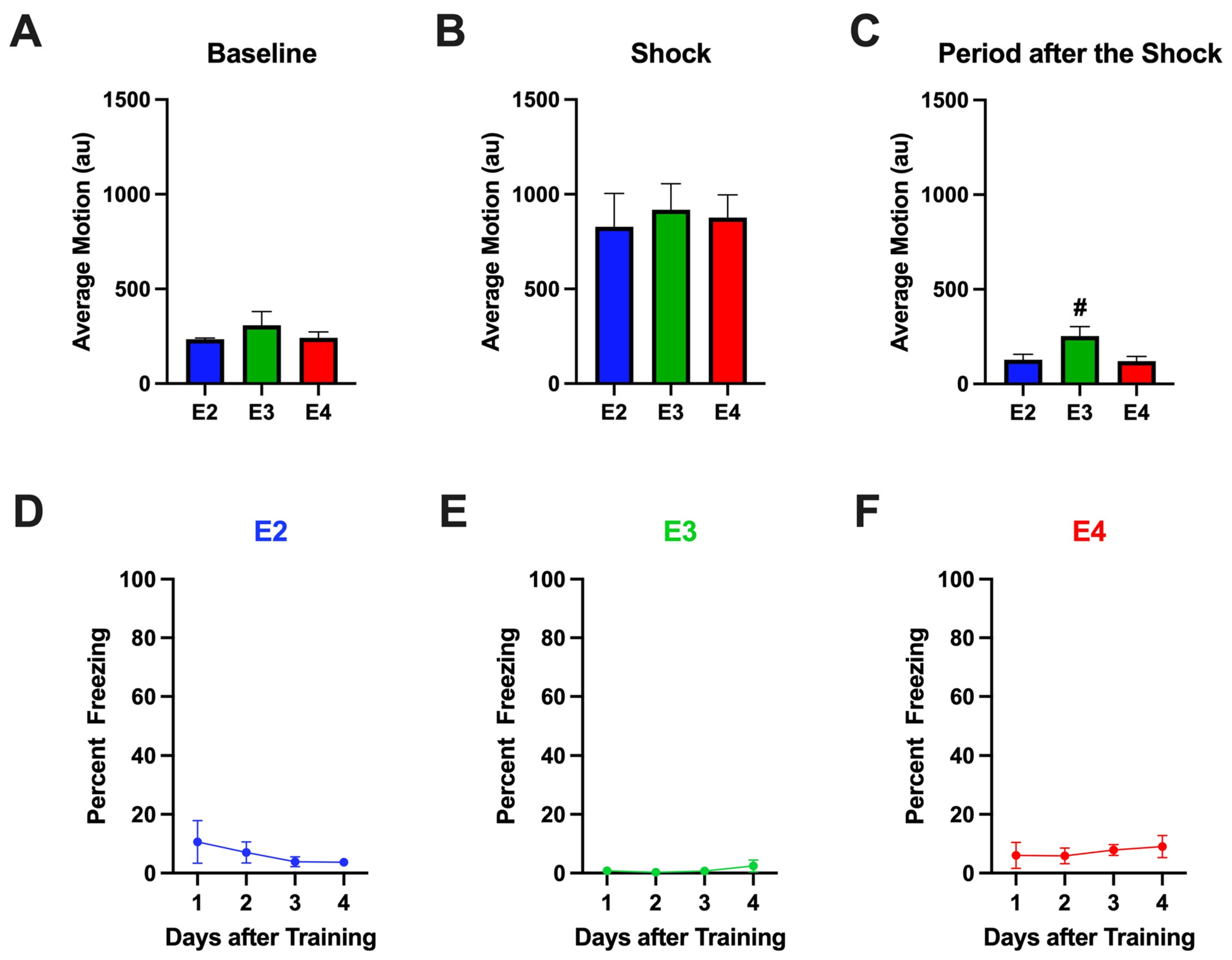
2.1.1. Baseline Activity Levels During Fear Learning
2.1.2. Response to Shocks During Fear Learning
2.1.3. Percent Freezing During the ISIs (Inter-Stimulus Intervals) During Fear Learning
2.1.4. Percent Freezing During the Extinction Days
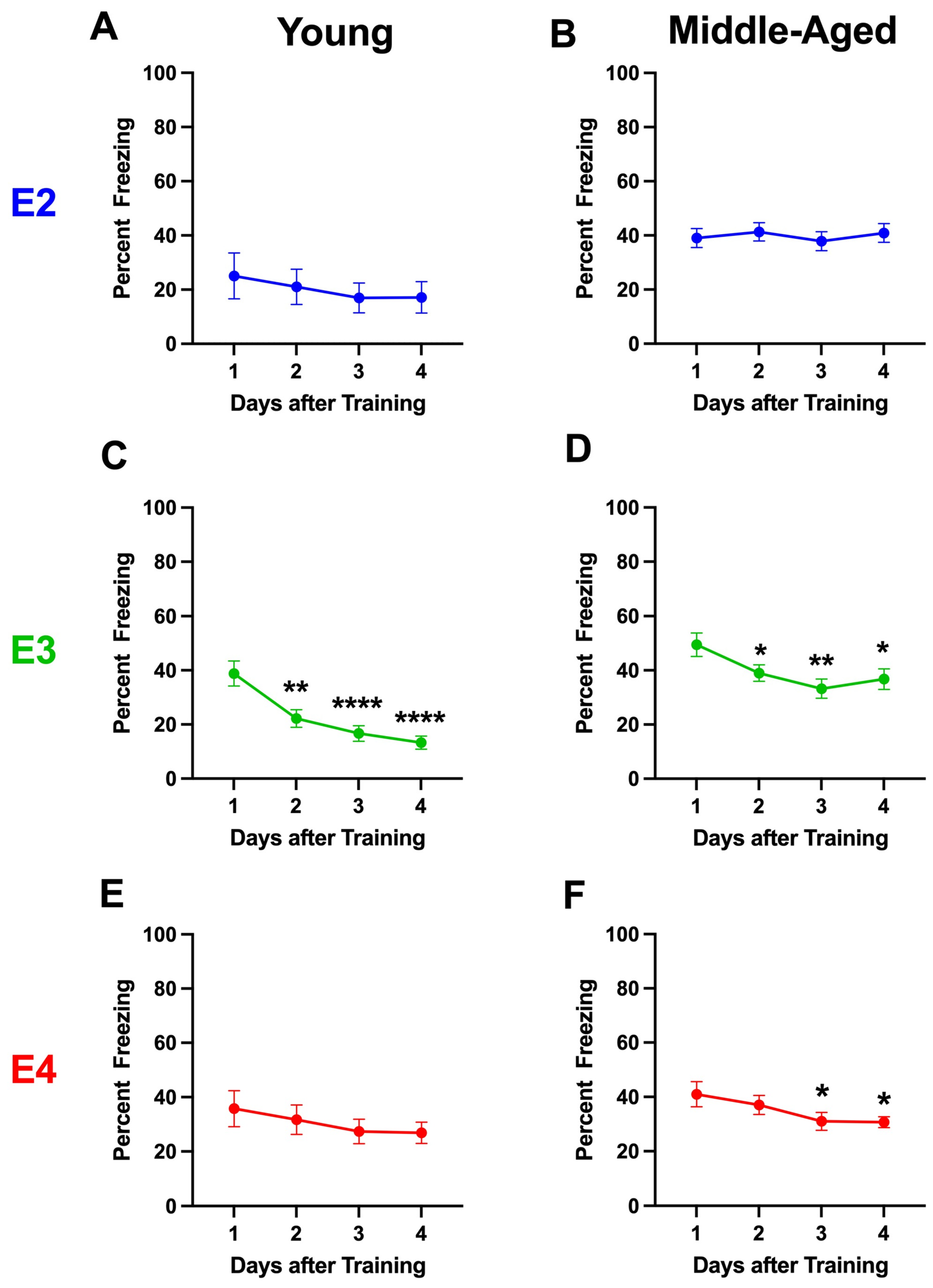
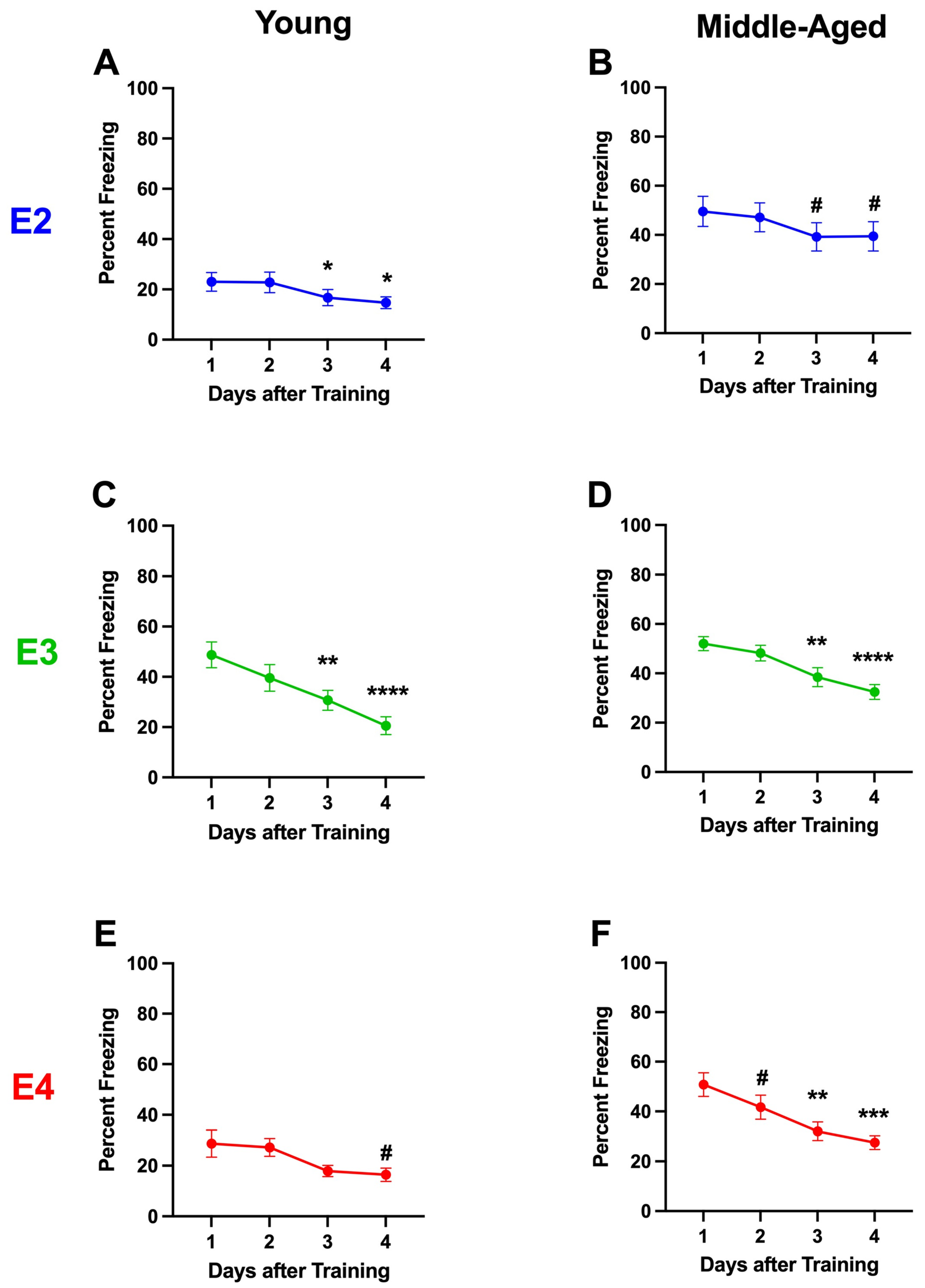
2.1.5. Analysis Broken Down by Housing
2.1.6. Analysis Broken Down by Genotype in Group-Housed Mice
2.1.7. Analysis Broken Down by Genotype in Single-Housed Mice
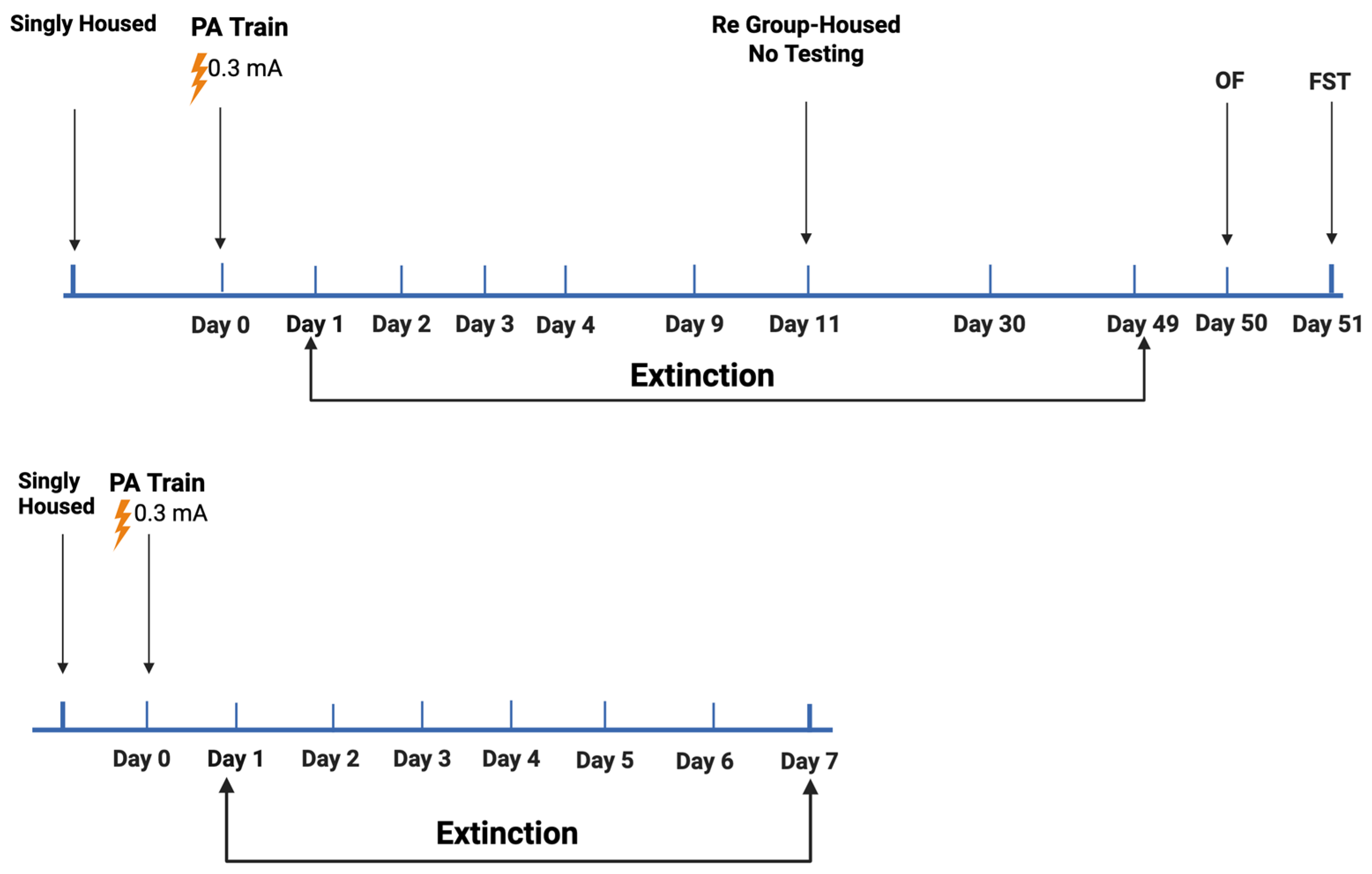
2.2. Study 2
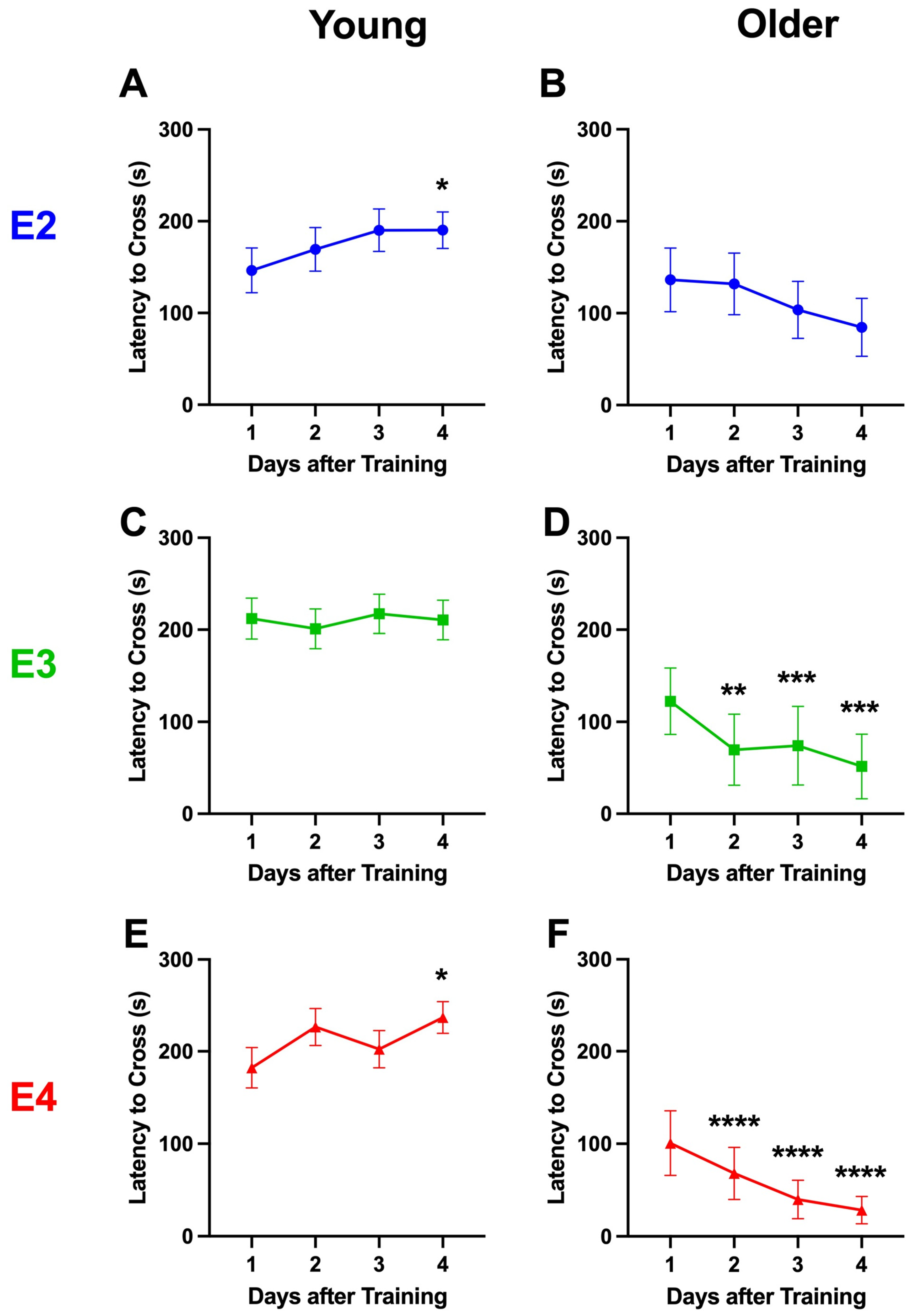
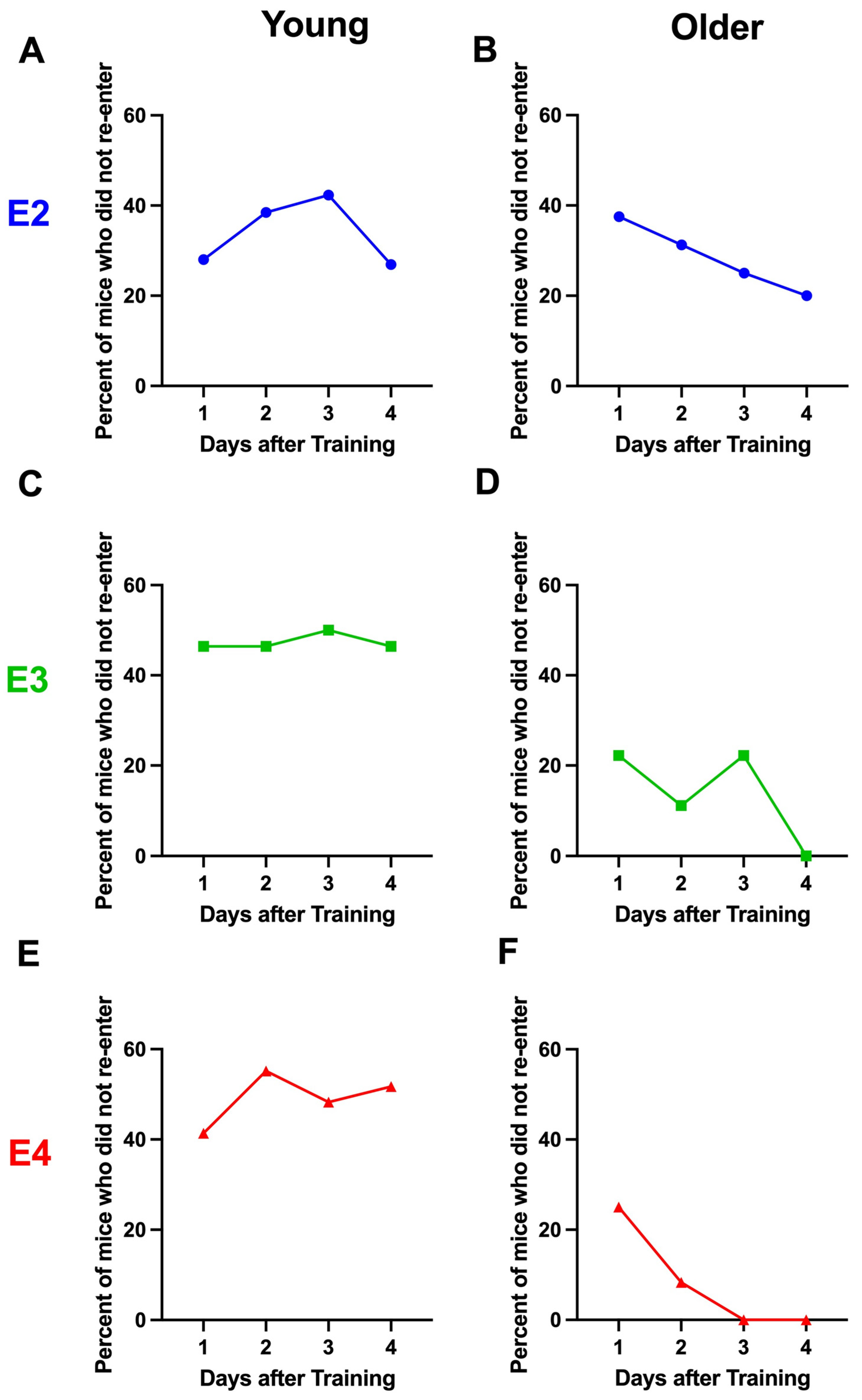
2.3. Study 3
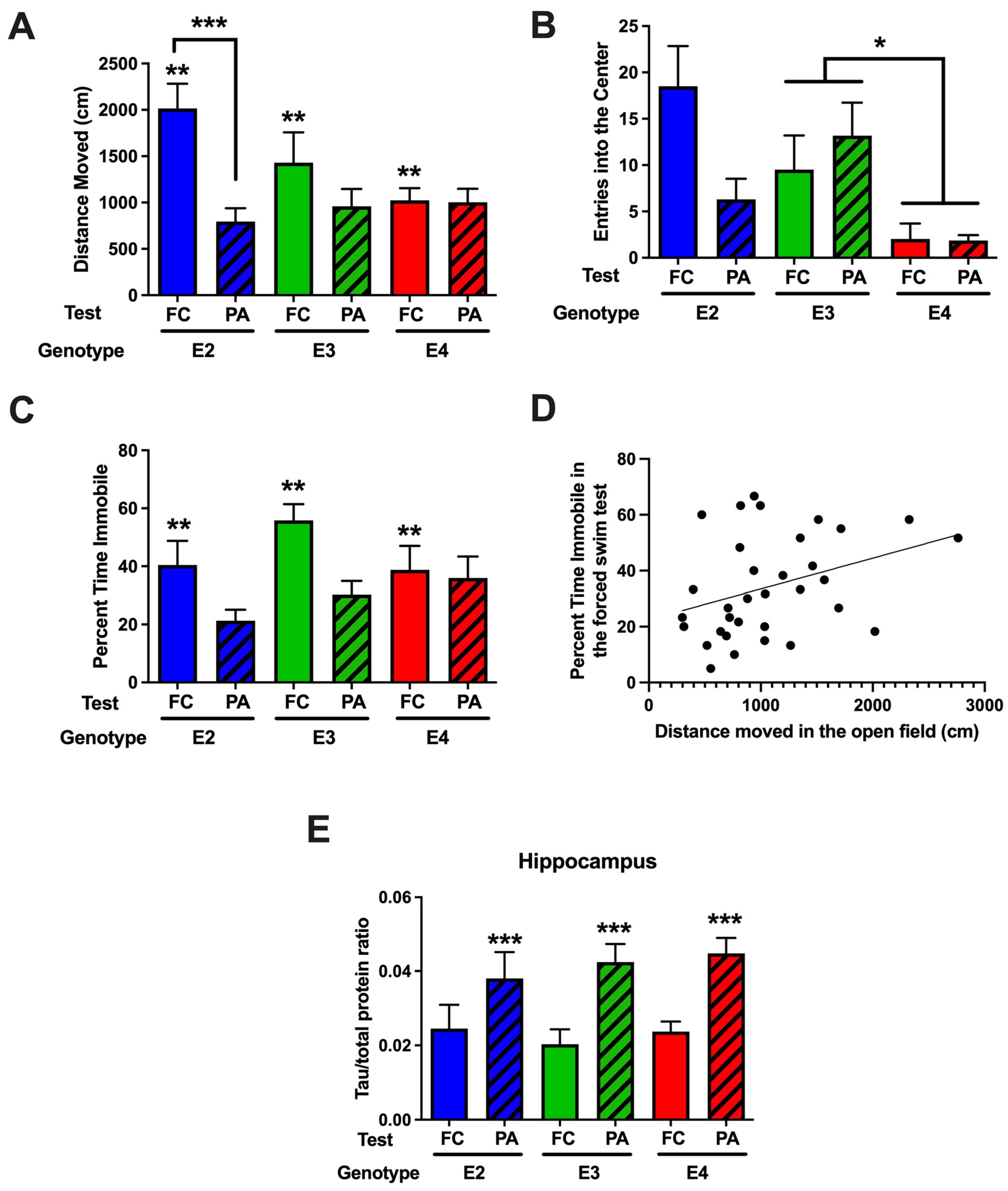
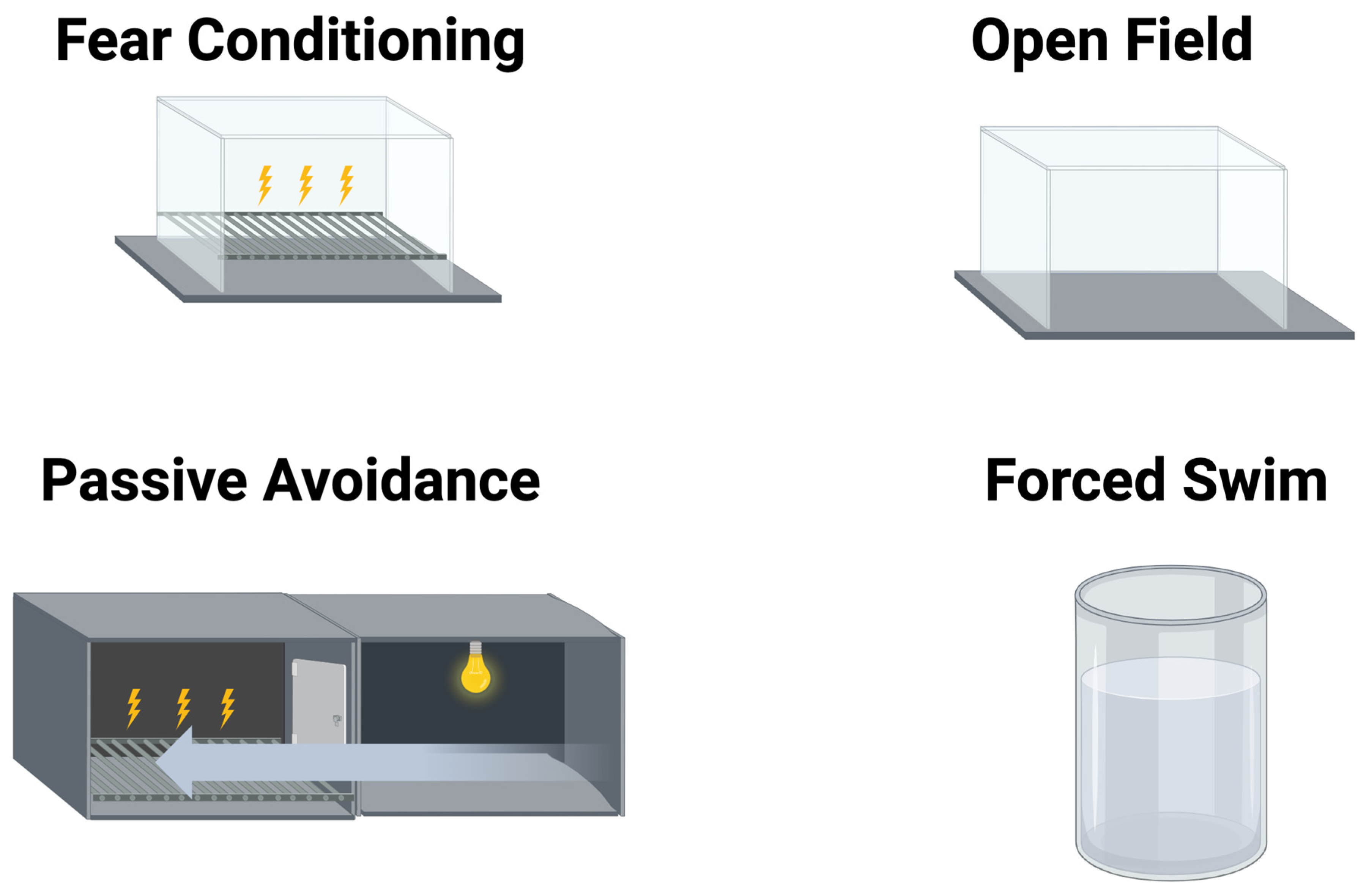
2.3.1. Comparison of Behavioral Performance in the Open-Field and Forced Swim Tests in Mice Tested for Passive Avoidance (Study 2) or Fear Conditioning (Study 3)
2.3.2. Comparison of Hippocampal MAP2, Syn, and Tau Levels in Mice Tested for Passive Avoidance (Study 2) or Fear Conditioning (Study 3)
3. Discussion
4. Materials and Methods
4.1. Open-Field Test
4.2. Forced Swim Test
4.3. Extinction of Passive Avoidance
4.4. Microtubule-Associated Protein 2 (MAP2) and Synaptophysin (Syn) ELISAs
4.5. Tau Dot Blot Analysis
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liberzon, I.; Taylor, S.F.; Amdur, R.; Jung, T.D.; Chamberlain, K.R.; Minoshima, S.; Koeppe, R.A.; Fig, L.M. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry 1999, 45, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Abelson, J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 2016, 92, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clin. Psychol. Rev. 2008, 28, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lokshina, Y.; Sheynin, J.; Vogt, G.; Liberson, I. Fear Extinction Learning in Posttraumatic Stress Disorder. Curr. Top. Behav. Neurosci. 2023, 64, 257–272. [Google Scholar]
- Salkovskis, P.; Clark, D.; Hackmann, A.F.; Wells, A.; Gelder, M. An experimental investigation of the role of safety-seeking behaviours in the maintenance of panic disorder with agoraphobia. Behav. Res. Ther. 1999, 37, 559–574. [Google Scholar] [CrossRef]
- Ledoux, J.; Gorman, J. A call to action: Overcoming anxiety through active coping. Am. J. Psychiatr. 2001, 158, 1953–1955. [Google Scholar] [CrossRef]
- Hofmann, S.; Hay, A. Rethinking Avoidance: Toward a Balanced Approach to Avoidance in Treating Anxiety Disorders. J. Anxiety Disord. 2018, 55, 14–21. [Google Scholar] [CrossRef]
- Milosevic, I.; Radomsky, A. Keep your eye on the target: Safety behavior reduces targeted threat beliefs following a behavioral experiment. Cogn. Ther. Res. 2013, 37, 557–571. [Google Scholar] [CrossRef]
- Rachman, S.; Radomsky, A.; Shafran, R. Safety behaviour: A reconsideration. Behav. Res. Ther. 2008, 46, 163–173. [Google Scholar] [CrossRef]
- Taylor, C.; Alden, L. To see ourselves as others see us: An experimental integration of the intra and interpersonal consequences of self-protection in social anxiety disorder. J. Abnorm. Psychol. 2011, 120, 129–141. [Google Scholar] [CrossRef]
- Telch, M.; Lancaster, C. Exposure therapy: Rethinking the model, refining the method. In Is There Room for Safety Behaviors in Exposure Therapy for Anxiety Disorders? Neudeck, P., Wittchen, H.U., Eds.; Springer: New York, NY, USA, 2012; pp. 313–334. [Google Scholar]
- Zuj, D.V.; Palmer, M.A.; Hsu, C.M.; Nicholson, E.L.; Cushing, P.J.; Gray, K.E.; Felmingham, K.L. Impaired Fear Extinction Associated with Ptsd Increases with Hours-since-Waking. Depress. Anxiety 2016, 33, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Briscione, M.A.; Jovanovic, T.; Norrholm, S.D. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Front. Psychiatry 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Poulos, A.M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005, 56, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; LeDoux, J. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiol. Learn. Mem. 1999, 72, 244–251. [Google Scholar] [CrossRef]
- Milad, M.; Orr, S.; Pittman, R.; Rauch, S. Context modulation of memory for fear extinction in humans. Psychophysiology 2005, 42, 456–464. [Google Scholar] [CrossRef]
- Milad, M.; Igoe, S.; Milad, M. Fear conditioning in rodents and humans. In Neuromethods: Animal Models of Behavioral Analysis; Humana Press: Totowa, NJ, USA, 2011; Volume 50, pp. 111–132. [Google Scholar]
- Milad, M.R.; Quirk, G.J. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef]
- Rosen, J.B.; Schulkin, J. From normal fear to pathological anxiety. Psychol. Rev. 1998, 105, 325–350. [Google Scholar] [CrossRef]
- Rauch, S.L.; Shin, L.M.; Phelps, E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research-past, present, and future. Biol. Psychiatry 2006, 60, 376–382. [Google Scholar] [CrossRef]
- Gilbertson, M.; Shenton, M.; Ciszewski, M.; Kasai, A.; Lasko, N.; Orr, S. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002, 5, 1242–1247. [Google Scholar] [CrossRef]
- Moustafa, A.; Gilbertson, M.; Orr, S.; Herzallah, M.; Servatius, R.J.; Myers, C. A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn. 2013, 81, 29–43. [Google Scholar] [CrossRef]
- Ratigan, H.; Krishnan, S.; Smith, S.; Sheffield, M. A thalamic-hippocampal CA1 signal for contextual fear memory suppression, extinction, and discrimination. Nat. Commun. 2023, 14, 3381. [Google Scholar] [CrossRef]
- Goedert, M.; Strittmatter, W.J.; Roses, A.D. Alzheimer’s disease: Risky apolipoprotein in brain. Nature 1994, 372, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. J. Am. Med. Assoc. 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Johnson, L.; Zuloaga, D.; Bidiman, E.; Marzulla, T.; Weber, S.; Wahbeh, H.; Raber, J. ApoE2 exaggerates PTSD-related behavioral, cognitive, and neuroendocrine alterations. NeuroPsychopharmacology 2015, 40, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.; Roca, V.; Guggenheim, F.; Kimbrell, T.; Griffin, W. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. Clin. Neurosci. 2005, 17, 541–543. [Google Scholar] [CrossRef]
- Kim, T.; Chung, H.; Shin, H.; Kim, S.; Choi, J.; Chung, M.; An, S.; Choi, T.; So, H.; Cho, H. Apolipoprotein E gene polymorphism, alcohol use, and their interactions in combat-related posttraumatic stress disorder. Depress. Anxiety 2013, 30, 194–201. [Google Scholar] [CrossRef]
- Kimbrel, N.; Hauser, M.; Garrett, M.; Ashley-Koch, A.; Liu, Y.; Dennis, M.; Klein, R. Effect of the APOE e4 allele and combat exposure on PTSD among Iraq.Afghanistan-era veterans. Depress. Anxiety 2015, 32, 307–315. [Google Scholar] [CrossRef]
- Lyons, M.; Genderson, M.; Grant, M.; Logue, M.; Zink, T.; McKenzie, R.; al, e. Gene- environment interaction of ApoE genotype and combat exposure on PTSD. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psych. Genet. 2013, 162b, 762–769. [Google Scholar] [CrossRef]
- Peterson, C.; James, L.; Anders, S.; Engdahl, B.; Georgopoulos, A. The number of cysteine residues per mole in Apolipoprotein E is associated with the severity of PTSD re-experiencing symptoms. J. Neuropsych. Clin. Neurosci. 2015, 27, 157–161. [Google Scholar] [CrossRef]
- Olsen, R.; Agam, M.; Davis, M.; Raber, J. ApoE isoform-dependent deficits in extinction of contextual fear conditioning. Genes. Brain Behav. 2012, 11, 806–812. [Google Scholar] [CrossRef]
- Prieto, S.; Nolan, K.E.; Moody, J.N.; Hayes, S.M.; Hayes, J.P. Posttraumatic stress symptom severity predicts cognitive decline beyond the effect of Alzheimer’s disease biomarkers in Veterans. Transl. Psychiatry 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Barer, Y.; Chodick, G.; Glaser Chodick, N.; Gurevich, T. Risk of Parkinson Disease Among Adults With vs. Without Posttraumatic Stress Disorder. JAMA Neurol. 2022, 5, e2225445. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.B.; Lahvis, G.P. Rodent empathy and affective neuroscience. Neurosci. Amp. Biobehav. Rev. 2011, 35, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Balba, N.; Lahvis, G. Integration of human eye-tracking responses and object recognition test perofrmance. In Handbook of Object Novelty Recognition; Ennaceur, A., De Souza Silva, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Langford, D.; Crager, S.; Shehzad, Z.; Smith, S.; Sotocinal, S.; Levenstadt, J.; Candra, M.; Levitin, D.; Mogil, J. Social Modulation of Pain as Evidence for Empathy in Mice. Science 2006, 312, 1967–1970. [Google Scholar] [CrossRef]
- Keysers, C.; Knapska, E.; Moita, M.; Gazzola, V. Emotional contagion and prosocial behavior in rodents. Cell 2022, 26, 688–706. [Google Scholar] [CrossRef]
- Chen, Q.; Panksepp, J.B.; Lahvis, G.P. Empathy is moderated by genetic background in mice. PLoS ONE 2009, 4, e4387. [Google Scholar] [CrossRef]
- Sanders, J.; Mayford, M.; Jeste, D. Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS ONE 2013, 8, e74609. [Google Scholar] [CrossRef]
- Panksepp, J.; Lahvis, G. Differential influence of social versus isolate housing on vicarious fear learning in adolescent mice. Behav. Neurosci. 2016, 130, 206–211. [Google Scholar] [CrossRef]
- Volkar, V.; Vasar, A.; Rauvala, H. Long-term individual housing in C57BL/6J and DBA/2 mice: Assessment of behavioral consequences. Genes. Brain Behav. 2004, 4, 240–252. [Google Scholar]
- Tribble, J.; Fanselow, M. Pair Housing Rats Does Not Protect From Behavioral Consequences of an Acute Traumatic Experience. Behav. Neurosci. 2020, 133, 232–239. [Google Scholar] [CrossRef]
- Buckinx, A.; Van Schuebeek, A.; Van Der Herrewegen, Y.; Smolders, I.; De Bundel, D. Exploring Refinement Strategies for Single Housing of Male C57BL/6JRj Mice: Effect of Cage Divider on Stress-Related Behavior and Hypothalamic-Pituitary-Adrenal-Axis Activity. Front. Behav. Neurosci. 2021, 15, 743959. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Martens, K.; Bashir, A.; Cheung, H.; Stukas, S.; Gibbs, E.; Namjoshi, D.R.; Button, E.B.; Wilkinson, A.; Barron, C.J.; et al. CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice. Alzheimer’s Res. Ther. 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Teng, J.; Takei, Y.; Oguchi, K.; Hirokawa, N. MAP-2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 2002, 158, 541–549. [Google Scholar] [CrossRef]
- Benice, T.; Rizk, A.; Pfankuch, T.; Kohama, S.; Raber, J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience 2006, 137, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Peister, A.; Zeitouni, S.; Pfankuch, T.; Prockop, D.; Raber, J. Novel object recognition in Apoe-/- mice improved by neonatal implantation of wildtype multipotent stromal cells. Exp. Neuro 2006, 201, 206–209. [Google Scholar] [CrossRef]
- Haley, G.; Kohama, S.; Urbanski, H.; Raber, J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus nacaque prefrontal cortex and hippocampus. AGE 2010, 32, 283–296. [Google Scholar] [CrossRef]
- Olsen, R.; Marzulla, T.; Raber, J. Impairment in extinction of contextual and cued fear following post-training whole body irradiation. Frontiers 2014, 8, 231. [Google Scholar] [CrossRef]
- Villasana, L.; Pfankuch, T.; Raber, J. Isoform-Dependent Effects of apoE on Doublecortin-Positive Cells and Microtubule-Associated Protein 2 Immunoreactivity following 137Cs Irradiation. Radiat. Environ. Biophys. 2010, 49, 421–426. [Google Scholar] [CrossRef]
- Haley, G.; Eghlidi, D.; Kohama, S.; Urbanski, H.; Raber, J. Association of microtubule associated protein-2, synaptophysin, and apolipoprotein E mRNA and protein levels with cognition and anxiety levels in aged female rhesus macaques. Behav. Brain Res. 2012, 232, 1–6. [Google Scholar] [CrossRef]
- Kimura, T.; Whitcomb, D.J.; Jihoon, J.; Regan, P.; Piers, T.; Heo, S.; Brown, C.; Hashikawa, T.; Murayama, M.; Seok, H.; et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos. Trans. R. Soc. B 2014, 369, 20130144. [Google Scholar] [CrossRef]
- Velazquez, R.; Ferriera, E.; Tran, A.H.; Turner, E.; Belfiore, R.; Branca, C.; Oddo, S. Acute tau knockdown in the hippocampus of adult mice causes learning and memory deficits. Aging Cell 2018, 17, e12775. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Z.; Yu, C.; Dong, H.; Wang, S.; Wang, G.; Wang, D. Tau aggravates stress-induced anxiety by inhibiting adult ventral hippocampal neurogenesis in mice. Cereb. Cortex 2023, 33, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Cominski, T.P.; Jiao, X.; Catuzzi, J.; Stewart, A.; Pang, K. The role of the hippocampus in avoidance learning and anxiety vulnerability. Front. Behav. Neurosci. 2014, 8, 00273. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Cho, J.-H. Encoding of contextual fear memory in hippocampal–amygdala circuit. Nat. Commun. 2020, 11, 1382. [Google Scholar] [CrossRef]
- Ji, J.; Maren, S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 2007, 758, 749–758. [Google Scholar] [CrossRef]
- Belovicova, K.; Bogi, E.; Casatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 19, 40–43. [Google Scholar] [CrossRef]
- White, D.; Kalinichev, E.; Holtzman, S. Locomotor response to novelty as a predictor of reactivity to aversive stimuli in the rat. Brain Res. 2007, 1149, 141–148. [Google Scholar] [CrossRef]
- Reardon, S. Pressure grows to ditch controversial forced swim test in rodent studies of depression. Science 2024, 383, 1279. [Google Scholar] [CrossRef]
- Gorkiewicz, T.; Danielewski, K.; Andraka, K.; Kondrakiewiicz, K.; Meyza, K.; Kaminsky, J.; Knapska, E. Social buffering diminishes fear response but does not equal improved fear extinction. Cereb. Cortex 2023, 33, 5007–5024. [Google Scholar] [CrossRef]
- Giaomucci, G.; Moschini, V.; Piazzesi, D.; Padiglioni, S.; Caruso, C.; Nuti, C.; Munarin, A.; Mazzeo, S.; Galdo, G.; Polito, C.; et al. Disentangling empathy impairment along Alzheimer’s disease continuum: From subjective cognitive decline to Alzheimer’s dementia. Cortex 2024, 172, 125–140. [Google Scholar] [CrossRef]
- Greenbert, M.; Tanev, K.; Marin, M.-F.; Pitman, R. Stress, PTSD, and dementia. Alzheimer’s Dement. 2014, 10, S155–S165. [Google Scholar]
- Pattinson, C.; Gill, J.; Lippa, S.; Brickell, T.; French, L.; Lange, R. Concurrent Mild Traumatic Brain Injury and Posttraumatic Stress Disorder Is Associated With Elevated Tau Concentrations in Peripheral Blood Plasma. J. Trauma. Stress 2019, 32, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Bogolovsky, T.; Wilson, D.A.; Chen, Y.; Hanlon, D.; Gill, J.; Jeromin, A.; Song, L.; Moore, C.; Gong, Y.; Kenney, K.; et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid β up to 90 days after traumatic brain injury. Neurotrauma 2017, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Merchant-Borna, K.; Jeromin, A.; Livingston, W.; Bazarian, J. Acute plasma tau relates to prolonged return to play after concussion. Neurology 2017, 88, 595–602. [Google Scholar] [CrossRef]
- Clouston, S.; Deri, Y.; Diminisch, E.; Kew, R.; Kotov, R.; Stewart, C.; Yang, X.; Gandy, S.; Sano, M.; Bromet, E.J.; et al. Posttraumatic stress disorder and total amyloid burden and amyloid-β 42/40 ratios in plasma: Results from a pilot study of World Trade Center responders. Alzheimer’s Dement. 2019, 11, 216–220. [Google Scholar] [CrossRef]
- Weinera, M.W.; Harvey, D.; Landaug, S.M.; Veitcha, D.P.; Neyland, T.C.; Grafmani, J.H.; Aisen, P.S.; Petersenk, R.C.; Jack, C.R., Jr.; Tosuna, D.; et al. Traumatic brain injury and post-traumatic stress disorder are not associated with Alzheimer’s disease pathology measured with biomarkers. Alzheimer’s Dement. 2022, 19, 884–895. [Google Scholar] [CrossRef]
- Cimino, N.; Kang, M.; Honig, L.; Rutherfor, B. Blood-Based Biomarkers for Alzheimer’s Disease in Older Adults with Posttraumatic Stress Disorder. J. Alzheimer’s Dis. Rep. 2022, 6, 49–56. [Google Scholar] [CrossRef]
- Taylor, T.; Caudle, W.; Shepherd, K.; Noorian, A.; Jackson, C.; Iuvone, P.M.; Weinschenker, D.; Greene, J.; Miller, G. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J. Neurosci. 2009, 29, 8103–8113. [Google Scholar] [CrossRef]
- Raber, J.; Fuentes Anaya, A.; Torres, E.; Lee, J.; Boutros, S.; Grygoryev, D.; Hammer, A.; Kasschau, K.; Sharpton, T.; Turker, M.; et al. Effects of Six Sequential Charged Particle Beams on Behavioral and Cognitive Performance in B6D2F1 Female and Male Mice. Front. Physiol. 2020, 11, 959. [Google Scholar] [CrossRef]
- Raber, J.; Perez, R.; Torres, E.; Krenik, D.; Boutros, S.; Patel, E.; Chlebowski, A.; Ramos Torres, E.; Perveen, Z.; Penn, A.; et al. Effects of chronic second-hand smoke (SHS) exposure on cognitive performance and metabolic pathways in the hippocampus of wild-type and human tau mice. Environ. Health Perspect. 2021, 129, 57009. [Google Scholar] [CrossRef]
| Contextual Fear Memory | E2 | E3 | E4 |
| Study 1, young group-housed mice | Extinction | ||
| Study 1, middle-aged group-housed mice | Extinction | Extinction | |
| Study 1, young single-housed mice | Extinction | Extinction | |
| Study 1, middle-aged single-housed mice | Extinction | Extinction | |
| Passive avoidance memory | E2 | E3 | E4 |
| Study 2, young mice | |||
| Study 2, older mice | Extinction | Extinction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saltonstall, E.; Pederson, A.; O’Niel, A.; Holden, S.; Kessler, K.; Torres, E.R.S.; Raber, J. ApoE Isoform-Dependent Effects on Extinction of Contextual Fear Memory and Passive Avoidance Memory. Int. J. Mol. Sci. 2025, 26, 5820. https://doi.org/10.3390/ijms26125820
Saltonstall E, Pederson A, O’Niel A, Holden S, Kessler K, Torres ERS, Raber J. ApoE Isoform-Dependent Effects on Extinction of Contextual Fear Memory and Passive Avoidance Memory. International Journal of Molecular Sciences. 2025; 26(12):5820. https://doi.org/10.3390/ijms26125820
Chicago/Turabian StyleSaltonstall, Elizabeth, Alexandra Pederson, Abigail O’Niel, Sarah Holden, Kat Kessler, Eileen Ruth Samson Torres, and Jacob Raber. 2025. "ApoE Isoform-Dependent Effects on Extinction of Contextual Fear Memory and Passive Avoidance Memory" International Journal of Molecular Sciences 26, no. 12: 5820. https://doi.org/10.3390/ijms26125820
APA StyleSaltonstall, E., Pederson, A., O’Niel, A., Holden, S., Kessler, K., Torres, E. R. S., & Raber, J. (2025). ApoE Isoform-Dependent Effects on Extinction of Contextual Fear Memory and Passive Avoidance Memory. International Journal of Molecular Sciences, 26(12), 5820. https://doi.org/10.3390/ijms26125820






