The Inflammatory Nexus: Unraveling Shared Pathways and Promising Treatments in Alzheimer’s Disease and Schizophrenia
Abstract
1. Introduction
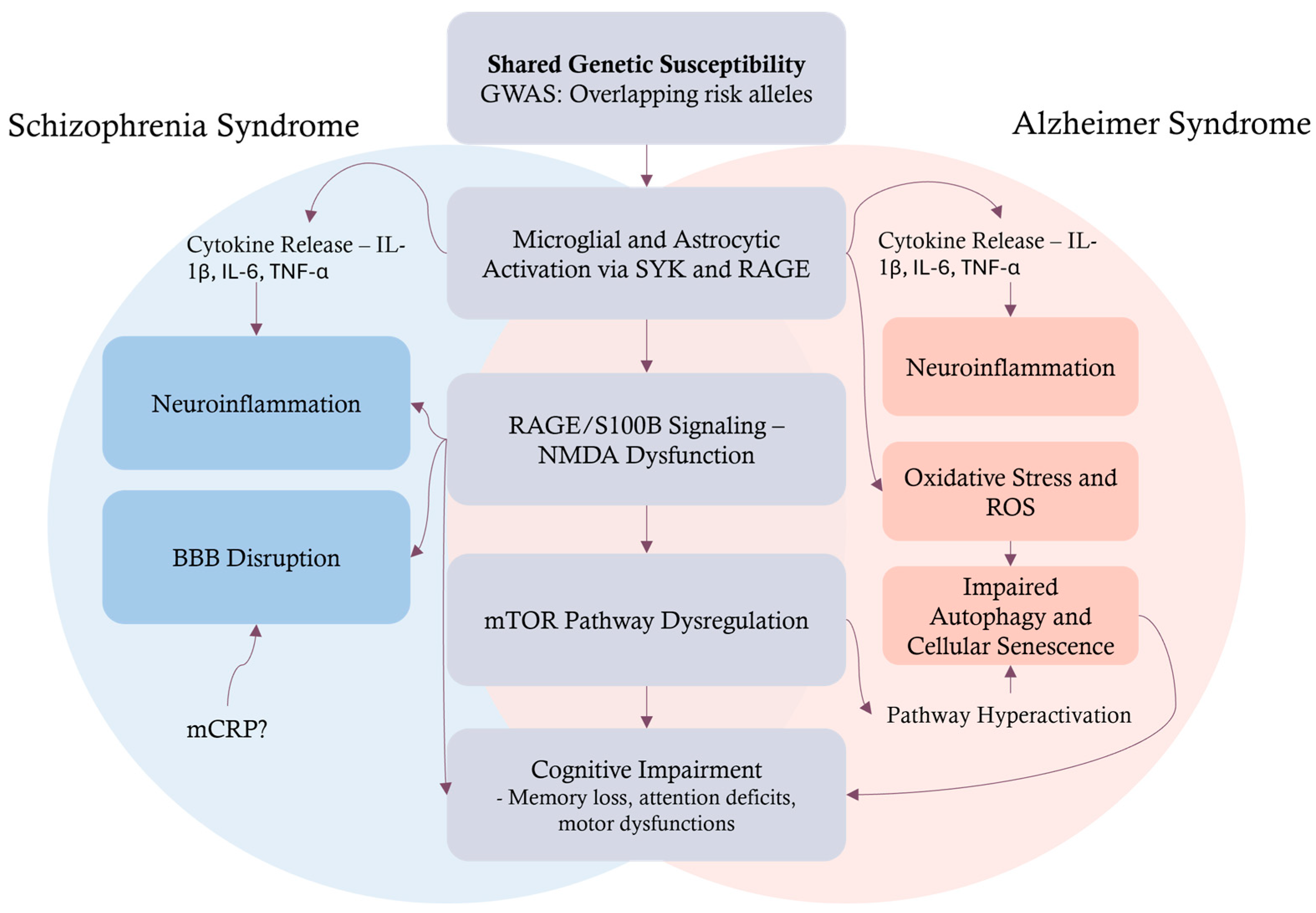
2. Neuropathological Processes in AD and Schizophrenia
2.1. Molecular and Morphological Signatures
2.2. Neuroinflammation and the Blood–Brain Barrier
2.3. Autophagy, Senescence, and Neurodegeneration
| Feature | Alzheimer Syndrome | Schizophrenia Syndrome |
|---|---|---|
| Main Pathology | Amyloid-β plaques, tau tangles [37,90] | E/I imbalance, PVI/PNN disruption [91,92] |
| Key Brain Regions | Hippocampus, cortex [93,94] | Prefrontal cortex, hippocampus [91] |
| Autophagy-Related Genes | ↓ BECN1, ↓ ULK2, ↓ ATG5 [85,95] | ↓ BECN1, ↓ ULK2, ↑ BCL2, ↑ ADNP [85] |
| Protein Aggregates | Extracellular Aβ, intracellular tau [94,96] | Synaptic/scaffolding protein buildup [85] |
| Neuroinflammation | Microglial activation, RAGE/NF-κB signaling [97] | MMP9/RAGE pathway, oxidative stress [63,92] |
| Cognitive Symptoms | Memory loss, executive dysfunction [98] | Working memory deficits, disorganized thinking [99] |
2.4. Divergent Cell Death Pathways in Schizophrenia and AD: From Apoptosis to Necroptosis
3. Biomarkers and Diagnostic Tools
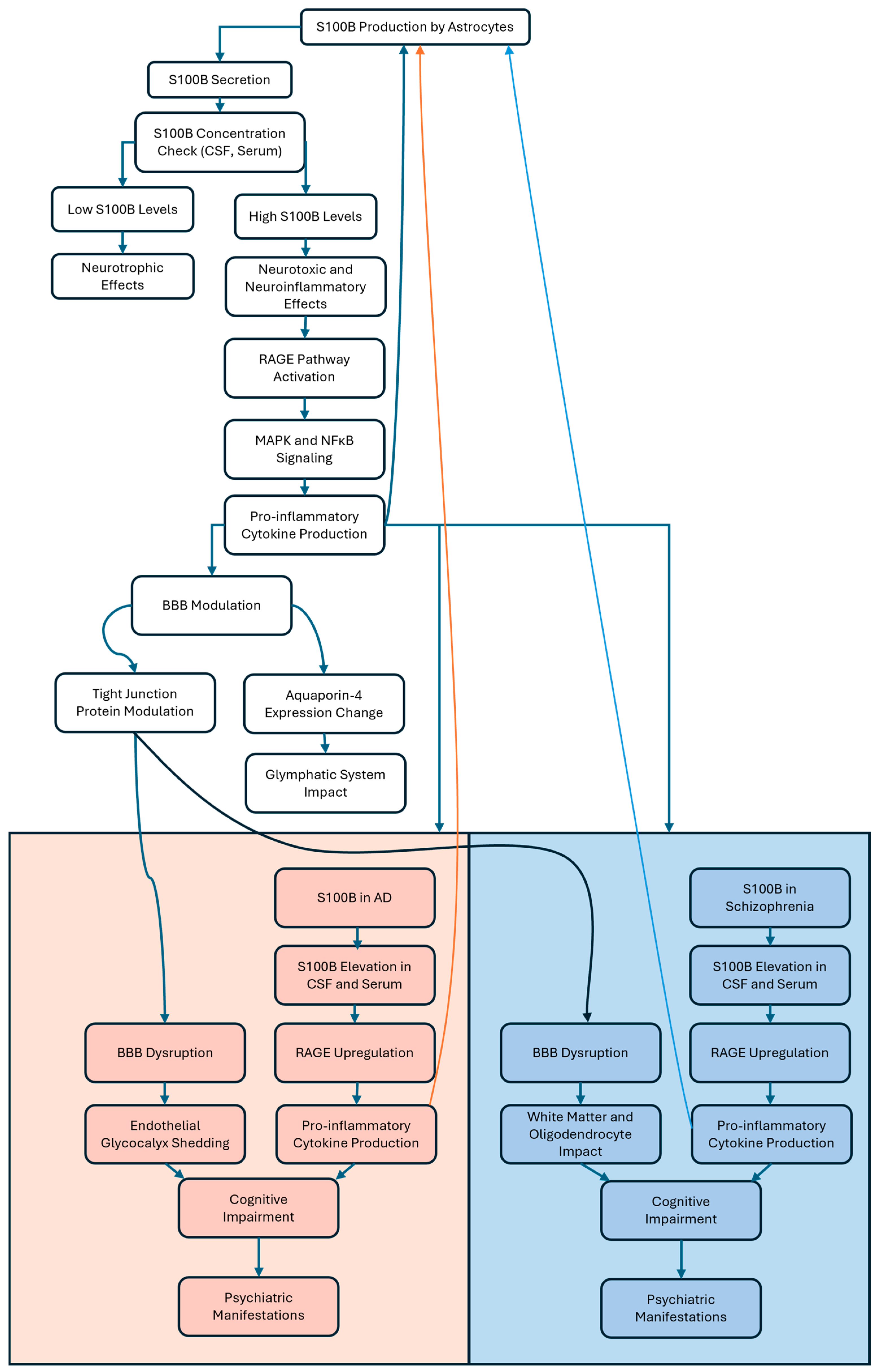
3.1. S100B in Neurodegeneration and RAGE Interaction
3.2. Cognitive Impairment in Alzheimer’s Disease and Schizophrenia
3.3. Psychiatric Manifestations as Early Indicators of Alzheimer’s Disease and Schizophrenia
3.4. Differential Cognitive Impairment in Schizophrenia and Alzheimer’s: Psychiatric and Psychological Perspectives
3.5. Diagnostic Challenges and the Need for Comprehensive Evaluation in Schizophrenia and AD
3.6. Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio
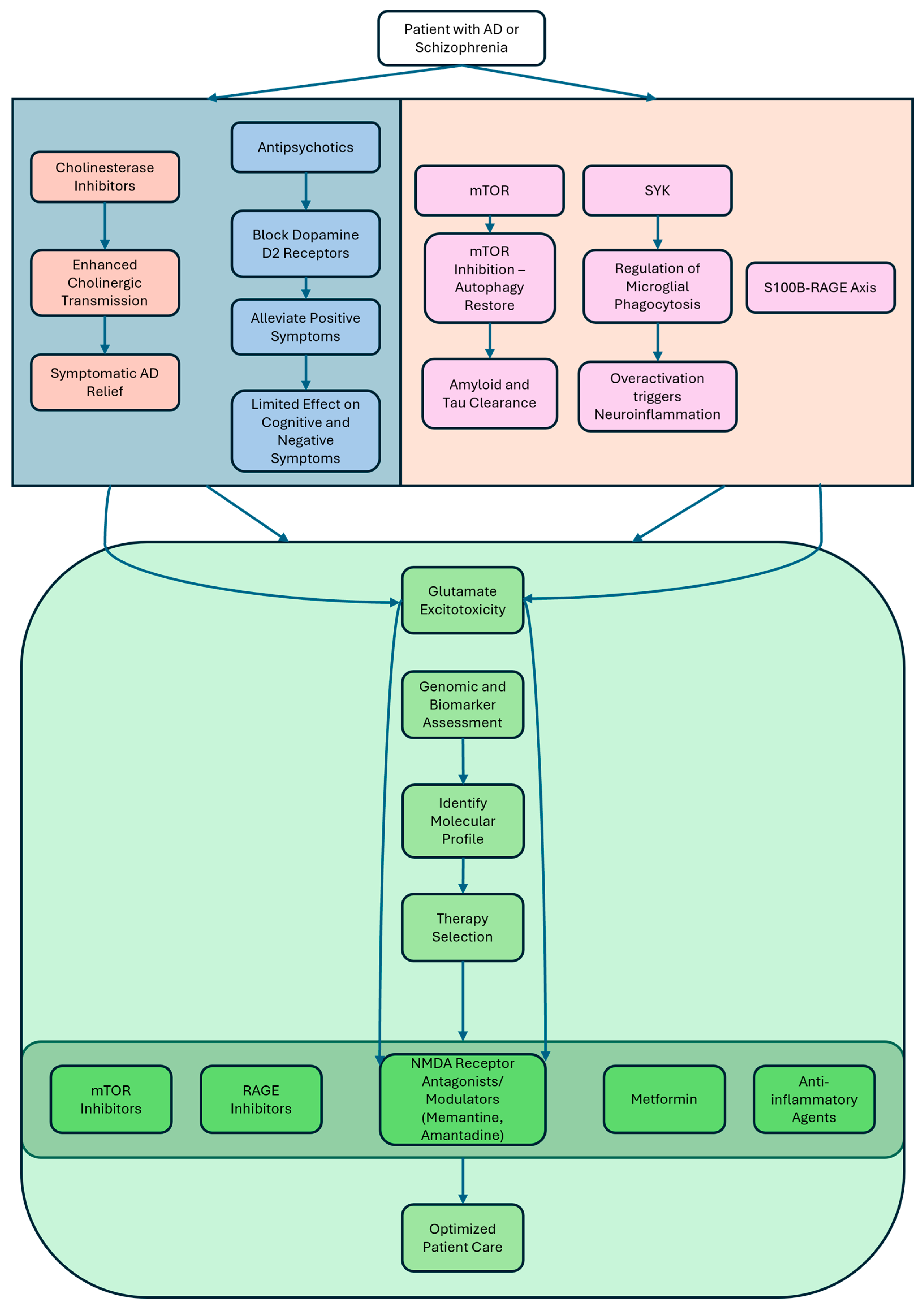
4. Therapeutic Strategies
4.1. Current Treatments: Antipsychotics, Metformin, and Cholinesterase Inhibitors
4.2. Beyond Genetics: The Role of Environment and Epigenetics
4.3. Emerging Targets: mTOR, SYK, and S100B-RAGE
4.4. The Roles of Psychiatry and Psychology in the Context of Schizophrenia and Alzheimer’s Management
4.5. Translational Outlook and Personalized Medicine
5. Conclusions, Research Gaps, and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT2A | 5-hydroxytryptamine receptor 2A |
| 5xFAD | 5-familial Alzheimer’s disease mutations |
| ABCA7 | Adenosine tri-phosphate binding cassette subfamily A member 7 |
| AD | Alzheimer’s disease |
| ADAM17 | A disintegrin and metalloproteinase 17 |
| ADNP | Activity-dependent neuroprotective protein |
| AGEs | Advanced glycation end-products |
| AIWG | Antipsychotic-induced weight gain |
| ALS | Amyotrophic lateral sclerosis |
| AMPK | AMP-activated protein kinase |
| APOE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| ATG5 | Autophagy protein 5 |
| Aβ | Amyloid-beta |
| Aβ1-42 | Amyloid beta protein fragment 1-42 |
| BBB | Blood–brain barrier |
| BCL2 | B-cell lymphoma 2 |
| BECN1 | Beclin-1 |
| BIN1 | Bridging integrator 1 |
| CACNA1C | Calcium voltage-gated channel subunit Alpha1 C |
| CCL2 | C-C motif chemokine ligand 2 |
| CD2AP | Cluster of differentiation 2-associated protein |
| CLU | Clusterin |
| CNS | Central nervous system |
| CR1 | Complement receptor 1 |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| DAMPs | Damage-associated molecular patterns |
| DISC1 | Disrupted in schizophrenia 1 |
| EEG | Electroencephalogram |
| EPHA1 | Ephrin type-A receptor 1 |
| FDA | Food and Drug Administration |
| FPS-ZM1 | 4-Chloro-N-cyclohexyl-N-(phenylmethyl)-benzamide |
| GABA | Gamma-aminobutyric acid |
| GLP-1 | Glucagon-like peptide-1 |
| GRIN2A | Glutamate ionotropic receptor NMDA type subunit 2A |
| GWAS | Genome-wide association studies |
| HMGB1 | High-mobility group box 1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin 6 |
| ITAMs | Immunoreceptor tyrosine-based activation motifs |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| LANDO | LC3-associated endocytosis |
| LRP1 | Lipoprotein receptor-related protein 1 |
| LRRK2 | Leucine-rich repeat kinase 2 |
| MAPK | Mitogen-activated protein kinase |
| MATRICS | Measurement and Treatment Research to Improve Cognition in Schizophrenia |
| MCP-1 | Monocyte chemoattractant protein-1 |
| mCRP | Monomeric C-reactive protein |
| MLKL | Mixed lineage kinase domain-like |
| MMP9 | Matrix metalloproteinase 9 |
| MS | Multiple sclerosis |
| MS4A | Membrane spanning 4-domains A |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| mTORC2 | Mammalian target of rapamycin complex 2 |
| NDDs | Neurodegenerative disorders |
| NF-κB | Nuclear factor kappa B |
| NMDA | N-methyl-D-aspartate |
| NMDARs | N-methyl-D-aspartate receptors |
| NRXN1 | Neurexin 1 |
| PAMPs | Pathogen-associated molecular patterns |
| PD | Parkinson’s disease |
| PDAPP | Platelet-derived growth factor promoter driving expression of human amyloid precursor protein |
| PET | Positron emission tomography |
| PICALM | Phosphatidylnositol binding clathrin assembly protein |
| PNN | Perineuronal nets |
| PSEN-1 | Presenilin-1 |
| PSEN-2 | Presenilin-2 |
| pTau217 | Phosphorylated tau protein at amino acid position 217 |
| PVI | Parvalbumin-positive interneuron |
| RAGE | Receptor for advanced glycation end-products |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| ROS | Reactive oxygen species |
| S100B | S100 calcium-binding protein B |
| SNCA | Synuclein alpha |
| SYK | Spleen tyrosine kinase |
| TNF-α | Tumor necrosis factor-alpha |
| ULK2 | Unc-51-like kinase 2 |
References
- Giri, P.M.; Banerjee, A.; Ghosal, A.; Layek, B. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3995. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kesari, K.K.; Rachamalla, M.; Mani, S.; Ashraf, G.M.; Jha, S.K.; Kumar, P.; Ambasta, R.K.; Dureja, H.; Devkota, H.P.; et al. CRISPR/Cas9 gene editing: New hope for Alzheimer’s disease therapeutics. J. Adv. Res. 2022, 40, 207–221. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Smith, E.E.; Geda, Y.; Sultzer, D.; Brodaty, H.; Smith, G.; Agüera-Ortiz, L.; Sweet, R.; Miller, D.; Lyketsos, C.G. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s Dement. 2016, 12, 195–202. [Google Scholar] [CrossRef]
- Wang, J.H.; Wu, Y.J.; Tee, B.L.; Lo, R.Y. Medical Comorbidity in Alzheimer’s Disease: A Nested Case-Control Study. J. Alzheimer’s Dis. 2018, 63, 773–781. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Nguyen, C.Q.N.; Ma, L.; Low, Y.L.C.; Tan, E.C.K.; Fowler, C.; Masters, C.L.; Jin, L.; Pan, Y. Exploring the link between comorbidities and Alzheimer’s dementia in the Australian Imaging, Biomarker & Lifestyle (AIBL) study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2024, 16, e12593. [Google Scholar] [CrossRef]
- Cai, L.; Huang, J. Schizophrenia and risk of dementia: A meta-analysis study. Neuropsychiatr. Dis. Treat. 2018, 14, 2047–2055. [Google Scholar] [CrossRef]
- Garcez, M.L.; Falchetti, A.C.B.; Mina, F.; Budni, J. Alzheimer’s disease associated with psychiatric comorbidities. An. Acad. Bras. Cienc. 2015, 87, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Lewine, R.; Hart, M. Schizophrenia spectrum and other psychotic disorders. Handb. Clin. Neurol. 2020, 175, 315–333. [Google Scholar] [CrossRef]
- Correll, C.U.; Arango, C.; Fagerlund, B.; Galderisi, S.; Kas, M.J.; Leucht, S. Identification and treatment of individuals with childhood-onset and early-onset schizophrenia. Eur. Neuropsychopharmacol. 2024, 82, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.L.; Kaltoft, K.; Wium-Andersen, I.K.; Wium-Andersen, M.K.; Osler, M. Association of early- and late-life bipolar disorder with incident dementia. A Danish cohort study. J. Affect. Disord. 2024, 367, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M. Aging, Cellular Senescence, and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef]
- Ribe, A.R.; Laursen, T.M.; Charles, M.; Katon, W.; Fenger-Grøn, M.; Davydow, D.; Chwastiak, L.; Cerimele, J.M.; Vestergaard, M. Long-term risk of dementia in persons with schizophrenia: A danish population-based cohort study. JAMA Psychiatry 2015, 72, 1095–1101. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Cue, J.M.; Patel, N.; Zheng, J.J.; Cummings, M.J.; Do, J. Genetic Relationship between Alzheimer’s Disease and Schizophrenia. Alzheimer’s Dement. 2022, 18, e065861. [Google Scholar] [CrossRef]
- Calero, M.; Gómez-Ramos, A.; Calero, O.; Soriano, E.; Avila, J.; Medina, M. Additional mechanisms conferring genetic susceptibility to Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, P.; Sweet, R.; Sims, R.; Harold, D.; Russo, G.; Abraham, R.; Stretton, A.; Jones, N.; Gerrish, A.; Chapman, J.; et al. Genome-wide association study of Alzheimer’s disease with psychotic symptoms. Mol. Psychiatry 2012, 17, 1316–1327. [Google Scholar] [CrossRef]
- Shen, L.; Jia, J. An Overview of Genome-Wide Association Studies in Alzheimer’s Disease. Neurosci. Bull. 2016, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Shokrgozar, A.; Rahimi, M.; Shoraka, S. Identification of key genes and pathways in schizophrenia: A bioinformatics analysis based on GWAS and GEO. Front. Psychiatry 2025, 16, 1464676. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.M.; Yang, P.; Chen, H.H.; Hao, R.H.; Dong, S.S.; Yao, S.; Chen, X.F.; Yan, H.; Zhang, Y.J.; Chen, Y.X.; et al. Comprehensive functional annotation of susceptibility SNPs prioritized 10 genes for schizophrenia. Transl. Psychiatry 2019, 9, 56. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Y.; Ji, Y.; Zhou, Y.; Xu, J.; Tang, J.; Liu, N.; Ding, H.; Qin, W.; Liu, F.; et al. Identification of genetic architecture shared between schizophrenia and Alzheimer’s disease. Transl. Psychiatry 2025, 15, 150. [Google Scholar] [CrossRef]
- Sousa, I.A.E.; Grotzinger, A.D.; Lawrence, J.M.; Breunig, S.; Marshall, C.R.; Korszun, A.; Foote, I.F. Examining the genetic relationship between Alzheimer’s disease, schizophrenia and their shared risk factors using genomic structural equation modelling. Brain Commun. 2025, 7, fcaf112. [Google Scholar] [CrossRef]
- Tani, M.; Akashi, N.; Hori, K.; Konishi, K.; Kitajima, Y.; Tomioka, H.; Inamoto, A.; Hirata, A.; Tomita, A.; Koganemaru, T.; et al. Anticholinergic Activity and Schizophrenia. Neurodegener. Dis. 2015, 15, 168–174. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Avram, M.; Grothe, M.J.; Meinhold, L.; Leucht, C.; Leucht, S.; Borgwardt, S.; Brandl, F.; Sorg, C. Lower cholinergic basal forebrain volumes link with cognitive difficulties in schizophrenia. Neuropsychopharmacology 2021, 46, 2320–2329. [Google Scholar] [CrossRef]
- Eickhoff, S.; Franzen, L.; Korda, A.; Rogg, H.; Trulley, V.-N.; Borgwardt, S.; Avram, M. The Basal Forebrain Cholinergic Nuclei and Their Relevance to Schizophrenia and Other Psychotic Disorders. Front. Psychiatry 2022, 13, 909961. [Google Scholar] [CrossRef]
- Kidambi, N.; Elsayed, O.H.; El-Mallakh, R.S. Xanomeline-Trospium and Muscarinic Involvement in Schizophrenia. Neuropsychiatr. Dis. Treat. 2023, 19, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.E.; Winston-Gray, C.; Barlow, J.W.; Rissman, R.A.; Jeste, D.V. Plasma Levels of Neuron- and Astrocyte-Derived Exosomal Amyloid Beta1-42, Amyloid Beta1-40, and Phosphorylated Tau Levels in Schizophrenia Patients and Non-psychiatric Comparison Subjects: Relationships with Cognitive Functioning and Psychopathology. Front. Psychiatry 2021, 11, 532624. [Google Scholar] [CrossRef]
- Prestia, A. Alzheimers disease and schizophrenia: Evidence of a specific, shared molecular background. Future Neurol. 2011, 6, 17–21. [Google Scholar] [CrossRef]
- Sæther, L.S.; Ueland, T.; Haatveit, B.; Maglanoc, L.A.; Szabo, A.; Djurovic, S.; Aukrust, P.; Roelfs, D.; Mohn, C.; Ormerod, M.B.E.G.; et al. Inflammation and cognition in severe mental illness: Patterns of covariation and subgroups. Mol. Psychiatry 2023, 28, 1284–1292. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef] [PubMed]
- Beaurain, M.; Salabert, A.S.; Payoux, P.; Gras, E.; Talmont, F. NMDA Receptors: Distribution, Role, and Insights into Neuropsychiatric Disorders. Pharmaceuticals 2024, 17, 1265. [Google Scholar] [CrossRef]
- Balu, D.T. The NMDA Receptor and Schizophrenia. From Pathophysiology to Treatment. Adv. Pharmacol. 2016, 76, 351–382. [Google Scholar] [CrossRef]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Colonna, M. The microglial immunoreceptor tyrosine-based motif-Syk signaling pathway is a promising target of immunotherapy for Alzheimer’s disease. Clin. Transl. Med. 2023, 13, e1200. [Google Scholar] [CrossRef] [PubMed]
- Ennerfelt, H.; Frost, E.L.; Shapiro, D.A.; Holliday, C.; Zengeler, K.E.; Voithofer, G.; Bolte, A.C.; Lammert, C.R.; Kulas, J.A.; Ulland, T.K.; et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 2022, 185, 4135–4152.E22. [Google Scholar] [CrossRef]
- Koper, O.M.; Kamińska, J.; Sawicki, K.; Kemona, H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv. Clin. Exp. Med. 2018, 27, 849–856. [Google Scholar] [CrossRef]
- Querfurth, H.; Lee, H.K. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol. Neurodegener. 2021, 16, 44. [Google Scholar] [CrossRef]
- Chen, L.; Saito, R.; Noda-Narita, S.; Kassai, H.; Aiba, A. Hyperactive mTORC1 in striatum dysregulates dopamine receptor expression and odor preference behavior. Front. Neurosci. 2024, 18, 1461178. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR–Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Ryskalin, L.; Limanaqi, F.; Frati, A.; Busceti, C.L.; Fornai, F. mTOR-related brain dysfunctions in neuropsychiatric disorders. Int. J. Mol. Sci. 2018, 19, 2226. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Lecue, I.; Diez-Alarcia, R.; Morentin, B.; Meana, J.J.; Callado, L.F.; Urigüen, L. Ribosomal Protein S6 Hypofunction in Postmortem Human Brain Links mTORC1-Dependent Signaling and Schizophrenia. Front. Pharmacol. 2020, 11, 344. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, W.; Wang, M.L.; Lin, X.Y. PI3K-AKT/mTOR Signaling in Psychiatric Disorders: A Valuable Target to Stimulate or Suppress? Int. J. Neuropsychopharmacol. 2024, 27, pyae010. [Google Scholar] [CrossRef]
- Kciuk, M.; Kruczkowska, W.; Gałęziewska, J.; Wanke, K.; Kałuzińska-Kołat, Ż.; Aleksandrowicz, M.; Kontek, R. Alzheimer’s Disease as Type 3 Diabetes: Understanding the Link and Implications. Int. J. Mol. Sci. 2024, 25, 11955. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V. Van Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Najem, D.; Bamji-Mirza, M.; Chang, N.; Liu, Q.Y.; Zhang, W. Insulin resistance, neuroinflammation, and Alzheimer’s disease. Rev. Neurosci. 2014, 25, 509–525. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, F.; Wang, J.; Liu, C.; Zhou, Y.; Xu, Z.; Zhang, C.; Sun, B.; Guan, Y. Pathological Mechanisms Linking Diabetes Mellitus and Alzheimer’s Disease: The Receptor for Advanced Glycation End Products (RAGE). Front. Aging Neurosci. 2020, 12, 217. [Google Scholar] [CrossRef]
- Wei, Z.; Koya, J.; Reznik, S.E. Insulin Resistance Exacerbates Alzheimer Disease via Multiple Mechanisms. Front. Neurosci. 2021, 15, 687157. [Google Scholar] [CrossRef]
- Datta, S.; Rahman, M.A.; Koka, S.; Boini, K.M. High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies. Cells 2024, 13, 1946. [Google Scholar] [CrossRef]
- Rojas, A.; Lindner, C.; Schneider, I.; Gonzalez, I.; Uribarri, J. The RAGE Axis: A Relevant Inflammatory Hub in Human Diseases. Biomolecules 2024, 14, 412. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Kocki, J.; Bogucki, J.; Bogucka-Kocka, A.; Czuczwar, S.J. LRP1 and RAGE Genes Transporting Amyloid and Tau Protein in the Hippocampal CA3 Area in an Ischemic Model of Alzheimer’s Disease with 2-Year Survival. Cells 2023, 12, 2763. [Google Scholar] [CrossRef]
- Derk, J.; MacLean, M.; Juranek, J.; Schmidt, A.M. The Receptor for Advanced Glycation Endproducts (RAGE) and Mediation of Inflammatory Neurodegeneration. J. Alzheimers Dis. Park. 2018, 8, 421. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Soni, P. RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 2023, 11, 1131. [Google Scholar] [CrossRef]
- Dwir, D.; Giangreco, B.; Xin, L.; Tenenbaum, L.; Cabungcal, J.H.; Steullet, P.; Goupil, A.; Cleusix, M.; Jenni, R.; Chtarto, A.; et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: A reverse translation study in schizophrenia patients. Mol. Psychiatry 2020, 25, 2889–2904. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Zhou, J.; Wu, X.; Zhao, J.; Zhang, J.; Guo, X.; Shao, M.; Song, M.; et al. Elevated plasma matrix metalloproteinase 9 in schizophrenia patients associated with poor antipsychotic treatment response and white matter density deficits. Schizophrenia 2024, 10, 71. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca2+ Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef]
- Ardura-Fabregat, A.; Boddeke, E.W.G.M.; Boza-Serrano, A.; Brioschi, S.; Castro-Gomez, S.; Ceyzériat, K.; Dansokho, C.; Dierkes, T.; Gelders, G.; Heneka, M.T.; et al. Targeting Neuroinflammation to Treat Alzheimer’s Disease. CNS Drugs 2017, 31, 1057–1082. [Google Scholar] [CrossRef]
- Lee, E.; Chang, Y. Modulating Neuroinflammation as a Prospective Therapeutic Target in Alzheimer’s Disease. Cells 2025, 14, 168. [Google Scholar] [CrossRef]
- Prestwood, T.R.; Asgariroozbehani, R.; Wu, S.; Agarwal, S.M.; Logan, R.W.; Ballon, J.S.; Hahn, M.K.; Freyberg, Z. Roles of inflammation in intrinsic pathophysiology and antipsychotic drug-induced metabolic disturbances of schizophrenia. Behav. Brain Res. 2021, 402, 113101. [Google Scholar] [CrossRef]
- Apweiler, M.; Saliba, S.W.; Sun, L.; Streyczek, J.; Normann, C.; Hellwig, S.; Bräse, S.; Fiebich, B.L. Modulation of neuroinflammation and oxidative stress by targeting GPR55—New approaches in the treatment of psychiatric disorders. Mol. Psychiatry 2024, 29, 3779–3788. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood–brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kato, N.; Yoshida, M.; Hiu, T.; Matsuo, T.; Mizukami, S.; Omata, D.; Suzuki, R.; Maruyama, K.; Mukai, H.; et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J. Control. Release 2022, 348, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Meng, X.; Yao, Y.; Xu, J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front. Endocrinol. 2022, 13, 1033479. [Google Scholar] [CrossRef]
- Liang, Y.; Doré, V.; Rowe, C.C.; Krishnadas, N. Clinical Evidence for GLP-1 Receptor Agonists in Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. Rep. 2024, 8, 777–789. [Google Scholar] [CrossRef]
- Kopp, K.O.; Glotfelty, E.J.; Li, Y.; Greig, N.H. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol. Res. 2022, 186, 106550. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Wang, X.; Han, M.; Fei, Y.; Wang, J. Blood–Brain Barrier Disruption in Schizophrenia: Insights, Mechanisms, and Future Directions. Int. J. Mol. Sci. 2025, 26, 873. [Google Scholar] [CrossRef]
- Moussiopoulou, J.; Yakimov, V.; Rauchmann, B.-S.; Toth, H.; Melcher, J.; Jäger, I.; Lutz, I.; Kallweit, M.; Papazov, B.; Seelos, K.; et al. Increased blood–brain barrier leakage in schizophrenia spectrum disorders compared to healthy controls in dynamic contrast-enhanced magnetic resonance imaging. medRxiv 2023. [Google Scholar] [CrossRef]
- Pastorello, Y.; Russo, A.P.; Bănescu, C.; Caprio, V.; Gáll, Z.; Potempa, L.; Cordoș, B.; Di Napoli, M.; Slevin, M. Brain Vascular Expression of Monomeric C-Reactive Protein Is Blocked by C10M Following Intraperitoneal Injection in an ApoE−/− Murine Model of Dyslipidemia: An Immunohistochemical Analysis. Cureus 2024, 16, e60682. [Google Scholar] [CrossRef]
- Eshraghi, M.; Adlimoghaddam, A.; Mahmoodzadeh, A.; Sharifzad, F.; Yasavoli-Sharahi, H.; Lorzadeh, S.; Albensi, B.C.; Ghavami, S. Alzheimer’s disease pathogenesis: Role of autophagy and mitophagy focusing in microglia. Int. J. Mol. Sci. 2021, 22, 3330. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Sun, J.; Shen, H.-M.; Wang, J.; Yang, C. Impairment of the autophagy–lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm. Sin. B 2022, 12, 1019–1040. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Wang, C.; Zou, C.; Zhu, H.; Zhang, W. Unraveling the potential of neuroinflammation and autophagy in schizophrenia. Eur. J. Pharmacol. 2025, 997, 177469. [Google Scholar] [CrossRef]
- Merenlender-Wagner, A.; Malishkevich, A.; Shemer, Z.; Udawela, M.; Gibbons, A.; Scarr, E.; Dean, B.; Levine, J.; Agam, G.; Gozes, I. Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry 2015, 20, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.L.; Miller, A.M.; Woesner, M.E. Autophagy and Schizophrenia: A Closer Look at How Dysregulation of Neuronal Cell Homeostasis Influences the Pathogenesis of Schizophrenia. Einstein J. Biol. Med. 2017, 31, 34. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Yamamoto, S.; Ting, T.; Fan, Y.; Sadleir, K.; Wang, Y.; Zhang, W.; Huang, S.; Levine, B.; Vassar, R.; et al. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genet. 2017, 13, e1006962. [Google Scholar] [CrossRef]
- Barmaki, H.; Nourazarian, A.; Khaki-Khatibi, F. Proteostasis and neurodegeneration: A closer look at autophagy in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1281338. [Google Scholar] [CrossRef]
- Guerrero, A.; De Strooper, B.; Arancibia-Cárcamo, I.L. Cellular senescence at the crossroads of inflammation and Alzheimer’s disease. Trends Neurosci. 2021, 44, 714–727. [Google Scholar] [CrossRef]
- Hendrickx, J.O.; Martinet, W.; Van Dam, D.; De Meyer, G.R.Y. Inflammation, Nitro-Oxidative Stress, Impaired Autophagy, and Insulin Resistance as a Mechanistic Convergence Between Arterial Stiffness and Alzheimer’s Disease. Front. Mol. Biosci. 2021, 8, 651215. [Google Scholar] [CrossRef]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef]
- Steullet, P.; Cabungcal, J.H.; Monin, A.; Dwir, D.; O’Donnell, P.; Cuenod, M.; Do, K.Q. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr. Res. 2016, 176, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Cabungcal, J.H.; Coyle, J.; Didriksen, M.; Gill, K.; Grace, A.A.; Hensch, T.K.; Lamantia, A.S.; Lindemann, L.; Maynard, T.M.; et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol. Psychiatry 2017, 22, 936–943. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and its involvement in Alzheimer’s disease: A review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Aksman, L.M.; Oxtoby, N.P.; Scelsi, M.A.; Wijeratne, P.A.; Young, A.L.; Alves, I.L.; Collij, L.E.; Vogel, J.W.; Barkhof, F.; Alexander, D.C.; et al. A data-driven study of Alzheimer’s disease related amyloid and tau pathology progression. Brain 2023, 146, 4935–4948. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Chen, B.; Zhang, J.P.; Ma, Y.Y. Up-regulation of autophagy-related gene 5 (ATG5) protects dopaminergic neurons in a zebrafish model of Parkinson’s disease. J. Biol. Chem. 2017, 292, 18062–18074. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive functions in Alzheimer disease: A systematic review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Mosolov, S.N.; Yaltonskaya, P.A. Primary and Secondary Negative Symptoms in Schizophrenia. Front. Psychiatry 2022, 12, 766692. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, K.; Shen, H.; Yao, X.; Sun, Q.; Chen, G. Necroptosis: A novel manner of cell death, associated with stroke (Review). Int. J. Mol. Med. 2018, 41, 624–630. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Colie, M.; Moore, T.; Schisler, J.C. Die hard: Necroptosis and its impact on age-dependent neuroinflammatory diseases. Front. Cell Death 2024, 3, 1348153. [Google Scholar] [CrossRef]
- Trépanier, M.O.; Hopperton, K.E.; Mizrahi, R.; Mechawar, N.; Bazinet, R.P. Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Mol. Psychiatry 2016, 21, 1009–1026. [Google Scholar] [CrossRef]
- Cahn, W.; Hulshoff Pol, H.E.; Lems, E.B.; van Haren, N.E.; Schnack, H.G.; van der Linden, J.A.; Schothorst, P.F.; van Engeland, H.; Kahn, R.S. Brain Volume Changes in First-Episode Schizophrenia A 1-Year Follow-up Study. Arch. Gen. Psychiatry 2002, 59, 1002–1010. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, W.; Li, R.; Tan, S.; Zhang, Y. Targeting necroptosis in Alzheimer’s disease: Can exercise modulate neuronal death? Front. Aging Neurosci. 2025, 17, 1499871. [Google Scholar] [CrossRef]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front. Mol. Neurosci. 2022, 15, 937133. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef] [PubMed]
- Gayger-Dias, V.; Vizuete, A.F.K.; Rodrigues, L.; Wartchow, K.M.; Bobermin, L.; Leite, M.C.; Quincozes-Santos, A.; Kleindienst, A.; Gonçalves, C.A. How S100B crosses brain barriers and why it is considered a peripheral marker of brain injury. Exp. Biol. Med. 2023, 248, 2109–2119. [Google Scholar] [CrossRef]
- Langeh, U.; Singh, S. Targeting S100B Protein as a Surrogate Biomarker and its Role in Various Neurological Disorders. Curr. Neuropharmacol. 2020, 19, 265–277. [Google Scholar] [CrossRef]
- Leclerc, E.; Sturchler, E.; Vetter, S.W. The S100B/RAGE axis in Alzheimer’s disease. Cardiovasc. Psychiatry Neurol. 2010, 2010, 539581. [Google Scholar] [CrossRef]
- Zou, Z.; Li, L.; Li, Q.; Zhao, P.; Zhang, K.; Liu, C.; Cai, D.; Maegele, M.; Gu, Z.; Huang, Q. The role of S100B/RAGE-enhanced ADAM17 activation in endothelial glycocalyx shedding after traumatic brain injury. J. Neuroinflamm. 2022, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Shen, C.; Yin, Q.; Sun, M.; Ma, Y.; Liu, X. Effects of RAGE-Specific Inhibitor FPS-ZM1 on Amyloid-β Metabolism and AGEs-Induced Inflammation and Oxidative Stress in Rat Hippocampus. Neurochem. Res. 2016, 41, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T.; Bargiel, W.; Grabarczyk, M.; Skibinska, M. Peripheral S100B Protein Levels in Five Major Psychiatric Disorders: A Systematic Review. Brain Sci. 2023, 13, 1334. [Google Scholar] [CrossRef]
- Schümberg, K.; Polyakova, M.; Steiner, J.; Schroeter, M.L. Serum s100b is related to illness duration and clinical symptoms in schizophrenia—A meta-regression analysis. Front. Cell Neurosci. 2016, 10, 46. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Sologova, S.S.; Mukhortova, P.; Levushkin, D.; Somasundaram, S.G.; Kirkland, C.E.; Bachurin, S.O.; Aliev, G. Alterations of astrocytes in the context of schizophrenic dementia. Front. Pharmacol. 2020, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Y.; Yang, Y.; Zhang, H.; Li, W.; Zhong, Z.; Lv, L. Serum S100B protein and white matter changes in schizophrenia before and after medication. Brain Res. Bull. 2024, 210, 110927. [Google Scholar] [CrossRef]
- Boccardi, M.; Monsch, A.U.; Ferrari, C.; Altomare, D.; Berres, M.; Bos, I.; Buchmann, A.; Cerami, C.; Didic, M.; Festari, C.; et al. Harmonizing neuropsychological assessment for mild neurocognitive disorders in Europe. Alzheimer’s Dement. 2022, 18, 29–42. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M.; Lu, S.; Chan, Y.; Perez, S.E.; Chen, K.; Mufson, E.J. Cognitive Domain Dispersion Association with Alzheimer’s Disease Pathology. J. Alzheimer’s Dis. 2017, 58, 575–583. [Google Scholar] [CrossRef]
- Chapman, R.M.; Mapstone, M.; Porsteinsson, A.P.; Gardner, M.N.; McCrary, J.W.; Degrush, E.; Reilly, L.A.; Sandoval, T.C.; Guillily, M.D. Diagnosis of Alzheimer’s disease using neuropsychological testing improved by multivariate analyses. J. Clin. Exp. Neuropsychol. 2010, 32, 793–808. [Google Scholar] [CrossRef]
- Uretsky, M.; Gibbons, L.E.; Mukherjee, S.; Trittschuh, E.H.; Fardo, D.W.; Boyle, P.A.; Keene, C.D.; Saykin, A.J.; Crane, P.K.; Schneider, J.A.; et al. Longitudinal cognitive performance of Alzheimer’s disease neuropathological subtypes. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12201. [Google Scholar] [CrossRef]
- Hazan, E.; Frankenburg, F.; Brenkel, M.; Shulman, K. The test of time: A history of clock drawing. Int. J. Geriatr. Psychiatry 2018, 33, e22–e30. [Google Scholar] [CrossRef] [PubMed]
- Brueggen, K.; Kasper, E.; Ochmann, S.; Pfaff, H.; Webel, S.; Schneider, W.; Teipel, S. Cognitive Rehabilitation in Alzheimer’s Disease: A Controlled Intervention Trial. J. Alzheimer’s Dis. 2017, 57, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Soria, I.; Iguacel, I.; Aguilar-Latorre, A.; Peralta-Marrupe, P.; Latorre, E.; Zaldívar, J.N.C.; Calatayud, E. Cognitive stimulation and cognitive results in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 104, 104807. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive impairment in schizophrenia: Aetiology, pathophysiology, and treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef] [PubMed]
- Mayeli, A.; Clancy, K.J.; Sonnenschein, S.; Sarpal, D.K.; Ferrarelli, F. A narrative review of treatment interventions to improve cognitive performance in schizophrenia, with an emphasis on at-risk and early course stages. Psychiatry Res. 2022, 317, 114926. [Google Scholar] [CrossRef]
- Vita, A.; Barlati, S.; Ceraso, A.; Deste, G.; Nibbio, G.; Wykes, T. Acceptability of cognitive remediation for schizophrenia: A systematic review and meta-analysis of randomized controlled trials. Psychol. Med. 2023, 53, 3661–3671. [Google Scholar] [CrossRef]
- Vita, A.; Barlati, S.; Ceraso, A.; Nibbio, G.; Ariu, C.; Deste, G.; Wykes, T. Effectiveness, Core Elements, and Moderators of Response of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2021, 78, 848–858. [Google Scholar] [CrossRef]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: Challenges and advances in diagnosis and treatment. Front. Neurosci. 2023, 17, 1263771. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Zhang, J.; Peng, W.; Li, T.; Zhang, J. Efficacy of selective serotonin reuptake inhibitors-related antidepressants in Alzheimer’s disease: A meta-analysis. Eur. J. Med. Res. 2024, 29, 438. [Google Scholar] [CrossRef]
- Westhoff, M.L.S.; Ladwig, J.; Heck, J.; Schülke, R.; Groh, A.; Deest, M.; Bleich, S.; Frieling, H.; Jahn, K. Early detection and prevention of schizophrenic psychosis—A review. Brain Sci. 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Hall, J. Schizophrenia—An anxiety disorder? Br. J. Psychiatry 2017, 211, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S. On the Origins of Schizophrenia. Am. J. Psychiatry 2020, 177, 291–297. [Google Scholar] [CrossRef]
- Di Luzio, M.; Pontillo, M.; Villa, M.; Attardi, A.G.; Bellantoni, D.; Di Vincenzo, C.; Vicari, S. Clinical features and comorbidity in very early-onset schizophrenia: A systematic review. Front. Psychiatry 2023, 14, 1270799. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Dursun, S.M.; Tusconi, M.; Hallak, J.E.C. Neuroinflammation and schizophrenia—Is there a link? Front. Psychiatry 2024, 15, 1356975. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.; Solano, R.; González De Chávez, M. Premorbid adjustment and previous personality in schizophrenic patients. Eur. J. Psychiatry 2005, 19, 243–254. [Google Scholar][Green Version]
- Ekstrøm, M.; Lykke Mortensen, E.; Sørensen, H.J.; Mednick, S.A. Premorbid personality in schizophrenia spectrum: A prospective study. Nord. J. Psychiatry 2006, 60, 417–422. [Google Scholar] [CrossRef]
- Henriques-Calado, J. Personality traits and disorders in Alzheimer’s disease. Brain Behav. 2023, 13, e2938. [Google Scholar] [CrossRef]
- Lopes, K.F.; Bahia, V.S.; Natividade, J.C.; Bastos, R.V.S.; Shiguti, W.A.; da Silva, K.E.R.; de Souza, W.C. Changes in personality traits in patients with Alzheimer’s Disease. Dement. Neuropsychol. 2022, 16, 187–193. [Google Scholar] [CrossRef]
- D’Iorio, A.; Garramone, F.; Piscopo, F.; Baiano, C.; Raimo, S.; Santangelo, G. Meta-Analysis of Personality Traits in Alzheimer’s Disease: A Comparison with Healthy Subjects. J. Alzheimer’s Dis. 2018, 62, 773–787. [Google Scholar] [CrossRef]
- Rouch, I.; Padovan, C.; Pongan, E.; Boublay, N.; Laurent, B.; Dorey, J.M.; Krolak-Salmon, P. Personality Traits are Related to Selective Cognitive Impairment in Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castañeda, P.; Santiago Molina, E.; Aguirre Loaiza, H.; Daza González, M.T. Positive symptoms of schizophrenia and their relationship with cognitive and emotional executive functions. Cogn. Res. Princ. Implic. 2022, 7, 78. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Endres, D.; Matysik, M.; Feige, B.; Venhoff, N.; Schweizer, T.; Michel, M.; Meixensberger, S.; Runge, K.; Maier, S.J.; Nickel, K.; et al. Diagnosing organic causes of schizophrenia spectrum disorders: Findings from a one-year cohort of the Freiburg diagnostic protocol in psychosis (FDPP). Diagnostics 2020, 10, 691. [Google Scholar] [CrossRef]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef]
- Munjal, S.; Ferrando, S.J.; Freyberg, Z. Neuropsychiatric Aspects of Infectious Diseases: An Update. Crit. Care Clin. 2017, 33, 681–712. [Google Scholar] [CrossRef]
- Xinyu, J.; Yong, L.; Guanglai, D.; Xiaoqian, D.; Xin, L. Comparison of Brain MRI Findings between Patients with Alzheimer’s Disease and Non-Dementia Psychiatric Disorders in the Elderly. Int. J. Radiol. Imaging Technol. 2023, 9, 115. [Google Scholar] [CrossRef]
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology 2023, 48, 151–167. [Google Scholar] [CrossRef]
- Bell, V.; Wilkinson, S.; Greco, M.; Hendrie, C.; Mills, B.; Deeley, Q. What is the functional/organic distinction actually doing in psychiatry and neurology? Wellcome Open Res. 2020, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Widing, L.; Simonsen, C.; Flaaten, C.B.; Haatveit, B.; Vik, R.K.; Wold, K.F.; Åsbø, G.; Ueland, T.; Melle, I. Symptom Profiles in Psychotic Disorder Not Otherwise Specified. Front. Psychiatry 2020, 11, 580444. [Google Scholar] [CrossRef]
- Widing, L.; Simonsen, C.; Bjella, T.; Engen, M.J.; Flaaten, C.B.; Gardsjord, E.; Haatveit, B.; Haug, E.; Lyngstad, S.H.; Svendsen, I.H.; et al. Long-term Outcomes of People With DSM Psychotic Disorder NOS. Schizophr. Bull. Open 2023, 4, sgad005. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, S.; Lan, G.; Lai, Y.J.; Wang, Q.H.; Chen, Y.; Xiao, Z.S.; Chen, X.; Bu, X.L.; Liu, Y.H.; et al. Diagnostic accuracy of plasma p-tau217/Aβ42 for Alzheimer’s disease in clinical and community cohorts. Alzheimer’s Dement. 2025, 21, e70038. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Rugarli, G.; Spinelli, E.G.; Ghirelli, A.; Zavarella, M.; Bottale, I.; Orlandi, F.; Santangelo, R.; Caso, F.; et al. Diagnostic accuracy of automated Lumipulse plasma pTau-217 in Alzheimer’s disease: A real-world study. J. Neurol. 2024, 271, 6739–6749. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Balázs, N.; Bereczki, D.; Kovács, T. Cholinesterase Inhibitors and Memantine for the Treatment of Alzheimer and Non-Alzheimer Dementias. Ideggyogy. Szle. 2021, 74, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.M.; Keating, G.M. Memantine: A Review of its Use in Alzheimer’s Disease. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in Moderate-to-Severe Alzheimer’s Disease. N. Engl. J. Med. 2003, 14, 1333–1374. [Google Scholar] [CrossRef]
- Volbracht, C.; Van Beek, J.; Zhu, C.; Blomgren, K.; Leist, M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur. J. Neurosci. 2006, 348, 1333–1341. [Google Scholar] [CrossRef]
- Weston-Green, K. Antipsychotic Drug Development: From Historical Evidence to Fresh Perspectives. Front. Psychiatry 2022, 13, 903156. [Google Scholar] [CrossRef]
- Stahl, S.M. Drugs for psychosis and mood: Unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 2017, 22, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Mailman, R.B.; Murthy, V. Third generation antipsychotic drugs: Partial agonism or receptor functional selectivity? Curr. Pharm. Des. 2010, 16, 488–501. [Google Scholar] [CrossRef]
- Zheng, L.; Mack, W.J.; Dagerman, K.S.; Hsiao, J.K.; Lebowitz, B.D.; Lyketsos, C.G.; Stroup, T.S.; Sultzer, D.L.; Tariot, P.N.; Vigen, C.; et al. Metabolic changes associated with second-generation antipsychotic use in Alzheimer’s disease patients: The CATIE-AD study. Am. J. Psychiatry 2009, 166, 583–590. [Google Scholar] [CrossRef]
- Marvanova, M. Strategies for prevention and management of second generation antipsychotic-induced metabolic side effects. Ment. Health Clin. 2013, 3, 154–161. [Google Scholar] [CrossRef]
- Beck, K.; Hindley, G.; Borgan, F.; Ginestet, C.; McCutcheon, R.; Brugger, S.; Driesen, N.; Ranganathan, M.; D’Souza, D.C.; Taylor, M.; et al. Association of Ketamine with Psychiatric Symptoms and Implications for Its Therapeutic Use and for Understanding Schizophrenia: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e204693. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T. NMDA receptor and schizophrenia: A brief history. Schizophr. Bull. 2012, 38, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Leenders, K.L.; Scharfetter, C.; Antonini, A.; Maguire, P.; Missimer, J.; Angst, J. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur. Neuropsychopharmacol. 1997, 7, 9–24. [Google Scholar] [CrossRef]
- Lahti, A.C.; Weiler, M.A.; Michaelidis, T.; Parwani, A.; Tamminga, C.A. Effects of Ketamine in Normal and Schizophrenic Volunteers. Neuropsychopharmacology 2001, 25, 455–467. [Google Scholar] [CrossRef]
- Carolan, A.; Hynes-Ryan, C.; Agarwal, S.M.; Bourke, R.; Cullen, W.; Gaughran, F.; Hahn, M.K.; Krivoy, A.; Lally, J.; Leucht, S.; et al. Metformin for the Prevention of Antipsychotic-Induced Weight Gain: Guideline Development and Consensus Validation. Schizophr. Bull. 2024, sbae205. [Google Scholar] [CrossRef]
- Stilo, S.A.; Murray, R.M. Non-Genetic Factors in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Bergen, S.E. Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front. Genet. 2021, 12, 686666. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 2011, 93, 23–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Q.; Yao, H.; Tan, J.; Liu, Z.; Zhou, Y.; Zou, Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 911635. [Google Scholar] [CrossRef]
- Hong, W.; Zhao, M.; Li, H.; Peng, F.; Wang, F.; Li, N.; Xiang, H.; Su, Y.; Huang, Y.; Zhang, S.; et al. Higher Plasma S100B Concentrations in Schizophrenia Patients, and Dependently Associated with Inflammatory Markers. Sci. Rep. 2016, 6, 27584. [Google Scholar] [CrossRef]
- Steiner, J.; Bernstein, H.-G.; Bogerts, B.; Gonçalves, C.-A. Potential roles of S100B in schizophrenia. Rev. Psiquiatr. Clín. 2013, 40, 35–40. [Google Scholar] [CrossRef]
- Mucci, A.; Kawohl, W.; Maria, C.; Wooller, A. Treating Schizophrenia: Open Conversations and Stronger Relationships Through Psychoeducation and Shared Decision-Making. Front. Psychiatry 2020, 11, 761. [Google Scholar] [CrossRef]
- Kuljiš, R.O.; Colom, L.V.; Rojo, L.E. Biological Basis for Cerebral Dysfunction in Schizophrenia in Contrast with Alzheimer’s Disease. Front. Psychiatry 2014, 4, 119. [Google Scholar] [CrossRef]
- Ting, C.; Rajji, T.K.; Ismail, Z.; Tang-Wai, D.F.; Apanasiewicz, N.; Miranda, D.; Mamo, D.; Mulsant, B.H. Differentiating the cognitive profile of schizophrenia from that of Alzheimer disease and depression in late life. PLoS ONE 2010, 5, e10151. [Google Scholar] [CrossRef]
- Pfammatter, M.; Junghan, U.M.; Brenner, H.D. Efficacy of psychological therapy in schizophrenia: Conclusions from meta-analyses. Schizophr. Bull. 2006, 32, S64–S80. [Google Scholar] [CrossRef]
- Lake, T.; Grossberg, G. The Role of the Psychiatrist in Alzheimer’s Disease. J. Clin. Psychiatry 1998, 59 (Suppl. S9), 3–6. [Google Scholar]
- Fan, P.; Kofler, J.; Ding, Y.; Marks, M.; Sweet, R.A.; Wang, L. Efficacy difference of antipsychotics in Alzheimer’s disease and schizophrenia: Explained with network efficiency and pathway analysis methods. Brief. Bioinform. 2022, 23, bbac394. [Google Scholar] [CrossRef] [PubMed]
- Beata, B.K.; Wojciech, J.; Johannes, K.; Piotr, L.; Barbara, M. Alzheimer’s Disease—Biochemical and Psychological Background for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1059. [Google Scholar] [CrossRef]
- Svensson, J.E.; Bolin, M.; Thor, D.; Williams, P.A.; Brautaset, R.; Carlsson, M.; Sörensson, P.; Marlevi, D.; Spin-Neto, R.; Probst, M.; et al. Evaluating the effect of rapamycin treatment in Alzheimer’s disease and aging using in vivo imaging: The ERAP phase IIa clinical study protocol. BMC Neurol. 2024, 24, 111. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.J.; Zhang, S.X.; Li, Y.; Xu, S.Y. Rapamycin Responds to Alzheimer’s Disease: A Potential Translational Therapy. Clin. Interv. Aging 2023, 18, 1629–1639. [Google Scholar] [CrossRef]
- Xie, P.L.; Zheng, M.Y.; Han, R.; Chen, W.X.; Mao, J.H. Pharmacological mTOR inhibitors in ameliorating Alzheimer’s disease: Current review and perspectives. Front. Pharmacol. 2024, 15, 1366061. [Google Scholar] [CrossRef]
- Lee, J.; Yanckello, L.M.; Ma, D.; Hoffman, J.D.; Parikh, I.; Thalman, S.; Bauer, B.; Hartz, A.M.S.; Hyder, F.; Lin, A.L. Neuroimaging Biomarkers of mTOR Inhibition on Vascular and Metabolic Functions in Aging Brain and Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 225. [Google Scholar] [CrossRef]
- Poor, S.R.; Ettcheto, M.; Cano, A.; Sanchez-Lopez, E.; Manzine, P.R.; Olloquequi, J.; Camins, A.; Javan, M. Metformin a potential pharmacological strategy in late onset Alzheimer’s disease treatment. Pharmaceuticals 2021, 14, 890. [Google Scholar] [CrossRef]
- Nashwan, A.J.; Elawfi, B. Beyond one-size-fits-all: The rise of personalized treatment in schizophrenia. Pers. Med. Psychiatry 2024, 43–44, 100118. [Google Scholar] [CrossRef]
- Buckley, P.F.; Miller, B.J. Personalized medicine for schizophrenia. npj Schizophr. 2017, 3, 2. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Personalised approaches to pharmacotherapy for schizophrenia. BJPsych Adv. 2016, 22, 78–86. [Google Scholar] [CrossRef]
- Češková, E.; Šilhán, P. From Personalized Medicine to Precision Psychiatry? Neuropsychiatr. Dis. Treat. 2021, 17, 3663–3668. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.F. The Case for Adjunctive Monoclonal Antibody Immunotherapy in Schizophrenia. Psychiatr. Clin. N. Am. 2016, 39, 187–198. [Google Scholar] [CrossRef]
- Chauhan, P.; Gurjit, K.; Rajendra, P.; Singh, H. Pharmacotherapy of schizophrenia: Immunological aspects and potential role of immunotherapy. Expert Rev. Neurother. 2021, 21, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Bannerman, D.M. GRIN2A (NR2A): A gene contributing to glutamatergic involvement in schizophrenia. Mol. Psychiatry 2023, 28, 3568–3572. [Google Scholar] [CrossRef] [PubMed]
- Herzog, L.E.; Wang, L.; Yu, E.; Choi, S.; Farsi, Z.; Song, B.J.; Pan, J.Q.; Sheng, M. Mouse mutants in schizophrenia risk genes GRIN2A and AKAP11 show EEG abnormalities in common with schizophrenia patients. Transl. Psychiatry 2023, 13, 92. [Google Scholar] [CrossRef]
- Shepard, N.; Baez-Nieto, D.; Iqbal, S.; Kurganov, E.; Budnik, N.; Campbell, A.J.; Pan, J.Q.; Sheng, M.; Farsi, Z. Differential functional consequences of GRIN2A mutations associated with schizophrenia and neurodevelopmental disorders. Sci. Rep. 2024, 14, 2798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.P.; Pastorello, Y.; Dénes, L.; Brînzaniuc, K.; Krupinski, J.; Slevin, M. The Inflammatory Nexus: Unraveling Shared Pathways and Promising Treatments in Alzheimer’s Disease and Schizophrenia. Int. J. Mol. Sci. 2025, 26, 6237. https://doi.org/10.3390/ijms26136237
Russo AP, Pastorello Y, Dénes L, Brînzaniuc K, Krupinski J, Slevin M. The Inflammatory Nexus: Unraveling Shared Pathways and Promising Treatments in Alzheimer’s Disease and Schizophrenia. International Journal of Molecular Sciences. 2025; 26(13):6237. https://doi.org/10.3390/ijms26136237
Chicago/Turabian StyleRusso, Aurelio Pio, Ylenia Pastorello, Lóránd Dénes, Klara Brînzaniuc, Jerzy Krupinski, and Mark Slevin. 2025. "The Inflammatory Nexus: Unraveling Shared Pathways and Promising Treatments in Alzheimer’s Disease and Schizophrenia" International Journal of Molecular Sciences 26, no. 13: 6237. https://doi.org/10.3390/ijms26136237
APA StyleRusso, A. P., Pastorello, Y., Dénes, L., Brînzaniuc, K., Krupinski, J., & Slevin, M. (2025). The Inflammatory Nexus: Unraveling Shared Pathways and Promising Treatments in Alzheimer’s Disease and Schizophrenia. International Journal of Molecular Sciences, 26(13), 6237. https://doi.org/10.3390/ijms26136237






