Comparative Evaluation of Urinary Biomarkers in Wilms Tumor Survivors and Children with Chronic Kidney Disease
Abstract
1. Introduction
2. Results
2.1. Biomarkers Patterns
2.2. Pattern Assessment of Normalized Urine Biomarkers Using Creatinine Calibration
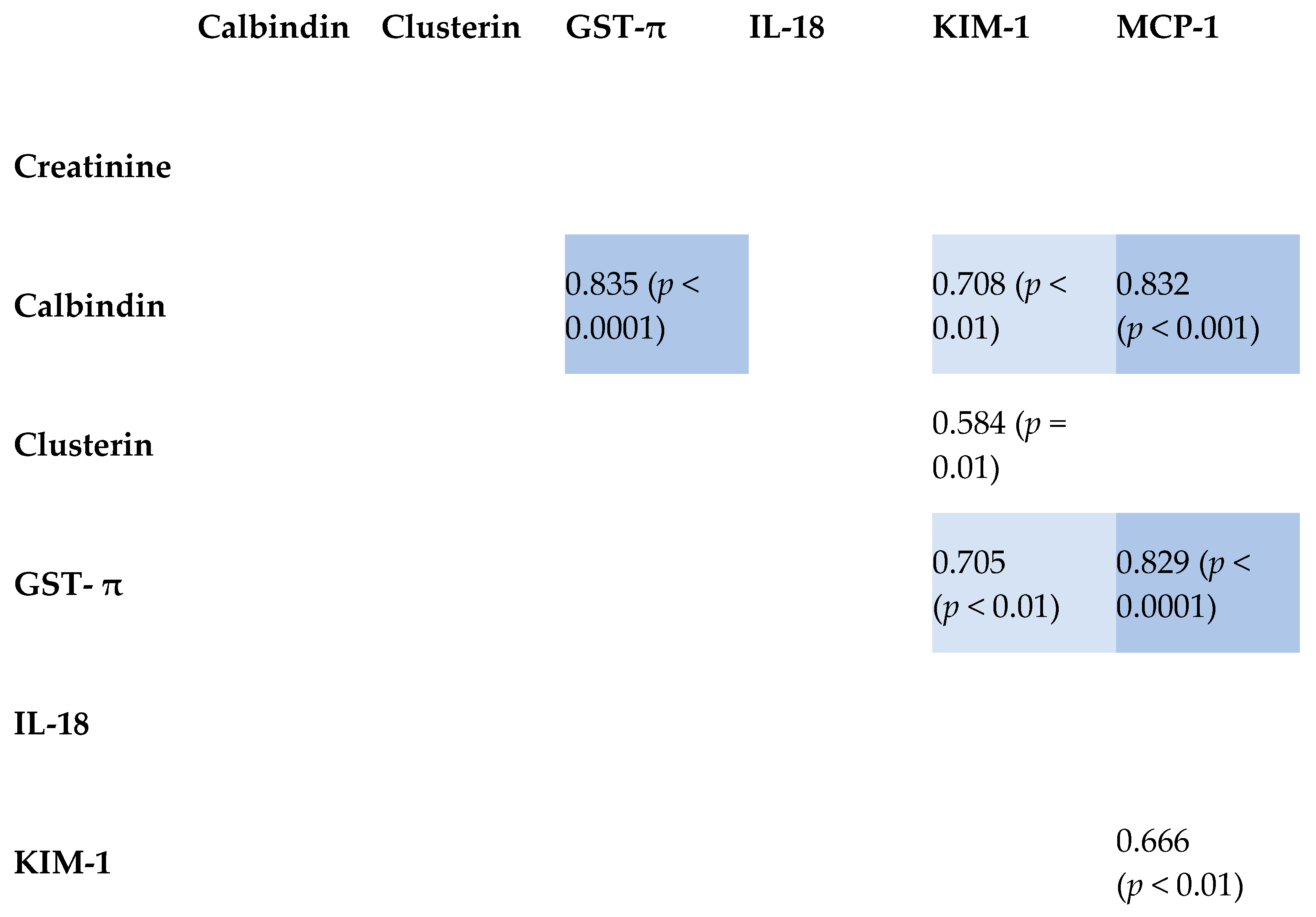
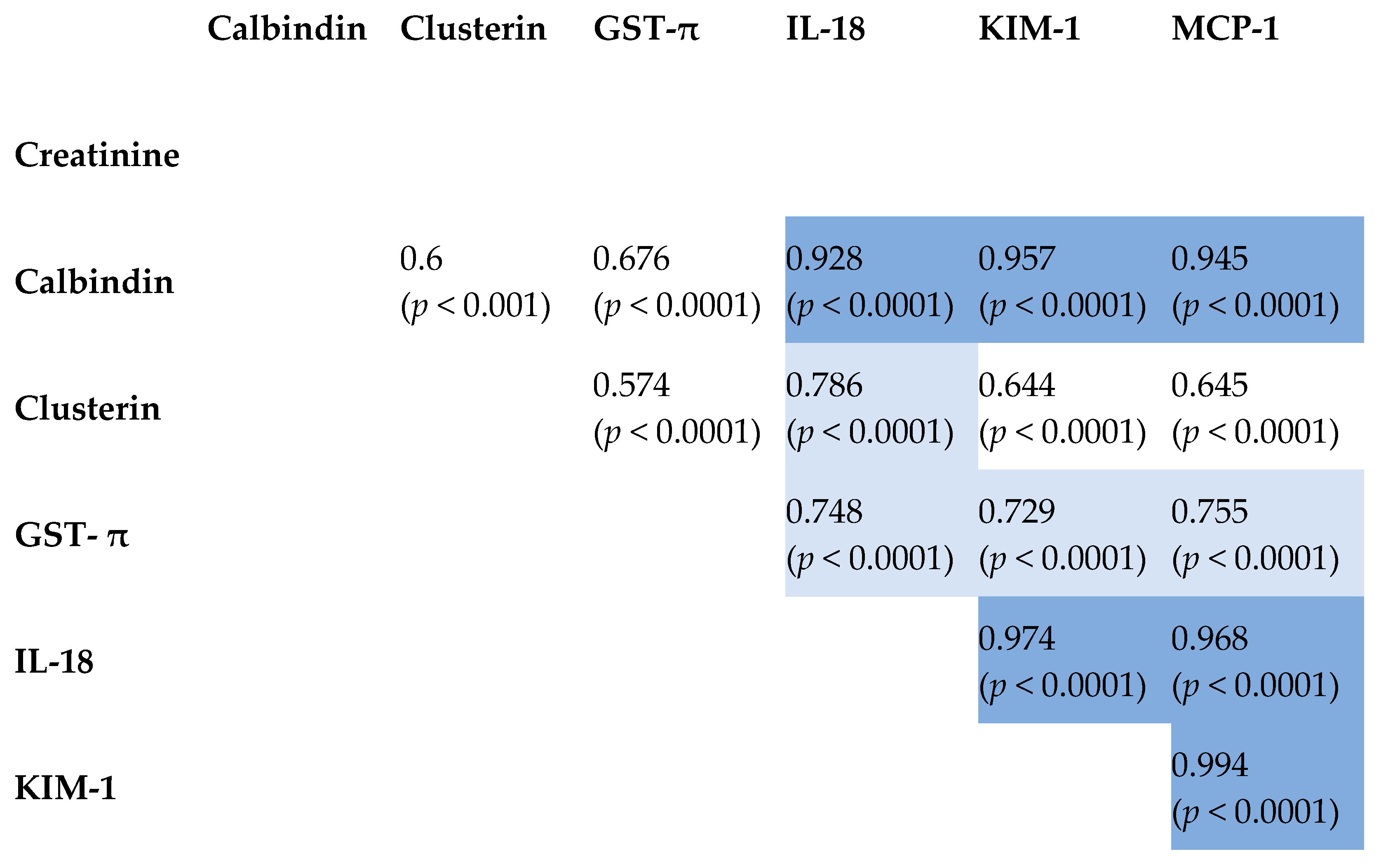
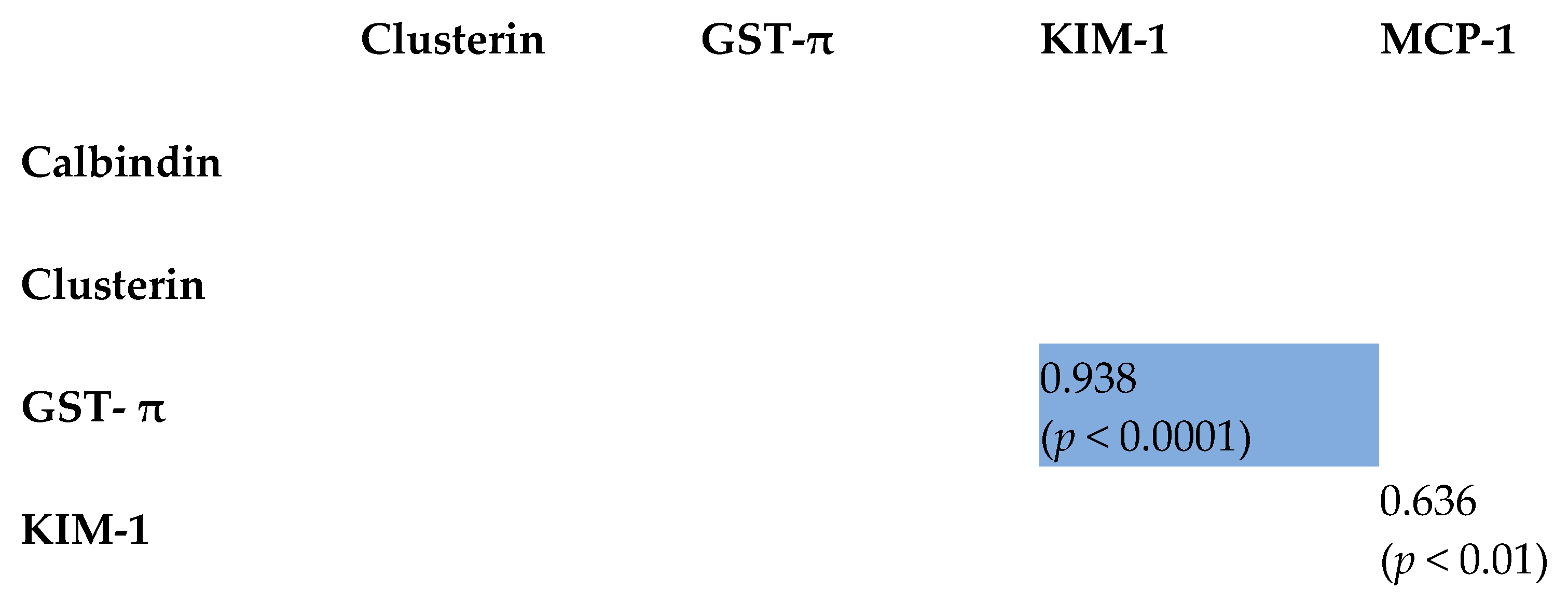
2.3. Correlation Between Tested Biomarkers
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dome, J.S.; Graf, N.; Geller, J.I.; Fernandez, C.V.; Mullen, E.A.; Spreafico, F.; Van den Heuvel-Eibrink, M.; Pritchard-Jones, K. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol. 2015, 33, 2999–3007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warady, B.A.; Abraham, A.G.; Schwartz, G.J.; Wong, C.S.; Muñoz, A.; Betoko, A.; Mitsnefes, M.; Kaskel, F.; Greenbaum, L.A.; Mak, R.H.; et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am. J. Kidney Dis. 2015, 66, 878–888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hains, D.S.; Sims-Lucas, S.; Carpenter, A.; Saha, M.; Murawski, I.; Kish, K.; Gupta, I.; McHugh, K.; Bates, C.M. High Incidence of Proteinuria in Focal Segmental Glomerulosclerosis with Coexistent Wilms Tumor: A Report of Two Cases. Case Rep. Nephrol. 2016, 2016, 8304096. [Google Scholar] [PubMed] [PubMed Central]
- Pritchard-Jones, K.; Bergeron, C.d.C.B.; Van Den Heuvel-Eibrink, M.M.; Acha, T.; Godzinski, J.; Oldenburger, F.; Boccon-Gibod, L.; Leuschner, I.; Vujanic, G.; Sandstedt, B.; et al. SIOP Renal Tumours Study Group. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms’ tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 2015, 385, 1301–1310. [Google Scholar] [PubMed] [PubMed Central]
- Mekahli, D.; Liutkus, A.; Ranchin, B.; Yu, A.; Bessenay, L.; Girardin, E.; Van Damme-Lombaerts, R.; Palcoux, J.B.; Cachat, F.; Lavocat, M.P.; et al. Long-term Outcome of Idiopathic Steroid-resistant Nephrotic Syndrome: A Multicenter Study. Pediatr. Nephrol. 2009, 24, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Robinson, B.H.; Chacko, J.; Cohen, A.J.; Carpenter, T.O.; Islam, M. Evidence for a renal tubular enzyme defect in patients with cystinosis. Kidney Int. 1994, 46, 1338–1344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oda, T.; Elkahloun, A.G.; Pike, B.L.; Okajima, K.; Krantz, I.D.; Genin, A.; Piccoli, D.A.; Meltzer, P.S.; Spinner, N.B.; Collins, F.S.; et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet. 1997, 17, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Quillinan, N.; Li, D.; Klopocki, E.; Hanchard, N.A.; Plagnol, V.; Bunyan, D.J.; Thakker, R.V.; Harrison, T.J.; Graham, A.; Halliday, D.; et al. A novel locus for X-linked recessive congenital talipes equinovarus maps to chromosome 2q24-25. Hum. Genet. 2008, 124, 125–130. [Google Scholar] [PubMed]
- Tucci, A.; Ciulli, S.; Saccone, P.; Polverari, S.; Papacci, P.; Della Rocca, M.T.; Stagni, K.; Di Capua, M.; Acampora, A.; Formica, M.; et al. Copy number variants associated with a mild and nonprogressive form of the autosomal dominant polycystic kidney disease. Gene 2013, 515, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermiin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Renal. Physiol. 2004, 286, F552–F563. [Google Scholar] [CrossRef]
- Boring, L.; Gosling, J.; Cleary, M.; Charo, I.F. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998, 394, 894–897. [Google Scholar] [CrossRef]
- Claudel, S.E.; Waikar, S.S. Chronic Kidney Disease of UnceRtain Etiology (CKDu) in Agricultural Communities (CURE) Research Consortium. Systematic Review of Kidney Injury Biomarkers for the Evaluation of CKD of Uncertain Etiology. Kidney Int. Rep. 2024, 9, 1614–1632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefanowicz, J.; Kosiak, M.; Kosiak, W.; Lipska-Ziętkiewicz, B.S. Chronic Kidney Disease in Wilms Tumour Survivors—What Do We Know Today? In Wilms Tumor [Internet]; Van den Heuvel-Eibrink, M.M., Ed.; Codon Publications: Brisbane, QLD, Australia, 2016; Chapter 9. [Google Scholar] [PubMed]
- Falcone, M.P.; Pritchard-Jones, K.; Brok, J.; Mifsud, W.; Williams, R.D.; Nakata, K.; Tugnait, S.; Al-Saadi, R.; Side, L.; Anderson, J.; et al. Long-term kidney function in children with Wilms tumour and constitutional WT1 pathogenic variant. Pediatr. Nephrol. 2022, 37, 821–832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, H.; Liu, J.; Pan, X.; Cui, X. Biomarkers for patients with Wilms tumor: A review. Front. Oncol. 2023, 13, 1137346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devarajan, P. Neutrophil Gelatinase-Associated Lipocalin: A Promising Biomarker for Human Acute Kidney Injury. Biomark. Med. 2010, 4, 265–280. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.E.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and Classification of Chronic Kidney Disease: A Position Statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Gatta, G.; Rossi, S.; Foschi, R.; Trama, A.; Marcos-Gragera, R.; Pastore, G.; Peris-Bonet, R.; Stiller, C.; Capocaccia, R.; EUROCARE Working Group. Survival and cure trends for European children, adolescents and young adults diagnosed with acute lymphoblastic leukemia from 1982 to 2002. Haematologica 2013, 98, 744–752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritchey, M.L.; Green, D.M.; Thomas, P.R.; Smith, G.R.; Haase, G.; Shochat, S.; Moksness, J.; Breslow, N.E. Renal failure in Wilms’ tumor patients: A report from the National Wilms’ Tumor Study Group. Med. Pediatr. Oncol. 1996, 26, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yang, H.Y.; Chien, H.P.; Tseng, M.H.; Huang, J.L. Urinary clusterin-a novel urinary biomarker associated with pediatric lupus renal histopathologic features and renal survival. Pediatr. Nephrol. 2018, 33, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- van Meer, L.; Moerland, M.; Cohen, A.F.; Burggraaf, J. Urinary kidney biomarkers for early detection of nephrotoxicity in clinical drug development. Br. J. Clin. Pharmacol. 2014, 77, 947–957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, K.J. Stress response proteins and renal ischemia. Minerva Urol. Nefrol. 2002, 54, 81–91. [Google Scholar] [PubMed]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Sig. Transduct. Target Ther. 2022, 7, 182. [Google Scholar] [CrossRef]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front. Med. 2021, 8, 639103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Canki, E.; Kho, E.; Hoenderop, J.G.J. Urinary biomarkers in kidney disease. Clin. Chim. Acta 2024, 555, 117798. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—When and why. Singapore Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2010, 25, 2872–2874. [Google Scholar] [CrossRef]
- Kayed, H.; Kleeff, J.; Keleg, S.; Buchler, M.W.; Friess, H. Calbindin-D28k is differentially expressed in pancreatic cancer and downregulated by hypoxia: Molecular mechanisms and clinical implications. Oncogene 2003, 23, 494–505. [Google Scholar]
- Christensen, E.I.; Birn, H. Megalin and cubilin: Multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 2011, 3, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.; Ozols, E.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in streptozotocin-induced diabetic nephropathy: Potential role in renal fibrosis. Nephrol. Dial. Transplant. 2004, 19, 2987–2996. [Google Scholar] [CrossRef]
- Rovin, B.H.; Song, H.; Birmingham, D.J.; Hebert, L.A.; Yu, C.Y.; Nagaraja, H.N. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. 2005, 16, 467–473. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Xu, F.; Sabbisetti, V.; Grgic, I.; Movahedi, N.; Parapuram, S.K.; Chen, G.; Xiao, S.; Patel, D.; Henderson, J.M.; et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Investig. 2013, 123, 4023–4035. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, S.C.; Upton, Z.; Bott, K.; Dargaville, T.R. Recent advances in chronic wound healing: Long-term healing in chronic wounds by fibroblast growth factors. Plast. Reconstr. Surg. 2009, 124, 540–550. [Google Scholar]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.-W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Humphreys, B.D.; Bonventre, J.V. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinase and juxtamembrane region. J. Am. Soc. Nephrol. 2007, 18, 2704–2714. [Google Scholar] [CrossRef]
- Tesch, G.H. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Nephrol. 2008, 28, 417–426. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ramirez, V.; Ichimura, T.; Bobadilla, N.A.; Bonventre, J.V. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal. Physiol. 2006, 290, F517–F529. [Google Scholar] [CrossRef]
| WT | CKD | Healthy Control | |
|---|---|---|---|
| Patients (n, %) | |||
| - Male | 7 (35%) | 18 (45%) | 10 (50%) |
| - Female | 13 (65%) | 22 (55%) | 10 (50%) |
| Median age (years, range) | 13 (4–18) | 9 (1–16) | NA |
| Disease advancement: | |||
| Stage of Wilms Tumor at diagnosis | |||
| - Total | 20 | ||
| - stage I | 7 (35%) | ||
| - stage II | 4 (20%) | ||
| - stage III | 5 (25%) | ||
| - stage VI | 4 (20%) | ||
| Stage of CKD among WT survivors (n, %) | |||
| - stage I | 4 (20%) | ||
| - stage II | 16 (80%) | ||
| Stage of Chronic Kidney Diseases among children with nephrological diseases (n, %) | |||
| - Total | 40 | ||
| - stage I | 14 (35%) | ||
| - stage II | 6 (15%) | ||
| - stage III | 4 (10%) | ||
| - stage IV | 4 (10%) | ||
| - stage V | 12 (30%) | ||
| Creatinine (mg/dL) | eGFR (mL/min/1.73 m2) | Calbindin (pg/mL) | Clusterin (pg/mL) | GST-π (pg/mL) | IL-18 (pg/mL) | KIM-1 (pg/mL) | MCP-1 (pg/mL) | |
|---|---|---|---|---|---|---|---|---|
| WT | 0.64 (0.26) | 131.6 (62.16) | 59.8 (57.1) | 40.8 (70) | 0.847 (1.3) | 0.012 (0.004) | 0.244 (0.314) | 0.128 (0.122) |
| CKD | 1.32 (1.9) | 62.35 (50.55) | 39.6 (81.8) | 349 (1310) | 5.55 (20.6) | 0.054 (0.249) | 0.378 (1.61) | 0.517 (0.288) |
| Control | 0.445 (0.11) | 139.94 (54.65) | 75.2 (87.4) | 18.1 (21) | 5.24 (10) | 0.04 (0.04) | 0.173 (0.168) | 0.169 (0.111) |
| p-value | <0.05 | <0.05 | 0.42 | 0.38 | 0.56 | NA | 0.8 | 0.52 |
| Calbindin/cr. | Clusterin/cr. | GST-π/cr. | IL-18/cr. | KIM-1/cr. | MCP-1/cr. | |
|---|---|---|---|---|---|---|
| WT | 0.237 (0.129; 0.808) | 0.114 (0.013; 0.872) | 0.007 (0.002; 0.013) | 0.0001 (0.00009; 0.0003) | 0.001 (0.0005; 0.0047) | 0.0009 (0.0007; 0.002) |
| CKD | 0.348 (0.086; 0.601) | 0.475 (0.109; 6.607) | 0.004 (0.002; 0.015) | 0.0002 (0.0001; 0.0004) | 0.003 (0.001; 0.004) | 0.003 (0.001; 0.013) |
| p | <0.001 | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubiela, P.; Taranta-Janusz, K.; Konończuk, K.; Konstantynowicz-Nowicka, K.; Chabowski, A.; Szymanska-Rozek, P.; Latoch, E. Comparative Evaluation of Urinary Biomarkers in Wilms Tumor Survivors and Children with Chronic Kidney Disease. Int. J. Mol. Sci. 2025, 26, 6238. https://doi.org/10.3390/ijms26136238
Dubiela P, Taranta-Janusz K, Konończuk K, Konstantynowicz-Nowicka K, Chabowski A, Szymanska-Rozek P, Latoch E. Comparative Evaluation of Urinary Biomarkers in Wilms Tumor Survivors and Children with Chronic Kidney Disease. International Journal of Molecular Sciences. 2025; 26(13):6238. https://doi.org/10.3390/ijms26136238
Chicago/Turabian StyleDubiela, Pawel, Katarzyna Taranta-Janusz, Katarzyna Konończuk, Karolina Konstantynowicz-Nowicka, Adrian Chabowski, Paulina Szymanska-Rozek, and Eryk Latoch. 2025. "Comparative Evaluation of Urinary Biomarkers in Wilms Tumor Survivors and Children with Chronic Kidney Disease" International Journal of Molecular Sciences 26, no. 13: 6238. https://doi.org/10.3390/ijms26136238
APA StyleDubiela, P., Taranta-Janusz, K., Konończuk, K., Konstantynowicz-Nowicka, K., Chabowski, A., Szymanska-Rozek, P., & Latoch, E. (2025). Comparative Evaluation of Urinary Biomarkers in Wilms Tumor Survivors and Children with Chronic Kidney Disease. International Journal of Molecular Sciences, 26(13), 6238. https://doi.org/10.3390/ijms26136238





