Abstract
Epilepsy affects over 12 million children worldwide, with approximately 30% classified as having drug-resistant epilepsy (DRE), often accompanied by neuropsychiatric comorbidities that severely impact quality of life. The endocannabinoid system (ECS) functions as a multifaceted neuromodulatory network regulating neuronal excitability, synaptic plasticity, and immune homeostasis from early life through adolescence and into aging. In pediatric epilepsies, alterations in ECS components, particularly CB1 receptor expression and endocannabinoid levels, reveal disorder-specific vulnerabilities and therapeutic opportunities. Cannabidiol (CBD), a non-psychoactive compound from Cannabis sativa, has shown strong preclinical and clinical efficacy in treating DRE and is approved for Dravet syndrome, Lennox–Gastaut syndrome, and Tuberous Sclerosis Complex. Other ECS-based strategies, such as the use of CB1 receptor-positive allosteric modulators, can selectively enhance endogenous cannabinoid signaling where and when it is active, potentially reducing seizures in conditions like Dravet and absence epilepsy. Similarly, FAAH and MAGL inhibitors may help restore ECS tone without directly activating CB1 receptors. Precision targeting of ECS components based on regional expression and syndrome-specific pathophysiology may optimize seizure control and associated comorbidities. Nonetheless, long-term pediatric use must be approached with caution, given the critical role of the ECS in brain development.
1. Introduction
Epilepsy is among the most common and disabling neurological conditions []. Due to the wide variety of epilepsy types, it is classified as a spectrum disorder []. A “seizure” is defined as a paroxysmal alteration in neurological function resulting from excessive, hypersynchronous neuronal discharge in the brain. Specifically, an “epileptic seizure” is caused by abnormal neuronal firing and must be distinguished from non-epileptic events, such as psychogenic seizures []. Epilepsy refers to a chronic condition of recurrent, unprovoked seizures caused by an underlying brain dysfunction []. In contrast, seizures resulting from reversible insults, such as fever, are classified as secondary and do not constitute epilepsy. The factors disrupting the normal balance between neuronal excitation and inhibition can be either genetic or acquired []. “Epilepsy syndrome” is characterized by a cluster of clinical features, including specific seizure types, characteristic EEG findings, triggers, prognosis, and responses to antiseizure medications (ASMs). Approximately 75% of epilepsy cases are first diagnosed in childhood, reflecting the heightened vulnerability of the developing brain to seizures []. Due to underlying physiological reasons, the developing brain is especially prone to seizures, but appears to be more “resistant” to the toxic effects of glutamate than the mature brain []. Excitatory synaptic function develops before inhibitory synaptic functions, favoring increased excitation and leading to seizure generation []. Additionally, GABA neurotransmission “induces” rather than “inhibits” in early life. GABA-releasing synapses are formed before glutamatergic contacts, and only the delayed expression of a chloride exporter leads to a negative shift in the reversal potential for chloride ions []. However, compared to those in the adult brain, seizures in the developing brain seem to cause less structural damage [].
The annual incidence of childhood epilepsy is approximately 35 per 100,000 individuals []. Of these, nearly 30% have drug-resistant epilepsy (DRE), meaning their seizures do not respond to conventional ASMs []. This pharmacoresistance profoundly impacts the quality of life of affected children and their families, leading to social isolation, physical limitations, and emotional distress [].
Despite the advances in ASM development, many forms of epilepsy remain challenging to treat, and the side effects of these drugs can further complicate management. In recent years, medicinal cannabis has emerged as a promising alternative therapy for epilepsy, particularly in children with DRE [].
The use of cannabis as a medical treatment is not a new phenomenon. Historically, cannabis has been used for medicinal purposes since ancient times []. However, in recent years, interest in cannabis-based treatments for epilepsy has surged, particularly with the growing body of evidence supporting its efficacy in treating drug-resistant seizures, although the first evidence dates back to the late 1970s []. This growing interest is largely due to the repressive laws surrounding cannabis use, including in the research, which have only recently begun to loosen []. Cannabinoids, the active compounds in cannabis, interact with the endocannabinoid system (ECS), which plays a key role in regulating neuronal activity and excitability []. Research has suggested that cannabinoids may help to reduce seizure frequency and severity by modulating a plethora of mechanisms from neurotransmitter release, inflammation, to ion channel activity in the brain and many others [].
Recent randomized clinical trials (RCTs) have provided robust evidence supporting the therapeutic efficacy of cannabinoids in the treatment of pediatric epilepsy. On 25 June 2018, the U.S. Food and Drug Administration (FDA) approved the first plant-derived, purified pharmaceutical-grade cannabidiol (CBD) medication, Epidiolex®, for patients aged 2 years and older with Dravet syndrome (DS) or Lennox–Gastaut syndrome (LGS) []. Epidiolex® has provided reassurance to parents, as it does not cause euphoric effects, and no cases of addiction have been reported. The approval of Epidiolex® represents a significant step forward in the medical use of cannabis-derived products [].
In Europe, Epidyolex® was approved by the European Commission on 19 September 2019 for adjunctive therapy in patients aged 2 years and older with seizures associated with DS or LGS, when used in conjunction with clobazam []. Successively, CBD was approved for treatment of seizures associated with Tuberous Sclerosis Complex (TSC), in patients aged 1 year and older in the USA [] and at least 2 years of age in Europe [].
Despite the promising findings, the use of cannabinoids in children with epilepsy remains a topic of controversy and debate. One of the key concerns is the potential long-term effects of cannabinoids use on brain development are not yet fully understood. Therefore, while cannabinoids may offer a valuable option for treatment-resistant epilepsy, further research is necessary to establish optimal treatment protocols, assess long-term safety, and address potential risks.
While many reviews have been written on cannabinoids and epilepsy [,,,,,,] and on pediatric epilepsy [,,,,,,], this review will cover new aspects, such as the alterations of the ECS in animal models and humans with pediatric epilepsy. A deeper understanding of cannabinoid mechanisms, particularly through the study of the impairment of the ECS in pediatric epilepsy, is crucial for advancing cannabinoid-based therapies as novel ASMs.
2. Pediatric Epilepsy
While a wide range of childhood epilepsies exist, as discussed in the next paragraph, we will focus here on those for which there is the most compelling evidence of involvement of the ECS. Specifically, we will briefly describe the pathophysiological features of febrile infection-related epilepsy syndrome (FIRES), DS, LGS, pediatric temporal lobe epilepsy, and childhood absence epilepsy (CAE). These epileptic syndromes are frequently associated with neuropsychiatric comorbidities, highlighting the importance of integrated and targeted therapeutic strategies [].
2.1. Classification of Pediatric Epilepsy
The recent updates by the International League Against Epilepsy (ILAE) in classifying seizures and epilepsies have significantly impacted the understanding and management of pediatric epilepsy. The new classifications continue to focus on clinical and electroencephalography (EEG) features but introduces several important changes in terminology and structure [,]. The updated seizure classification includes four main categories: focal, generalized, unknown, and unclassified []. It distinguishes classifiers, which reflect biological and clinical significance, from descriptors, which detail additional features. Focal and unknown seizures are further categorized based on the patient’s consciousness during the event, assessed clinically. Generalized seizures are divided into absence, generalized tonic–clonic, and other types, now including negative myoclonus. A basic version notes the presence or absence of visible signs, while an expanded version outlines the seizure’s chronological semiology. With 21 defined seizure types, the classification emphasizes global usability, aiming to create a unified language for clinicians, patients, and caregivers across diverse settings [] (Table 1).

Table 1.
Updated seizure classification based on [,].
Febrile infection-related epilepsy syndrome (FIRES), which primarily affects children and leads to refractory seizures following a febrile illness, is classified under unknown and immune causes, depending on the suspected immunological mechanisms []. Dravet syndrome, a severe genetic epilepsy syndrome that begins in infancy, is categorized under genetic causes, specifically developmental and epileptic encephalopathies. Similarly, Lennox–Gastaut syndrome, which is characterized by multiple seizure types and cognitive impairment, is also a genetic and structural epilepsy syndrome.
Pediatric temporal lobe epilepsy (TLE) often falls under structural causes, where focal onset seizures, typically starting in the temporal lobe, are associated with a variety of brain abnormalities, such as hippocampal sclerosis. Absence seizures, frequently observed in children, are classified as generalized onset seizures with a strong genetic basis, commonly linked to genetic syndromes, like childhood absence epilepsy.
The refined ILAE classification system, coupled with advances in genetic and structural diagnostics, facilitates more accurate diagnoses and individualized treatment plans. This approach, outlined in the 2022 update, underscores the importance of a comprehensive, etiology-based understanding of pediatric epilepsy for both the clinicians and researchers [] (Table 2).

Table 2.
Epilepsy syndromes with onset in childhood [].
2.2. Febrile Infection-Related Epilepsy Syndrome (FIRES)
FIRES is a rare, devastating epileptic encephalopathy affecting previously healthy school-aged children. It manifests as explosive onset status epilepticus [], followed by chronic refractory epilepsy with cognitive decline []. First described in 1986 [], FIRES is characterized by fever preceding seizures [], focal seizures involving the perisylvian and frontotemporal regions, and severe cognitive and behavioral impairments []. Current treatments, including barbiturates and immunotherapy, show limited efficacy [].
2.3. Dravet and Lennox Gastaut Syndrome
DS is an early-onset treatment-resistant epilepsy. It typically presents during the first year of life with prolonged febrile and afebrile hemiclonic or generalized clonic seizures []. The syndrome affects males twice as often as females, causing epileptic encephalopathy. In 70% to 80% of patients, de novo mutations of the SCN1A gene encoding Nav1.1 were identified [].
In children between one and four years of age, additional seizure types develop, including myoclonic and atypical absences, focal seizures, and generalized tonic–clonic seizures. Over time, seizures develop to become less frequent and more severe in adolescence; however, fever sensitivity persists [].
In both the convulsive and nonconvulsive types, status epilepticus commonly occurs in patients with DS. This state may be life threatening, while its symptoms may be subtle and difficult to identify. The most common adult seizure type is generalized tonic–clonic. This may have a focal onset but it occurs mainly during sleep. Common treatments include valproic acid, clobazam, and a ketogenic diet []. LGS is considered to be an epileptic encephalopathy or a second network epilepsy. It is defined by a triad of drug-resistant seizure types. A typical EEG pattern shows bursts of slow spike–wave complexes or generalized paroxysmal fast activity and intellectual disability. The etiology of LGS varies among genetic, structural, or metabolic factors and is due to an unknown cause []. LGS is usually drug resistant, and complete seizure control is not possible. Beyond recurrent seizures, DS is associated with a high rate of premature mortality, affecting approximately a fifth of patients [].
Valproate, lamotrigine, and topiramate are considered first-line therapeutic drugs for reducing the frequency of seizures. Long-term outcomes are usually poor since the syndrome is associated with long-term adverse effects on intellectual development, independent living, and social functioning []. Multiple genetic animal models exist for studying DS, which aim to replicate SCN1A loss-of-function observed in DS []. Homozygous Scn1a knockout mice develop ataxia and die at 15 days postnatal, whereas heterozygous Scn1a-deficient mice show seizure activity and early mortality starting at 3 weeks of age [,]. The hybrid heterozygous Scn1a+/− mouse is among the most established. Created by crossing 129S heterozygous males with C57BL/6 wild-type females, these mice exhibit hallmark DS features, including spontaneous seizures, early mortality, and behavioral comorbidities, such as social deficits, anxiety, and cognitive impairments []. Their consistent phenotype makes them a standard preclinical model for testing therapies targeting both seizures and associated neuropsychiatric symptoms [,].
Neuropsychiatric Comorbidities
Individuals with this condition often face a wide range of debilitating comorbidities, including delayed psychomotor development, abnormal gait, hyperactivity, attentional difficulties, autism spectrum features, disrupted sleep, anxiety, depression, language delays, and profound cognitive impairments, all of which significantly diminish quality of life []. Current therapeutic approaches typically involve polytherapy with ASMs, such as valproate and clobazam []. Nevertheless, seizure control remains inadequate for many patients, and these treatments frequently lead to serious neurological and psychiatric side effects, including increased anxiety, depressive symptoms, and cognitive deficits such as memory loss [].
2.4. Pediatric Temporal Lobe Epilepsy
Pediatric temporal lobe epilepsy (TLE) represents a distinct clinical entity, differing significantly from adult-onset TLE in terms of semiology, neurodevelopmental implications, and outcomes []. The incidence of pediatric epilepsy ranges between 33 and 82 per 100,000 annually, with TLE accounting for approximately 8% of cases, often associated with focal seizures [].
In children under six, TLE’s semiology often lacks classical features observed in adults, such as automatisms and aura, complicating localization. Instead, generalized symptoms, like behavioral arrest and ictal motor manifestations, predominate, reflecting the immature central nervous system’s incomplete myelination []. Older children (6+ years old) show semiology closer to adults, including dystonic posturing and oroalimentary automatisms, aiding in lateralization [].
Hippocampal sclerosis, cortical dysplasia, and low-grade tumors are common etiologies of medically intractable TLE. Although antiepileptic drugs (AEDs) remain first-line treatments, intractable cases often require surgical intervention. Early surgical evaluations, leveraging MRI, EEG, and advanced imaging techniques, like PET and SISCOM, are crucial for optimizing outcomes []. Post-surgical seizure freedom rates are in the range of 58–91%, with cognitive benefits often observed, especially in younger children []. However, neurodevelopmental and psychiatric challenges, including memory impairments and behavioral disorders, frequently accompany pediatric TLE, necessitating comprehensive management approaches [].
Overall, pediatric TLE requires prompt diagnosis and tailored interventions to mitigate its impact on cognitive development and quality of life [].
2.5. Childhood Absence Epilepsy (CAE)
Poupart in 1705 first described childhood absence epilepsy (CAE) and Esquirol soon after the term “petit mal” was introduced []. In 1854, Delasiauve classified absences as a seizure type with lower severity []. Childhood absence seizures (ASs) are genetically determined, but the exact mode of inheritance of the involved genes has not been determined []. Typical ASs are classified by the ILAE among generalized nonmotor seizures []. ASs have a higher prevalence in females compared to males and typically develop in childhood between the ages of 4 and 10 years, although seizures with later onset have also been recorded []. Typical ASs persist for 6.6 years, disappearing between the ages of 10.5 and 14 years, with the age of onset and medication efficacy being strong determinants of seizure course, though the tendency for ASs to cease is present at all ages, not just at puberty []. ASs on EEG show generalized 2.5–4 Hz spike-and-wave discharges (SWDs), often triggered by hyperventilation and, less commonly, photic stimulation, with brief lapses of consciousness, eye closure, eyelid movements, and oral automatisms [,,]. Depending on the context of epilepsy, typical ASs may occur as the only seizure type or as generalized tonic–clonic seizures or myoclonic seizures. The presence of perioral myoclonia, single violent jerks, multiple spikes, or spikes coexisting with myoclonic jerks during the ictus of an AS indicates a worse prognosis []. ASs result from abnormal corticothalamic network activity, leading to synchronous SWDs and transient consciousness impairments [,]. While enhanced thalamic tonic inhibition via extrasynaptic GABAA receptors contributes to ASs [] recent studies highlight the critical role of cortical tonic inhibition [] and parvalbumin-expressing interneurons []. McCafferty et al. [] demonstrated that cortical and thalamic neurons show rhythmic but decreased firing during ASs.
Typical ASs of idiopathic generalized epilepsies consist of sudden, brief periods of a loss of consciousness, which are accompanied by synchronous, generalized spike-and-wave discharges (SWDs) in the EEG [,]. Similar ASs are exhibited by diverse genetic rat models, including the Genetic Absence Epilepsy Rats from Strasbourg (GAERS) [] and Wistar Albino Glaxo/Rij (WAG/Rij) rats [] and mice models, such as stargazer (STG) [] and GABAγ2(R43Q) []. SWDs originate from abnormal firing in thalamic and cortical networks, and GABAA inhibition is integral to their appearance [,]. Moreover, an important AS modulation occurs via the basal ganglia [], and changes in the firing of GABAergic nigral neurons modulate ASs both indirectly via the nigra/superior colliculus/thalamic projection [] and directly via the nigro-thalamic pathway []. Despite the progress made in advancing our understanding of the neuropathological mechanisms underlying AS [], there are still many unanswered questions, including the mechanism of action of the gold-standard anti-absence drugs, such as ethosuximide (ETX), valproate, and lamotrigine. Failure of monotherapy with gold-standard anti-absence drugs in >50% of childhood/juvenile absence epilepsy [], the high neuropsychiatric comorbidity rate even after seizure control [], and the risk of developing generalized seizures [] increase the demand novel therapeutic approaches.
Neuropsychiatric Comorbidities
ASs in children and teenagers were in the past considered relatively benign because of their nonconvulsive nature and high remittance rate in early adulthood. However, recent studies on large cohorts of drug-naïve CAE patients have now conclusively demonstrated that 30% of children with AS are pharmacoresistant [,], and 60% suffer from various neuropsychiatric comorbidities. These include anxiety, depression, attention-deficit/hyperactivity disorder (ADHD), and learning deficits that may precede epilepsy diagnosis, persist, and even be aggravated after full pharmacological control of the seizures (see [] and references within).
For instance, 61% of children with CAE had a psychiatric diagnosis, with 43% experiencing linguistic difficulties and 25% exhibiting subtle cognitive deficits. The severity of these comorbidities was associated with factors such as epilepsy duration, seizure frequency, and antiepileptic drug treatment. However, only 23% of the affected children were receiving interventions for these issues []. These findings underscore the importance of the early identification and management of neuropsychiatric comorbidities in children with CAE, highlighting the need for holistic treatments that address both seizures and associated behavioral and cognitive impairments [].
3. Cannabinoids
Cannabinoids are bioactive compounds that interact with the ECS, influencing various physiological processes. They are classified into three main types: phytocannabinoids, which are naturally derived from cannabis plants, such as Δ9-tetrahydrocannabinol (∆9-THC) and cannabidiol (CBD) []; synthetic cannabinoids, which are artificially produced to mimic or enhance cannabinoid effects, including nabilone and JWH-018 []; and endocannabinoids (eCBs), which are endogenous lipid-based neurotransmitters synthesized within the body, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG) []. These compounds play crucial roles in pain modulation, appetite regulation, and neuroprotection.
3.1. Phytocannabinoids
Over 120 natural cannabinoids have been found in Cannabis sativa. Seven of these compounds are classified as CBD-like compounds, including cannabidiol []. The two most relevant therapeutic and abundant components of the plant are ∆9-THC and CBD []. Cannabinoids share a heterocyclic terpene–phenolic structure. They readily cross the brain barrier and are very lipophilic. Hence, cannabinoids are distributed to lipid-based tissues, including neuronal cell membranes and brain parenchyma. Because lipids can be stored in lipid-rich tissues for weeks, they may be gradually released into the bloodstream over time []. Among non-psychoactive phytocannabinoids, the most well-known is CBD, which has demonstrated therapeutic potential for epilepsy, anxiety, and inflammation []. Other notable non-psychoactive cannabinoids include cannabigerol (CBG), which exhibits neuroprotective and anti-inflammatory properties; cannabichromene (CBC), known for its analgesic and anti-depressant effects; and cannabidiolic acid (CBDA), a precursor to CBD with potential anti-nausea benefits. Other cannabinoids with therapeutic potentials include cannabichromenic acid (CBCA) and cannabichromevarinic acid (CBCVA), as well as cannabinoid-like compounds from non-cannabis plants [,]. Unlike ∆9-THC, these compounds exhibit a low affinity for CB1 receptors, reducing their psychoactive potential while retaining their medicinal properties [].
3.2. Cannabidiol (CBD)
Growing evidence highlights the multitarget effects of CBD in the human body. CBD modulates the ECS through both direct and indirect CB1R interactions. Although it has low affinity for CB1R, it antagonizes Δ9-THC and AEA, enhances eCB tone by inhibiting AEA breakdown and transport, and increases 2-AG levels. It may also act as a negative allosteric modulator (PAN) at a distinct CB1R site.
CBD also affects non-CB pathways, modulating inflammation, cell proliferation, and various ion channels (5-HT1AR, adenosine A1R, CaV, PPARγ, GABAAR, and TRP channels). Recent studies have identified potential targets that may explain CBD’s effects on nervous system hyperexcitability. However, further pharmacological research is needed to pinpoint the precise targets and underlying mechanisms [,,,,,,,]. Given the ECS’s role in brain development, CBD may produce beneficial or harmful effects in infants depending on the dose and context. Its actions on other targets at low concentrations make its full mechanism unclear (Figure 1).
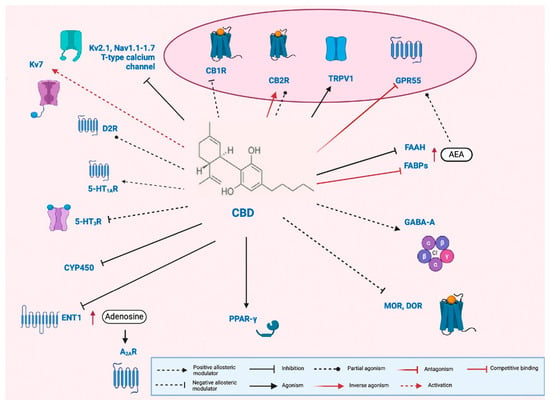
Figure 1.
Pleiotropic mechanism of cannabidiol (CBD). Cannabidiol (CBD) acts as a negative allosteric modulator at type 1 cannabinoid receptors (CB1Rs) [], as well as at μ- and δ-opioid receptors (MOR and DOR, respectively) [], and serotonin 5-HT3 receptors (5-HT3R) []. At type 2 cannabinoid receptors (CB2Rs), CBD has been reported to function both as a partial agonist [] and an inverse agonist []. Additionally, CBD serves as an agonist at transient receptor potential vanilloid 1 (TRPV1) channels [] and peroxisome proliferator-activated receptor gamma (PPAR-γ) [], and as a partial agonist at dopamine D2 receptors (D2Rs) []. It also acts as a positive allosteric modulator at serotonin 5-HT1A receptors (5-HT1ARs) [] and GABAA receptors []. Furthermore, CBD antagonizes the G-protein-coupled receptor 55 (GPR55) []. It may elevate anandamide (AEA) levels by inhibiting fatty acid amide hydrolase (FAAH) [] and competing with AEA for binding to fatty acid binding proteins (FABPs) [], thereby indirectly enhancing the activation of CB1R, CB2R, TRPV1, and GPR55. CBD also inhibits the equilibrative nucleoside transporter 1 (ENT1) [], leading to increased extracellular adenosine and subsequent indirect activation of adenosine A2A receptors (A2ARs). In addition to its receptor-level actions, CBD modulates ion channels by activating voltage-gated potassium channel Kv7 [], while inhibiting Kv2.1, several voltage-gated sodium channels (Nav1.1 to Nav1.7), and neuronal T-type calcium channels []. Finally, in hepatic metabolism, CBD inhibits multiple cytochrome P450 (CYP450) isoforms, raising the potential for drug–drug interactions []. Figure modified from [].
3.3. Tetrahydrocannabinol (∆9-THC)
∆9-THC has two chiral centers in a tricyclic 21-carbon structure in the trans-configuration [,]. ∆9-THC has low bioavailability (6–10%), which may be variable due to extensive first-pass metabolism and gastric degradation [].
Through the oxidation of ∆9-THC, the active metabolite 11-hydroxy-THC is formed by cytochrome P450, 2C9, 2C19, and 3A4 []. 11-OH-D9-THC is further oxidized to the inactive THC COOH []. THC acts as a partial agonist of CB1Rs in the central nervous system (CNS) [] and CB2Rs in the immune system []. Hence, THC works similarly to eCBs naturally produced by the brain. It has psychotropic effects that involve behavior, cognitive ability, and stability. This effect may be avoided by pretreatment with a recombinant CB1R antagonist [].
3.4. Endocannabinoids
The targets of ∆9-THC and CBD include the ECS but also other systems []. The ECS includes two main cannabinoid receptors, CB1/CB2 [], and endogenous ligands, eCBs, and the enzymes responsible for their synthesis and degradation []. The ECS is elaborate and complex; CBRs and other targets respond to endogenous eCBs and to exogenous substances produced by the cannabis plant []. The ECS may be activated by three types of ligands. These can be either eCBs produced within the organism or phytocannabinoids produced by the cannabis plant or synthetic cannabinoids if synthesized in the laboratory []. 2-arachidonoylglycerol (2-AG) [] and N-arachidonolethanolamide (AEA, anandamide) [] are both endogenous ligands that activate CB1R and CB2R. 2-AG is found centrally and peripherally in the hippocampus, brainstem, striatum, and medulla. AEA is found at high concentrations in the brain, mainly in the striatum, hippocampus, and brainstem, and to a lesser extent in the cerebral cortex and cerebellum. To synthesize 2-AG, the enzyme diacylglycerol lipase (DAGL) must catalyze a reaction involving diacylglycerol. Alternatively, 2-AG can be broken down by the substance fatty acid amide hydrolase (FAAH) or by monoacylglycerol lipase (MAGL) into arachidonic acid and glycerol. The synthesis of AEA requires a hydrolysis reaction and phospholipase D [].
4. The Endocannabinoid System in Relation to Pediatric Epilepsy
The ECS can modify excitatory and inhibitory synaptic transmission within the nervous system. eCBs are known to be “produced on demand”, meaning they are produced in response to physiological needs [,]. Recently, however, it has also been shown that 2-AG is “released on demand” and accumulates within microvesicles []. Hence, eCBs mediate the depolarized-induced suppression of excitation (DSE) or inhibition (DSI) [,]. The severity of seizure has been shown to correlate with eCB concentrations in the brain [,]. Disruption of ECS signaling is believed to contribute to epileptogenesis; however, it remains unclear whether the resulting molecular changes drive proepileptogenic processes, seizures, or reactive adaptations [] (Figure 2).
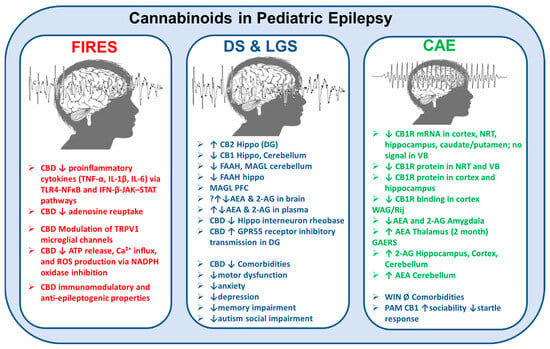
Figure 2.
Cannabinoid system alterations in and effects on pediatric epilepsy syndromes. This schematic illustrates the pathophysiological changes in the endocannabinoid system (ECS) and the modulatory actions of cannabidiol (CBD) and other cannabinoids across three major pediatric epilepsy syndromes: febrile infection-related epilepsy syndrome (FIRES), Dravet syndrome (DS), and Lennox–Gastaut syndrome (LGS), and childhood absence epilepsy (CAE). FIRES (left panel): CBD reduces the release of proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) via TLR4–NFκB and IFN-β–JAK–STAT signaling pathways, inhibits adenosine reuptake, modulates TRPV1 channels on microglia, and suppresses ATP release, Ca2+ influx, and reactive oxygen species (ROS) production through NADPH oxidase inhibition. These mechanisms underlie CBD’s anti-inflammatory, immunomodulatory, and antiepileptogenic properties. DS and LGS (center panel): Observed alterations include the upregulation of CB2 receptors in the hippocampus and CB1 receptors in the hippocampus and cerebellum, along with a decreased expression of FAAH and MAGL enzymes in various brain regions. CBD reduces hippocampal interneuron rheobase and enhances inhibitory transmission via GPR55 receptors in the dentate gyrus. Clinically, CBD ameliorates associated comorbidities, such as motor dysfunction, anxiety, depression, memory impairment, and autism-related social deficits. CAE (right panel): There is a significant downregulation of CB1R mRNA and protein in the cortex, hippocampus, and thalamic regions (nucleus reticularis thalami [NRT] and ventrobasal complex [VB]). In Wistar Albino Glaxo/Rijswijk (WAG/Rij) rats and Genetic Absence Epilepsy Rats from Strasbourg (GAERS), two well-established rodent models of CAE, region-specific alterations in endocannabinoid levels have been reported. These include increased anandamide (AEA) concentrations in the thalamus and cerebellum, and elevated levels of 2-arachidonoylglycerol (2-AG) in the hippocampus, cortex, and cerebellum. While the synthetic CB1 receptor agonist WIN 55,212-2 has shown no significant effects on associated neuropsychiatric comorbidities, positive allosteric modulators (PAMs) of CB1 receptors have been demonstrated to enhance sociability and reduce exaggerated startle responses (see main text for references). The symbol ↓ indicates a decrease in expression, concentration, or function; ↑ indicates an increase in expression, concentration, or function; ↔ denotes no significant change or an unclear direction; and Ø represents the absence or lack of effect.
4.1. The Endocannabinoid System and Cannabinoids in Febrile Infection-Related Epilepsy Syndrome (FIRES)
Evidence for ECS dysfunction in FIRES remains limited. However, CBD has demonstrated efficacy as an adjunctive treatment during both the acute and chronic stages of the disease, acting as an immune modulator and an antiepileptogenic agent [,]. Despite these findings, CBD is not currently included in the treatments in the acute phase of FIRES [], and therefore, it is not recommended as a first-line treatment. A recent case report [] described a young patient with acute-phase FIRES, refractory to standard treatments, who achieved complete seizure control and successful recovery with a combination of vagal nerve stimulation (VNS) and CBD, suggesting that CBD may serve as a potential adjunctive therapy also to VNS.
The role of the ECS and its receptors in mediating the effects of CBD on FIRES has not yet been elucidated. However, CBD’s modulatory effects on neuroexcitability are linked to its ability to target several key molecules involved in the pathogenesis of FIRES and its outcomes. CBD reduced microglia-mediated neuroinflammation by suppressing proinflammatory cytokines and chemokines, including TNF-α, IL-1β, and IL-6, through the inhibition of the TLR4-NFκB and IFN-β-JAK–STAT pathways [,]. In addition, its anti-inflammatory effects are linked to the inhibition of adenosine reuptake, which contributes to decreased neuronal excitability [,].
Additionally, CBD activates and subsequently desensitizes microglial TRPV1 channels, thereby mitigating sustained neuroinflammation [,]. It also reduces ATP release, intracellular calcium influx, and the production of reactive oxygen species (ROS) through the inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity [] (Figure 1).
4.2. The Endocannabinoid System and Cannabinoids in Dravet Syndrome (DS) and Lennox–Gastaut Syndrome (LGS)
4.2.1. Clinical Evidence of Cannabinoid Use in DS and LGS
CBD medication, Epidiolex®, has been approved for patients aged 1 year and older with DS or LGS and TSC, in conjunction with clobazam []. CBD approval was based on six randomized controlled trials (RCTs) demonstrating a 40 to 50% reduction in seizure frequency among Epidiolex-treated patients [,,,,]. A recent retrospective multicenter chart review in Germany observed a reduction in seizure frequency and sustained treatment retention for up to 12 months across different age groups, among patients with severe, treatment-refractory LGS or DS receiving adjunctive CBD and clobazam simultaneously []. Several meta-analyses have been published on CBD use in DRE among the adult [] and pediatric population [,,]. Adding CBD benefits most children with pharmacoresistant epilepsy. While 20 mg/kg/day offers better seizure control than 10 mg/kg/day, the lower dose remains effective and a viable treatment option [,,]. Common adverse effects of CBD include drowsiness, lethargy, diarrhea, loss of appetite, and weight loss [,]. DS is a channelopathy mainly linked to SCN1A and SCN1B mutations, with additional contributions from potassium and calcium channel gene variants. These disrupt excitatory/inhibitory balance through altered channel function []. Thus, the modulation of these channels may underlie CBD’s therapeutic mechanism, although current support comes primarily from preclinical studies []. CBD may also exert therapeutic effects in DS and LGS by modulating dysregulated components of the ECS, although ECS alterations in these conditions remain poorly characterized. For instance, Rubio et al. [] reported an increased expression of CB2Rs in lymphocytes derived from DS patients, potentially indicating a compensatory or pathological upregulation in peripheral immune cells. Notably, no significant changes were detected in plasma levels of key endocannabinoid, such as AEA or 2-AG, suggesting that peripheral eCB levels may not accurately represent central ECS dysfunction in these syndromes [].
4.2.2. Animal Evidence of Cannabinoid Use in DS and LGS
CBD has been shown to attenuate hyperthermia-induced seizures in Scn1a+/− mice, which closely mirrors the clinical features of DS [,], and reduce the frequency and severity of spontaneous seizures []. CBD’s anticonvulsant effects were initially thought to stem from pharmacokinetic and pharmacodynamic interactions with clobazam []. However, the preclinical evidence suggests that CBD’s efficacy goes beyond elevated clobazam levels, likely involving the positive modulation of GABAARs and multimodal engagement of anticonvulsant pathways in DS [].
Several other cannabinoids have shown efficacy against thermal-induced seizures in Scn1a+/− mice. Treatment with phytocannabinoids, such as CBCA and CBCVA [] CBGA, CBDVA, and CBGA [], as well as the cannabinoid-like compound magnolol from non-cannabis plant Magnolia officinalis [], reduced spontaneous seizures and improved survival in the Scn1a+/− mouse model of DS.
Impairments in the ECS have been proved in the same Scn1a+/− mouse model [,]. Anderson et al. (2022) [] identified Cnr1, the gene encoding CB1R, as a genetic modifier of epilepsy in Scn1a+/− mice, with a deficiency of eCBs serving the pathological background. Reduced hippocampal CB1R expression was observed in this model, while cortical CB1 levels and brain concentrations of major endocannabinoids (AEA and 2-AG) remained unchanged (Anderson et al., 2022). However, lesser-studied monoacylglycerols, such as 2-linoleoylglycerol (2-LG) and 1-linoleoylglycerol (1-LG), were significantly elevated following hyperthermia-induced seizures in Scn1a+/− mice []. The delayed timing of sampling (5 min post-seizure) in these studies [,] may have limited the detection of transient changes in eCB levels. For instance, in vivo two-photon imaging demonstrated significant increases in 2-AG concentrations in the hippocampal CA1 region contextually to electrically induced seizures, while AEA levels remained unchanged []. In support of a deficiency of ECS in DS, the positive allosteric modulation (PAM) of CB1 receptor GAT229 and enhancement of brain 2-AG concentrations using ABX-1431, a MAGL inhibitor, both produced significant anticonvulsant effects against hyperthermia-induced seizures in Scn1a+/− mice [].
A novel DS model involving heterozygous, conditional, knock-in mice with a missense mutation (A1783V) in the SCN1A gene, expressed exclusively in CNS neurons (Syn-Cre/SCN1AWT/A1783V), revealed elevated CB2R levels in the hippocampus, particularly in the dentate gyrus []. This upregulation may represent an endogenous protective response aimed at reducing neuroinflammation in DS. Additionally, this model exhibited other alterations in the ECS, including the downregulation of CB1Rs in the cerebellum and hippocampus, changes that may reflect impaired synaptic function and correlate with the motor and memory deficits observed in these mice. Notably, reductions in eCB-inactivating enzymes were detected in specific brain regions: both MAGL and FAAH in the cerebellum, MAGL alone in the prefrontal cortex, and FAAH alone in the hippocampus. These enzymatic changes suggest region-specific elevations in endocannabinoid levels, particularly 2-AG, given the prominent downregulation of MAGL []. These findings underscore the potential of targeting the ECS for next-generation anticonvulsant therapies. However, the therapeutic utility of MAGL inhibition may be constrained by its narrow therapeutic range, as subchronic treatment with high doses of ABX-1431 exacerbated spontaneous seizures. This highlights the need for dose optimization and further investigation into the long-term safety and efficacy of this approach [].
Animal studies on LGS are instead limited, but a promising model has recently emerged: the Gabrb3+/D120N mouse, a heterozygous knock-in for the β3 subunit of the GABAAR []. Acute administration of magnolol significantly reduced the number and duration of atypical ASs in the Gabrb3+/D120N mouse model, consistent with an antiseizure effect []. In vitro, magnolol inhibited all T-type calcium channel subtypes, similar to other phytocannabinoids [], but showed no activation of CB1 or CB2Rs [].
In conclusion, cannabinoid efficacy in DS extends beyond interactions with clobazam, involving the direct modulation of GABAARs and multiple anticonvulsant mechanisms. Additionally, PAM and elevating eCBs show antiseizure effects through CB1 and CB2R modulation and T-type calcium channel inhibition, underscoring the therapeutic potential of targeting the broader endocannabinoid system.
4.2.3. Cannabinoid Use for Neuropsychiatric Comorbidities in DS and LGS
No direct data are available on the involvement of alterations in ECS in epilepsy-associated comorbidities. However, indirect evidence comes from studies investigating the long-term effects (followed for up to 2 years) of CBD treatment in patients with DRE, where CBD improved mood [,] without impairing cognition []. Furthermore, in Scn1a−/− and Scn1a+/− mouse models of DS, CBD administered at anticonvulsant doses improved their welfare by reducing pain and enhancing general health [,]. In heterozygous mice, it also alleviated several complex behavioral comorbidities, including motor dysfunction, anxiety-like behavior (assessed in the elevated plus maze), and depression-like behavior (evaluated with the sucrose preference test) []. Furthermore, CBD improved spatial learning and memory in the eight-arm radial maze [] as well as deficits in autism-like social interactions [,].
CBD exerts dual actions in the hippocampus of DS mice, contributing to the restoration of excitatory–inhibitory balance. First, it lowers the rheobase of interneurons, thereby enhancing their excitability and addressing deficits in GABAergic firing [,]. Second, CBD acts through the GPR55 receptor to enhance inhibitory synaptic transmission onto dentate gyrus granule cells (DGCs), leading to a reduction in their spontaneous firing. Additionally, CBD directly reduces DGC excitability, which may underlie its capacity to decrease both seizure frequency and severity [].
These findings underscore the complex role of the ECS system in DS and LGS, highlighting its potential as a therapeutic target. With a favorable side-effect profile, emerging evidence supports CBD’s efficacy in treatment-resistant epilepsies and their associated comorbidities. However, further research is required to better understand the underlying mechanisms and the broader therapeutic implications (Figure 1).
4.3. The Endocannabinoid System in Other Refractory Pediatric Epilepsies
Several developmental and encephalopathies beyond DS and LGS exhibit DRE, including TSC, Infantile Spasms and Epileptic Spasms, CDKL5 Deficiency and Aicardi, Doose Syndrome, Dup15q Syndrome, and Sturge–Weber Syndrome []. These conditions have been treated with pharmaceutical-grade CBD, with reports of reduced seizure severity []. A recent systematic review of preliminary open-label studies [] indicated that purified CBD may be effective in managing otherwise refractory childhood epilepsies. Among these, the most robust evidence supports CBD’s efficacy in TSC, where it has demonstrated seizure reduction as both monotherapy [] and as adjunctive therapy with clobazam [], leading to its FDA approval for use in TSC, alongside DS and LGS. However, conventional RCTs often face limitations in rare epilepsy syndromes due to low patient numbers and heterogeneity. As such, novel and adaptive clinical trial designs should be considered to rigorously evaluate the efficacy and safety of CBD in these intractable pediatric epilepsies [].
4.4. The Endocannabinoid System and Cannabinoids in Pediatric Temporal Lobe Epilepsy
Research specifically focusing on the ECS in pediatric TLE remains limited, with no direct data currently available in the pediatric population. Despite this, studies on TLE animal models offer valuable insights into the potential role of the ECS in seizure modulation and the pathophysiology of TLE, which may inform future research in pediatric cases []. The effects of CBD have not been investigated in patients with TLE yet, although in vitro evidence from human samples suggests that high concentrations of CBD interacts with 5-HT1A receptors, acting as an inverse agonist []. Recent findings show altered receptor-expression hippocampal and cortical microvasculature, CB1, and CB2Rs may offer a potential therapeutic target to preserve blood–brain barrier integrity in drug-resistant mesial temporal lobe epilepsy (DR-MTLE) patients []. In DR-MTLE patients, CB1R-induced G-protein signaling microvasculature is increased, along with higher levels of ANA, and reduced levels of 2-AG. These changes are more pronounced in patients without mood disorders, suggesting that altered eCB levels in the hippocampus and temporal neocortex may contribute to MTLE pathophysiology. This supports the idea that increased eCB activity could explain the absence of mood disorders in some MTLE patients, highlighting the complex interplay between the ECS and neurological and psychological aspects [].
On the other hand, drug-naive TLE patients show increased glutamatergic activity [] and reduced AEA levels in the cerebrospinal fluid (CSF), while 2-AG levels remain unchanged []. Reduced CB1R mRNA and protein expression have been detected in the hippocampus of pharmacoresistant TLE patients []. However, PET imaging reveals increased CB1R availability in the temporal lobe ipsilateral to the epileptic focus in MTLE patients [], suggesting enhanced CB1R binding despite low mRNA and protein expression. This indicates increased CB1R-induced neurotransmission with potential inhibitory effects in epilepsy, though severe hippocampal damage in pharmacoresistant MTLE suggests hypoactive eCB signaling []. Hypoactivity of the ECS is linked to anxiety and depression [], and as pharmacoresistant MTLE often coexists with these conditions [], hypoactivity may contribute to their comorbidity, though direct evidence is lacking.
Another comorbidity of MTLE is memory impairment []; the modulation of hippocampal synaptic plasticity through ECS targeting provides additional therapeutic insights. Notably, the inhibition of FAAH by URB597 suppresses maximal dentate after-discharges [], restores seizure-induced impairments in short- and long-term plasticity, and avoids the detrimental memory effects associated with synthetic cannabinoid receptor agonists []. Moreover, AEA signaling augmentation restores the phasic control of eCB signaling over GABAergic activity and plasticity in the basolateral amygdala (BLA), mitigating seizure-induced alterations in fear memory []. This evidence supports the selective enhancement of eCB tone over broad CB1R activation as a promising approach for managing seizures and cognitive impairments in epilepsy. Moreover, interactions between cannabinoid and serotonin systems, as evidenced in TLE models, reveal synergistic neuroprotective effects, further advancing our understanding of their potential [,] (Colangeli et al., 2019). Together, these findings highlight the intricate interplay of cannabinoids, eCBs, and serotonin in shaping neuronal excitability and therapeutic strategies for epilepsy and related disorders [].
4.5. The Endocannabinoid System in Childhood Absence Epilepsy (CAE)
4.5.1. Clinical Evidence of Cannabinoid Use in CAE
As far as CAE is concerned, the evidence regarding the involvement of the ECS in human ASs remains conflicting. However, a recent prospective pilot study [] demonstrated the effect of pharmaceutical-grade CBD on a small cohort of 14 patients with typical CAE. CBD treatment (8–20 mg/kg/day) administered over 90 days appeared ineffective for typical absence seizures and may have even exacerbate them, as several patients exhibited a clinically significant increase in SWDs. Despite this, CBD was generally well tolerated, with only mild side effects reported. However, an anti-absence effect of CBD was observed in 5 out of the 14 (35%) patients enrolled, with 3 patients receiving CBD in combination with ETX and 2 patients on CBD monotherapy []. This anti-absence effect was also reported in the pooled analysis of the CBD Expanded Access Program (EAP), which provided treatment to 892 patients with DRE for up to 33 months [], as well as in 4 out of 5 patients enrolled in a separate EAP conducted in Massachusetts involving 50 patients treated for up to 60 months []. However, definitive conclusions from these EAP studies cannot be drawn, as they did not distinguish between typical and atypical CAE, and changes in seizure frequency were not confirmed by EEG monitoring [].
Two clinical trials (ClinicalTrials.gov Identifiers: NCT03355300 and NCT03336242) were initiated in 2017 to evaluate the antiepileptic efficacy of a CBD oral solution in pediatric patients with treatment-resistant childhood absence seizures. However, both trials were terminated before completion, and no conclusive results were obtained []. From the limited information available, it appears that an effect of CBD was observed only at low concentrations (approximately 20 mg/kg/day) in the small cohort of patients enrolled []. No studies have specifically investigated the effect of ∆9-THC on absence seizures (ASs), except for a small prospective study on childhood epilepsy that included three patients with idiopathic generalized epilepsy treated with oral cannabis extracts containing both CBD and ∆9-THC []. The study yielded inconclusive results regarding whether the combination of ∆9-THC and CBD offers better seizure control, or at what ratio, compared to CBD alone, but it highlighted that the risk–benefit profile may be less favorable than that of pharmaceutical-grade CBD []. The major outcomes are summarized in Table 3.

Table 3.
Summary of the preclinical pharmacological evidence on the effects of cannabinoids in animal models of absence epilepsy.
4.5.2. Animal Evidence of Cannabinoid Use in CAE
Although the experimental evidence remains inconsistent, more data are available on the role of cannabinoids in ASs from animal experimental models of the disease compared to other pediatric epilepsies (Table 3). One of the earliest investigations on ∆9-THC and CBD SWDs photically evoked showed a lack of effect by CBD and conversely pro-absence activity by ∆9-THC []. Of note, by analyzing cannabinoids in other animal models of epilepsy, it was proposed that cannabinoids were useful against convulsive seizures but not CAE []. For the last few decades, the cannabis research has been hindered by law restrictions and its criminalization in the USA Controlled Substances Act of 1970 [] determining that cannabis therapeutic potentials were forgotten until recently. In 2010, a study on epileptic WAG/Rij rats [] showed a complex biphasic modulation of SWDs by a synthetic CB1/2R agonist, WIN 55,212-2 []. The authors showed that the acute general administration of high doses (3–12 mg/kg, s.c.) of cannabinoids biphasically modulated SWDs. The doses of 6 and 12 mg/kg decreased the incidence of SWDs in the first 2 and 3 h, respectively, but produced an elongation of the seizures after 3 h, with single SWDs reaching up to 100 s or more at the 6 h mark. The CB1R antagonist/inverse agonist AM251 was ineffective at 6 and 12 mg/kg, but the latter dose blocked the early inhibition in SWDs, the late increase in mean SWD duration, and produced at the 4th and 5th hours an increase in the incidence of SWDs. These data are puzzling, especially since incredibly high doses of cannabinoids were used. WIN 55,212-2 loses affinity for CB1Rs at high doses, and an inhibition of motor activity was shown, which might have caused the early decrease in SWDs. A 12 mg/kg dose of WIN 55,212-2 completely suppressed SWDs in a WAG/Rij rat that developed generalized convulsive seizures after cannabinoid treatment [], suggesting that CB1R activation may facilitate the transition from nonconvulsive to generalized convulsive seizures.
Nevertheless, more recent investigations did not replicate the anti-absence effect of WIN 55,212-2 [,]. Indeed, both acute and subchronic administrations of 6 mg/kg WIN 55,212-2 did not modify the incidence of SWDs, but similarly to the previous study [] elongated the duration of the SWDs, likely by inhibiting NRT neurons known to be involved in the stopping mechanism of SWDs [].
Sysoeva et al. (2023) explored the effect of cannabinoids on absence epileptic networks, revealing that WIN55,212-2 modulated the network strength in the frontal and hippocampal areas. It decreased SWD frequency but increased its duration, indicating a complex interaction with epileptic networks and neuronal coupling [].
Recently, Howland and colleagues reported a contrasting effect of agonist PAMs (ago-PAMs) for CB1Rs [,] and the phytocannabinoids ∆9-THC and CBD [] in GAERS. Systemic administrations of ago-PAMs GAT229 and GAT211 reduced SWD incidence and duration by 50% and 40%, respectively [], while the more powerful ago-PAM GAT591 and GAT593 only decreased the total time spent in seizures by 36% and 34%, respectively, without affecting SWD incidence, average duration, or oscillatory frequency []. The anti-absence effects of CB1R PAMs appear CB1R-dependent, as SR141716A, a CB1R antagonist/inverse agonist, blocked GAT211’s effect []. In contrast, purified ∆9-THC (3–10 mg/kg) exerted a pro-absence effect on male and female GAERS, increasing SWD incidence, duration, and total seizure time, while reducing SWD frequency []. Conversely, purified CBD (30–100 mg/kg) did not alter SWD incidence, but reduced seizure duration by shortening the average SWD length. Inhalation of smoke from a high-∆9-THC cannabis strain increased the total and average SWD duration, while leaving the frequency unchanged. These effects were likely not CB1R-dependent, as they were not blocked by SR141716A. On the other hand, exposure to smoke from a CBD-rich cannabis strain had no effect on any SWDs, likely because the CBD plasma concentrations did not reach effective levels [] (Table 3).
4.5.3. Cannabinoids Infusion in Brain Areas in CAE Animal Models
In WAG/Rij rats, both intracerebroventricular (i.c.v.) and intraperitoneal (i.p.) administrations of AEA and its analog N-palmitoylethanolamine (PEA) reduced SWD incidence and duration. PEA’s effects were blocked by pre-treatment with SR141716 and the PPAR-α antagonist GW6471, whereas AEA’s i.c.v. anti-absence effects were blocked only by SR141716A []. Further studies showed that AEA and the CB1R agonist WIN55,212-2, when injected into the thalamic nuclei (NRT, VB), the somatosensory cortex (S1po), or administered i.c.v., also suppressed SWDs. Interestingly, SR141716A alone did not induce a significant pro-absence effect [].
In GAERS, the infusion of the ago-PAM GAT229 into the motor cortex, a region not directly involved in SWD generation, reduced SWD incidence, average duration, and total duration; this effect was prevented by systemic SR141716A, which alone had no effect [] (Table 3).
4.5.4. Cannabinoid Concentration in Brain Areas in CAE Animal Models
eCB concentrations in specific brain regions were assessed in 2- and 6-month-old WAG/Rij rats, with ACE rats and normal Wistars as controls []. In 6-month-old WAG/Rij rats, the amygdala showed reduced levels of AEA, 2-AG, and PEA, while the cortex exhibited higher levels of PEA, and no significant changes were detected in the thalamus compared to ACE rats []. In contrast, adult, symptomatic GAERS exhibited increased 2-AG levels in the cortex, hippocampus, and cerebellum, but not in the thalamus, and elevated AEA only in the cerebellum [].
4.5.5. Cannabinoid Receptor Expression in CAE Animal Models
In situ hybridization in age-matched WAG/Rij and ACI rats revealed reduced CB1R mRNA expression in the cortex, NRT, hippocampus, and caudate/putamen of WAG/Rij rats, with no detectable signal in the VB []. However, Western blot (WB) analysis indicated decreased CB1R protein levels only in the NRT and VB []. In GAERS, CB1R expression was examined in the cortex, thalamus, hippocampus, and cerebellum, showing reduced protein levels in the cortex and hippocampus []. This cortical downregulation was further confirmed by [3H]SR141716A binding analysis via liquid scintillation spectrometry [].
4.5.6. Modulators of Cannabinoid Synthesis and Breakdown in CAE Animal Models
The two major eCBs, AEA and 2-AG, are both synthesized postsynaptically but act on presynaptic CB1Rs to inhibit neurotransmitter release []. Given the adverse effects observed with direct-acting CB1R agonists and antagonists in preclinical rodent models of seizures, several studies have investigated whether enhancing endogenous cannabinoid tone might reduce seizure occurrence and severity []. This type of pharmacological intervention has not yet been investigated in CAE; however, our preliminary studies using PF-04457845, a novel FAAH inhibitor [], have shown a promising anti-absence effect in GAERS (unpublished observations).
4.5.7. Cannabinoids and Neuropsychiatric Comorbidities in CAE Animal Models
Alterations in the ECS have been implicated in anxiety and mood disorders, and the modulation of ECS components may provide therapeutic benefits []. Therefore, the neuropsychiatric comorbidities observed in CAE [] may be responsive to cannabinoid modulators. Nevertheless, studies on GAERS may have limited the ability to provide further insights, as recent evidence shows that NEC rats exhibit higher anxiety-like behavior and neophobia compared to GAERS [,,], contrary to previous assumptions []. Additionally, the effects of cannabinoid receptor agonists, such as WIN 55,212-2, appear to be strain-dependent: this compound reduces anxiety in NEC rats but not in GAERS, where it instead produces sedative effects []. These behavioral outcomes were paralleled by monoaminergic alterations. GAERS exhibited lower baseline 5-HT levels in the hippocampus and substantia nigra, as well as reduced NA levels in the entopeduncular nucleus, potentially contributing to their hypermotility and reduced anxiety phenotype. Moreover, ECS activation modulated monoamine levels, further implicating ECS involvement in the observed behavioral differences []. However, other evidence suggests that the effect of cannabinoid treatment may not be uniform and could be dependent on the behavioral test used. For example, WIN 55,212-2 induced hyperlocomotion in GAERS and did not improve anxiety in the elevated plus maze in NEC rats and GAERS [] both of which exhibited comparable levels of anxiety-like behavior [,]. Consistently, Roebuck et al. [] also found that CB1R PAM GAT211, while ineffective on anxiety-like behavior in elevated plus-maze and open-field tests or locomotory activity, showed increase sociability and reduced the elevated startle response, particularly in female GAERS [].
Together, these findings suggest that the GAERS model does not fully replicate the anxiety observed in CAE, and cannabinoids do not significantly alter the emotional state of GAERS. This limits the ability to draw definitive conclusions about the efficacy of cannabinoids in treating comorbid anxiety in children with AS. While the anxiolytic effects of cannabinoids have been consistently observed in normal rats [], the apparent unresponsiveness in GAERS may be due to impairments in ECS signaling, complicating interpretation. However, the observed effects on sociability and startle response in female GAERS [] are intriguing, suggesting a potential therapeutic role for cannabinoids in addressing other neuropsychiatric comorbidities. These findings highlight the importance of investigating sex differences in ECS modulation. Further studies are needed to clarify the mechanisms and therapeutic windows of ECS-targeted interventions for both seizures and associated psychiatric symptoms. Critically, the development or selection of more appropriate animal models that better reflect both the seizure phenotype and comorbidities is essential.
Comparing GAERS to WAG/Rij rats reveals a different form of cannabinoid control of SWDs [,,], both in terms of their generation and termination, further increasing the number of differences already described between these two epileptic strains [].
CB1Rs at GABAergic synapses act as strategically placed control points for the activity-dependent regulation of dynamically changing normal and pathological oscillatory network activity []. The proepileptic effects of CB1Rs may also involve other brain regions, such as the output structures of the basal ganglia, particularly the substantia nigra pars reticulata (SNr), as shown in a study on normal mice []. CB1R activation induced thalamocortical high-voltage spindles (HVSs) (putative SWDs) by selectively increasing the activity in the nigro-thalamic pathway []. This may lead to increased GABA release in the thalamus and enhanced GABAAR receptor-mediated tonic inhibition, contributing to ASs [], highlighting a potential risk of CAE induced by cannabis abuse (Figure 3).
Both NRT and TC VB neurons can synthesize and release 2-AG; moderate DAGLα expression in the NRT and VB of adult Wistar rats was observed []. Nevertheless, a synapse target-dependent effect was observed in the thalamus, as DSI occurs only at intra-NRT synapses, not to NRT to VB []. This 2-AG-mediated DSI may increase NRT neuronal discharges, reducing burst firing due to membrane depolarization [], or Ca2+ influx during a low-threshold spike (LTS) may elevate intracellular 2-AG via DAGLα, depressing LTS burst spikes itself []. Summing up, eCBs modulate synaptic strength at specific inhibitory thalamic pathways, dynamically influencing thalamic–cortical synchrony. NRT neurons inhibit either VB neurons, promoting rhythmic oscillations, or neighboring TRN neurons, limiting synchrony. Therefore, the emergence of ASs may result from reduced eCB signaling in the NRT, supported by a reduction of both CB1R mRNA and protein expression [] (but not in GAERS, in which no thalamic ECS alterations were detected []), which weakens intra-TRN inhibition and enhances thalamic synchrony []. The long-lasting increase in long SWDs observed after acute and subchronic WIN administrations in adult WAG/Rij rats is likely driven by CB1R activation and a consequent reduction in GABA availability within the NRT []. Another brain region implicated in the ictogenesis of ASs is the peri-oral region of the somatosensory cortex (poS1) [,], where the dysregulation of the ECS has been observed. In GAERS, reduced CB1R levels in the cortex likely create a permissive environment for increased excitability and seizure propagation [], whereas no changes in CB1R mRNA expression were detected in WAG/Rij rats []. These ECS alterations may contribute to seizure onset during development and their exacerbation during adolescence []. The hippocampus (HPC) shows decreased CB1R protein [] and mRNA expression levels [], whereas 2-AG levels are elevated in GAERS compared to the controls []. These findings are relevant considering the recent evidence of HPC involvement in CAE and the marked decrease in HPC frontal-cortex unidirectional coupling exclusively during SWDs, which was reversed by WIN55,212-2 treatment that effectively reduced ASs []. These changes in the ECS in the HPC may help explain the cognitive and behavioral alterations seen in GAERS [,,,,,] and patients with CAE [].
The effects of CB1R-dependent activation, whether through agonists or ago-PAMs, or CB1R-independent effects mediated by CBD administration on modulating SWDs remain unclear. The pro-absence effects observed after acute ∆9-THC exposure in GAERS [], and following both acute and subchronic administrations of WIN55,212-2 in WAG/Rij rats [] limit the therapeutic potential of CB1R orthosteric agonists. In contrast, CB1R ago-PAMs appear more promising, as they do not lead to the overactivation of CB1Rs or associated pro-absence, such as elongated SWD duration [], nor do they produce psychotropic effects. Since ago-PAMs require the presence of eCBs to exert their action, they provide an intrinsic ceiling effect that reduces the risk of overstimulation.
Although ago-PAM anti-absence effects are blocked by CB1R antagonism, the eCB may also have mixed effects. These include the modulation of TRPV1 channels, inhibition of L- and T-type Ca2+ channels [,], or direct activation of extrasynaptic GABAARs [,] by binding to their β2 subunit []. Synaptic GABAAR-mediated inhibition has also been reported []. Notably, enhanced tonic GABAAR inhibition via the δ-containing extrasynaptic GABAA receptor in VB TC neurons is both necessary and sufficient for the expression of ASs in different rodent models, while phasic GABAAR inhibition remains unaltered []. These observations support the hypothesis of a potential hyper-eCB signal contribution to the pathophysiology of ASs and suggest limitations in the use of cannabinoids, given their contrasting effects on GABAARs and other targets, for this type of childhood epilepsy. However, further research is needed to explore ECS involvement in both seizures and neurobehavioral comorbidities in CAE (Figure 2 and Figure 3).
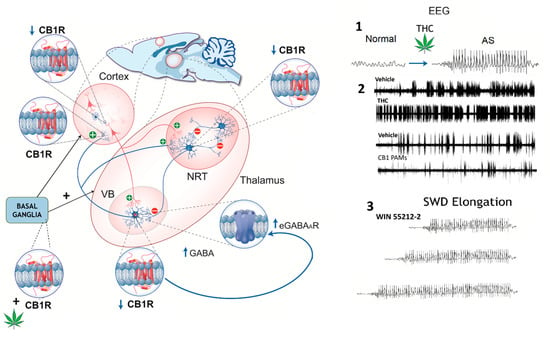
Figure 3.
Schematic representation of cannabinoid receptor type 1 (CB1R) distribution in the rat thalamocortical circuit, illustrating region-specific changes in expression (↑ or ↓) and their impact on GABAergic signaling. CB1R expression is decreased in the cortex, thalamic relay neurons (VBs), basal ganglia, and key inhibitory synapses. In the thalamic reticular nucleus (NRT), reduced CB1R expression may contribute to thalamocortical hyperexcitability. Enhanced GABA release in the VB could increase extrasynaptic GABA_A receptor (eGABAAR)-mediated tonic inhibition, facilitating the generation of SWDs. Right panels—EEG recordings: 1. Phytocannabinoids (∆9-THC): THC-induced SWDs are hypothesized to result from CB1R activation at striatonigral synapses, which enhances GABA release and disinhibits oscillation-promoting neurons in the nigro-thalamic pathway, triggering SWDs even in previously non-epileptic animals. 2. In GAERS, ∆9-THC exacerbates SWDs by increasing their frequency and duration, while CB1R-positive allosteric modulators (PAMs) reduce SWD incidence. Cannabidiol (CBD) has been reported to suppress SWDs in animal models, although human studies suggest it may paradoxically increase SWD activity in some cases. 3. In WAG/Rij rats, the synthetic CB1R agonist WIN 55212-2 disrupts the termination (“stop”) mechanism of SWDs, likely via effects on the NRT, resulting in prolonged discharges without altering their incidence (see main text for references). Figure modified with permission from [].
5. Limitations, Risks, and Future Directions for the Research and Treatment of Cannabinoid Use in Pediatric Epilepsy
The ECS functions as a multifaceted neuromodulatory network that regulates neuronal excitability, synaptic plasticity, and immune homeostasis [], from early life through adolescence and into aging []. Therefore, is not surprising that in various pediatric epilepsies, alterations in ECS components, particularly CB1R expression and brain eCB content, highlight both disorder-specific vulnerabilities and therapeutic opportunities [].
In the developing brain, the ECS plays a critical role in regulating neural progenitor cell survival, proliferation, differentiation, and migration primarily through CB1 receptor signaling []. This system helps shape brain development by modulating synaptic plasticity and maintaining the balance between excitation and inhibition, which is essential for normal cortical formation []. ECS activity influences key developmental processes in regions like the cortex, hippocampus, and amygdala [,]. Disruptions in ECS signaling during early life, such as exposure to exogenous cannabinoids, can impair neuronal connectivity and lead to long-lasting effects on motor function and seizure susceptibility []. The ECS also supports the maturation of stress responses and emotional behaviors during adolescence, contributing to proper neurodevelopmental trajectories [].
Current preclinical and clinical data indicate that only purified CBD shows consistent efficacy in treating DRE and related neuropsychiatric comorbidities in children. However, given CBD’s potential to modulate the ECS, further studies are necessary to clarify its mechanisms of action and developmental impact. Notably, the long-term effects of chronic CBD exposure on the ECS and brain maturation remain insufficiently understood, highlighting the urgent need for extended follow-up in both clinical and animal studies to determine safe dosing, treatment duration, and the developmental stages at which its use is both effective and safe. Future studies should employ appropriate experimental models focusing on pure CBD and its synthetic derivatives to establish safe and effective dosages, define precise therapeutic targets, and identify analogs that preserve therapeutic benefits while minimizing risks to the developing brain [].
A different therapeutic approach, supported by preclinical studies, suggests that CB1R ago-PAMs are promising, as they enhance endogenous CB1R signaling preferentially via AEA over 2-AG [], in an activity-dependent manner, reducing seizure burden in DS and CAE without overstimulation. In contrast, broad CB1R agonism (e.g., THC, WIN552122) often results in mixed or adverse effects, particularly in CAE, where CB1R activation prolongs SWD duration and facilitates seizure generalization. Additionally, FAAH and MAGL inhibitors or dual FAAH MAGL inhibitors, which elevate AEA, 2AG, or both, may restore ECS tone without direct CB1R activation [,,], offering another potential therapeutic strategy for pediatric patients with DRE and CAE.
6. Conclusions
In conclusion, alterations in the ECS are likely involved in the pathophysiology of childhood epilepsy. While therapeutic modulation of the ECS holds promise, it is inherently complex and must be approached with caution, as both inhibition and overactivation can cause adverse effects, especially during critical periods of brain development. A deeper understanding of the physiological roles of the ECS is essential to anticipate the consequences of its modulation in pediatric patients and to develop safer therapeutic strategies. Precision targeting of ECS components, considering regional CB1R density, fluctuating eCB levels, and syndrome-specific ECS pathophysiology, may offer a more rational and safer strategy for pediatric epilepsy cases with multifactorial etiologies. This approach may optimize seizure control and address neuropsychiatric comorbidities, particularly in syndromes such as DS, LGS, other DRE, and potentially well-defined subgroups of CAE, but it requires deep mechanistic insights and individualized profiling to avoid therapeutic missteps. However, given the roles of eCBs in development, the long-term use in children warrants caution and further investigation.
Author Contributions
G.M. and G.D.G. performed the literature review and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.; Tinuper, P.; Perucca, E.; Moshé, S.L. Introduction to the epilepsy syndrome papers. Epilepsia 2022, 63, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; D’souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, S.D. The etiologic classification of epilepsy. Epilepsia 2011, 52, 1052–1057. [Google Scholar] [CrossRef]
- Camfield, P.; Camfield, C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015, 17, 117–123. [Google Scholar] [CrossRef]
- Holmes, G.L.; Ben-Ari, Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr. Res. 2001, 49, 320–325. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Holmes, G.L. Effects of seizures on developmental processes in the immature brain. Lancet. Neurol. 2006, 5, 1055–1063. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef]
- Holmes, G.L.; Ben-Ari, Y. Seizures in the developing brain: Perhaps not so benign after all. Neuron 1998, 21, 1231–1234. [Google Scholar] [CrossRef]
- Wirrell, E.C.; Grossardt, B.R.; Wong-Kisiel, L.C.; Nickels, K.C. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: A population-based study. Epilepsy Res. 2011, 95, 110–118. [Google Scholar] [CrossRef]
- Wirrell, E.C. Predicting pharmacoresistance in pediatric epilepsy. Epilepsia 2013, 54, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Conway, L.; Smith, M.L.; Ferro, M.A.; Speechley, K.N.; Connoly, M.B.; Snead, O.C.; Widjaja, E. Correlates of health-related quality of life in children with drug resistant epilepsy. Epilepsia 2016, 57, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Samuelraj, S.; Lee-Archer, P.; Bowers, A.; Herbert, A. Medicinal Cannabinoids in Children: A Scoping Review. Integr. Med. Rep. 2025, 4, 9–43. [Google Scholar] [CrossRef]
- Devinsky, O.; Jones, N.A.; Cunningham, M.O.; Jayasekera, B.A.P.; Devore, S.; Whalley, B.J. Cannabinoid treatments in epilepsy and seizure disorders. Physiol. Rev. 2024, 104, 591–649. [Google Scholar] [CrossRef]
- Mechoulam, R.; Carlini, E.A. Toward drugs derived from cannabis. Naturwissenschaften 1978, 65, 174–179. [Google Scholar] [CrossRef]
- Riney, K.; Bogacz, A.; Somerville, E.; Hirsch, E.; Nabbout, R.; Scheffer, I.E.; Zuberi, S.M.; Alsaadi, T.; Jain, S.; French, J. International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1443–1474. [Google Scholar] [CrossRef]
- Di Marzo, V.; De Petrocellis, L.; Bisogno, T.; Melck, D. Metabolism of anandamide and 2-arachidonoylglycerol: An historical overview and some recent developments. Lipids 1999, 34, S319–S325. [Google Scholar] [CrossRef]
- Abu-Sawwa, R.; Stehling, C. Epidiolex (Cannabidiol) Primer: Frequently Asked Questions for Patients and Caregivers. J. Pediatr. Pharmacol. Ther. JPPT Off. J. PPAG 2020, 25, 75–77. [Google Scholar] [CrossRef]
- Di Giovanni, G. Cannabis medicine offers hope for severe paediatric epilepsies. Xjenza 2018, 6, 62–64. [Google Scholar]
- European Medicines Agency. Epidyolex: Assessment Report; European Medicines Agency: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Biosciences, G. Epidiolex®(Cannabidiol) Oral Solution [Package Insert]; US Food and Drug Administration Website: Silver Spring, MD, USA, 2021. [Google Scholar]
- Blair, R.E.; Deshpande, L.S.; DeLorenzo, R.J. Cannabinoids: Is there a potential treatment role in epilepsy? Expert Opin. Pharmacother. 2015, 16, 1911–1914. [Google Scholar] [CrossRef]
- De Caro, C.; Leo, A.; Citraro, R.; De Sarro, C.; Russo, R.; Calignano, A.; Russo, E. The potential role of cannabinoids in epilepsy treatment. Expert Rev. Neurother. 2017, 17, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Billakota, S.; Devinsky, O.; Marsh, E. Cannabinoid therapy in epilepsy. Curr. Opin. Neurol. 2019, 32, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Detyniecki, K.; Hirsch, L. Marijuana Use in Epilepsy: The Myth and the Reality. Curr. Neurol. Neurosci. Rep. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.C.; Patra, P.H.; Whalley, B.J. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. EB 2017, 70, 319–327. [Google Scholar] [CrossRef]
- Massey, S.; Quigley, A.; Rochfort, S.; Christodoulou, J.; Van Bergen, N.J. Cannabinoids and Genetic Epilepsy Models: A Review with Focus on CDKL5 Deficiency Disorder. Int. J. Mol. Sci. 2024, 25, 10768. [Google Scholar] [CrossRef]
- DeGasperis, S.M.; Webster, R.; Pohl, D. Cannabis Treatment in Children with Epilepsy: Practices of Canadian Neurologists. Can J. Neurol. Sci. 2020, 47, 511–518. [Google Scholar] [CrossRef]
- Elliott, J.; DeJean, D.; Clifford, T.; Coyle, D.; Potter, B.; Skidmore, B.; Alexander, C.; Repetski, A.E.; McCoy, B.; Wells, G.A. Cannabis for pediatric epilepsy: Protocol for a living systematic review. Syst. Rev. 2018, 7, 95. [Google Scholar] [CrossRef]
- Filloux, F.M. Cannabinoids for pediatric epilepsy? Up in smoke or real science? Transl. Pediatr. 2015, 4, 271–282. [Google Scholar] [CrossRef]
- Huntsman, R.J.; Tang-Wai, R.; Shackelford, A.E. Cannabis for Pediatric Epilepsy. J. Clin. Neurophysiol. 2020, 37, 2–8. [Google Scholar] [CrossRef]
- Knupp, K.G.; Rice, J.D.; Helmkamp, L.J.; Galinkin, J.; Sempio, C.; Jost, K.; Chapman, K.E. Prospective evaluation of oral cannabis extracts in children with epilepsy. Seizure 2019, 72, 23–27. [Google Scholar] [CrossRef]
- Ayoub, D.; Al-Hajje, A.; Salameh, P.; Jost, J.; Hmaimess, G.; Jaafar, F.; Halabi, T.; Boumediene, F.; Beydoun, A. Beyond Seizures: Psychiatric comorbidities in children with epilepsy. Epilepsy Behav. 2025, 163, 110234. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Trinka, E.; Wirrell, E.; Abdulla, F.; Al Baradie, R.; Alonso Vanegas, M.; Auvin, S.; Singh, M.B.; Blumenfeld, H.; Bogacz Fressola, A.; et al. Updated classification of epileptic seizures: Position paper of the International League Against Epilepsy. Epilepsia 2025, 66, 1804–1823. [Google Scholar] [CrossRef]
- Specchio, N.; Wirrell, E.C.; Scheffer, I.E.; Nabbout, R.; Riney, K.; Samia, P.; Guerreiro, M.; Gwer, S.; Zuberi, S.M.; Wilmshurst, J.M. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1398–1442. [Google Scholar] [CrossRef] [PubMed]
- Kramer, U.; Chi, C.S.; Lin, K.L.; Specchio, N.; Sahin, M.; Olson, H.; Nabbout, R.; Kluger, G.; Lin, J.J.; van Baalen, A. Febrile infection–related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome: A multicenter study on 77 children. Epilepsia 2011, 52, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.B.; Katanyuwong, K.; Mackay, M.T.; Bailey, C.A.; Scheffer, I.E.; Freeman, J.L.; Berkovic, S.F.; Harvey, A.S. Long-term follow-up of febrile infection–related epilepsy syndrome. Epilepsia 2012, 53, 101–110. [Google Scholar] [CrossRef]
- Oguni, H.; Fukuyama, Y. Pathogenesis of epilepsy in childhood. Psychiatry Clin. Neurosci. 1986, 40, 293–299. [Google Scholar] [CrossRef]
- Van Baalen, A.; Häusler, M.; Boor, R.; Rohr, A.; Sperner, J.; Kurlemann, G.; Panzer, A.; Stephani, U.; Kluger, G. Febrile infection–related epilepsy syndrome (FIRES): A nonencephalitic encephalopathy in childhood. Epilepsia 2010, 51, 1323–1328. [Google Scholar] [CrossRef]
- Mikaeloff, Y.; Jambaqué, I.; Hertz-Pannier, L.; Zamfirescu, A.; Adamsbaum, C.; Plouin, P.; Dulac, O.; Chiron, C. Devastating epileptic encephalopathy in school-aged children (DESC): A pseudo encephalitis. Epilepsy Res. 2006, 69, 67–79. [Google Scholar] [CrossRef]
- Nabbout, R.; Mazzuca, M.; Hubert, P.; Peudennier, S.; Allaire, C.; Flurin, V.; Aberastury, M.; Silva, W.; Dulac, O. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia 2010, 51, 2033–2037. [Google Scholar] [CrossRef]
- Connolly, M.B. Dravet syndrome: Diagnosis and long-term course. Can. J. Neurol. Sci. 2016, 43, S3–S8. [Google Scholar] [CrossRef]
- Dravet, C. Dravet syndrome history. Dev. Med. Child Neurol. 2011, 53, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, R.H.; Fejerman, N. Dravet syndrome: A study of 53 patients. Epilepsy Res. 2006, 70, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Villas, N.; Meskis, M.A.; Goodliffe, S. Dravet syndrome: Characteristics, comorbidities, and caregiver concerns. Epilepsy Behav. 2017, 74, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A. Lennox-Gastaut syndrome: A comprehensive review. Neurol. Sci. 2018, 39, 403–414. [Google Scholar] [CrossRef]
- Dravet, C.; Bureau, M.; Oguni, H.; Fukuyama, Y.; Cokar, O. Severe myoclonic epilepsy in infancy (Dravet syndrome). Epileptic Syndr. Infancy Child. Adolesc. 2005, 4, 89–113. [Google Scholar]
- Asadi-Pooya, A.A.; Bazrafshan, M.; Farazdaghi, M. Long-term medical and social outcomes of patients with Lennox-Gastaut syndrome. Epilepsy Res. 2021, 178, 106813. [Google Scholar] [CrossRef]
- Griffin, A.; Hamling, K.R.; Hong, S.; Anvar, M.; Lee, L.P.; Baraban, S.C. Preclinical animal models for Dravet syndrome: Seizure phenotypes, comorbidities and drug screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K. Nav1. 1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef]
- Han, S.; Tai, C.; Westenbroek, R.E.; Yu, F.H.; Cheah, C.S.; Potter, G.B.; Rubenstein, J.L.; Scheuer, T.; De La Iglesia, H.O.; Catterall, W.A. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012, 489, 385–390. [Google Scholar] [CrossRef]
- Anderson, L.L.; Hawkins, N.A.; Thompson, C.H.; Kearney, J.A.; George, A.L., Jr. Unexpected efficacy of a novel sodium channel modulator in Dravet syndrome. Sci. Rep. 2017, 7, 1682. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- Genton, P.; Velizarova, R.; Dravet, C. Dravet syndrome: The long-term outcome. Epilepsia 2011, 52, 44–49. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Chen, B.; Choi, H.; Hirsch, L.J.; Katz, A.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017, 76, 24–31. [Google Scholar] [CrossRef]
- Nickels, K.C.; Wong-Kisiel, L.C.; Moseley, B.D.; Wirrell, E.C. Temporal lobe epilepsy in children. Epilepsy Res. Treat. 2012, 2012, 849540. [Google Scholar] [CrossRef]
- Fogarasi, A.; Tuxhorn, I.; Janszky, J.; Janszky, I.; Rásonyi, G.; Kelemen, A.; Halász, P. Age-dependent seizure semiology in temporal lobe epilepsy. Epilepsia 2007, 48, 1697–1702. [Google Scholar] [CrossRef]
- Olbrich, A.; Urak, L.; Gröppel, G.; Serles, W.; Novak, K.; Porsche, B.; Benninger, F.; Czech, T.; Baumgartner, C.; Feucht, M. Semiology of temporal lobe epilepsy in children and adolescents value in lateralizing the seizure onset zone. Epilepsy Res. 2002, 48, 103–110. [Google Scholar] [CrossRef]
- Cascino, G.D. Surgical treatment for epilepsy. Epilepsy Res. 2004, 60, 179–186. [Google Scholar] [CrossRef]
- Skirrow, C.; Cross, J.H.; Cormack, F.; Harkness, W.; Vargha-Khadem, F.; Baldeweg, T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology 2011, 76, 1330–1337. [Google Scholar] [CrossRef]
- Guerrini, R.; Conti, V.; Mantegazza, M.; Balestrini, S.; Galanopoulou, A.S.; Benfenati, F. Developmental and epileptic encephalopathies: From genetic heterogeneity to phenotypic continuum. Physiol. Rev. 2023, 103, 433–513. [Google Scholar] [CrossRef] [PubMed]
- Brigo, F.; Trinka, E.; Lattanzi, S.; Bragazzi, N.L.; Nardone, R.; Martini, M. A brief history of typical absence seizures—Petit mal revisited. Epilepsy Behav. 2018, 80, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Leresche, N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat. Rev. 2002, 3, 371–382. [Google Scholar] [CrossRef]
- Reichsoellner, J.; Larch, J.; Unterberger, I.; Dobesberger, J.; Kuchukhidze, G.; Luef, G.; Bauer, G.; Trinka, E. Idiopathic generalised epilepsy of late onset: A separate nosological entity? J. Neurol. Neurosurg. Psychiatry 2010, 81, 1218–1222. [Google Scholar] [CrossRef]
- Posner, E. Absence seizures in children. BMJ Clin. Evid. 2013, 2013, 0317. [Google Scholar]
- Crunelli, V.; Lőrincz, M.L.; McCafferty, C.; Lambert, R.C.; Leresche, N.; Di Giovanni, G.; David, F. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain A J. Neurol. 2020, 143, 2341–2368. [Google Scholar] [CrossRef]
- Panayiotopoulos, C.P. Idiopathic generalized epilepsies: A review and modern approach. Epilepsia 2005, 46, 1–6. [Google Scholar] [CrossRef]
- Leresche, N.; Crunelli, V. Corticothalamic network abnormalities underlying absence seizures. In The Cerebral Cortex and Thalamus; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Cope, D.W.; Di Giovanni, G.; Fyson, S.J.; Orban, G.; Errington, A.C.; Lorincz, M.L.; Gould, T.M.; Carter, D.A.; Crunelli, V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat. Med. 2009, 15, 1392–1398. [Google Scholar] [CrossRef]
- Mangan, K.P.; Nelson, A.B.; Petrou, S.; Cirelli, C.; Jones, M.V. Cortical Tonic Inhibition Gates the Expression of Spike-and-Wave Discharges Associated with Absence Epilepsy. J. Integr. Neurosci. 2024, 23, 24. [Google Scholar] [CrossRef]
- Meyer, J.; Maheshwari, A. Cortical and Thalamic PV+ Interneuron Dysfunction in the Pathogenesis of Absence Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies; Avoli, M., de Curtis, M., Bernard, C., Soltesz, I., Noebels, J.L., Avoli, M., Rogawski, M.A., Vezzani, A., Delgado-Escueta, A.V., Eds.; Oxford University Press: Oxford, UK, 2024. [Google Scholar]
- McCafferty, C.; David, F.; Venzi, M.; Lorincz, M.L.; Delicata, F.; Atherton, Z.; Recchia, G.; Orban, G.; Lambert, R.C.; Di Giovanni, G.; et al. Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat. Neurosci. 2018, 21, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Depaulis, A.; David, O.; Charpier, S. The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J. Neurosci. Methods 2016, 260, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.; Van Luijtelaar, E.L. Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- Noebels, J.L.; Qiao, X.; Bronson, R.T.; Spencer, C.; Davisson, M.T. Stargazer: A new neurological mutant on chromosome 15 in the mouse with prolonged cortical seizures. Epilepsy Res. 1990, 7, 129–135. [Google Scholar] [CrossRef]
- Tan, H.O.; Reid, C.A.; Single, F.N.; Davies, P.J.; Chiu, C.; Murphy, S.; Clarke, A.L.; Dibbens, L.; Krestel, H.; Mulley, J.C.; et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc. Natl. Acad. Sci. USA 2007, 104, 17536–17541. [Google Scholar] [CrossRef]
- Deransart, C.; Depaulis, A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord 2002, 4 (Suppl. S3), S61–S72. [Google Scholar] [CrossRef]
- Paz, J.T.; Chavez, M.; Saillet, S.; Deniau, J.M.; Charpier, S. Activity of ventral medial thalamic neurons during absence seizures and modulation of cortical paroxysms by the nigrothalamic pathway. J. Neurosci. 2007, 27, 929–941. [Google Scholar] [CrossRef]
- Glauser, T.A.; Cnaan, A.; Shinnar, S.; Hirtz, D.G.; Dlugos, D.; Masur, D.; Clark, P.O.; Adamson, P.C. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy: Initial monotherapy outcomes at 12 months. Epilepsia 2013, 54, 141–155. [Google Scholar] [CrossRef]
- Terra, V.C.; de Paola, L.; Silvado, C.E. Are children affected by epileptic neuropsychiatric comorbidities? Epilepsy Behav. EB 2014, 38, 8–12. [Google Scholar] [CrossRef]
- Tenney, J.R.; Glauser, T.A. The current state of absence epilepsy: Can we have your attention? Epilepsy Curr. 2013, 13, 135–140. [Google Scholar] [CrossRef]
- Cnaan, A.; Shinnar, S.; Arya, R.; Adamson, P.C.; Clark, P.O.; Dlugos, D.; Hirtz, D.G.; Masur, D.; Glauser, T.A. Second monotherapy in childhood absence epilepsy. Neurology 2017, 88, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Caplan, R.; Siddarth, P.; Stahl, L.; Lanphier, E.; Vona, P.; Gurbani, S.; Koh, S.; Sankar, R.; Shields, W.D. Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia 2008, 49, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Fratta, W. Beyond THC: The new generation of cannabinoid designer drugs. Front. Behav. Neurosci. 2011, 5, 60. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoid signaling in the brain: Biosynthetic mechanisms in the limelight. Nat. Neurosci. 2011, 14, 9–15. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Notcutt, W. Cannabinoids. Br. J. Hosp. Med. (Lond. Engl. 2005) 2016, 77, C78–C80. [Google Scholar] [CrossRef]
- Manzoni, O.J.; Manduca, A.; Trezza, V. Therapeutic potential of cannabidiol polypharmacology in neuropsychiatric disorders. Trends Pharmacol. Sci. 2025, 46, 145–162. [Google Scholar] [CrossRef]
- Mirlohi, S.; Bladen, C.; Santiago, M.J.; Arnold, J.C.; McGregor, I.; Connor, M. Inhibition of human recombinant T-type calcium channels by phytocannabinoids in vitro. Br. J. Pharmacol. 2022, 179, 4031–4043. [Google Scholar] [CrossRef]
- Rempel, V.; Fuchs, A.; Hinz, S.; Karcz, T.; Lehr, M.; Koetter, U.; Müller, C.E. Magnolia Extract, Magnolol, and Metabolites: Activation of Cannabinoid CB2 Receptors and Blockade of the Related GPR55. ACS Med. Chem. Lett. 2013, 4, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, M.; Carona, A.; Castanheira, S.; Falcão, A.; Bicker, J.; Fortuna, A. Pharmacokinetics of Non-Psychotropic Phytocannabinoids. Pharmaceutics 2025, 17, 236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, C.; Jin, S.; Wang, Y.; Peng, Y.; Wang, X. Cannabidiol alleviates neuroinflammation and attenuates neuropathic pain via targeting FKBP5. Brain Behav. Immun. 2023, 111, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, N.; Gómez-Acero, L.; Sarasola, L.I.; Argerich, J.; Chevigné, A.; Jacobson, K.A.; Ciruela, F.; Fernández-Dueñas, V.; Aso, E. Cannabidiol negatively modulates adenosine A2A receptor functioning in living cells. Acta Neuropsychiatr. 2023, 36, 320–324. [Google Scholar] [CrossRef]
- Alexander, C.; Jeon, J.; Nickerson, K.; Hassler, S.; Vasefi, M. CBD and the 5-HT1A receptor: A medicinal and pharmacological review. Biochem. Pharmacol. 2025, 233, 116742. [Google Scholar] [CrossRef]
- Lima, I.V.d.A.; Bellozi, P.M.Q.; Batista, E.M.; Vilela, L.R.; Brandão, I.L.; Ribeiro, F.M.; Moraes, M.F.D.; Moreira, F.A.; de Oliveira, A.C.P. Cannabidiol anticonvulsant effect is mediated by the PI3Kγ pathway. Neuropharmacology 2020, 176, 108156. [Google Scholar] [CrossRef]
- Starkus, J.; Jansen, C.; Shimoda, L.M.N.; Stokes, A.J.; Small-Howard, A.L.; Turner, H. Diverse TRPV1 responses to cannabinoids. Channels 2019, 13, 172–191. [Google Scholar] [CrossRef]
- Tsien, R.W.; Rosenberg, E.C. Ion channels and G protein-coupled receptors: Cannabidiol actions on disorders of excitability and synaptic excitatory-inhibitory ratio. Mol. Pharmacol. 2025, 107, 100017. [Google Scholar] [CrossRef]
- Singh, K.; Bhushan, B.; Chanchal, D.K.; Sharma, S.K.; Rani, K.; Yadav, M.K.; Porwal, P.; Kumar, S.; Sharma, A.; Virmani, T. Emerging therapeutic potential of cannabidiol (CBD) in neurological disorders: A comprehensive review. Behav. Neurol. 2023, 2023, 8825358. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-H.; Galadari, S.; Isaev, D.; Petroianu, G.; Shippenberg, T.S.; Oz, M. The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J. Pharmacol. Exp. Ther. 2010, 333, 547–554. [Google Scholar] [CrossRef]
- Thomas, A.; Baillie, G.; Phillips, A.; Razdan, R.; Ross, R.A.; Pertwee, R. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Khosropoor, S.; Alavi, M.S.; Etemad, L.; Roohbakhsh, A. Cannabidiol goes nuclear: The role of PPARγ. Phytomedicine 2023, 114, 154771. [Google Scholar] [CrossRef]
- Seeman, P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl. Psychiatry 2016, 6, e920. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef]
- Bakas, T.; Van Nieuwenhuijzen, P.; Devenish, S.; McGregor, I.; Arnold, J.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009, 30, 156–163. [Google Scholar] [CrossRef]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Drummond-Main, C.; Greening, D.; Yao, J.; Chen, S.; Appendino, J.; Au, P.B.; Turner, R.W. Cannabidiol counters the effects of a dominant-negative pathogenic Kv7. 2 variant. iScience 2022, 25, 105092. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.R. Cannabinoid interactions with ion channels and receptors. Channels 2019, 13, 162–167. [Google Scholar] [CrossRef]
- Smith, S.A.; Le, G.H.; Teopiz, K.M.; Kwan, A.T.; Rhee, T.G.; Ho, R.C.; Wu, J.; Cao, B.; Ceban, F.; McIntyre, R.S. Effects of cannabidiol and Δ9-tetrahydrocannabinol on cytochrome P450 enzymes: A systematic review. Drug Metab. Rev. 2024, 56, 164–174. [Google Scholar] [CrossRef]
- Chen, P.X.; Rogers, M.A. Opportunities and challenges in developing orally administered cannabis edibles. Curr. Opin. Food Sci. 2019, 28, 7–13. [Google Scholar] [CrossRef]
- Johansson, E.; Halldin, M.M. Urinary excretion half-life of Δ1-tetrahydrocannabinol-7-oic acid in heavy marijuana users after smoking. J. Anal. Toxicol. 1989, 13, 218–223. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.-H. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Howlett, A.; Barth, F.; Bonner, T.; Cabral, G.; Casellas, P.; Devane, W.; Felder, C.; Herkenham, M.; Mackie, K.; Martin, B. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Tanimura, A.; Yamazaki, M.; Hashimotodani, Y.; Uchigashima, M.; Kawata, S.; Abe, M.; Kita, Y.; Hashimoto, K.; Shimizu, T.; Watanabe, M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase α mediates retrograde suppression of synaptic transmission. Neuron 2010, 65, 320–327. [Google Scholar] [CrossRef]
- Gao, Y.; Vasilyev, D.V.; Goncalves, M.B.; Howell, F.V.; Hobbs, C.; Reisenberg, M.; Shen, R.; Zhang, M.-Y.; Strassle, B.W.; Lu, P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 2010, 30, 2017–2024. [Google Scholar] [CrossRef]
- Straub, V.M.; Barti, B.; Tandar, S.T.; Stevens, A.F.; van Egmond, N.; van der Wel, T.; Zhu, N.; Rüegger, J.; van der Horst, C.; Heitman, L.H. The endocannabinoid 2-arachidonoylglycerol is released and transported on demand via extracellular microvesicles. Proc. Natl. Acad. Sci. USA 2025, 122, e2421717122. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Regehr, W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef]
- Ohno-Shosaku, T.; Maejima, T.; Kano, M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 2001, 29, 729–738. [Google Scholar] [CrossRef]
- Martínez-Aguirre, C.; Cinar, R.; Rocha, L. Targeting Endocannabinoid System in Epilepsy: For Good or for Bad. Neuroscience 2022, 482, 172–185. [Google Scholar] [CrossRef]
- Fetta, A.; Crotti, E.; Campostrini, E.; Bergonzini, L.; Cesaroni, C.A.; Conti, F.; Di Pisa, V.; Gentile, V.; Mondardini, M.C.; Vezzoli, C. Cannabidiol in the acute phase of febrile infection-related epilepsy syndrome (FIRES). Epilepsia Open 2023, 8, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Gofshteyn, J.S.; Wilfong, A.; Devinsky, O.; Bluvstein, J.; Charuta, J.; Ciliberto, M.A.; Laux, L.; Marsh, E.D. Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J. Child Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, R.; Taraschenko, O.; Dilena, R.; Payne, E.T.; Specchio, N.; Nabbout, R.; Koh, S.; Gaspard, N.; Hirsch, L.J.; Group, I.N.C. International consensus recommendations for management of new onset refractory status epilepticus including febrile infection-related epilepsy syndrome: Statements and supporting evidence. Epilepsia 2022, 63, 2840–2864. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, C.M.; Furlanis, G.M.; Toldo, I.; Guarrera, B.; Luisi, C.; Pettenazzo, A.; Nosadini, M.; Boniver, C.; Sartori, S.; Landi, A. Myoclonic super-refractory status epilepticus with favourable evolution in a teenager with FIRES: Is the association of vagus nerve stimulation and cannabidiol effective? Brain Dev. 2023, 45, 293–299. [Google Scholar] [CrossRef]
- Sermet, S.; Li, J.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Cannabidiol selectively modulates interleukin (IL)-1β and IL-6 production in toll-like receptor activated human peripheral blood monocytes. Toxicology 2021, 464, 153016. [Google Scholar] [CrossRef]
- Yousaf, M.; Chang, D.; Liu, Y.; Liu, T.; Zhou, X. Neuroprotection of cannabidiol, its synthetic derivatives and combination preparations against microglia-mediated neuroinflammation in neurological disorders. Molecules 2022, 27, 4961. [Google Scholar] [CrossRef]
- Gray, R.A.; Whalley, B.J. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord. 2020, 22, 10–15. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V. Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Thiele, E.; Marsh, E.; Mazurkiewicz-Beldzinska, M. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia 2019, 60, 419–428. [Google Scholar] [CrossRef]
- Privitera, M.; Bhathal, H.; Wong, M.; Cross, J.H.; Wirrell, E.; Marsh, E.D.; Mazurkiewicz-Beldzinska, M.; Villanueva, V.; Checketts, D.; Knappertz, V. Time to onset of cannabidiol (CBD) treatment effect in Lennox–Gastaut syndrome: Analysis from two randomized controlled trials. Epilepsia 2021, 62, 1130–1140. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Schubert-Bast, S.; von Podewils, F.; Knake, S.; Mayer, T.; Klotz, K.A.; Buhleier, E.; Herold, L.; Immisch, I.; Kurlemann, G.; et al. Real-world experience of cannabidiol in conjunction with clobazam for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome: Results from a retrospective multicentre chart review in Germany. Epilepsy Behav. 2025, 166, 110302. [Google Scholar] [CrossRef]
- Ong, M.J.Y.; Abd Rahman, M.S.H.; Lee, V.L.L.; Lee, K.H.; Chang, C.J.Y.; Khoo, C.S.; Hod, R.; Tan, H.J.; Trinka, E. The use of cannabidiol as adjunctive therapy in adult patients with drug-resistant epilepsy: A systematic review and meta-analysis. Ther. Adv. Neurol. Disord. 2025, 18, 17562864251313914. [Google Scholar] [CrossRef]
- de Oliveira, V.G.; de Almeida, N.B.; Radmann, G.C.; de Oliveira Santos, B.F. The efficacy of cannabidiol for seizures reduction in pharmacoresistant epilepsy: A systematic review and meta-analysis. Acta Epileptol. 2025, 7, 20. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.; Xiao, W.; Wang, C.; Liang, R. Efficacy and safety of pharmacological and non-pharmacological therapies in Lennox-Gastaut syndrome: A systematic review and network meta-analysis. Front. Pharmacol. 2025, 16, 1522543. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, C. Efficacy and safety of antiseizure medication for Lennox–Gastaut syndrome: A systematic review and network meta-analysis. Dev. Med. Child Neurol. 2022, 64, 305–313. [Google Scholar] [CrossRef]
- Chesney, E.; Oliver, D.; Green, A. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799–1806. [Google Scholar] [CrossRef]
- Fazlollahi, A.; Zahmatyar, M.; ZareDini, M. Adverse events of cannabidiol use in patients with epilepsy: A systematic review and meta-analysis. JAMA Netw. Open 2023, 6, e239126. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Gozal, D.; Carney, P. Channelopathy of Dravet Syndrome and Potential Neuroprotective Effects of Cannabidiol. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211048045. [Google Scholar] [CrossRef]
- Rubio, M.; Valdeolivas, S.; Piscitelli, F.; Verde, R.; Satta, V.; Barroso, E.; Montolio, M.; Aras, L.M.; Di Marzo, V.; Sagredo, O. Analysis of endocannabinoid signaling elements and related proteins in lymphocytes of patients with Dravet syndrome. Pharmacol. Res. Perspect. 2016, 4, e00220. [Google Scholar] [CrossRef]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef]
- Anderson, L.L.; Ametovski, A.; Lin Luo, J.; Everett-Morgan, D.; McGregor, I.S.; Banister, S.D.; Arnold, J.C. Cannabichromene, related phytocannabinoids, and 5-fluoro-cannabichromene have anticonvulsant properties in a mouse model of Dravet Syndrome. ACS Chem. Neurosci. 2021, 12, 330–339. [Google Scholar] [CrossRef]
- Anderson, L.L.; Heblinski, M.; Absalom, N.L.; Hawkins, N.A.; Bowen, M.T.; Benson, M.J.; Zhang, F.; Bahceci, D.; Doohan, P.T.; Chebib, M. Cannabigerolic acid, a major biosynthetic precursor molecule in cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br. J. Pharmacol. 2021, 178, 4826–4841. [Google Scholar] [CrossRef]
- Yip, K.L.; Udoh, M.; Sharman, L.A.; Harman, T.; Bedoya-Pérez, M.; Anderson, L.L.; Banister, S.D.; Arnold, J.C. Cannabinoid-like compounds found in non-cannabis plants exhibit antiseizure activity in genetic mouse models of drug-resistant epilepsy. Epilepsia 2025, 66, 303–314. [Google Scholar] [CrossRef]
- Miller, A.R.; Hawkins, N.A.; McCollom, C.E.; Kearney, J.A. Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes Brain Behav. 2014, 13, 163–172. [Google Scholar] [CrossRef]
- Bahceci, D.; Anderson, L.L.; Kevin, R.C.; Doohan, P.T.; Arnold, J.C. Hyperthermia-Induced Seizures Enhance Brain Concentrations of the Endocannabinoid-Related Linoleoyl Glycerols in a Scn1a+/− Mouse Model of Dravet Syndrome. Cannabis Cannabinoid Res. 2023, 8, 495–504. [Google Scholar] [CrossRef]
- Anderson, L.L.; Doohan, P.T.; Hawkins, N.A.; Bahceci, D.; Garai, S.; Thakur, G.A.; Kearney, J.A.; Arnold, J.C. The endocannabinoid system impacts seizures in a mouse model of Dravet syndrome. Neuropharmacology 2022, 205, 108897. [Google Scholar] [CrossRef]
- Anderson, L.L.; Etchart, M.G.; Bahceci, D.; Golembiewski, T.A.; Arnold, J.C. Cannabis constituents interact at the drug efflux pump BCRP to markedly increase plasma cannabidiolic acid concentrations. Sci. Rep. 2021, 11, 14948. [Google Scholar] [CrossRef]
- Farrell, J.S.; Colangeli, R.; Dong, A.; George, A.G.; Addo-Osafo, K.; Kingsley, P.J.; Morena, M.; Wolff, M.D.; Dudok, B.; He, K. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 2021, 109, 2398–2403. e2394. [Google Scholar] [CrossRef]
- Satta, V.; Alonso, C.; Díez, P.; Martín-Suárez, S.; Rubio, M.; Encinas, J.M.; Fernández-Ruiz, J.; Sagredo, O. Neuropathological characterization of a Dravet syndrome knock-in mouse model useful for investigating cannabinoid treatments. Front. Mol. Neurosci. 2021, 13, 602801. [Google Scholar] [CrossRef]
- Qu, S.; Catron, M.; Zhou, C.; Janve, V.; Shen, W.; Howe, R.K.; Macdonald, R.L. GABAA receptor β3 subunit mutation D120N causes Lennox–Gastaut syndrome in knock-in mice. Brain Commun. 2020, 2, fcaa028. [Google Scholar] [CrossRef]
- Gaston, T.E.; Ampah, S.B.; Bebin, E.M.; Grayson, L.P.; Cutter, G.R.; Hernando, K.; Szaflarski, J.P. Long-term safety and efficacy of highly purified cannabidiol for treatment refractory epilepsy. Epilepsy Behav. 2021, 117, 107862. [Google Scholar] [CrossRef]
- Gaston, T.E.; Szaflarski, M.; Hansen, B.; Bebin, E.M.; Szaflarski, J.P. Quality of life in adults enrolled in an open-label study of cannabidiol (CBD) for treatment-resistant epilepsy. Epilepsy Behav. 2019, 95, 10–17. [Google Scholar] [CrossRef]
- Martin, R.C.; Gaston, T.E.; Thompson, M.; Ampah, S.B.; Cutter, G.; Bebin, E.M.; Szaflarski, J.P. Cognitive functioning following long-term cannabidiol use in adults with treatment-resistant epilepsy. Epilepsy Behav. 2019, 97, 105–110. [Google Scholar] [CrossRef]
- Patra, P.H.; Serafeimidou-Pouliou, E.; Bazelot, M.; Whalley, B.J.; Williams, C.M.; McNeish, A.J. Cannabidiol improves survival and behavioural co-morbidities of Dravet syndrome in mice. Br. J. Pharmacol. 2020, 177, 2779–2792. [Google Scholar] [CrossRef]
- Cheah, C.S.; Yu, F.H.; Westenbroek, R.E.; Kalume, F.K.; Oakley, J.C.; Potter, G.B.; Rubenstein, J.L.; Catterall, W.A. Specific deletion of NaV1. 1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 14646–14651. [Google Scholar] [CrossRef]
- Raucci, U.; Pietrafusa, N.; Paolino, M.C.; Di Nardo, G.; Villa, M.P.; Pavone, P.; Terrin, G.; Specchio, N.; Striano, P.; Parisi, P. Cannabidiol Treatment for Refractory Epilepsies in Pediatrics. Front. Pharmacol. 2020, 11, 586110. [Google Scholar] [CrossRef]
- Lattanzi, S.; Trinka, E.; Striano, P.; Rocchi, C.; Salvemini, S.; Silvestrini, M.; Brigo, F. Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox-Gastaut Syndrome. CNS Drugs 2021, 35, 265–281. [Google Scholar] [CrossRef]
- Hess, E.J.; Moody, K.A.; Geffrey, A.L.; Pollack, S.F.; Skirvin, L.A.; Bruno, P.L.; Paolini, J.L.; Thiele, E.A. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 2016, 57, 1617–1624. [Google Scholar] [CrossRef]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D. Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: A placebo-controlled randomized clinical trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef]
- Ong, K.S.; Carlin, J.B.; Fahey, M.; Freeman, J.L.; Scheffer, I.E.; Gillam, L.; Anderson, M.; Huque, M.H.; Legge, D.; Dirnbauer, N.; et al. Protocol for a single patient therapy plan: A randomised, double-blind, placebo-controlled N-of-1 trial to assess the efficacy of cannabidiol in patients with intractable epilepsy. J. Paediatr. Child Health 2020, 56, 1918–1923. [Google Scholar] [CrossRef]
- Martínez-Aguirre, C.; Carmona-Cruz, F.; Velasco, A.L.; Velasco, F.; Aguado-Carrillo, G.; Cuéllar-Herrera, M.; Rocha, L. Cannabidiol Acts at 5-HT(1A) Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy. Front. Behav. Neurosci. 2020, 14, 611278. [Google Scholar] [CrossRef]
- Nuñez-Lumbreras, M.d.l.Á.; Castañeda-Cabral, J.L.; Valle-Dorado, M.G.; Sánchez-Valle, V.; Orozco-Suárez, S.; Guevara-Guzmán, R.; Martínez-Juárez, I.; Alonso-Vanegas, M.; Walter, F.; Deli, M.A.; et al. Drug-Resistant Temporal Lobe Epilepsy Alters the Expression and Functional Coupling to Gαi/o Proteins of CB1 and CB2 Receptors in the Microvasculature of the Human Brain. Front. Behav. Neurosci. 2021, 14, 611780. [Google Scholar] [CrossRef]
- Rocha, L.; Cinar, R.; Guevara-Guzmán, R.; Alonso-Vanegas, M.; San-Juan, D.; Martínez-Juárez, I.; Castañeda-Cabral, J.L.; Carmona-Cruz, F. Endocannabinoid System and Cannabinoid 1 Receptors in Patients With Pharmacoresistant Temporal Lobe Epilepsy and Comorbid Mood Disorders. Front. Behav. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef]
- During, M.J.; Spencer, D.D. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 1993, 341, 1607–1610. [Google Scholar] [CrossRef]
- Romigi, A.; Bari, M.; Placidi, F.; Marciani, M.G.; Malaponti, M.; Torelli, F.; Izzi, F.; Prosperetti, C.; Zannino, S.; Corte, F. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia 2010, 51, 768–772. [Google Scholar] [CrossRef]
- Ludányi, A.; Erőss, L.; Czirják, S.; Vajda, J.; Halász, P.; Watanabe, M.; Palkovits, M.; Maglóczky, Z.; Freund, T.F.; Katona, I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008, 28, 2976–2990. [Google Scholar] [CrossRef]
- Goffin, K.; Van Paesschen, W.; Van Laere, K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain A J. Neurol. 2011, 134, 1033–1040. [Google Scholar] [CrossRef]
- Nearing, K.; Madhavan, D.; Devinsky, O. Temporal lobe epilepsy: A progressive disorder? Rev. Neurol. Dis. 2007, 4, 122–127. [Google Scholar]
- Colangeli, R.; Teskey, G.C.; Di Giovanni, G. Endocannabinoid-serotonin systems interaction in health and disease. Prog. Brain Res. 2021, 259, 83–134. [Google Scholar] [CrossRef]
- Nogueira, M.H.; Yasuda, C.L.; Coan, A.C.; Kanner, A.M.; Cendes, F. Concurrent mood and anxiety disorders are associated with pharmacoresistant seizures in patients with MTLE. Epilepsia 2017, 58, 1268–1276. [Google Scholar] [CrossRef]
- Vinti, V.; Dell’Isola, G.B.; Tascini, G.; Mencaroni, E.; Cara, G.D.; Striano, P.; Verrotti, A. Temporal Lobe Epilepsy and Psychiatric Comorbidity. Front. Neurol. 2021, 12, 775781. [Google Scholar] [CrossRef]
- Colangeli, R.; Pierucci, M.; Benigno, A.; Campiani, G.; Butini, S.; Di Giovanni, G. The FAAH inhibitor URB597 suppresses hippocampal maximal dentate afterdischarges and restores seizure-induced impairment of short and long-term synaptic plasticity. Sci. Rep. 2017, 7, 11152. [Google Scholar] [CrossRef]
- Colangeli, R.; Morena, M.; Pittman, Q.J.; Hill, M.N.; Teskey, G.C. Anandamide Signaling Augmentation Rescues Amygdala Synaptic Function and Comorbid Emotional Alterations in a Model of Epilepsy. J. Neurosci. 2020, 40, 6068–6081. [Google Scholar] [CrossRef]
- Colangeli, R.; Di Maio, R.; Pierucci, M.; Deidda, G.; Casarrubea, M.; Di Giovanni, G. Synergistic action of CB1 and 5-HT2B receptors in preventing pilocarpine-induced status epilepticus in rats. Neurobiol. Dis. 2019, 125, 135–145. [Google Scholar] [CrossRef]
- Di Maio, R.; Colangeli, R.; Di Giovanni, G. WIN 55,212-2 Reverted Pilocarpine-Induced Status Epilepticus Early Changes of the Interaction among 5-HT2C/NMDA/CB1 Receptors in the Rat Hippocampus. ACS Chem. Neurosci. 2019, 10, 3296–3306. [Google Scholar] [CrossRef]
- Azevedo, M.; Benbadis, S.R. Efficacy of highly purified cannabidiol (CBD) in typical absence seizures: A pilot study. Epilepsy Behav. 2023, 149, 109512. [Google Scholar] [CrossRef]
- Flamini, R.J.; Comi, A.M.; Bebin, E.M.; Chez, M.G.; Clark, G.; Devinsky, O.; Hussain, S.A.; Lyons, P.D.; Patel, A.D.; Rosengard, J.L.; et al. Efficacy of cannabidiol in convulsive and nonconvulsive seizure types associated with treatment-resistant epilepsies in the Expanded Access Program. Epilepsia 2023, 64, e156–e163. [Google Scholar] [CrossRef]
- Patel, S.; Grinspoon, R.; Fleming, B.; Skirvin, L.A.; Wade, C.; Wolper, E.; Bruno, P.L.; Thiele, E.A. The long-term efficacy of cannabidiol in the treatment of refractory epilepsy. Epilepsia 2021, 62, 1594–1603. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Cannabidiol Oral Solution in Pediatric Participants with Treatment-Resistant Childhood Absence Seizures. Available online: https://clinicaltrials.gov/study/NCT03336242 (accessed on 2 April 2025).
- Turkanis, S.A.; Chiu, P.; Borys, H.K.; Karler, R. Influence of Δ9 and cannabidiol on photically evoked after-discharge potentials. Psychopharmacology 1977, 52, 207–212. [Google Scholar] [CrossRef]
- Roebuck, A.J.; Greba, Q.; Onofrychuk, T.J.; McElroy, D.L.; Sandini, T.M.; Zagzoog, A.; Simone, J.; Cain, S.M.; Snutch, T.P.; Laprairie, R.B.; et al. Dissociable changes in spike and wave discharges following exposure to injected cannabinoids and smoked cannabis in Genetic Absence Epilepsy Rats from Strasbourg. Eur. J. Neurosci. 2022, 55, 1063–1078. [Google Scholar] [CrossRef]
- Sales-Carbonell, C.; Rueda-Orozco, P.E.; Soria-Gomez, E.; Buzsaki, G.; Marsicano, G.; Robbe, D. Striatal GABAergic and cortical glutamatergic neurons mediate contrasting effects of cannabinoids on cortical network synchrony. Proc. Natl. Acad. Sci. USA 2013, 110, 719–724. [Google Scholar] [CrossRef]
- van Rijn, C.M.; Gaetani, S.; Santolini, I.; Badura, A.; Gabova, A.; Fu, J.; Watanabe, M.; Cuomo, V.; van Luijtelaar, G.; Nicoletti, F.; et al. WAG/Rij rats show a reduced expression of CB(1) receptors in thalamic nuclei and respond to the CB(1) receptor agonist, R(+)WIN55,212-2, with a reduced incidence of spike-wave discharges. Epilepsia 2010, 51, 1511–1521. [Google Scholar] [CrossRef]
- Perescis, M.F.J.; Flipsen, N.A.R.; van Luijtelaar, G.; van Rijn, C.M. Altered SWD stopping mechanism in WAG/Rij rats subchronically treated with the cannabinoid agonist R(+)WIN55,212-2. Epilepsy Behav. EB 2020, 102, 106722. [Google Scholar] [CrossRef]
- Citraro, R.; Russo, E.; Ngomba, R.T.; Nicoletti, F.; Scicchitano, F.; Whalley, B.J.; Calignano, A.; De Sarro, G. CB1 agonists, locally applied to the cortico-thalamic circuit of rats with genetic absence epilepsy, reduce epileptic manifestations. Epilepsy Res. 2013, 106, 74–82. [Google Scholar] [CrossRef]
- Roebuck, A.J.; Greba, Q.; Smolyakova, A.M.; Alaverdashvili, M.; Marks, W.N.; Garai, S.; Baglot, S.L.; Petrie, G.; Cain, S.M.; Snutch, T.P.; et al. Positive allosteric modulation of type 1 cannabinoid receptors reduces spike-and-wave discharges in Genetic Absence Epilepsy Rats from Strasbourg. Neuropharmacology 2021, 190, 108553. [Google Scholar] [CrossRef]
- Citraro, R.; Russo, E.; Scicchitano, F.; van Rijn, C.M.; Cosco, D.; Avagliano, C.; Russo, R.; D’Agostino, G.; Petrosino, S.; Guida, F.; et al. Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-α receptor activation in a genetic model of absence epilepsy. Neuropharmacology 2013, 69, 115–126. [Google Scholar] [CrossRef]
- McElroy, D.L.; Roebuck, A.J.; Greba, Q.; Garai, S.; Brandt, A.L.; Yilmaz, O.; Cain, S.M.; Snutch, T.P.; Thakur, G.A.; Laprairie, R.B.; et al. The type 1 cannabinoid receptor positive allosteric modulators GAT591 and GAT593 reduce spike-and-wave discharges in Genetic Absence Epilepsy Rats from Strasbourg. IBRO Neurosci. Rep. 2022, 12, 121–130. [Google Scholar] [CrossRef]
- USA Controlled Substances Act. USA Controlled Substances Act of 1970. Available online: https://uscode.house.gov/view.xhtml?path=/prelim@title21/chapter13&edition=prelim (accessed on 20 June 2022).
- Sysoeva, M.V.; Kuznetsova, G.D.; Sysoev, I.V.; Ngomba, R.T.; Vinogradova, L.V.; Grishchenko, A.A.; van Rijn, C.M.; van Luijtelaar, G. Network analysis reveals a role of the hippocampus in absence seizures: The effects of a cannabinoid agonist. Epilepsy Res. 2023, 192, 107135. [Google Scholar] [CrossRef]
- Lüttjohann, A.; Schoffelen, J.-M.; Van Luijtelaar, G. Termination of ongoing spike-wave discharges investigated by cortico–thalamic network analyses. Neurobiol. Dis. 2014, 70, 127–137. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Smith, S.E.; Liimatta, M.B.; Beidler, D.; Sadagopan, N.; Dudley, D.T.; Young, T.; Wren, P.; Zhang, Y.; Swaney, S. Mechanistic and pharmacological characterization of PF-04457845: A highly potent and selective FAAH inhibitor that reduces inflammatory and noninflammatory pain. J. Pharmacol. Exp. Ther. 2011, 338, 114–124. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdère, P.; Casarrubea, M.; Cassar, D.; Radic, M.; Puginier, E.; Chagraoui, A.; Crescimanno, G.; Crunelli, V.; Di Giovanni, G. Cannabinoid 1/2 receptor activation induces strain-dependent behavioral and neurochemical changes in genetic absence epilepsy rats from Strasbourg and non-epileptic control rats. Front. Cell. Neurosci. 2022, 16, 886033. [Google Scholar] [CrossRef]
- Neuparth-Sottomayor, M.; Pina, C.C.; Morais, T.P.; Farinha-Ferreira, M.; Abreu, D.S.; Solano, F.; Mouro, F.; Good, M.; Sebastião, A.M.; Di Giovanni, G.; et al. Cognitive comorbidities of experimental absence seizures are independent of anxiety. Neurobiol. Dis. 2023, 186, 106275. [Google Scholar] [CrossRef]
- Casarrubea, M.; Radic, M.; Morais, T.P.; Mifsud, E.; Cuboni, E.; Aiello, S.; Crescimanno, G.; Crunelli, V.; Di Giovanni, G. A quantitative and T-pattern analysis of anxiety-like behavior in male GAERS, NEC, and Wistar rats bred under the same conditions, against a commercially available Wistar control group in the hole board and elevated plus maze tests. CNS Neurosci. Ther. 2024, 30, e14443. [Google Scholar] [CrossRef]
- Cassar, D.; Radic, M.; Casarrubea, M.; Crunelli, V.; Di Giovanni, G. The effect of cannabinoid receptor agonist WIN 55,212–2 on anxiety-like behavior and locomotion in a genetic model of absence seizures in the elevated plus-maze. CNS Neurosci. Ther. 2022, 28, 1268. [Google Scholar] [CrossRef]
- Roebuck, A.J. Modulation of the Endocannabinoid System in Genetic Absence Epilepsy Rats from Strasbourg: New Avenues for Treatment of Absence Seizures and Behavioural Comorbidities. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2021. [Google Scholar]
- Depaulis, A.; Charpier, S. Pathophysiology of absence epilepsy: Insights from genetic models. Neurosci. Lett. 2018, 667, 53–65. [Google Scholar] [CrossRef]
- Sugaya, Y.; Kano, M. Control of excessive neural circuit excitability and prevention of epileptic seizures by endocannabinoid signaling. Cell. Mol. Life Sci. CMLS 2018, 75, 2793–2811. [Google Scholar] [CrossRef]
- Suárez, J.; Ortíz, O.; Puente, N.; Bermúdez-Silva, F.J.; Blanco, E.; Fernández-Llebrez, P.; Grandes, P.; de Fonseca, F.R.; Moratalla, R. Distribution of diacylglycerol lipase alpha, an endocannabinoid synthesizing enzyme, in the rat forebrain. Neuroscience 2011, 192, 112–131. [Google Scholar] [CrossRef]
- Sun, Y.G.; Wu, C.S.; Lu, H.C.; Beierlein, M. Target-dependent control of synaptic inhibition by endocannabinoids in the thalamus. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 9222–9230. [Google Scholar] [CrossRef]
- Aguirre-Rodríguez, C.A.; Delgado, A.; Alatorre, A.; Oviedo-Chávez, A.; Martínez-Escudero, J.R.; Barrientos, R.; Querejeta, E. Local activation of CB1 receptors by synthetic and endogenous cannabinoids dampens burst firing mode of reticular thalamic nucleus neurons in rats under ketamine anesthesia. Exp. Brain Res. 2024, 242, 2137–2157. [Google Scholar] [CrossRef]
- Zhang, L.; Kolaj, M.; Renaud, L.P. Endocannabinoid 2-AG and intracellular cannabinoid receptors modulate a low-threshold calcium spike-induced slow depolarizing afterpotential in rat thalamic paraventricular nucleus neurons. Neuroscience 2016, 322, 308–319. [Google Scholar] [CrossRef]
- Marks, W.N.; Cavanagh, M.E.; Greba, Q.; Cain, S.M.; Snutch, T.P.; Howland, J.G. The G enetic A bsence E pilepsy R ats from S trasbourg model of absence epilepsy exhibits alterations in fear conditioning and latent inhibition consistent with psychiatric comorbidities in humans. Eur. J. Neurosci. 2016, 43, 25–40. [Google Scholar] [CrossRef]
- Marks, W.N.; Cain, S.M.; Snutch, T.P.; Howland, J.G. The T-type calcium channel antagonist Z944 rescues impairments in crossmodal and visual recognition memory in Genetic Absence Epilepsy Rats from Strasbourg. Neurobiol. Dis. 2016, 94, 106–115. [Google Scholar] [CrossRef]
- Marks, W.N.; Zabder, N.K.; Greba, Q.; Cain, S.M.; Snutch, T.P.; Howland, J.G. The T-type calcium channel blocker Z944 reduces conditioned fear in Genetic Absence Epilepsy Rats from Strasbourg and the non-epileptic control strain. Eur. J. Neurosci. 2019, 50, 3046–3059. [Google Scholar] [CrossRef]
- Roebuck, A.J.; An, L.; Marks, W.N.; Sun, N.; Snutch, T.P.; Howland, J.G. Cognitive impairments in touchscreen-based visual discrimination and reversal learning in genetic absence epilepsy rats from Strasbourg. Neuroscience 2020, 430, 105–112. [Google Scholar] [CrossRef]
- Chemin, J.; Monteil, A.; Perez-Reyes, E.; Nargeot, J.; Lory, P. Direct inhibition of T-type calcium channels by the endogenous cannabinoid anandamide. EMBO J. 2001, 20, 7033–7040. [Google Scholar] [CrossRef]
- van der Stelt, M.; Trevisani, M.; Vellani, V.; De Petrocellis, L.; Schiano Moriello, A.; Campi, B.; McNaughton, P.; Geppetti, P.; Di Marzo, V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J. 2005, 24, 3026–3037. [Google Scholar] [CrossRef]
- Sigel, E.; Baur, R.; Rácz, I.; Marazzi, J.; Smart, T.G.; Zimmer, A.; Gertsch, J. The major central endocannabinoid directly acts at GABAA receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 18150–18155. [Google Scholar] [CrossRef]
- Golovko, T.; Min, R.; Lozovaya, N.; Falconer, C.; Yatsenko, N.; Tsintsadze, T.; Tsintsadze, V.; Ledent, C.; Harvey, R.J.; Belelli, D.; et al. Control of Inhibition by the Direct Action of Cannabinoids on GABAA Receptors. Cereb. Cortex 2014, 25, 2440–2455. [Google Scholar] [CrossRef]
- Baur, R.; Kielar, M.; Richter, L.; Ernst, M.; Ecker, G.F.; Sigel, E. Molecular analysis of the site for 2-arachidonylglycerol (2-AG) on the β2 subunit of GABAA receptors. J. Neurochem. 2013, 126, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, G.; Chagraoui, A.; Bharatiya, R.; De Deurwaerdère, P. Chapter 10—Serotonergic control of excitability: From neuron to networks. In Handbook of Behavioral Neuroscience; Müller, C.P., Cunningham, K.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 197–215. [Google Scholar]
- Schonhofen, P.; Bristot, I.J.; Crippa, J.A.; Hallak, J.E.C.; Zuardi, A.W.; Parsons, R.B.; Klamt, F. Cannabinoid-Based Therapies and Brain Development: Potential Harmful Effect of Early Modulation of the Endocannabinoid System. CNS Drugs 2018, 32, 697–712. [Google Scholar] [CrossRef]
- Harkany, T.; Guzmán, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef]
- Dow-Edwards, D.; Silva, L. Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 2017, 1654, 157–164. [Google Scholar] [CrossRef]
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology 2018, 43, 21–33. [Google Scholar] [CrossRef]
- de Salas-Quiroga, A.; Díaz-Alonso, J.; García-Rincón, D.; Remmers, F.; Vega, D.; Gómez-Cañas, M.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. USA 2015, 112, 13693–13698. [Google Scholar] [CrossRef]
- Lovelace, J.W.; Corches, A.; Vieira, P.A.; Hiroto, A.S.; Mackie, K.; Korzus, E. An animal model of female adolescent cannabinoid exposure elicits a long-lasting deficit in presynaptic long-term plasticity. Neuropharmacology 2015, 99, 242–255. [Google Scholar] [CrossRef]
- Green, H.M.; Manning, J.J.; Greig, I.R.; Ross, R.A.; Finlay, D.B.; Glass, M. Positive allosteric modulation of the cannabinoid CB1 receptor potentiates endocannabinoid signalling and changes ERK1/2 phosphorylation kinetics. Br. J. Pharmacol. 2024, 181, 3642–3662. [Google Scholar] [CrossRef]
- Papa, A.A.-O.; Pasquini, S.A.-O.; Contri, C.A.-O.; Gemma, S.A.-O.; Campiani, G.; Butini, S.A.-O.; Varani, K.A.-O.; Vincenzi, F.A.-O. Polypharmacological Approaches for CNS Diseases: Focus on Endocannabinoid Degradation Inhibition. Cells 2022, 11, 471. [Google Scholar] [CrossRef]
- Grillo, A.; Fezza, F.; Chemi, G.A.-O.; Colangeli, R.; Brogi, S.A.-O.; Fazio, D.; Federico, S.A.-O.; Papa, A.; Relitti, N.A.-O.; Di Maio, R.; et al. Selective Fatty Acid Amide Hydrolase Inhibitors as Potential Novel Antiepileptic Agents. ACS Chem. Neurosci. 2021, 12, 1716–1736. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).