Connecting the Dots: Mitochondrial Dysfunction, PCOS, and Insulin Resistance—Insights and Therapeutic Advances
Abstract
1. Introduction
2. Mitochondrial Biology
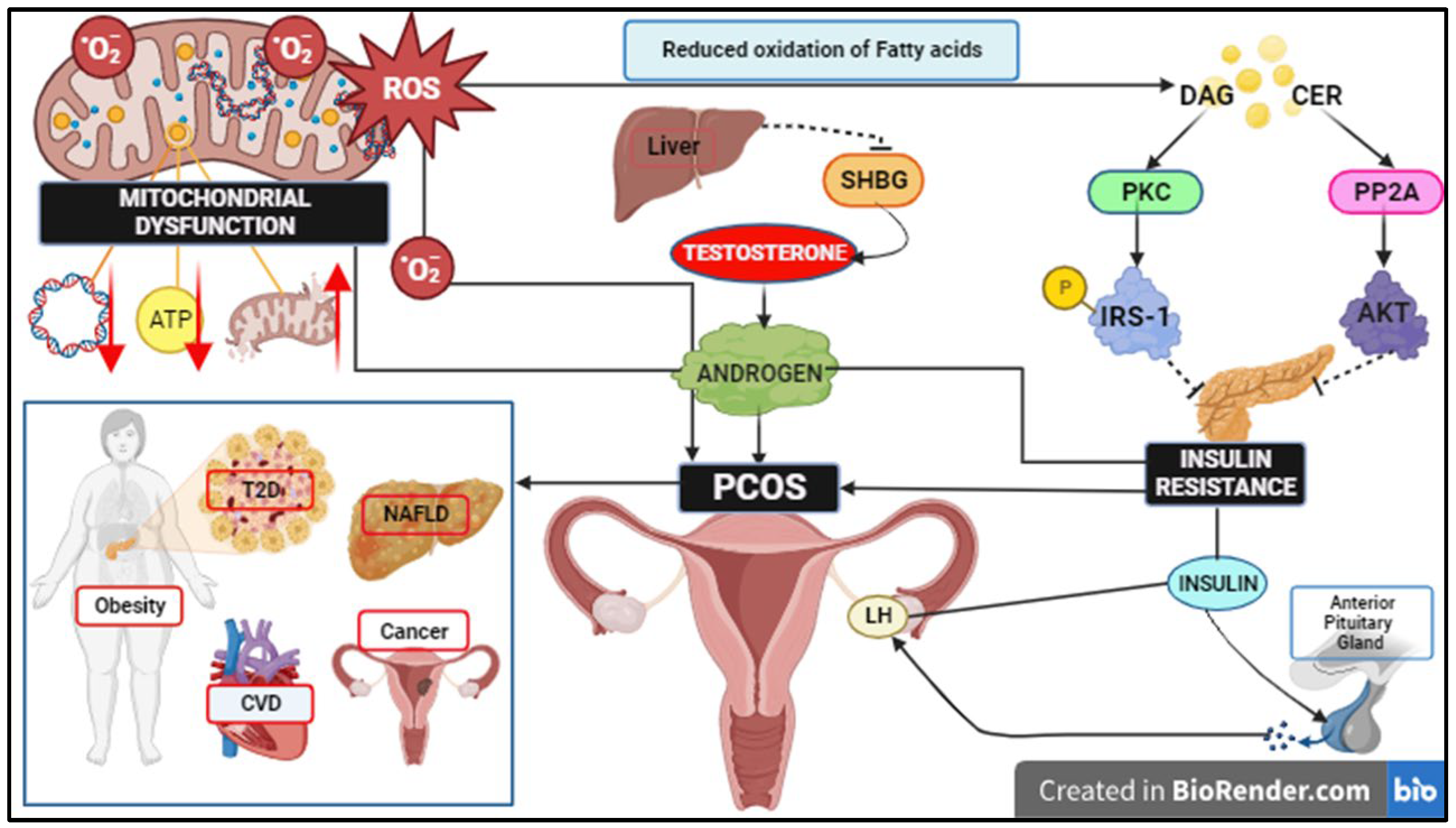
3. Role of Free Fatty Acids, ROS Signalling, and Mitochondrial Dysfunction in IR
4. IR in the Pathophysiology of PCOS
5. Mitochondrial Dysregulation in the Pathophysiology of PCOS
6. Variants Associated with IR in mtDNA Genes and Nuclear-Related mtDNA Genes in PCOS
| Gene | Locus | Mutation | Nucleotide Positions in tRNA/Protein Change | Type | Homoplasmy/ Heteroplasmy | Associated Disease | Reference |
|---|---|---|---|---|---|---|---|
| tRNA Glu | mtDNA | A14693G | 54 | Mutation | Homoplasmy | Deafness, HTN MELAS, LHON | [56,118,121] |
| tRNA Lys | mtDNA | A8343G | 54 | Mutation | Homoplasmy | MetS, PD risk factor, deafness | [56,118,121,152] |
| tRNA Leu (UUR) | mtDNA | C3275T | 44 | Mutation | Homoplasmy | MetS, LHON | [56,118,121,152] |
| tRNA Leu (UUR) | mtDNA | A3302G | 71 | Mutation | Heteroplasmy | MELAS, MM | [56,118,121,152] |
| tRNA Gln | mtDNA | T4363C | 38 | Mutation | Homoplasmy | HTN, MetS, possibly deafness, HTN, LHON | [56,118,121,152] |
| tRNA Gln | mtDNA | T4395C | 6 | Mutation | Homoplasmy | HTN | [56,118,121,152] |
| tRNA Arg | mtDNA | T10454C | 55 | Mutation | Homoplasmy | Possibly deafness, HTN | [56,118,121,152] |
| tRNA Asp | mtDNA | A7543G | 29 | Mutation | Heteroplasmy | MEPR | [56,118,121,152] |
| tRNA Ser (UCN) | mtDNA | C7492T | 26 | Mutation | Homoplasmy | CPEO, HTN, deafness risk factor | [56,118,121,152] |
| NC7 | mtDNA | 9-bp deletion | Deletion | Homoplasmy | [16,56,152] | ||
| ND1 | mtDNA | T3394C | Y30H | Mutation | LHON, diabetes | [56,152] | |
| ND2 | mtDNA | C5178A | L237M | Mutation | Acute MI, Diabetes, atherosclerosis | [56] | |
| ND5 | mtDNA | T12338C | M1T | Mutation | Homoplasmy | EH, LHON, MIDD | [56,152] |
| ND5 | mtDNA | T12811C | Y159H | Mutation | Homoplasmy | Possible LHON factor | [56,152] |
| D-loop | mtDNA | T16126C | SNP | [137] | |||
| D-loop | mtDNA | A16203G | SNP | [137] | |||
| D-loop | mtDNA | T16217C | SNP | Endometriosis | [151] | ||
| D-loop | mtDNA | A16316G | SNP | [151] | |||
| D-loop | mtDNA | A16203G | SNP | [151] | |||
| PGC-1α | nDNA | rs8192678 | G482S | SNP | Risk factor for diabetes | [55,135] |
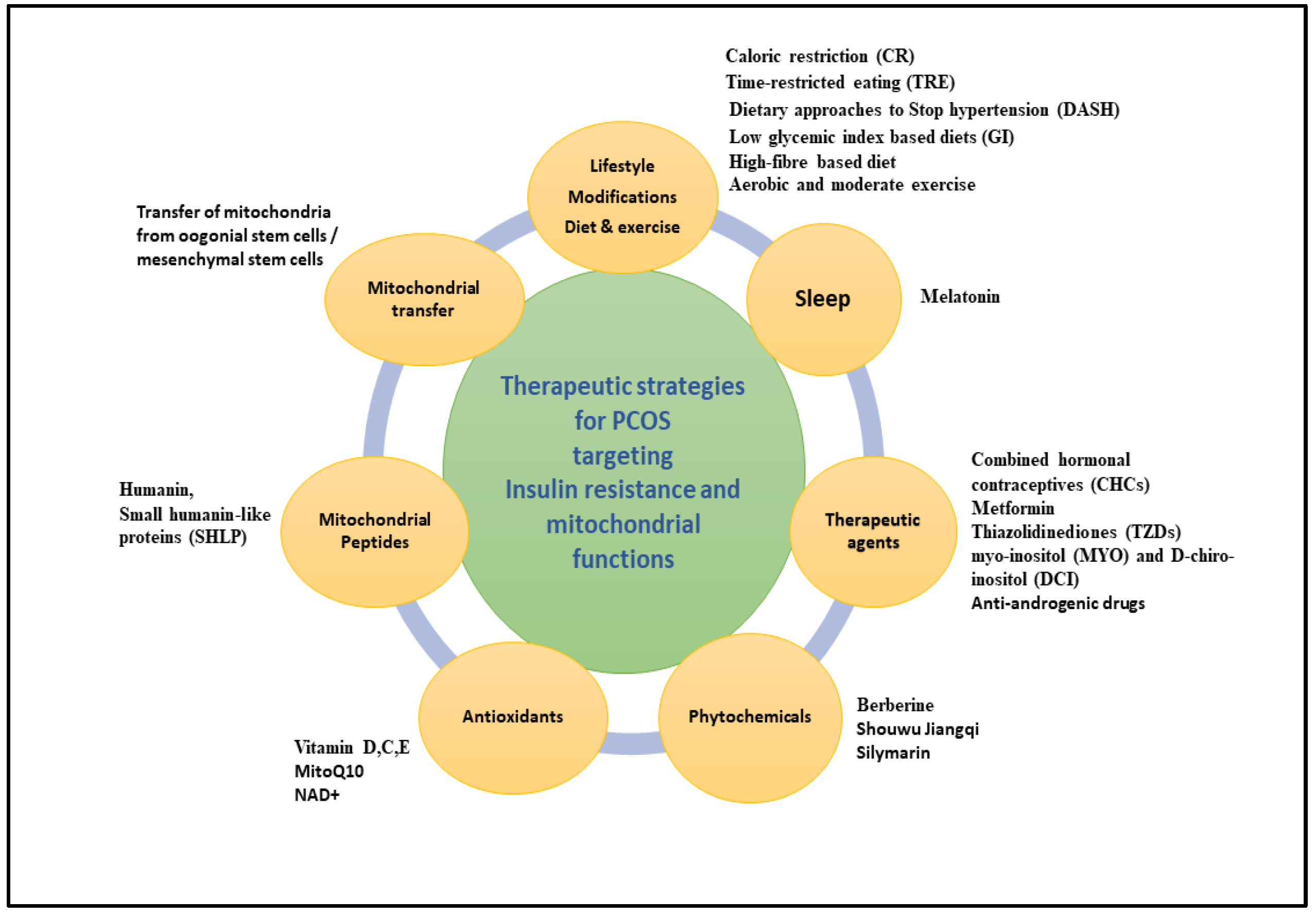
7. Therapeutic Interventions Targeting IR and Mitochondrial Dysfunction to Ameliorate PCOS
7.1. Exercise
7.2. Diet
7.3. Therapeutic Agents
7.4. Phytochemicals
7.5. Antioxidants
7.6. Mitochondrial Peptides
7.7. Sleep and Mental Health Management
7.8. Mitochondrial Transfer: Future Therapeutic Approach in IVF
8. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCOS | Polycystic Ovary Syndrome |
| IR | Insulin Resistance |
| T2D | Type 2 Diabetes |
| CVD | Cardiovascular Disease |
| EC | Endometrial Cancer |
| LH | Luteinizing Hormone |
| FSH | Follicle-Stimulating Hormone |
| SHBG | Sex Hormone-Binding Globulin |
| ROS | Reactive Oxygen Species |
| OS | Oxidative Stress |
| mtDNA | Mitochondrial DNA |
| ETC | Electron Transport Chain |
| OXPHOS | Oxidative Phosphorylation |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha |
| NRF-1 | Nuclear Respiratory Factor 1 |
| NRF-2 | Nuclear Respiratory Factor 2 |
| ERR-α | Oestrogen-Related Receptor Alpha |
| TFAM | Transcription Factor A, Mitochondrial |
| Mfn1/2 | Mitofusin 1/Mitofusin 2 |
| Opa1 | Optic Atrophy 1 |
| DRP1 | Dynamin-Related Protein 1 |
| Fis1 | Mitochondrial Fission 1 Protein |
| DAG | Diacylglycerol |
| CER | Ceramide |
| PKC | Protein Kinase C |
| AKT | Protein Kinase B |
| IRS1 | Insulin Receptor Substrate 1 |
| GLUT4 | Glucose Transporter Type 4 |
| FFAs | Free Fatty Acids |
| PA | Palmitate |
| AIF | Apoptosis-Inducing Factor |
| NOX4 | NADPH Oxidase 4 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| JNK | c-Jun N-terminal Kinase |
| NF-κB | Nuclear Factor Kappa B |
| MAPK | Mitogen-Activated Protein Kinase |
| FOXO1 | Forkhead Box Protein O1 |
| HMOX1 | Heme Oxygenase 1 |
| NOX2 | NADPH Oxidase 2 |
| RAC1 | Ras-Related C3 Botulinum Toxin Substrate 1 |
| P47PHOX | 47 kDa Subunit of NADPH Oxidase |
| CaMKII | Calcium/Calmodulin-Dependent Protein Kinase II |
| Myo1c | Myosin-1c |
| MAMs | Mitochondria-Associated ER Membranes |
| mTOR | Mammalian Target of Rapamycin |
| AMPK | AMP-Activated Protein Kinase |
| SIRT1 | Sirtuin 1 |
| DNMT1 | DNA Methyltransferase 1 |
| PSEN2 | Presenilin 2 |
| COX-1 | Cytochrome c Oxidase Subunit 1 |
| MPO | Myeloperoxidase |
| SDHD | Succinate Dehydrogenase Complex Subunit D |
| UCRC | Ubiquinol–Cytochrome c Reductase Core Protein |
| COX7C | Cytochrome c Oxidase Subunit 7C |
| GLUT1 | Glucose Transporter Type 1 |
| GLUT3 | Glucose Transporter Type 3 |
| iPSC | Induced Pluripotent Stem Cell |
| ND1 | NADH Dehydrogenase 1 |
| ND2 | NADH Dehydrogenase 2 |
| 8-OHDG | 8-Hydroxy-2′-Deoxyguanosine |
| ND5 | NADH Dehydrogenase 5 |
| LHON | Leber’s Hereditary Optic Neuropathy |
| TFAM | Transcription Factor A, Mitochondrial |
| NRF-1 | Nuclear Respiratory Factor 1 |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| NFKBI α | Nuclear Factor of Kappa Light Polypeptide Gene Enhancer in B-Cells Inhibitor Alpha |
| MAPK3 | Mitogen-Activated Protein Kinase 3 |
| SIRT1 | Sirtuin 1 |
| GLUT4 | Glucose Transporter Type 4 |
| HNG | Humanin Analogue S14G |
| TOS | Total Oxidant Status |
| BCL-2 | B-Cell Lymphoma 2 |
| SGLT2-i | Sodium-Glucose Cotransporter 2 Inhibitor |
| MYO | Myo-Inositol |
| DCI | D-Chiro-Inositol |
| MOTS-c | Mitochondrial Open Reading Frame of 12S rRNA-c |
| SHLP | Small Humanin-Like Protein |
| ART | Assisted Reproductive Technology |
| MSC | Mesenchymal Stem Cell |
| NR | Nicotinamide Riboside |
| SHBG | Sex Hormone-Binding Globulin |
| OSA | Obstructive Sleep Apnoea |
References
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of Endometrial, Ovarian and Breast Cancer in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Gunning, M.N.; Petermann, T.S.; Crisosto, N.; Van Rijn, B.B.; De Wilde, M.A.; Christ, J.P.; Uiterwaal, C.S.P.M.; De Jager, W.; Eijkemans, M.J.C.; Kunselman, A.R.; et al. Cardiometabolic Health in Offspring of Women with PCOS Compared to Healthy Controls: A Systematic Review and Individual Participant Data Meta-Analysis. Hum. Reprod. Update 2020, 26, 104–118. [Google Scholar] [CrossRef]
- Kakoly, N.S.; Earnest, A.; Teede, H.J.; Moran, L.J.; Joham, A.E. The Impact of Obesity on the Incidence of Type 2 Diabetes Among Women with Polycystic Ovary Syndrome. Diabetes Care 2019, 42, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Reaven, G.M. The Metabolic Syndrome: Requiescat in Pace. Clin. Chem. 2005, 51, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Brower, M.A.; Hai, Y.; Jones, M.R.; Guo, X.; Chen, Y.D.I.; Rotter, J.I.; Krauss, R.M.; Legro, R.S.; Azziz, R.; Goodarzi, M.O. Bidirectional Mendelian Randomization to Explore the Causal Relationships between Body Mass Index and Polycystic Ovary Syndrome. Hum. Reprod. 2019, 34, 127–136. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Jiang, X.; Li, Z.; Zhao, S.; Cui, L.; Chen, Z.J. Metabolic Disturbances in Non-Obese Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Fertil. Steril. 2019, 111, 168–177. [Google Scholar] [CrossRef]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of Diet on Insulin Resistance in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 3346–3360. [Google Scholar] [CrossRef]
- Morgante, G.; Darino, I.; Spanò, A.; Luisi, S.; Luddi, A.; Piomboni, P.; Governini, L.; De Leo, V. PCOS Physiopathology and Vitamin D Deficiency: Biological Insights and Perspectives for Treatment. J. Clin. Med. 2022, 11, 4509. [Google Scholar] [CrossRef]
- Marx, T.L.; Mehta, A.E. Polycystic Ovary Syndrome: Pathogenesis and Treatment over the Short and Long Term. Cleve Clin. J. Med. 2003, 70, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Carmina, E.; Azziz, R. DHEA, DHEAS and PCOS. J. Steroid Biochem. Mol. Biol. 2015, 145, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Deswal, R.; Yadav, A.; Dang, A.S. Sex Hormone Binding Globulin—An Important Biomarker for Predicting PCOS Risk: A Systematic Review and Meta-Analysis. Syst. Biol. Reprod. Med. 2018, 64, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, Z.E.C.; Sattar, N.; Fleming, R.; Greer, I.A. Polycystic Ovarian Syndrome: The Metabolic Syndrome Comes to Gynaecology. BMJ 1998, 317, 329–332. [Google Scholar] [CrossRef]
- Ashkar, F.; Rezaei, S.; Salahshoornezhad, S.; Vahid, F.; Gholamalizadeh, M.; Dahka, S.M.; Doaei, S. The Role of Medicinal Herbs in Treatment of Insulin Resistance in Patients with Polycystic Ovary Syndrome: A Literature Review. Biomol. Concepts 2020, 11, 57–75. [Google Scholar] [CrossRef]
- Moosa, A.; Ghani, M.; O’Neill, H.C. Genetic Associations with Polycystic Ovary Syndrome: The Role of the Mitochondrial Genome; a Systematic Review and Meta-Analysis. J. Clin. Pathol. 2022, 75, 815–824. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Shukla, P.; Mukherjee, S. Mitochondrial Dysfunction: An Emerging Link in the Pathophysiology of Polycystic Ovary Syndrome. Mitochondrion 2020, 52, 24–39. [Google Scholar] [CrossRef]
- Cook, K.L.; Soto-Pantoja, D.R.; Clarke, P.A.G.; Cruz, M.I.; Zwart, A.; Wärri, A.; Hilakivi-Clarke, L.; Roberts, D.D.; Clarke, R. Endoplasmic Reticulum Stress Protein GRP78 Modulates Lipid Metabolism to Control Drug Sensitivity and Antitumor Immunity in Breast Cancer. Cancer Res. 2016, 76, 5657–5670. [Google Scholar] [CrossRef]
- Tuppen, H.A.L.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA Mutations and Human Disease. Biochim. Biophys. Acta 2010, 1797, 113–128. [Google Scholar] [CrossRef]
- Schon, E.A.; Dimauro, S.; Hirano, M. Human Mitochondrial DNA: Roles of Inherited and Somatic Mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Singh, A.; Sharma, C.; Jain, S.K.; Singh, N. Mutations in the Mitochondrial DNA D-Loop Region Are Frequent in Cervical Cancer. Cancer Cell Int. 2005, 5, 34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Popov, L.D. Mitochondrial Biogenesis: An Update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Siemers, K.M.; Klein, A.K.; Baack, M.L. Mitochondrial Dysfunction in PCOS: Insights into Reproductive Organ Pathophysiology. Int. J. Mol. Sci. 2023, 24, 13123. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial Dysfunction and Insulin Resistance: An Update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial Fusion and Fission in Cell Life and Death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Twig, G.; Hyde, B.; Shirihai, O.S. Mitochondrial Fusion, Fission and Autophagy as a Quality Control Axis: The Bioenergetic View. Biochim. Biophys. Acta 2008, 1777, 1092–1097. [Google Scholar] [CrossRef]
- Cipolat, S.; De Brito, O.M.; Dal Zilio, B.; Scorrano, L. OPA1 Requires Mitofusin 1 to Promote Mitochondrial Fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 15927–15932. [Google Scholar] [CrossRef]
- Hales, K.G.; Fuller, M.T. Developmentally Regulated Mitochondrial Fusion Mediated by a Conserved, Novel, Predicted GTPase. Cell 1997, 90, 121–129. [Google Scholar] [CrossRef]
- Hales, K.G. The Machinery of Mitochondrial Fusion, Division, and Distribution, and Emerging Connections to Apoptosis. Mitochondrion 2004, 4, 285–308. [Google Scholar] [CrossRef]
- Martin, S.D.; McGee, S.L. The Role of Mitochondria in the Aetiology of Insulin Resistance and Type 2 Diabetes. Biochim. Biophys. Acta 2014, 1840, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Westermeier, F.; Navarro-Marquez, M.; López-Crisosto, C.; Bravo-Sagua, R.; Quiroga, C.; Bustamante, M.; Verdejo, H.E.; Zalaquett, R.; Ibacache, M.; Parra, V.; et al. Defective Insulin Signaling and Mitochondrial Dynamics in Diabetic Cardiomyopathy. Biochim. Biophys. Acta 2015, 1853, 1113–1118. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin Resistance: Review of the Underlying Molecular Mechanisms. J. Cell Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Schmitz-Peiffer, C.; Craig, D.L.; Biden, T.J. Ceramide Generation Is Sufficient to Account for the Inhibition of the Insulin-Stimulated PKB Pathway in C2C12 Skeletal Muscle Cells Pretreated with Palmitate. J. Biol. Chem. 1999, 274, 24202–24210. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Xie, X.H.; Chen, C.H.; Peng, X.; Zhang, P.; Yang, C.; Wang, Y.T. Molecular Regulation Mechanisms and Interactions Between Reactive Oxygen Species and Mitophagy. DNA Cell Biol. 2019, 38, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Malamouli, M.; Levinger, I.; McAinch, A.J.; Trewin, A.J.; Rodgers, R.J.; Moreno-Asso, A. The Mitochondrial Profile in Women with Polycystic Ovary Syndrome: Impact of Exercise. J. Mol. Endocrinol. 2022, 68, R11–R23. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-Induced Insulin Resistance: Unravelling the Mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Turner, N.; Kowalski, G.M.; Leslie, S.J.; Risis, S.; Yang, C.; Lee-Young, R.S.; Babb, J.R.; Meikle, P.J.; Lancaster, G.I.; Henstridge, D.C.; et al. Distinct Patterns of Tissue-Specific Lipid Accumulation during the Induction of Insulin Resistance in Mice by High-Fat Feeding. Diabetologia 2013, 56, 1638–1648. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Dufour, S.; Befroy, D.; Frattini, J.; Shatzkes, N.; Neschen, S.; White, M.F.; Bilz, S.; Sono, S.; et al. Reduced Mitochondrial Density and Increased IRS-1 Serine Phosphorylation in Muscle of Insulin-Resistant Offspring of Type 2 Diabetic Parents. J. Clin. Investig. 2005, 115, 3587–3593. [Google Scholar] [CrossRef]
- Lepretti, M.; Martucciello, S.; Aceves, M.A.B.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic Review Series: Adipocyte Biology. Adipose Tissue Function and Plasticity Orchestrate Nutritional Adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Iossa, S.; Venditti, P. Skeletal Muscle Insulin Resistance: Role of Mitochondria and Other ROS Sources. J. Endocrinol. 2017, 233, R15–R42. [Google Scholar] [CrossRef]
- Mancini, G.; Pirruccio, K.; Yang, X.; Blücher, M.; Rodeheffer, M.; Horvath, T.L. Mitofusin 2 in Mature Adipocytes Controls Adiposity and Body Weight. Cell Rep. 2019, 26, 2849–2858.e4. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojo, C.; Vargas-Vargas, M.A.; Olmos-Orizaba, B.E.; Rodríguez-Orozco, A.R.; Calderón-Cortés, E. Interplay between NADH Oxidation by Complex I, Glutathione Redox State and Sirtuin-3, and Its Role in the Development of Insulin Resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165801. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Joza, N.; Pospisilik, J.A.; Hangen, E.; Hanada, T.; Modjtahedi, N.; Penninger, J.M.; Kroemer, G. AIF: Not Just an Apoptosis-Inducing Factor. Ann. N. Y. Acad. Sci. 2009, 1171, 2–11. [Google Scholar] [CrossRef]
- Pospisilik, J.A.; Knauf, C.; Joza, N.; Benit, P.; Orthofer, M.; Cani, P.D.; Ebersberger, I.; Nakashima, T.; Sarao, R.; Neely, G.; et al. Targeted Deletion of AIF Decreases Mitochondrial Oxidative Phosphorylation and Protects from Obesity and Diabetes. Cell 2007, 131, 476–491. [Google Scholar] [CrossRef]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of Carnitine Palmitoyltransferase-1 in Skeletal Muscle Is Sufficient to Enhance Fatty Acid Oxidation and Improve High-Fat Diet-Induced Insulin Resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef]
- Han, C.Y.; Umemoto, T.; Omer, M.; Den Hartigh, L.J.; Chiba, T.; LeBoeuf, R.; Buller, C.L.; Sweet, I.R.; Pennathur, S.; Abel, E.D.; et al. NADPH Oxidase-Derived Reactive Oxygen Species Increases Expression of Monocyte Chemotactic Factor Genes in Cultured Adipocytes. J. Biol. Chem. 2012, 287, 10379–10393. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, K.; Motoshima, H.; Wu, X.; Ruddy, J.M.; Arnold, R.S.; Cheng, G.; Lambeth, J.D.; Goldstein, B.J. The NAD(P)H Oxidase Homolog Nox4 Modulates Insulin-Stimulated Generation of H2O2 and Plays an Integral Role in Insulin Signal Transduction. Mol. Cell Biol. 2004, 24, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Roos, G.; Messens, J. Protein Sulfenic Acid Formation: From Cellular Damage to Redox Regulation. Free Radic. Biol. Med. 2011, 51, 314–326. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic Ovary Syndrome and Mitochondrial Dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef]
- Dong, X.C.; Liu, C.; Zhuo, G.C.; Ding, Y. Potential Roles of MtDNA Mutations in PCOS-IR: A Review. Diabetes Metab. Syndr. Obes. 2023, 16, 139–149. [Google Scholar] [CrossRef]
- Cheng, Z.; Tseng, Y.; White, M.F. Insulin Signaling Meets Mitochondria in Metabolism. Trends Endocrinol. Metab. 2010, 21, 589–598. [Google Scholar] [CrossRef]
- Balteau, M.; Tajeddine, N.; De Meester, C.; Ginion, A.; Des Rosiers, C.; Brady, N.R.; Sommereyns, C.; Horman, S.; Vanoverschelde, J.L.; Gailly, P.; et al. NADPH Oxidase Activation by Hyperglycaemia in Cardiomyocytes Is Independent of Glucose Metabolism but Requires SGLT1. Cardiovasc. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef]
- Yip, M.F.; Ramm, G.; Larance, M.; Hoehn, K.L.; Wagner, M.C.; Guilhaus, M.; James, D.E. CaMKII-Mediated Phosphorylation of the Myosin Motor Myo1c Is Required for Insulin-Stimulated GLUT4 Translocation in Adipocytes. Cell Metab. 2008, 8, 384–398. [Google Scholar] [CrossRef]
- Townsend, L.K.; Medak, K.D.; Peppler, W.T.; Meers, G.M.; Scott Rector, R.; LeBlanc, P.J.; Wright, D.C. High-Saturated-Fat Diet-Induced Obesity Causes Hepatic Interleukin-6 Resistance via Endoplasmic Reticulum Stress. J. Lipid Res. 2019, 60, 1236–1249. [Google Scholar] [CrossRef]
- Tubbs, E.; Theurey, P.; Vial, G.; Bendridi, N.; Bravard, A.; Chauvin, M.A.; Ji-Cao, J.; Zoulim, F.; Bartosch, B.; Ovize, M.; et al. Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Is Required for Insulin Signaling and Is Implicated in Hepatic Insulin Resistance. Diabetes 2014, 63, 3279–3294. [Google Scholar] [CrossRef] [PubMed]
- Rieusset, J.; Fauconnier, J.; Paillard, M.; Belaidi, E.; Tubbs, E.; Chauvin, M.A.; Durand, A.; Bravard, A.; Teixeira, G.; Bartosch, B.; et al. Disruption of Calcium Transfer from ER to Mitochondria Links Alterations of Mitochondria-Associated ER Membrane Integrity to Hepatic Insulin Resistance. Diabetologia 2016, 59, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Brunetta, H.S.; Mori, M.A.S. Mitochondria-Associated ER Membranes in Glucose Homeostasis and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E1053–E1060. [Google Scholar] [CrossRef]
- Ye, J. Mechanism of Insulin Resistance in Obesity: A Role of ATP. Front. Med. 2021, 15, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Luo, M.; Wang, R.; Ye, J.; Wang, X. Mitochondria in Sex Hormone-Induced Disorder of Energy Metabolism in Males and Females. Front. Endocrinol. 2021, 12, 749451. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated Reduction of Genes of Oxidative Metabolism in Humans with Insulin Resistance and Diabetes: Potential Role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-Gamma Coactivator 1 Alpha (PGC-1 Alpha): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Vondra, K.; Rath, R.; Bass, A.; Slabochová, Z.; Teisinger, J.; Vítek, V. Enzyme Activities in Quadriceps Femoris Muscle of Obese Diabetic Male Patients. Diabetologia 1977, 13, 527–529. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Shock, L.S.; Thakkar, P.V.; Peterson, E.J.; Moran, R.G.; Taylor, S.M. DNA Methyltransferase 1, Cytosine Methylation, and Cytosine Hydroxymethylation in Mammalian Mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 3630–3635. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, S.; Copps, K.; Dong, X.; Kollipara, R.; Rodgers, J.T.; Depinho, R.A.; Puigserver, P.; White, M.F. Foxo1 Integrates Insulin Signaling with Mitochondrial Function in the Liver. Nat. Med. 2009, 15, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- De Kreutzenberg, S.V.; Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Semplicini, A.; Dalla Man, C.; Cobelli, C.; Fadini, G.P.; Avogaro, A. Downregulation of the Longevity-Associated Protein Sirtuin 1 in Insulin Resistance and Metabolic Syndrome: Potential Biochemical Mechanisms. Diabetes 2010, 59, 1006–1015. [Google Scholar] [CrossRef]
- O’Hagan, H.M.; Wang, W.; Sen, S.; DeStefano Shields, C.; Lee, S.S.; Zhang, Y.W.; Clements, E.G.; Cai, Y.; Van Neste, L.; Easwaran, H.; et al. Oxidative Damage Targets Complexes Containing DNA Methyltransferases, SIRT1, and Polycomb Members to Promoter CpG Islands. Cancer Cell 2011, 20, 606–619. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, H.M.; Mohammad, H.P.; Baylin, S.B. Double Strand Breaks Can Initiate Gene Silencing and SIRT1-Dependent Onset of DNA Methylation in an Exogenous Promoter CpG Island. PLoS Genet. 2008, 4, e1000155. [Google Scholar] [CrossRef]
- Peng, L.; Yuan, Z.; Ling, H.; Fukasawa, K.; Robertson, K.; Olashaw, N.; Koomen, J.; Chen, J.; Lane, W.S.; Seto, E. SIRT1 Deacetylates the DNA Methyltransferase 1 (DNMT1) Protein and Alters Its Activities. Mol. Cell Biol. 2011, 31, 4720–4734. [Google Scholar] [CrossRef]
- Zheng, L.D.; Linarelli, L.E.; Liu, L.; Wall, S.S.; Greenawald, M.H.; Seidel, R.W.; Estabrooks, P.A.; Almeida, F.A.; Cheng, Z. Insulin Resistance Is Associated with Epigenetic and Genetic Regulation of Mitochondrial DNA in Obese Humans. Clin. Epigenetics 2015, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A.; et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef]
- Jiang, N.X.; Li, X.L. The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms. Reprod. Sci. 2022, 29, 2465–2476. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Baillargeon, J.P.; Carpentier, A. Role of Insulin in the Hyperandrogenemia of Lean Women with Polycystic Ovary Syndrome and Normal Insulin Sensitivity. Fertil. Steril. 2007, 88, 886–893. [Google Scholar] [CrossRef]
- Baillargeon, J.P.; Nestler, J.E. Commentary: Polycystic Ovary Syndrome: A Syndrome of Ovarian Hypersensitivity to Insulin? J. Clin. Endocrinol. Metab. 2006, 91, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; Falcon de Vargas, A.; Brik, C.; Quintero, N.; Medina, F. Insulin Stimulates Testosterone Biosynthesis by Human Thecal Cells from Women with Polycystic Ovary Syndrome by Activating Its Own Receptor and Using Inositolglycan Mediators as the Signal Transduction System. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A Direct Effect of Hyperinsulinemia on Serum Sex Hormone-Binding Globulin Levels in Obese Women with the Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Plymate, S.R.; Matej, L.A.; Jones, R.E.; Friedl, K.E. Inhibition of Sex Hormone-Binding Globulin Production in the Human Hepatoma (Hep G2) Cell Line by Insulin and Prolactin. J. Clin. Endocrinol. Metab. 1988, 67, 460–464. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic Dysfunction in Polycystic Ovary Syndrome: Pathogenic Role of Androgen Excess and Potential Therapeutic Strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Panidis, D.; Rousso, D.; Koliakos, G.; Kourtis, A.; Katsikis, I.; Farmakiotis, D.; Votsi, E.; Diamanti-Kandarakis, E. Plasma Metastin Levels Are Negatively Correlated with Insulin Resistance and Free Androgens in Women with Polycystic Ovary Syndrome. Fertil. Steril. 2006, 85, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Polak, K.; Czyzyk, A.; Simoncini, T.; Meczekalski, B. New Markers of Insulin Resistance in Polycystic Ovary Syndrome. J. Endocrinol. Investig. 2017, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shahin, L.; Hyassat, D.; Batieha, A.; Khader, Y.; El-Khateeb, M.; Ajlouni, K. Insulin Sensitivity Indices in Patients with Polycystic Ovary Syndrome with Different Body Mass Index Categories. Curr. Diabetes Rev. 2020, 16, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Vrbíková, J.; Cibula, D.; Dvořáková, K.; Stanická, S.; Šindelka, G.; Hill, M.; Fanta, M.; Vondra, K.; Škrha, J. Insulin Sensitivity in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 2942–2945. [Google Scholar] [CrossRef]
- Cozzolino, M.; Seli, E. Mitochondrial Function in Women with Polycystic Ovary Syndrome. Curr. Opin. Obstet. Gynecol. 2020, 32, 205–212. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, K.; Patel, S.; Gupta, A.; Varshney, S.; Gupta, S.; Rajan, S.; Srivastava, A.; Vishwakarma, A.L.; Gaikwad, A.N. Chronic Hyperinsulinemia Promotes Meta-Inflammation and Extracellular Matrix Deposition in Adipose Tissue: Implications of Nitric Oxide. Mol. Cell Endocrinol. 2018, 477, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Oróstica, L.; García, P.; Vera, C.; García, V.; Romero, C.; Vega, M. Effect of TNF-α on Molecules Related to the Insulin Action in Endometrial Cells Exposed to Hyperandrogenic and Hyperinsulinic Conditions Characteristics of Polycystic Ovary Syndrome. Reprod. Sci. 2018, 25, 1000–1009. [Google Scholar] [CrossRef]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal Diet-Induced Obesity Alters Mitochondrial Activity and Redox Status in Mouse Oocytes and Zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mohanty, P.; Ghanim, H.; Aljada, A.; Browne, R.; Hamouda, W.; Prabhala, A.; Afzal, A.; Garg, R. The Suppressive Effect of Dietary Restriction and Weight Loss in the Obese on the Generation of Reactive Oxygen Species by Leukocytes, Lipid Peroxidation, and Protein Carbonylation. J. Clin. Endocrinol. Metab. 2001, 86, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, T.; Vural, H.; Harma, M.; Harma, M. Oxidative Stress in Polycystic Ovary Syndrome and Its Contribution to the Risk of Cardiovascular Disease. Clin. Biochem. 2001, 34, 407–413. [Google Scholar] [CrossRef]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive Oxygen Species-Induced Oxidative Stress in the Development of Insulin Resistance and Hyperandrogenism in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin Resistance (HOMA-IR) Cut-off Values and the Metabolic Syndrome in a General Adult Population: Effect of Gender and Age: EPIRCE Cross-Sectional Study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef]
- Shimizu, I.; Yoshida, Y.; Katsuno, T.; Tateno, K.; Okada, S.; Moriya, J.; Yokoyama, M.; Nojima, A.; Ito, T.; Zechner, R.; et al. P53-Induced Adipose Tissue Inflammation Is Critically Involved in the Development of Insulin Resistance in Heart Failure. Cell Metab. 2012, 15, 51–64. [Google Scholar] [CrossRef]
- Anagnostis, P.; Tarlatzis, B.C.; Kauffman, R.P. Polycystic Ovarian Syndrome (PCOS): Long-Term Metabolic Consequences. Metabolism 2018, 86, 33–43. [Google Scholar] [CrossRef]
- Kelley, C.E.; Brown, A.J.; Diehl, A.M.; Setji, T.L. Review of Nonalcoholic Fatty Liver Disease in Women with Polycystic Ovary Syndrome. World J. Gastroenterol. 2014, 20, 14172–14184. [Google Scholar] [CrossRef]
- Nandi, A.; Chen, Z.; Patel, R.; Poretsky, L. Polycystic Ovary Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Guo, H.; Li, M. Hyperandrogenemia and Insulin Resistance: The Chief Culprit of Polycystic Ovary Syndrome. Life Sci. 2019, 236, 123–147. [Google Scholar] [CrossRef]
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered Lipid Metabolism and the Pathogenesis of Insulin Resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef]
- Pirwany, I.R.; Fleming, R.; Greer, I.A.; Packard, C.J.; Sattar, N. Lipids and Lipoprotein Subfractions in Women with PCOS: Relationship to Metabolic and Endocrine Parameters. Clin. Endocrinol. 2001, 54, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Essah, P.A.; Nestler, J.E.; Carmina, E. Differences in Dyslipidemia between American and Italian Women with Polycystic Ovary Syndrome. J. Endocrinol. Investig. 2008, 31, 35–41. [Google Scholar] [CrossRef]
- Tziomalos, K.G.; Athyros, V.; Karagiannis, A. Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes: Pathogenesis and Treatment Options. Curr. Vasc. Pharmacol. 2012, 10, 162–172. [Google Scholar] [CrossRef]
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the Polycystic Ovary Syndrome. Diabetes Res. Clin. Pract. 2017, 130, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Tran, T.P.; Afendy, A.; Wang, L.; Shamsaddini, A.; Mehta, R.; Chandhoke, V.; Birerdinc, A.; Younossi, Z.M. Molecular Signature of Adipose Tissue in Patients with Both Non-Alcoholic Fatty Liver Disease (NAFLD) and Polycystic Ovarian Syndrome (PCOS). J. Transl. Med. 2013, 11, 133. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Manson, J.E.; Li, J.; Harris, T.G.; Rohan, T.E.; Xue, X.N.; Ho, G.Y.F.; et al. A Prospective Evaluation of Insulin and Insulin-like Growth Factor-I as Risk Factors for Endometrial Cancer. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 921–929. [Google Scholar] [CrossRef]
- Rivero, R.; Garin, C.A.; Ormazabal, P.; Silva, A.; Carvajal, R.; Gabler, F.; Romero, C.; Vega, M. Protein Expression of PKCZ (Protein Kinase C Zeta), Munc18c, and Syntaxin-4 in the Insulin Pathway in Endometria of Patients with Polycystic Ovary Syndrome (PCOS). Reprod. Biol. Endocrinol. 2012, 10, 17. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, D.J.; Lee, H.S.; Kim, T.J.; Kim, M.H.; Jeong, H.J.; Im, J.A.; Lee, D.C.; Lee, J.W. Mitochondrial DNA Copy Number in Peripheral Blood in Polycystic Ovary Syndrome. Metabolism 2011, 60, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.V.; Govatati, S.; Deenadayal, M.; Sisinthy, S.; Bhanoori, M. Impact of Mitochondrial DNA Copy Number and Displacement Loop Alterations on Polycystic Ovary Syndrome Risk in South Indian Women. Mitochondrion 2019, 44, 35–40. [Google Scholar] [CrossRef]
- Pruett, J.E.; Everman, S.J.; Hoang, N.H.; Salau, F.; Taylor, L.C.; Edwards, K.S.; Hosler, J.P.; Huffman, A.M.; Romero, D.G.; Yanes Cardozo, L.L. Mitochondrial Function and Oxidative Stress in White Adipose Tissue in a Rat Model of PCOS: Effect of SGLT2 Inhibition. Biol. Sex. Differ. 2022, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Shukla, P.; Dange, P.; Mohanty, B.S.; Gadewal, N.; Chaudhari, P.; Sarin, R. ARID2 Suppression Promotes Tumor Progression and Upregulates Cytokeratin 8, 18 and β-4 Integrin Expression in TP53-Mutated Tobacco-Related Oral Cancer and Has Prognostic Implications. Cancer Gene Ther. 2022, 29, 1908–1917. [Google Scholar] [CrossRef]
- Tauffenberger, A.; Magistretti, P.J. Reactive Oxygen Species: Beyond Their Reactive Behavior. Neurochem. Res. 2021, 46, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Mitochondrial DNA copy number variation and gene mutations mediate polycystic ovary syndrome: Research progress. Am. J. Transl. Res. 2024, 16, 6303–6313. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, B.H.; Zhang, C.J.; Zhuo, G.C. Mutations in Mitochondrial TRNA Genes May Be Related to Insulin Resistance in Women with Polycystic Ovary Syndrome. Am. J. Transl. Res. 2017, 9, 2984. [Google Scholar]
- Lai, Q.; Xiang, W.; Li, Q.; Zhang, H.; Li, Y.; Zhu, G.; Xiong, C.; Jin, L. Oxidative Stress in Granulosa Cells Contributes to Poor Oocyte Quality and IVF-ET Outcomes in Women with Polycystic Ovary Syndrome. Front. Med. 2018, 12, 518–524. [Google Scholar] [CrossRef]
- Ernst, E.H.; Lykke-Hartmann, K. Transcripts Encoding Free Radical Scavengers in Human Granulosa Cells from Primordial and Primary Ovarian Follicles. J. Assist. Reprod. Genet. 2018, 35, 1787–1798. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, Q.; Long, S.L.; Zhong, Q.; Mo, Z. Mitochondrial Dysfunction in Polycystic Ovary Syndrome. DNA Cell Biol. 2020, 39, 1401–1409. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Li, T.; Li, M.; Li, J.; Li, R.; Liu, P.; Yu, Y.; Qiao, J. Metabolism alteration in follicular niche: The nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015, 86, 295–307. [Google Scholar] [CrossRef]
- Luca, T.; Pezzino, S.; Puleo, S.; Castorina, S. Lesson on obesity and anatomy of adipose tissue: New models of study in the era of clinical and translational research. J. Transl. Med. 2024, 22, 1–18. [Google Scholar] [CrossRef]
- Horvath, T.L.; Andrews, Z.B.; Diano, S. Fuel Utilization by Hypothalamic Neurons: Roles for ROS. Trends Endocrinol. Metab. 2009, 20, 78–87. [Google Scholar] [CrossRef]
- Malin, S.K.; Kirwan, J.P.; Sia, C.L.; González, F. Glucose-Stimulated Oxidative Stress in Mononuclear Cells Is Related to Pancreatic β-Cell Dysfunction in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 322–329. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear Factor-KappaB: A Pivotal Transcription Factor in Chronic Inflammatory Diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef]
- Anusree, S.S.; Nisha, V.M.; Priyanka, A.; Raghu, K.G. Insulin Resistance by TNF-α Is Associated with Mitochondrial Dysfunction in 3T3-L1 Adipocytes and Is Ameliorated by Punicic Acid, a PPARγ Agonist. Mol. Cell Endocrinol. 2015, 413, 120–128. [Google Scholar] [CrossRef]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; De Marañon, A.M.; Rios-Navarro, C.; Alvarez, A.; Gomez, M.; Rocha, M.; Hernández-Mijares, A. Insulin Resistance in PCOS Patients Enhances Oxidative Stress and Leukocyte Adhesion: Role of Myeloperoxidase. PLoS ONE 2016, 11, e0151960. [Google Scholar] [CrossRef]
- Konopka, A.R.; Asante, A.; Lanza, I.R.; Robinson, M.M.; Johnson, M.L.; Man, C.D.; Cobelli, C.; Amols, M.H.; Irving, B.A.; Nair, K.S. Defects in Mitochondrial Efficiency and H2O2 Emissions in Obese Women Are Restored to a Lean Phenotype with Aerobic Exercise Training. Diabetes 2015, 64, 2104–2115. [Google Scholar] [CrossRef]
- Skov, V.; Glintborg, D.; Knudsen, S.; Jensen, T.; Kruse, T.A.; Tan, Q.; Brusgaard, K.; Beck-Nielsen, H.; Højlund, K. Reduced Expression of Nuclear-Encoded Genes Involved in Mitochondrial Oxidative Metabolism in Skeletal Muscle of Insulin-Resistant Women with Polycystic Ovary Syndrome. Diabetes 2007, 56, 2349–2355. [Google Scholar] [CrossRef]
- Boots, C.E.; Boudoures, A.; Zhang, W.; Drury, A.; Moley, K.H. Obesity-Induced Oocyte Mitochondrial Defects Are Partially Prevented and Rescued by Supplementation with Co-Enzyme Q10 in a Mouse Model. Hum. Reprod. 2016, 31, 2090–2097. [Google Scholar] [CrossRef]
- Rabøl, R.; Svendsen, P.F.; Skovbro, M.; Boushel, R.; Schjerling, P.; Nilas, L.; Madsbad, S.; Dela, F. Skeletal Muscle Mitochondrial Function in Polycystic Ovarian Syndrome. Eur. J. Endocrinol. 2011, 165, 631–637. [Google Scholar] [CrossRef]
- Liu, X.; Trakooljul, N.; Hadlich, F.; Murani, E.; Wimmers, K.; Ponsuksili, S. Mitochondrial-Nuclear Crosstalk, Haplotype and Copy Number Variation Distinct in Muscle Fiber Type, Mitochondrial Respiratory and Metabolic Enzyme Activities. Sci. Rep. 2017, 7, 14024. [Google Scholar] [CrossRef]
- De Marco, G.; Garcia-Garcia, A.B.; Real, J.T.; Gonzalez-Albert, V.; Briongos-Figuero, L.S.; Cobos-Siles, M.; Lago-Sampedro, A.; Corbaton, A.; Teresa Martinez-Larrad, M.; Carmena, R.; et al. Respiratory Chain Polymorphisms and Obesity in the Spanish Population, a Cross-Sectional Study. BMJ Open 2019, 9, e027004. [Google Scholar] [CrossRef]
- Reddy, T.V.; Govatati, S.; Deenadayal, M.; Shivaji, S.; Bhanoori, M. Polymorphisms in the TFAM and PGC1-α Genes and Their Association with Polycystic Ovary Syndrome among South Indian Women. Gene 2018, 641, 129–136. [Google Scholar] [CrossRef]
- Tharayil, S.P.; Rasal, S.; Gawde, U.; Mukherjee, S.; Patil, A.; Joshi, B.; Idicula-Thomas, S.; Shukla, P. Relation of mitochondrial DNA copy number and variants with the clinical characteristics of polycystic ovary syndrome. Mol. Cell Endocrinol. 2024, 594, 112386. [Google Scholar] [CrossRef]
- Deng, X.; Ji, D.; Li, X.; Xu, Y.; Cao, Y.; Zou, W.; Liang, C.; Lee Marley, J.; Zhang, Z.; Wei, Z.; et al. Polymorphisms and Haplotype of Mitochondrial DNA D-Loop Region Are Associated with Polycystic Ovary Syndrome in a Chinese Population. Mitochondrion 2021, 57, 173–181. [Google Scholar] [CrossRef]
- Zhuo, G.; Ding, Y.; Feng, G.; Yu, L.; Jiang, Y. Analysis of Mitochondrial DNA Sequence Variants in Patients with Polycystic Ovary Syndrome. Arch. Gynecol. Obstet. 2012, 286, 653–659. [Google Scholar] [CrossRef]
- Zhu, J.; Vinothkumar, K.R.; Hirst, J. Structure of Mammalian Respiratory Complex I. Nature 2016, 536, 354–358. [Google Scholar] [CrossRef]
- Kokaze, A.; Ishikawa, M.; Matsunaga, N.; Yoshida, M.; Sekine, Y.; Sekiguchi, K.; Harada, M.; Satoh, M.; Teruya, K.; Takeda, N.; et al. Longevity-Associated Mitochondrial DNA 5178 A/C Polymorphism and Blood Pressure in the Japanese Population. J. Hum. Hypertens. 2004, 18, 41–45. [Google Scholar] [CrossRef]
- Mukae, S.; Aoki, S.; Itoh, S.; Sato, R.; Nishio, K.; Iwata, T.; Katagiri, T. Mitochondrial 5178A/C Genotype Is Associated with Acute Myocardial Infarction. Circ. J. 2003, 67, 16–20. [Google Scholar] [CrossRef]
- Jiang, Z.; Teng, L.; Zhang, S.; Ding, Y. Mitochondrial ND1 T4216C and ND2 C5178A Mutations Are Associated with Maternally Transmitted Diabetes Mellitus. Mitochondrial DNA Part A 2021, 32, 59–65. [Google Scholar] [CrossRef]
- Ding, Y.; Zhuo, G.; Zhang, C.; Leng, J. Point Mutation in Mitochondrial TRNA Gene Is Associated with Polycystic Ovary Syndrome and Insulin Resistance. Mol. Med. Rep. 2016, 13, 3169–3172. [Google Scholar] [CrossRef]
- Jiang, Z.; Cai, X.; Kong, J.; Zhang, R.; Ding, Y. Maternally Transmitted Diabetes Mellitus May Be Associated with Mitochondrial ND5 T12338C and TRNAAla T5587C Variants. Ir. J. Med. Sci. 2022, 191, 2625–2633. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Y.; Lu, Y.; Fu, R.; Xu, M.; Liu, X.; Guan, M.X. Leber’s Hereditary Optic Neuropathy (LHON)-Associated ND5 12338T > C Mutation Altered the Assembly and Function of Complex I, Apoptosis and Mitophagy. Hum. Mol. Genet. 2018, 27, 1999–2011. [Google Scholar] [CrossRef]
- Huoponen, K.; Lamminen, T.; Juvonen, V.; Aula, P.; Nikoskelainen, E.; Savontaus, M.L. The Spectrum of Mitochondrial DNA Mutations in Families with Leber Hereditary Optic Neuroretinopathy. Hum. Genet. 1993, 92, 379–384. [Google Scholar] [CrossRef]
- Rinaldi, T.; Lande, R.; Bolotin-Fukuhara, M.; Frontali, L. Additional Copies of the Mitochondrial Ef-Tu and Aspartyl-TRNA Synthetase Genes Can Compensate for a Mutation Affecting the Maturation of the Mitochondrial TRNAAsp. Curr. Genet. 1997, 31, 494–496. [Google Scholar] [CrossRef]
- Belostotsky, R.; Frishberg, Y.; Entelis, N. Human Mitochondrial TRNA Quality Control in Health and Disease: A Channelling Mechanism? RNA Biol. 2012, 9, 33–39. [Google Scholar] [CrossRef]
- Ye, M.; Hu, B.; Shi, W.; Guo, F.; Xu, C.; Li, S. Mitochondrial DNA 4977 Bp in Peripheral Blood Is Associated with Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 675581. [Google Scholar] [CrossRef]
- Saeed, N.A.H.A.A.H.; Hamzah, I.H.; Al-Gharrawi, S.A.R. Polycystic Ovary Syndrome Dependency on MtDNA Mutation; Copy Number and Its Association with Insulin Resistance. BMC Res. Notes 2019, 12, 455. [Google Scholar] [CrossRef]
- He, S.; Ji, D.; Liu, Y.; Deng, X.; Zou, W.; Liang, D.; Du, Y.; Zong, K.; Jiang, T.; Li, M.; et al. Polymorphisms of MtDNA in the D-Loop Region Moderate the Associations of BMI with HOMA-IR and HOMA-β among Women with Polycystic Ovary Syndrome: A Cross-Sectional Study. J. Assist. Reprod. Genet. 2023, 40, 1983–1993. [Google Scholar] [CrossRef]
- Ilie, I.R. Advances in PCOS Pathogenesis and Progression-Mitochondrial Mutations and Dysfunction. Adv. Clin. Chem. 2018, 86, 127–155. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Andersen, M.; Azziz, R.; et al. Recommendations from the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Samadi, Z.; Bambaeichi, E.; Valiani, M.; Shahshahan, Z. Evaluation of Changes in Levels of Hyperandrogenism, Hirsutism and Menstrual Regulation After a Period of Aquatic High Intensity Interval Training in Women with Polycystic Ovary Syndrome. Int. J. Prev. Med. 2019, 10, 187. [Google Scholar] [CrossRef]
- Shele, G.; Genkil, J.; Speelman, D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J. Funct. Morphol. Kinesiol. 2020, 5, 35. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Nikiforov, N.G.; Eid, A.H.; Nedosugova, L.V.; Starodubova, A.V.; Popkova, T.V.; Bezsonov, E.E.; Orekhov, A.N. Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 3923. [Google Scholar] [CrossRef]
- Silva Dantas, W.; Antonio Miguel Marcondes, J.; Katsuyuki Shinjo, S.; Augusto Perandini, L.; Olzon Zambelli, V.; Das Neves, W.; Roberto Grimaldi Barcellos, C.; Patrocínio Rocha, M.; Dos Reis Vieira Yance, V.; Tavares Dos Santos Pereira, R.; et al. GLUT4 Translocation Is Not Impaired after Acute Exercise in Skeletal Muscle of Women with Obesity and Polycystic Ovary Syndrome. Obesity 2015, 23, 2207–2215. [Google Scholar] [CrossRef]
- Dantas, W.S.; Murai, I.H.; Perandini, L.A.; Azevedo, H.; Moreira-Filho, C.A.; Camara, N.O.S.; Roschel, H.; Gualano, B. Acute Exercise Elicits Differential Expression of Insulin Resistance Genes in the Skeletal Muscle of Patients with Polycystic Ovary Syndrome. Clin. Endocrinol. 2017, 86, 688–697. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Almenning, I.; Rieber-Mohn, A.; Lundgren, K.M.; Løvvik, T.S.; Garnæs, K.K.; Moholdt, T. Effects of High Intensity Interval Training and Strength Training on Metabolic, Cardiovascular and Hormonal Outcomes in Women with Polycystic Ovary Syndrome: A Pilot Study. PLoS ONE 2015, 10, e0138793. [Google Scholar] [CrossRef]
- García-Prieto, C.F.; Fernández-Alfonso, M.S. Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders. Nutrients 2016, 8, 370. [Google Scholar] [CrossRef]
- Dengel, D.R.; Pratley, R.E.; Hagberg, J.M.; Rogus, E.M.; Goldberg, A.P. Distinct Effects of Aerobic Exercise Training and Weight Loss on Glucose Homeostasis in Obese Sedentary Men. J. Appl. Physiol. 1996, 81, 318–325. [Google Scholar] [CrossRef]
- Weiss, E.P.; Racette, S.B.; Villareal, D.T.; Fontana, L.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O. Improvements in Glucose Tolerance and Insulin Action Induced by Increasing Energy Expenditure or Decreasing Energy Intake: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2006, 84, 1033–1042. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Alfonso, A.; Ravussin, E. Effect of Calorie Restriction with or without Exercise on Insulin Sensitivity, Beta-Cell Function, Fat Cell Size, and Ectopic Lipid in Overweight Subjects. Diabetes Care 2006, 29, 1337–1344. [Google Scholar] [CrossRef]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Tsuda, S.I.; Kanasaki, K.; Koya, D. Calorie Restriction in Overweight Males Ameliorates Obesity-Related Metabolic Alterations and Cellular Adaptations through Anti-Aging Effects, Possibly Including AMPK and SIRT1 Activation. Biochim. Biophys. Acta 2013, 1830, 4820–4827. [Google Scholar] [CrossRef]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 Enhances Skeletal Muscle Insulin Sensitivity in Mice during Caloric Restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef]
- Shukla, P.; Melkani, G.C. Mitochondrial Epigenetic Modifications and Nuclear-Mitochondrial Communication: A New Dimension towards Understanding and Attenuating the Pathogenesis in Women with PCOS. Rev. Endocr. Metab. Disord. 2023, 24, 317–326. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Zhou, F.; Wu, Q.; Ma, C.; Zhang, Y.; Ding, J.; Hua, K. Life Modifications and PCOS: Old Story But New Tales. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Koh, E.S.; Kim, J.E. Lowering Breakfast Glycemic Index and Glycemic Load Attenuates Postprandial Glycemic Response: A Systematically Searched Meta-Analysis of Randomized Controlled Trials. Nutrition 2020, 71, 110634. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Long-Term Effects of Low Glycemic Index/Load vs. High Glycemic Index/Load Diets on Parameters of Obesity and Obesity-Associated Risks: A Systematic Review and Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Biltoft-Jensen, A.; Tetens, I.; Michaelsen, K.F.; Lind, M.V.; Astrup, A.; Landberg, R. Whole-Grain Intake, Reflected by Dietary Records and Biomarkers, Is Inversely Associated with Circulating Insulin and Other Cardiometabolic Markers in 8- to 11-Year-Old Children. J. Nutr. 2017, 147, 816–824. [Google Scholar] [CrossRef]
- Heikkilä, H.M.; Krachler, B.; Rauramaa, R.; Schwab, U.S. Diet, Insulin Secretion and Insulin Sensitivity--the Dose-Responses to Exercise Training (DR’s EXTRA) Study (ISRCTN45977199). Br. J. Nutr. 2014, 112, 1530–1541. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE Restriction Ameliorates Insulin Resistance in Obese Individuals with the Metabolic Syndrome: A Randomised Controlled Trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.S.; Beverley, R.; Barnard, E.; Baradaran-Shoraka, M.; Sanfilippo, J.S. Polycystic Ovary Syndrome in Adolescents. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 103–114. [Google Scholar] [CrossRef]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef] [PubMed]
- Homburg, R.; Eshel, A.; Abdalla, H.I.; Jacobs, H.S. Growth Hormone Facilitates Ovulation Induction by Gonadotrophins. Clin. Endocrinol. 1988, 29, 113–117. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Jin, S.; Zhu, H.; Deng, T.; Quan, Y.; Huang, W. Growth Hormone Alleviates Oxidative Stress and Improves Oocyte Quality in Chinese Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Sci. Rep. 2020, 10, 18769. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The Management of Anovulatory Infertility in Women with Polycystic Ovary Syndrome: An Analysis of the Evidence to Support the Development of Global WHO Guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Luz, M.; Albarracín, G.; Yibby, A.; Torres, F.; Albarracin, G. Adiponectin and Leptin Adipocytokines in Metabolic Syndrome: What Is Its Importance? Dubai Diabetes Endocrinol. J. 2020, 26, 93–102. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Donà, G.; Sabbadin, C.; Fiore, C.; Bragadin, M.; Giorgino, F.L.; Ragazzi, E.; Clari, G.; Bordin, L.; Armanini, D. Inositol Administration Reduces Oxidative Stress in Erythrocytes of Patients with Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2012, 166, 703–710. [Google Scholar] [CrossRef]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public. Health 2019, 16, 2965. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic-Radosevic, J.; Cigrovski Berkovic, M.; Kruezi, E.; Bilic-Curcic, I.; Mrzljak, A. Exploring New Treatment Options for Polycystic Ovary Syndrome: Review of a Novel Antidiabetic Agent SGLT2 Inhibitor. World J. Diabetes 2021, 12, 932–938. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, T.; Sheng, Y.; Li, R.; Xu, H. The Effects of Letrozole and Metformin Combined with Targeted Nursing Care on Ovarian Function, LH, and FSH in Infertile Patients with Polycystic Ovary Syndrome. J. Healthc. Eng. 2022, 2022, 3712166. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of Insulin and Insulin Resistance in Androgen Excess Disorders. World J. Diabetes 2021, 12, 616. [Google Scholar] [CrossRef]
- Patel, S. Polycystic Ovary Syndrome (PCOS), an Inflammatory, Systemic, Lifestyle Endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Anderson, R.A.; Graham, G.M.; Chu, M.C.; Sauer, M.V.; Guarnaccia, M.M.; Lobo, R.A. The Effect of Cinnamon Extract on Insulin Resistance Parameters in Polycystic Ovary Syndrome: A Pilot Study. Fertil. Steril. 2007, 88, 240–243. [Google Scholar] [CrossRef]
- Nabiuni, M.; Kayedpoor, P.; Mohammadi, S.; Karimzadeh, L. Effect of Silymarin on Estradiol Valerate- Induced Polycystic Ovary Syndrome. Med. Sci. J. Islam. Azad Univesity—Tehran Med. Branch 2015, 25, 16–26. [Google Scholar]
- Azhar, A.; Haider, G.; Naseem, Z.; Farooqui, N.; Farooqui, M.U.; Rehman, R. Morphological Changes in the Experimental Model of Polycystic Ovary Syndrome and Effects of Vitamin D Treatment. J. Obstet. Gynaecol. Res. 2021, 47, 1164–1171. [Google Scholar] [CrossRef]
- Safaei, Z.; Bakhshalizadeh, S.; Nasr-Esfahani, M.H.; Akbari Sene, A.; Najafzadeh, V.; Soleimani, M.; Shirazi, R. Vitamin D3 Affects Mitochondrial Biogenesis through Mitogen-Activated Protein Kinase in Polycystic Ovary Syndrome Mouse Model. J. Cell Physiol. 2020, 235, 6113–6126. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, Y.; Wu, G.; Zheng, L. Oxidative Stress and Mitochondrial Dysfunction of Granulosa Cells in Polycystic Ovarian Syndrome. Front. Med. 2023, 10, 1193749. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Z.; Xia, B.; Zhang, L.; Zhang, C.; Leng, J. Mitochondria-Targeted Antioxidant Therapy for an Animal Model of PCOS-IR. Int. J. Mol. Med. 2019, 43, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Q.; Wang, H.; Zhu, J.; Cong, L.; Li, H.; Sun, Y. NAD+ Deficiency and Mitochondrial Dysfunction in Granulosa Cells of Women with Polycystic Ovary Syndrome‡. Biol. Reprod. 2021, 105, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, X.; Liu, W.; Chen, Z.; Xiao, X.; Deng, G. Supplementation with NAD+ and Its Precursors: A Rescue of Female Reproductive Diseases. Biochem. Biophys. Rep. 2024, 38, 101715. [Google Scholar] [CrossRef]
- Yen, K.; Lee, C.; Mehta, H.; Cohen, P. The Emerging Role of the Mitochondrial-Derived Peptide Humanin in Stress Resistance. J. Mol. Endocrinol. 2013, 50, R11–R19. [Google Scholar] [CrossRef]
- Kim, S.J.; Xiao, J.; Wan, J.; Cohen, P.; Yen, K. Mitochondrially Derived Peptides as Novel Regulators of Metabolism. J. Physiol. 2017, 595, 6613–6621. [Google Scholar] [CrossRef] [PubMed]
- Kuliawat, R.; Klein, L.; Gong, Z.; Nicoletta-Gentile, M.; Nemkal, A.; Cui, L.; Bastie, C.; Su, K.; Huffman, D.; Surana, M.; et al. Potent Humanin Analog Increases Glucose-Stimulated Insulin Secretion through Enhanced Metabolism in the β Cell. FASEB J. 2013, 27, 4890–4898. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, R.H.; Huffman, D.M.; Atzmon, G.; Buettner, C.; Cobb, L.J.; Fishman, S.; Budagov, T.; Cui, L.; Einstein, F.H.; Poduval, A.; et al. Humanin: A Novel Central Regulator of Peripheral Insulin Action. PLoS ONE 2009, 4, e6334. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Zhao, S.; Tang, L.; Yan, J.; Li, N.; Zou, L.; Fan, X.; Xu, C.; Huang, J.; et al. Humanin Alleviates Insulin Resistance in Polycystic Ovary Syndrome: A Human and Rat Model-Based Study. Endocrinology 2021, 162, bqab056. [Google Scholar] [CrossRef]
- Lee, C. Nuclear Transcriptional Regulation by Mitochondrial-Encoded MOTS-c. Mol. Cell Oncol. 2019, 6, 1–2. [Google Scholar] [CrossRef]
- Cobb, L.J.; Lee, C.; Xiao, J.; Yen, K.; Wong, R.G.; Nakamura, H.K.; Mehta, H.H.; Gao, Q.; Ashur, C.; Huffman, D.M.; et al. Naturally Occurring Mitochondrial-Derived Peptides Are Age-Dependent Regulators of Apoptosis, Insulin Sensitivity, and Inflammatory Markers. Aging 2016, 8, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.F.; Myatt, L. Placental Mitochondrial Dysfunction with Metabolic Diseases: Therapeutic Approaches. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165967. [Google Scholar] [CrossRef]
- Kolhe, J.V.; Chhipa, A.S.; Butani, S.; Chavda, V.; Patel, S.S. PCOS and Depression: Common Links and Potential Targets. Reprod. Sci. 2022, 29, 3106–3123. [Google Scholar] [CrossRef]
- Patel, A.; Dewani, D.; Jaiswal, A.; Yadav, P.; Reddy, L.S. Exploring Melatonin’s Multifaceted Role in Polycystic Ovary Syndrome Management: A Comprehensive Review. Cureus 2023, 15, e48929. [Google Scholar] [CrossRef]
- Sam, S.; Tasali, E. Role of obstructive sleep apnea in metabolic risk in PCOS. Curr. Opin. Endocr. Metab. Res. 2021, 17, 46–51. [Google Scholar] [CrossRef]
- He, J.; Ruan, X.; Li, J. Polycystic ovary syndrome in obstructive sleep apnea-hypopnea syndrome: An updated meta-analysis. Front. Endocrinol. 2024, 15, 1418933. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Gamage, U.S.K.; Yamochi, T.; Saeki, N.; Morimoto, N.; Yamanaka, M.; Koike, A.; Miyamoto, Y.; Tanaka, K.; Fukuda, A.; et al. Mitochondrial Transfer into Human Oocytes Improved Embryo Quality and Clinical Outcomes in Recurrent Pregnancy Failure Cases. Int. J. Mol. Sci. 2023, 24, 2738. [Google Scholar] [CrossRef]
- Abdi, A.; Ranjbaran, M.; Amidi, F.; Akhondzadeh, F.; Seifi, B. The Effect of Adipose-Derived Mesenchymal Stem Cell Transplantation on Ovarian Mitochondrial Dysfunction in Letrozole-Induced Polycystic Ovary Syndrome in Rats: The Role of PI3K-AKT Signaling Pathway. J. Ovarian Res. 2024, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharayil, S.P.; Shukla, P. Connecting the Dots: Mitochondrial Dysfunction, PCOS, and Insulin Resistance—Insights and Therapeutic Advances. Int. J. Mol. Sci. 2025, 26, 6233. https://doi.org/10.3390/ijms26136233
Tharayil SP, Shukla P. Connecting the Dots: Mitochondrial Dysfunction, PCOS, and Insulin Resistance—Insights and Therapeutic Advances. International Journal of Molecular Sciences. 2025; 26(13):6233. https://doi.org/10.3390/ijms26136233
Chicago/Turabian StyleTharayil, Samia Palat, and Pallavi Shukla. 2025. "Connecting the Dots: Mitochondrial Dysfunction, PCOS, and Insulin Resistance—Insights and Therapeutic Advances" International Journal of Molecular Sciences 26, no. 13: 6233. https://doi.org/10.3390/ijms26136233
APA StyleTharayil, S. P., & Shukla, P. (2025). Connecting the Dots: Mitochondrial Dysfunction, PCOS, and Insulin Resistance—Insights and Therapeutic Advances. International Journal of Molecular Sciences, 26(13), 6233. https://doi.org/10.3390/ijms26136233






