Multispectral Pulsed Photobiomodulation Enhances Diabetic Wound Healing via Focal Adhesion-Mediated Cell Migration and Extracellular Matrix Remodeling
Abstract
1. Introduction
2. Result
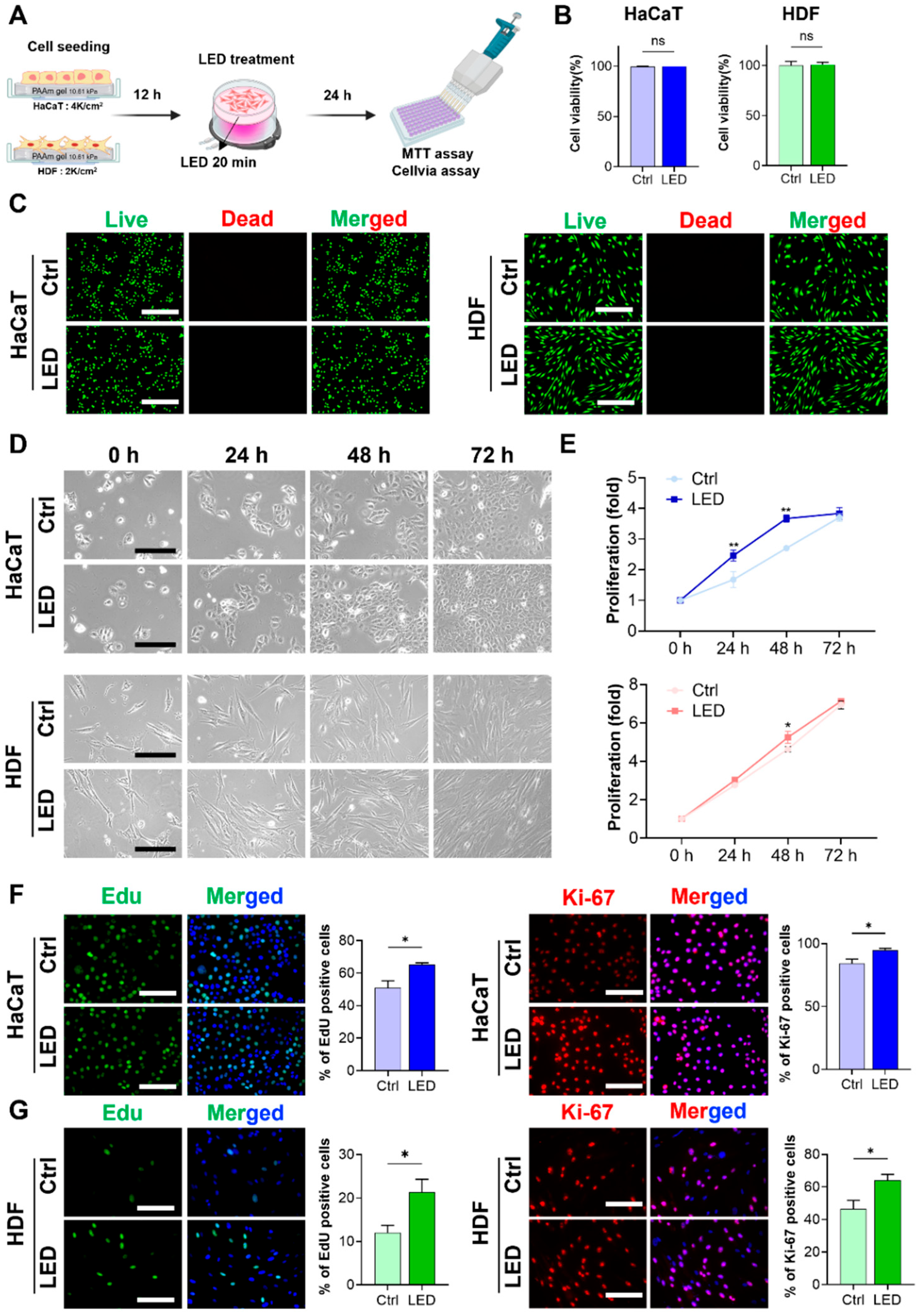
2.1. LED Photostimulation Enhances Keratinocyte and Fibroblast Proliferation
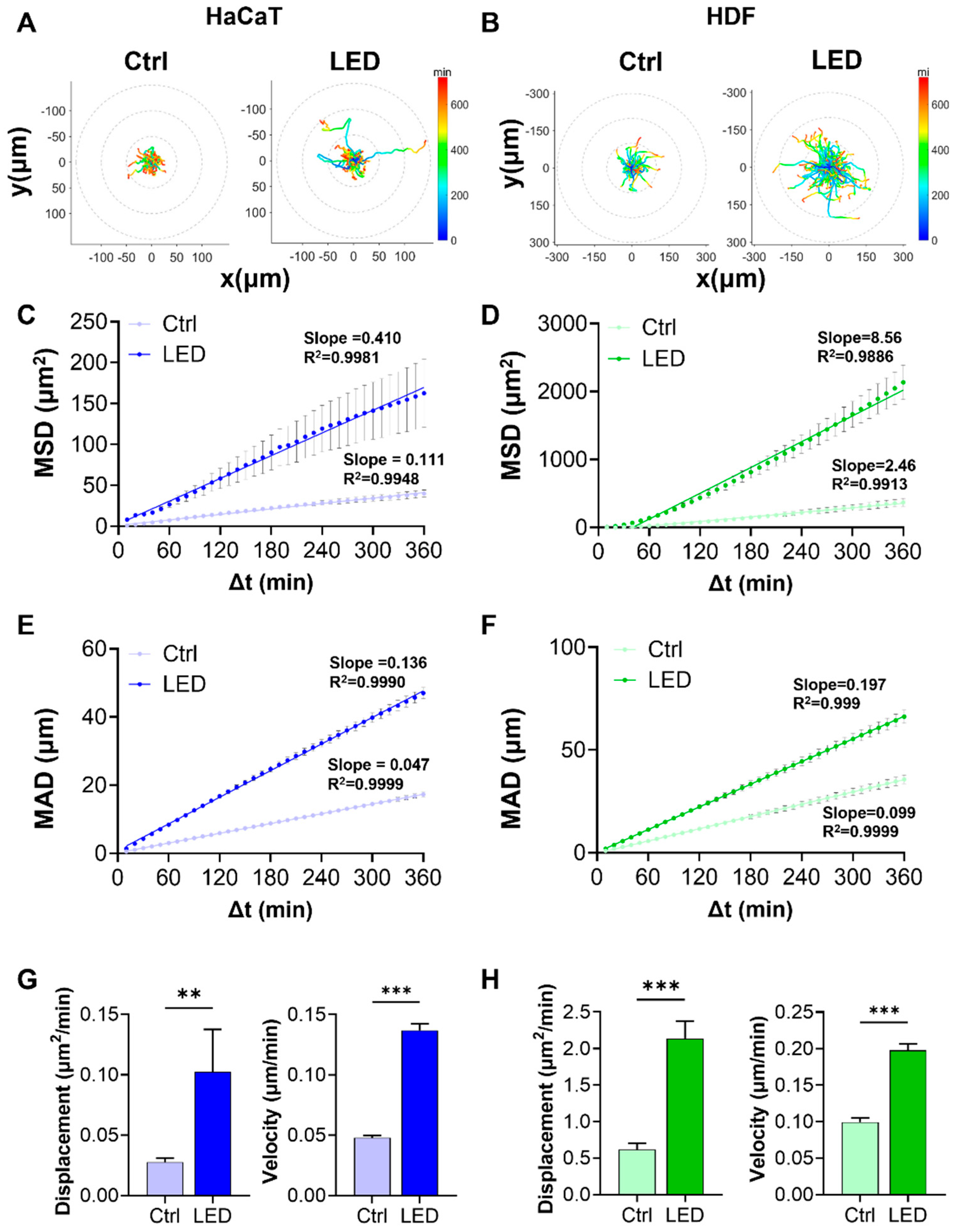
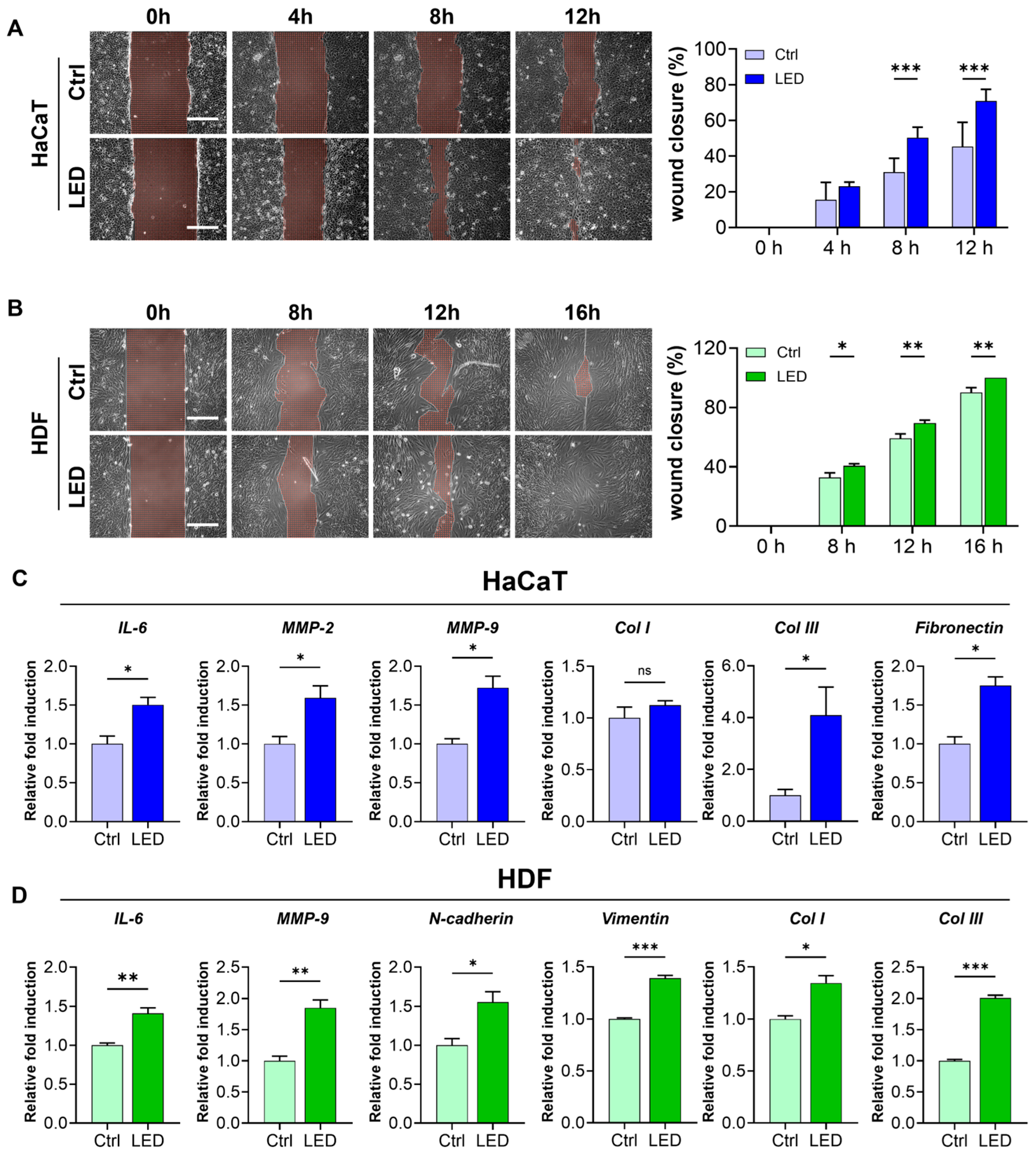
2.2. LED Photostimulation Enhances Cell Migration
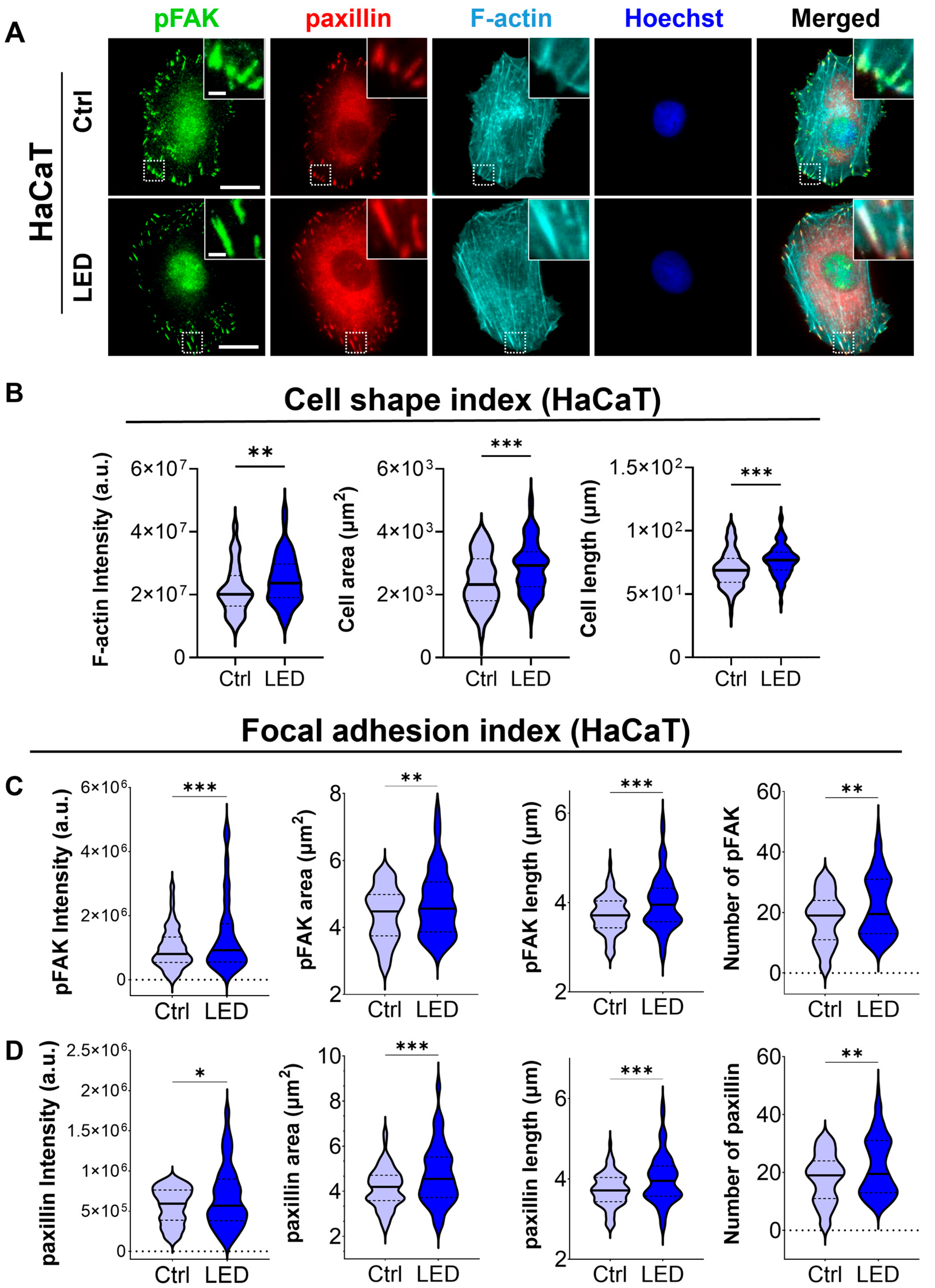
2.3. LED Photostimulation Enhances Focal Adhesion Signaling and Cytoskeletal Remodeling
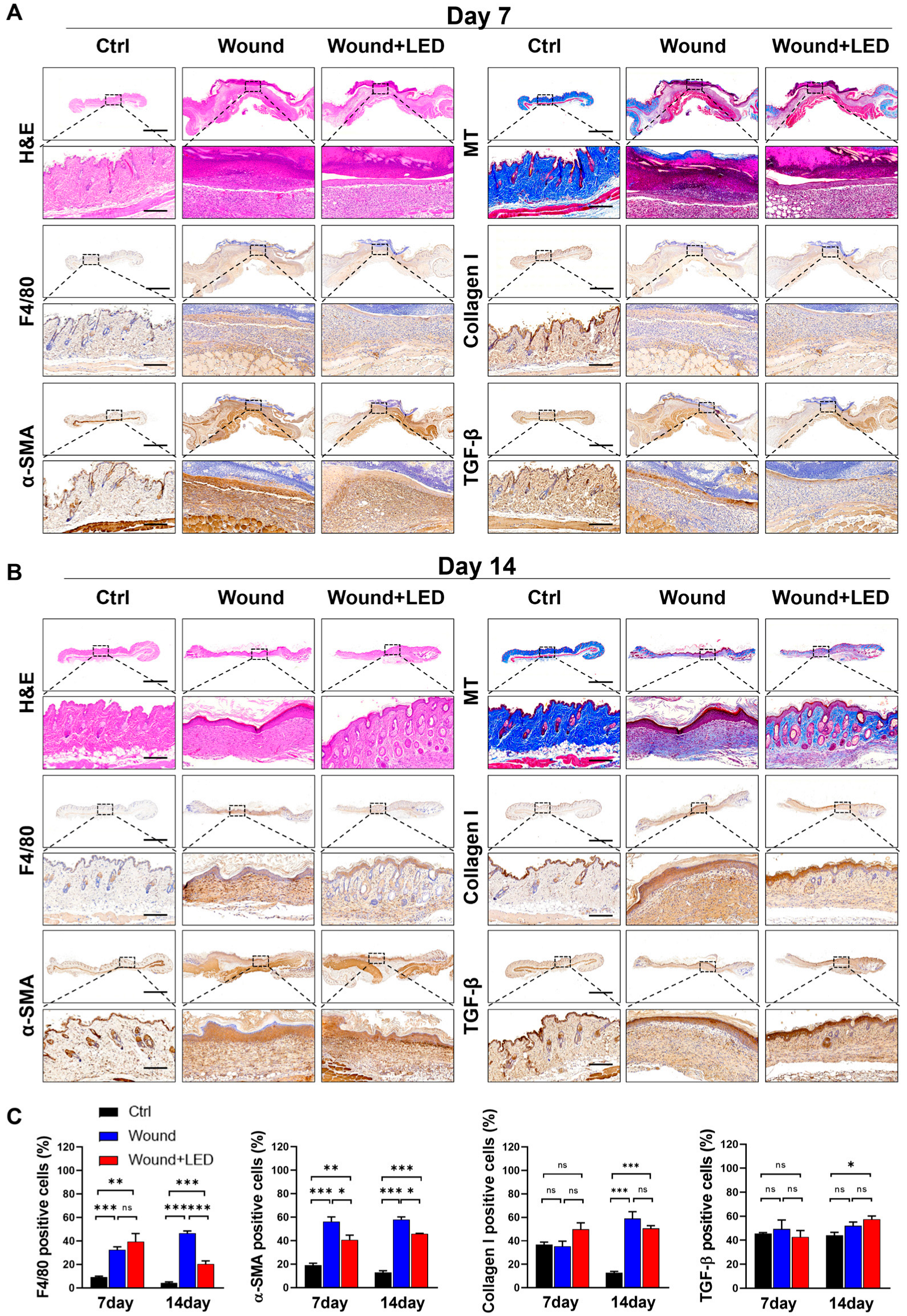
2.4. LED Photobiomodulation Accelerates Wound Healing and Modulates Fibrotic Remodeling in an STZ-Induced Diabetic Model
2.5. LED Photobiomodulation Modulates ECM Remodeling and Myofibroblast Activation During Late-Stage Wound Healing
3. Discussion
4. Materials and Methods
4.1. Photomodulation Device
4.2. Preparation of Polyacrylamide (PAA) Gels
4.3. Cell Culture and LED Treatment
4.4. Single-Cell Migration Assay
4.5. Collective Cell Migration Assay
4.6. Cell Viability Assay
4.7. Cell Proliferation Analysis
4.8. RNA Isolation and Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Streptozotocin (STZ)-Induced Mouse Model of Diabetes
4.10. Treatment of Diabetic Mouse Skin with LED
4.11. Measurement of Wound Closure
4.12. Histological Analysis of Skin Tissues
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef] [PubMed]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Vizely, K.; Wagner, K.T.; Mandla, S.; Gustafson, D.; Fish, J.E.; Radisic, M. Angiopoietin-1 derived peptide hydrogel promotes molecular hallmarks of regeneration and wound healing in dermal fibroblasts. iScience 2023, 26, 105984. [Google Scholar] [CrossRef]
- Kim, H.; Anggradita, L.D.; Lee, S.J.; Hur, S.S.; Bae, J.; Hwang, N.S.; Nam, S.M.; Hwang, Y. Ameliorating Fibrotic Phenotypes of Keloid Dermal Fibroblasts through an Epidermal Growth Factor-Mediated Extracellular Matrix Remodeling. Int. J. Mol. Sci. 2021, 22, 2198. [Google Scholar] [CrossRef]
- Subramaniam, M.D.; Bae, J.S.; Son, J.; Anggradita, L.D.; Kim, M.K.; Lee, M.Y.; Jang, S.; Choi, K.; Lee, J.C.; Nam, S.M.; et al. Floating electrode-dielectric barrier discharge-based plasma promotes skin regeneration in a full-thickness skin defect mouse model. Biomed. Eng. Lett. 2024, 14, 605–616. [Google Scholar] [CrossRef]
- Ahmad, J. The diabetic foot. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 48–60. [Google Scholar]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37–53. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. Epma J. 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.A.; Jere, S.W.; Houreld, N.N. Current Therapeutic Modalities for the Management of Chronic Diabetic Wounds of the Foot. J. Diabetes Res. 2023, 2023, 1359537. [Google Scholar] [CrossRef]
- Edwards, J.; Stapley, S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010, 2010, Cd003556. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Li, H.; Chen, X.; Feng, S.; Mei, Z. How Effective are Nano-Based Dressings in Diabetic Wound Healing? A Comprehensive Review of Literature. Int. J. Nanomed. 2022, 17, 2097–2119. [Google Scholar] [CrossRef]
- Mansouri, V.; Arjmand, B.; Rezaei Tavirani, M.; Razzaghi, M.; Rostami-Nejad, M.; Hamdieh, M. Evaluation of Efficacy of Low-Level Laser Therapy. J. Lasers Med. Sci. 2020, 11, 369–380. [Google Scholar] [CrossRef]
- Chung, E.H.; Son, J.W.; Eun, Y.S.; Yang, N.G.; Kim, J.Y.; Lee, S.; Heo, N.H.; Rhee, J.; Lee, S.Y.; Hwang, Y.; et al. A Novel, Hand-Held, and Low-Level Light Therapy Device for the Treatment of Acne Vulgaris: A Single-Arm, Prospective Clinical Study. Dermatol. Ther. 2023, 2023, 8846620. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, H.J.; Kim, J.Y.; Ban, M.J.; Son, J.; Hwang, Y.; Cho, S.B. Immediate and Late Effects of Pulse Widths and Cycles on Bipolar, Gated Radiofrequency-Induced Tissue Reactions in in vivo Rat Skin. Clin. Cosmet. Investig. Dermatol. 2023, 16, 721–729. [Google Scholar] [CrossRef]
- Wiegand, C.; Dirksen, A.; Tittelbach, J. Treatment with a red-laser-based wound therapy device exerts positive effects in models of delayed keratinocyte and fibroblast wound healing. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12926. [Google Scholar] [CrossRef] [PubMed]
- Mathioudaki, E.; Rallis, M.; Politopoulos, K.; Alexandratou, E. Photobiomodulation and Wound Healing: Low-Level Laser Therapy at 661 nm in a Scratch Assay Keratinocyte Model. Ann. Biomed. Eng. 2024, 52, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Carpena, N.; Kang, B.; Lee, M.Y. Effects of Photobiomodulation on Stem Cells Important for Regenerative Medicine. Med. Lasers 2020, 9, 134–141. [Google Scholar] [CrossRef]
- Khadra, M.; Lyngstadaas, S.P.; Haanaes, H.R.; Mustafa, K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 2005, 26, 3503–3509. [Google Scholar] [CrossRef]
- Mineroff, J.; Maghfour, J.; Ozog, D.M.; Lim, H.W.; Kohli, I.; Jagdeo, J. Photobiomodulation CME part II: Clinical applications in dermatology. J. Am. Acad. Dermatol. 2024, 91, 805–815. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Houreld, N.N.; Abrahamse, H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol. Ther. 2012, 14, 1110–1117. [Google Scholar] [CrossRef]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Li, Z.; Zhang, C.; Wang, L.; Zhang, Q.; Dong, Y.; Sha, X.; Wang, B.; Zhu, Z.; Wang, W.; Wang, Y.; et al. Chitooligosaccharides promote diabetic wound healing by mediating fibroblast proliferation and migration. Sci. Rep. 2025, 15, 556. [Google Scholar] [CrossRef]
- Chen, S.; Luo, Y.; He, Y.; Li, M.; Liu, Y.; Zhou, X.; Hou, J.; Zhou, S. In-situ-sprayed therapeutic hydrogel for oxygen-actuated Janus regulation of postsurgical tumor recurrence/metastasis and wound healing. Nat. Commun. 2024, 15, 814. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Xiang, Y.; Chen, Y.; Shi, Y.; Ge, X.; Zeng, B.; Shen, J. Hyperthermia-enhanced immunoregulation hydrogel for oxygenation and ROS neutralization in diabetic foot ulcers. Cell Biomater. 2025, 1, 100020. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Simões, A.; Corrêa, L.; Aranha, A.C.; Giudice, F.S.; Hamblin, M.R.; Sousa, S.C. Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J. Biophotonics 2015, 8, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and Molecular Processes in Wound Healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Ye, R.; He, Y.; Ni, W.; Zhang, Y.; Zhu, Y.; Cao, M.; He, R.; Yao, M. LLLT accelerates experimental wound healing under microgravity conditions via PI3K/AKT-CCR2 signal axis. Front. Bioeng. Biotechnol. 2024, 12, 1387474. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In Vitro Wound Healing Potential of Stem Extract of Alternanthera sessilis. Evid. Based Complement. Altern. Med. 2018, 2018, 3142073. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci. Rep. 2020, 10, 16192. [Google Scholar] [CrossRef] [PubMed]
- Raja; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineerging 2021, 8, 63. [Google Scholar] [CrossRef]
- Wang, L.; Xu, C.; Liu, X.; Yang, Y.; Cao, L.; Xiang, G.; Liu, F.; Wang, S.; Liu, J.; Meng, Q.; et al. TGF-β1 stimulates epithelial-mesenchymal transition and cancer-associated myoepithelial cell during the progression from in situ to invasive breast cancer. Cancer Cell Int. 2019, 19, 343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Christ, S.; Correa-Gallegos, D.; Ramesh, P.; Kalgudde Gopal, S.; Wannemacher, J.; Mayr, C.H.; Lupperger, V.; Yu, Q.; Ye, H.; et al. Injury triggers fascia fibroblast collective cell migration to drive scar formation through N-cadherin. Nat. Commun. 2020, 11, 5653. [Google Scholar] [CrossRef]
- Sabino, F.; auf dem Keller, U. Matrix metalloproteinases in impaired wound healing. Met. Med. 2015, 2, 1–8. [Google Scholar]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Abate, M.; Citro, M.; Pisanti, S.; Caputo, M.; Martinelli, R. Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol. Int. J. Mol. Sci. 2021, 22, 2438. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2023, 24, 641. [Google Scholar] [CrossRef]
- Lee, Y.B.; Lee, D.H.; Kim, Y.C.; Bhang, S.H. Enhancing Skin Regeneration Efficacy of Human Dermal Fibroblasts Using Carboxymethyl Cellulose-Coated Biodegradable Polymer. Tissue Eng. Regen. Med. 2025, 22, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Ko, E.; Ryu, Y.H.; Lee, S.J.; Jun, Y.J. Hyaluronic Acid Based Adipose Tissue-Derived Extracellular Matrix Scaffold in Wound Healing: Histological and Immunohistochemical Study. Tissue Eng. Regen. Med. 2024, 21, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Mavrakis, M.; Juanes, M.A. The compass to follow: Focal adhesion turnover. Curr. Opin. Cell Biol. 2023, 80, 102152. [Google Scholar] [CrossRef]
- Kuo, J.C. Mechanotransduction at focal adhesions: Integrating cytoskeletal mechanics in migrating cells. J. Cell Mol. Med. 2013, 17, 704–712. [Google Scholar] [CrossRef]
- Nifontova, G.; Safaryan, S.; Khristidis, Y.; Smirnova, O.; Vosough, M.; Shpichka, A.; Timashev, P. Advancing wound healing by hydrogel-based dressings loaded with cell-conditioned medium: A systematic review. Stem Cell Res. Ther. 2024, 15, 371. [Google Scholar] [CrossRef]
- Riaz, A.; Ali, S.; Summer, M.; Noor, S.; Nazakat, L.; Aqsa; Sharjeel, M. Exploring the underlying pharmacological, immunomodulatory, and anti-inflammatory mechanisms of phytochemicals against wounds: A molecular insight. Inflammopharmacology 2024, 32, 2695–2727. [Google Scholar] [CrossRef]
- Huang, X.H.; Ma, Y.; Lou, H.; Chen, N.; Zhang, T.; Wu, L.Y.; Chen, Y.J.; Zheng, M.M.; Lou, Y.L.; Xie, D.L. The Role of TSC1 in the Macrophages Against Vibrio vulnificus Infection. Front. Cell Infect. Microbiol. 2020, 10, 596609. [Google Scholar] [CrossRef]
- Jones, G.E.; Prigmore, E.; Calvez, R.; Hogan, C.; Dunn, G.A.; Hirsch, E.; Wymann, M.P.; Ridley, A.J. Requirement for PI 3-kinase gamma in macrophage migration to MCP-1 and CSF-1. Exp. Cell Res. 2003, 290, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D. Macrophage Polarization: A Novel Target and Strategy for Pathological Scarring. Tissue Eng. Regen. Med. 2024, 21, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Fleckner, M.; Döhmen, N.K.; Salz, K.; Christophers, T.; Windolf, J.; Suschek, C.V.; Oezel, L. Exposure of Primary Human Skin Fibroblasts to Carbon Dioxide-Containing Solution Significantly Reduces TGF-β-Induced Myofibroblast Differentiation In Vitro. Int. J. Mol. Sci. 2024, 25, 3013. [Google Scholar] [CrossRef]
- Wang, L.; Lin, B.; Zhai, M.; Hull, L.; Cui, W.; Xiao, M. Endothelial Dysfunction and Impaired Wound Healing Following Radiation Combined Skin Wound Injury. Int. J. Mol. Sci. 2024, 25, 2498. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakajima, S.; Ogasawara, K.; Asano, R.; Nakae, Y.; Sakata, I.; Iizuka, H. Photodynamic therapy using a novel irradiation source, LED lamp, is similarly effective to photodynamic therapy using diode laser or metal-halide lamp on DMBA- and TPA-induced mouse skin papillomas. J. Dermatol. 2014, 41, 729–731. [Google Scholar] [CrossRef]
- Wang, T.; Song, Y.; Yang, L.; Liu, W.; He, Z.; Shi, Y.; Song, B.; Yu, Z. Photobiomodulation Facilitates Rat Cutaneous Wound Healing by Promoting Epidermal Stem Cells and Hair Follicle Stem Cells Proliferation. Tissue Eng. Regen. Med. 2024, 21, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.-H.; Lai, Y.-Y.; Chen, C.-L.; Wang, H.-Y.; Chang, Y.-N.; Lin, Y.-C.; Yan, Y.-T.; Lai, C.-H.; Cheng, B. Cobalt protoporphyrin promotes human keratinocyte migration under hyperglycemic conditions. Mol. Med. 2022, 28, 71. [Google Scholar] [CrossRef]
- Abedin-Do, A.; Zhang, Z.; Douville, Y.; Méthot, M.; Bernatchez, J.; Rouabhia, M. Engineering diabetic human skin equivalent for in vitro and in vivo applications. Front. Bioeng. Biotechnol. 2022, 10, 989888. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Y.; Zhang, S.; Chen, D.X.; Gao, R.; Qin, P.; Yang, C.; Li, Q. KRT17 From Keratinocytes with High Glucose Stimulation Inhibit Dermal Fibroblasts Migration Through Integrin α11. J. Endocr. Soc. 2024, 8, bvad176. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Li, R.; Park, C.Y.; Fredberg, J.J.; Janmey, P.A. A novel method to make viscoelastic polyacrylamide gels for cell culture and traction force microscopy. APL Bioeng. 2020, 4, 036104. [Google Scholar] [CrossRef]
- Syed, S.; Karadaghy, A.; Zustiak, S. Simple polyacrylamide-based multiwell stiffness assay for the study of stiffness-dependent cell responses. J. Vis. Exp. 2015, 97, e52643. [Google Scholar] [CrossRef]
- Wang, Y.; Jeong, Y.; Jhiang, S.M.; Yu, L.; Menq, C.H. Quantitative characterization of cell behaviors through cell cycle progression via automated cell tracking. PLoS ONE 2014, 9, e98762. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, K.N.; Kim, J.H.; Noh, K.; Hur, S.S.; Kim, Y.; Hong, M.; Chung, J.C.; Park, J.H.; Lee, J.; et al. Self-organized insulin-producing beta-cells differentiated from human omentum-derived stem cells and their in vivo therapeutic potential. Biomater. Res. 2023, 27, 82. [Google Scholar] [CrossRef]


| Primer | Sequences (5′–3′) |
|---|---|
| Gapdh_Forward | CACTCCACCTTTGACGC |
| Gapdh_Reverse | GGTCCAGGGGTCTTACTCC |
| IL-6_Forward | ACTCACCTCTTCAGAACGAATTG |
| IL-6_Reverse | CCATCTTTGGAAGGTTCAGGTTG |
| MMP2_Forward | GATACCCCTTTGACGGTAAGGA |
| MMP2_Reverse | CCTTCTCCCAAGGTCCATAGC |
| MMP9_Forward | GGGACGCAGACATCGTCATC |
| MMP9_Reverse | TCGTCATCGTCGAAATGGGC |
| Col I_Forward | CAAGACAG TGATTGAATACAAAACCA |
| Col I_Reverse | ACGTCGAAGCCGAATTCCT |
| Col III_Forward | ACACGCAAGGCTGTGAGACT |
| Col III_Reverse | TGTCGGTCACTTGCACTGGT |
| Fibronectin_Forward | AGGAAGCCGAGGTTTTAACTG |
| Fibronectin_Reverse | AGGACGCTCATAAGTGTCACC |
| Primer | Sequences (5′–3′) |
|---|---|
| Gapdh_Forward | AAGGTCATCCCAGAGCTGAA |
| Gapdh_Reverse | CTGCTTCACCACCTTCTTGA |
| Col I_Forward | GCT CCT CTT AGG GGC CAC T |
| Col I_Reverse | CCT TTGTCA GAA TAC TGA GCA GC |
| IL-6_Forward | CCGGAGAGGAGACTTCACAG |
| IL-6_Reverse | CAGAATTGCCATTGCACAAC |
| TGF-β1_Forward | ATGACATGAACCGACCCTTC |
| TGF-β1_Reverse | ACTTCCAACCCAGGTCCTTC |
| Krt1_Forward | TGGGAGATTTTCAGGAGGAGG |
| Krt1_Reverse | GCCACACTCTTGGAGATGCTC |
| MMP9_Forward | GGGACGCAGACATCGTCATC |
| MMP9_Reverse | CCCACATTTGACGTCCAGAGAAGAA |
| Ki-67_Forward | CTGCCTCAGATGGCTCAAAGA |
| Ki-67_Reverse | GAAGACTTCGGTTCCCTGTAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Ban, M.J.; Gil, C.H.; Hur, S.S.; Anggradita, L.D.; Kim, M.-K.; Son, J.W.; Kim, J.E.; Hwang, Y. Multispectral Pulsed Photobiomodulation Enhances Diabetic Wound Healing via Focal Adhesion-Mediated Cell Migration and Extracellular Matrix Remodeling. Int. J. Mol. Sci. 2025, 26, 6232. https://doi.org/10.3390/ijms26136232
Choi J, Ban MJ, Gil CH, Hur SS, Anggradita LD, Kim M-K, Son JW, Kim JE, Hwang Y. Multispectral Pulsed Photobiomodulation Enhances Diabetic Wound Healing via Focal Adhesion-Mediated Cell Migration and Extracellular Matrix Remodeling. International Journal of Molecular Sciences. 2025; 26(13):6232. https://doi.org/10.3390/ijms26136232
Chicago/Turabian StyleChoi, Jihye, Myung Jin Ban, Chan Hee Gil, Sung Sik Hur, Laurensia Danis Anggradita, Min-Kyu Kim, Ji Won Son, Jung Eun Kim, and Yongsung Hwang. 2025. "Multispectral Pulsed Photobiomodulation Enhances Diabetic Wound Healing via Focal Adhesion-Mediated Cell Migration and Extracellular Matrix Remodeling" International Journal of Molecular Sciences 26, no. 13: 6232. https://doi.org/10.3390/ijms26136232
APA StyleChoi, J., Ban, M. J., Gil, C. H., Hur, S. S., Anggradita, L. D., Kim, M.-K., Son, J. W., Kim, J. E., & Hwang, Y. (2025). Multispectral Pulsed Photobiomodulation Enhances Diabetic Wound Healing via Focal Adhesion-Mediated Cell Migration and Extracellular Matrix Remodeling. International Journal of Molecular Sciences, 26(13), 6232. https://doi.org/10.3390/ijms26136232







