The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Comprehensive Analysis of PreDM and T2DM Patients Presenting with Metabolic Syndrome: Demographic and Clinical Characteristics, Medical History, and Laboratory Assessments
2.2. A Comparative Examination of Clinical Features, Anthropometric Measurements, Metabolic Indices, Lipid Profiles, and Metabolic Syndrome-Related Parameters in Individuals with PreDM and T2DM
2.3. Comparative Analysis of the Two Phenotypes of Cardiometabolic Syndrome: MUHNW and MUHO in Individuals with PreDM and T2DM
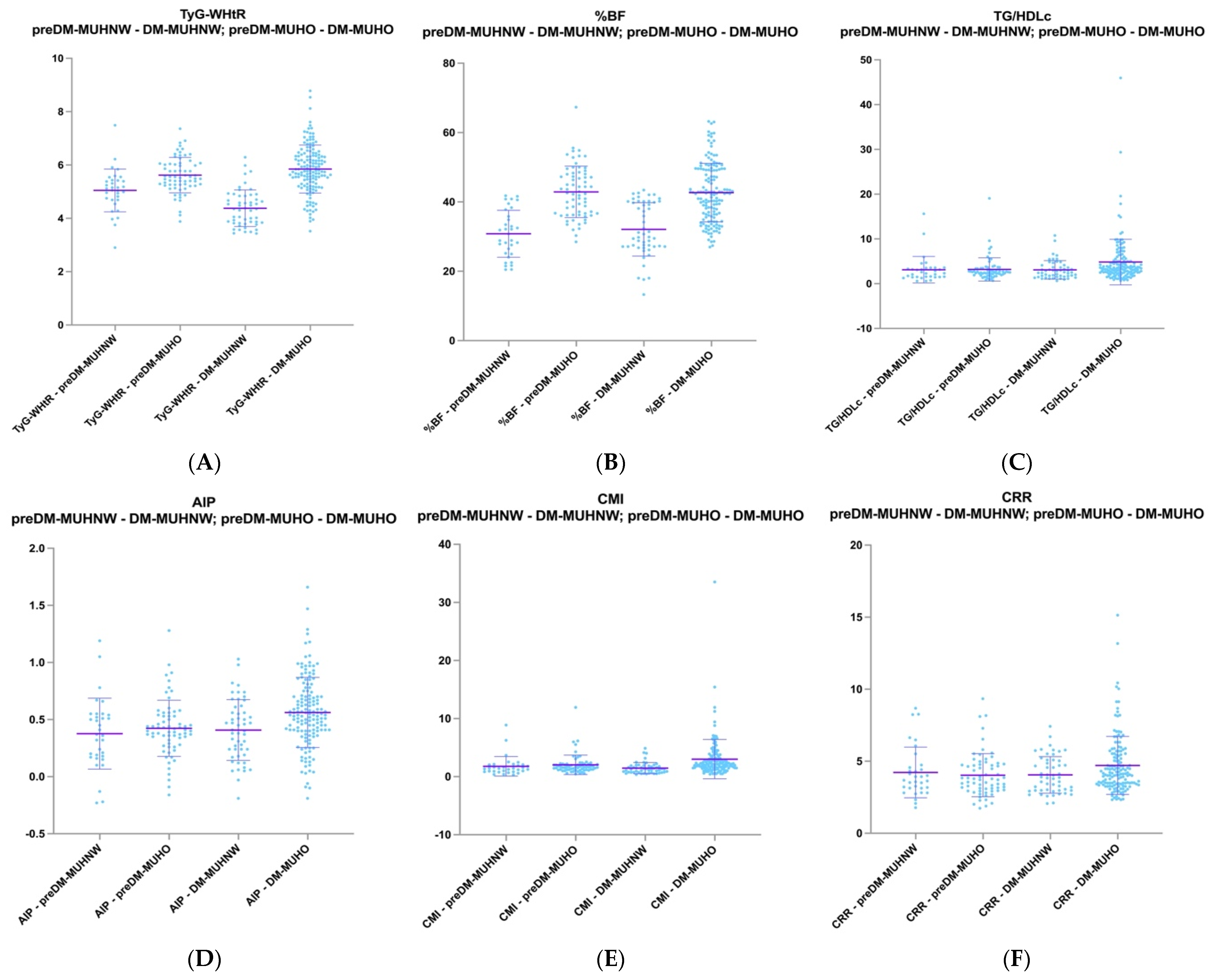
2.4. Comparative Analysis of PreDM MUHNW Patients with T2DM MUHNW Patients and PreDM MUHO Patients with T2DM MUHO Patients
2.5. Connections of TyG and TyG-Related Indices with BMI, WHR, WHtR, and Body Fat Percentage in the PreDM and T2DM Groups
2.6. Connections of AIP, CMI, and CRR with BMI, WHR, WHtR, and %BF in the PreDM and T2DM Groups
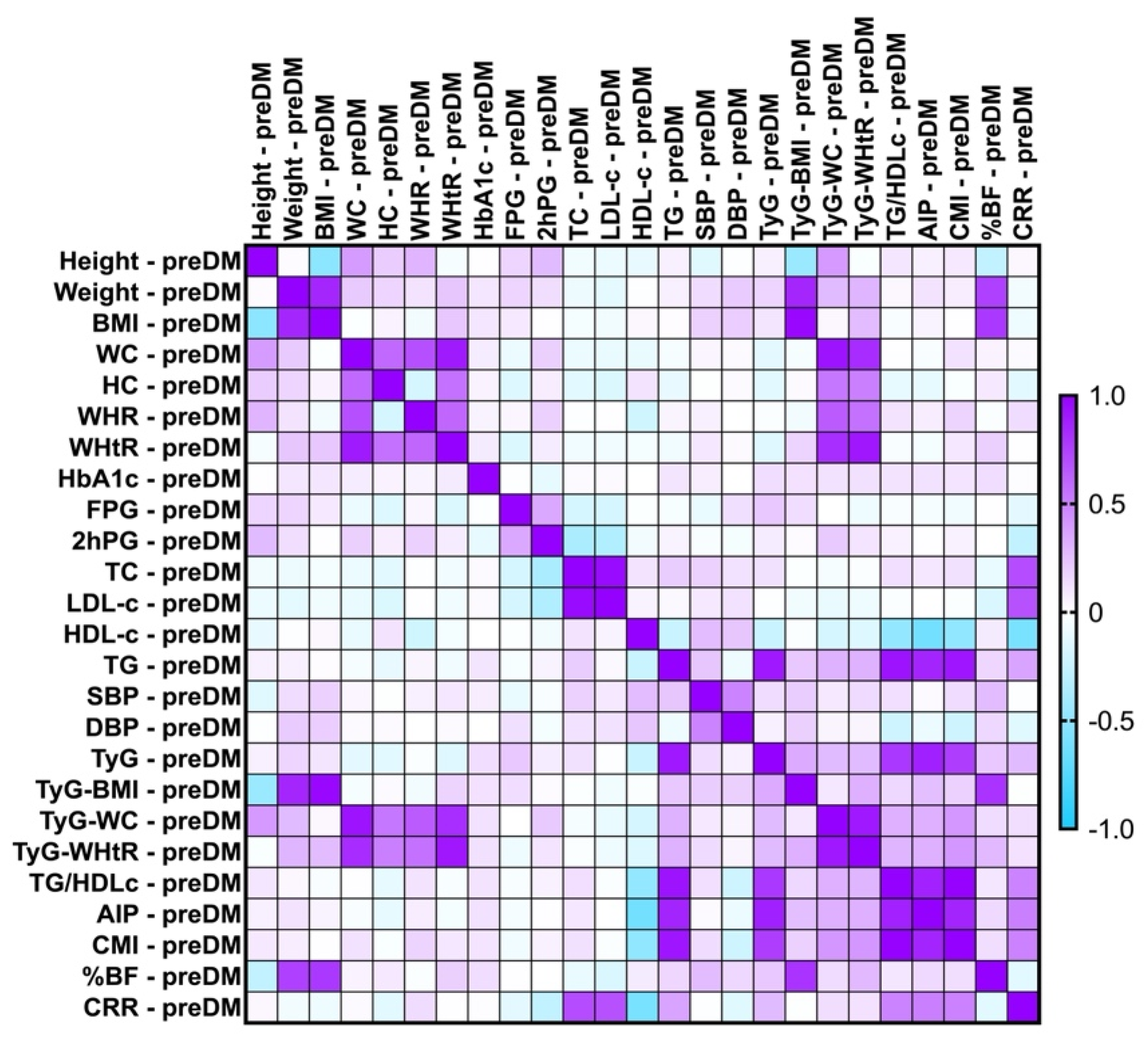
2.7. Correlations Between Metabolic Syndrome-Related Indices
2.7.1. Correlations Between Metabolic Syndrome-Related Indices Concerning PreDM and T2DM Patients
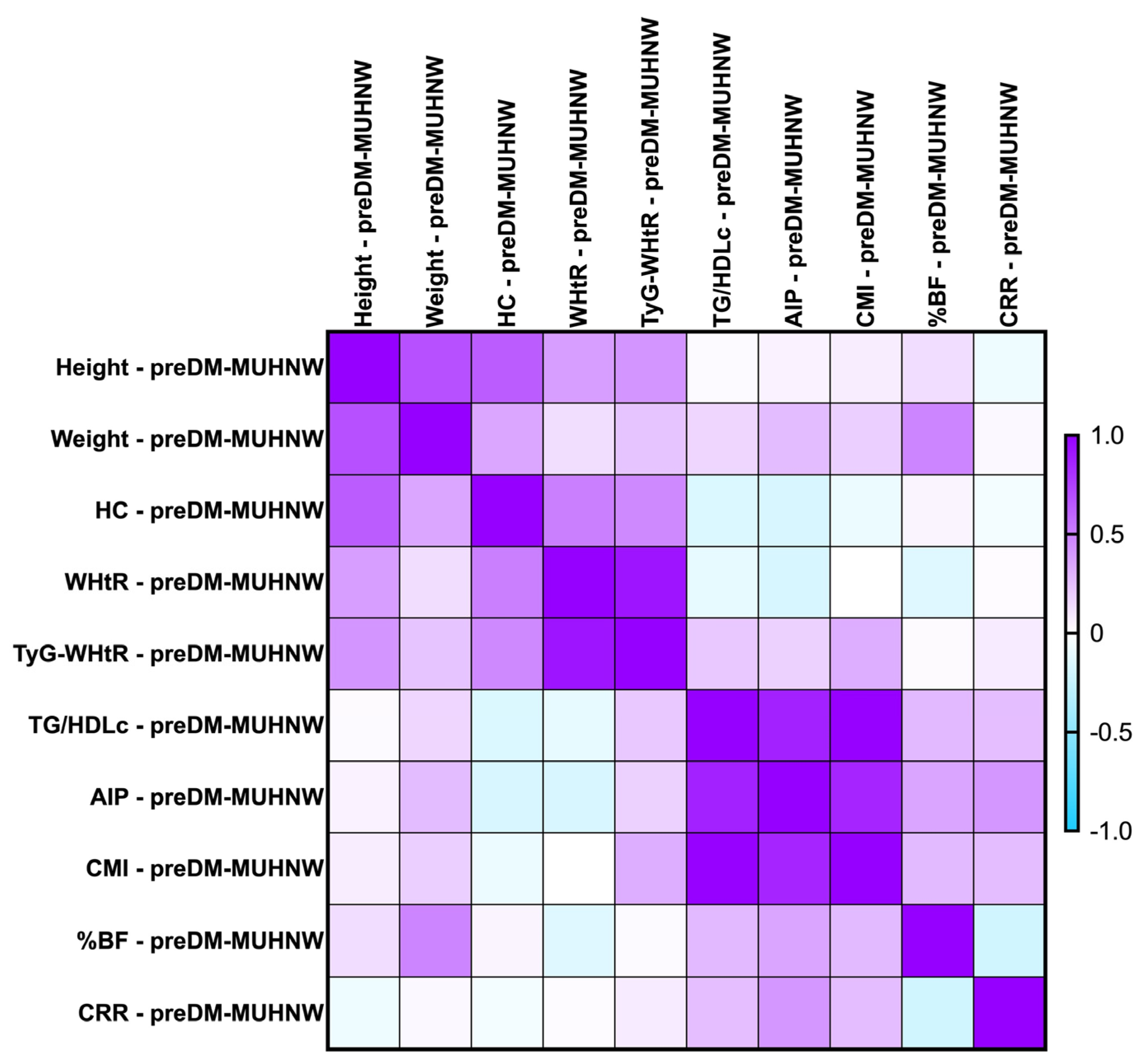
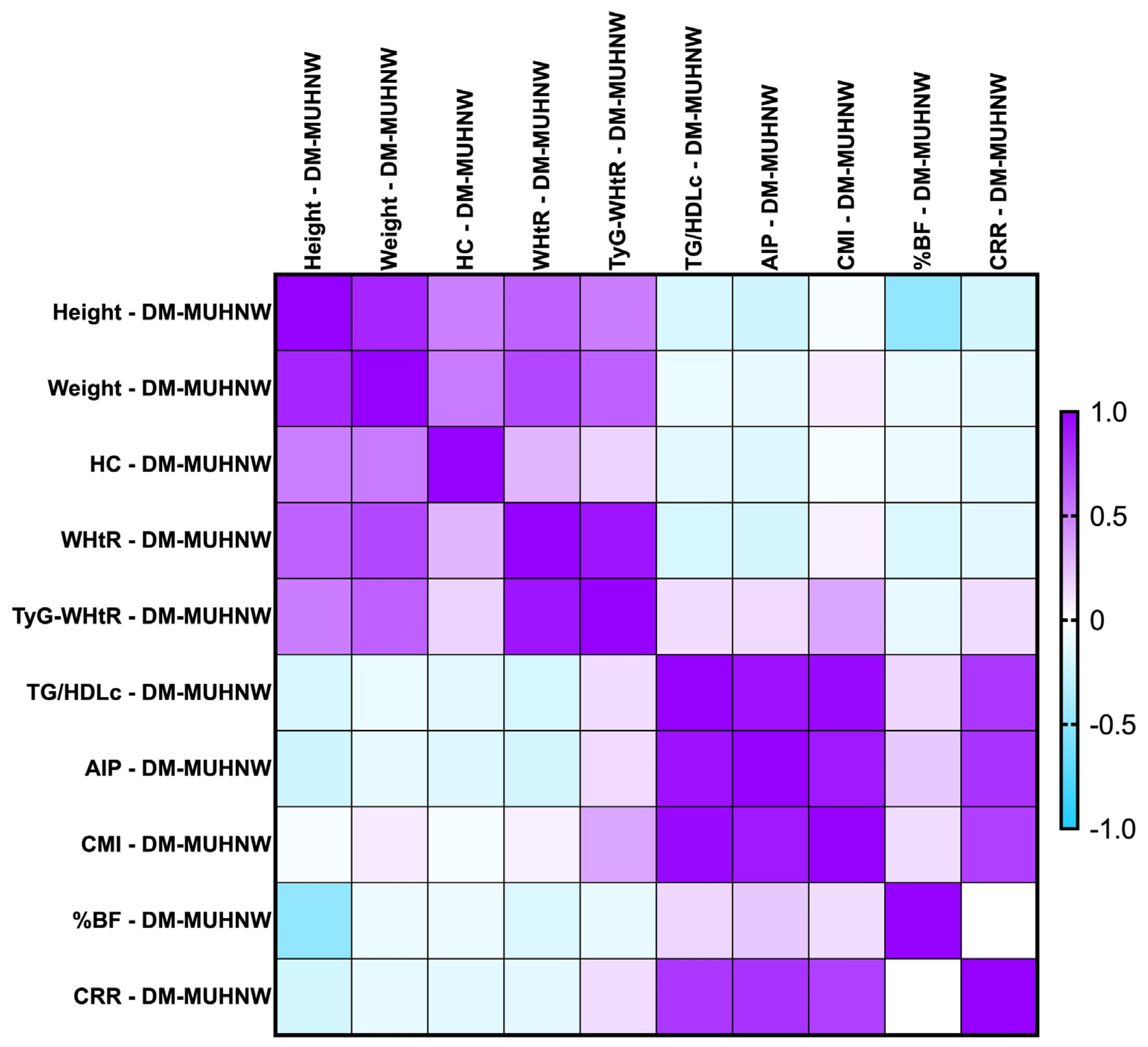
2.7.2. Correlations Between Metabolic Syndrome-Related Indices Concerning PreDM Patients After the MUHNW and MUHO Phenotypes
- -
- PreDM MUHNW:
- CMI correlated positively, strongly with AIP (rho = 0.856, p-value < 0.0001);
- TG/HDL-c correlated positively and strongly with the CMI (rho = 0.992, p-value = 0.0001);
- TyG-WHtR correlated weakly with the CMI (rho = 0.314, p-value of 0.007).
- -
- PreDM MUHO:
- AIP presented statistically significant correlations with the CMI (rho = 0.870, p-value = 0.0001), and TG/HDL-c ratio (rho = 0.874, p-value < 0.0001)—strong and positive;
- TG/HDL-c correlated positively and strongly with the CMI (rho = 0.991, p-value = 0.0001);
- CRR correlated moderately with the CMI (rho = 0.638, p-value < 0.0001).
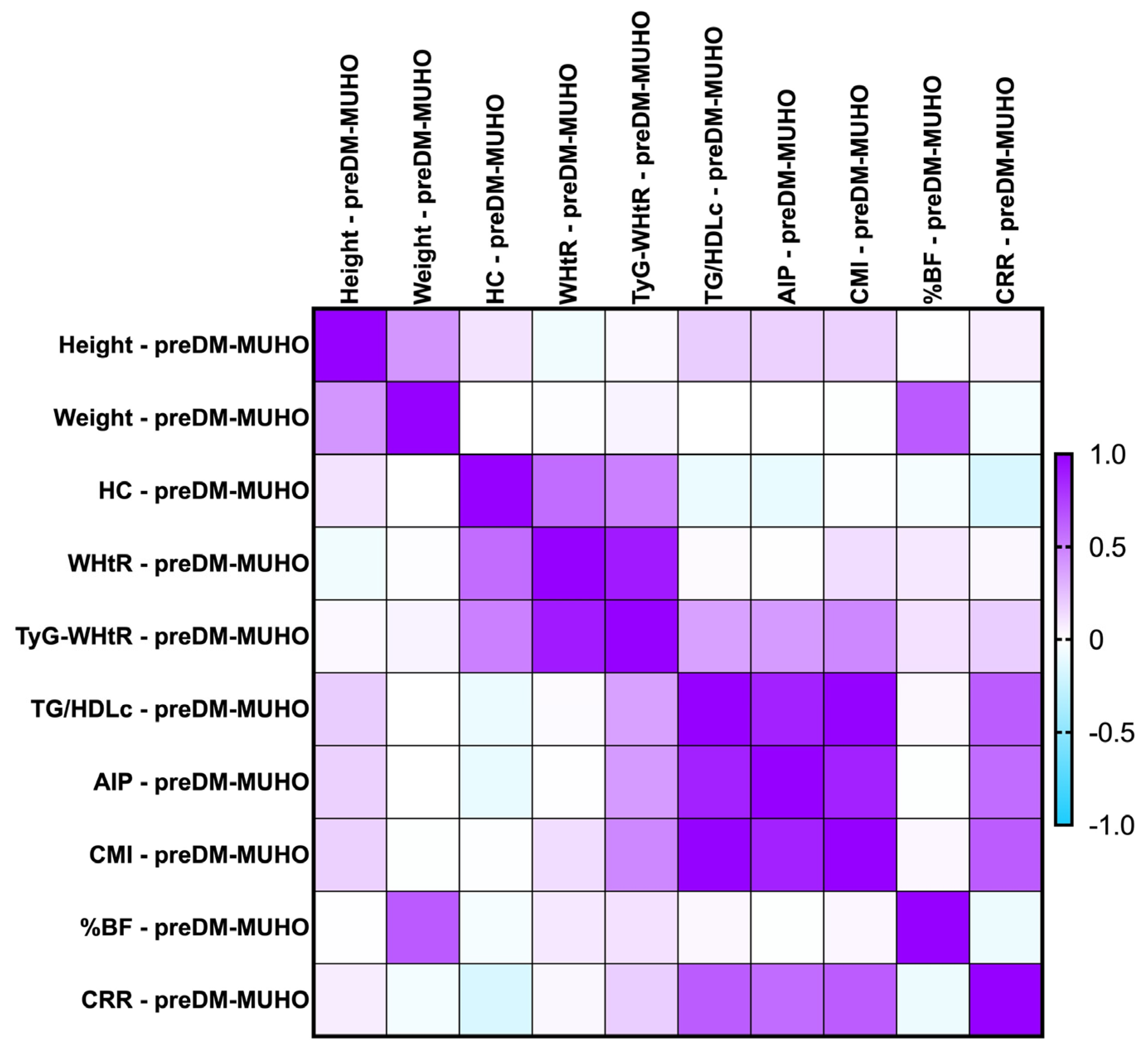
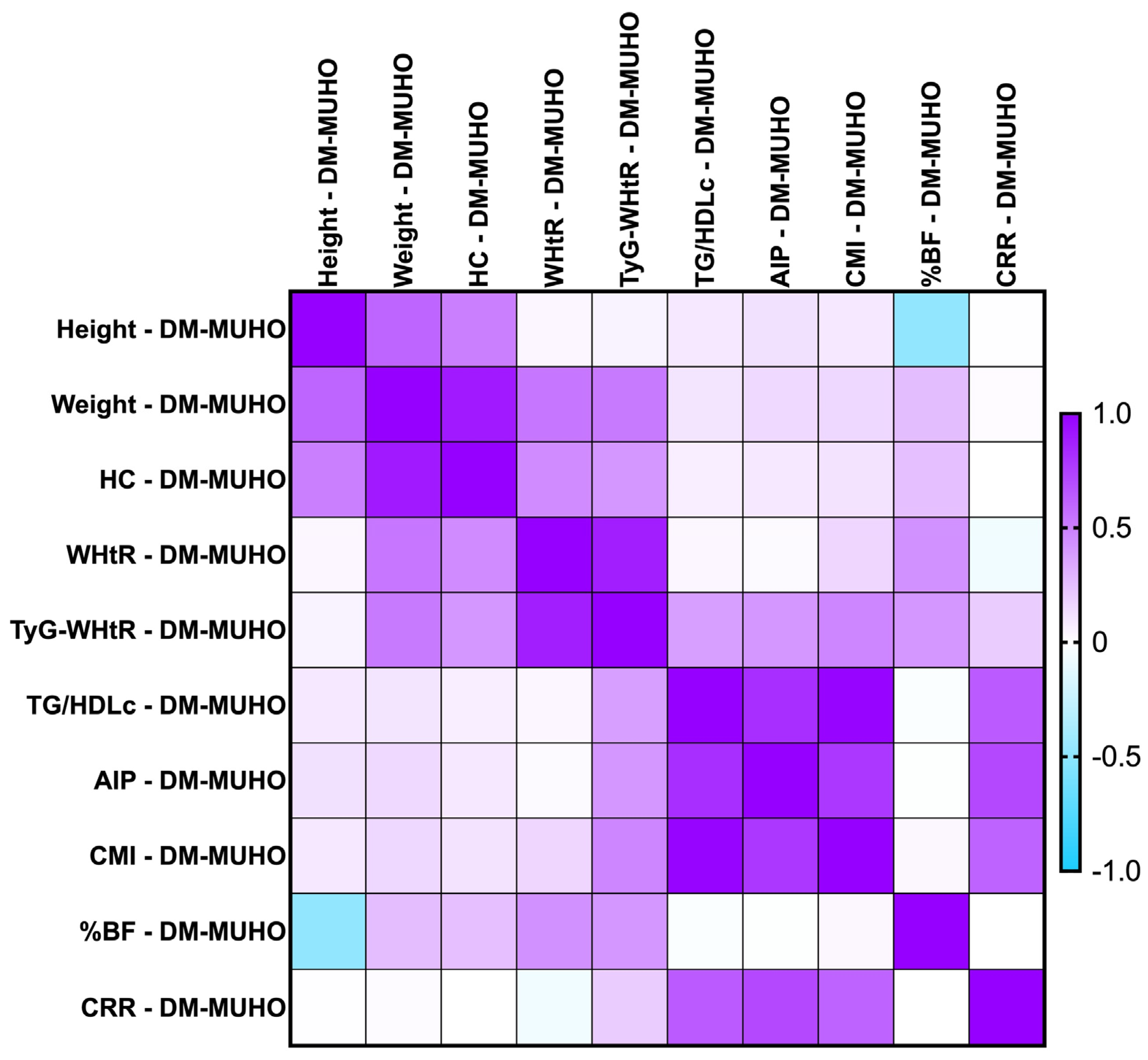
2.7.3. Correlations Between Metabolic Syndrome-Related Indices Concerning PreDM and T2DM Patients for the MUHNW and MUHO Phenotypes
- -
- T2DM MUHNW:
- AIP presented statistically significant correlations with the CMI (rho = 0. 904, p-value = 0.0001)—strong and positive;
- The CMI correlated positively and strongly with TG/HDL-c (rho = 0.964, p-value = 0.0001);
- The CRR correlated strongly with AIP (rho = 0.802, p-value < 0.0001).
- -
- T2DM MUHO:
- AIP presented statistically significant correlations with the CRR (rho = 0.716, p-value = 0.0001)—moderate and positive;
- The CMI correlated positively and strongly with TG/HDL-c (rho = 0.983, p-value = 0.0001);
- The CRR correlated positively and moderately with TG/HDL-c (rho = 0.646, p-value = 0.0001).
2.8. Comparative Analysis of Metabolic Syndrome-Related Indices Among Subgroups of Females and Males in PreDM and T2DM Groups
2.9. Diagnostic Accuracy of Different Indices and Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patient Selection, Review of Clinical History, Evaluation of Biometric Metrics, and Compilation of Demographic Information
4.2. Assessment of T2DM and PreDM Patients
4.3. MetS Definition and Cardiometabolic Phenotypes
4.4. Evaluation of Various MetS-Related Indices (TyG, TyG-Related Indices, AIP, CMI, and CRR)
- TyG-BMI = TyG × BMI
- TyG-WHtR = TyG × WHtR
- TyG-WC = TyG × WC
4.5. Laboratory Investigations
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ştefan, A.G.; Clenciu, D.; Mitrea, A.; Vladu, I.M.; Protasiewicz-Timofticiuc, D.C.; Roşu, M.M.; Maria, D.T.; Dinu, I.R.; Gheonea, T.C.; Vladu, B.E.; et al. Metabolic Syndrome and Insulin Resistance in Romania. Int. J. Mol. Sci. 2025, 26, 2389. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Ayoobi, F.; Jalali, Z.; Bidaki, R.; Lotfi, M.A.; Esmaeili-Nadimi, A.; Khalili, P. Metabolic syndrome: A population-based study of prevalence and risk factors. Sci. Rep. 2024, 14, 3987. [Google Scholar] [CrossRef]
- Badawy, M.; Elsayes, K.M.; Lubner, M.G.; Shehata, M.A.; Fowler, K.; Kaoud, A.; Pickhardt, P.J. Metabolic syndrome: Imaging features and clinical outcomes. Br. J. Radiol. 2024, 97, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Vesa, C.M.; Zaha, D.C.; Bungău, S.G. Molecular Mechanisms of Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 5452. [Google Scholar] [CrossRef]
- Tirandi, A.; Carbone, F.; Montecucco, F.; Liberale, L. The role of metabolic syndrome in sudden cardiac death risk: Recent evidence and future directions. Eur. J. Clin. Investig. 2022, 52, e13693. [Google Scholar] [CrossRef] [PubMed]
- Scurt, F.G.; Ganz, M.J.; Herzog, C.; Bose, K.; Mertens, P.R.; Chatzikyrkou, C. Association of metabolic syndrome and chronic kidney disease. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2024, 25, e13649. [Google Scholar] [CrossRef]
- Park, I.R.; Chung, Y.G.; Chung, S.M.; Moon, J.S.; Yoon, J.S.; Won, K.C. Association between obstructive sleep apnea risk and atherosclerosis: A nationwide cross-sectional study in the Korean population. PLoS ONE 2025, 20, e0322897. [Google Scholar] [CrossRef]
- Zhu, X.M.; Xu, Y.; Zhang, J. Cardiometabolic Index is associated with heart failure: A cross-sectional study based on NHANES. Front. Med. 2024, 11, 1507100. [Google Scholar] [CrossRef]
- Singh, M.; Pathak, M.S.; Paul, A. A Study on Atherogenic Indices of Pregnancy Induced Hypertension Patients as Compared to Normal Pregnant Women. J. Clin. Diagn. Res. JCDR 2015, 9, BC5–BC8. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Pomar, M.D.; Rodríguez-Urgellés, E.; Sastre-Belío, M.; Martín-Lorenzo, A.; Schnecke, V.; Segú, L.; Brosa, M.; Vilarrasa, N. Assessment of the Potential Clinical and Economic Impact of Weight Loss in the Adult Population with Obesity and Associated Comorbidities in Spain. Adv. Ther. 2025, 42, 1265–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, C.; Li, M.; Yan, G.; Tang, C. Sex Differences in the Associations among Insulin Resistance Indexes with Metabolic Syndrome: A Large Cross-Sectional Study. Int. J. Endocrinol. 2024, 2024, 3352531. [Google Scholar] [CrossRef]
- Paniagua, J.A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J. Diabetes 2016, 7, 483–514. [Google Scholar] [CrossRef]
- Dichi, I.; Simão, A.N.; Vannucchi, H.; Curi, R.; Calder, P.C. Metabolic syndrome: Epidemiology, pathophysiology, and nutrition intervention. J. Nutr. Metab. 2012, 2012, 584541. [Google Scholar] [CrossRef]
- He, X.; Zhu, J.; Liang, W.; Yang, X.; Ning, W.; Zhao, Z.; Chen, J.; He, Q. Association of body roundness index with cardiovascular disease in patients with cardiometabolic syndrome: A cross-sectional study based on NHANES 2009–2018. Front. Endocrinol. 2025, 16, 1524352. [Google Scholar] [CrossRef]
- Liu, C.; Liang, D.; Xiang, G.; Zhao, X.; Xiao, K.; Xie, L. Association of the triglyceride glucose index with all cause and CVD mortality in the adults with diabetes aged < 65 years without cardiovascular disease. Sci. Rep. 2025, 15, 2745. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Toda, H.; Ejiri, K.; Yoshida, M.; Nanba, Y.; Yoshida, M.; Nakamura, K.; et al. Prognostic value of non-alcoholic fatty liver disease for predicting cardiovascular events in patients with diabetes mellitus with suspected coronary artery disease: A prospective cohort study. Cardiovasc. Diabetol. 2021, 20, 8. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Non-alcoholic Fatty Liver Disease Study Group; Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2015, 47, 997–1006. [Google Scholar] [CrossRef]
- Stepanova, M.; Younossi, Z.M. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012, 10, 646–650. [Google Scholar] [CrossRef]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Mielke, C.H.; Shields, J.P.; Broemeling, L.D. Coronary artery calcium, coronary artery disease, and diabetes. Diabetes Res. Clin. Pract. 2001, 53, 55–61. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Vermund, S.H. Advantages and Limitations of the Body Mass Index (BMI) to Assess Adult Obesity. Int. J. Environ. Res. Public Health 2024, 21, 757. [Google Scholar] [CrossRef] [PubMed]
- Adab, P.; Pallan, M.; Whincup, P.H. Is BMI the best measure of obesity? BMJ 2018, 360, k1274. [Google Scholar] [CrossRef]
- Soheilifard, S.; Faramarzi, E.; Mahdavi, R. Relationship between dietary intake and atherogenic index of plasma in cardiometabolic phenotypes: A cross-sectional study from the Azar cohort population. J. Health Popul. Nutr. 2025, 44, 28. [Google Scholar] [CrossRef]
- Mathew, H.; Farr, O.M.; Mantzoros, C.S. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metab. Clin. Exp. 2016, 65, 73–80. [Google Scholar] [CrossRef]
- Stefan, N. Metabolically Healthy and Unhealthy Normal Weight and Obesity. Endocrinol. Metab. 2020, 35, 487–493. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Sun, S.; Li, F.; Zhao, W.; Yang, H.; Wu, X. The predictive value of atherogenic index of plasma for cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention with LDL-C below 1.8 mmol/L. Cardiovasc. Diabetol. 2023, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Avagimyan, A.; Pogosova, N.; Fogacci, F.; Aghajanova, E.; Djndoyan, Z.; Patoulias, D.; Sasso, L.L.; Bernardi, M.; Faggiano, A.; Mohammadifard, N.; et al. Triglyceride-glucose index (TyG) as a novel biomarker in the era of cardiometabolic medicine. Int. J. Cardiol. 2025, 418, 132663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Q.; Zheng, M.; Zuo, Z.; Zhang, G.; Shi, R.; Wu, T. Comparative study on the predictive value of TG/HDL-C, TyG and TyG-BMI indices for 5-year mortality in critically ill patients with chronic heart failure: A retrospective study. Cardiovasc. Diabetol. 2024, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Chen, T.; Li, Y.; Li, X.; Du, R. Comparison of prognostic value of triglyceride-glucose index and atherogenic index of plasma in patients with acute coronary syndrome: A retrospective study. JRSM Cardiovasc. Dis. 2025, 14, 20480040251341155. [Google Scholar] [CrossRef]
- Sbriscia, M.; Colombaretti, D.; Giuliani, A.; Di Valerio, S.; Scisciola, L.; Rusanova, I.; Bonfigli, A.R.; Olivieri, F.; Sabbatinelli, J. Triglyceride glucose index predicts long-term mortality and major adverse cardiovascular events in patients with type 2 diabetes. Cardiovasc. Diabetol. 2025, 24, 115. [Google Scholar] [CrossRef]
- Ali, M.K.; Seiglie, J.A.; Narayan, K.M.V. Progress in diabetes prevention or epidemiology-or both, or neither? Lancet Diabetes Endocrinol. 2021, 9, 190–191. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Diagnosis of non-alcoholic fatty liver disease (NAFLD). Diabetologia 2016, 59, 1104–1111. [Google Scholar] [CrossRef]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Son, D.H.; Lee, H.S.; Lee, Y.J.; Lee, J.H.; Han, J.H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 596–604. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Gamboa-Gómez, C.I.; Aradillas-García, C.; Rodríguez-Morán, M.; Guerrero-Romero, F. The triglyceride and glucose index is a useful biomarker to recognize glucose disorders in apparently healthy children and adolescents. Eur. J. Pediatr. 2020, 179, 953–958. [Google Scholar] [CrossRef]
- Ran, L.; Han, Y.; Zhaohu, H.; Hailin, S. Correlation Between Triglyceride-Glucose Index and Microvascular Complications in Patients With Early- Onset of Type 2 Diabetes Mellitus. Endocrinol. Diabetes Metab. 2025, 8, e70027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, W.; Xu, B.; Wang, S.; Du, Z.; Cheng, W. Association of triglyceride glucose index and triglyceride glucose-body mass index with sudden cardiac arrest in the general population. Cardiovasc. Diabetol. 2024, 23, 173. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, L.; He, S. Triglyceride-glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: A 15-year prospective study. Endocrine 2021, 72, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Cao, R.; Wan, Q. Association between the plasma atherogenic index and type 2 diabetes in Chinese population: Prospective cohort study based on 4C study. Front. Endocrinol. 2025, 16, 1571602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, M.; Zhang, H.; Tan, Q.; Ji, J.; Cheng, Y.; Lu, F. Relationship between plasma atherogenic index and incidence of cardiovascular diseases in Chinese middle-aged and elderly people. Sci. Rep. 2025, 15, 8775. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, R.; Cao, H.; Zhang, J.; Li, S.; Kang, X.; Ma, Y.; Wu, Y.; Guo, Y.; Feng, L. AIP, fatty liver, and HbA1c as modifiers of the C-index and diabetes risk relationship. Lipids Health Dis. 2025, 24, 129. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, C.; Yan, Z.; Li, Q. Association between cardiac metabolic index and diabetic kidney disease: A cross-sectional study of NHANES 1999–2018. J. Health Popul. Nutr. 2025, 44, 105. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Sun, J.; Jiang, Y.; Tang, Y. Identification of metabolic syndrome using lipid accumulation product and cardiometabolic index based on NHANES data from 2005 to 2018. Nutr. Metab. 2024, 21, 96. [Google Scholar] [CrossRef]
- Mansoori, A.; Allahyari, M.; Mirvahabi, M.S.; Tanbakuchi, D.; Ghoflchi, S.; Derakhshan-Nezhad, E.; Azarian, F.; Ferns, G.; Esmaily, H.; Ghayour-Mobarhan, M. Predictive properties of novel anthropometric and biochemical indexes for prediction of cardiovascular risk. Diabetol. Metab. Syndr. 2024, 16, 304. [Google Scholar] [CrossRef]
- Czernichow, S.; Kengne, A.P.; Stamatakis, E.; Hamer, M.; Batty, G.D. Body mass index, waist circumference and waist-hip ratio: Which is the better discriminator of cardiovascular disease mortality risk?: Evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, 680–687. [Google Scholar] [CrossRef]
- García Samuelsson, M.; Tárraga López, P.J.; López-González, Á.A.; Paublini, H.; Martínez-Almoyna Rifá, E.; Ramírez-Manent, J.I. Assessment of the Risk of Insulin Resistance in Workers Classified as Metabolically Healthy Obese. Nutrients 2025, 17, 1345. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Song, J.; Singer, K. Age and Sex: Impact on adipose tissue metabolism and inflammation. Mech. Ageing Dev. 2021, 199, 111563. [Google Scholar] [CrossRef]
- Bogdan, M.; Meca, A.D.; Boldeanu, M.V.; Gheorghe, D.N.; Turcu-Stiolica, A.; Subtirelu, M.S.; Boldeanu, L.; Blaj, M.; Botnariu, G.E.; Vlad, C.E.; et al. Possible Involvement of Vitamin C in Periodontal Disease-Diabetes Mellitus Association. Nutrients 2020, 12, 553. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 12. Retinopa-thy, n., and foot care: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S231–S243. [Google Scholar] [CrossRef] [PubMed]
- Ahrițculesei, R.V.; Boldeanu, L.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Cîmpeanu, R.C.; Mustață, M.L.; Siloși, I.; Boldeanu, M.V.; Vere, C.C. Correlation Between Prognostic Nutritional Index, Glasgow Prognostic Score, and Different Obesity-Related Indices in People with Diabetes or Prediabetes. Diagnostics 2024, 14, 2661. [Google Scholar] [CrossRef]
- Ahrițculesei, R.V.; Boldeanu, L.; Caragea, D.C.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Ungureanu, A.M.; Văduva, C.C.; Dijmărescu, A.L.; Popescu, A.I.S.; et al. Association Between Pentraxins and Obesity in Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus Patients. Int. J. Mol. Sci. 2025, 26, 3661. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Assani, M.Z.; Novac, M.B.; Dijmărescu, A.L.; Văduva, C.C.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Ahrițculesei, R.V.; Stroe-Ionescu, A.Ș.; Assani, A.D.; et al. Potential Association Between Atherogenic Coefficient, Prognostic Nutritional Index, and Various Obesity Indices in Diabetic Nephropathy. Nutrients 2025, 17, 1339. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S20–S42. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; Hadziosmanovic, N.; Aylward, P.E.; Hagström, E.; Hochman, J.S.; Stewart, R.A.H.; White, H.D.; Wallentin, L. Body Mass Index and Association With Cardiovascular Outcomes in Patients With Stable Coronary Heart Disease—A STABILITY Substudy. J. Am. Heart Assoc. 2022, 11, e023667. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Lin, X.; Huang, S. Atherogenic Index of Plasma mediates the association between Life’s Crucial 9 with overactive bladder: A secondary data analysis from NHANES. Front. Endocrinol. 2025, 16, 1505712. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yang, D.; Xia, H.; Ren, Z.; Chen, J.; Yao, S. Cardiometabolic index: A new predictor for metabolic associated fatty liver disease in Chinese adults. Front. Endocrinol. 2022, 13, 1004855. [Google Scholar] [CrossRef]
- Kubalová, K.; Porvazník, I.; Majherová, M.; Demková, L.; Piotrowska, A.; Mydlárová Blaščáková, M. Lipid Levels and Atherogenic Indices as Important Predictive Parameters in the Assessment of Cardiovascular Risk in Patients with Pulmonary Tuberculosis-Slovak Pilot Study. Medicina 2025, 61, 365. [Google Scholar] [CrossRef]
- Shan, Y.; Lin, M.; Gu, F.; Li, D.; Zhu, Q.; Chen, Z.; Zhang, W.; Fu, G.; Wang, M. The prognostic association of triglyceride-glucose index and its derived indicators with stable coronary artery disease patients undergoing percutaneous coronary intervention. Front. Endocrinol. 2025, 16, 1465376. [Google Scholar] [CrossRef]
- Xu, J.; Cai, D.; Jiao, Y.; Liao, Y.; Shen, Y.; Shen, Y.; Han, W. Insights into the complex relationship between triglyceride glucose-waist height ratio index, mean arterial pressure, and cardiovascular disease: A nationwide prospective cohort study. Cardiovasc. Diabetol. 2025, 24, 93. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, R.; Wang, C.; Song, Y.; Wang, X.; Zeng, F. TyG-WC Index as a Superior Predictor of Hyperuricemia Risk in the Hypertensive Population: A Prospective Cohort Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2025, 18, 679–689. [Google Scholar] [CrossRef]
- MDRD GFR Equation. Available online: https://www.mdcalc.com/calc/76/mdrd-gfr-equation (accessed on 25 April 2025).






| Parameters | PreDM Cohort (n = 100) | T2DM Cohort (n = 200) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test/ Mann–Whitney Test |
|---|---|---|---|
| Demographic and Clinical Features—Medical History | |||
| Age (years) (mean ± SD) | 56.51 ± 11.52 | 62.91 ± 12.32 | <0.0001 * |
| Gender Male/Female (n) | 47/53 | 101/99 | 0.56 |
| Residence Urban/Rural (n) | 61/39 | 119/81 | 0.75 |
| Education Yes/No (n) | 68/32 | 130/70 | 0.60 |
| Drinking Yes/No (n) | 42/58 | 60/140 | 0.03 * |
| Smoking Yes/No (n) | 47/53 | 66/134 | 0.01 * |
| Hepatoseatosis n (%) | 66 (66%) | 148 (74%) | 0.14 |
| Dyslipidemia n (%) | 85 (85%) | 176 (88%) | 0.46 |
| Hypertension n (%) | 93 (93%) | 181 (90%) | 0.46 |
| Hyperuricemia n (%) | 12 (12%) | 35 (17%) | 0.21 |
| Laboratory examination | |||
| HbA1c (%) (mean ± SD) | 5.85 ± 0.36 | 9.36 ± 2.24 | <0.0001 * |
| FPG (mg/dL) (mean ± SD) | 99.47 ± 13.45 | 163.70 ± 41.00 | <0.0001 * |
| 2h-PG (mg/dL) (mean ± SD) | 162.40 ± 13.91 | 259.90 ± 86.22 | <0.0001 * |
| ESR (mm/1st hour) [median (range)] | 30.00 (5.00–115.00) | 30.00 (4.00–140.00) | 0.02 * |
| CRP (mg/dL) [median (range)] | 0.46 (0.01–30.42) | 20.00 (3.20–98.80) | <0.0001 * |
| AST (mg/dL) [median (range)] | 19.51 (9.85–75.08) | 22.47 (9.85–146.20) | 0.003 * |
| ALT (mg/dL) [median (range)] | 19.50 (7.00–90.00) | 23.00 (7.00–261.00) | 0.0004 * |
| Creatinine (mg/dL) [median (range)] | 0.77 (0.36–1.75) | 0.78 (0.40–7.42) | 0.90 |
| e-GFR (mL/min/1.73 m2) MDRD equation (mean ± SD) | 89.20 ± 33.67 | 88.34 ± 35.43 | 0.84 |
| WBC (×103/μL) (mean ± SD) | 7.82 ± 2.10 | 8.83 ± 3.14 | 0.003 * |
| HGB (g/dL) (mean ± SD) | 12.99 ± 2.06 | 13.26 ± 2.01 | 0.27 |
| PLT (×103/μL) (mean ± SD) | 255.00 ± 75.48 | 254.80 ± 70.93 | 0.87 |
| Parameters | PreDM Cohort (n = 100) | T2DM Cohort (n = 200) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test/ Mann–Whitney Test |
|---|---|---|---|
| Clinical and Anthropometric Features—Various indices | |||
| SBP (mmHg) (mean ± SD) | 137.00 ± 17.91 | 136.3 ± 17.13 | 0.74 |
| DBP (mmHg) (mean ± SD) | 79.74 ± 14.41 | 80.86 ± 11.94 | 0.34 |
| Height (cm) (mean ± SD) | 166.3 ± 10.57 | 166.5 ± 9.70 | 0.66 |
| Weight (kg) [median (range)] | 82.00 (50.00–140.50) | 80.00 (45.00–147.00) | 0.77 |
| WC (cm) [median (range)] | 101.00 (58.00–171.00) | 101.00 (52.00–133.00) | 0.12 |
| HC (cm) [median (range)] | 108.50 (75.00–147.00) | 108.00 (75.00–145.00) | 0.93 |
| WHR (mean ± SD) | 0.93 (0.59–1.64) | 0.92 (0.54–1.28) | 0.052 ** |
| WHtR [median (range)] | 0.61 (0.34–0.95) | 0.60 (0.35–0.85) | 0.02 * |
| BMI (kg/m2) [median (range)] | 30.40 (16.72–58.48) | 29.55 (18.21–46.85) | 0.80 |
| %BF (mean ± SD) | 39.10 ± 9.13 | 40.17 ± 9.35 | 0.34 |
| Lipemic specter | |||
| TC (mg/dL) (mean ± SD) | 206.60 ± 58.78 | 187.5 ± 60.73 | 0.001 * |
| LDL-c (mg/dL) (mean ± SD) | 125.10 ± 52.47 | 110.00 ± 47.71 | 0.005 * |
| HDL-c (mg/dL) (mean ± SD) | 53.59 ± 13.59 | 44.12 ± 13.34 | <0.0001 * |
| TG (mg/dL) [median (range)] | 128.50 (45.00–610.00) | 144.50 (44.00–1563.00) | 0.34 |
| Metabolic Syndrome-Related Indices | |||
| TyG (mean ± SD) | 8.78 ± 0.50 | 9.31 ± 0.67 | <0.0001 * |
| TyG-BMI (mean ± SD) | 268.10 ± 64.33 | 284.60 ± 59.59 | 0.02 * |
| TyG-WC (mean ± SD) | 903.10 ± 137.10 | 910.10 ± 182.10 | 0.43 |
| TyG-WHtR (mean ± SD) | 5.43 ± 0.75 | 5.46 ± 1.06 | 0.77 |
| TG/HDL-c [median (range)] | 2.53 (0.59–19.06) | 3.23 (0.64–45.97) | 0.0005 * |
| AIP (mean ± SD) range | 0.40 ± 0.26 (−0.23–1.28) | 0.52 ± 0.30 (−0.19–1.66) | 0.0005 * |
| CMI [median (range)] | 1.59 (0.33–11.92) | 1.89 (0.37–33.53) | 0.008 * |
| CRR [median (range)] | 3.71 (1.74–9.34) | 4.06 (2.06–15.14) | 0.02 * |
| Parameters | MUHNW-PreDM Cohort (n = 33) | MUHO-PreDM Cohort (n = 67) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test/Mann–Whitney Test | MUHNW-T2DM Cohort (n = 52) | MUHO-T2DM Cohort (n = 148) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test/Mann–Whitney Test |
|---|---|---|---|---|---|---|
| Demographic and Clinical Features—Medical History | ||||||
| Age (years) (mean ± SD) | 54.67 ± 11.46 | 57.42 ± 11.53 | 0.26 | 64.58 ± 13.41 | 62.32 ± 11.90 | 0.057 ** |
| Gender Male/Female (n) | 11/22 | 36/31 | 0.05 * | 28/24 | 73/75 | 0.57 |
| Residence Urban/Rural (n) | 22/11 | 39/28 | 0.41 | 35/17 | 84/64 | 0.18 |
| Education Yes/No (n) | 26/7 | 42/25 | 0.10 | 40/12 | 90/58 | 0.03 * |
| Drinking Yes/No (n) | 12/21 | 30/37 | 0.42 | 21/31 | 39/109 | 0.054 ** |
| Smoking Yes/No (n) | 14/19 | 33/34 | 0.52 | 19/33 | 47/101 | 0.52 |
| Hepatoseatosis n (%) | 26 (78%) | 40 (59%) | 0.05 * | 27 (51%) | 121 (81%) | <0.0001 * |
| Hyperuricemia n (%) | 3 (9%) | 9 (13%) | 0.74 | 6 (11%) | 29 (19%) | 0.18 |
| Anthropometric features and various indices | ||||||
| Height (cm) (mean ± SD) | 174.00 ± 10.70 | 162.60 ± 8.26 | <0.0001 * | 169.40 ± 9.48 | 165.50 ± 9.60 | 0.01 * |
| Weight (kg) [median (range)] | 67.00 (50.00–94.00) | 88.00 (65.00–140.5) | <0.0001 * | 68.50 (45.00–87.00) | 86.00 (62.00–147.00) | <0.0001 * |
| HC (cm) [median (range)] | 104.00 (75.00–147.00) | 111.00 (82.00–147.00) | 0.11 | 103.50 (82.00–130.00) | 110.00 (75.00–145.00) | <0.0001 * |
| WHtR [median (range)] | 0.57 (0.34–0.95) | 0.63 (0.45–0.88) | 0.001 * | 0.46 (0.35–0.70) | 0.63 (0.43–0.85) | <0.0001 * |
| %BF (mean ± SD) | 30.82 ± 6.77 | 42.90 ± 7.40 | <0.0001 * | 32.10 ± 7.70 | 42.68 ± 8.42 | <0.0001 * |
| Laboratory examination | ||||||
| ESR (mm/1st hour) [median (range)] | 30.00 (10.00–115.00) | 29.00 (5.00–115.00) | 0.30 | 30.00 (4.00–110.00) | 32.00 (4.00–140.00) | 0.33 |
| CRP (mg/dL) [median (range)] | 19.50 (7.00–80.00) | 20 (3.20–98.80) | 0.71 | 0.30 (0.01–19.65) | 0.51 (0.01–30.42) | 0.01 * |
| AST (mg/dL) [median (range)] | 19.35 (9.85–32.11) | 19.62 (9.85–75.08) | 0.41 | 21.28 (9.85–65.43) | 22.64 (12.00–146.20) | 0.05 * |
| ALT (mg/dL) [median (range)] | 20.00 (7.00–28.00) | 19.00 (7.00–90.00) | 0.08 | 18.50 (7.00–60.00) | 24.50 (9.00–261.00) | <0.0001 * |
| Creatinine (mg/dL) [median (range)] | 0.74 (0.47–1.75) | 0.82 (0.36–1.66) | 0.61 | 0.71 (0.40–2.83) | 0.82 (0.44–7.42) | 0.15 |
| e-GFR (mL/min/1.73 m2) MDRD equation (mean ± SD) | 88.81 ± 37.25 | 89.39 ± 32.05 | 0.93 | 96.33 ± 41.93 | 85.53 ± 32.54 | 0.05 * |
| WBC (×103/μL) (mean ± SD) | 7.98 ± 2.28 | 7.73 ± 2.01 | 0.57 | 8.22 ± 2.77 | 9.04 ± 3.25 | 0.10 |
| HGB (g/dL) (mean ± SD) | 13.19 ± 1.86 | 12.89 ± 2.16 | 0.49 | 12.64 ± 1.87 | 13.48 ± 2.02 | 0.009 * |
| PLT (×103/μL) (mean ± SD) | 275.10 ± 89.42 | 245.10 ± 66.09 | 0.06 | 243.90 ± 78.36 | 258.70 ± 68.00 | 0.19 |
| Metabolic Syndrome-Related Indices | ||||||
| TyG-WHtR (mean ± SD) | 5.04 ± 0.80 | 5.62 ± 0.66 | 0.0003 * | 4.37 ± 0.68 | 5.84 ± 0.90 | <0.0001 * |
| TG/HDL-c [median (range)] | 2.35 (0.59–15.61) | 2.54 (0.69–19.06) | 0.91 | 2.55 (0.64–10.75) | 3.47 (0.65–45.97) | 0.001 * |
| AIP (mean ± SD) range | 0.37 ± 0.31 (−0.23–1.19) | 0.42 ± 0.24 (−0.16–1.28) | 0.41 | 0.40 ± 0.26 (−0.19–1.03) | 0.56 ± 0.30 (−0.19–1.66) | 0.001 * |
| CMI [median (range)] | 1.40 (0.33–8.98) | 1.67 (0.45–11.92) | 0.10 | 1.27 (0.36–4.89) | 2.16 (0.43–33.53) | <0.0001 * |
| CRR [median (range)] | 3.68 (1.78–8.68) | 3.71 (1.74–9.34) | 0.57 | 3.85 (2.06–7.42) | 4.15 (2.33–15.14) | 0.04 * |
| Parameters | MUHNW-PreDM Cohort (n = 33) | MUHNW-T2DM Cohort (n = 52) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test/Mann–Whitney Test | MUHO-PreDM Cohort (n = 67) | MUHO-T2DM Cohort (n = 148) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test/Mann–Whitney Test |
|---|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 54.67 ± 11.46 | 64.58 ± 13.41 | 0.0007 * | 57.42 ± 11.53 | 62.32 ± 11.90 | 0.005 * |
| Gender Male/Female (n) | 11/22 | 28/24 | 0.06 | 36/31 | 73/75 | 0.54 |
| Residence Urban/Rural (n) | 22/11 | 35/17 | 1 | 39/28 | 84/64 | 0.84 |
| Drinking Yes/No (n) | 12/21 | 21/31 | 0.70 | 30/37 | 39/109 | 0.007 * |
| Smoking Yes/No (n) | 14/19 | 19/33 | 0.59 | 33/34 | 47/101 | 0.01 * |
| Hepatoseatosis n (%) | 26 (78%) | 27 (51%) | 0.01 * | 40 (59%) | 121 (81%) | 0.0005 * |
| Height (cm) (mean ± SD) | 174.00 ± 10.70 | 169.40 ± 9.48 | 0.04 * | 162.60 ± 8.26 | 165.50 ± 9.60 | 0.03 * |
| Weight (kg) [median (range)] | 67.00 (50.00–94.00) | 68.50 (45.00–87.00) | 0.65 | 88.00 (65.00–140.5) | 86.00 (62.00–147.00) | 0.54 |
| HC (cm) [median (range)] | 104.00 (75.00–147.00) | 103.50 (82.00–130.00) | 0.18 | 111.00 (82.00–147.00) | 110.00 (75.00–145.00) | 0.64 |
| WHtR [median (range)] | 0.57 (0.34–0.95) | 0.46 (0.35–0.70) | <0.0001 * | 0.63 (0.45–0.88) | 0.63 (0.43–0.85) | 0.28 |
| %BF (mean ± SD) | 30.82 ± 6.77 | 32.10 ± 7.70 | 0.43 | 42.90 ± 7.40 | 42.68 ± 8.42 | 0.85 |
| e-GFR (mL/min/1.73 m2) MDRD equation (mean ± SD) | 88.81 ± 37.25 | 96.33 ± 41.93 | 0.40 | 89.39 ± 32.05 | 85.53 ± 32.54 | 0.41 |
| TyG-WHtR (mean ± SD) | 5.04 ± 0.80 | 4.37 ± 0.68 | <0.0001 * | 5.62 ± 0.66 | 5.84 ± 0.90 | 0.059 ** |
| TG/HDL-c [median (range)] | 2.35 (0.59–15.61) | 2.55 (0.64–10.75) | 0.59 | 2.54 (0.69–19.06) | 3.47 (0.65–45.97) | 0.0002 * |
| AIP (mean ± SD) range | 0.37 ± 0.31 (−0.23–1.19) | 0.40 ± 0.26 (−0.19–1.03) | 0.61 | 0.42 ± 0.24 (−0.16–1.28) | 0.56 ± 0.30 (−0.19–1.66) | 0.001 * |
| CMI [median (range)] | 1.40 (0.33–8.98) | 1.27 (0.36–4.89) | 0.46 | 1.67 (0.45–11.92) | 2.16 (0.43–33.53) | 0.0008 * |
| CRR [median (range)] | 3.68 (1.78–8.68) | 3.85 (2.06–7.42) | 0.91 | 3.71 (1.74–9.34) | 4.15 (2.33–15.14) | 0.01 * |
| Variables (Mean ± SD) | PreDM Cohort (n = 100) | |||||||
|---|---|---|---|---|---|---|---|---|
| TyG | p-Value from Kruskal–Wallis/ One-Way ANOVA | TyG-BMI | p-Value from Kruskal–Wallis/ One-Way ANOVA | TyG-WC | p-Value from Kruskal–Wallis/ One-Way ANOVA | TyG-WHtR | p-Value from Kruskal–Wallis/ One-Way ANOVA | |
| BMI category (kg/m2) | ||||||||
| Normal weight (18.5–24.9 kg/m2) | 8.57 ± 0.55 | 0.05 * | 185.20 ± 22.08 | <0.0001 * | 884.50 ± 196.40 | 0.47 | 5.01 ± 0.96 | 0.002 * |
| Overweight (25–29.9 kg/m2) | 8.92 ± 0.53 | 242.60 ± 21.38 | 886.60 ± 107.80 | 5.32 ± 0.57 | ||||
| Obese (≥30 kg/m2) | 8.80 ± 0.45 | 313.30 ± 49.42 | 919.20 ± 119.70 | 5.65 ± 0.66 | ||||
| WHR | ||||||||
| Q 1 (0.59–0.86) | 8.65 ± 0.42 | 0.17 | 286.40 ± 77.51 | 0.25 | 785.00 ± 122.30 | <0.0001 * | 4.90 ± 0.78 | <0.0001 * |
| Q 2 (0.87–0.92) | 8.80 ± 0.40 | 260.00 ± 53.20 | 919.00 ± 118.90 | 5.57 ± 0.62 | ||||
| Q 3 (0.93–0.97) | 8.96 ± 0.59 | 250.10 ± 50.78 | 905.90 ± 99.05 | 5.34 ± 0.57 | ||||
| Q 4 (0.98–1.64) | 8.73 ± 0.55 | 268.70 ± 70.74 | 990.50 ± 119.80 | 5.85 ± 0.70 | ||||
| WHtR | ||||||||
| Q 1 (0.34–0.56) | 8.86 ± 0.61 | 0.51 | 242.90 ± 78.24 | 0.09 | 775.40 ± 130.00 | <0.0001 * | 4.59 ± 0.64 | <0.0001 * |
| Q 2 (0.57–0.60) | 8.86 ± 0.42 | 261.60 ± 53.64 | 880.20 ± 73.76 | 5.25 ± 0.29 | ||||
| Q 3 (0.61–0.66) | 8.71 ± 0.54 | 272.70 ± 46.13 | 914.00 ± 80.58 | 5.55 ± 0.35 | ||||
| Q 4 (0.67–0.95) | 8.70 ± 0.40 | 289.70 ± 77.33 | 1051.00 ± 113.30 | 6.34 ± 0.50 | ||||
| %BF | ||||||||
| Q 1 (20.49–32.58) | 8.72 ± 0.59 | 0.22 | 201.10 ± 38.88 | <0.0001 * | 894.80 ± 173.80 | 0.04 * | 5.16 ± 0.84 | 0.004 * |
| Q 2 (32.59–39.04) | 8.64 ± 0.41 | 254.80 ± 32.06 | 848.90 ± 91.73 | 5.26 ± 0.58 | ||||
| Q 3 (39.05–45.54) | 8.89 ± 0.43 | 277.60 ± 46.77 | 913.50 ± 114.90 | 5.43 ± 0.62 | ||||
| Q 4 (45.55–67.32) | 8.88 ± 0.55 | 333.40 ± 58.28 | 955.10 ± 140.10 | 5.86 ± 0.79 | ||||
| Variables (Mean ± SD) | T2DM Cohort (n = 200) | |||||||
|---|---|---|---|---|---|---|---|---|
| TyG | p-Value from Kruskal–Wallis/ One-Way ANOVA | TyG-BMI | p-Value from Kruskal–Wallis/ One-Way ANOVA | TyG-WC | p-Value from Kruskal–Wallis/ One-Way ANOVA | TyG-WHtR | p-Value from Kruskal–Wallis/ One-Way ANOVA | |
| BMI category (kg/m2) | ||||||||
| Normal weight (18.5–24.9 kg/m2) | 9.06 ± 0.56 | 0.02 * | 212.10 ± 18.53 | <0.0001 * | 724.30 ± 132.40 | <0.0001 * | 4.26 ± 0.60 | <0.0001 * |
| Overweight (25–29.9 kg/m2) | 9.37 ± 0.63 | 259.30 ± 24.71 | 880.70 ± 152.30 | 5.25 ± 0.79 | ||||
| Obese (≥30 kg/m2) | 9.39 ± 0.72 | 328.10 ± 51.19 | 1009.00 ± 145.70 | 6.11 ± 0.83 | ||||
| WHR | ||||||||
| Q 1 (0.54–0.79) | 9.27 ± 0.70 | 0.88 | 240.60 ± 48.19 | <0.0001 * | 680.60 ± 105.90 | <0.0001 * | 4.18 ± 0.56 | <0.0001 * |
| Q 2 (0.80–0.91) | 9.34 ± 0.69 | 291.50 ± 59.88 | 916.30 ± 122.00 | 5.41 ± 0.70 | ||||
| Q 3 (0.92–0.97) | 9.28 ± 0.54 | 298.20 ± 54.85 | 979.70 ± 109.10 | 5.84 ± 0.64 | ||||
| Q 4 (0.98–1.28) | 9.36 ± 0.73 | 300.00 ± 61.56 | 1060.00 ± 124.00 | 6.39 ± 0.82 | ||||
| WHtR | ||||||||
| Q 1 (0.35–0.50) | 9.24 ± 0.60 | 0.54 | 229.40 ± 33.32 | <0.0001 * | 672.60 ± 80.79 | <0.0001 * | 4.12 ± 0.42 | <0.0001 * |
| Q 2 (0.51–0.59) | 9.30 ± 0.76 | 266.20 ± 43.21 | 897.50 ± 96.82 | 5.24 ± 0.49 | ||||
| Q 3 (0.60–0.65) | 9.29 ± 0.59 | 294.50 ± 42.18 | 983.30 ± 86.65 | 5.84 ± 0.37 | ||||
| Q 4 (0.66–0.85) | 9.43 ± 0.74 | 344.00 ± 59.91 | 1097.00 ± 116.80 | 6.71 ± 0.70 | ||||
| %BF | ||||||||
| Q 1 (13.27–33.11) | 9.19 ± 0.72 | 0.15 | 235.10 ± 40.51 | <0.0001 * | 859.30 ± 172.50 | <0.0001 * | 4.96 ± 0.91 | <0.0001 * |
| Q 2 (33.12–40.41) | 9.36 ± 0.57 | 272.60 ± 38.09 | 894.90 ± 170.40 | 5.28 ± 0.86 | ||||
| Q 3 (40.42–46.29) | 9.23 ± 0.70 | 283.00 ± 50.31 | 877.40 ± 200.50 | 5.33 ± 1.09 | ||||
| Q 4 (46.30–63.23) | 9.46 ± 0.66 | 343.70 ± 57.67 | 1009.00 ± 151.90 | 6.30 ± 0.91 | ||||
| Variables (Mean ± SD) [Median (Range)] | PreDM Cohort (n = 100) | T2DM Cohort (n = 200) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIP | p-Value from Kruskal–Wallis/ One-Way ANOVA | CMI | p-Value from Kruskal–Wallis/ One-Way ANOVA | CRR | p-Value from Kruskal–Wallis/ One-Way ANOVA | AIP | p-Value from Kruskal–Wallis/ One-Way ANOVA | CMI | p-Value from Kruskal–Wallis/ One-Way ANOVA | CRR | p-Value from Kruskal–Wallis/ One-Way ANOVA | ||
| BMI category (kg/m2) | |||||||||||||
| Normal weight (18.5–24.9 kg/m2) | 0.30 ± 0.30 (−0.23–1.19) | 0.06 | 1.26 (0.33–8.89) | 0.01 * | 3.60 (2.07–8.68) | 0.49 | 0.37 ± 0.23 (−0.03–0.98) | 0.001 * | 1.09 (0.47–4.13) | <0.0001 * | 3.50 (2.06–7.42) | 0.01 * | |
| Overweight (25–29.9 kg/m2) | 0.47 ± 0.25 (0.02–1.05) | 1.85 (0.59–6.26) | 4.09 (1.78–8.26) | 0.55 ± 0.27 (−0.19–1.06) | 2.02 (0.36–7.08) | 4.38 (2.10–10.17) | |||||||
| Obese (≥30 kg/m2) | 0.41 ± 0.24 (−0.16–1.28) | 1.65 (0.45–11.92) | 3.61 (1.74–9.34) | 0.56 ± 0.32 (−0.19–1.66) | 2.21 (0.43–33.53) | 4.16 (2.36–15.14) | |||||||
| WHR | |||||||||||||
| PreDM Value T2DM Value | Q 1 (0.59–0.86) (0.54–0.79) | 0.31 ± 0.21 (−0.23–0.75) | 0.04 * | 1.23 (0.33–3.38) | 0.02 * | 3.19 (1.74–6.64) | 0.19 | 0.49 ± 0.33 (−0.06–1.47) | 0.001 * | 1.28 (0.47–15.43) | 0.03 * | 4.02 (2.06–8.14) | 0.48 |
| PreDM Value T2DM Value | Q 2 (0.87–0.92) (0.80–0.91) | 0.39 ± 0.19 (−0.03–0.89) | 1.62 (0.58–5.53) | 3.52 (1.78–8.26) | 0.53 ± 0.29 (−0.19 ± 1.29) | 1.99 (0.43–11.24) | 4.46 (2.33–15.14) | ||||||
| PreDM Value T2DM Value | Q 3 (0.93–0.97) (0.92–0.97) | 0.52 ± 0.30 (−0.13–1.19) | 1.88 (0.41–8.89) | 3.93 (2.08–6.06) | 1.04 ± 1.52 (−0.19–8.81) | 1.95 (0.36–6.66) | 3.91 (2.10–10.17) | ||||||
| PreDM Value T2DM Value | Q 4 (0.98–1.64) (0.98–1.28) | 0.39 ± 0.29 (−0.22–1.28) | 1.73 (0.39–11.92) | 3.95 (2.07–9.34) | 0.54 ± 0.33 (−0.03–1.66) | 2.21 (0.59–33.53) | 3.90 (2.33–10.44) | ||||||
| WHtR | |||||||||||||
| PreDM Value T2DM Value | Q 1 (0.34–0.56) (0.35–0.50) | 0.42 ± 0.30 (−0.23–1.19) | 0.92 | 1.39 (0.33–8.89) | 0.47 | 3.62 (2.72–8.26) | 0.57 | 0.48 ± 0.27 (0.04–1.03) | 0.41 | 1.35 (0.47–4.89) | <0.0001 * | 4.12 (2.06–8.14) | 0.60 |
| PreDM Value T2DM Value | Q 2 (0.57–0.60) (0.51–0.59) | 0.42 ± 0.17 (0.02–0.78) | 1.62 (0.59–3.54) | 3.52 (2.08–8.24) | 0.49 ± 0.32 (−0.19–1.47) | 1.82 (0.36–15.43) | 4.09 (2.10–15.14) | ||||||
| PreDM Value T2DM Value | Q 3 (0.61–0.66) (0.60–0.65) | 0.39 ± 0.33 (−0.22–1.28) | 1.53 (0.39–11.92) | 3.84 (1.78–9.34) | 0.53 ± 0.27 (−0.03–1.18) | 2.10 (0.59–9.42) | 3.80 (2.46–13.17) | ||||||
| PreDM Value T2DM Value | Q 4 (0.67–0.95) (0.66–0.85) | 0.38 ± 0.20 (0.07–0.91) | 1.73 (0.82–5.99) | 3.77 (1.74–8.68) | 0.57 ± 0.33 (−0.19–1.66) | 2.50 (0.43–33.53) | 4.22 (2.33–10.44) | ||||||
| %BF | |||||||||||||
| PreDM Value T2DM Value | Q 1 (20.49–32.58) (13.27–33.11) | 0.38 ± 0.32 (−0.23 ± 1.19) | 0.48 | 1.44 (0.33–8.89) | 0.27 | 4.29 (2.07–8.68) | 0.34 | 0.46 ± 0.32 (−0.19–1.47) | 0.53 | 1.58 (0.36–15.43) | 0.06 | 4.06 (2.10–7.42) | 0.68 |
| PreDM Value T2DM Value | Q 2 (32.59–39.04) (33.12–40.41) | 0.35 ± 0.22 (−0.03–1.05) | 1.41 (0.58–6.26) | 3.63 (1.78–7.29) | 0.54 ± 0.27 (0.03–1.18) | 2.02 (0.48–9.42) | 3.92 (2.06–13.17) | ||||||
| PreDM Value T2DM Value | Q 3 (39.05–45.54) (40.42–46.29) | 0.45 ± 0.20 (0.17–0.89) | 1.70 (0.73–5.53) | 3.93 (2.08–6.53) | 0.53 ± 0.30 (−0.09–1.66) | 1.82 (0.47–33.53) | 4.32 (2.42–10.44) | ||||||
| PreDM Value T2DM Value | Q 4 (45.55–67.32) (46.30–63.23) | 0.43 ± 0.30 (−0.16–1.28) | 1.73 (0.45–11.92) | 3.37 (1.74–9.34) | 0.54 ± 0.31 (−0.19 ± 1.29) | 2.17 (0.43–11.92) | 3.92 (2.37–15.14) | ||||||
| Metabolic Syndrome- Related Indices | Male—PreDM (n = 47) | Male—T2DM (n = 101) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test | Female—PreDM (n = 53) | Female—T2DM (n = 99) | p-Value from Pearson’s Chi-Squared/ Student’s t-Test |
|---|---|---|---|---|---|---|
| TyG (mean ± SD) | 8.70 ± 0.40 | 9.26 ± 0.64 | <0.0001 * | 8.85 ± 0.57 | 9.37 ± 0.69 | <0.0001 * |
| TyG-BMI (mean ± SD) | 279.7 ± 70.10 | 272.80 ± 54.60 | 0.51 | 255.30 ± 58.77 | 292.90 ± 65.96 | 0.0007 * |
| TyG-WC (mean ± SD) | 886.20 ± 136.50 | 937.70 ± 153.00 | 0.05 * | 918.10 ± 137.10 | 882.00 ± 204.60 | 0.25 |
| TyG-WHtR (mean ± SD) | 5.46 ± 0.74 | 5.44 ± 0.84 | 0.87 | 5.40 ± 0.77 | 5.49 ± 1.25 | 0.64 |
| TG/HDL-c [median (range)] | 2.42 (0.60–9.59) | 3.41 (0.64–29.35) | 0.001 * | 2.58 (0.59–19.06) | 3.19 (0.65–45.97) | 0.07 |
| AIP (mean ± SD) range | 0.37 ± 0.21 (−0.22–0.98) | 0.51 ± 0.29 (−0.19–1.47) | 0.004 * | 0.43 ± 0.30 (−0.23–1.28) | 0.52 ± 0.31 (−0.19–1.66) | 0.08 |
| CMI [median (range)] | 1.60 (0.39–6.16) | 2.02 (0.36–15.43) | 0.009 * | 1.58 (0.33–11.92) | 1.82 (0.43–33.53) | 0.27 |
| CRR [median (range)] | 3.91 (2.07–8.68) | 4.05 (2.10–13.17) | 0.63 | 3.52 (1.74–9.34) | 4.14 (2.06–15.14) | 0.01 * |
| %BF (mean ± SD) | 35.69 ± 9.31 | 33.54 ± 6.65 | 0.11 | 41.77 ± 8.08 | 46.45 ± 7.14 | 0.0003 * |
| Parameter | AUC | Std. Error | Cut-Off Values | Sensitivity (%) | Specificity (%) | Youden Index | p-Value |
|---|---|---|---|---|---|---|---|
| PreDM-T2DM | |||||||
| AIP | 0.623 | 0.033 | 0.49 | 54.00 | 67.00 | 0.21 | 0.0005 * |
| TG/HDLc | 0.622 | 0.033 | 2.79 | 62.50 | 61.00 | 0.24 | 0.0005 * |
| CMI | 0.592 | 0.033 | 1.71 | 54.00 | 59.00 | 0.13 | 0.009 * |
| WHtR | 0.579 | 0.033 | 0.60 | 50.50 | 57.00 | 0.08 | 0.02 * |
| CRR | 0.577 | 0.035 | 3.79 | 53.00 | 59.00 | 0.12 | 0.02 * |
| %BF | 0.527 | 0.035 | 40.05 | 53.50 | 54.00 | 0.08 | 0.435 |
| TyG-WHtR | 0.517 | 0.033 | 5.56 | 50.00 | 64.00 | 0.14 | 0.628 |
| MUHNW-PreDM—MUHNW-T2DM | |||||||
| WHtR | 0.798 | 0.050 | 0.52 | 71.15 | 84.85 | 0.56 | <0.0001 * |
| TyG-WHtR | 0.765 | 0.054 | 4.78 | 73.08 | 69.70 | 0.43 | <0.0001 * |
| CRR | 0.549 | 0.063 | 3.46 | 42.31 | 63.64 | 0.06 | 0.443 |
| CMI | 0.547 | 0.064 | 1.39 | 57.69 | 51.52 | 0.09 | 0.462 |
| TG/HDLc | 0.534 | 0.064 | 2.37 | 55.77 | 51.52 | 0.07 | 0.594 |
| AIP | 0.533 | 0.064 | 0.37 | 55.77 | 51.52 | 0.07 | 0.604 |
| %BF | 0.506 | 0.065 | 29.14 | 57.69 | 48.48 | 0.06 | 0.917 |
| MUHO-PreDM—MUHO-T2DM | |||||||
| TG/HDLc | 0.659 | 0.038 | 2.92 | 63.51 | 68.66 | 0.32 | 0.0002 * |
| AIP | 0.659 | 0.038 | 0.44 | 67.57 | 64.18 | 0.32 | 0.0002 * |
| CMI | 0.641 | 0.039 | 1.79 | 65.54 | 61.19 | 0.27 | 0.0009 * |
| %BF | 0.607 | 0.042 | 42.55 | 51.53 | 50.75 | 0.02 | 0.01 * |
| CRR | 0.591 | 0.043 | 3.87 | 64.19 | 58.21 | 0.22 | 0.03 * |
| TyG-WHtR | 0.587 | 0.040 | 5.61 | 61.49 | 58.21 | 0.19 | 0.03 * |
| WHtR | 0.545 | 0.041 | 0.63 | 56.76 | 47.76 | 0.05 | 0.287 |
| Criteria | Cut-Off Values |
|---|---|
| Waist circumference | >102 cm (male); >88 cm (female) |
| Serum FPG | ≥100 mg/dL or use of hypoglycemic drugs |
| Serum tryglicerides | ≥150 mg/dL or use of triglyceride-lowering drugs |
| Reduced HDL-c values | ≤40 mg/dL (males) or ≤50 mg/dL (females), or use of HDL-C-raising drugs |
| Elevated blood pressure | SBP ≥ 130 mmHg or DBP ≥ 85 mmHg, or use of blood pressure-lowering drugs |
| Cardiometabolic Phenotype | Criteria | |||
|---|---|---|---|---|
| BMI < 25.0 kg/m2 | BMI ≥ 25.0 kg/m2 | <3 MetS Criteria | ≥3 MetS Criteria | |
| MHNW | + | − | + | − |
| MUHNW | − | + | − | + |
| MHO | + | − | + | − |
| MUHO | − | + | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitroi Sakizlian, D.D.; Boldeanu, L.; Mitrea, A.; Clenciu, D.; Vladu, I.M.; Ciobanu Plasiciuc, A.E.; Șarla, A.V.; Siloși, I.; Boldeanu, M.V.; Assani, M.-Z.; et al. The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 6227. https://doi.org/10.3390/ijms26136227
Mitroi Sakizlian DD, Boldeanu L, Mitrea A, Clenciu D, Vladu IM, Ciobanu Plasiciuc AE, Șarla AV, Siloși I, Boldeanu MV, Assani M-Z, et al. The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus. International Journal of Molecular Sciences. 2025; 26(13):6227. https://doi.org/10.3390/ijms26136227
Chicago/Turabian StyleMitroi Sakizlian, Daniela Denisa, Lidia Boldeanu, Adina Mitrea, Diana Clenciu, Ionela Mihaela Vladu, Alina Elena Ciobanu Plasiciuc, Andra Veronica Șarla, Isabela Siloși, Mihail Virgil Boldeanu, Mohamed-Zakaria Assani, and et al. 2025. "The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus" International Journal of Molecular Sciences 26, no. 13: 6227. https://doi.org/10.3390/ijms26136227
APA StyleMitroi Sakizlian, D. D., Boldeanu, L., Mitrea, A., Clenciu, D., Vladu, I. M., Ciobanu Plasiciuc, A. E., Șarla, A. V., Siloși, I., Boldeanu, M. V., Assani, M.-Z., & Ciobanu, D. (2025). The Interplay of Cardiometabolic Syndrome Phenotypes and Cardiovascular Risk Indices in Patients Diagnosed with Diabetes Mellitus. International Journal of Molecular Sciences, 26(13), 6227. https://doi.org/10.3390/ijms26136227




.jpg)





