Sperm-Derived Dysfunction of Human Embryos: Molecular Mechanisms and Clinical Resolution
Abstract
1. Introduction
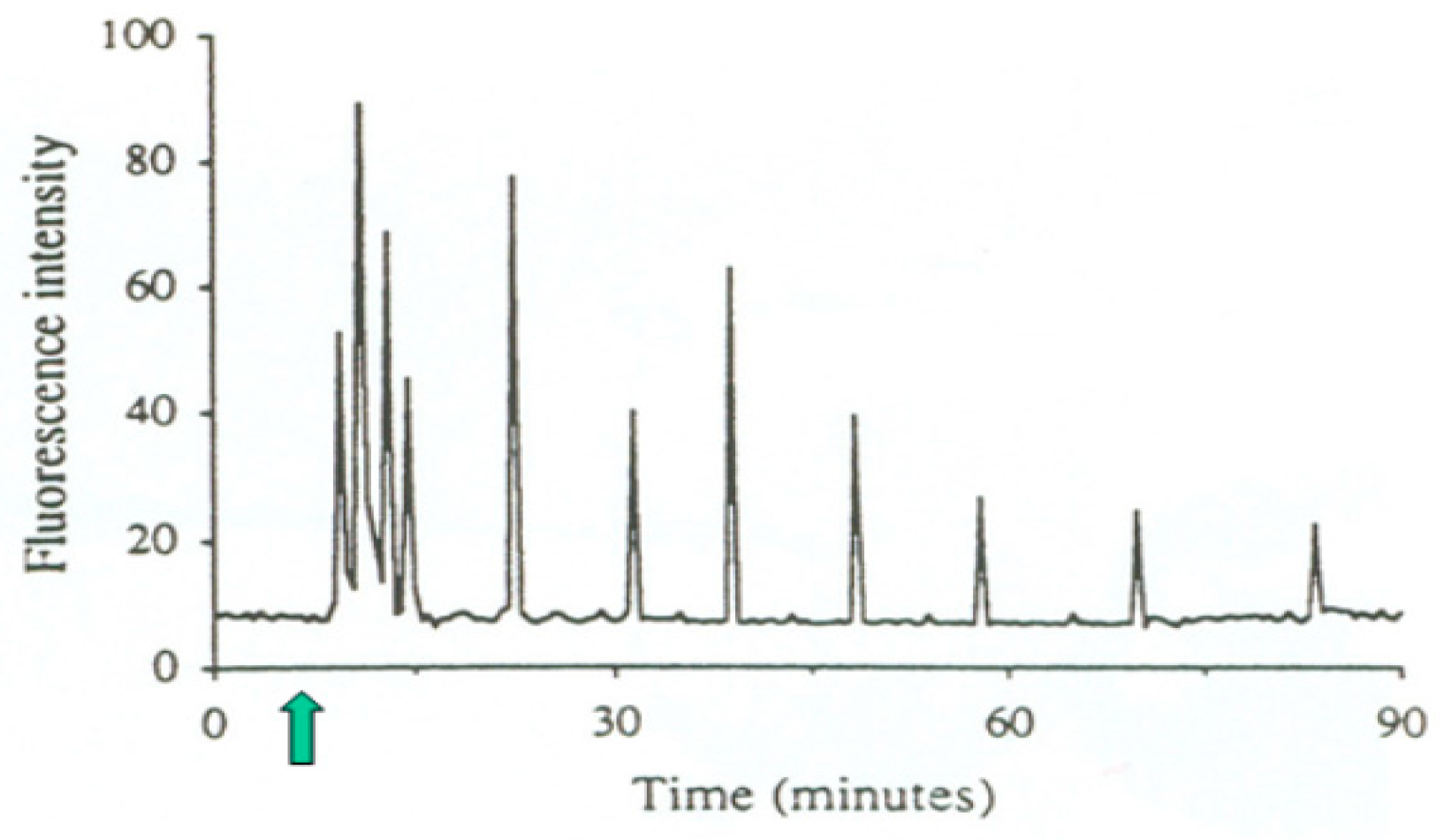
2. Sperm Oocyte-Activating Factor
2.1. Molecular Basis
2.2. Clinical Resolution
2.2.1. Diagnostic Methods
2.2.2. Treatment Options
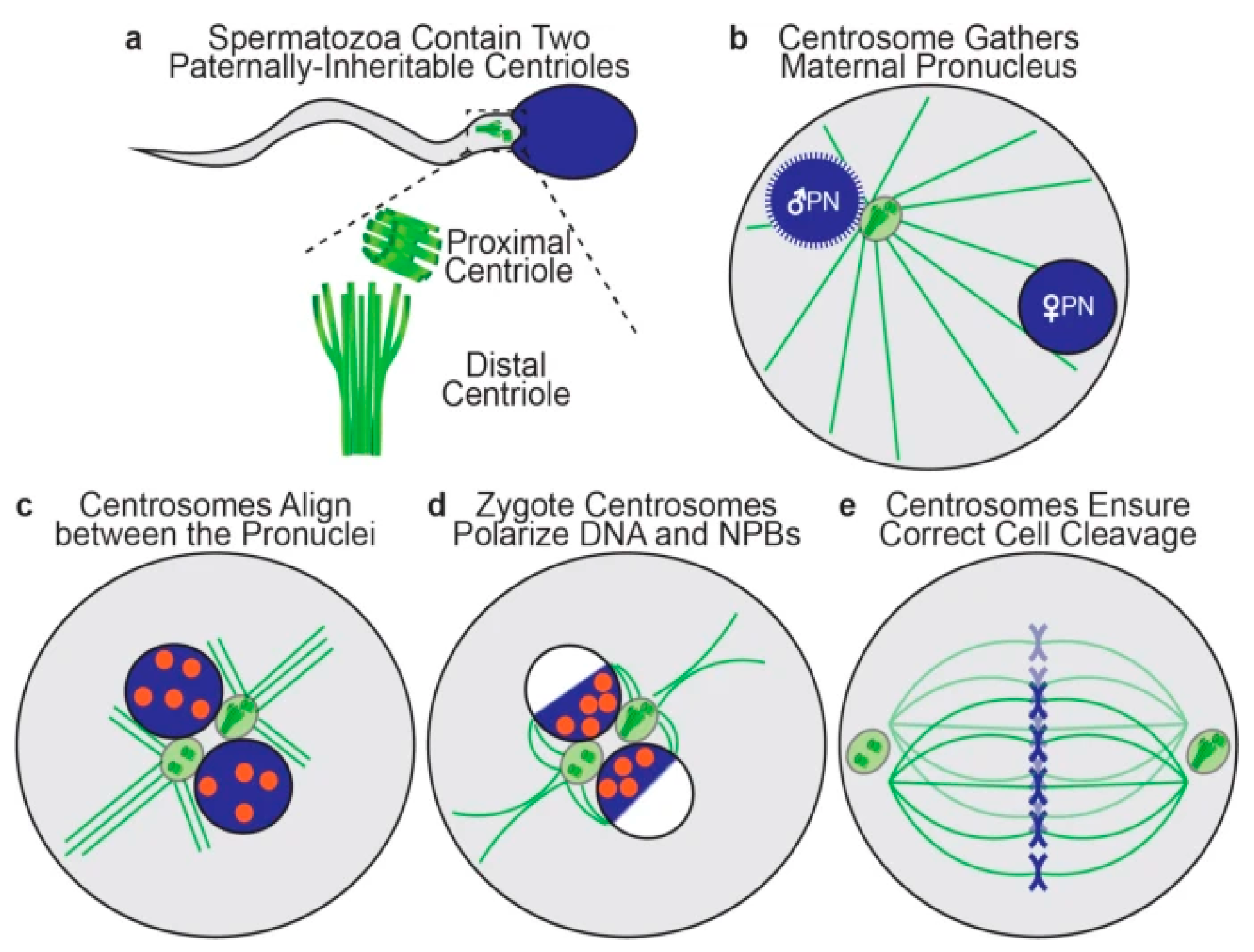
3. Sperm Centrioles
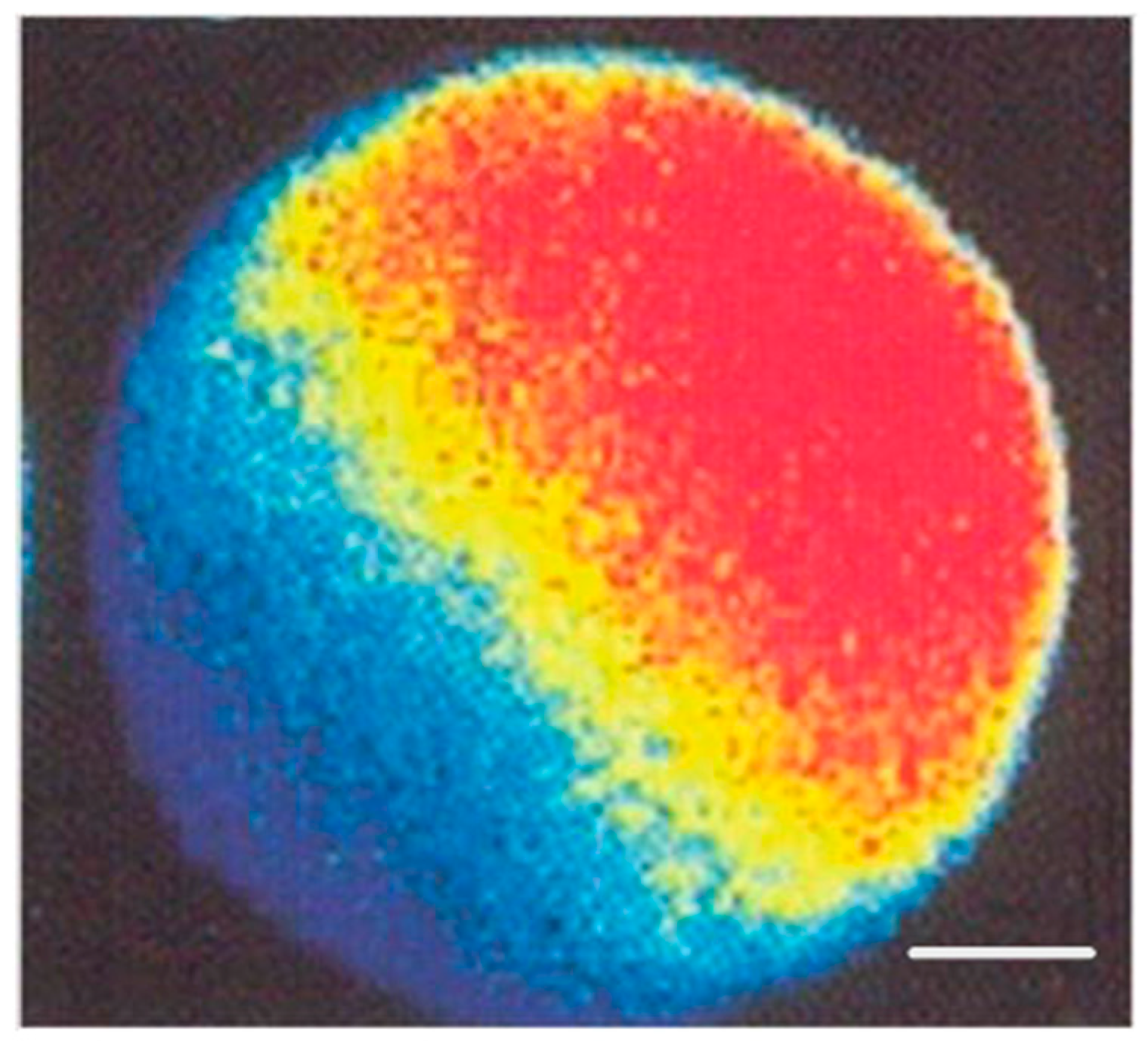
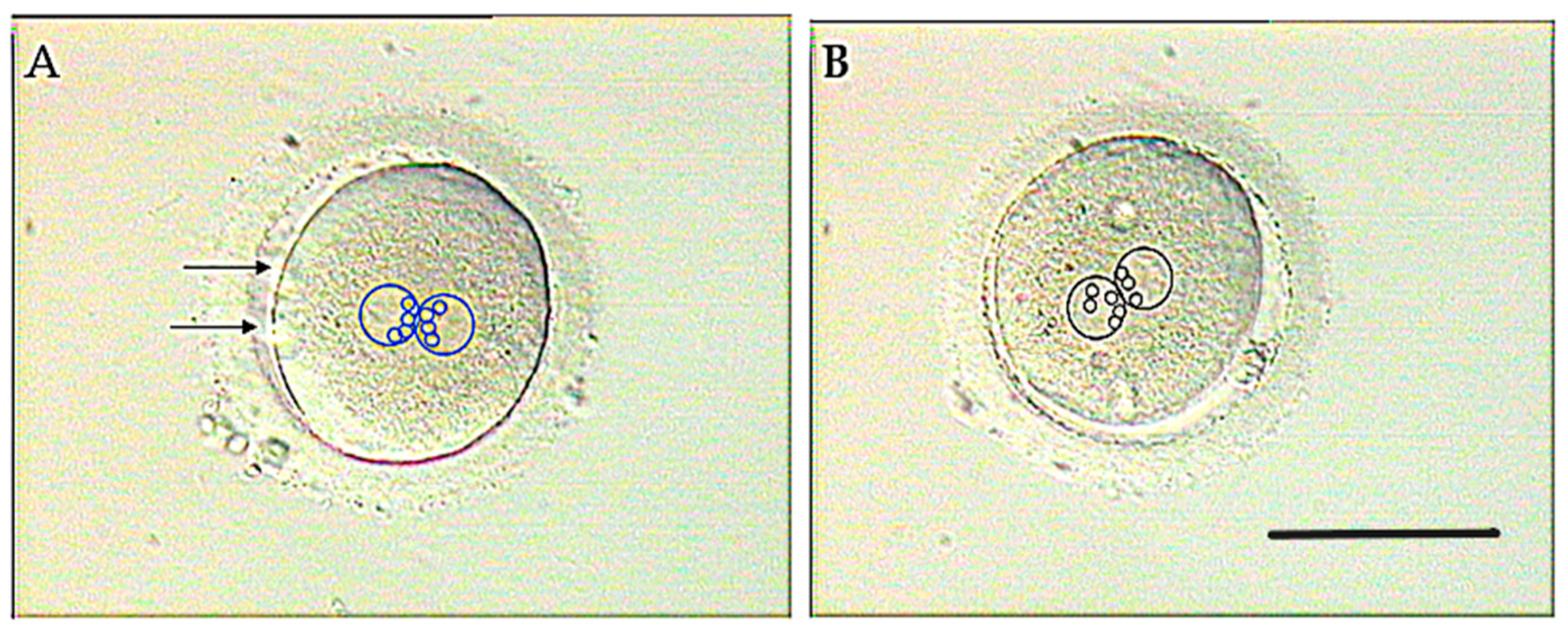
3.1. Molecular Basis
3.2. Clinical Resolution
3.2.1. Diagnostic Methods
3.2.2. Treatment Options
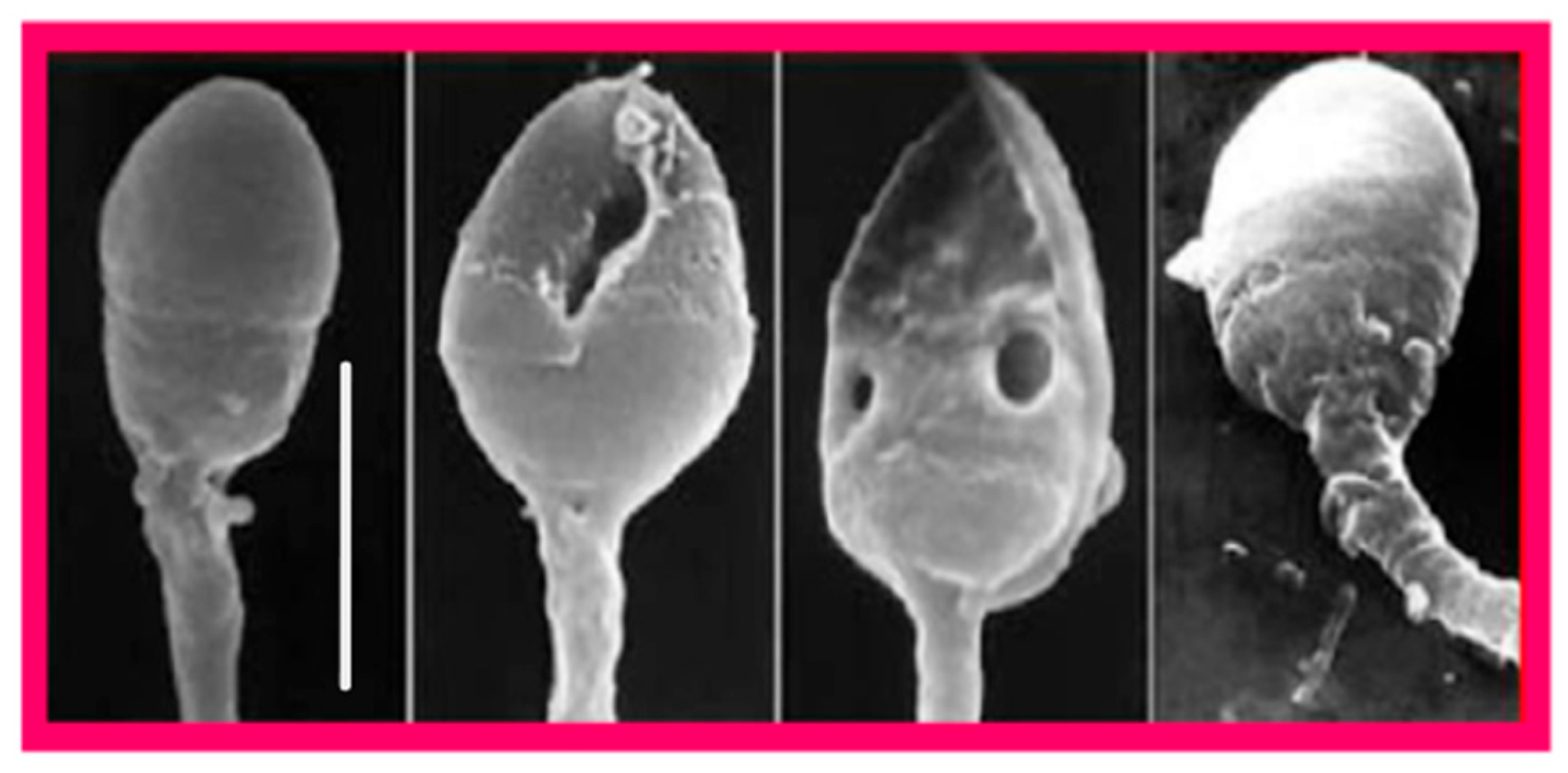
4. Sperm DNA
4.1. Molecular Basis
4.2. Clinical Resolution
4.2.1. Diagnostic Methods
4.2.2. Treatment Options
5. Sperm RNA
5.1. Molecular Basis
5.2. Clinical Resolution
5.2.1. Diagnostic Methods
5.2.2. Treatment Options
6. Sperm Proteins
6.1. Molecular Basis
6.2. Clinical Resolution
6.2.1. Diagnostic Methods
6.2.2. Treatment Options
7. General Considerations of Gamete Complementarity and Individual Susceptibility
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, P.C.; Edwards, R.G. Birth after reimplantation of a human embryo. Lancet 1978, 2, 366. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.G.; Steptoe, P.C. Current status of in-vitro fertilisation and implantation of human embryos. Lancet 1983, 2, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Tesarik, J. Assisted Reproduction: New Challenges and Future Prospects. In 40 Years After In Vitro Fertilisation; Tesarik, J., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; pp. 269–286. [Google Scholar]
- Tesarik, J.; Mendoza, C.; Testart, J. Viable embryos from injection of round spermatids into oocytes. N. Engl. J. Med. 1995, 333, 525. [Google Scholar] [CrossRef]
- Hanson, B.M.; Kohn, T.P.; Pastuszak, A.W.; Scott, R.T., Jr.; Cheng, P.J.; Hotaling, J.M. Round spermatid injection into human oocytes: A systematic review and meta-analysis. Asian J. Androl. 2021, 23, 363–369. [Google Scholar] [CrossRef]
- Loutradi, K.E.; Tarlatzis, B.C.; Goulis, D.G.; Zepiridis, L.; Pagou, T.; Chatziioannou, E.; Grimbizis, G.F.; Papadimas, I.; Bontis, I. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2006, 23, 69–74. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Maziotis, E.; Karantzali, E.; Kokkini, G.; Grigoriadis, S.; Pantou, A.; Giannelou, P.; Petroutsou, K.; Markomichali, C.; Fakiridou, M.; et al. Molecular drivers of developmental arrest in the human preimplantation embryo: A systematic review and critical analysis leading to mapping future research. Int. J. Mol. Sci. 2021, 22, 8353. [Google Scholar] [CrossRef]
- Vallet-Buisan, M.; Mecca, R.; Jones, C.; Coward, K.; Yeste, M. Contribution of semen to early embryo development: Fertilization and beyond. Hum. Reprod. Updat. 2023, 29, 395–433. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Qin, J.; Zhang, M.; He, X.; Ma, B.; Wan, Y.; Gao, Y.; Wang, M.; Hong, Z. Unraveling the mysteries of early embryonic arrest: Genetic factors and molecular mechanisms. J. Assist. Reprod. Genet. 2024, 41, 3301–3316. [Google Scholar] [CrossRef]
- Sousa, M.; Cunha, M.; Pereira, M.; Silva, J.; Gonçalves, A.; Viana, P.; Barros, N.; Pinto, D.; Geraldo, M.; Teixeira da Silva, J.; et al. Clinical outcomes of 127 patients with recurrent implantation failure treated with testicular sperm aspiration (TESA). Hum. Reprod. 2022, 37 (Suppl. 1), deac107.060. [Google Scholar] [CrossRef]
- Gkeka, K.; Symeonidis, E.N.; Tsampoukas, G.; Moussa, M.; Issa, H.; Kontogianni, E.; Almusafer, M.; Katsouri, A.; Mykoniatis, I.; Dimitriadis, F.; et al. Recurrent miscarriage and male factor infertility: Diagnostic and therapeutic implications. A narrative review. Cent. Eur. J. Urol. 2023, 76, 336–346. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.; Li, M.; Ma, X. Sperm DNA fragmentation index affect pregnancy outcomes and offspring safety in assisted reproductive technology. Sci. Rep. 2024, 14, 356. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Liew, F.F.; Dhawan, V.; Das, B.; Mottola, F.; Slama, P.; Rocco, L.; Roychoudhury, S. Environmental and genetic traffic in the journey from sperm to offspring. Biomolecules 2023, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.T.Y.; Lee, B.K.M.; Lee, C.L.; Tian, X.; Lam, K.K.W.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Ou, J.P.; Chiu, P.C.N. The role of spermatozoa-zona pellucida interaction in selecting fertilization-competent spermatozoa in humans. Front. Endocrinol. 2023, 14, 1135973. [Google Scholar] [CrossRef]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLC zeta: A sperm-specific trigger of Ca(2+) oscillations in eggs and embryo Development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jellerette, T.; Salicioni, A.M.; Lee, H.C.; Yoo, M.S.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.B.; Visconti, P.E.; et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo Development. J. Clin. Investig. 2008, 118, 3671–3681. [Google Scholar] [CrossRef]
- Escoffier, J.; Yassine, S.; Lee, H.C.; Martinez, G.; Delaroche, J.; Coutton, C.; Karaouzène, T.; Zouari, R.; Metzler-Guillemain, C.; Pernet-Gallay, K.; et al. Subcellular localization of phospholipase Cζ in human sperm and its absence in DPY19L2-deficient sperm are consistent with its role in oocyte activation. Mol. Hum. Reprod. 2015, 21, 157–168. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Z.; Dong, S.; Ding, M.; Li, J.; Wang, M.; Zeng, X.; Zhang, X.; Sun, X. Calcium signaling in oocyte quality and functionality and its application. Front. Endocrinol. 2024, 15, 1411000. [Google Scholar] [CrossRef]
- Modarres, P.; Tavalaee, M.; Ghaedi, K.; Nasr-Esfahani, M.H. An overview of the globozoospermia as a multigenic identified syndrome. Int. J. Fertil. Steril. 2019, 12, 273–277. [Google Scholar] [CrossRef]
- Cheung, S.; Parrella, A.; Tavares, D.; Keating, D.; Xie, P.; Rosenwaks, Z.; Palermo, G.D. Single-center thorough evaluation and targeted treatment of globozoospermic men. J. Assist. Reprod. Genet. 2021, 38, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Medrano, L.; Aizpurua, J.; Gómez-Torres, M.J. Phospholipase C zeta in human spermatozoa: A systematic review on current development and clinical application. Int. J. Mol. Sci. 2024, 25, 1344. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Vogiatzi, P.; Saleh, R.; Shah, R.; Agarwal, A. Sperm morphology value in assisted reproduction: Dismantling an enigma and key takeaways for the busy clinician. Ther. Adv. Reprod. Health 2024, 18, 26334941241303888. [Google Scholar] [CrossRef]
- Sousa, M.; Barros, A.; Tesarik, J. The role of ryanodine-sensitive Ca2+ stores in the Ca2+ oscillation machine of human oocytes. Mol. Hum. Reprod. 1996, 2, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J. Paternal Effects on Embryonic, Fetal and Offspring Health: The Role of Epigenetics in the ICSI and ROSI Era. In Innovations In Assisted Reproduction Technology; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Tesarik, J. Calcium signaling in human preimplantation development: A review. J. Assist. Reprod. Genet. 1999, 16, 216–220. [Google Scholar] [CrossRef]
- Xin, A.; Qu, R.; Chen, G.; Zhang, L.; Chen, J.; Tao, C.; Fu, J.; Tang, J.; Ru, Y.; Chen, Y.; et al. Disruption in ACTL7A causes acrosomal ultrastructural defects in human and mouse sperm as a novel male factor inducing early embryonic arrest. Sci. Adv. 2020, 6, eaaz4796. [Google Scholar] [CrossRef]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.Y.; Fissore, R.A.; Hamer, R.; Deane, C.M.; Ruas, M.; et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum. Reprod. 2009, 24, 2417–2428. [Google Scholar] [CrossRef]
- Yang, T.Y.; Chen, Y.; Chen, G.W.; Sun, Y.S.; Li, Z.C.; Shen, X.R.; Zhang, Y.N.; He, W.; Zhou, D.; Shi, H.J.; et al. Sperm-specific protein ACTL7A as a biomarker for fertilization outcomes of assisted reproductive technology. Asian J. Androl. 2022, 24, 260–265. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, T.; Guo, J.; Zhou, Q.; Gu, Y.; Zhang, J.; Hu, L.; Zong, Y.; Song, J.; Zhang, S.; et al. Homozygous pathogenic variants in ACTL9 cause fertilization failure and male infertility in humans and mice. Am. J. Hum. Genet. 2021, 108, 469–481. [Google Scholar] [CrossRef]
- Dai, J.; Li, Q.; Zhou, Q.; Zhang, S.; Chen, J.; Wang, Y.; Guo, J.; Gu, Y.; Gong, F.; Tan, Y.; et al. IQCN disruption causes fertilization failure and male infertility due to manchette assembly defect. EMBO Mol. Med. 2022, 14, e16501. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, Y.; Deng, K.; Shen, J.; Cui, Y.; Liu, J.; Yang, X.; Diao, F. Mutations in PLCZ1 induce male infertility associated with polyspermy and fertilization failure. J. Assist. Reprod. Genet. 2023, 40, 53–64. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.; Li, B.; Zhang, T.; Niu, Y.; Hu, S.; Ding, Y.; Yao, G.; Wei, Z.; Yao, N.; et al. Novel mutations in PLCZ1 lead to early embryonic arrest as a male factor. Front. Cell Dev. Biol. 2023, 11, 1193248. [Google Scholar] [CrossRef]
- Tesarik, J.; Testart, J. Treatment of sperm-injected human oocytes with Ca2+ ionophore supports the development of Ca2+ oscillations. Biol. Reprod. 1994, 51, 385–391. [Google Scholar] [CrossRef][Green Version]
- Sousa, M.; Mendoza, C.; Barros, A.; Tesarik, J. Calcium responses of human oocytes after intracytoplasmic injection of leukocytes, spermatocytes and round spermatids. Mol. Hum. Reprod. 1996, 2, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Cardona Barberán, A.; Boel, A.; Vanden Meerschaut, F.; Stoop, D.; Heindryckx, B. Diagnosis and treatment of male infertility-related fertilization failure. J. Clin. Med. 2020, 9, 3899. [Google Scholar] [CrossRef] [PubMed]
- Rybouchkin, A.; Dozortsev, D.; de Sutter, P.; Qian, C.; Dhont, M. Intracytoplasmic injection of human spermatozoa into mouse oocytes: A useful model to investigate the oocyte-activating capacity and the karyotype of human spermatozoa. Hum. Reprod. 1995, 10, 1130–1135. [Google Scholar] [CrossRef]
- Heindryckx, B.; Van der Elst, J.; De Sutter, P.; Dhont, M. Treatment option for sperm- or oocyte-related fertilization failure: Assisted oocyte activation following diagnostic heterologous ICSI. Hum. Reprod. 2005, 20, 2237–2241. [Google Scholar] [CrossRef] [PubMed]
- Vanden Meerschaut, F.; Leybaert, L.; Nikiforaki, D.; Qian, C.; Heindryckx, B.; De Sutter, P. Diagnostic and prognostic value of calcium oscillatory pattern analysis for patients with ICSI fertilization failure. Hum. Reprod. 2013, 28, 87–98. [Google Scholar] [CrossRef]
- Ahmadi, A.; Bongso, A.; Ng, S.C. Intracytoplasmic injection of human sperm into the hamster oocyte (hamster ICSI assay) as a test for fertilizing capacity of the severe male-factor sperm. J. Assist. Reprod. Genet. 1996, 13, 647–651. [Google Scholar] [CrossRef]
- Taylor, S.L.; Yoon, S.Y.; Morshedi, M.S.; Lacey, D.R.; Jellerette, T.; Fissore, R.A.; Oehninger, S. Complete globozoospermia associated with PLCζ deficiency treated with calcium ionophore and ICSI results in pregnancy. Reprod. Biomed. Online 2010, 20, 559–564. [Google Scholar] [CrossRef]
- Heindryckx, B.; De Gheselle, S.; Gerris, J.; Dhont, M.; De Sutter, P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod. Biomed. Online 2008, 17, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Montag, M.; Köster, M.; van der Ven, K.; Bohlen, U.; van der Ven, H. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod. Biomed. Online 2012, 24, 521–526. [Google Scholar] [CrossRef]
- Ebner, T.; Montag, M.; Oocyte Activation Study Group; Van der Ven, K.; Van der Ven, H.; Ebner, T.; Shebl, O.; Oppelt, P.; Hirchenhain, J.; Krüssel, J.; et al. Live birth after artificial oocyte activation using a ready-to-use ionophore: A prospective multicentre study. Reprod. Biomed. Online 2015, 30, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Saso, S.; Jones, B.P.; Bracewell-Milnes, T.; Athanasiou, T.; Mania, A.; Serhal, P.; Ben-Nagi, J. Does the use of calcium ionophore during artificial oocyte activation demonstrate an effect on pregnancy rate? A meta-analysis. Fertil. Steril. 2017, 108, 468–482.e3. [Google Scholar] [CrossRef]
- Bonte, D.; Ferrer-Buitrago, M.; Dhaenens, L.; Popovic, M.; Thys, V.; De Croo, I.; De Gheselle, S.; Steyaert, N.; Boel, A.; Vanden Meerschaut, F.; et al. Assisted oocyte activation significantly increases fertilization and pregnancy outcome in patients with low and total failed fertilization after intracytoplasmic sperm injection: A 17-year retrospective study. Fertil. Steril. 2019, 112, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, H.; Lv, J.; Dong, Y.; Zhao, M.; Sui, X.; Cui, R.; Liu, B.; Wu, K. Calcium ionophore improves embryonic development and pregnancy outcomes in patients with previous developmental problems in ICSI cycles. BMC Pregnancy Childbirth 2022, 22, 894. [Google Scholar] [CrossRef]
- Ruan, J.L.; Liang, S.S.; Pan, J.P.; Chen, Z.Q.; Teng, X.M. Artificial oocyte activation with Ca2+ ionophore improves reproductive outcomes in patients with fertilization failure and poor embryo development in previous ICSI cycles. Front. Endocrinol. 2023, 14, 1244507. [Google Scholar] [CrossRef]
- Tesarik, J.; Rienzi, L.; Ubaldi, F.; Mendoza, C.; Greco, E. Use of a modified intracytoplasmic sperm injection technique to overcome sperm-borne and oocyte-borne oocyte activation failures. Fertil. Steril. 2002, 78, 619–624. [Google Scholar] [CrossRef]
- Mansour, R.; Fahmy, I.; Tawab, N.A.; Kamal, A.; El-Demery, Y.; Aboulghar, M.; Serour, G. Electrical activation of oocytes after intracytoplasmic sperm injection: A controlled randomized study. Fertil. Steril. 2009, 91, 133–139. [Google Scholar] [CrossRef]
- Capalbo, A.; Ottolini, C.S.; Griffin, D.K.; Ubaldi, F.M.; Handyside, A.H.; Rienzi, L. Artificial oocyte activation with calcium ionophore does not cause a widespread increase in chromosome segregation errors in the second meiotic division of the oocyte. Fertil. Steril. 2016, 105, 807–814.e2. [Google Scholar] [CrossRef]
- Vanden Meerschaut, F.; D’Haeseleer, E.; Gysels, H.; Thienpont, Y.; Dewitte, G.; Heindryckx, B.; Oostra, A.; Roeyers, H.; Van Lierde, K.; De Sutter, P. Neonatal and neurodevelopmental outcome of children aged 3–10 years born following assisted oocyte activation. Reprod. Biomed. Online 2014, 28, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Deemeh, M.R.; Tavalaee, M.; Nasr-Esfahani, M.H. Health of children born through artificial oocyte activation: A pilot study. Reprod. Sci. 2015, 22, 322–328. [Google Scholar] [CrossRef]
- Mateizel, I.; Verheyen, G.; Van de Velde, H.; Tournaye, H.; Belva, F. Obstetric and neonatal outcome following ICSI with assisted oocyte activation by calcium ionophore treatment. J. Assist. Reprod. Genet. 2018, 35, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, Y.; Yan, Z.; Li, M.; Xue, S.; Cai, R.; Fu, Y.; Hong, Q.; Long, H.; Yin, M.; et al. Pregnancy and neonatal outcomes of artificial oocyte activation in patients undergoing frozen-thawed embryo transfer: A 6-year population-based retrospective study. Arch. Gynecol. Obstet. 2019, 300, 1083–1092. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Mazur, M.; Fishman, E.L.; Sindhwani, P. The role of sperm centrioles in human reproduction—The known and the unknown. Front. Cell Dev. Biol. 2019, 7, 188. [Google Scholar] [CrossRef]
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210. [Google Scholar] [CrossRef]
- Kai, Y.; Iwata, K.; Iba, Y.; Mio, Y. Diagnosis of abnormal human fertilization status based on pronuclear origin and/or centrosome number. J. Assist. Reprod. Genet. 2015, 32, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Kluczynski, D.F.; Jaiswal, A.; Xu, M.; Nadiminty, N.; Saltzman, B.; Schon, S.; Avidor-Reiss, T. Spermatozoa centriole quality determined by FRAC may correlate with zygote nucleoli polarization-a pilot study. J. Assist. Reprod. Genet. 2025. [Google Scholar] [CrossRef]
- Hinduja, I.; Baliga, N.B.; Zaveri, K. Correlation of human sperm centrosomal proteins with fertility. J. Hum. Reprod. Sci. 2010, 3, 95–101. [Google Scholar] [CrossRef]
- Tesarik, J. Noninvasive biomarkers of human embryo developmental potential. Int. J. Mol. Sci. 2025, 26, 4928. [Google Scholar] [CrossRef]
- Oh, H.S.; Jang, J.M.; Yoon, H.J.; Choo, C.W.; Lim, K.S.; Lim, J.H.; Cheon, Y.P. The kinetics of nucleolar precursor bodies clustering at the pronuclei interface: Positive correlations with the morphokinetic characteristics of cleaving embryos and euploidy in preimplantation genetic testing programs. Clin. Exp. Reprod. Med. 2025, 52, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Y.; Schatten, H. Centrosome inheritance after fertilization and nuclear transfer in mammals. Adv. Exp. Med. Biol. 2007, 591, 58–71. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Khire, A.; Fishman, E.L.; Jo, K.H. Atypical centrioles during sexual reproduction. Front. Cell Dev. Biol. 2015, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Nakamura, S.; Simerly, C.; Hewitson, L.; Murakami, T.; Yaegashi, N.; Okamura, K.; Schatten, G. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs: A new male factor infertility assay. Mol. Reprod. Dev. 2004, 67, 360–365. [Google Scholar] [CrossRef]
- Nakamura, S.; Terada, Y.; Horiuchi, T.; Emuta, C.; Murakami, T.; Yaegashi, N.; Okamura, K. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo-driven pipette: A novel assay for human sperm centrosomal function. Biol. Reprod. 2001, 65, 1359–1363. [Google Scholar] [CrossRef]
- Nakamura, S.; Terada, Y.; Horiuchi, T.; Emuta, C.; Murakami, T.; Yaegashi, N.; Okamura, K. Analysis of the human sperm centrosomal function and the oocyte activation ability in a case of globozoospermia, by ICSI into bovine oocytes. Hum. Reprod. 2002, 17, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.A.; Fishman, E.L.; Asadullah, M.; Ott, B.; Dusza, P.; Shah, T.A.; Sindhwani, P.; Nadiminty, N.; Molinari, E.; Patrizio, P.; et al. Fluorescence-Based Ratiometric Analysis of Sperm Centrioles (FRAC) finds patient age and sperm morphology are associated with centriole quality. Front. Cell Dev. Biol. 2021, 9, 658891. [Google Scholar] [CrossRef]
- Nakamura, S.; Terada, Y.; Rawe, V.Y.; Uehara, S.; Morito, Y.; Yoshimoto, T.; Tachibana, M.; Murakami, T.; Yaegashi, N.; Okamura, K. A trial to restore defective human sperm centrosomal function. Hum. Reprod. 2005, 20, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J. Reprod. Fertil. 1986, 78, 463–470. [Google Scholar] [CrossRef]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation Development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecny, V.; Plachot, M.; Mandelbaum, J. Early morphological signs of embryonic genome expression in human preimplantation development as revealed by quantitative electron microscopy. Dev. Biol. 1988, 128, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Sousa, M. Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum. Genes 2023, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Belva, F.; Bonduelle, M.; Buysse, A.; Van den Bogaert, A.; Hes, F.; Roelants, M.; Verheyen, G.; Tournaye, H.; Keymolen, K. Chromosomal abnormalities after ICSI in relation to semen parameters: Results in 1114 fetuses and 1391 neonates from a single center. Hum. Reprod. 2020, 35, 2149–2162. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Baldi, E. Sperm DNA fragmentation: Mechanisms of origin. Adv. Exp. Med. Biol. 2019, 1166, 75–85. [Google Scholar] [CrossRef]
- Tesarik, J. Acquired sperm DNA modifications: Causes, consequences, and potential solutions. Eur. Med J. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Leem, J.; Bai, G.Y.; Oh, J.S. The capacity to repair sperm DNA damage in zygotes is enhanced by inhibiting WIP1 activity. Front. Cell Dev. Biol. 2022, 10, 841327. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza-Tesarik, R. Molecular clues to understanding causes of human assisted reproduction treatment failures and possible treatment options. Int. J. Mol. Sci. 2022, 23, 10357. [Google Scholar] [CrossRef]
- Musson, R.; Gąsior, Ł.; Bisogno, S.; Ptak, G.E. DNA damage in preimplantation embryos and gametes: Specification, clinical relevance and repair strategies. Hum. Reprod. Updat. 2022, 28, 376–399. [Google Scholar] [CrossRef] [PubMed]
- Newman, H.; Catt, S.; Vining, B.; Vollenhoven, B.; Horta, F. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in ART: A systematic review. Mol. Hum. Reprod. 2022, 28, gaab071. [Google Scholar] [CrossRef]
- Martin, R.H. Mechanisms of nondisjunction in human spermatogenesis. Cytogenet. Genome Res. 2005, 111, 245–249. [Google Scholar] [CrossRef]
- Levron, J.; Aviram-Goldring, A.; Madgar, I.; Raviv, G.; Barkai, G.; Dor, J. Sperm chromosome abnormalities in men with severe male factor infertility who are undergoing in vitro fertilization with intracytoplasmic sperm injection. Fertil. Steril. 2001, 76, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Carrell, D.T.; Wilcox, A.L.; Udoff, L.C.; Thorp, C.; Campbell, B. Chromosome 15 aneuploidy in the sperm and conceptus of a sibling with variable familial expression of round-headed sperm syndrome. Fertil. Steril. 2001, 76, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Burrello, N.; Vicari, E.; Shin, P.; Agarwal, A.; De Palma, A.; Grazioso, C.; D’Agata, R.; Calogero, A.E. Lower sperm aneuploidy frequency is associated with high pregnancy rates in ICSI programmes. Hum. Reprod. 2003, 18, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Carrell, D.T. Epigenetics of the male gamete. Fertil. Steril. 2012, 97, 267–274. [Google Scholar] [CrossRef]
- Hackett, J.A.; Surani, M.A. Beyond DNA: Programming and inheritance of parental methylomes. Cell 2013, 153, 737–739. [Google Scholar] [CrossRef]
- Hackett, J.A.; Sengupta, R.; Zylicz, J.J.; Murakami, K.; Lee, C.; Down, T.A.; Surani, M.A. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science 2013, 339, 448–452. [Google Scholar] [CrossRef]
- Lismer, A.; Kimmins, S. Emerging evidence that the mammalian sperm epigenome serves as a template for embryo Development. Nat. Commun. 2023, 14, 2142. [Google Scholar] [CrossRef]
- Cambiasso, M.Y.; Romanato, M.; Gotfryd, L.; Valzacchi, G.R.; Calvo, L.; Calvo, J.C.; Fontana, V.A. Sperm histone modifications may predict success in human assisted reproduction: A pilot study. J. Assist. Reprod. Genet. 2024, 41, 3147–3159. [Google Scholar] [CrossRef]
- Bogliotti, Y.S.; Ross, P.J. Mechanisms of histone H3 lysine 27 trimethylation remodeling during early mammalian Development. Epigenetics 2012, 7, 976–981. [Google Scholar] [CrossRef]
- Yuan, S.; Zhan, J.; Zhang, J.; Liu, Z.; Hou, Z.; Zhang, C.; Yi, L.; Gao, L.; Zhao, H.; Chen, Z.-J.; et al. Human zygotic genome activation is initiated from paternal genome. Cell Discov. 2023, 9, 13. [Google Scholar] [CrossRef]
- Sotomayor-Lugo, F.; Iglesias-Barrameda, N.; Castillo-Aleman, Y.M.; Casado-Hernandez, I.; Villegas-Valverde, C.A.; Bencomo-Hernandez, A.A.; Ventura-Carmenate, Y.; Rivero-Jimenez, R.A. The dynamics of histone modifications during mammalian zygotic genome activation. Int. J. Mol. Sci. 2024, 25, 1459. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Khalafiyan, A.; Zare, M.; Karimzadeh, H.; Bahrami, B.; Hammami, B.; Kazemi, M. Sperm epigenetics and male infertility: Unraveling the molecular puzzle. Hum. Genomics 2024, 18, 57. [Google Scholar] [CrossRef]
- Evgeni, E.; Sabbaghian, M.; Saleh, R.; Gül, M.; Vogiatzi, P.; Durairajanayagam, D.; Jindal, S.; Parmegiani, L.; Boitrelle, F.; Colpi, G.; et al. Sperm DNA fragmentation test: Usefulness in assessing male fertility and assisted reproductive technology outcomes. Panminerva Med. 2023, 65, 135–147. [Google Scholar] [CrossRef]
- Tesarik, J. Lifestyle and environmental factors affecting male fertility, individual predisposition, prevention, and intervention. Int. J. Mol. Sci. 2025, 26, 2797. [Google Scholar] [CrossRef] [PubMed]
- Adel Domínguez, M.A.; Cardona Maya, W.D.; Mora Topete, A. Sperm DNA fragmentation: Focusing treatment on seminal transport fluid beyond sperm production. Arch. Ital. Urol. Androl. 2025, 97, 13128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male infertility due to testicular disorders. J. Clin. Endocrinol. Metab. 2021, 106, e442–e459. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef]
- Greco, E.; Romano, S.; Iacobelli, M.; Ferrero, S.; Baroni, E.; Minasi, M.G.; Ubaldi, F.; Rienzi, L.; Tesarik, J. ICSI in cases of sperm DNA damage: Beneficial effect of oral antioxidant treatment. Hum. Reprod. 2005, 20, 2590–2594. [Google Scholar] [CrossRef]
- Hazout, A.; Dumont-Hassan, M.; Junca, A.M.; Cohen Bacrie, P.; Tesarik, J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod. Biomed. Online 2006, 12, 19–25. [Google Scholar] [CrossRef]
- Greco, E.; Scarselli, F.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Franco, G.; Anniballo, N.; Mendoza, C.; Tesarik, J. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum. Reprod. 2005, 20, 226–230. [Google Scholar] [CrossRef]
- Tesarik, J.; Thébault, A.; Testart, J. Effect of pentoxifylline on sperm movement characteristics in normozoospermic and asthenozoospermic specimens. Hum. Reprod. 1992, 7, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza, C. Sperm treatment with pentoxifylline improves the fertilizing ability in patients with acrosome reaction insufficiency. Fertil. Steril. 1993, 60, 141–148. [Google Scholar] [CrossRef]
- Bendayan, M.; Caceres, L.; Saïs, E.; Swierkowski-Blanchard, N.; Alter, L.; Bonnet-Garnier, A.; Boitrelle, F. Human Sperm Morphology as a Marker of Its Nuclear Quality and Epigenetic Pattern. Cells 2022, 11, 1788. [Google Scholar] [CrossRef]
- Johnson, G.D.; Lalancette, C.; Linnemann, A.K.; Leduc, F.; Boissonneault, G.; Krawetz, S.A. The sperm nucleus: Chromatin, RNA, and the nuclear matrix. Reproduction 2011, 141, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Howl, J.; Santos, M.A.S.; Fardilha, M. All you need to know about sperm RNAs. Hum. Reprod. Updat. 2021, 28, 67–91. [Google Scholar] [CrossRef]
- Hamilton, M.; Russell, S.; Swanson, G.M.; Krawetz, S.A.; Menezes, K.; Moskovtsev, S.I.; Librach, C. A comprehensive analysis of spermatozoal RNA elements in idiopathic infertile males undergoing fertility treatment. Sci. Rep. 2024, 14, 10316. [Google Scholar] [CrossRef] [PubMed]
- Leggio, L.; Paternò, G.; Cavallaro, F.; Falcone, M.; Vivarelli, S.; Manna, C.; Calogero, A.E.; Cannarella, R.; Iraci, N. Sperm epigenetics and sperm RNAs as drivers of male infertility: Truth or myth? Mol. Cell. Biochem. 2025, 480, 659–682. [Google Scholar] [CrossRef]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Pérez, J.A.; Rando, O.J. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell. 2018, 46, 470–480.e3. [Google Scholar] [CrossRef]
- Sharma, U.; Sun, F.; Conine, C.C.; Reichholf, B.; Kukreja, S.; Herzog, V.A.; Ameres, S.L.; Rando, O.J. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev. Cell. 2018, 46, 481–494.e6. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, Q.X.; Liu, J.C.; Yeung, W.S.; Zhang, J.V.; Duan, Y.G. Role of small RNAs harbored by sperm in embryonic development and offspring phenotype. Andrology 2023, 11, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Latham, K.E. Paternal effects in mammalian reproduction: Functional, environmental, and clinical relevance of sperm components in early embryos and beyond. Mol. Reprod. Dev. 2025, 92, e70020. [Google Scholar] [CrossRef] [PubMed]
- González, B.; González, C.R. Sperm-borne mRNAs: Potential roles in zygote genome activation and epigenetic inheritance. Open Biol. 2025, 15, 240321. [Google Scholar] [CrossRef]
- Estill, M.; Hauser, R.; Nassan, F.L.; Moss, A.; Krawetz, S.A. The effects of di-butyl phthalate exposure from medications on human sperm RNA among men. Sci. Rep. 2019, 9, 12397. [Google Scholar] [CrossRef] [PubMed]
- Jodar, M.; Sendler, E.; Moskovtsev, S.I.; Librach, C.L.; Goodrich, R.; Swanson, S.; Hauser, R.; Diamond, M.P.; Krawetz, S.A. Absence of sperm RNA elements correlates with idiopathic male infertility. Sci. Transl. Med. 2015, 7, 295re6. [Google Scholar] [CrossRef]
- Hamilton, M.; Russell, S.; Menezes, K.; Moskovtsev, S.I.; Librach, C. Assessing spermatozoal small ribonucleic acids and their relationship to blastocyst development in idiopathic infertile males. Sci. Rep. 2022, 12, 20010. [Google Scholar] [CrossRef]
- Joshi, M.; Andrabi, S.W.; Singh, V.; Bansal, S.K.; Makker, G.C.; Mishra, G.; Gupta, G.; Rajender, S. Coding and regulatory transcriptome comparisons between fertile and infertile spermatozoa identify RNA signatures of male infertility. Andrologia 2022, 54, e14437. [Google Scholar] [CrossRef]
- Joshi, M.; Andrabi, S.W.; Yadav, R.K.; Sankhwar, S.N.; Gupta, G.; Rajender, S. Qualitative and quantitative assessment of sperm miRNAs identifies hsa-miR-9-3p, hsa-miR-30b-5p and hsa-miR-122-5p as potential biomarkers of male infertility and sperm quality. Reprod. Biol. Endocrinol. 2022, 20, 122. [Google Scholar] [CrossRef]
- Castillo, J.; Jodar, M.; Oliva, R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Updat. 2018, 24, 535–555. [Google Scholar] [CrossRef]
- Schneider, S.; Shakeri, F.; Trötschel, C.; Arévalo, L.; Kruse, A.; Buness, A.; Poetsch, A.; Steger, K.; Schorle, H. Protamine-2 deficiency initiates a reactive oxygen species (ROS)-mediated destruction cascade during epididymal sperm maturation in mice. Cells 2020, 9, 1789. [Google Scholar] [CrossRef]
- Corda, P.O.; Moreira, J.; Howl, J.; Oliveira, P.F.; Fardilha, M.; Silva, J.V. Differential proteomic analysis of human sperm: A systematic review to identify candidate targets to monitor sperm quality. World J. Mens Health 2024, 42, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Tully, C.A.; Alesi, S.; McPherson, N.O.; Sharkey, D.J.; Teong, X.T.; Tay, C.T.; Silva, T.R.; Puglisi, C.; Barsby, J.P.; Moran, L.J.; et al. Assessing the influence of preconception diet on male fertility: A systematic scoping review. Hum. Reprod. Updat. 2024, 30, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The effect of nutrients and dietary supplements on sperm quality parameters: A systematic review and meta-analysis of randomized clinical trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef] [PubMed]

| Infertility Phenotype | Genes |
|---|---|
| Asthenospermia | AKAP3, AKAP4, AXDND1, CATSPER1, CATSPER2, CATSPER3, CATSPER4, CCDC103, CCDC40, CFAP43, CFAP44, CFAP70, COPS7A, CRHR1, CUL3, DEFB126, DNAAF1, DNAAF6, DNAH6, DNAH11, DNAH17, DNAH5, DNAH8, DNAH9, DNAI1, DNAJB13, DNHD1, DRC1, HIP1, HTX11, INSL6 IQCG, IQUB, KLHL7, KRT34, LRRC6, MT-C03, NEDD4, NSUN7, QRICH2, RSPH3, RSPH6A, SEPTIN4, SLC26A8, SPAG17, SPATA33, TEKT2, ZMYND10 |
| Multiple morphological anomalies of the flagella (MMAF) | BRWD1, CCDC34, CCDC39, CEP135, CFAP251, CFAP58, CFAP61, CFAP69, CFAP74, DNAH1, DNAH10, DNAH17, DNAH2, DNAH5, DNAH6, DNAH7, DNAH8, DZIP1, DZP1, FSIP2, MAATS1, ODF2, QRICH2, SPAG6, SPATA16, SPEF2, TTC21A, TTC29, WDR19, WDR66 |
| Nonobstructive azoospermia | AR, ABLIM1, AHRR, ART3, ATM, AZFa, AZFb, AZFc, BCL2, BPDY2, BPY2, CCDC34, CDC42BPA, CDY2A, DAZ1, DBX3Y, DMC1, DMRT1, DNMT3B, EPSTI1, ETV5, FANCM, GNAO1, HLA-DRA, HSF2, HSFY1, KLHL10, M1AP, MCM8, MEIOB, MLH3, MSMB, MTHFR, NANOS1, NPAS2, NR5A1, PACRG, PIWIL2, PNLDC1, PYGO2, RBMX, RBYMIAI, REC8, SIRPG, SOHLH1, SOX5, SPINK2, SRSF6, STAG3, STX2, SYCE1, SYCE1L, SYCP3, TAF4B, TDRD9, TEX11, TEX14, TEX15, USP9Y, WT1, XRCC2, ZMYND15 |
| Obstructive azoospermia | ADGRG2, CFTR |
| Oligozoospermia | AXDND1, DAZ1, DAZ2, DICER1, DNMT1, EPHX2, GSTM1, GSTT1, KIT, KITLG, NR0B1, NR5A1, OR2W3, PARP1, PIWIL3, PIWIL4, PLK4, PON1, PON2, PRM1, PSAT1, SIRPA, SOX6, USP8, ZMYND15 |
| Teratozoospermia | AURKC, BSCL2, CCIN, CCDC90B, CCDC91, DPY19L2, SPATA20, SPA17, CYP1A1, FBXO43, PPP2R3C, SEPTIN12, ZPBP, DPY19L2, PICK1, SPATA16, SEPTIN4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Mendoza Tesarik, R. Sperm-Derived Dysfunction of Human Embryos: Molecular Mechanisms and Clinical Resolution. Int. J. Mol. Sci. 2025, 26, 6217. https://doi.org/10.3390/ijms26136217
Tesarik J, Mendoza Tesarik R. Sperm-Derived Dysfunction of Human Embryos: Molecular Mechanisms and Clinical Resolution. International Journal of Molecular Sciences. 2025; 26(13):6217. https://doi.org/10.3390/ijms26136217
Chicago/Turabian StyleTesarik, Jan, and Raquel Mendoza Tesarik. 2025. "Sperm-Derived Dysfunction of Human Embryos: Molecular Mechanisms and Clinical Resolution" International Journal of Molecular Sciences 26, no. 13: 6217. https://doi.org/10.3390/ijms26136217
APA StyleTesarik, J., & Mendoza Tesarik, R. (2025). Sperm-Derived Dysfunction of Human Embryos: Molecular Mechanisms and Clinical Resolution. International Journal of Molecular Sciences, 26(13), 6217. https://doi.org/10.3390/ijms26136217







