Abstract
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are linked by shared genetic mutations and overlapping clinical features, forming a clinical spectrum. This systematic review and meta-analysis analysed 97 studies, including 3212 patients with key ALS/FTD gene mutations, to identify gene-specific behavioural profiles. Chromosome 9 open reading frame 72 (C9orf72) mutations were strongly associated with psychotic symptoms and aggression, while superoxide dismutase 1 (SOD1) mutations had minimal cognitive effects. Progranulin (PGRN) mutations correlated with apathy and hallucinations, microtubule-associated protein tau (MAPT) mutations with disinhibition, and charged multivesicular body protein 2B (CHMP2B) with social impairments. Fused in sarcoma (FUS) mutations caused early sleep disturbances, TANK-binding kinase 1 (TBK1) led to disinhibition, and presenilin 1 and 2 (PSEN1/2) was linked to severe aggression. Prodromal cognitive changes in PGRN, MAPT, and CHMP2B mutations suggested early disease onset. Despite overlapping symptoms and clinical heterogeneity, understanding gene-specific patterns could inform tailored care strategies to enhance the quality of life for ALS and FTD patients. This study calls for refined guidelines integrating genetic behavioural profiles to improve patient and family support.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a relentlessly progressive neurodegenerative disease characterised primarily by motor neuron degeneration [1], leading to muscle wasting, weakness, and spasticity. Frontotemporal lobar degeneration (FTLD), in contrast, is a neurodegenerative syndrome encompassing a spectrum of neurodegenerative syndromes, including frontotemporal dementia (FTD), semantic dementia (SD), and progressive non-fluent aphasia (PNFA) [2]. The behavioural variant of FTD (bvFTD) is primarily characterised by marked changes in personality, behaviour, and executive dysfunction [3,4]. Semantic dementia (SD) presents with fluent yet empty speech, impaired word comprehension, and a progressive loss of semantic knowledge. Progressive non-fluent aphasia (PNFA) is mainly defined by effortful, non-fluent speech, agrammatism, and motor speech deficits, while single-word comprehension remains relatively preserved in the early stages [5]. Its diagnostic criteria include cognitive impairment, alongside the presence of at least three Rascovsky symptoms, such as disinhibition, apathy or inertia, loss of sympathy or empathy, perseverative or compulsive behaviours, hyperorality, and executive dysfunction [6]. A diagnosis of FTD may also be made in cases where there is a loss of insight and/or the presence of psychotic features accompanied by at least two Rascovsky symptoms, or when language impairment consistent with semantic dementia is present [6]. Moreover, FTD frequently presents with overlapping clinical features of other neurodegenerative conditions, most notably amyotrophic lateral sclerosis (ALS-FTD) or parkinsonism (FTD-P) [7].
Traditionally, ALS was understood as a purely motor disorder. However, accumulating evidence indicates that behavioural and cognitive manifestations are also present in ALS. Neuropsychological screening has demonstrated that 14–40% of ALS patients experience behavioural disturbances, with or without dementia [8]. Approximately 10–15% of ALS patients meet criteria for the behavioural variant of FTD (bvFTD), being diagnosed as ALS-FTD. The remaining ALS patients who do not develop dementia but develop apathy and other behavioural symptoms are classified as ALS with behavioural impairment (ALSbi) [9].
Genetic mutations underlie the pathogenesis of approximately 10% of familial ALS (FALS) and 90% of sporadic ALS (SALS) cases [10]. Mutations in the microtubule associated protein tau (MAPT), progranulin (PGRN), chromosome 9 open reading frame 72 (C9orf72seq), valosin containing protein (VCP) and charged multivesicular body protein 2B (CHMP2B) are principally associated with FTD. ALS-FTD is mainly correlated with C9orf72seq mutations [11]. Transactive response DNA-binding protein (TARDBP), Ubiquilin 2 (UBQLN2) and VCP mutations are usually linked to ALS. However, although rare, TARDBP mutations are also reported in ALS-FTD or FTD cases [12].
Across ALS-FTD and FTD patients, behavioural alterations such as disinhibition, apathy, loss of sympathy/empathy, perseverative or stereotypical behaviour, and dietary alterations are frequently observed [13]. Some features, such as apathy, self-centredness, and irritability, are more prominent in ALS; stereotypies and dietary alterations are more typical in FTD [9]. Despite attempts to distinguish between the behavioural profiles of ALS and FTD, substantial overlap has been observed at clinical, neuropathological, and genetic levels. These findings support the concept of a disease continuum between ALS and FTD [14].
Previous studies have attempted genotype–phenotype correlations for some ALS/FTD-related genes [15,16]. However, substantial gaps remain in our understanding of how specific mutations influence behavioural and cognitive features [17]. Drawing definitive conclusions regarding the differential effects of genetic mutations on patients’ behavioural profiles is challenging, largely due to methodological limitations in the existing studies. These include small sample sizes, variability and inconsistency in assessment tools, and a lack of appropriately matched control groups [10,17]. It is therefore essential to interpret the findings of such studies with caution, particularly as many do not provide quantitative data. Furthermore, identical or distinct mutations within the same gene can result in markedly variable phenotypes, [17] the underlying mechanisms of which remain to be elucidated.
In this systematic review and meta-analysis, we critically examined the differential impact of established ALS- and FTD-related genetic mutations on behavioural and cognitive changes, providing a comprehensive quantitative synthesis of the available evidence.
2. Materials and Methods
2.1. Search Strategy
A systematic bibliographic search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Independent searches for original research articles related to genetic mutations in ALS or FTD patients, and healthy patients, and their relationship with cognitive deficits assessed in neuropsychological evaluation were performed in four electronic databases: PubMed, Web of Science (WoS), Science Direct, and Scopus. The search was carried out by two researchers (AJG, NA) on 26 August 2024, using the following terms and search combinations: [“Amyotrophic Lateral Sclerosis”], [“Frontotemporal Dementia OR Frontotemporal Lobar Degeneration”], [“SOD1”], [“FUS”], [“C9orf72”], [“TARDBP”], [“PGRN”], [“ATXN2”], [“TBK1”], [“SQSTM1”], [“UBQLN2”], [“VCP”], [“Tau”], [“MAPT”], [“CHMP2B”], [“OPTN”], [“PSEN1/2”], [“ANXA2”], [“DCTN1”], [“TIA1”], [“Mutation”], [“Behavioural OR Behaviour”], [“Neuropsychiatric”], [“Depression OR Depressive”], [“Anxiety OR Anxious”], [“Social Behaviour”], [“Executive Function”], [“Cognitive OR Cognition”], [“Dementia”]. No chronological or methodological filters were applied to the search engines, apart from filtering by titles, keywords, and abstracts. The search strategy was adjusted for all databases. Reference lists of eligible studies were also scanned to include additional publications. All results were compiled into an Excel spreadsheet, and duplicates were discarded.
2.2. Literature Selection
Following the removal of duplicates, titles and abstracts of articles retrieved through database searching were screened for eligibility. Studies which did not specifically pertain to the effects of ALS/FTD-related genetic mutations on behavioural changes were deemed ineligible. After the initial screening phase, the full-text articles of selected studies were assessed against the inclusion criteria. For a study to be included in this review, it had to (i) include a study population diagnosed with ALS/FTD according to validated clinical criteria; (ii) report at least one outcome of interest (behavioural changes due to mutations of SOD1/FUS/C9orf72/TARDBP/PGRN/ATXN2/TBK1/SQSTM1/UBQLN2/VCP/MAPT/Tau/OPTN/TIA1/DCTN1/CHMP2B/PSEN1/PSEN2/ANXA2 genes); (iii) report unique cohorts, and for multiple articles reporting data from the same cohort, the article with the largest sample size was selected to avoid repetition; (iv) be a full-text article with original research; (v) be published in English; and (vi) be published in a peer-reviewed journal.
Exclusion criteria included articles with no abstracts or full texts, editorial publications, systematic reviews, case reports, meta-analyses, and commentaries.
2.3. Data Extraction
A total of 20 studies were included in the meta-analysis to evaluate the differential impact of known ALS- and FTD-related genetic mutations on behavioural effects. A continuous random effects model with a standard mean difference was employed to measure the impact of these genetic mutations on behavioural outcomes. Of the 97 studies included in this systematic review, 77 were excluded from the meta-analysis for the following reasons: A total of 39 studies lacked data for the control group, either because a healthy control group was not included, or the controls were not assessed using neuropsychological tests. Additionally, 30 studies were excluded as they were case reports, and 8 studies were excluded due to not reporting the means and standard deviations necessary for the meta-analysis. Statistical significance did not influence the inclusion of these studies in the meta-analysis, and studies reporting null findings were also included.
2.4. Statitical Analysis
In the meta-analysis, we evaluated the differential impact of known ALS- and FTD-related genetic mutations on behavioural outcomes. A continuous random effects model was employed using the standard mean difference (SMD) for continuous variables and the odds ratio (OR) for binary variables, both with 95% confidence intervals (CIs).
Statistical heterogeneity between studies was evaluated using the I2 test. A fixed effect model was applied when the heterogeneity was low (I2 < 50%, p > 0.1), while a random effects model was chosen for higher heterogeneity (I2 > 50%, p < 0.1). Subgroup and meta-regression analyses were conducted to explore potential sources of heterogeneity. For publication bias, Egger’s test was used when the number of included studies was ten or greater.
All data were analysed using R Studio (version 4.3.1) software. The ‘metafor’ package in R was used to calculate the SMD, log odds ratios, CIs, and heterogeneity, while the ‘meta’ package generated the forest plot. Statistical significance was set at p < 0.05 for all analyses.
3. Results
3.1. Study Selection
A total of 129,372 studies were identified through searches in WoS, PubMed, SCOPUS, and Science Direct. Initially, 118,346 studies were automatically excluded by the database tools, leaving 10,438 studies. Subsequently, 588 duplicates were removed, reducing the number to 9850. An additional 60 studies were excluded due to language restrictions, resulting in 9790 studies. Following this, 9046 studies were excluded for not being empirical, leaving 744 studies. Finally, after applying additional inclusion and exclusion criteria, a total of 97 studies were included in this systematic review. Studies were excluded due to being outside of the intended date range, missing gene data, and lacking information on cognitive symptoms (see Figure 1).
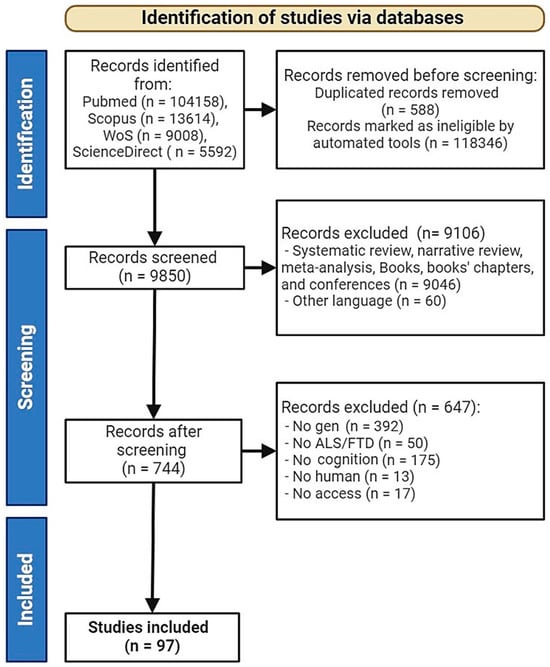
Figure 1.
Flowchart of the literature search according to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). ALS—Amyotrophic Lateral Sclerosis, FTD—Frontotemporal Dementia.
3.2. General Characteristics of Selected Studies
The studies included in this systematic review were published between 2002 and 2024, comprising a mix of cohort and retrospective studies. A total of 97 studies were selected, evaluating the impact of various genetic mutations on cognitive symptoms in patients with frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). The participants included in these studies had one of the two diagnoses mentioned, commonly either bvFTD or the presence of motor neuron disease (MND).
Across these studies, a total of 3814 patients were studied, encompassing a range of widely researched and novel gene mutations for comparison. The mean age of the participants across these studies was approximately 59 years, aligning with the diverse sample groups analysed. Most studies included database samples of between 200 and 800 participants, while some studies were single-case investigations or had smaller sample sizes, ranging from 10 to 20 participants. Additionally, 30 studies were case reports, including between one and five participants.
The most frequently gene studied was C9orf72seq, with 67 studies evaluating 1438 patients. MAPT was the focus of 38 studies, involving 906 patients, followed by GRN in 35 studies with 644 individuals. TARDBP was studied in eight studies, covering 91 patients, while FUS was examined in nine studies involving 18 patients. SOD1 was evaluated in six studies with 98 patients, and TBK1 was investigated in five studies with 17 patients. Additionally, a variety of other less common genes linked to FTD, including PSEN1, SQSTM1, VCP, and ANXA11, were assessed in smaller patient cohorts across several studies.
A detailed description of all the features is provided in Table 1.

Table 1.
Description of the different cognitive changes and the related mutations in ALS and FTD patients.
3.2.1. Evaluation of Global Cognition
The results indicate that carriers of C9orf72seq, GRN, and MAPT mutations exhibit a significant global cognitive impairment compared to healthy controls. Notably, C9orf72seq mutation carriers demonstrated lower scores on the Mini-Mental State Examination (MMSE) (n = 33), the Montreal Cognitive Assessment (MoCA) (n = 11), the Addenbrooke’s Cognitive Examination—Revised (ACE-R) (n = 1) or ACE-III (n = 2), and the Frontotemporal Dementia Rating Scale (FRS) (n = 3). This cognitive decline is consistently significant when compared to other genetic groups. GRN mutation carriers also display a substantial global cognitive decline, with lower MMSE and Frontal Assessment Battery (FAB) scores (n = 4); the Wisconsin Card Sorting Test (WCST) (n = 3) is particularly sensitive to executive dysfunction in these individuals. MAPT mutation carriers show lower scores on both MMSE and MoCA, with significant global cognitive impairment, although this is generally less severe than in GRN carriers. Commonly used tests for assessing global cognition include the MMSE, MoCA, and Clinical Dementia Rating (CDR) (n = 20). Similar levels of cognitive performance to controls were observed in presymptomatic cases [3,4,25,36,37,44,56,89,96,107].
TARDBP patients exhibit a complex profile of cognitive, behavioural, and functional impairments. In terms of global cognition, initial MMSE scores were comparable to healthy controls but declined over time, reflecting the progressive nature of the disease. Memory performance, particularly in immediate recall tasks like the Rey Figure, was notably poor, while other memory functions, such as story recall, showed only minor fluctuations.
Global cognitive decline in FUS patients is evident, with MMSE and Addenbrooke’s Mental Test Scores (AMTS) showing a gradual decrease over time, reflecting a worsening cognitive profile. SOD1 mutation carriers show a trend toward better performance in ALS-specific cognitive scores compared to sporadic ALS and C9orf72seq carriers, with statistically significant differences observed only between SOD1 carriers and sporadic ALS patients.
In patients with TBK1-related FTD, global cognitive function initially appeared normal, as evidenced by perfect scores on the MMSE and strong performance on episodic memory tests. However, over time, there was a noticeable decline in cognitive scores, reflecting the progressive nature of the condition.
In individuals carrying the PSEN1 mutation, a progressive decline in global cognitive functioning was observed, evidenced by a marked reduction in Short Test of Mental Status (STMS) and Mattis Dementia Rating Scale scores, progressing from mild impairment at age 57 to severe dementia by age 59.
Among patients with SQSTM1 mutations, cognitive impairment was variable. One case exhibited mild cognitive impairment, as indicated by an MMSE score of 27/30, with pronounced short-term memory deficits but relatively preserved long-term memory.
In those with VCP mutations, cognitive impairment was present in 25.5% of cases, with frontotemporal dementia (FTD) being the most common presentation. One specific case demonstrated global cognitive decline, with an MMSE score of 25, revealing deficits in orientation, attention, recall, and repetition. Frontal lobe dysfunction was further supported by a score of 12/18 on the Frontal Assessment Battery, indicating impaired executive functions. Other neurological findings were unremarkable; however, mixed cognitive impairments, including FTD, were frequently observed in this population.
Finally, carriers of the ANXA11 mutation presented with a combined phenotype of ALS and behavioural variant frontotemporal dementia (bvFTD). Cognitive assessment revealed significant impairment, with one patient scoring 20/30 on the MMSE and 15/30 on the MoCA, alongside behavioural symptoms such as irritability and socially inappropriate behaviour. Across patients, considerable variability was observed in cognitive and behavioural symptoms, with MMSE scores ranging from 19 to 27 and MoCA scores from 18 to 25.
3.2.2. Evaluation of Language
In the language domain, the Boston Naming Test (BNT) (n = 9) and category fluency, such as naming animals or words beginning with the letter (e.g., ‘D’) within 1 min (n = 7), are commonly employed, along with other letter fluency assessments. C9orf72seq mutation carriers exhibited relatively better performance on naming tests such as the BNT compared to MAPT mutation carriers. However, difficulties in verbal fluency and comprehension are evident in both groups, with notably lower scores compared to controls. GRN mutation carriers experience more severe deficits in language, including reduced verbal fluency and word retrieval, particularly on the BNT and category fluency tests. MAPT mutation carriers also displayed significant language deficits, including poor performance on the BNT and category fluency assessments.
Language abilities, particularly action naming, declined significantly in TARDBP mutation carriers, whereas noun naming showed milder but still observable impairments. In contrast, verbal fluency did not differ significantly from that of controls. Similarly, language performance, including tasks such as verbal fluency and the Graded Naming Test, appears comparable between SOD1 FALS patients and controls, with no notable deficits in action or noun naming. However, patients with the FUS mutation demonstrated a marked deterioration, particularly in word recall and phonemic fluency, with evident anomia and semantic errors, although prosopagnosia was not observed. Verbal fluency is notably better preserved in SOD1 mutation carriers compared to sporadic ALS patients, while no significant differences are found between C9orf72seq expansion carriers and sporadic ALS cases.
Language abilities, including naming and vocabulary, progressively declined in TBK1 mutation carriers, indicating a worsening language impairment over time. A comparable deterioration was observed in PSEN1 mutation carriers, with naming and verbal fluency impairments evident through performance on the Boston Naming Test and COWAT. In individuals with SQSTM1 mutations, language was reduced to single-word expressions and was accompanied by mild impairment in verbal fluency.
3.2.3. Evaluation of Visuospatial Skills
Tests commonly used for assessing visuospatial skills include the Rey–Osterrieth Complex Figure Test (n = 3) and the Clock Drawing Test (n = 3). C9orf72seq mutation carriers demonstrate difficulties accurately reproducing complex visual information, as seen in the Rey–Osterrieth Complex Figure Test. However, their performance on basic visual perception tasks, assessed by the Visual Object and Space Perception Battery (VOSP), is generally preserved. GRN mutation carriers have relatively preserved visuospatial skills in the early stages but develop significant deficits in advanced stages. MAPT mutation carriers generally maintain visuospatial abilities, with few significant deficits observed in tasks such as Clock Drawing and visual construction tests. No changes in visuospatial skills were appreciated in TARDBP mutation carriers. In contrast, visuoperceptual skills deteriorated significantly in TBK1 patients, demonstrating severe impairment by the end of the assessment period. Visuoconstructive skills, measured by the Rey–Osterreith Complex Figure test, and visuospatial skills both showed notable declines in PSEN1 mutation carriers, though line orientation judgement remained intact. Visuospatial difficulties were noted in SQSTM1 patients, particularly in handling objects and navigating spaces. However, patients with this mutation also exhibited difficulties in visuoconstruction.
3.2.4. Evaluation of Executive Functions
Common tests for assessing executive functions include the Trail Making Test (TMT) (n = 5), the WCST (n = 2), and the Stroop Colour and Word Test (SCWT) (n = 8). Executive functions are significantly affected across all groups, particularly in C9orf72seq and GRN mutation carriers. C9orf72seq carriers show marked deficits in processing speed and verbal fluency, with the SCWT being particularly sensitive to executive dysfunction. GRN carriers exhibit difficulties in planning, organisation, and cognitive flexibility, as indicated by the Trail Making Test and the WCST. MAPT carriers also display severe executive function deficits, with issues in planning, organisation, and cognitive flexibility detected by the Trail Making Test and the WCST. TARDBP subjects’ executive functions remain relatively stable.
Patients with FUS-associated FTD exhibit a pronounced executive dysfunction, characterised by difficulties in planning, task shifting, and perseveration. In atypical frontotemporal dementia with ubiquitin inclusions (aFTLD-U), these executive impairments manifest as slowed task initiation, poor engagement, and subcortical–frontal dysfunction, without significant cortical symptoms such as aphasia, agnosia, or spatial disorientation. Conversely, in neuronal intermediate filament inclusion disease (NIFID), memory loss, aphasia, and apraxia are prominent, with more pronounced cortical involvement, leading to potential misdiagnosis as Alzheimer’s disease.
Executive functions, assessed using the Hayling Sentence Completion Test, are comparable between SOD1 FALS patients and controls. Executive functions are notably better in SOD1 mutation carriers compared to sporadic ALS patients, with no significant differences between C9orf72seq carriers and sporadic ALS patients. Attention and executive functions also exhibited notable declines in TBK1 mutations carriers, as seen in tasks such as colour–word interference and trail making.
Attention and concentration, assessed through the Trail Making Test and Stroop Test, deteriorated in PSEN1 mutation carriers, with increased completion times and difficulty handling conflicting information.
Finally, memory, language, and executive functions were variably affected, with measures such as the Trail Making Test (TMT-B), Verbal Fluency Test (VFT), and Stroop Test showing a wide range of performances, indicating differing levels of executive processing speed and cognitive control across the patient cohort.
3.2.5. Evaluation of Memory
For memory assessment, the WAIS-III Digit Span (n = 4) and the California Verbal Learning Test—Short Form (CVLT-SF) (n = 2) are commonly used. C9orf72seq carriers experience impairment in episodic and semantic memory, although short-term memory tends to be relatively preserved. GRN carriers have significant issues with episodic memory, including immediate recall and word list memory. MAPT carriers show substantial deficits in episodic memory, with poor performance on tests such as the CVLT-SF and word recognition.
Memory impairments are also pronounced in patients with FUS mutations, particularly affecting episodic recall, although short-term memory remains relatively preserved. In carriers of TBK1 mutations, memory performance, especially episodic memory, declined significantly, with impairments in word and face recognition becoming apparent after two years. Furthermore, PSEN1 mutation carriers exhibited severe deficits in memory and learning in both verbal and visual memory tasks.
3.2.6. Evaluation of Attention and Processing Speed
Attention has been assessed using the Trail Making Test (TMT), the Rey Auditory Verbal Learning Test (RAVLT), and the Digit Span. C9orf72seq carriers exhibit attention difficulties, evidenced by poorer performance in the Digit Span Backward and sustained attention tasks such as the Modified Trails. GRN carriers also show a reduced processing speed and attention, with poor performance on the Number Span Forward test. MAPT carriers exhibit deficits in attention and processing speed, with poorer results on tests such as the RAVLT and the Rivermead Behavioural Memory Test (RBMT).
3.2.7. Evaluation of Psychiatric Symptoms
Psychiatric symptoms were evaluated using the Neuropsychiatric Inventory (NPI). In case reports, specific clinical evaluations and psychopathological scales were employed. Perceptual disorders, such as hallucinations and delusions, are more frequent and severe in C9orf72seq and FTLD-TDP carriers compared to controls and other genetic groups. GRN and MAPT carriers exhibit fewer psychotic symptoms but still experience emotional issues such as anxiety and depression. FTLD-TDP patients report high levels of disinhibition and apathy, with notable occurrences of hallucinations. In the TARDBP patients, depression and mania were less frequent compared to other groups, but hallucinations and delusions were more prevalent.
3.2.8. Behaviour and Emotion
Emotional issues, such as anxiety and depression, have been commonly assessed using the Hospital Anxiety and Depression Scale (HADS), although other scales like the State–Trait Anxiety Inventory (STAI) or the Beck Depression Inventory have also been used. Clinical behavioural assessments are commonly employed to measure these symptoms. Behavioural changes, including apathy, disinhibition, and repetitive behaviours, are prominent in C9orf72seq and FTLD-TDP carriers, alongside emotional symptoms such as anxiety, depression, and hallucinations. GRN and MAPT carriers also display behavioural changes, though these are generally less severe compared to C9orf72seq and FTLD-TDP carriers. In TARDBP mutation carriers, behavioural symptoms are marked by high levels of disinhibition, apathy, and hallucinations, with functional abilities in daily living tasks deteriorating progressively, leading to complete dependence. Behaviourally, FUS patients experience increasing disinhibition, impulsivity, and obsessive behaviours, often accompanied by social withdrawal and personality changes characteristic of behavioural variant frontotemporal dementia (bvFTD). High levels of disinhibition (85.7%) and apathy (85.7%) were observed in the FTLD-FUS group, alongside perseveration and hyperorality in 71.4% of cases. Depression is significantly more common in the FTLD-FUS group compared to the FTLD-TDP and FTLD-tau groups, affecting 71.4% of patients, with a lower occurrence of mania and the absence of hallucinations and delusions. As the disease progresses, patients experience worsening dysarthria and dysphagia, a loss of insight, and increasingly compulsive behaviours.
Behavioural measures reveal higher levels of apathy in SOD1 FALS patients compared to controls, although this is attributed more to the physical limitations of the disease rather than cognitive decline. No significant differences are observed in other behavioural aspects, such as frontal behaviour changes. Emotional lability is elevated in both SOD1 and sporadic ALS (SALS) patients relative to controls, suggesting that emotional changes can occur independently of cognitive impairment in ALS.
Severe apathy, aggression, and compulsive behaviour were observed in SQSTM1 mutation carriers. Progressive behavioural changes, including disinhibition, impulsivity, and paranoia, were reported alongside a lack of empathy and emotional expression in other case studies for the same gene.
3.3. Meta-Analyses
Of the included papers, a meta-analysis was conducted on cognitive impairment, memory, attention, visuospatial construction, and language. Also, emotional response (depression and anxiety symptoms) and patients’ neurobehavioural and psychiatric symptoms were analysed.
3.3.1. Global Cognition
From the included articles, a meta-analysis was conducted on cognitive impairment, assessed using the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), the Clinical Dementia Rating (CDR) plus the National Alzheimer’s Coordinating Center Sum of Boxes (NACC FTLD), the Addenbrooke’s Cognitive Examination (ACE-III), the Frontal Assessment Battery (FAB) and the Frontotemporal Dementia Rating Scale (FRS).
A meta-analysis was conducted using the random effects model to assess cognitive decline, evaluated with the Mini-Mental State Examination (MMSE). A total of 31 studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(30) = 319.4069, p < 0.0001, tau2 = 0.5777, I2 = 92.48%). Significant differences were found in the MMSE (Z = −15.0143; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = −15.5048; p < 0.0001), GRN (Z = −18.1524; p < 0.0001), and MAPT (Z = −5.3780; p < 0.0001).
A meta-analysis was conducted to assess cognitive decline, evaluated with the Montreal Cognitive Assessment (MoCA). A total of three studies were included in the analysis. The true outcomes appear not to be heterogeneous (Q(2) = 1.4841, p = 0.4761, tau2 = 0.00, I2 = 0.00%). Significant differences were found in the MoCA (Z = −3.5622; p = 0.0004).
A meta-analysis was conducted to assess cognitive impairment, evaluated with the Clinical Dementia Rating (CDR) plus the National Alzheimer’s Coordinating Center (NACC FTLD) Sum of Boxes. A total of 22 studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(21) = 1696.6578, p < 0.0001, tau2 = 3.0817, I2 = 98.48%). Significant differences were found in the CDR plus NACC FTLD (Z = 11.1972; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 8.2216; p < 0.0001), GRN (Z = 5.8247; p < 0.0001), and MAPT (Z = 4.3178; p = 0.0003).
A meta-analysis was conducted to assess the cognitive impairment, evaluated with the Addenbrooke’s Cognitive Examination (ACE-III). A total of five studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(4) = 78.5611, p < 0.0001, tau2 = 1.4138, I2 = 90.05%). Significant differences were found in ACE-III (Z = −4.0817; p < 0.0001). All the participants who were assessed with the ACE-III had a mutation in the C9orf72 gene. No other mutations were included in this analysis.
A meta-analysis was conducted to assess cognitive impairment, evaluated with the Frontal Assessment Battery (FAB). A total of three studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(2) = 7.6701, p = 0.0216, tau2 = 0.2992, I2 = 76.14%). Significant differences were found in the FAB (Z = −2.7302; p = 0.0063).
A meta-analysis was conducted to evaluate cognitive impairment using the Frontotemporal Dementia Rating Scale (FRS). A total of six studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(5) = 461.3421, p < 0.0001, tau2 = 3.2109, I2 = 99.32%). Significant differences in FRS scores were found overall (Z = −3.5050; p = 0.0005). However, when examining the data by specific genes, no significant differences were observed for c9orf72seq (Z = −1.7672; p = 0.0772), GRN (Z = −1.4732; p = 0.1407), or MAPT (Z = −1.6227; p = 0.1046). This suggests that while there is overall evidence of cognitive impairment, individual genetic factors do not show distinct differences in this analysis.
3.3.2. Memory and Visuospatial Skills
Memory was analysed using the memory subscale of the CBI and the Benson Recall.
A meta-analysis was conducted to assess memory, evaluated with the subscale of the CBI. A total of six studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(22) = 63.1966, p < 0.0001, tau2 = 0.5934, I2 = 92.82%). Significant differences were found in the memory subscale of the CBI (Z = 10.5079; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 4.5844; p < 0.0001), GRN (Z = 11.2308; p < 0.0001), and MAPT (Z = 7.2684; p < 0.0001).
A meta-analysis was conducted to assess visual memory, evaluated with the Benson Recall. A total of five studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(4) = 95.9081, p = 0.1106, tau2 = 0.7607, I2 = 94.08%; Figure 2A). Significant differences were found in the Benson Recall (Z = −3.1151; p = 0.0018). However, sub-analyses performed according to the gene affected in the studies included in the meta-analysis were significant for c9orf72seq (Z = −2.1455; p = 0.0319) and GRN (Z = −2.6333; p = 0.0085) but not for MAPT (Z = −0.8878; p = 0.3747; Figure 2B).
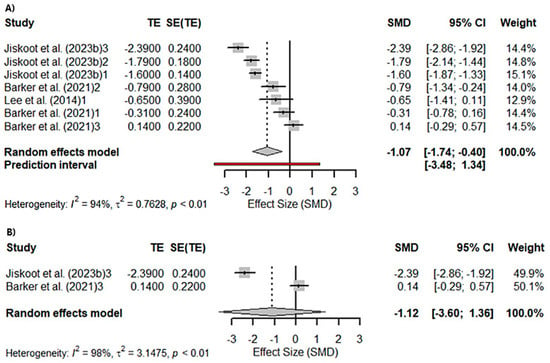
Figure 2.
Meta-analysis using a random effects model of selected studies relating to visuospatial memory assessed with the Benson Recall in all patients (p = 0.0018) (A) and only the MAPT mutation patients (p = 0.37) (B) [21,56,64]. The plot shows the effect estimates and corresponding confidence intervals (CIs) for each study included in the meta-analysis. The relative weight or contribution of each study to the overall effect estimate is also included in percentages. The overall weighted effect is indicated by a diamond at the bottom of the figure. The labels “1”, “2”, and “3” indicate the genetic groups included in the analysis: 1 = C9orf72 mutation carriers; 2 = GRN mutation carriers; 3 = MAPT mutation carriers The figure was generated with R software version 4.3.1.
A meta-analysis was conducted to assess visuoconstructive skills, evaluated with Benson Copy. A total of four studies were included in the analysis. The true outcomes appear not to be heterogeneous (Q(3) = 4.0585, p = 0.2552, tau2 = 0.0000, I2 = 0.01%). Significant differences were found in Benson Copy (Z = −13.1915; p < 0.0001). Similarly, sub-analyses performed according to the gene affected in the studies included in the meta-analysis were significant for c9orf72seq (Z = −2.7351; p = 0.0062).
3.3.3. Attention
Attention was analysed using the Digit Spam (DS) Forward and Backward, and the Trail Making Test Part A (TMT-A) and B (TMT-B).
A meta-analysis was conducted to assess attention, evaluated with DS Forward. A total of four studies were included in the analysis. The true outcomes appear not to be heterogeneous (Q(3) = 3.8627, p = 0.2767, tau2 = 0.0222, I2 = 17.40%). No significant differences were found in DS Forward (Z = −1.2189; p = 0.2229). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being not significant for c9orf72seq (Z = −1.6057; p = 0.1083) or MAPT (Z = −0.0587; p = 0.9532).
A meta-analysis was conducted to assess attention, evaluated with DS Backward. A total of seven studies were included in the analysis. The true outcomes appear to be highly heterogeneous (Q(6) = 47.0155, p < 0.0001, tau2 = 8.6971, I2 = 98.74%). No significant differences were found in DS Backward (Z = 0.6606; p = 0.5089). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being not significant for c9orf72seq (Z = 0.7360; p = 0.4617) or MAPT (Z = 0.5211; p = 0.6023).
A meta-analysis was conducted to assess sustained attention, evaluated with TMT-A. A total of three studies were included in the analysis (k = 6). The true outcomes appear to be moderate heterogeneous (Q(2) = 7.8111, p = 0.0201, tau2 = 0.1667, I2 = 72.86%). Significant differences were found in TMT-A (Z = 2.2225; p = 0.0262).
A meta-analysis was conducted to assess executive functioning, evaluated with TMT-B. A total of three studies were included in the analysis (k = 3). The true outcomes appear to be heterogeneous (Q(2) = 9.7358, p = 0.0077, tau2 = 0.2430, I2 = 79.64%). Significant differences were found in TMT-B (Z = 2.7108; p = 0.0067).
3.3.4. Language
Language was analysed using the Boston Naming Test (BNT) to measure confrontational word retrieval. On the other hand, semantic impairment was assessed by the Camel and Cactus Test and by requesting the writing of as many animals as they could think of for 1 min.
A meta-analysis was conducted to assess naming, measured with the BNT. A total of four studies were included in the analysis. The true outcomes appear to be highly heterogeneous (Q(3) = 23.1580, p < 0.0001, tau2 = 1.0237, I2 = 89.34%). Significant differences were found in BNT (Z = −3.7154; p = 0.0002). Sub-analyses performed according to the gene affected in the studies included in the meta-analysis were significant for c9orf72seq (Z = −7.0829; p < 0.0001).
A meta-analysis was conducted to assess language, evaluated through semantic frequency (animals in 1 min). A total of seven studies were included in the analysis. The true outcomes appear to be heterogeneous (Q(4) = 46.6999, p < 0.0001, tau2 = 0.5801, I2 = 87.47%). Significant differences were found in semantic fluency (Z = −3.9284; p < 0.0001). Sub-analyses performed according to the gene affected in the studies included in the meta-analysis were significant for c9orf72seq (Z = −2.6964; p = 0.0070) but not for GRN (Z = −1.5330; p = 0.1253) or MAPT (Z = −1.4691; p = 0.1418). On the other hand, a total of six studies were included in the analysis of semantic impairment assessed with the Camel and Cactus Test. The true outcomes appear to be highly heterogeneous (Q(5) = 66.7411, p < 0.0001, tau2 = 0.4644, I2 = 90.00%). Significant differences were found in semantic impairment (Z = −7.4356; p < 0.0001; Figure 3A). Similarly, sub-analyses performed according to the gene affected in the studies included in the meta-analysis were significant for c9orf72seq (Z = −3.7858; p = 0.0002), GRN (Z = −7.2035; p < 0.0001; Figure 3B), and MAPT (Z = −7.6944; p < 0.0001; Figure 3C).
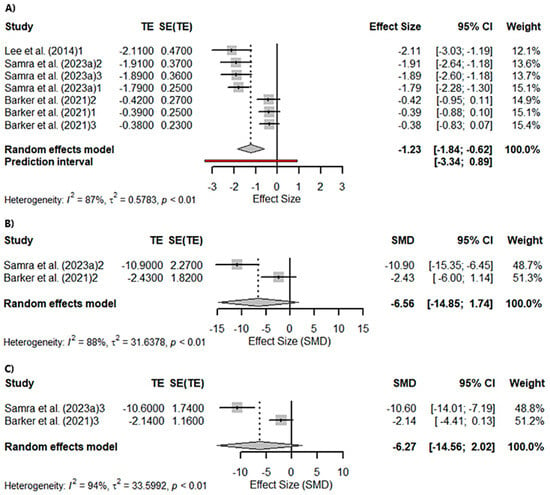
Figure 3.
Meta-analysis using a random effects model of selected studies relating to semantic fluency assessed with the “animals in 1 min” in all patients (p < 0.0001) (A), only the GRN mutation patients (p = 0.13) (B), and only the MAPT mutation patients (p = 0.14) (C) [21,64,82]. The plot shows the effect estimates and corresponding confidence intervals (CIs) for each study included in the meta-analysis. The relative weight or contribution of each study to the overall effect estimate is also included in percentages. The overall weighted effect is indicated by a diamond at the bottom of the figure. The labels “1”, “2”, and “3” indicate the genetic groups included in the analysis: 1 = C9orf72 mutation carriers; 2 = GRN mutation carriers; 3 = MAPT mutation carriers. The figure was generated with R software version 4.3.1.
3.3.5. Emotion
On the other hand, the emotional response of the patients was analysed using the Hospital Anxiety and Depression Scale (HADS).
A meta-analysis was conducted to assess depression, measured with the HADS. A total of three studies were included in the analysis. The true outcomes appear not to be heterogeneous (Q(2) = 0.7812, p = 0.6766, tau2 = 0, I2 = 0.00%). Significant differences were found in depression (Z = 3.0057; p = 0.0027). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, which was significant for SOD-1 (Z = 2.8566; p = 0.0043).
A meta-analysis was conducted to assess anxiety, measured with the HADS. A total of three studies were included in the analysis. The true outcomes appear not to be heterogeneous (Q(2) = 3.1005, p = 0.2122, tau2 = 0.0445, I2 = 31.45%). Significant differences were found in anxiety (Z = 0.7236; p = 0.4693). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, which was not significant for SOD-1 (Z = 0.8113; p = 0.4172).
3.3.6. Neurobehavioural and Psychiatric Symptoms
Patients’ neurobehavioral and psychiatric symptoms were also analysed, including daily living skills, self-care skills, mood changes, bizarre beliefs, eating habits, abnormal behaviours, sleep, stereotyped motor behaviours, and reduced motivation from the subscales of the Cambridge Behavioural Inventory Revised (CBI).
A total of six studies were included in the analysis of everyday skills. The true outcomes appear to be heterogeneous (Q(5) = 30.6372, p < 0.0001, tau2 = 0.2080, I2 = 83.41%). Significant differences were found in everyday skills (Z = 12.8595; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 5.1538; p < 0.0001), GRN (Z = 13.7429; p < 0.0001), and MAPT (Z = 5.8062; p < 0.0001).
A total of six studies were included in the analysis of self-care skills. The true outcomes appear not to be heterogeneous (Q(5) = 2.9616, p = 0.7059, tau2 = 0, I2 = 0.00%). Significant differences were found in self-care skills (Z = 10.8165; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 7.9004; p < 0.0001), GRN (Z = 5.4006; p = 0.0043), and MAPT (Z = 5.3070; p = 0.0001).
A total of six studies were included in the analysis of mood changes. The true outcomes appear not to be heterogeneous (Q(5) = 5.0131, p = 0.4143, tau2 = 0.0021, I2 = 6.20%). Significant differences were found in mood changes (Z = 17.7143; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 12.2727; p < 0.0001), GRN (Z = 6.6881; p < 0.0001), and MAPT (Z = 9.2339; p < 0.0001).
A total of six studies were included in the analysis of odd beliefs. The true outcomes appear not to be heterogeneous (Q(5) = 1.7272, p = 0.8855, tau2 = 0, I2 = 0.00%). Significant differences were found in odd beliefs (Z = 7.9739; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 5.0640; p < 0.0001), GRN (Z = 2.8566; p = 0.0043), and MAPT (Z = 3.7976; p = 0.0001).
A total of six studies were included in the analysis of eating habits. The true outcomes appear to be heterogeneous (Q(5) = 48.7983, p < 0.0001, tau2 = 0.4332, I2 = 91.59%). Significant differences were found in eating habits (Z = 9.1995; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 5.7407; p < 0.0001), GRN (Z = 6.1056; p < 0.0001), and MAPT (Z = 6.1907; p < 0.0001).
A total of six studies were included in the analysis of abnormal behaviour. The true outcomes appear to be heterogeneous (Q(5) = 91.1562, p < 0.0001, tau2 = 0.3120, I2 = 93.19%). Significant differences were found in abnormal behaviour (Z = 10.1224; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = −7.8000; p < 0.0001), GRN (Z = 6.3129; p < 0.0001), and MAPT (Z = 16.8402; p < 0.0001).
A total of six studies were included in the analysis of sleep. The true outcomes appear not to be heterogeneous (Q(5) = 2.5827, p = 0.7640, tau2 = 0, I2 = 0.00%). Significant differences were found in sleep (Z = 9.1693; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 6.2292; p < 0.0001), GRN (Z = 5.5088; p = 0.0043), and MAPT (Z = 3.0225; p = 0.0025).
A total of six studies were included in the analysis of stereotypic and motor behaviours. The true outcomes appear to be heterogeneous (Q(5) = 14.9692, p = 0.0105, tau2 = 0.4321, I2 = 68.21%). Significant differences were found in stereotypic and motor behaviours (Z = 8.1324; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 3.6949; p < 0.0001), GRN (Z = 6.3292; p < 0.0001), and MAPT (Z = 3.0101; p = 0.0026).
A total of six studies were included in the analysis of reduced motivation. The true outcomes appear to be heterogeneous (Q(5) = 17.4170, p = 0.0038, tau2 = 0.5156, I2 = 71.67%). Significant differences were found in reduced motivation (Z = 7.6225; p < 0.0001). Sub-analyses were performed according to the gene affected in the studies included in the meta-analysis, being significant for c9orf72seq (Z = 8.6801; p < 0.0001), GRN (Z = 3.7614; p = 0.0002), and MAPT (Z = 7.4340; p < 0.0001).
4. Discussion
This systematic review and meta-analysis evaluate the complex and distinct behavioural profiles associated with different genetic mutations within the ALS-FTD spectrum. By examining cognitive and behavioural outcomes in relation to specific gene mutations, significant variability was observed, reinforcing the notion that genetic subtypes within ALS and FTD are characterised by unique clinical presentations. These findings underscore the importance of considering genetic underpinnings when evaluating and managing patients within this clinical continuum. The results are discussed by gene, highlighting the salient behavioural and cognitive features identified.
4.1. C9orf72seq
Patients with the C9orf72seq repeat expansion consistently exhibited the most pronounced cognitive and behavioural impairments across multiple domains. In terms of global cognition, these patients showed significantly greater deterioration domains compared to those with GRN and MAPT mutations, as evidenced by lower scores on the MMSE, CDR-FTD-Sum of Boxes, and the FRS. Although memory was not the most severely impaired domain, C9orf72seq carriers still demonstrated notable deficits, particularly in specific recall tasks [32,64,65,79]. Attention was also significantly affected, with substantial difficulties observed on the TMT, indicating considerable attentional dysfunction. However, the DS Backward and Forward tests failed to detect significant differences, suggesting that attentional deficits in C9orf72seq patients may be more nuanced and dependent on task complexity [64].
In the language domain, C9orf72 repeat expansion carriers were the most severely affected, consistently demonstrating poor performance on the BNT, semantic fluency tasks, and the Camel and Cactus Test, indicating a broad and pervasive language impairment [64]. While semantic fluency assessments, such as generating animal names in one minute, did not differentiate GRN and MAPT mutation carriers from healthy controls, they clearly identified deficits in C9orf72seq patients [82].
With respect to neurobehavioural and psychiatric symptomatology, C9orf72seq mutation carriers again stood out, particularly in areas such as self-care abilities, mood dysregulation, sleep disturbances, unusual beliefs, and diminished motivation. This constellation of symptoms suggests a unique behavioural phenotype characterised by cognitive deterioration, pronounced language dysfunction, and severe psychiatric features, collectively resulting in substantial impairments in everyday functioning and quality of life [48,73].
The C9orf72seq repeat expansion is associated with widespread and severe neurodegeneration, particularly affecting the frontal and temporal lobes. This mutation leads to significant disruptions in global cognitive performance, with patients exhibiting pronounced deficits across multiple cognitive domains, including memory, attention, and language. The profound impact on global cognition is likely due to extensive neuroanatomical involvement, particularly within the prefrontal cortex, which is crucial for executive function, working memory, and attention.
The marked language difficulties observed in tasks like the BNT and semantic fluency may be linked to degeneration in the left perisylvian language network, including Broca’s area and temporal regions involved in semantic processing. The severe attention deficits detected on the TMT, but not in simpler tasks like DS Backward and Forward, suggest that the C9orf72seq-related pathology may disproportionately affect more complex, multitasking cognitive functions, possibly due to prefrontal cortical degeneration.
The pronounced neurobehavioural and psychiatric symptoms, such as mood changes, sleep difficulties, and reduced motivation, likely reflect the widespread involvement of the limbic system and other subcortical structures, which are critical for emotional regulation and motivation. The broad spectrum of impairments in C9orf72seq carriers underscores the extensive and multifaceted impact of this mutation on both cognitive and behavioural functions.
4.2. Granulin
Patients with GRN mutations exhibited a distinct profile of cognitive and behavioural impairments, generally less severe than those observed in C9orf72 expansion carriers. In terms of global cognition, GRN mutation carriers demonstrated moderate impairment, mirroring the decline seen in C9orf72 patients, albeit to a lesser extent. The most striking finding in this group was in the domain of memory, with GRN patients exhibiting the most pronounced impairments, as reflected in a poor performance on both the CBI and Benson Recall task [21,33]. The Benson Recall task appeared particularly sensitive to memory impairment in GRN mutation carriers yet failed to identify similar deficits in MAPT patients, highlighting the specificity of the memory dysfunction associated with GRN’s pathology.
With respect to attention, GRN patients did not show the same level of impairment as C9orf72seq patients, and, consistent with other groups, performance on DS (Backwards and Forwards) was of limited diagnostic value. Language abilities in GRN patients were also comparatively preserved, with no significant differences observed in semantic fluency tasks relative to healthy controls. These findings suggest that while GRN mutations do affect language, the impact is relatively mild and more selective than that observed in C9orf72 mutation carriers [83,102].
In terms of neurobehavioural and psychiatric symptoms, GRN patients displayed significant impairments, particularly in everyday skills, stereotypic motor behaviours, and reduced motivation. These findings indicate that while GRN carriers experience considerable behavioural difficulties, these are distinct from those observed in C9orf72seq carriers, with a more pronounced impact on routine activities and motor functions [83].
Overall, patients with GRN mutations present a cognitive–behavioural profile characterised by severe memory impairment, alongside moderate difficulties in other domains. The significant memory deficits, particularly in tasks like the CBI and Benson Recall, suggest that GRN mutations may lead to pronounced hippocampal and parietal lobe atrophy, regions crucial for memory consolidation and spatial navigation.
The relatively preserved global cognition and language functions in GRN patients could be due to a more selective neurodegeneration, sparing the frontal and temporal regions to a greater extent than in C9orf72seq mutation carriers. The moderate impact on attention, with less severe impairment than seen in C9orf72seq carriers, also supports the idea of more focal neurodegenerative processes in GRN-related pathology.
Patients with GRN mutations demonstrate impairments in everyday functioning, stereotyped motor behaviours, and apathy, features associated with dysfunction in the frontoparietal networks essential for motor planning and goal-directed activity. Degeneration in the parietal lobe disrupts sensory integration and visuospatial coordination, thereby impairing the execution of daily tasks. Concurrently, involvement of the frontal lobe, critical for executive planning and inhibitory control, accounts for the presence of repetitive behaviours and a diminished ability to adjust motor responses, alongside reduced motivation or apathy.
In contrast to C9orf72seq expansion carriers, who typically present with more widespread cognitive impairment across multiple domains, carriers of GRN mutations display a more circumscribed pattern of neurodegeneration, with certain cognitive functions, such as language and aspects of memory, relatively preserved. This selective frontoparietal involvement highlights the importance of network-based brain organisation in mediating the cognitive and behavioural phenotypes observed in neurodegenerative conditions.
4.3. MAPT
The behavioural and cognitive profile of patients with MAPT mutations differed considerably from those with C9orf72seq and GRN mutations. In terms of global cognition, MAPT patients were the least impaired among the three groups, with relatively preserved scores across the MMSE, CDR-FTD-Sum of Boxes, and FRS [21]. Notably, memory was also less affected in MAPT carriers, as evidenced by the Benson Recall task, which failed to detect significant deficits in this group—sharply contrasting with the findings in GRN mutation carriers [96].
Regarding attention, MAPT mutation carriers did not exhibit the same level of impairment seen in C9orf72seq carriers, and the DS Backward and Forward tasks again were not sensitive enough to highlight any differences compared to controls. Language abilities in MAPT patients were similarly preserved, with semantic fluency tasks, including the generation of animal names, failing to distinguish MAPT patients from controls [82]. This suggests that while cognitive and behavioural changes do occur in MAPT carriers, they are less severe and more specific compared to the other groups.
In the assessment of neurobehavioural and psychiatric symptoms, MAPT mutation carriers were most impaired in abnormal behaviours, which distinguished them from the other groups. Although they showed significant differences from controls, the impairment profile was distinct, with less impact on daily skills and motivation but a greater presence of behavioural abnormalities, aligning with the recognised phenotype associated with MAPT mutations [36].
Overall, the MAPT mutation is associated with the least severe cognitive impairment among the three genetic subtypes, with global cognitive functioning largely preserved and notable deficits confined to specific areas, such as abnormal behaviour. The relative preservation of memory functions, as evidenced by performance on the Benson Recall task, suggests that MAPT-related pathology may primarily affect the anterior temporal and frontal lobes, with limited involvement of the hippocampal structures critical for memory consolidation.
The absence of marked deficits in attention and language tasks, particularly in tasks like DS (Backwards and Forwards) and semantic fluency, may reflect the predominant localisation of tau pathology within the frontal and temporal cortices, sparing regions more directly implicated in basic attentional processes and core language functions. The selective disruption in behavioural domains points towards a focal impact of MAPT mutations on neural circuits subserving social cognition and behavioural regulation, notably within the orbitofrontal cortex and anterior cingulate gyrus.
The relatively preserved cognitive profile in MAPT mutation carriers suggests that tau pathology may initially affect brain regions involved in behavioural regulation and social cognition, with less immediate impact on core cognitive functions. This could explain why MAPT mutation carriers do not exhibit the same breadth of cognitive impairments seen in C9orf72seq or GRN mutations.
4.4. TARDBP
Patients carrying mutations in the TARDBP gene, which has been associated with both ALS and FTD, frequently exhibit early deterioration in executive function [3,26,34,67,84,95,110]. This is most notably manifested as difficulties in planning, cognitive flexibility, and perseveration—hallmark features of subcortical–frontal dysfunction. Affected individuals may appear disengaged and demonstrate reduced initiative, often observed as vacant staring or an inability to complete tasks. Memory performance in TARDBP mutation carriers is often affected indirectly, with inefficiencies that align with the executive impairments, but overall memory remains intact relative to the profound frontal dysfunction [26,95]. As the disease progresses, these patients may exhibit behavioural disinhibition, impulsivity, and obsessive–compulsive tendencies, leading to the significant personality changes that characterise bvFTD. Hallucinations, apathy, and compulsive behaviours are common, and motor symptoms associated with ALS can emerge as well, adding to the complexity of their condition [26].
4.5. FUS
The FUS mutation is characterised by executive function deficits and subcortical–frontal dysfunction. These patients particularly struggle with planning and task shifting, often becoming stuck on repetitive actions or thought patterns. Interestingly, FUS-related FTD patients do not typically show early cortical symptoms like aphasia or apraxia, which can make the presentation more subtle initially compared to other forms of FTD [29]. Memory impairments in FUS-related cases are generally secondary to executive dysfunction rather than primary memory loss [29,51]. Over time, however, patients exhibit behavioural changes such as disinhibition and impulsivity, leading to social withdrawal, loss of decorum, and obsessive behaviours [50]. Depression is highly prevalent in these patients, alongside hyperorality and compulsive behaviour. FUS mutation carriers show a particularly high degree of disinhibition and emotional dysregulation, often leading to more severe behavioural disturbances than in other forms of FTD [110].
4.6. SOD1
Patients with SOD1 mutations display a cognitive profile that is relatively preserved compared to other ALS-linked genes [67,101,106]. Many SOD1 mutation carriers perform similarly to healthy controls on cognitive tasks, showing no significant memory impairment, language deficits, or executive dysfunction. However, SOD1 mutation carriers exhibit a distinct emotional profile, with higher levels of emotional lability, suggesting that while cognitive functions remain intact, emotional regulation is significantly affected [105,106]. Apathy is also more common in these patients, but it is thought to be related to physical limitations rather than intrinsic cognitive decline [67].
4.7. PSEN1, SQSTM1, VCP, and ANXA11
The remaining ALS/FTD cases with mutations in different genes, as presented in Table 1 (e.g., PSEN1, Tau, VAPB, TBK1, KIF5A, etc.), did not have a sufficient sample size for a comprehensive comparison. This limitation hindered our ability to draw reliable conclusions. Mutations in these genes are rare, and this study was constrained by the challenge of obtaining an adequate number of cases for a meta-analysis. However, it is important to mention that the results from patients with PSEN1, SQSTM1, VCP, and ANXA11 mutations highlight distinct patterns of cognitive impairment, reflecting both shared and gene-specific features of neurodegeneration. All these mutations are linked to varying degrees of cognitive impairment, though the onset and severity differ. In PSEN1 mutation carriers, a rapid and severe cognitive decline is prominent, as shown by the significant drop from mild to severe dementia within a two-year period. This reflects the aggressive nature of PSEN1-associated familial Alzheimer’s disease (AD), known for its early onset and rapid progression [98]. In contrast, SQSTM1 and ANXA11 mutations present with a milder initial impairment but progressively worsen, showing a variability in cognitive symptoms even in the early stages of FTD associated with these mutations [61].
Interestingly, patients with VCP mutations also exhibit a substantial prevalence of cognitive impairment; however, the predominance of FTD as the primary cognitive phenotype suggests that these patients may initially present with a more focal executive dysfunction, in contrast to the more global cognitive decline observed in PSEN1 mutation carriers [87].
Memory impairment is a key feature in PSEN1 mutation carriers, with both verbal and visual memory severely affected. The memory deficits in SQSTM1 and ANXA11 mutations are less pronounced early on, but SQSTM1 cases still show short-term memory loss, while long-term memory remains intact [61]. In ANXA11 mutation carriers, the range of memory deficits is broader, with scores on memory tasks showing variability [104]. These findings suggest that while PSEN1 mutations follow a more typical Alzheimer’s-like memory impairment, SQSTM1 and ANXA11 patients may experience more selective or progressive memory deficits that are reflective of FTD’s pathology.
Executive dysfunction is a hallmark feature across all mutations, though the degree and nature vary. PSEN1 carriers show marked impairments in planning, problem-solving, and cognitive flexibility. This highlights the early involvement of the frontal lobes in PSEN1-related dementia, despite its strong Alzheimer’s phenotype. SQSTM1, VCP, and ANXA11 mutations, linked to FTD, also show substantial executive dysfunction, with patients displaying deficits in attention, task switching, and inhibition control. Also, the VCP-related FTD results supported the impact of the mutation on executive function at early stages.
Regarding language impairments, PSEN1 carriers show significant difficulty with naming, verbal fluency, and language retrieval. In SQSTM1, VCP, and ANXA11 mutations, language impairment appears more variable, with some patients exhibiting speech apraxia or grammatical issues [27,61,98]. This reflects the heterogeneity of language deficits in FTD, which may range from non-fluent aphasia to more semantic language impairments, depending on the specific mutation and its effects on the temporal and frontal lobes.
Visuospatial skills are notably impaired in PSEN1 mutation carriers [98]. In contrast, SQSTM1, VCP, and ANXA11 mutations show a more mixed pattern of visuospatial decline. For example, VCP mutation carriers often have preserved visuospatial skills early on, but visuoconstructional abilities may deteriorate as the disease progresses [87,111]. The variability in visuospatial decline across FTD-related mutations indicates that while some patients experience early impairments, others may retain these abilities until later stages.
Finally, behavioural disturbances are a common feature across SQSTM1, VCP, and ANXA11 mutations, all of which are linked to FTD syndromes. SQSTM1 patients, in particular, showed severe apathy, disinhibition, and aggressive behaviour, alongside compulsive tendencies [61]. Similarly, VCP mutation carriers often develop behavioural variant FTD (bvFTD), characterised by social withdrawal, irritability, and compulsive behaviours [3,50]. These behavioural features are less prominent in PSEN1 mutation carriers, where personality changes tend to occur later in the disease’s course, likely due to the primarily cognitive nature of AD [98]. The presence of significant emotional and behavioural changes in FTD-associated mutations underscores the involvement of the frontal lobes, particularly in social cognition and impulse control.
4.8. Limitations and Future Directions
While our findings indicate that specific behavioural impairments may be significantly increased in certain subgroups of ALS or FTD patients, we also acknowledge several limitations of the present meta-analysis. A major limitation lies in the inability to evaluate certain genes due to a lack of studies meeting the inclusion criteria. This highlights an uneven distribution of research attention across the genetic mutations implicated in ALS and FTD, potentially biasing our understanding of the broader genetic and behavioural spectrum of these disorders.
While C9orf72seq, GRN, and MAPT mutations have been extensively associated with behavioural dysregulation, other genes such as CHMP2B, FUS, and TBK1 remain under-investigated. While individuals with mutations in these genes often present with cognitive and behavioural deficits resembling FTD phenotypes, the limited number of patients with these mutations and the lack of research on less commonly studied mutations hinder comprehensive meta-analytic comparisons. This hampers our understanding of the behavioural and cognitive phenotypes associated with these mutations. Additionally, the lack of robust data may contribute to diagnostic bias, where certain genetic mutations are under-recognised or misinterpreted due to the absence of well-established behavioural and cognitive markers.
This research gap highlights a critical need for more comprehensive investigations into these underrepresented genetic mutations. Future studies should incorporate well-matched control groups and standardised behavioural and cognitive assessment tools to provide a more holistic understanding of the ALS-FTD spectrum. Such an expansion of the research focus would facilitate more accurate diagnoses and the development of tailored therapeutic strategies.
Another limitation of this meta-analysis is the relative paucity of studies that include healthy control groups, thereby limiting the scope of cross-sectional comparisons across behavioural and cognitive domains. This shortcoming reinforces the necessity for future investigations to include appropriately matched controls to enable a more accurate delineation of the specific effects of genetic mutations on neuropsychological functioning. The observed clinical heterogeneity within identical mutations, along with phenotypic overlap between different genetic variants, further complicates the identification of distinct behavioural profiles. These challenges underscore the importance of employing refined assessment tools tailored to genetic subtypes within the ALS–FTD continuum.
Additionally, a significant challenge encountered in this meta-analysis was the limited ability to evaluate tests like the ECAS (Edinburgh Cognitive and Behavioural ALS Screen), which are specifically designed for ALS and FTD patients. The ECAS is essential in clinical settings for detecting cognitive and behavioural changes in individuals with ALS. However, its utility in broader comparative analyses is constrained by the fact that such tests are rarely, if ever, administered to healthy control groups. This limitation presents a significant barrier to meta-analytic comparisons, as meta-analyses rely on synthesising data across studies to establish generalisable conclusions about the effectiveness or diagnostic utility of various tests. Without control group data, it becomes impossible to determine the specificity and sensitivity of tests like the ECAS relative to the general population, limiting our ability to compare the cognitive profiles of ALS/FTD patients with those of unaffected individuals and to establish normative data.
The lack of normative data for the ECAS further limits the interpretability of its outcomes. Although the ECAS is invaluable for monitoring cognitive decline in ALS/FTD, without data from non-affected individuals, it remains challenging to determine whether the detected impairments are specific to these conditions or may reflect broader neurodegenerative processes or even normal ageing. This gap in the literature underscores the need for studies that administer the ECAS or similar ALS/FTD-specific tools to healthy controls. Such data would allow researchers to refine their understanding of these tests’ discriminatory power and place within the broader context of neuropsychological assessment.
5. Conclusions
In conclusion, this systematic review and meta-analysis have identified that specific behavioural outcomes are frequently observed in subtypes of ALS/FTD cases, segregated by genes. Validated diagnostic investigations and biomarkers, as well as confounding phenotypes with other conditions, may delay the diagnosis of ALS and, thus, the treatment. Given the link between genetic mutations and behavioural outcomes, refining patient care guidelines to consider specific behavioural characterisations based on the underlying genetic mutations may improve the overall quality of life for patients.
Author Contributions
Conceptualization, A.L.N. and N.A.; methodology, A.M.J.-G.; formal analysis, A.M.J.-G.; investigation, A.M.J.-G. and M.E.T.; resources, N.A.; data curation, A.M.J.-G.; writing—original draft preparation, A.M.J.-G., M.E.T., A.L.N. and N.A.; writing—review and editing, A.M.J.-G., A.L.N. and N.A.; visualization, N.A.; supervision, N.A.; project administration, N.A.; funding acquisition, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Innovation, grant number PID2023-151715OB-IOO, and by the European Union NextGeneration EU/PRTR, grant numbers PLEC2022-009464 and CPP2022-009646. The APC was funded by the Ministry of Science and Innovation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The figures included in this manuscript were created using BioRender.com and R 4.3.1. software.
Conflicts of Interest
Author N.A. was employed by the company INEUROPA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE-III | Addenbrooke’s Cognitive Examination III |
| ACE-R | Addenbrooke’s Cognitive Examination—Revised |
| ALS | Amyotrophic Lateral Sclerosis |
| ANXA11 | Annexin A11 |
| BNT | Boston Naming Test |
| bvFTD | Behavioural variant Frontotemporal Dementia |
| C9orf72seq | Chromosome 9 Open Reading Frame 72 |
| CBI | Cambridge Behavioural Inventory |
| CBI-R | Cambridge Behavioural Inventory Revised |
| CCNF | Cyclin F |
| CDR | Clinical Dementia Rating |
| CDR-FTD | Clinical Dementia Rating–Frontotemporal Dementia version |
| CHMP2B | Charged Multivesicular Body Protein 2B |
| CVLT-SF | California Verbal Learning Test–Short Form |
| DCTN1 | Dynactin 1 |
| DS | Digit Span |
| ECAS | Edinburgh Cognitive and Behavioural ALS Screen |
| FAB | Frontal Assessment Battery |
| FALS | Familial Amyotrophic Lateral Sclerosis |
| FDG-PET | Fluorodeoxyglucose Positron Emission Tomography |
| FBI | Frontal Behavioural Inventory |
| FRS | Frontotemporal Dementia Rating Scale |
| FTD | Frontotemporal Dementia |
| FTLD | Frontotemporal Lobar Degeneration |
| FUS | Fused in Sarcoma |
| GRN | Progranulin gene mutation |
| HADS | Hospital Anxiety and Depression Scale |
| HNRNPA1 | Heterogeneous Nuclear Ribonucleoprotein A1 |
| MAPT | Microtubule Associated Protein Tau |
| MINT | Multilingual Naming Test |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| NPI | Neuropsychiatric Inventory |
| PGRN | Progranulin |
| PPA | Primary Progressive Aphasia |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin 2 |
| RMT | Recognition Memory Test |
| SALS | Sporadic Amyotrophic Lateral Sclerosis |
| SOD1 | Superoxide Dismutase 1 |
| SQSTM1 | Sequestosome 1 |
| STAI | State–Trait Anxiety Inventory |
| TBK1 | TANK-Binding Kinase 1 |
| TARDBP | Transactive Response DNA Binding Protein |
| TMT | Trail Making Test |
| TREM2 | Triggering Receptor Expressed on Myeloid Cells 2 |
| UBQLN2 | Ubiquilin 2 |
| VCP | Valosin-Containing Protein |
References
- Gordon, P.H. Amyotrophic Lateral Sclerosis: An Update for 2013 Clinical Features, Pathophysiology, Management and Therapeutic Trials. Aging Dis. 2013, 4, 295–310. [Google Scholar] [CrossRef]
- Mohandas, E.; Rajmohan, V. Frontotemporal Dementia: An Updated Overview. Indian. J. Psychiatry 2009, 51 (Suppl. S1), S65–S69. [Google Scholar]
- Gossye, H.; Van Mossevelde, S.; Sieben, A.; Bjerke, M.; Hendrickx Van de Craen, E.; van der Zee, J.; De Deyn, P.P.; De Bleecker, J.; Versijpt, J.; van den Ende, J.; et al. Patients Carrying the Mutation p.R406W in MAPT Present with Non-Conforming Phenotypic Spectrum. Brain 2023, 146, 1624–1636. [Google Scholar] [CrossRef]
- Gendron, T.F.; Heckman, M.G.; White, L.J.; Veire, A.M.; Pedraza, O.; Burch, A.R.; Bozoki, A.C.; Dickerson, B.C.; Domoto-Reilly, K.; Foroud, T.; et al. Comprehensive Cross-Sectional and Longitudinal Analyses of Plasma Neurofilament Light across FTD Spectrum Disorders. Cell Rep. Med. 2022, 3, 100607. [Google Scholar] [CrossRef]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain 2011, 134 Pt 9, 2456–2477. [Google Scholar] [CrossRef]
- Neary, D.; Snowden, J.S.; Mann, D.M. Classification and Description of Frontotemporal Dementias. Ann. N. Y. Acad. Sci. 2000, 920, 46–51. [Google Scholar] [CrossRef]
- Xu, Z.; Alruwaili, A.R.S.; Henderson, R.D.; McCombe, P.A. Screening for Cognitive and Behavioural Impairment in Amyotrophic Lateral Sclerosis: Frequency of Abnormality and Effect on Survival. J. Neurol. Sci. 2017, 376, 16–23. [Google Scholar] [CrossRef]
- Gibbons, Z.C.; Richardson, A.; Neary, D.; Snowden, J.S. Behaviour in Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2008, 9, 67–74. [Google Scholar] [CrossRef]
- Kim, E.; White, M.A.; Phillips, B.U.; Lopez-Cruz, L.; Kim, H.; Heath, C.J.; Lee, J.E.; Saksida, L.M.; Sreedharan, J.; Bussey, T.J. Coexistence of Perseveration and Apathy in the TDP-43Q331K Knock-in Mouse Model of ALS–FTD. Transl. Psychiatry 2020, 10, 377. [Google Scholar] [CrossRef]
- Van Langenhove, T.; van der Zee, J.; Gijselinck, I.; Engelborghs, S.; Vandenberghe, R.; Vandenbulcke, M.; De Bleecker, J.; Sieben, A.; Versijpt, J.; Ivanoiu, A.; et al. Distinct Clinical Characteristics of C9orf72 Expansion Carriers Compared with GRN, MAPT, and Nonmutation Carriers in a Flanders-Belgian FTLD Cohort. JAMA Neurol. 2013, 70, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Benajiba, L.; Le Ber, I.; Camuzat, A.; Lacoste, M.; Thomas-Anterion, C.; Couratier, P.; Legallic, S.; Salachas, F.; Hannequin, D.; Decousus, M.; et al. TARDBP Mutations in Motoneuron Disease with Frontotemporal Lobar Degeneration. Ann. Neurol. 2009, 65, 470–473. [Google Scholar] [CrossRef]
- Saxon, J.A.; Thompson, J.C.; Harris, J.M.; Richardson, A.M.; Langheinrich, T.; Rollinson, S.; Pickering-Brown, S.; Chaouch, A.; Ealing, J.; Hamdalla, H.; et al. Cognition and Behaviour in Frontotemporal Dementia with and without Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.R.; Halliday, G.M.; Kril, J.J.; Ittner, L.M.; Götz, J.; Kiernan, M.C.; Hodges, J.R. The Frontotemporal Dementia-Motor Neuron Disease Continuum. Lancet 2016, 388, 919–931. [Google Scholar] [CrossRef] [PubMed]
- McCann, E.P.; Williams, K.L.; Fifita, J.A.; Tarr, I.S.; O’Connor, J.; Rowe, D.B.; Nicholson, G.A.; Blair, I.P. The Genotype-Phenotype Landscape of Familial Amyotrophic Lateral Sclerosis in Australia. Clin. Genet. 2017, 92, 259–266. [Google Scholar] [CrossRef]
- Millecamps, S.; Salachas, F.; Cazeneuve, C.; Gordon, P.; Bricka, B.; Camuzat, A.; Guillot-Noël, L.; Russaouen, O.; Bruneteau, G.; Pradat, P.-F.; et al. SOD1, ANG, VAPB, TARDBP, and FUS Mutations in Familial Amyotrophic Lateral Sclerosis: Genotype-Phenotype Correlations. J. Med. Genet. 2010, 47, 554–560. [Google Scholar] [CrossRef]
- Connolly, O.; Le Gall, L.; McCluskey, G.; Donaghy, C.G.; Duddy, W.J.; Duguez, S. A Systematic Review of Genotype-Phenotype Correlation across Cohorts Having Causal Mutations of Different Genes in ALS. J. Pers. Med. 2020, 10, 58. [Google Scholar] [CrossRef]
- Abbate, C.; Arosio, B.; Galimberti, D.; Nicolini, P.; Chiara, L.R.; Rossi, P.D.; Ferri, E.; Gussago, C.; Deriz, M.; Fenoglio, C.; et al. Phenotypic Variability Associated with the C9ORF72 Hexanucleotide Repeat Expansion: A Sporadic Case of Frontotemporal Lobar Degeneration with Prodromal Hyposmia and Predominant Semantic Deficits. J. Alzheimers Dis. 2014, 40, 849–855. [Google Scholar] [CrossRef]
- Alcolea, D.; Vilaplana, E.; Suárez-Calvet, M.; Illán-Gala, I.; Blesa, R.; Clarimón, J.; Lladó, A.; Sánchez-Valle, R.; Molinuevo, J.L.; García-Ribas, G.; et al. CSF SAPPβ, YKL-40, and Neurofilament Light in Frontotemporal Lobar Degeneration. Neurology 2017, 89, 178–188. [Google Scholar] [CrossRef]
- Arighi, A.; Fumagalli, G.G.; Jacini, F.; Fenoglio, C.; Ghezzi, L.; Pietroboni, A.M.; De Riz, M.; Serpente, M.; Ridolfi, E.; Bonsi, R.; et al. Early Onset Behavioral Variant Frontotemporal Dementia Due to the C9ORF72 Hexanucleotide Repeat Expansion: Psychiatric Clinical Presentations. J. Alzheimers Dis. 2012, 31, 447–452. [Google Scholar] [CrossRef]
- Barker, M.S.; Manoochehri, M.; Rizer, S.J.; Appleby, B.S.; Brushaber, D.; Dev, S.I.; Devick, K.L.; Dickerson, B.C.; Fields, J.A.; Foroud, T.M.; et al. Recognition Memory and Divergent Cognitive Profiles in Prodromal Genetic Frontotemporal Dementia. Cortex 2021, 139, 99–115. [Google Scholar] [CrossRef]
- Beck, J.; Rohrer, J.D.; Campbell, T.; Isaacs, A.; Morrison, K.E.; Goodall, E.F.; Warrington, E.K.; Stevens, J.; Revesz, T.; Holton, J.; et al. A Distinct Clinical, Neuropsychological and Radiological Phenotype Is Associated with Progranulin Gene Mutations in a Large UK Series. Brain 2008, 131 Pt 3, 706–720. [Google Scholar] [CrossRef]
- Benussi, A.; Premi, E.; Gazzina, S.; Brattini, C.; Bonomi, E.; Alberici, A.; Jiskoot, L.; van Swieten, J.C.; Sanchez-Valle, R.; Moreno, F.; et al. Progression of Behavioral Disturbances and Neuropsychiatric Symptoms in Patients With Genetic Frontotemporal Dementia. JAMA Netw. Open 2021, 4, e2030194. [Google Scholar] [CrossRef]
- Block, N.R.; Sha, S.J.; Karydas, A.M.; Fong, J.C.; De May, M.G.; Miller, B.L.; Rosen, H.J. Frontotemporal Dementia and Psychiatric Illness: Emerging Clinical and Biological Links in Gene Carriers. Am. J. Geriatr. Psychiatry 2016, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Todd, E.G.; Bouzigues, A.; Cash, D.M.; Nicholas, J.M.; Convery, R.S.; Russell, L.L.; Thomas, D.L.; Malone, I.B.; Iglesias, J.E.; et al. Structural MRI Predicts Clinical Progression in Presymptomatic Genetic Frontotemporal Dementia: Findings from the GENetic Frontotemporal Dementia Initiative Cohort. Brain Commun. 2023, 5, fcad061. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Bonvicini, C.; Alberici, A.; Buratti, E.; Agosti, C.; Archetti, S.; Papetti, A.; Stuani, C.; Di Luca, M.; Gennarelli, M.; et al. Mutation within TARDBP Leads to Frontotemporal Dementia without Motor Neuron Disease. Hum. Mutat. 2009, 30, E974–E983. [Google Scholar] [CrossRef] [PubMed]
- Boutoleau-Bretonnière, C.; Camuzat, A.; Le Ber, I.; Bouya-Ahmed, K.; Guerreiro, R.; Deruet, A.-L.; Evrard, C.; Bras, J.; Lamy, E.; Auffray-Calvier, E.; et al. A Phenotype of Atypical Apraxia of Speech in a Family Carrying SQSTM1 Mutation. J. Alzheimers Dis. 2015, 43, 625–630. [Google Scholar] [CrossRef]
- Bouzigues, A.; Russell, L.L.; Peakman, G.; Bocchetta, M.; Greaves, C.V.; Convery, R.S.; Todd, E.; Rowe, J.B.; Borroni, B.; Galimberti, D.; et al. Anomia Is Present Pre-Symptomatically in Frontotemporal Dementia Due to MAPT Mutations. J. Neurol. 2022, 269, 4322–4332. [Google Scholar] [CrossRef]
- Bradfield, N.I.; McLean, C.; Drago, J.; Darby, D.G.; Ames, D. Rapidly Progressive Fronto-Temporal Dementia (FTD) Associated with Frontotemporal Lobar Degeneration (FTLD) in the Presence of Fused in Sarcoma (FUS) Protein: A Rare, Sporadic, and Aggressive Form of FTD. Int. Psychogeriatr. 2017, 29, 1743–1746. [Google Scholar] [CrossRef]
- Bussy, A.; Levy, J.P.; Best, T.; Patel, R.; Cupo, L.; Van Langenhove, T.; Nielsen, J.E.; Pijnenburg, Y.; Waldö, M.L.; Remes, A.M.; et al. Cerebellar and Subcortical Atrophy Contribute to Psychiatric Symptoms in Frontotemporal Dementia. Hum. Brain Mapp. 2023, 44, 2684–2700. [Google Scholar] [CrossRef]
- Byrne, S.; Elamin, M.; Bede, P.; Shatunov, A.; Walsh, C.; Corr, B.; Heverin, M.; Jordan, N.; Kenna, K.; Lynch, C.; et al. Cognitive and Clinical Characteristics of Patients with Amyotrophic Lateral Sclerosis Carrying a C9orf72 Repeat Expansion: A Population-Based Cohort Study. Lancet Neurol. 2012, 11, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Agosta, F.; Magnani, G.; Cardamone, R.; Borghesani, V.; Miller, Z.; Riva, N.; La Joie, R.; Coppola, G.; Grinberg, L.T.; et al. Temporal Variant of Frontotemporal Dementia in C9orf72 Repeat Expansion Carriers: Two Case Studies. Brain Imaging Behav. 2020, 14, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Castelnovo, V.; Canu, E.; Domi, T.; Pozzi, L.; Vignaroli, F.; Spinelli, E.G.; Ghirelli, A.; Tondo, G.; Comi, C.; Riva, N.; et al. A Novel GRN Mutation in an Italian Patient with Non-Fluent Variant of Primary Progressive Aphasia at Onset: A Longitudinal Case Report. Front. Neurosci. 2023, 17, 1204504. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Brunetti, M.; Barberis, M.; Iazzolino, B.; Montuschi, A.; Ilardi, A.; Cammarosano, S.; Canosa, A.; Moglia, C.; Calvo, A. The Role of APOE in the Occurrence of Frontotemporal Dementia in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2016, 73, 425–430. [Google Scholar] [CrossRef]
- Christidi, F.; Kleinerova, J.; Tan, E.L.; Delaney, S.; Tacheva, A.; Hengeveld, J.C.; Doherty, M.A.; McLaughlin, R.L.; Hardiman, O.; Siah, W.F.; et al. Limbic Network and Papez Circuit Involvement in ALS: Imaging and Clinical Profiles in GGGGCC Hexanucleotide Carriers in C9orf72 and C9orf72-Negative Patients. Biology 2024, 13, 504. [Google Scholar] [CrossRef]
- Chu, C.; Li, T.; Yu, L.; Li, Y.; Li, M.; Guo, M.; Zhao, J.; Zhai, Q.; Tian, F.; Chen, W. A Low-Protein, High-Carbohydrate Diet Exerts a Neuroprotective Effect on Mice with 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease by Regulating the Microbiota-Metabolite–Brain Axis and Fibroblast Growth Factor 21. J. Agric. Food Chem. 2023, 71, 8877–8893. [Google Scholar] [CrossRef]
- Clarke, M.T.M.; St-Onge, F.; Beauregard, J.-M.; Bocchetta, M.; Todd, E.; Cash, D.M.; Rohrer, J.D.; Laforce, R. Early Anterior Cingulate Involvement Is Seen in Presymptomatic MAPT P301L Mutation Carriers. Alzheimers Res. Ther. 2021, 13, 42. [Google Scholar] [CrossRef]
- Colombo, E.; Poletti, B.; Maranzano, A.; Peverelli, S.; Solca, F.; Colombrita, C.; Torre, S.; Tiloca, C.; Verde, F.; Bonetti, R.; et al. Motor, Cognitive and Behavioural Profiles of C9orf72 Expansion-Related Amyotrophic Lateral Sclerosis. J. Neurol. 2023, 270, 898–908. [Google Scholar] [CrossRef]
- Devenney, E.; Hornberger, M.; Irish, M.; Mioshi, E.; Burrell, J.; Tan, R.; Kiernan, M.C.; Hodges, J.R. Frontotemporal Dementia Associated with the C9ORF72 Mutation: A Unique Clinical Profile. JAMA Neurol. 2014, 71, 331–339. [Google Scholar] [CrossRef]
- Devenney, E.M.; Landin-Romero, R.; Irish, M.; Hornberger, M.; Mioshi, E.; Halliday, G.M.; Kiernan, M.C.; Hodges, J.R. The Neural Correlates and Clinical Characteristics of Psychosis in the Frontotemporal Dementia Continuum and the C9orf72 Expansion. Neuroimage Clin. 2017, 13, 439–445. [Google Scholar] [CrossRef]
- Devenney, E.M.; Tu, S.; Caga, J.; Ahmed, R.M.; Ramsey, E.; Zoing, M.; Kwok, J.; Halliday, G.M.; Piguet, O.; Hodges, J.R.; et al. Neural Mechanisms of Psychosis Vulnerability and Perceptual Abnormalities in the ALS-FTD Spectrum. Ann. Clin. Transl. Neurol. 2021, 8, 1576–1591. [Google Scholar] [CrossRef]
- Dong, L.; Wang, J.; Liu, C.; Li, J.; Mao, C.; Huang, X.; Chu, S.; Peng, B.; Cui, L.; Gao, J. Genetic Spectrum and Clinical Heterogeneity of Chinese Frontotemporal Dementia Patients: Data from PUMCH Dementia Cohort. J. Alzheimers Dis. 2022, 89, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Downey, L.E.; Fletcher, P.D.; Golden, H.L.; Mahoney, C.J.; Agustus, J.L.; Schott, J.M.; Rohrer, J.D.; Beck, J.; Mead, S.; Rossor, M.N.; et al. Altered Body Schema Processing in Frontotemporal Dementia with C9ORF72 Mutations. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.; Malik, R.; Bocchetta, M.; Coleman, K.; Graff, C.; Borroni, B.; Masellis, M.; Laforce, R.; Greaves, C.V.; Russell, L.L.; et al. Neurodevelopmental Effects of Genetic Frontotemporal Dementia in Young Adult Mutation Carriers. Brain 2023, 146, 2120–2131. [Google Scholar] [CrossRef]
- Floeter, M.K.; Danielian, L.E.; Braun, L.E.; Wu, T. Longitudinal Diffusion Imaging across the C9orf72 Clinical Spectrum. J. Neurol. Neurosurg. Psychiatry 2018, 89, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Borghero, G.; Cannas, A.; Di Stefano, F.; Ruiu, E.; Murru, M.R.; Corongiu, D.; Cuccu, S.; Tranquilli, S.; Sardu, C.; et al. Constructional Apraxia in Frontotemporal Dementia Associated with the C9orf72 Mutation: Broadening the Clinical and Neuropsychological Phenotype. Amyotroph. Lateral Scler. Frontotemporal Degener. 2015, 16, 8–15. [Google Scholar] [CrossRef]
- Foster, P.H.; Russell, L.L.; Peakman, G.; Convery, R.S.; Bouzigues, A.; Greaves, C.V.; Bocchetta, M.; Cash, D.M.; van Swieten, J.C.; Jiskoot, L.C.; et al. Examining Empathy Deficits across Familial Forms of Frontotemporal Dementia within the GENFI Cohort. Cortex 2022, 150, 12–28. [Google Scholar] [CrossRef]
- Foxe, D.; Elan, E.; Burrell, J.R.; Leslie, F.V.C.; Devenney, E.; Kwok, J.B.; Halliday, G.M.; Hodges, J.R.; Piguet, O. Intrafamilial Phenotypic Variability in the C9orf72 Gene Expansion: 2 Case Studies. Front. Psychol. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Gabryelewicz, T.; Masellis, M.; Berdynski, M.; Bilbao, J.M.; Rogaeva, E.; St George-Hyslop, P.; Barczak, A.; Czyzewski, K.; Barcikowska, M.; Wszolek, Z.; et al. Intra-Familial Clinical Heterogeneity Due to FTLD-U with TDP-43 Proteinopathy Caused by a Novel Deletion in Progranulin Gene (PGRN). J. Alzheimers Dis. 2010, 22, 1123–1133. [Google Scholar] [CrossRef]
- García-Roldán, E.; Rivas-Infante, E.; Medina-Rodríguez, M.; Arriola-Infante, J.E.; Rodrigo-Herrero, S.; Paradas, C.; Rábano-Gutiérrez, A.; Franco-Macías, E. Lessons Learned from a Sporadic FUSopathy in a Young Man: A Case Report. BMC Neurol. 2023, 23, 55. [Google Scholar] [CrossRef]
- Gowell, M.; Baker, I.; Ansorge, O.; Husain, M. Young-Onset Frontotemporal Dementia with FUS Pathology. Pract. Neurol. 2020, 21, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, C.; Cantello, R.; Terazzi, E.; Carecchio, M.; D’Alfonso, S.; Chieppa, N.; Ressico, F.; Rizza, M.C.; Zeppegno, P. Early Onset Frontotemporal Dementia with Psychiatric Presentation Due to the C9ORF72 Hexanucleotide Repeat Expansion: A Case Report. BMC Neurol. 2014, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.W.; Wang, P.; Rascovsky, K.; Wolf, A.; Appleby, B.; Bove, J.; Bordelon, Y.; Brannelly, P.; Brushaber, D.E.; Caso, C.; et al. Comparison of Sporadic and Familial Behavioral Variant Frontotemporal Dementia (FTD) in a North American Cohort. Alzheimers Dement. 2020, 16, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hokelekli, F.O.; Whitwell, J.L.; Machulda, M.M.; Jones, D.T.; Uitti, R.J.; Pham, N.T.T.; Giannini, C.; Baker, M.; Lowe, V.J.; Dickson, D.W.; et al. Underlying Pathology Identified after 20 Years of Disease Course in Two Cases of Slowly Progressive Frontotemporal Dementia Syndromes. Neurocase 2021, 27, 212–222. [Google Scholar] [CrossRef]
- Iazzolino, B.; Peotta, L.; Zucchetti, J.P.; Canosa, A.; Manera, U.; Vasta, R.; Grassano, M.; Palumbo, F.; Brunetti, M.; Barberis, M.; et al. Differential Neuropsychological Profile of Patients With Amyotrophic Lateral Sclerosis With and Without C9orf72 Mutation. Neurology 2021, 96, e141–e152. [Google Scholar] [CrossRef]
- Jiskoot, L.C.; van den Berg, E.; Laenen, S.A.A.M.; Poos, J.M.; Giannini, L.A.A.; Satoer, D.D.; van Hemmen, J.; Pijnenburg, Y.A.L.; Vonk, J.M.J.; Seelaar, H. Longitudinal Changes in Qualitative Aspects of Semantic Fluency in Presymptomatic and Prodromal Genetic Frontotemporal Dementia. J. Neurol. 2023, 270, 5418–5435. [Google Scholar] [CrossRef]
- Kertesz, A.; Ang, L.C.; Jesso, S.; MacKinley, J.; Baker, M.; Brown, P.; Shoesmith, C.; Rademakers, R.; Finger, E.C. Psychosis and Hallucinations in Frontotemporal Dementia with the C9ORF72 Mutation: A Detailed Clinical Cohort. Cogn. Behav. Neurol. 2013, 26, 146–154. [Google Scholar] [CrossRef]
- Kobayashi, T.; Mori, H.; Okuma, Y.; Dickson, D.W.; Cookson, N.; Tsuboi, Y.; Motoi, Y.; Tanaka, R.; Miyashita, N.; Anno, M.; et al. Contrasting Genotypes of the Tau Gene in Two Phenotypically Distinct Patients with P301L Mutation of Frontotemporal Dementia and Parkinsonism Linked to Chromosome 17. J. Neurol. 2002, 249, 669–675. [Google Scholar] [CrossRef]
- Kobayashi, R.; Naruse, H.; Kawakatsu, S.; Iseki, C.; Suzuki, Y.; Koyama, S.; Morioka, D.; Ishiura, H.; Mitsui, J.; Ohta, Y.; et al. Valosin-Containing Protein Asp395Gly Mutation in a Patient with Frontotemporal Dementia: A Case Report. BMC Neurol. 2022, 22, 406. [Google Scholar] [CrossRef]
- Koriath, C.A.M.; Bocchetta, M.; Brotherhood, E.; Woollacott, I.O.C.; Norsworthy, P.; Simón-Sánchez, J.; Blauwendraat, C.; Dick, K.M.; Gordon, E.; Harding, S.R.; et al. The Clinical, Neuroanatomical, and Neuropathologic Phenotype of TBK1-Associated Frontotemporal Dementia: A Longitudinal Case Report. Alzheimers Dement. 2017, 6, 75–81. [Google Scholar] [CrossRef]
- Kovacs, G.G.; van der Zee, J.; Hort, J.; Kristoferitsch, W.; Leitha, T.; Höftberger, R.; Ströbel, T.; Van Broeckhoven, C.; Matej, R. Clinicopathological Description of Two Cases with SQSTM1 Gene Mutation Associated with Frontotemporal Dementia. Neuropathology 2016, 36, 27–38. [Google Scholar] [CrossRef]
- Laszlo, Z.I.; Hindley, N.; Sanchez Avila, A.; Kline, R.A.; Eaton, S.L.; Lamont, D.J.; Smith, C.; Spires-Jones, T.L.; Wishart, T.M.; Henstridge, C.M. Synaptic Proteomics Reveal Distinct Molecular Signatures of Cognitive Change and C9ORF72 Repeat Expansion in the Human ALS Cortex. Acta Neuropathol. Commun. 2022, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.A.; Gough, A.; Rideout, A.L.; Dyack, S.; Singh, K.; MacNeil, M. Atypical Neuropsychiatric Presentation of FTD-ALS Caused by a Pathogenic Repeat Expansion in C9orf72: A Case Report. J. Geriatr. Psychiatry Neurol. 2024, 37, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Khazenzon, A.M.; Trujillo, A.J.; Guo, C.C.; Yokoyama, J.S.; Sha, S.J.; Takada, L.T.; Karydas, A.M.; Block, N.R.; Coppola, G.; et al. Altered Network Connectivity in Frontotemporal Dementia with C9orf72 Hexanucleotide Repeat Expansion. Brain 2014, 137 Pt 11, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.P.; Bocti, C.; Elie, D.; Paquet, N.; Soucy, J.-P.; Ducharme, S. Bifrontal Hypermetabolism on Brain FDG-PET in a Case of C9orf72-Related Behavioral Variant of Frontotemporal Dementia. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 92–94. [Google Scholar] [CrossRef]
- Mahoney, C.J.; Beck, J.; Rohrer, J.D.; Lashley, T.; Mok, K.; Shakespeare, T.; Yeatman, T.; Warrington, E.K.; Schott, J.M.; Fox, N.C.; et al. Frontotemporal Dementia with the C9ORF72 Hexanucleotide Repeat Expansion: Clinical, Neuroanatomical and Neuropathological Features. Brain 2012, 135 Pt 3, 736–750. [Google Scholar] [CrossRef]
- Manini, A.; Casiraghi, V.; Brusati, A.; Maranzano, A.; Gentile, F.; Colombo, E.; Bonetti, R.; Peverelli, S.; Invernizzi, S.; Gentilini, D.; et al. Association of the Risk Factor UNC13A with Survival and Upper Motor Neuron Involvement in Amyotrophic Lateral Sclerosis. Front. Aging Neurosci. 2023, 15, 1067954. [Google Scholar] [CrossRef]
- McDade, E.; Boeve, B.F.; Burrus, T.M.; Boot, B.P.; Kantarci, K.; Fields, J.; Lowe, V.J.; Peller, P.; Knopman, D.; Baker, M.; et al. Similar Clinical and Neuroimaging Features in Monozygotic Twin Pair with Mutation in Progranulin. Neurology 2012, 78, 1245–1249. [Google Scholar] [CrossRef]
- Melis, M.; Defazio, G.; Casaglia, E.; Melas, V.; Floris, G. Early Juvenile Reading Epilepsy and Later Frontotemporal Dementia (FTD): Expanding the Clinical Phenotype of C9ORF72 Mutation? Amyotroph. Lateral Scler. Frontotemporal Degener. 2022, 23, 139–142. [Google Scholar] [CrossRef]
- Mendez, M.F. Manic Behavior and Asymmetric Right Frontotemporal Dementia from a Novel Progranulin Mutation. Neuropsychiatr. Dis. Treat. 2018, 14, 657–662. [Google Scholar] [CrossRef]
- Moore, K.; Convery, R.; Bocchetta, M.; Neason, M.; Cash, D.M.; Greaves, C.; Russell, L.L.; Clarke, M.T.M.; Peakman, G.; van Swieten, J.; et al. A Modified Camel and Cactus Test Detects Presymptomatic Semantic Impairment in Genetic Frontotemporal Dementia within the GENFI Cohort. Appl. Neuropsychol. Adult 2022, 29, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Russell, L.L.; Peakman, G.; Convery, R.S.; Bouzigues, A.; Greaves, C.V.; Bocchetta, M.; Cash, D.M.; van Swieten, J.C.; Jiskoot, L.; et al. The CBI-R Detects Early Behavioural Impairment in Genetic Frontotemporal Dementia. Ann. Clin. Transl. Neurol. 2022, 9, 644–658. [Google Scholar] [CrossRef]
- Ogonowski, N.; Santamaria-Garcia, H.; Baez, S.; Lopez, A.; Laserna, A.; Garcia-Cifuentes, E.; Ayala-Ramirez, P.; Zarante, I.; Suarez-Obando, F.; Reyes, P.; et al. Frontotemporal Dementia Presentation in Patients with Heterozygous p.H157Y Variant of TREM2. J. Med. Genet. 2023, 60, 894–904. [Google Scholar] [CrossRef]
- Olney, N.T.; Ong, E.; Goh, S.-Y.M.; Bajorek, L.; Dever, R.; Staffaroni, A.M.; Cobigo, Y.; Bock, M.; Chiang, K.; Ljubenkov, P.; et al. Clinical and Volumetric Changes with Increasing Functional Impairment in Familial Frontotemporal Lobar Degeneration. Alzheimers Dement. 2020, 16, 49–59. [Google Scholar] [CrossRef]
- Olszewska, D.A.; Fallon, E.M.; Pastores, G.M.; Murphy, K.; Blanco, A.; Lynch, T.; Murphy, S.M. Autosomal Dominant Gene Negative Frontotemporal Dementia-Think of SCA17. Cerebellum 2019, 18, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Peakman, G.; Russell, L.L.; Convery, R.S.; Nicholas, J.M.; Van Swieten, J.C.; Jiskoot, L.C.; Moreno, F.; Sanchez-Valle, R.; Laforce, R.; Graff, C.; et al. Comparison of Clinical Rating Scales in Genetic Frontotemporal Dementia within the GENFI Cohort. J. Neurol. Neurosurg. Psychiatry 2022, 93, 158–168. [Google Scholar] [CrossRef]
- Pengo, M.; Alberici, A.; Libri, I.; Benussi, A.; Gadola, Y.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Borroni, B. Sex Influences Clinical Phenotype in Frontotemporal Dementia. Neurol. Sci. 2022, 43, 5281–5287. [Google Scholar] [CrossRef] [PubMed]
- Pletnikova, O.; Sloane, K.L.; Renton, A.E.; Traynor, B.J.; Crain, B.J.; Reid, T.; Zu, T.; Ranum, L.P.W.; Troncoso, J.C.; Rabins, P.V.; et al. Hippocampal Sclerosis Dementia with the C9ORF72 Hexanucleotide Repeat Expansion. Neurobiol. Aging 2014, 35, 2419.e17. [Google Scholar] [CrossRef]
- Poos, J.M.; Jiskoot, L.C.; Leijdesdorff, S.M.J.; Seelaar, H.; Panman, J.L.; van der Ende, E.L.; Mol, M.O.; Meeter, L.H.H.; Pijnenburg, Y.A.L.; Donker Kaat, L.; et al. Cognitive Profiles Discriminate between Genetic Variants of Behavioral Frontotemporal Dementia. J. Neurol. 2020, 267, 1603–1612. [Google Scholar] [CrossRef]
- Poos, J.M.; MacDougall, A.; van den Berg, E.; Jiskoot, L.C.; Papma, J.M.; van der Ende, E.L.; Seelaar, H.; Russell, L.L.; Peakman, G.; Convery, R.; et al. Longitudinal Cognitive Changes in Genetic Frontotemporal Dementia Within the GENFI Cohort. Neurology 2022, 99, e281–e295. [Google Scholar] [CrossRef]
- Russell, L.L.; Greaves, C.V.; Bocchetta, M.; Nicholas, J.; Convery, R.S.; Moore, K.; Cash, D.M.; van Swieten, J.; Jiskoot, L.; Moreno, F.; et al. Social Cognition Impairment in Genetic Frontotemporal Dementia within the GENFI Cohort. Cortex 2020, 133, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Samra, K.; MacDougall, A.M.; Bouzigues, A.; Bocchetta, M.; Cash, D.M.; Greaves, C.V.; Convery, R.S.; van Swieten, J.C.; Seelaar, H.; Jiskoot, L.; et al. Language Impairment in the Genetic Forms of Behavioural Variant Frontotemporal Dementia. J. Neurol. 2023, 270, 1976–1988. [Google Scholar] [CrossRef] [PubMed]
- Samra, K.; Macdougall, A.; Peakman, G.; Bouzigues, A.; Bocchetta, M.; Cash, D.M.; Greaves, C.V.; Convery, R.S.; van Swieten, J.C.; Jiskoot, L.C.; et al. Neuropsychiatric Symptoms in Genetic Frontotemporal Dementia: Developing a New Module for Clinical Rating Scales. J. Neurol. Neurosurg. Psychiatry 2023, 94, 357–368. [Google Scholar] [CrossRef]
- Santamaría-García, H.; Ogonowsky, N.; Baez, S.; Palacio, N.; Reyes, P.; Schulte, M.; López, A.; Matallana, D.; Ibanez, A. Neurocognitive Patterns across Genetic Levels in Behavioral Variant Frontotemporal Dementia: A Multiple Single Cases Study. BMC Neurol. 2022, 22, 454. [Google Scholar] [CrossRef]
- Sassi, C.; Capozzo, R.; Hammer, M.; Zecca, C.; Federoff, M.; Blauwendraat, C.; Bernstein, N.; Ding, J.; Gibbs, J.R.; Price, T.; et al. Exploring Dementia and Neuronal Ceroid Lipofuscinosis Genes in 100 FTD-like Patients from 6 Towns and Rural Villages on the Adriatic Sea Cost of Apulia. Sci. Rep. 2021, 11, 6353. [Google Scholar] [CrossRef]
- Scarioni, M.; Gami-Patel, P.; Peeters, C.F.W.; de Koning, F.; Seelaar, H.; Mol, M.O.; van Swieten, J.C.; Netherlands Brain Bank; Rozemuller, A.J.M.; Hoozemans, J.J.M.; et al. Psychiatric Symptoms of Frontotemporal Dementia and Subcortical (Co-)Pathology Burden: New Insights. Brain 2023, 146, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Schiava, M.; Ikenaga, C.; Villar-Quiles, R.N.; Caballero-Ávila, M.; Topf, A.; Nishino, I.; Kimonis, V.; Udd, B.; Schoser, B.; Zanoteli, E.; et al. Genotype-Phenotype Correlations in Valosin-Containing Protein Disease: A Retrospective Muticentre Study. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1099–1111. [Google Scholar] [CrossRef]
- Seelaar, H.; Papma, J.M.; Garraux, G.; de Koning, I.; Reijs, A.E.; Valkema, R.; Rozemuller, A.J.M.; Salmon, E.; van Swieten, J.C. Brain Perfusion Patterns in Familial Frontotemporal Lobar Degeneration. Neurology 2011, 77, 384–392. [Google Scholar] [CrossRef]
- Sellami, L.; Bocchetta, M.; Masellis, M.; Cash, D.M.; Dick, K.M.; van Swieten, J.; Borroni, B.; Galimberti, D.; Tartaglia, M.C.; Rowe, J.B.; et al. Distinct Neuroanatomical Correlates of Neuropsychiatric Symptoms in the Three Main Forms of Genetic Frontotemporal Dementia in the GENFI Cohort. J. Alzheimers Dis. 2018, 65, 147–163. [Google Scholar] [CrossRef]
- Shinagawa, S.; Naasan, G.; Karydas, A.M.; Coppola, G.; Pribadi, M.; Seeley, W.W.; Trojanowski, J.Q.; Miller, B.L.; Grinberg, L.T. Clinicopathological Study of Patients With C9ORF72-Associated Frontotemporal Dementia Presenting With Delusions. J. Geriatr. Psychiatry Neurol. 2015, 28, 99–107. [Google Scholar] [CrossRef]
- Silva-Spínola, A.; Lima, M.; Leitão, M.J.; Durães, J.; Tábuas-Pereira, M.; Almeida, M.R.; Santana, I.; Baldeiras, I. Serum Neurofilament Light Chain as a Surrogate of Cognitive Decline in Sporadic and Familial Frontotemporal Dementia. Eur. J. Neurol. 2022, 29, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.S.; Pickering-Brown, S.M.; Mackenzie, I.R.; Richardson, A.M.T.; Varma, A.; Neary, D.; Mann, D.M.A. Progranulin Gene Mutations Associated with Frontotemporal Dementia and Progressive Non-Fluent Aphasia. Brain 2006, 129, 3091–3102. [Google Scholar] [CrossRef]
- Snowden, J.S.; Hu, Q.; Rollinson, S.; Halliwell, N.; Robinson, A.; Davidson, Y.S.; Momeni, P.; Baborie, A.; Griffiths, T.D.; Jaros, E.; et al. The Most Common Type of FTLD-FUS (AFTLD-U) Is Associated with a Distinct Clinical Form of Frontotemporal Dementia but Is Not Related to Mutations in the FUS Gene. Acta Neuropathol. 2011, 122, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Solje, E.; Aaltokallio, H.; Koivumaa-Honkanen, H.; Suhonen, N.M.; Moilanen, V.; Kiviharju, A.; Traynor, B.; Tienari, P.J.; Hartikainen, P.; Remes, A.M. The Phenotype of the C9ORF72 Expansion Carriers According to Revised Criteria for BvFTD. PLoS ONE 2015, 10, e0131817. [Google Scholar] [CrossRef]
- Spinelli, E.G.; Ghirelli, A.; Riva, N.; Canu, E.; Castelnovo, V.; Domi, T.; Pozzi, L.; Carrera, P.; Silani, V.; Chiò, A.; et al. Profiling Morphologic MRI Features of Motor Neuron Disease Caused by TARDBP Mutations. Front. Neurol. 2022, 13, 931006. [Google Scholar] [CrossRef] [PubMed]
- Staffaroni, A.M.; Quintana, M.; Wendelberger, B.; Heuer, H.W.; Russell, L.L.; Cobigo, Y.; Wolf, A.; Goh, S.-Y.M.; Petrucelli, L.; Gendron, T.F.; et al. Temporal Order of Clinical and Biomarker Changes in Familial Frontotemporal Dementia. Nat. Med. 2022, 28, 2194–2206. [Google Scholar] [CrossRef]
- Tan, H.H.G.; Westeneng, H.-J.; van der Burgh, H.K.; van Es, M.A.; Bakker, L.A.; van Veenhuijzen, K.; van Eijk, K.R.; van Eijk, R.P.A.; Veldink, J.H.; van den Berg, L.H. The Distinct Traits of the UNC13A Polymorphism in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2020, 88, 796–806. [Google Scholar] [CrossRef]
- Tang-Wai, D.; Lewis, P.; Boeve, B.; Hutton, M.; Golde, T.; Baker, M.; Hardy, J.; Michels, V.; Ivnik, R.; Jack, C.; et al. Familial Frontotemporal Dementia Associated with a Novel Presenilin-1 Mutation. Dement. Geriatr. Cogn. Disord. 2002, 14, 13–21. [Google Scholar] [CrossRef]
- Temp, A.G.M.; Kasper, E.; Vielhaber, S.; Machts, J.; Hermann, A.; Teipel, S.; Prudlo, J. Loss of “Insight” into Behavioral Changes in ALS: Differences across Cognitive Profiles. Brain Behav. 2022, 12, e2439. [Google Scholar] [CrossRef]
- Tipton, P.W.; Deutschlaender, A.B.; Savica, R.; Heckman, M.G.; Brushaber, D.E.; Dickerson, B.C.; Gavrilova, R.H.; Geschwind, D.H.; Ghoshal, N.; Graff-Radford, J.; et al. Differences in Motor Features of C9orf72, MAPT, or GRN Variant Carriers With Familial Frontotemporal Lobar Degeneration. Neurology 2022, 99, e1154–e1167. [Google Scholar] [CrossRef]
- Tondo, G.; Mazzini, L.; Caminiti, S.P.; Sarnelli, M.F.; Corrado, L.; Matheoud, R.; D’Alfonso, S.; Cantello, R.; Sacchetti, G.M.; Perani, D.; et al. Clinical Relevance of Single-Subject Brain Metabolism Patterns in Amyotrophic Lateral Sclerosis Mutation Carriers. Neuroimage Clin. 2022, 36, 103222. [Google Scholar] [CrossRef]
- Van Deerlin, V.M.; Wood, E.M.; Moore, P.; Yuan, W.; Forman, M.S.; Clark, C.M.; Neumann, M.; Kwong, L.K.; Trojanowski, J.Q.; Lee, V.M.-Y.; et al. Clinical, Genetic, and Pathologic Characteristics of Patients with Frontotemporal Dementia and Progranulin Mutations. Arch. Neurol. 2007, 64, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, G.; Gallingani, C.; Zucchi, E.; Martinelli, I.; Gianferrari, G.; Simonini, C.; Bedin, R.; Chiari, A.; Zamboni, G.; Mandrioli, J. Young Onset Alzheimer’s Disease Associated with C9ORF72 Hexanucleotide Expansion: Further Evidence for a Still Unsolved Association. Genes 2023, 14, 930. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, X.; Zhou, X.; Wang, R.; Zhang, X.; Cao, Z.; Wang, X.; Zhou, Z.; Sun, Y.; Peng, D. ANXA11 Mutations Are Associated with Amyotrophic Lateral Sclerosis-Frontotemporal Dementia. Front. Neurol. 2022, 13, 886887. [Google Scholar] [CrossRef] [PubMed]
- Wicks, P.; Abrahams, S.; Papps, B.; Al-Chalabi, A.; Shaw, C.E.; Leigh, P.N.; Goldstein, L.H. SOD1 and Cognitive Dysfunction in Familial Amyotrophic Lateral Sclerosis. J. Neurol. 2009, 256, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wiesenfarth, M.; Günther, K.; Müller, K.; Witzel, S.; Weiland, U.; Mayer, K.; Herrmann, C.; Brenner, D.; Schuster, J.; Freischmidt, A.; et al. Clinical and Genetic Features of Amyotrophic Lateral Sclerosis Patients with C9orf72 Mutations. Brain Commun. 2023, 5, fcad087. [Google Scholar] [CrossRef]
- Wilke, C.; Reich, S.; van Swieten, J.C.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; Graff, C.; Galimberti, D.; Rowe, J.B.; et al. Stratifying the Presymptomatic Phase of Genetic Frontotemporal Dementia by Serum NfL and PNfH: A Longitudinal Multicentre Study. Ann. Neurol. 2022, 91, 33–47. [Google Scholar] [CrossRef]
- Wood, R.; Moodley, K.; Hodges, J.R.; Allinson, K.; Spillantini, M.G.; Chan, D. Slowly Progressive Behavioural Presentation in Two UK Cases with the R406W MAPT Mutation. Neuropathol. Appl. Neurobiol. 2016, 42, 291–295. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Liu, Q.; Liu, L.; Li, J.; Cai, Z.; Zhang, K.; Liu, S.; He, D.; Shen, D.; et al. Mutation Spectrum of Chinese Amyotrophic Lateral Sclerosis Patients with Frontotemporal Dementia. Orphanet J. Rare Dis. 2022, 17, 404. [Google Scholar] [CrossRef]
- Scarioni, M.; Gami-Patel, P.; Timar, Y.; Seelaar, H.; van Swieten, J.C.; Rozemuller, A.J.M.; Dols, A.; Scarpini, E.; Galimberti, D.; Netherlands Brain Bank; et al. Frontotemporal Dementia: Correlations Between Psychiatric Symptoms and Pathology. Ann. Neurol. 2020, 87, 950–961. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J. Therapeutic Potential of Bifidobacterium Breve Strain A1 for Preventing Cognitive Impairment in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).