Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
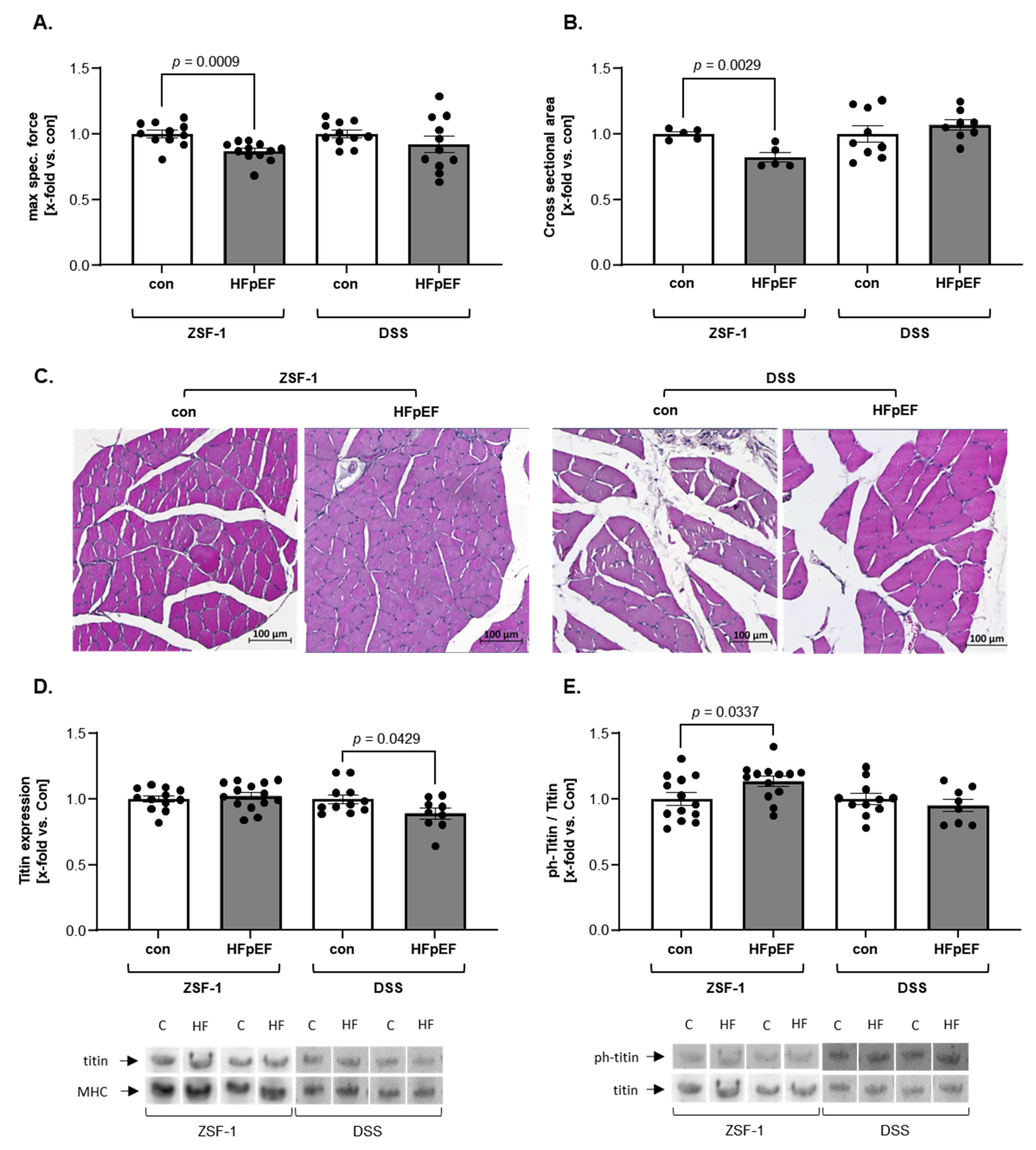
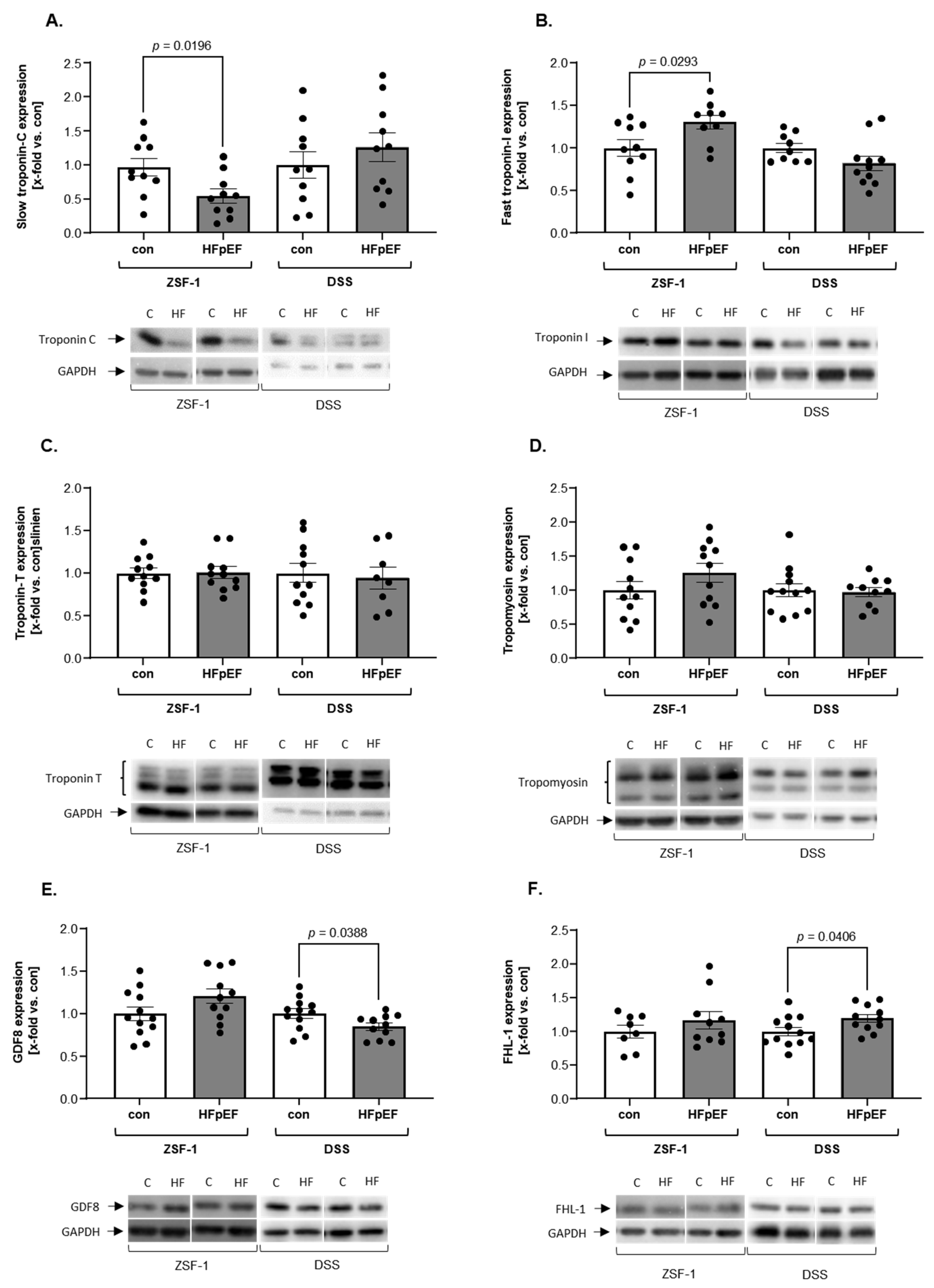
2. Results
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Animals
4.2. Echocardiography
4.3. Invasive Haemodynamic Measurements
4.4. Skeletal Muscle Function
4.5. Histological Analyses
4.6. Titin Analysis
4.7. Protein Analysis
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| Con | Control |
| CSA | Cross-sectional area |
| DSS | Dahl salt-sensitive |
| EDL | Extensor digitorum longus |
| E/é | Early mitral inflow velocity/early diastolic mitral annular velocity |
| FHL1 | Four and a half lim protein 1 |
| GDF8 | Myostatin |
| HF | Heart failure |
| HFpEF | Heart failure with preserved ejection fraction |
| HS | High salt |
| LS | Low salt |
| LVEDP | Left ventricular end-diastolic pressure |
| LVEF | Left ventricular ejection fraction |
| nd | Not determined |
| ph | Phospho |
| SKM | Skeletal muscle |
| TL | Tibia length |
| Tm | Tropomyosin |
| TnC | Troponin C |
| TnI | Troponin I |
| TnT | Troponin T |
| ZSF-1 | Zucker fatty/spontaneously hypertensive heart failure F1 hybrid |
References
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur. J. Heart Fail. 2020, 22, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Loescher, C.M.; Hobbach, A.J.; Linke, W.A. Titin (TTN): From molecule to modifications, mechanics, and medical significance. Cardiovasc. Res. 2022, 118, 2903–2918. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Arena, R.; Borlaug, B.A.; Carbone, S.; Canada, J.M.; Kirkman, D.L.; Garten, R.; Rodriguez-Miguelez, P.; Guazzi, M.; Lavie, C.J.; et al. Exercise Intolerance in Patients With Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2209–2225. [Google Scholar] [CrossRef]
- Vahle, B.; Heilmann, L.; Schauer, A.; Augstein, A.; Jarabo, M.-E.P.; Barthel, P.; Mangner, N.; Labeit, S.; Bowen, T.S.; Linke, A.; et al. Modulation of Titin and Contraction-Regulating Proteins in a Rat Model of Heart Failure with Preserved Ejection Fraction: Limb vs. Diaphragmatic Muscle. Int. J. Mol. Sci. 2024, 25, 6618. [Google Scholar] [CrossRef]
- Goto, K.; Schauer, A.; Augstein, A.; Methawasin, M.; Granzier, H.; Halle, M.; Van Craenenbroeck, E.M.; Rolim, N.; Gielen, S.; Pieske, B.; et al. Muscular changes in animal models of heart failure with preserved ejection fraction: What comes closest to the patient? ESC Heart Fail. 2021, 8, 139–150. [Google Scholar] [CrossRef]
- Koser, F.; Hobbach, A.J.; Abdellatif, M.; Herbst, V.; Türk, C.; Reinecke, H.; Krüger, M.; Sedej, S.; Linke, W.A. Acetylation and phosphorylation changes to cardiac proteins in experimental HFpEF due to metabolic risk reveal targets for treatment. Life Sci. 2022, 309, 120998. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Amthor, H.; Otto, A.; Vulin, A.; Rochat, A.; Dumonceaux, J.; Garcia, L.; Mouisel, E.; Hourdé, C.; Macharia, R.; Friedrichs, M.; et al. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc. Natl. Acad. Sci. USA 2009, 106, 7479–7484. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lori, D.; Wells, D.J.; Kemp, P.R. FHL1 activates myostatin signalling in skeletal muscle and promotes atrophy. FEBS Open Bio 2015, 5, 753–762. [Google Scholar] [CrossRef]
- Gueneau, L.; Bertrand, A.T.; Jais, J.-P.; Salih, M.A.; Stojkovic, T.; Wehnert, M.; Hoeltzenbein, M.; Spuler, S.; Saitoh, S.; Verschueren, A.; et al. Mutations of the FHL1 gene cause Emery-Dreifuss muscular dystrophy. Am. J. Hum. Genet. 2009, 85, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Jin, J.-P. Troponin Variants as Markers of Skeletal Muscle Health and Diseases. Front. Physiol. 2021, 12, 747214. [Google Scholar] [CrossRef]
- Saw, E.L.; Ramachandran, S.; Valero-Muñoz, M.; Sam, F. Skeletal muscle (dys)function in heart failure with preserved ejection fraction. Curr. Opin. Cardiol. 2021, 36, 219–226. [Google Scholar] [CrossRef]
- Bowen, T.S.; Rolim, N.P.L.; Fischer, T.; Bækkerud, F.H.; Medeiros, A.; Werner, S.; Brønstad, E.; Rognmo, O.; Mangner, N.; Linke, A.; et al. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur. J. Heart Fail. 2015, 17, 263–272. [Google Scholar] [CrossRef]
- Stolina, M.; Luo, X.; Dwyer, D.; Han, C.-Y.; Chen, R.; Zhang, Y.; Xiong, Y.; Chen, Y.; Yin, J.; Shkumatov, A.; et al. The evolving systemic biomarker milieu in obese ZSF1 rat model of human cardiometabolic syndrome: Characterization of the model and cardioprotective effect of GDF15. PLoS ONE 2020, 15, e0231234. [Google Scholar] [CrossRef]
- Badole, S.L.; Jangam, G.B. Chapter 14—Animal Models of Diabetic Cardiomyopathy. In Glucose Intake and Utilization in Pre-Diabetes and Diabetes: Implications for Cardiovascular Disease, 1st ed.; Watson, R.R., Dokken, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–190. [Google Scholar]
- Ogihara, T.; Asano, T.; Ando, K.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; Abe, M.; et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension 2002, 40, 83–89. [Google Scholar] [CrossRef]
- Nogueira-Ferreira, R.; Santos, I.; Ferreira, R.; Fontoura, D.; Sousa-Mendes, C.; Falcão-Pires, I.; Lourenço, A.P.; Leite-Moreira, A.; Duarte, I.F.; Moreira-Gonçalves, D. Exercise training impacts skeletal muscle remodelling induced by metabolic syndrome in ZSF1 rats through metabolism regulation. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166709. [Google Scholar] [CrossRef]
- Winzer, E.B.; Schauer, A.; Langner, E.; Augstein, A.; Goto, K.; Männel, A.; Barthel, P.; Jannasch, A.; Labeit, S.; Mangner, N.; et al. Empagliflozin Preserves Skeletal Muscle Function in a HFpEF Rat Model. Int. J. Mol. Sci. 2022, 23, 989. [Google Scholar] [CrossRef]
- Cheng, R.K.; Cox, M.; Neely, M.L.; Heidenreich, P.A.; Bhatt, D.L.; Eapen, Z.J.; Hernandez, A.F.; Butler, J.; Yancy, C.W.; Fonarow, G.C. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am. Heart J. 2014, 168, 721–730. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B.; et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, W. Detection and quantification of the giant protein titin by SDS-agarose gel electrophoresis. MethodsX 2017, 4, 320–327. [Google Scholar] [CrossRef] [PubMed]
| Parameter | ZSF-1 | DSS | ||
|---|---|---|---|---|
| Control (n = 13) | HFpEF (n = 14) | Control (n = 12) | HFpEF (n = 11) | |
| Body weight (g) | 264 ± 4 | 562 ± 6 *** | 292 ± 5 | 288 ± 6 |
| Heart weight (mg/mm TL) | 23.7 ± 0.3 | 35.5 ± 0.5 *** | 22.8 ± 0.5 | 31.3 ± 1.1 *** |
| Mean arterial BP (mmHg) | 104.8 ± 5.5 | 135.1 ± 3.2 *** | 128.5 ± 4 | 178.8 ± 6 *** |
| LVEF (%) | 78.8 ± 1.1 | 73.9 ± 1.2 | 82.7 ± 1.1 | 68.7 ± 2.5 ** |
| E/é ratio | 21.7 ± 0.4 | 27.7 ± 0.7 *** | 10.7 ± 1.5 | 19.8 ± 1.4 * |
| LVEDP (mmHg) | 1.3 ± 0.4 | 6.9 ± 0.5 *** | 5.6 ± 0.9 | 14.8 ± 3.1 ** |
| NT-pro-BNP (pg/mL) | 96.9 ± 9.4 | 209.0 ± 38.2 * | 19.7 ± 5.0 | 65.0 ± 16.8 * |
| Glucose (mM) | 17.8 ± 1.1 | 31.4 ± 1.2 *** | 12.8 ± 0.9 | 12.6 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahle, B.; Klädtke, R.; Schauer, A.; Bowen, T.S.; Wisløff, U.; Linke, A.; Adams, V. Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2025, 26, 6196. https://doi.org/10.3390/ijms26136196
Vahle B, Klädtke R, Schauer A, Bowen TS, Wisløff U, Linke A, Adams V. Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences. 2025; 26(13):6196. https://doi.org/10.3390/ijms26136196
Chicago/Turabian StyleVahle, Beatrice, Romy Klädtke, Antje Schauer, T. Scott Bowen, Ulrik Wisløff, Axel Linke, and Volker Adams. 2025. "Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction" International Journal of Molecular Sciences 26, no. 13: 6196. https://doi.org/10.3390/ijms26136196
APA StyleVahle, B., Klädtke, R., Schauer, A., Bowen, T. S., Wisløff, U., Linke, A., & Adams, V. (2025). Skeletal Muscle Alterations in Different Phenotypes of Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences, 26(13), 6196. https://doi.org/10.3390/ijms26136196





