Development of an HPLC-FLD Method for Estradiol and Metabolites: Application of Solid-Phase Microextraction
Abstract
1. Introduction
1.1. Estrogen in Cancer Development
1.2. Estrogen Metabolites
1.3. Estrogen Derivatization
1.4. Quantitative Analysis of Estrogen in Biological Samples
1.5. HPLC-FLD
2. Results
2.1. Optimization of the Chromatographic Conditions
2.2. Optimization of Derivatization Procedure
2.3. Stability of Dansylated Estrogens
2.4. Liquid–Liquid Extraction Optimization
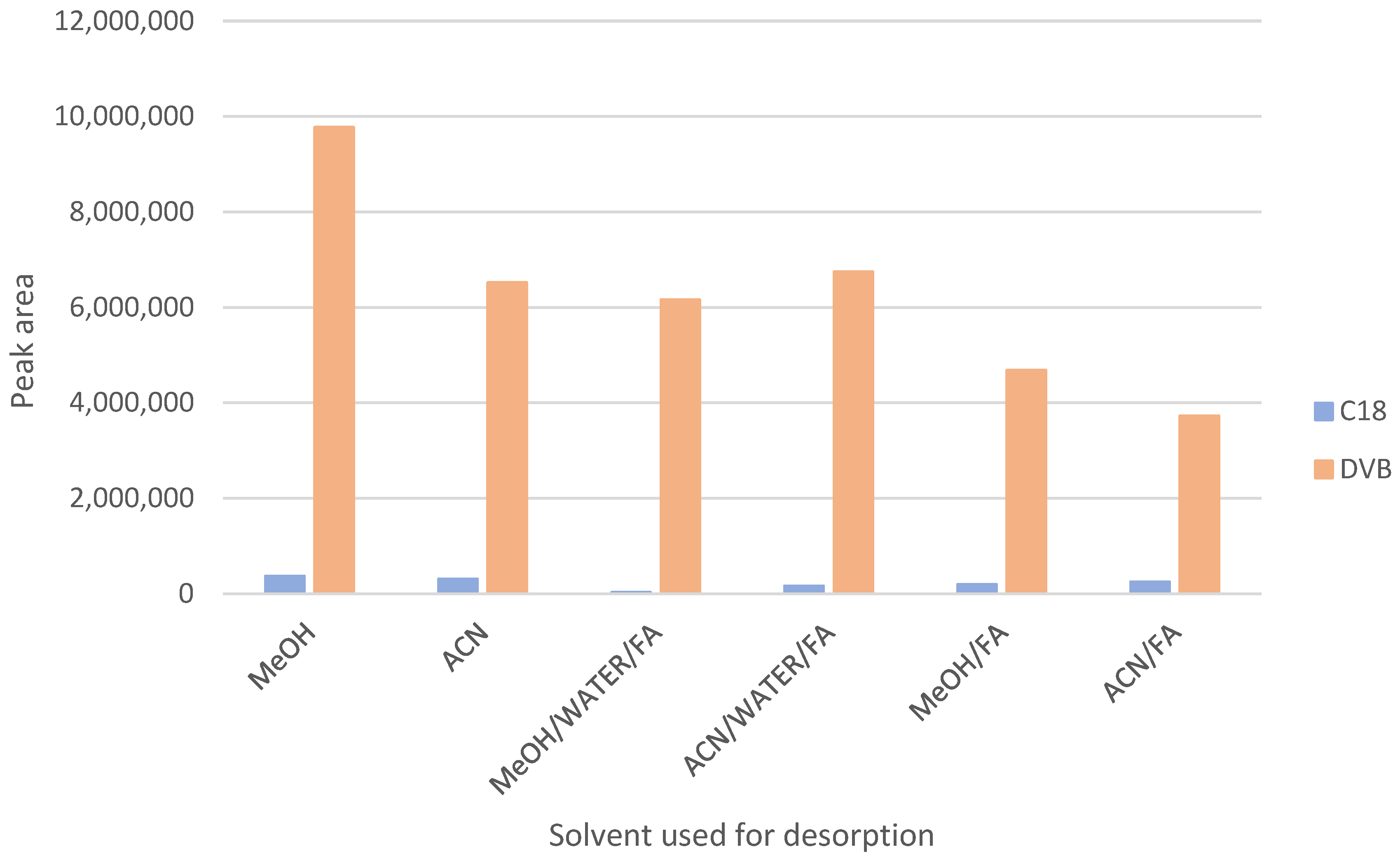
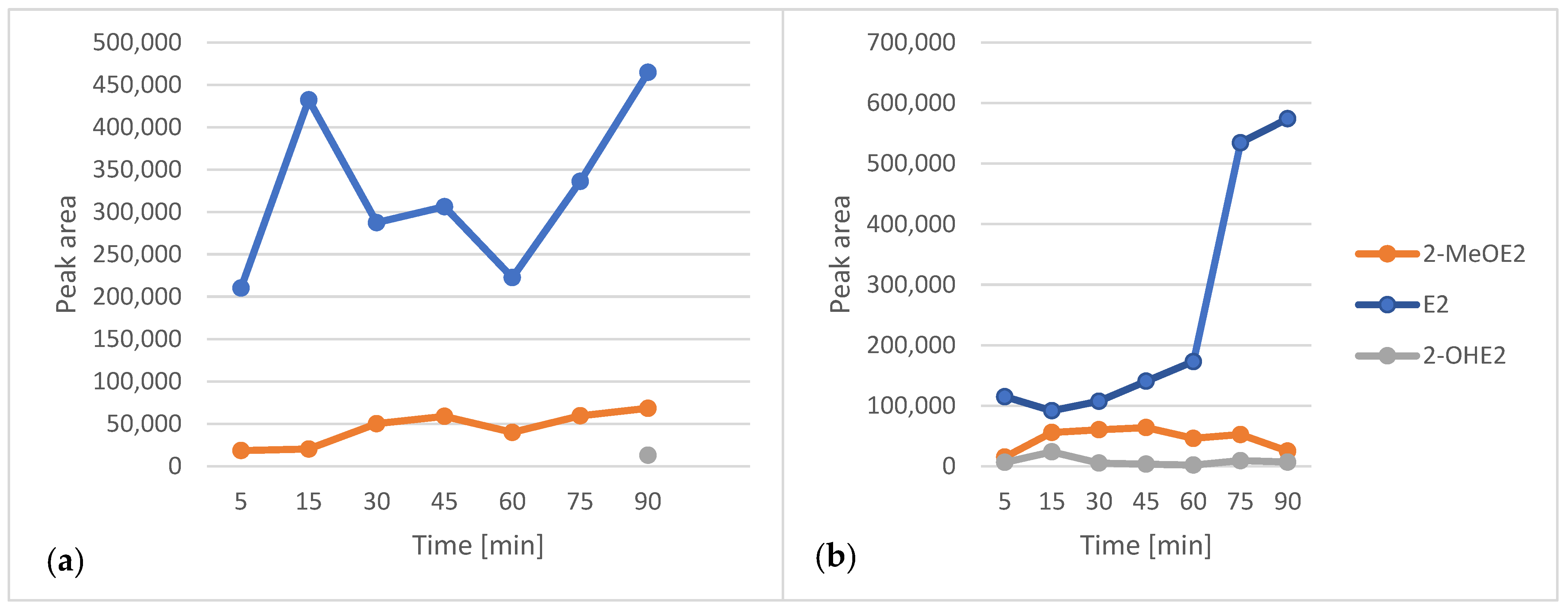
2.5. Solid-Phase Microextraction Optimization
2.6. Calibration Curves
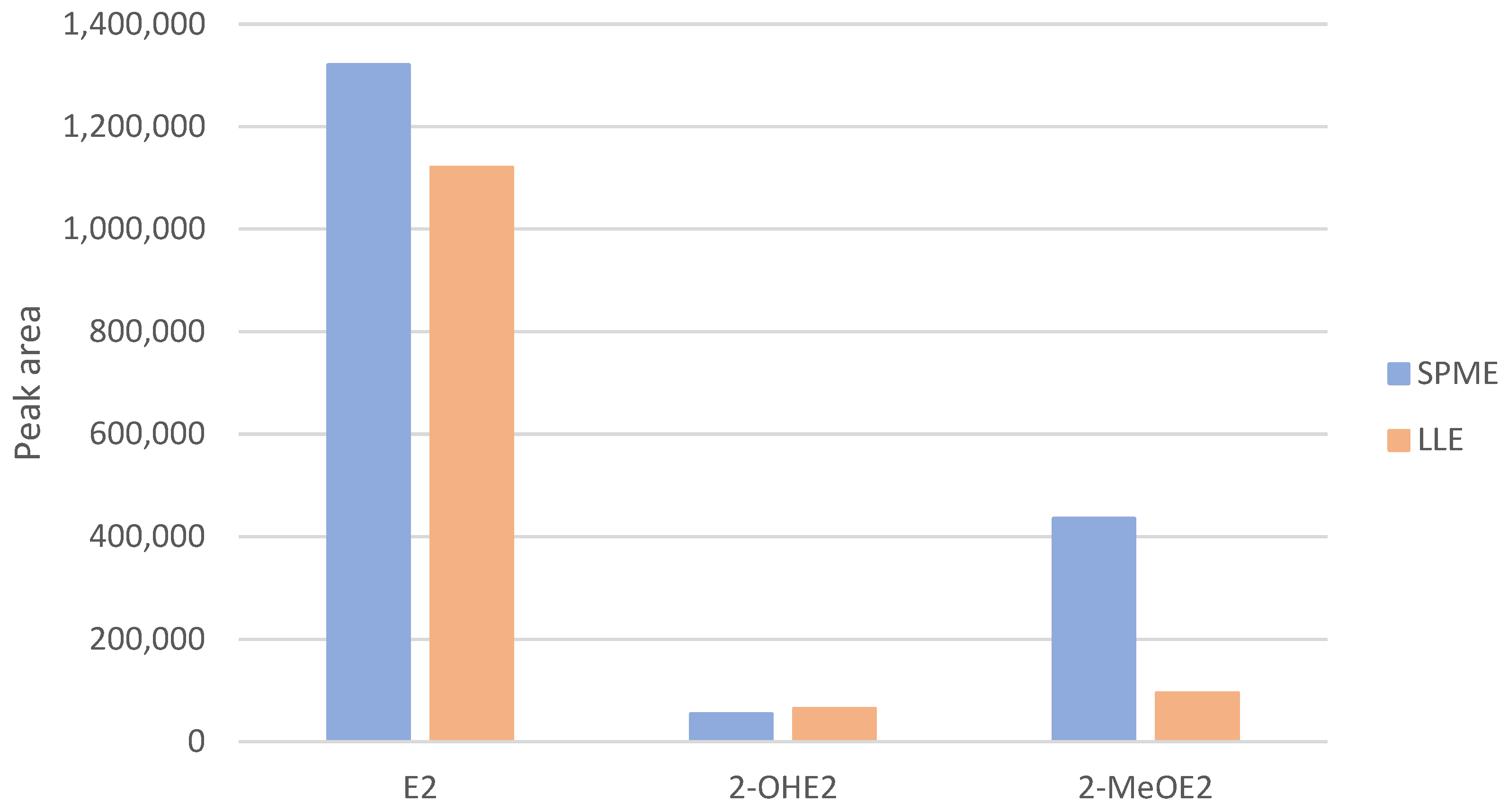
2.7. LLE and SPME Comparison
2.8. LC-MS Analysis
2.9. Patient Samples
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instrumentation and Chromatographic Conditions
4.3. Samples
4.4. Sample Preparation
4.4.1. Liquid–Liquid Extraction
4.4.2. Solid-Phase Microextraction
4.5. Derivatization Procedure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-MeOE2 | 2-Methoxyestradiol |
| 2-OHE2 | 2-Hydroxyestardiol |
| ACN | Acetonitrile |
| DNS-Cl | Dansyl chloride |
| DCHM | Dichloromethane |
| DVB | Divinylbenzene |
| E1 | Estrone |
| E2 | Estradiol |
| EtOH | Ethanol |
| FA | Formic acid |
| FLD | Fluorescence detector/detection |
| HPLC | High-performance liquid chromatography |
| HRT | Hormone replacement therapy |
| LC-MS | Liquid chromatography-mass spectrometry |
| LLE | Liquid–liquid extraction |
| LLOQ | Lower limit of quantification |
| LOC | Lifetime number of ovulatory cycles |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MeOH | Methanol |
| MTBE | Methyl tert-butyl ether |
| NSCLC | Non-small-cell lung cancer |
| PBS | Phosphate-buffered saline |
| ROS | Reactive oxygen species |
| SPE | Solid-phase extraction |
| SPME | Solid-phase microextraction |
References
- Kelsey, J.; Gammon, M.D.; John, E.M. Reproductive factors and breast cancer. Epidemiol. Rev. 1993, 15, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hawazie, A.; Druce, M. Breast cancer risk and management in the endocrine clinic: A comprehensive review. Clin. Epidemiol. 2025, 1, 1–25. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Au, C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Mogers, R.; Brown, K. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid. Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bukato, K.; Kostrzewa, T.; Gammazza, A.M.; Górska-Ponikowska, M.; Sawicki, S. Endogenous estrogen metabolites as oxidative stress mediators and endometrial cancer biomarkers. Cell. Commun. Signal. 2024, 22, 205. [Google Scholar] [CrossRef]
- Caldon, C. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front. Oncol. 2004, 4, 106. [Google Scholar] [CrossRef]
- Trabert, B.; O’Brien, S.T.K.; Towsend, M.; Fronter, R.; Iversen, E.; Hartage, P.; Wihite, P.; Amiano, P.; Arslan, A. The risk of ovarian cancer increases with an increase in the lifetime number of ovulatory cycles: An analysis from the Ovarian Cancer Cohort Consorium (OC3). Cancer Res. 2020, 80, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.; Gertz, J. Estrogen signaling in endometrial cancer: A key oncogenic pathway with several open questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- MacLean, J.A.; Hayashi, K. Progesterone actions and resistance in gynecological disorders. Cells 2022, 11, 647. [Google Scholar] [CrossRef]
- Zazzo, E.; Galasso, G.; Givannelli, P.; Donato, M.; Castoria, G. Estrogens and their receptors in prostate cancer: Therapeutic implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef]
- Diao, M.; Wang, Y.; Wu, S.; Liao, Y. Estrogen, estrogen receptor and the tumor microenvironment of NSCLC. Int. J. Cancer 2025, 156, 1501–1508. [Google Scholar] [CrossRef]
- Falk, R.T.; Brinton, L.A.; Dorgan, J.F.; Fuhrman, B.J.; Veenstra, T.D.; Xu, X.; Gierach, G.L. Relationship of serum estrogen metabolites to postmenopausal breast cancer risk: A nested case-control study. Breast Cancer Res. 2013, 15, R34. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.J.; Schairer, C.; Gail, M.; Boyd-Morin, J.; Xu, X.; Sue, L.Y.; Buys, S.S.; Isaacs, C.; Keefer, L.K.; Veenstra, T.D.; et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2012, 104, 326–329. [Google Scholar] [CrossRef]
- Fotsis, T.; Zhang, Y.; Pepper, M.S.; Adlercreut, H.; Montesano, R.; Nawroth, P.P.; Schweigerer, L. The endogenous oestrogen metabolite 2-methoxyestradiol inhibits angiogenesis and suppresses tumor growth. Nature 1994, 368, 237–239. [Google Scholar] [CrossRef]
- Chander, S.K.; Foster, P.A.; Leese, M.P.; Newman, S.P.; Potter, B.V.L.; Purohit, A.; Reed, M.J. In vivo inhibition of angiogenesis by sulfamoylated derivatives of 2-methoxyestradiol. Br. J. Cancer 2007, 96, 136–137. [Google Scholar] [CrossRef][Green Version]
- Ba, M.; Duan, Y. Advance of 2-methoxyestradiol as a promising anticancer agent for cancer therapy. Future Med. Chem. 2020, 12, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Batth, I.S.; Huang, S.B.; Viallareal, M.; Gong, J.; Chakravarthy, D.; Keppler, B.; Jayamohan, S.; Osmulski, P.; Xie, J.; Rivas, P.; et al. Evidence for 2-methoxyestradiol-mediated inhibition of receptor tyrosine kinase RON in the management of prostate cancer. Int. J. Mol. Sci. 2021, 22, 1852. [Google Scholar] [CrossRef]
- Górska-Ponikowska, M.; Kuban-Jankowska, A.; Eisler, S.A.; Perricone, U.; Lo Bosco, G.; Barone, G.; Nussberger, S. 2-methoxyestradiol affects mitochondrial biogenesis pathway and succinate dehydrogenase complex flavoprotein subunit a in osteosarcoma cancer cells. Cancer Genom. Proteom. 2018, 15, 73–89. [Google Scholar] [CrossRef]
- Sawicka, E.; Saczko, J.; Roik, J.; Kulbacka, J.; Piwowar, A. Effect of interaction between 17β-estradiol, 2-methoxyestardiol and 16α-hydroxyestrone with chromium (VI) on ovary cancer line SKOV-3: Preliminary study. Molecules 2020, 25, 5214. [Google Scholar] [CrossRef] [PubMed]
- Romero, Y.; Castillejos-Lopez, M.; Romero-Garcia, S.; Aguayo, A.S.; Herrera, I.; Garcia-Martin, M.O.; Torres-Espindola, L.M.; Negrete-Garcia, M.C.; Olvera, A.C.; Huerta-Cruz, J.C.; et al. Antitumor therapy under hypoxic microenvironment by the combination of 2-methoxyestradiol and sodium dichloroacetate on human non-small-cell lung cancer. Oxid. Med. Cell Longev. 2020, 2020, 3176375. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Al-Rabia, M.W.; Asfour, H.Z.; Alshehri, S.; Alharbi, W.S.; Halawani, A.; Alamoudi, A.J.; Noor, A.O.; Bannan, D.F.; Fahmy, U.A.; et al. 2-Methoxy-estradiol loaded alpha lipoic acid nanoparticles augment cytotoxicity in MCF-7 breast cancer cells. Dose Response 2021, 19, 15593258211055023. [Google Scholar] [CrossRef]
- Zhu, B.T.; Conney, A.H. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 1998, 19, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef] [PubMed]
- Hmeedat, F.; Hawamdeh, S.; Alnabulsi, S.; Zayed, A. High performance liquid chromatography (HPLC) with fluorescence detection for quantification of steroids in clinical, pharmaceutical, and environmental samples: A review. Molecules 2022, 27, 1807. [Google Scholar] [CrossRef]
- Xu, F.; Zou, L.; Zhang, Z.; Ong, C.N. Enhancement of the capabilities of liquid chromatography-mass spectrometry with derivatization: General principles and applications. Mass Spectrom. Rev. 2011, 30, 1143–1172. [Google Scholar] [CrossRef]
- Keski-Rahkonen, P.; Desai, R.; Jimenez, M.; Hardwood, D.T.; Handelsman, D.J. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization agent. Anal. Chem. 2015, 87, 7180–7186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Kellermann, G. Simultaneous determination of three estrogens in human saliva without derivatization or liquid-liquid extraction for routine testing via miniaturized solid phase extraction with LC-MS/MS detection. Talanta 2018, 178, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.; Alahdal, A.M. Liquid chromatography-tandem mass spectrometric analysis of ten estrogen metabolites at sub-picogram levels in breast cancer women. J. Chromatogr. B 2016, 1031, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Nascimento do Carmo, S.; Merib, J.; Carasek, E. Bract as a novel extraction phase in thin-film SPME combine with 96-well plate system for the high-throughput determination of estrogens in human urine by liquid chromatography coupled to fluorescence detection. J. Chromatogr. B 2019, 1118, 17–24. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Li, L.; Gatson, J.W.; Maass, D.; Wigginton, J.G.; Simpkins, J.W.; Schug, K.A. Simultaneous quantification of four native estrogen hormones at trace levels in human cerebrospinal fluid using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 54, 830–837. [Google Scholar] [CrossRef]
- Yang, W.C.; Regnier, F.E.; Sliva, D.; Adamec, J. Stable isotope-coded quarterization for comparative quantification of estrogen metabolites by high-performace liquid-chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B 2008, 870, 233–240. [Google Scholar] [CrossRef]
- Yamashita, K.; Okuyama, M.; Watanabe, Y.; Honma, S.; Kobayashi, S.; Numazawa, M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids 2007, 72, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Hakkinen, M.R.; Heinosalo, T.; Saarinen, N.; Linnanen, T.; Voutilainen, R.; Lakka, T.; Jaaskelainen, J.; Poutanen, M.; Auriola, S. Analysis by LC-MS/MS of endogenous steroids from human serum, plasma, endometrium and endometriotic tissue. J. Pharm. Biomed. Anal. 2018, 152, 165–172. [Google Scholar] [CrossRef]
- Yuan, T.F.; Le, J.; Cui, Y.; Pang, R.; Wang, S.T.; Li, Y. An LC-MS/MS analysis for seven sex hormones in serum. J. Pharm. Biomed. Anal. 2019, 162, 34–40. [Google Scholar] [CrossRef]
- Mao, L.; Sun, C.; Zhang, H.; Li, Y.; Wu, D. Determination of environmental estrogens in human urine b yhigh performance liquid chromatography after fluorescence derivatization with p-nitrobenzoyl chloride. Anal. Chem. Acta 2004, 522, 241–246. [Google Scholar] [CrossRef]
- Li, X.; Franke, A.A. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids 2015, 99, 84–90. [Google Scholar] [CrossRef]
- Fishman, S. Determination of estrogens in dosage forms by fluorescence using dansyl chloride. J. Pharm. Sci. 1975, 64, 674–680. [Google Scholar] [CrossRef]
- Schmidt, G.J.; Vandemark, F.L.; Slavin, W. Estrogen determination using liquid chromatography with precolumn fluorescence labelling. Anal. Biochem. 1978, 91, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Liz, M.; Amaral, B.; Stats, S.; Nagata, N.; Peralta-Zamora, P. Sensitive estrogens determination in wastewater samples by HPLC and fluorescence detection. J. Braz. Chem. Soc. 2017, 28, 1453–1460. [Google Scholar] [CrossRef]
- Cerrato, A.; Aita, S.E.; Cavaliere, C.; Laganà, A.; Montone, C.M.; Piovesana, S.; Taglioni, E.; Capriotti, A.L. Preparation of Monolith for Online Extraction and LC–MS Analysis of β-Estradiol in Serum Via a Simple Multicomponent Reaction. Anal. Chem. 2024, 96, 4639–4646. [Google Scholar] [CrossRef]
- Yi, X.; Huang, X.; Luo, H.; Liang, R.; Xiong, Y.; Wu, Y. Selective quantification of estrogens in serum with aptamer-affinity and LC-MS/MS analysis. Microchem. J. 2024, 207, 111845. [Google Scholar] [CrossRef]
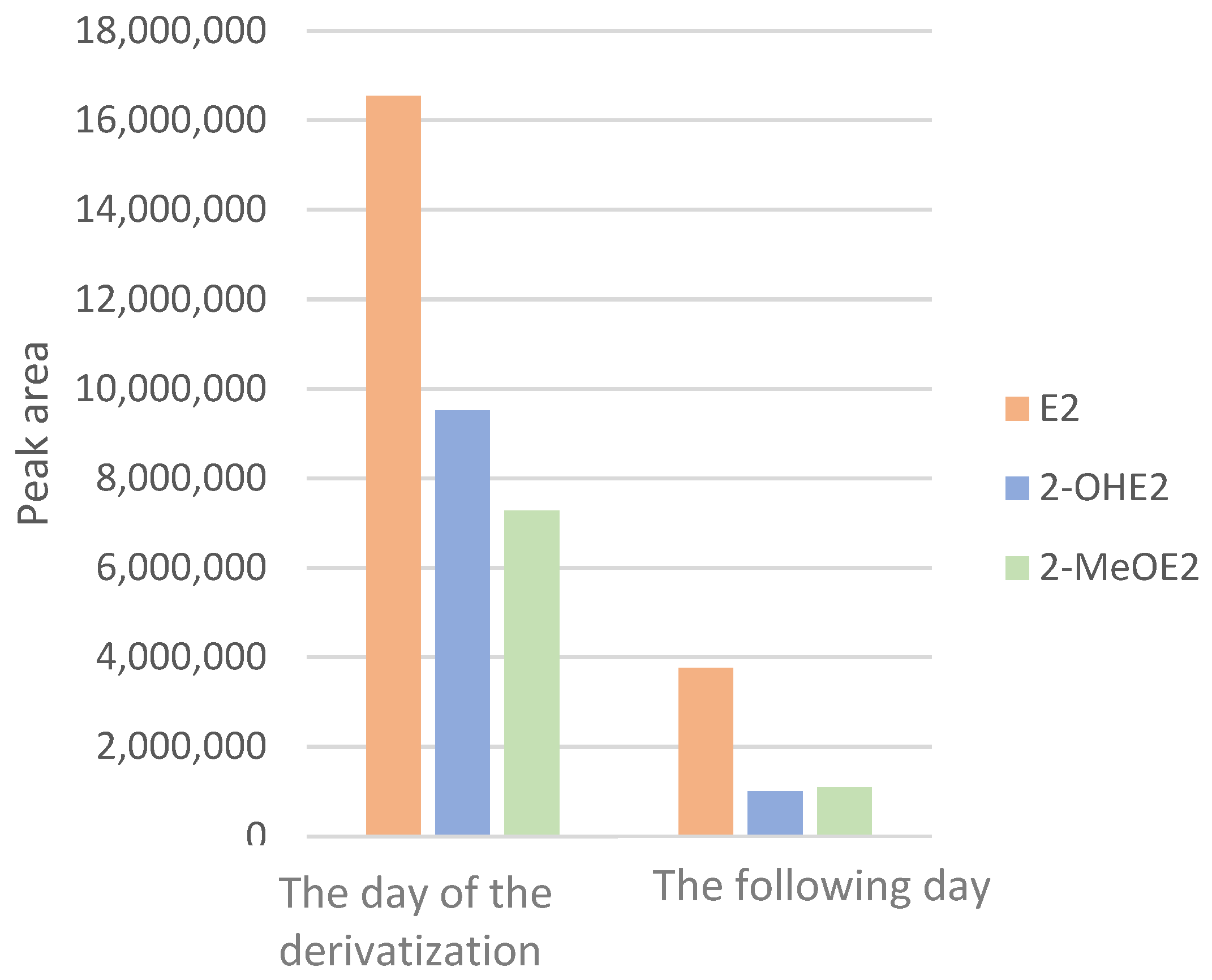
| Biological Material | Extraction | Solvent Used for Extraction | Derivatizing Agent | Quantitative Technique | LLOQ | Source | ||
|---|---|---|---|---|---|---|---|---|
| E2 | 2-OHE2 | 2-MeOE2 | ||||||
| Blood serum | LLE | DCHM | N-methyl-nicotinic acid Nhydroxysuccinimide ester | LC-MS/MS | 0.44 ng/mL * | 0.69 ng/mL * | 0.37 ng/mL * | [30] |
| Blood serum | LLE | MTBE | 1,2-dimethylimidazole-5-sulfonyl chloride | LC-MS/MS | 0.5 pg/mL | - | - | [25] |
| Saliva | SPE | MeOH | - | LC-MS/MS | 1 pg/mL | - | - | [26] |
| Blood plasma | SPE | MeOH:CHI3 | 3-bromomethyl-propyphenazone | LC-MS/MS | 0.3 pg/mL | 2.4 pg/mL | 0.9 pg/mL | [27] |
| Blood serum | LLE | MTBE | DNS-Cl | LC-MS/MS | 5 pg/mL | - | - | [33] |
| Blood serum and plasma | LLE | Toluene | - | LC-MS/MS | 6.6 pmol/L | - | - | [32] |
| Blood serum | LLE | MTBE | DNS-Cl | LC-MS/MS (Orbitrap) | 2.5 pg/mL | 50 pg/mL | 4.4 pg/mL | [35] |
| Urine | SPE | DCHM | p-Nitrobenzoyl chloride | HPLC-FLD | 2.7 ng/mL * | - | - | [34] |
| Cerebrospinal fluid | LLE | Ethyl acetate | DNS-Cl | LC-MS/MS | 26 pg/mL * | - | - | [29] |
| Column | Organic Phase | Mobile Phase [% Organic Component] | Was the Separation Successful? | |
|---|---|---|---|---|
| Discovery C18 (150 × 4.6 mm, 5 µm) | ACN | 50% | No | |
| Discovery C18 (150 × 4.6 mm, 5 µm) | ACN | 40% | No | |
| Discovery C18 (150 × 4.6 mm, 5 µm) | ACN | 0 min 10 min 10.1 min 14 min | 30% 100% 30% 30% | No |
| Phenomenex C18 (250 × 2.0 mm, 4 µm) | ACN | 0 min 12 min 12.1 min 15 min | 40% 10% 40% 40% | No |
| Phenomenex C18 (250 × 2.0 mm, 4 µm) | ACN | 0 min 10 min 10.1 min 14 min | 50% 100% 50% 50% | No |
| Phenomenex C18 (250 × 2.0 mm, 4 µm) | ACN | 0 min 8 min 8.1 min 11 min | 70% 100% 70% 70% | No |
| InfinityLab Proshell 120 EC-C18 (100 × 2.1 mm, 2.7 µm) | ACN | 0 min 8 min 8.1 min 11 min | 70% 100% 70% 70% | No |
| InfinityLab Proshell 120 EC-C18 (100 × 2.1 mm, 2.7 µm) | ACN | 0 min 10 min 10.1 min 14 min | 70% 100% 70% 70% | No |
| InfinityLab Proshell 120 EC-C18 (100 × 2.1 mm, 2.7 µm) | ACN | 0 min 10 min 10.1 min 14 min | 70% 100% 70% 70% | No |
| InfinityLab Proshell 120 EC-C18 (100 × 2.1 mm, 2.7 µm) | MeOH | 0 min 10 min 10.1 min 14 min | 70% 100% 70% 70% | No |
| InfinityLab Proshell 120 EC-C18 (100 × 2.1 mm, 2.7 µm) | MeOH | 0 min 8 min 8.1 min 11 min | 76% 100% 76% 76% | Yes |
| Scheme | Solvent | Peak Area | ||
|---|---|---|---|---|
| E2 | 2-OHE2 | 2-MeOE2 | ||
| 2-fold | MeOH | 805775 | 57456 | 6268 |
| MeOH + 0.1% FA | 1382545 | 10794 | 15868 | |
| DCHM | 1125206 | 33379 | 65759 | |
| 4-fold | MeOH | 257809 | 35261 | 99468 |
| MeOH + 0.1% FA | 590494 | 30525 | 15609 | |
| DCHM | 1120908 | 65138 | 95586 | |
| Analyte | Calibration Curve for Ethanol Solution | Calibration Curve for Serum (SPME) | Calibration Curve for Serum (LLE) | Calibration Curve for Saliva (SPME) | Calibration Curve for Saliva (LLE) |
|---|---|---|---|---|---|
| E2 | Y = 13,996x + 400,161 R2 = 0.9995 | Y = 2814.6x – 20,608 R2 = 0.9993 | Y = 3186.2x – 13,139 R2 = 0.9893 | Y = 2666.8x + 18,280 R2 = 0.9992 | Y = 2655.9x + 20,183 R2 = 0.994 |
| 2-OHE2 | Y = 2663x + 31,812 R2 = 0.9991 | Y = 840.38x + 2684.9 R2 = 0.9979 | Y = 728.46x − 1882.7 R2 = 0.9947 | Y = 173.4 + 19,348 R2 = 0.9988 | Y = 2468.5x + 3884.5 R2 = 0.9988 |
| 2-MeOE2 | Y = 10,759x + 389,649 R2 = 0.9985 | Y = 311.85x + 4717.3 R2 = 0.9995 | Y = 314.98x + 836.28 R2 = 0.9995 | Y = 245.13x + 50,221 R2 = 0.9984 | Y = 507.78x + 1985.4 R2 = 0.9987 |
| Accuracy of determinations | |||||
| E2 | 96.89% | 99.12% | 101.85% | 100.12% | 99.32% |
| 2-OHE2 | 97.49% | 99.01% | 99.65% | 100.93% | 99.55% |
| 2-MeOE2 | 96.05% | 99.38% | 99.78% | 99.21% | 100.82% |
| E2 | 2-OHE2 | 2-MeOE2 | |
|---|---|---|---|
| Calibration curve | Y = 4096.47x + 330.64 | Y = 890.32x + 3613.9 | Y = 2217.1x + 6459.4 |
| R2 | 0.9996 | 0.993 | 0.99971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliszewska, A.; Struczyński, P.; Bączek, T.; Konieczna, L. Development of an HPLC-FLD Method for Estradiol and Metabolites: Application of Solid-Phase Microextraction. Int. J. Mol. Sci. 2025, 26, 6194. https://doi.org/10.3390/ijms26136194
Kaliszewska A, Struczyński P, Bączek T, Konieczna L. Development of an HPLC-FLD Method for Estradiol and Metabolites: Application of Solid-Phase Microextraction. International Journal of Molecular Sciences. 2025; 26(13):6194. https://doi.org/10.3390/ijms26136194
Chicago/Turabian StyleKaliszewska, Anna, Piotr Struczyński, Tomasz Bączek, and Lucyna Konieczna. 2025. "Development of an HPLC-FLD Method for Estradiol and Metabolites: Application of Solid-Phase Microextraction" International Journal of Molecular Sciences 26, no. 13: 6194. https://doi.org/10.3390/ijms26136194
APA StyleKaliszewska, A., Struczyński, P., Bączek, T., & Konieczna, L. (2025). Development of an HPLC-FLD Method for Estradiol and Metabolites: Application of Solid-Phase Microextraction. International Journal of Molecular Sciences, 26(13), 6194. https://doi.org/10.3390/ijms26136194






