Phage-Microbiota Crosstalk: Implications for Central Nervous System Disorders
Abstract
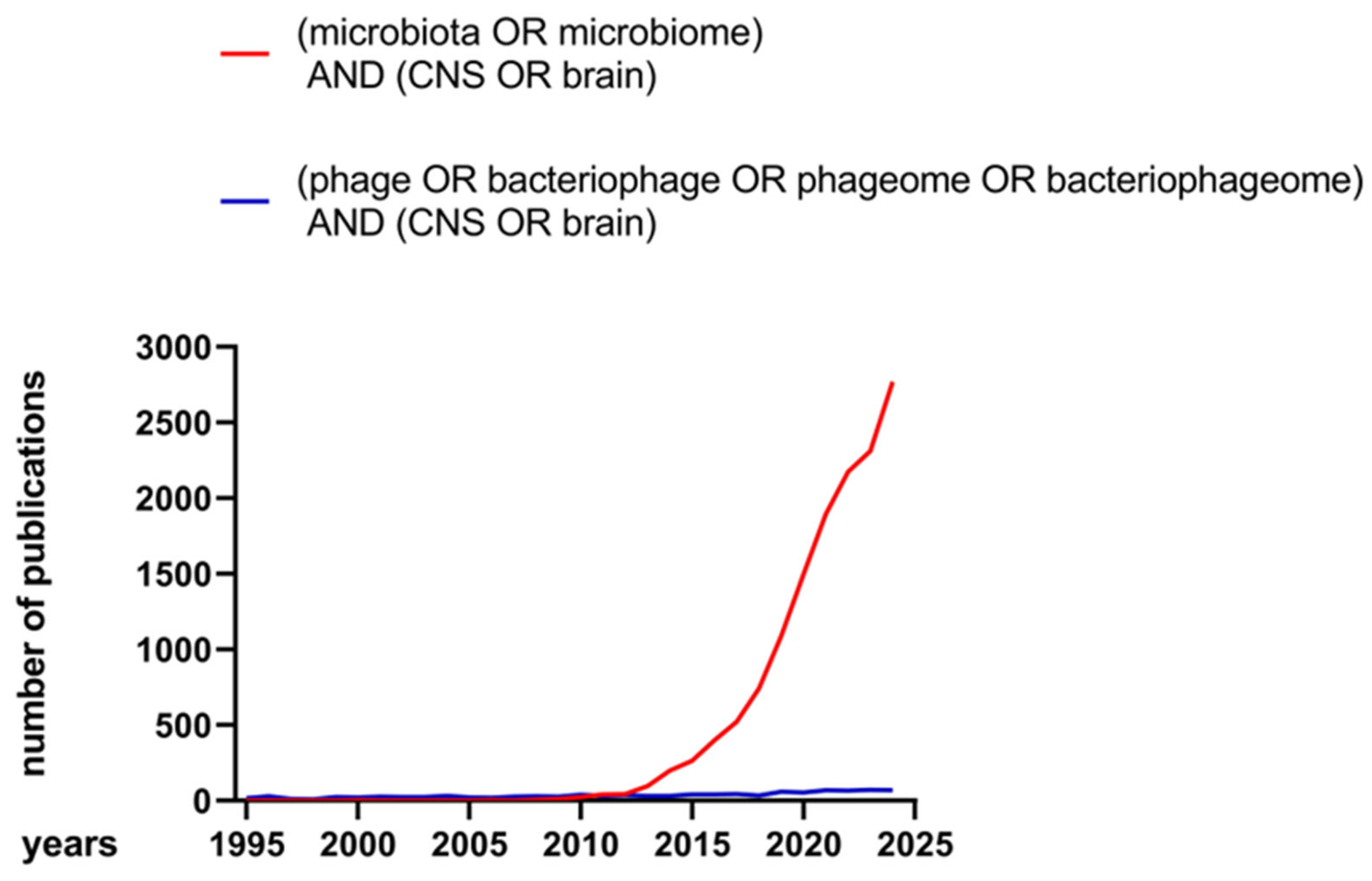
1. Introduction
2. Phages and Their Presence in the Human Body
3. Therapeutic Use of Phages
4. The Microbiota–Gut–Brain Axis
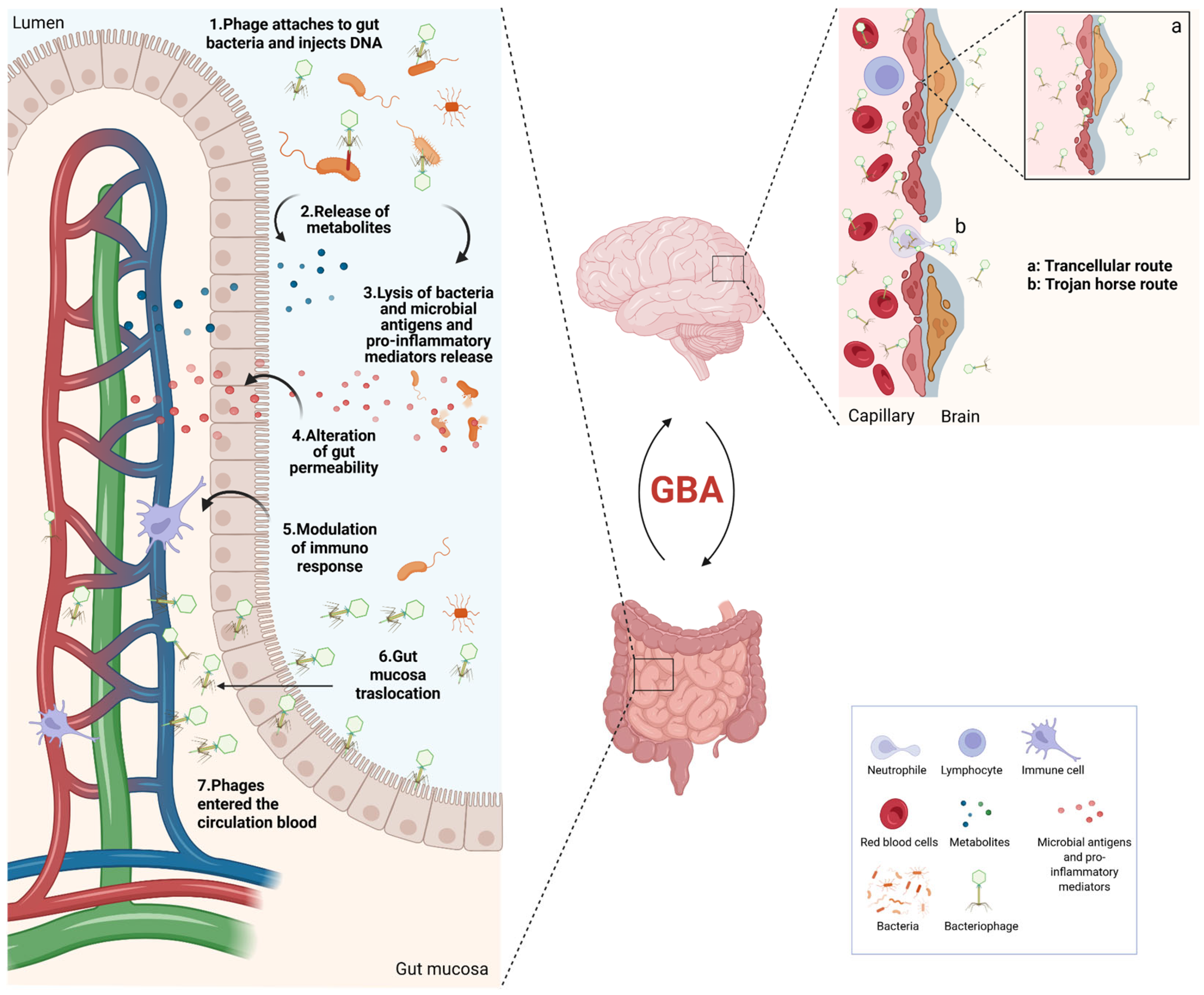
5. Phages and the CNS
5.1. Phages and Schizophrenia
5.2. Phages and Depression
5.3. Phages and Parkinson’s Disease
5.4. Phages and Alzheimer’s Disease
5.5. Phages and Huntington’s Disease
5.6. Phages and Amyotrophic Lateral Sclerosis
5.7. Phages and Multiple Sclerosis
5.8. Phages and Stroke
5.9. Phages and Epilepsy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| ANS | Autonomic nervous system |
| Aβ | Amyloid-beta |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| ENS | Enteric nervous system |
| MGBA | Microbiota–Gut–brain axis |
| HD | Huntington’s disease |
| HPA | Hypothalamic–pituitary–adrenal |
| i.v. | Intravenous |
| L-DOPA | Orally administered levodopa |
| MCAO | Middle cerebral artery occlusion |
| MDD | Major depressive disorder |
| MS | Multiple sclerosis |
| OAE | Onchocerciasis-associated epilepsy |
| PD | Parkinson disease |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SLE | Systemic lupus erythematosus |
| SURG | Surgical resections for epilepsy |
| TDC | Tyrosine decarboxylase |
References
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L., 4th; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-Derived Metabolites as Drivers of Gut-Brain Communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef] [PubMed]
- Shkoporov, A.N.; Turkington, C.J.; Hill, C. Mutualistic Interplay between Bacteriophages and Bacteria in the Human Gut. Nat. Rev. Microbiol. 2022, 20, 737–749. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Matthewman, C.; Narin, A.; Huston, H.; Hopkins, C.E. Systems to Model the Personalized Aspects of Microbiome Health and Gut Dysbiosis. Mol. Aspects Med. 2023, 91, 101115. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Reyes, A.; Blanton, L.V.; Cao, S.; Zhao, G.; Manary, M.; Trehan, I.; Smith, M.I.; Wang, D.; Virgin, H.W.; Rohwer, F.; et al. Gut DNA Viromes of Malawian Twins Discordant for Severe Acute Malnutrition. Proc. Natl. Acad. Sci. USA 2015, 112, 11941–11946. [Google Scholar] [CrossRef]
- Minot, S.; Bryson, A.; Chehoud, C.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Rapid Evolution of the Human Gut Virome. Proc. Natl. Acad. Sci. USA 2013, 110, 12450–12455. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Stockdale, S.R.; Lavelle, A.; Kondova, I.; Heuston, C.; Upadrasta, A.; Khokhlova, E.V.; van der Kamp, I.; Ouwerling, B.; Draper, L.A.; et al. Viral Biogeography of the Mammalian Gut and Parenchymal Organs. Nat. Microbiol. 2022, 7, 1301–1311. [Google Scholar] [CrossRef]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and Their Potential to Modulate the Microbiome and Immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel Phage Lysin Capable of Killing the Multidrug-Resistant Gram-Negative Bacterium Acinetobacter Baumannii in a Mouse Bacteremia Model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.W.; Shin, T.H.; Kim, J.H.; Shin, S.P.; Han, J.E.; Heo, G.J.; De Zoysa, M.; Shin, G.W.; Chai, J.Y.; Park, S.C. Bacteriophage Therapy of a Vibrio Parahaemolyticus Infection Caused by a Multiple-Antibiotic-Resistant O3:K6 Pandemic Clinical Strain. J. Infect. Dis. 2014, 210, 72–78. [Google Scholar] [CrossRef]
- Nale, J.Y.; Spencer, J.; Hargreaves, K.R.; Buckley, A.M.; Trzepiński, P.; Douce, G.R.; Clokie, M.R.J. Bacteriophage Combinations Significantly Reduce Clostridium Difficile Growth in Vitro and Proliferation in Vivo. Antimicrob. Agents Chemother. 2016, 60, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, S.; Duan, H.; Shao, H.; Duan, Y. Bacteriophages and Their Potential for Treatment of Metabolic Diseases. J. Diabetes 2024, 16, e70024. [Google Scholar] [CrossRef]
- Ackermann, H.-W.; Prangishvili, D. Prokaryote Viruses Studied by Electron Microscopy. Arch. Virol. 2012, 157, 1843–1849. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage Diversity, Genomics and Phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Smith, M.C.; Burns, R.N.; Ford, M.E.; Hatfull, G.F. Evolutionary Relationships among Diverse Bacteriophages and Prophages: All the World’s a Phage. Proc. Natl. Acad. Sci. USA 1999, 96, 2192–2197. [Google Scholar] [CrossRef]
- Harrison, E.; Brockhurst, M.A. Ecological and Evolutionary Benefits of Temperate Phage: What Does or Doesn’t Kill You Makes You Stronger. Bioessays 2017, 39, 1700112. [Google Scholar] [CrossRef]
- Spriewald, S.; Stadler, E.; Hense, B.A.; Münch, P.C.; McHardy, A.C.; Weiss, A.S.; Obeng, N.; Müller, J.; Stecher, B. Evolutionary Stabilization of Cooperative Toxin Production through a Bacterium-Plasmid-Phage Interplay. mBio 2020, 11. [Google Scholar] [CrossRef]
- Gummalla, V.S.; Zhang, Y.; Liao, Y.-T.; Wu, V.C.H. The Role of Temperate Phages in Bacterial Pathogenicity. Microorganisms 2023, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The Human Virome: Assembly, Composition and Host Interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Minot, S.; Sinha, R.; Chen, J.; Li, H.; Keilbaugh, S.A.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. The Human Gut Virome: Inter-Individual Variation and Dynamic Response to Diet. Genome Res. 2011, 21, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The Human Gut Microbiota and Virome: Potential Therapeutic Implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguière, A.; Manuguerra, J.-C.; et al. Human Skin Microbiota: High Diversity of DNA Viruses Identified on the Human Skin by High Throughput Sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef]
- Pride, D.T.; Salzman, J.; Haynes, M.; Rohwer, F.; Davis-Long, C.; White, R.A., 3rd; Loomer, P.; Armitage, G.C.; Relman, D.A. Evidence of a Robust Resident Bacteriophage Population Revealed through Analysis of the Human Salivary Virome. ISME J. 2012, 6, 915–926. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Zhao, F. Phage–bacteria Interaction Network in Human Oral Microbiome. Environ. Microbiol. 2016, 18, 2143–2158. [Google Scholar] [CrossRef]
- Edlund, A.; Santiago-Rodriguez, T.M.; Boehm, T.K.; Pride, D.T. Bacteriophage and Their Potential Roles in the Human Oral Cavity. J. Oral Microbiol. 2015, 7, 27423. [Google Scholar] [CrossRef]
- Dickson, R.P.; Huffnagle, G.B. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015, 11, e1004923. [Google Scholar] [CrossRef] [PubMed]
- Willner, D.; Furlan, M.; Haynes, M.; Schmieder, R.; Angly, F.E.; Silva, J.; Tammadoni, S.; Nosrat, B.; Conrad, D.; Rohwer, F. Metagenomic Analysis of Respiratory Tract DNA Viral Communities in Cystic Fibrosis and Non-Cystic Fibrosis Individuals. PLoS ONE 2009, 4, e7370. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, T.M.; Ly, M.; Bonilla, N.; Pride, D.T. The Human Urine Virome in Association with Urinary Tract Infections. Front. Microbiol. 2015, 6, 14. [Google Scholar] [CrossRef]
- Elsayed, N.S.; Wolfe, A.J.; Burk, R.D. Urine Microbiome in Individuals with an Impaired Immune System. Front. Cell. Infect. Microbiol. 2023, 13, 1308665. [Google Scholar] [CrossRef]
- Brüssow, H.; Canchaya, C.; Hardt, W.-D. Phages and the Evolution of Bacterial Pathogens: From Genomic Rearrangements to Lysogenic Conversion. Microbiol. Mol. Biol. Rev. 2004, 68, 560–602. [Google Scholar] [CrossRef]
- Li, S.-K.; Leung, R.K.-K.; Guo, H.-X.; Wei, J.-F.; Wang, J.-H.; Kwong, K.-T.; Lee, S.-S.; Zhang, C.; Tsui, S.K.-W. Detection and Identification of Plasma Bacterial and Viral Elements in HIV/AIDS Patients in Comparison to Healthy Adults. Clin. Microbiol. Infect. 2012, 18, 1126–1133. [Google Scholar] [CrossRef]
- Dinakaran, V.; Rathinavel, A.; Pushpanathan, M.; Sivakumar, R.; Gunasekaran, P.; Rajendhran, J. Elevated Levels of Circulating DNA in Cardiovascular Disease Patients: Metagenomic Profiling of Microbiome in the Circulation. PLoS ONE 2014, 9, e105221. [Google Scholar] [CrossRef]
- Moustafa, A.; Xie, C.; Kirkness, E.; Biggs, W.; Wong, E.; Turpaz, Y.; Bloom, K.; Delwart, E.; Nelson, K.E.; Venter, J.C.; et al. The Blood DNA Virome in 8,000 Humans. PLoS Pathog. 2017, 13, e1006292. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Clement, F.; Merabishvili, M.; Lavigne, R.; Vaneechoutte, M. Pro- and Anti-Inflammatory Responses of Peripheral Blood Mononuclear Cells Induced by Staphylococcus Aureus and Pseudomonas Aeruginosa Phages. Sci. Rep. 2017, 7, 8004. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Park, E.-J.; Roh, S.W.; Bae, J.-W. Diversity and Abundance of Single-Stranded DNA Viruses in Human Feces. Appl. Environ. Microbiol. 2011, 77, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Manrique, P.; Dills, M.; Young, M. The Human Gut Phage Community and Its Implications for Health and Disease. Viruses 2017, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Carroll-Portillo, A.; Lin, D.M.; Lin, H.C. The Diversity of Bacteriophages in the Human Gut. Methods Mol. Biol. 2024, 2738, 17–30. [Google Scholar]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Breitbart, M.; Haynes, M.; Kelley, S.; Angly, F.; Edwards, R.A.; Felts, B.; Mahaffy, J.M.; Mueller, J.; Nulton, J.; Rayhawk, S.; et al. Viral Diversity and Dynamics in an Infant Gut. Res. Microbiol. 2008, 159, 367–373. [Google Scholar] [CrossRef]
- Lim, E.S.; Zhou, Y.; Zhao, G.; Bauer, I.K.; Droit, L.; Ndao, I.M.; Warner, B.B.; Tarr, P.I.; Wang, D.; Holtz, L.R. Early Life Dynamics of the Human Gut Virome and Bacterial Microbiome in Infants. Nat. Med. 2015, 21, 1228–1234. [Google Scholar] [CrossRef]
- Venkataraman, S.; Shahgolzari, M.; Yavari, A.; Hefferon, K. Bacteriophages as Targeted Therapeutic Vehicles: Challenges and Opportunities. Bioengineering 2025, 12, 469. [Google Scholar] [CrossRef]
- Yao, M.; Zhu, Y.; Duan, J.-A.; Xiao, P. Phage Therapy: A Novel Approach to Combat Drug-Resistant Pathogens. Microbiol. Res. 2025, 298, 128228. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage Transfer during Faecal Microbiota Transplantation in Clostridium Difficile Infection Is Associated with Treatment Outcome. Gut 2018, 67, 634–643. [Google Scholar]
- Khoruts, A.; Sadowsky, M.J. Therapeutic Transplantation of the Distal Gut Microbiota. Mucosal Immunol. 2011, 4, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Maronek, M.; Link, R.; Ambro, L.; Gardlik, R. Phages and Their Role in Gastrointestinal Disease: Focus on Inflammatory Bowel Disease. Cells 2020, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Suh, G.A.; Cullen, G.D.; Perez Rodriguez, S.; Dharmaraj, T.; Chang, T.H.W.; Li, Z.; Chen, Q.; Green, S.I.; Lavigne, R.; et al. Bacteriophage Therapy for Multidrug-Resistant Infections: Current Technologies and Therapeutic Approaches. J. Clin. Investig. 2025, 135, e187996. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Mudaliar, S.B.; Bhat, V.G.; Chakraborty, I.; Prasad, A.S.B.; Mazumder, N. Phage Therapy: A Novel Approach against Multidrug-Resistant Pathogens. 3 Biotech 2024, 14, 256. [Google Scholar] [CrossRef]
- Marchi, J.; Zborowsky, S.; Debarbieux, L.; Weitz, J.S. The Dynamic Interplay of Bacteriophage, Bacteria and the Mammalian Host during Phage Therapy. iScience 2023, 26, 106004. [Google Scholar] [CrossRef]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage Therapy: A Different Approach to Fight Bacterial Infections. Biologics 2022, 16, 173–186. [Google Scholar]
- Egido, J.E.; Costa, A.R.; Aparicio-Maldonado, C.; Haas, P.-J.; Brouns, S.J.J. Mechanisms and Clinical Importance of Bacteriophage Resistance. FEMS Microbiol. Rev. 2022, 46, fuab048. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Derovs, A.; Laivacuma, S.; Krumina, A. Targeting Microbiota: What Do We Know about It at Present? Medicina 2019, 55, 459. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-Gut-Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [PubMed]
- Kurhaluk, N.; Kamiński, P.; Bilski, R.; Kołodziejska, R.; Woźniak, A.; Tkaczenko, H. Role of Antioxidants in Modulating the Microbiota-Gut-Brain Axis and Their Impact on Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 3658. [Google Scholar] [CrossRef] [PubMed]
- Stolzer, I.; Scherer, E.; Süß, P.; Rothhammer, V.; Winner, B.; Neurath, M.F.; Günther, C. Impact of Microbiome-Brain Communication on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 14925. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Our Mental Health Is Determined by an Intrinsic Interplay between the Central Nervous System, Enteric Nerves, and Gut Microbiota. Int. J. Mol. Sci. 2023, 25, 38. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K.K. Gut-Organ Axis: A Microbial Outreach and Networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal Afferent Responses to Fatty Acids of Different Chain Length in the Rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef]
- de La Serre, C.B.; de Lartigue, G.; Raybould, H.E. Chronic Exposure to Low Dose Bacterial Lipopolysaccharide Inhibits Leptin Signaling in Vagal Afferent Neurons. Physiol. Behav. 2015, 139, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Strader, A.D.; Woods, S.C. Gastrointestinal Hormones and Food Intake. Gastroenterology 2005, 128, 175–191. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef]
- Ye, L.; Bae, M.; Cassilly, C.D.; Jabba, S.V.; Thorpe, D.W.; Martin, A.M.; Lu, H.-Y.; Wang, J.; Thompson, J.D.; Lickwar, C.R.; et al. Enteroendocrine Cells Sense Bacterial Tryptophan Catabolites to Activate Enteric and Vagal Neuronal Pathways. Cell Host Microbe 2021, 29, 179–196.e9. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors That Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and Characterization of Gut Microbiota Decarboxylases That Can Produce the Neurotransmitter Tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Hong, D.; Zhang, C.; Wu, W.; Lu, X.; Zhang, L. Modulation of the Gut-Brain Axis via the Gut Microbiota: A New Era in Treatment of Amyotrophic Lateral Sclerosis. Front. Neurol. 2023, 14, 1133546. [Google Scholar] [CrossRef] [PubMed]
- Juruena, M.F.; Bocharova, M.; Agustini, B.; Young, A.H. Atypical Depression and Non-Atypical Depression: Is HPA Axis Function a Biomarker? A Systematic Review. J. Affect. Disord. 2018, 233, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Crawford, M. ’sa; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the Gut Microbiota: Considering Structural Variation. FEBS Lett. 2022, 596, 849–875. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-M.; Lee, K.-E.; Lee, H.-J.; Kim, D.-H. Immobilization Stress-Induced Escherichia Coli Causes Anxiety by Inducing NF-κB Activation through Gut Microbiota Disturbance. Sci. Rep. 2018, 8, 13897. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Chukkapalli, S.; Rivera, M.; Velsko, I.; Kesavalu, L.; Crean, S. Active Invasion of Porphyromonas Gingivalis and Infection-Induced Complement Activation in ApoE-/- Mice Brains. J. Alzheimers. Dis. 2015, 43, 67–80. [Google Scholar] [CrossRef]
- Brouwers, N.; Van Cauwenberghe, C.; Engelborghs, S.; Lambert, J.-C.; Bettens, K.; Le Bastard, N.; Pasquier, F.; Montoya, A.G.; Peeters, K.; Mattheijssens, M.; et al. Alzheimer Risk Associated with a Copy Number Variation in the Complement Receptor 1 Increasing C3b/C4b Binding Sites. Mol. Psychiatry 2012, 17, 223–233. [Google Scholar] [CrossRef]
- Jędrusiak, A.; Fortuna, W.; Majewska, J.; Górski, A.; Jończyk-Matysiak, E. Phage Interactions with the Nervous System in Health and Disease. Cells 2023, 12, 1720. [Google Scholar] [CrossRef]
- Podlacha, M.; Węgrzyn, G.; Węgrzyn, A. Bacteriophages-Dangerous Viruses Acting Incognito or Underestimated Saviors in the Fight against Bacteria? Int. J. Mol. Sci. 2024, 25, 2107. [Google Scholar] [CrossRef]
- Emencheta, S.C.; Olovo, C.V.; Eze, O.C.; Kalu, C.F.; Berebon, D.P.; Onuigbo, E.B.; Vila, M.M.D.C.; Balcão, V.M.; Attama, A.A. The Role of Bacteriophages in the Gut Microbiota: Implications for Human Health. Pharmaceutics 2023, 15, 2416. [Google Scholar] [CrossRef]
- Mills, S.; Shanahan, F.; Stanton, C.; Hill, C.; Coffey, A.; Ross, R.P. Movers and Shakers: Influence of Bacteriophages in Shaping the Mammalian Gut Microbiota. Gut Microbes 2013, 4, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, L.A.; Jones, B.V. The Human Gut Virome: A Multifaceted Majority. Front. Microbiol. 2015, 6, 918. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.K.; Maurice, C.F. Ménage à Trois in the Human Gut: Interactions between Host, Bacteria and Phages. Nat. Rev. Microbiol. 2017, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.M.; Kelly, L.; Muscatt, G.; Box, J.D.; Hargraves, N.; Lilley, D.; Jameson, E. The Human Gut Phageome: Origins and Roles in the Human Gut Microbiome. Front. Cell. Infect. Microbiol. 2021, 11, 643214. [Google Scholar] [CrossRef]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the Immune System. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The Gut Virome: A New Microbiome Component in Health and Disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Rodriguez-Valera, F.; Martin-Cuadrado, A.-B.; Rodriguez-Brito, B.; Pasić, L.; Thingstad, T.F.; Rohwer, F.; Mira, A. Explaining Microbial Population Genomics through Phage Predation. Nat. Rev. Microbiol. 2009, 7, 828–836. [Google Scholar] [CrossRef]
- Scanlan, P.D. Bacteria–bacteriophage Coevolution in the Human Gut: Implications for Microbial Diversity and Functionality. Trends Microbiol. 2017, 25, 614–623. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Li, S.C. Understanding Horizontal Gene Transfer Network in Human Gut Microbiota. Gut Pathog. 2020, 12, 33. [Google Scholar] [CrossRef]
- Kirsch, J.M.; Brzozowski, R.S.; Faith, D.; Round, J.L.; Secor, P.R.; Duerkop, B.A. Bacteriophage-Bacteria Interactions in the Gut: From Invertebrates to Mammals. Annu. Rev. Virol. 2021, 8, 95–113. [Google Scholar] [CrossRef]
- Furuse, K.; Osawa, S.; Kawashiro, J.; Tanaka, R.; Ozawa, A.; Sawamura, S.; Yanagawa, Y.; Nagao, T.; Watanabe, I. Bacteriophage Distribution in Human Faeces: Continuous Survey of Healthy Subjects and Patients with Internal and Leukaemic Diseases. J. Gen. Virol. 1983, 64 Pt 9, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Lepage, P.; Colombet, J.; Marteau, P.; Sime-Ngando, T.; Doré, J.; Leclerc, M. Dysbiosis in Inflammatory Bowel Disease: A Role for Bacteriophages? Gut 2008, 57, 424–425. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the Mucosal Barrier to Infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Schluter, J.; Foster, K.R. The Evolution of Mutualism in Gut Microbiota via Host Epithelial Selection. PLoS Biol. 2012, 10, e1001424. [Google Scholar] [CrossRef]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage Adhering to Mucus Provide a Non-Host-Derived Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef]
- Almeida, G.M.F.; Laanto, E.; Ashrafi, R.; Sundberg, L.-R. Bacteriophage Adherence to Mucus Mediates Preventive Protection against Pathogenic Bacteria. mBio 2019, 10. [Google Scholar] [CrossRef]
- Bille, E.; Meyer, J.; Jamet, A.; Euphrasie, D.; Barnier, J.-P.; Brissac, T.; Larsen, A.; Pelissier, P.; Nassif, X. A Virulence-Associated Filamentous Bacteriophage of Neisseria Meningitidis Increases Host-Cell Colonisation. PLoS Pathog. 2017, 13, e1006495. [Google Scholar] [CrossRef]
- Sinha, A.; Maurice, C.F. Bacteriophages: Uncharacterized and Dynamic Regulators of the Immune System. Mediators Inflamm. 2019, 2019, 3730519. [Google Scholar] [CrossRef]
- de Souza, E.B.; Pinto, A.R.; Fongaro, G. Bacteriophages as Potential Clinical Immune Modulators. Microorganisms 2023, 11, 2222. [Google Scholar] [CrossRef]
- Kurzepa, A.; Dabrowska, K.; Skaradziński, G.; Górski, A. Bacteriophage Interactions with Phagocytes and Their Potential Significance in Experimental Therapy. Clin. Exp. Med. 2009, 9, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Carroll-Portillo, A.; Lin, H.C. Bacteriophage and the Innate Immune System: Access and Signaling. Microorganisms 2019, 7, 625. [Google Scholar] [CrossRef]
- Miernikiewicz, P.; Dąbrowska, K. Endocytosis of Bacteriophages. Curr. Opin. Virol. 2022, 52, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Harjai, K.; Chhibber, S. Bacteriophage-Aided Intracellular Killing of Engulfed Methicillin-Resistant Staphylococcus Aureus (MRSA) by Murine Macrophages. Appl. Microbiol. Biotechnol. 2014, 98, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Miedzybrodzki, R.; Switala-Jelen, K.; Fortuna, W.; Weber-Dabrowska, B.; Przerwa, A.; Lusiak-Szelachowska, M.; Dabrowska, K.; Kurzepa, A.; Boratynski, J.; Syper, D.; et al. Bacteriophage Preparation Inhibition of Reactive Oxygen Species Generation by Endotoxin-Stimulated Polymorphonuclear Leukocytes. Virus Res. 2008, 131, 233–242. [Google Scholar] [CrossRef]
- Miernikiewicz, P.; Kłopot, A.; Soluch, R.; Szkuta, P.; Kęska, W.; Hodyra-Stefaniak, K.; Konopka, A.; Nowak, M.; Lecion, D.; Kaźmierczak, Z.; et al. T4 Phage Tail Adhesin Gp12 Counteracts LPS-Induced Inflammation in Vivo. Front. Microbiol. 2016, 7, 1112. [Google Scholar] [CrossRef]
- Weber-Dabrowska, B.; Zimecki, M.; Mulczyk, M. Effective Phage Therapy Is Associated with Normalization of Cytokine Production by Blood Cell Cultures. Arch. Immunol. Ther. Exp. 2000, 48, 31–37. [Google Scholar]
- Zhang, L.; Hou, X.; Sun, L.; He, T.; Wei, R.; Pang, M.; Wang, R. Corrigendum: Staphylococcus Aureus Bacteriophage Suppresses LPS-Induced Inflammation in MAC-T Bovine Mammary Epithelial Cells. Front. Microbiol. 2018, 9, 2511. [Google Scholar] [CrossRef]
- Bichet, M.C.; Adderley, J.; Avellaneda-Franco, L.; Magnin-Bougma, I.; Torriero-Smith, N.; Gearing, L.J.; Deffrasnes, C.; David, C.; Pepin, G.; Gantier, M.P.; et al. Mammalian Cells Internalize Bacteriophages and Use Them as a Resource to Enhance Cellular Growth and Survival. PLoS Biol. 2023, 21, e3002341. [Google Scholar] [CrossRef]
- Zimecki, M.; Artym, J.; Kocieba, M.; Weber-Dabrowska, B.; Borysowski, J.; Górski, A. Effects of Prophylactic Administration of Bacteriophages to Immunosuppressed Mice Infected with Staphylococcus Aureus. BMC Microbiol. 2009, 9, 169. [Google Scholar] [CrossRef]
- Secor, P.R.; Michaels, L.A.; Smigiel, K.S.; Rohani, M.G.; Jennings, L.K.; Hisert, K.B.; Arrigoni, A.; Braun, K.R.; Birkland, T.P.; Lai, Y.; et al. Filamentous Bacteriophage Produced by Pseudomonas Aeruginosa Alters the Inflammatory Response and Promotes Noninvasive Infection in Vivo. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kamme, C. Antibodies against Staphylococcal Bacteriophages in Human Sera. I. Assay of Antibodies in Healthy Individuals and in Patients with Staphylococcal Infections. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 1973, 81, 741–748. [Google Scholar]
- Górski, A.; Międzybrodzki, R.; Borysowski, J.; Dąbrowska, K.; Wierzbicki, P.; Ohams, M.; Korczak-Kowalska, G.; Olszowska-Zaremba, N.; Łusiak-Szelachowska, M.; Kłak, M.; et al. Phage as a Modulator of Immune Responses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2012; pp. 41–71. ISBN 9780123944382. [Google Scholar]
- Dąbrowska, K.; Miernikiewicz, P.; Piotrowicz, A.; Hodyra, K.; Owczarek, B.; Lecion, D.; Kaźmierczak, Z.; Letarov, A.; Górski, A. Immunogenicity Studies of Proteins Forming the T4 Phage Head Surface. J. Virol. 2014, 88, 12551–12557. [Google Scholar] [CrossRef]
- Ghose, C.; Ly, M.; Schwanemann, L.K.; Shin, J.H.; Atab, K.; Barr, J.J.; Little, M.; Schooley, R.T.; Chopyk, J.; Pride, D.T. The Virome of Cerebrospinal Fluid: Viruses Where We Once Thought There Were None. Front. Microbiol. 2019, 10, 2061. [Google Scholar] [CrossRef]
- Barr, J.J. A Bacteriophages Journey through the Human Body. Immunol. Rev. 2017, 279, 106–122. [Google Scholar] [CrossRef]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism to Cross Epithelial Cell Layers. mBio 2017, 8. [Google Scholar] [CrossRef]
- Møller-Olsen, C.; Ross, T.; Leppard, K.N.; Foisor, V.; Smith, C.; Grammatopoulos, D.K.; Sagona, A.P. Bacteriophage K1F Targets Escherichia Coli K1 in Cerebral Endothelial Cells and Influences the Barrier Function. Sci. Rep. 2020, 10, 8903. [Google Scholar] [CrossRef]
- Zhu, Y.; Shang, J.; Peng, C.; Sun, Y. Phage Family Classification under Caudoviricetes: A Review of Current Tools Using the Latest ICTV Classification Framework. Front Microbiol. 2022, 13, 1032186. [Google Scholar] [CrossRef]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of Morphology-Based Taxa and Change to Binomial Species Names: 2022 Taxonomy Update of the ICTV Bacterial Viruses Subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Garre-Olmo, J.; Puig, J.; Ramos, R.; Martínez-Hernández, F.; Burokas, A.; Coll, C.; Moreno-Navarrete, J.M.; et al. Caudovirales Bacteriophages Are Associated with Improved Executive Function and Memory in Flies, Mice, and Humans. Cell Host Microbe 2022, 30, 340–356.e8. [Google Scholar] [CrossRef]
- Fernández, L.; Duarte, A.C.; Rodríguez, A.; García, P. The Relationship between the Phageome and Human Health: Are Bacteriophages Beneficial or Harmful Microbes? Benef. Microbes 2021, 12, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Anpilogov, K.; Dhuli, K.; Paolacci, S.; Benedetti, S.; Manara, E.; Guerri, G.; Dautaj, A.; Beccari, T.; Dundar, M.; et al. Naturally-Occurring and Cultured Bacteriophages in Human Therapy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 101–107. [Google Scholar] [PubMed]
- Tao, S.; Wu, Y.; Xiao, L.; Huang, Y.; Wang, H.; Tang, Y.; Liu, S.; Liu, Y.; Ma, Q.; Yin, Y.; et al. Alterations in Fecal Bacteriome Virome Interplay and Microbiota-Derived Dysfunction in Patients with Schizophrenia. Transl. Psychiatry 2025, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of Bacterial and Metabolic Signatures and Their Interaction in Major Depressive Disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef]
- Tetz, G.; Brown, S.M.; Hao, Y.; Tetz, V. Parkinson’s Disease and Bacteriophages as Its Overlooked Contributors. Sci. Rep. 2018, 8, 10812. [Google Scholar] [CrossRef]
- Duru, I.C.; Lecomte, A.; Laine, P.; Shishido, T.K.; Suppula, J.; Paulin, L.; Scheperjans, F.; Pereira, P.A.B.; Auvinen, P. Comparison of Phage and Plasmid Populations in the Gut Microbiota between Parkinson’s Disease Patients and Controls. Sci. Rep. 2025, 15, 13723. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, C.; Li, N.; He, Q.; Gao, G.; Sun, Z. Longitudinal and Multi-Kingdom Gut Microbiome Alterations in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 11472. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ferreira, D.; Maioli, S. A Metagenomic Study of Gut Viral Markers in Amyloid-Positive Alzheimer’s Disease Patients. Alzheimers. Res. Ther. 2023, 15, 141. [Google Scholar] [CrossRef]
- Tomofuji, Y.; Kishikawa, T.; Maeda, Y.; Ogawa, K.; Nii, T.; Okuno, T.; Oguro-Igashira, E.; Kinoshita, M.; Yamamoto, K.; Sonehara, K.; et al. Whole Gut Virome Analysis of 476 Japanese Revealed a Link between Phage and Autoimmune Disease. Ann. Rheum. Dis. 2022, 81, 278–288. [Google Scholar] [CrossRef]
- Chelluboina, B.; Kieft, K.; Breister, A.; Anantharaman, K.; Vemuganti, R. Gut Virome Dysbiosis Following Focal Cerebral Ischemia in Mice. J. Cereb. Blood Flow Metab. 2022, 42, 1597–1602. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, K.; Zhou, H. Characteristics of gut virome and microbiome in patients with stroke. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 862–869. [Google Scholar]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- McOmish, C.E.; Burrows, E.L.; Hannan, A.J. Identifying Novel Interventional Strategies for Psychiatric Disorders: Integrating Genomics, “Enviromics” and Gene-Environment Interactions in Valid Preclinical Models. Br. J. Pharmacol. 2014, 171, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological Insights from 108 Schizophrenia-Associated Genetic Loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and Immunity in Schizophrenia: Implications for Pathophysiology and Treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef]
- Williams, J.A.; Burgess, S.; Suckling, J.; Lalousis, P.A.; Batool, F.; Griffiths, S.L.; Palmer, E.; Karwath, A.; Barsky, A.; Gkoutos, G.V.; et al. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry 2022, 79, 498–507. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Diaz-Pedrero, R.; Lopez-Gonzalez, L.; Monserrat, J.; Barrena-Blázquez, S.; Alvarez-Mon, M.A.; Lahera, G.; Alvarez-Mon, M. Understanding Immune System Dysfunction and Its Context in Mood Disorders: Psychoneuroimmunoendocrinology and Clinical Interventions. Mil. Med. Res. 2024, 11, 80. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Guo, R.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Metagenome-Wide Association of Gut Microbiome Features for Schizophrenia. Nat. Commun. 2020, 11, 1612. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H.; et al. The Gut Microbiome from Patients with Schizophrenia Modulates the Glutamate-Glutamine-GABA Cycle and Schizophrenia-Relevant Behaviors in Mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; et al. A Systematic Review of Gut Microbiota Composition in Observational Studies of Major Depressive Disorder, Bipolar Disorder and Schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of Microbiota from Drug-Free Patients with Schizophrenia Causes Schizophrenia-like Abnormal Behaviors and Dysregulated Kynurenine Metabolism in Mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.C.; Fineran, P.C. A Century of the Phage: Past, Present and Future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Aggarwala, V.; Liang, G.; Bushman, F.D. Viral Communities of the Human Gut: Metagenomic Analysis of Composition and Dynamics. Mob. DNA 2017, 8, 12. [Google Scholar] [CrossRef]
- Yolken, R.H.; Severance, E.G.; Sabunciyan, S.; Gressitt, K.L.; Chen, O.; Stallings, C.; Origoni, A.; Katsafanas, E.; Schweinfurth, L.A.B.; Savage, C.L.G.; et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals with Schizophrenia Differs from that of Controls. Schizophr. Bull. 2015, 41, 1153–1161. [Google Scholar] [CrossRef]
- Selle, K.; Klaenhammer, T.R. Genomic and Phenotypic Evidence for Probiotic Influences of Lactobacillus Gasseri on Human Health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef]
- Luongo, D.; Miyamoto, J.; Bergamo, P.; Nazzaro, F.; Baruzzi, F.; Sashihara, T.; Tanabe, S.; Rossi, M. Differential Modulation of Innate Immunity in Vitro by Probiotic Strains of Lactobacillus Gasseri. BMC Microbiol. 2013, 13, 298. [Google Scholar] [CrossRef]
- Muzaffar, K.; Jan, R.; Ahmad Bhat, N.; Gani, A.; Ahmed Shagoo, M. Commercially Available Probiotics and Prebiotics Used in Human and Animal Nutrition. In Advances in Probiotics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 417–435. ISBN 9780128229095. [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Reyes-Martínez, S.; Segura-Real, L.; Gómez-García, A.P.; Tesoro-Cruz, E.; Constantino-Jonapa, L.A.; Amedei, A.; Aguirre-García, M.M. Neuroinflammation, Microbiota-Gut-Brain Axis, and Depression: The Vicious Circle. J. Integr. Neurosci. 2023, 22, 65. [Google Scholar] [CrossRef]
- Pan, J.-X.; Xia, J.-J.; Deng, F.-L.; Liang, W.-W.; Wu, J.; Yin, B.-M.; Dong, M.-X.; Chen, J.-J.; Ye, F.; Wang, H.-Y.; et al. Diagnosis of Major Depressive Disorder Based on Changes in Multiple Plasma Neurotransmitters: A Targeted Metabolomics Study. Transl. Psychiatry 2018, 8, 130. [Google Scholar] [CrossRef]
- Zheng, P.; Gao, H.C.; Li, Q.; Shao, W.H.; Zhang, M.L.; Cheng, K.; Yang, D.Y.; Fan, S.H.; Chen, L.; Fang, L.; et al. Plasma Metabonomics as a Novel Diagnostic Approach for Major Depressive Disorder. J. Proteome Res. 2012, 11, 1741–1748. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The Microbiome-Gut-Brain Axis in Parkinson Disease—From Basic Research to the Clinic. Nat. Rev. Neurol. 2022, 18, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, C.-C.; Chiang, H.-L.; Liou, J.-M.; Chang, C.-M.; Lu, T.-P.; Chuang, E.Y.; Tai, Y.-C.; Cheng, C.; Lin, H.-Y.; et al. Altered Gut Microbiota and Inflammatory Cytokine Responses in Patients with Parkinson’s Disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Chen, D.; Xu, X.; Li, W.; Li, K.; He, J.; Su, W.; Luo, Q. The Alteration of Intestinal Mucosal α-Synuclein Expression and Mucosal Microbiota in Parkinson’s Disease. Appl. Microbiol. Biotechnol. 2023, 107, 1917–1929. [Google Scholar] [CrossRef]
- Metcalfe-Roach, A.; Cirstea, M.S.; Yu, A.C.; Ramay, H.R.; Coker, O.; Boroomand, S.; Kharazyan, F.; Martino, D.; Sycuro, L.K.; Appel-Cresswell, S.; et al. Metagenomic Analysis Reveals Large-Scale Disruptions of the Gut Microbiome in Parkinson’s Disease. Mov. Disord. 2024, 39, 1740–1751. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. 2020, 35, 1626–1635. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Meng, L.; Jin, L.; Liu, C.; Liang, Y.; Ren, L.; Liu, Y.; Liu, Y.; Liu, S.; et al. Novel Naturally Occurring Autoantibodies Attenuate α-Synuclein Pathology in a Mouse Model of Parkinson’s Disease. Neuropathol. Appl. Neurobiol. 2023, 49, e12860. [Google Scholar] [CrossRef]
- Hong, J.-P.; Shin, S.; Chung, S.H.; Song, M.-C.; Shim, J.-G.; Kim, Y.; Lee, B.; Yeom, M.; Park, H.-J.; Jung, K.-H.; et al. Bacteriophages Targeting Enterococcus Faecalis Enhance the Therapeutic Efficacy of Levodopa in an MPTP-Induced Parkinson’s Disease Mouse Model with E. Faecalis Gut Colonization. Sci. Rep. 2024, 14, 26146. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- Seo, D.-O.; Holtzman, D.M. Current Understanding of the Alzheimer’s Disease-Associated Microbiome and Therapeutic Strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Winslow, W.; Mifflin, M.A. Choline as a Prevention for Alzheimer’s Disease. Aging 2020, 12, 2026–2027. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Zhao, L. L-Lactate Administration Improved Synaptic Plasticity and Cognition in Early 3xTg-AD Mice. Int. J. Mol. Sci. 2025, 26, 1486. [Google Scholar] [CrossRef]
- Veloz Castillo, M.F.; Magistretti, P.J.; Calì, C. L-Lactate: Food for Thoughts, Memory and Behavior. Metabolites 2021, 11, 548. [Google Scholar] [CrossRef]
- Mohammadi, G.; Dargahi, L.; Peymani, A.; Mirzanejad, Y.; Alizadeh, S.A.; Naserpour, T.; Nassiri-Asl, M. The Effects of Probiotic Formulation Pretreatment (Lactobacillus Helveticus R0052 and Bifidobacterium Longum R0175) on a Lipopolysaccharide Rat Model. J. Am. Coll. Nutr. 2019, 38, 209–217. [Google Scholar] [CrossRef]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does Severity of Alzheimer’s Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota Modulation Counteracts Alzheimer’s Disease Progression Influencing Neuronal Proteolysis and Gut Hormones Plasma Levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Leblhuber, F.; Egger, M.; Schuetz, B.; Fuchs, D. Commentary: Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2018, 10, 54. [Google Scholar] [CrossRef]
- Dimant, H.; Sharon, N.; Solomon, B. Modulation Effect of Filamentous Phage on α-Synuclein Aggregation. Biochem. Biophys. Res. Commun. 2009, 383, 491–496. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Gao, H.; Qing, G. Phage Display Derived Peptides for Alzheimer’s Disease Therapy and Diagnosis. Theranostics 2022, 12, 2041–2062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Handley, R.R.; Lehnert, K.; Snell, R.G. From Pathogenesis to Therapeutics: A Review of 150 Years of Huntington’s Disease Research. Int. J. Mol. Sci. 2023, 24, 13021. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Yang, T.; Xu, S.; Li, X.; Liu, L.; Zhou, G.; Yang, S.; Yin, S.; Li, X.-J.; Li, S. Huntington’s Disease: Complex Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 3845. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Cao, K.-A.L.; Judd, L.M.; Li, S.; Renoir, T.; Hannan, A.J. Microbiome Profiling Reveals Gut Dysbiosis in a Transgenic Mouse Model of Huntington’s Disease. Neurobiol. Dis. 2020, 135, 104268. [Google Scholar] [CrossRef]
- Wasser, C.I.; Mercieca, E.-C.; Kong, G.; Hannan, A.J.; McKeown, S.J.; Glikmann-Johnston, Y.; Stout, J.C. Gut Dysbiosis in Huntington’s Disease: Associations among Gut Microbiota, Cognitive Performance and Clinical Outcomes. Brain Commun. 2020, 2, fcaa110. [Google Scholar] [CrossRef]
- Sharma, G.; Biswas, S.S.; Mishra, J.; Navik, U.; Kandimalla, R.; Reddy, P.H.; Bhatti, G.K.; Bhatti, J.S. Gut Microbiota Dysbiosis and Huntington’s Disease: Exploring the Gut-Brain Axis and Novel Microbiota-Based Interventions. Life Sci. 2023, 328, 121882. [Google Scholar] [CrossRef]
- Wronka, D.; Karlik, A.; Misiorek, J.O.; Przybyl, L. What the Gut Tells the Brain-Is There a Link between Microbiota and Huntington’s Disease? Int. J. Mol. Sci. 2023, 24, 4477. [Google Scholar] [CrossRef]
- Gubert, C.; Choo, J.M.; Love, C.J.; Kodikara, S.; Masson, B.A.; Liew, J.J.M.; Wang, Y.; Kong, G.; Narayana, V.K.; Renoir, T.; et al. Faecal Microbiota Transplant Ameliorates Gut Dysbiosis and Cognitive Deficits in Huntington’s Disease Mice. Brain Commun. 2022, 4, fcac205. [Google Scholar] [CrossRef]
- Wasser, C.I.; Mercieca, E.-C.; Kong, G.; Hannan, A.J.; Allford, B.; McKeown, S.J.; Stout, J.C.; Glikmann-Johnston, Y. A Randomized Controlled Trial of Probiotics Targeting Gut Dysbiosis in Huntington’s Disease. J. Huntingtons Dis. 2023, 12, 43–55. [Google Scholar] [CrossRef]
- Khoshnan, A. Gut Microbiota as a Modifier of Huntington’s Disease Pathogenesis. J. Huntingtons Dis. 2024, 13, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Tucker, T.; Ren, H.; Kenan, D.J.; Henderson, B.S.; Keene, J.D.; Strittmatter, W.J.; Burke, J.R. Inhibition of Polyglutamine Protein Aggregation and Cell Death by Novel Peptides Identified by Phage Display Screening. J. Biol. Chem. 2000, 275, 10437–10442. [Google Scholar] [CrossRef] [PubMed]
- Kenan, D.J.; Strittmatter, W.J.; Burke, J.R. Phage Display Screening for Peptides That Inhibit Polyglutamine Aggregation. In Amyloid, Prions, and Other Protein Aggregates, Part C; Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 253–273. ISBN 9780121828189. [Google Scholar]
- Kolkwitz, P.E.; Mohrlüder, J.; Willbold, D. Inhibition of Polyglutamine Misfolding with D-Enantiomeric Peptides Identified by Mirror Image Phage Display Selection. Biomolecules 2022, 12, 157. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin. Proc. 2018, 93, 1617–1628. [Google Scholar] [CrossRef]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic Lateral Sclerosis: A Neurodegenerative Disorder Poised for Successful Therapeutic Translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef]
- Ghadge, G.D.; Kay, B.K.; Drigotas, C.; Roos, R.P. Single Chain Variable Fragment Antibodies Directed against SOD1 Ameliorate Disease in Mutant SOD1 Transgenic Mice. Neurobiol. Dis. 2019, 121, 131–137. [Google Scholar] [CrossRef]
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A Prospective Longitudinal Study on the Microbiota Composition in Amyotrophic Lateral Sclerosis. BMC Med. 2020, 18, 153. [Google Scholar] [CrossRef]
- Federici, S.; Kredo-Russo, S.; Valdés-Mas, R.; Kviatcovsky, D.; Weinstock, E.; Matiuhin, Y.; Silberberg, Y.; Atarashi, K.; Furuichi, M.; Oka, A.; et al. Targeted Suppression of Human IBD-Associated Gut Microbiota Commensals by Phage Consortia for Treatment of Intestinal Inflammation. Cell 2022, 185, 2879–2898.e24. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Ford, H. Clinical Presentation and Diagnosis of Multiple Sclerosis. Clin. Med. 2020, 20, 380–383. [Google Scholar] [CrossRef]
- Metaxakis, A.; Petratou, D.; Tavernarakis, N. Molecular Interventions towards Multiple Sclerosis Treatment. Brain Sci. 2020, 10, 299. [Google Scholar] [CrossRef]
- Glenn, J.D.; Mowry, E.M. Emerging Concepts on the Gut Microbiome and Multiple Sclerosis. J. Interferon Cytokine Res. 2016, 36, 347–357. [Google Scholar] [CrossRef]
- Chu, F.; Shi, M.; Lang, Y.; Shen, D.; Jin, T.; Zhu, J.; Cui, L. Gut Microbiota in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis: Current Applications and Future Perspectives. Mediators Inflamm. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Mowry, E.M.; Glenn, J.D. The Dynamics of the Gut Microbiome in Multiple Sclerosis in Relation to Disease. Neurol. Clin. 2018, 36, 185–196. [Google Scholar] [CrossRef]
- Ma, Y.; Sannino, D.; Linden, J.R.; Haigh, S.; Zhao, B.; Grigg, J.B.; Zumbo, P.; Dündar, F.; Butler, D.; Profaci, C.P.; et al. Epsilon Toxin-Producing Clostridium Perfringens Colonize the Multiple Sclerosis Gut Microbiome Overcoming CNS Immune Privilege. J. Clin. Investig. 2023, 133, e163239. [Google Scholar] [CrossRef]
- Gupta, V.K.; Janda, G.S.; Pump, H.K.; Lele, N.; Cruz, I.; Cohen, I.; Ruff, W.E.; Hafler, D.A.; Sung, J.; Longbrake, E.E. Alterations in Gut Microbiome-Host Relationships after Immune Perturbation in Patients with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200355. [Google Scholar] [CrossRef]
- Yu, X.; Gilden, D.H.; Ritchie, A.M.; Burgoon, M.P.; Keays, K.M.; Owens, G.P. Specificity of Recombinant Antibodies Generated from Multiple Sclerosis Cerebrospinal Fluid Probed with a Random Peptide Library. J. Neuroimmunol. 2006, 172, 121–131. [Google Scholar] [CrossRef]
- Vargas-Sanchez, K.; Vekris, A.; Petry, K.G. DNA Subtraction of in Vivo Selected Phage Repertoires for Efficient Peptide Pathology Biomarker Identification in Neuroinflammation Multiple Sclerosis Model. Biomark. Insights 2016, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Boiziau, C.; Nikolski, M.; Mordelet, E.; Aussudre, J.; Vargas-Sanchez, K.; Petry, K.G. A Peptide Targeting Inflammatory CNS Lesions in the EAE Rat Model of Multiple Sclerosis. Inflammation 2018, 41, 932–947. [Google Scholar] [CrossRef] [PubMed]
- Cortini, A.; Bembich, S.; Marson, L.; Cocco, E.; Edomi, P. Identification of Novel Non-Myelin Biomarkers in Multiple Sclerosis Using an Improved Phage-Display Approach. PLoS ONE 2019, 14, e0226162. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Meka, R.R.; Venkatesha, S.H.; Lees, J.R.; Teesalu, T.; Moudgil, K.D. A Novel CNS-Homing Peptide for Targeting Neuroinflammatory Lesions in Experimental Autoimmune Encephalomyelitis. Mol. Cell. Probes 2020, 51, 101530. [Google Scholar] [CrossRef]
- Li, Y.; Yang, K.-D.; Kong, D.-C.; Li, X.-M.; Duan, H.-Y.; Ye, J.-F. Harnessing Filamentous Phages for Enhanced Stroke Recovery. Front. Immunol. 2023, 14, 1343788. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar]
- Hong, H.-Y.; Choi, J.S.; Kim, Y.J.; Lee, H.Y.; Kwak, W.; Yoo, J.; Lee, J.-T.; Kwon, T.-H.; Kim, I.-S.; Han, H.-S.; et al. Detection of Apoptosis in a Rat Model of Focal Cerebral Ischemia Using a Homing Peptide Selected from in Vivo Phage Display. J. Control. Release 2008, 131, 167–172. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Lei, F.; Wang, Y.; Yang, M.; Mao, C. Highly Effective Stroke Therapy Enabled by Genetically Engineered Viral Nanofibers. Adv. Mater. 2022, 34, e2201210. [Google Scholar] [CrossRef]
- Song, M.; Wu, D.; Hu, Y.; Luo, H.; Li, G. Characterization of an Enterococcus Faecalis Bacteriophage vB_EfaM_LG1 and Its Synergistic Effect with Antibiotic. Front. Cell. Infect. Microbiol. 2021, 11, 698807. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE Classification of the Epilepsies: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Xie, K.; Royer, J.; Rodriguez-Cruces, R.; Horwood, L.; Ngo, A.; Arafat, T.; Auer, H.; Sahlas, E.; Chen, J.; Zhou, Y.; et al. Temporal Lobe Epilepsy Perturbs the Brain-Wide Excitation-Inhibition Balance: Associations with Microcircuit Organization, Clinical Parameters, and Cognitive Dysfunction. Adv. Sci. 2025, 12, e2406835. [Google Scholar] [CrossRef] [PubMed]
- Darch, H.; McCafferty, C.P. Gut Microbiome Effects on Neuronal Excitability & Activity: Implications for Epilepsy. Neurobiol. Dis. 2022, 165, 105629. [Google Scholar]
- Mengoni, F.; Salari, V.; Kosenkova, I.; Tsenov, G.; Donadelli, M.; Malerba, G.; Bertini, G.; Del Gallo, F.; Fabene, P.F. Gut Microbiota Modulates Seizure Susceptibility. Epilepsia 2021, 62, e153–e157. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Cantu, A.; Vieri, M.K.; Cotten, M.; Kellam, P.; Phan, M.; van der Hoek, L.; Mandro, M.; Tepage, F.; Mambandu, G.; et al. No Evidence Known Viruses Play a Role in the Pathogenesis of Onchocerciasis-Associated Epilepsy. An Explorative Metagenomic Case-Control Study. Pathogens 2021, 10, 787. [Google Scholar] [CrossRef]
- Branton, W.G.; Ellestad, K.K.; Maingat, F.; Wheatley, B.M.; Rud, E.; Warren, R.L.; Holt, R.A.; Surette, M.G.; Power, C. Brain Microbial Populations in HIV/AIDS: α-Proteobacteria Predominate Independent of Host Immune Status. PLoS ONE 2013, 8, e54673. [Google Scholar] [CrossRef]
- Gazarian, K.; Rowlay, M.; Gazarian, T.; Vazquez Buchelli, J.E.; Hernández Gonzáles, M. Mimotope Peptides Selected from Phage Display Combinatorial Library by Serum Antibodies of Pigs Experimentally Infected with Taenia Solium as Leads to Developing Diagnostic Antigens for Human Neurocysticercosis. Peptides 2012, 38, 381–388. [Google Scholar] [CrossRef]
| Pathology | Model | Findings | Reference |
|---|---|---|---|
| Schizophrenia | human (patients) | ↓ Suoliviridae, Rountreeviridae Pachyviridae, and Schitoviridae-bacterial correlation profile | [134] |
| Depression | human (patients) | ↓ Clostridium_phage_phi8074-B1 ↓ Klebsiella_phage_vB_KpnP_SU552A ↑ Escherichia_phage_ECBP5 | [135] |
| PD | human (patients) | ↓ Lactococcus spp. ↑ Microviridae ↑ Tectiviridae | [136,137] |
| AD | mice (APP/PS-a model) | ↑ Lahndsivirus rarus (at 3 months) ↑ Lactobacillus prophage Lj771 (at 6 months) ↑ Lactobacillus phage KC5a (at 6 months) ↑ Lactobacillus phage phi jlb1 (at 6 months) ↓ Enquatrovirus N4 (at 3 and 4 months) ↓ Eneladusvirus BF (at 3 and 4 months) ↓ Lactococcus | [138] |
| human (patients) | ↓ Siphoviridae ↓ Lactococcus | [139] | |
| MS | human (patients) | no difference between healthy subjects and patients | [140] |
| Stroke | mice (MCAO model) | ↓ Siphoviridae_u_t ↓ Lactobacillus prophage Lj771_u_t | [141] |
| human (patients) | ↑ Bacteroides phage B40_8 ↑ Cronobacter phage CS01 | [15,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salari, V.; Parrella, E.; Mengoni, F.; Cintra, L.; Bertini, G.; Fabene, P.F. Phage-Microbiota Crosstalk: Implications for Central Nervous System Disorders. Int. J. Mol. Sci. 2025, 26, 6183. https://doi.org/10.3390/ijms26136183
Salari V, Parrella E, Mengoni F, Cintra L, Bertini G, Fabene PF. Phage-Microbiota Crosstalk: Implications for Central Nervous System Disorders. International Journal of Molecular Sciences. 2025; 26(13):6183. https://doi.org/10.3390/ijms26136183
Chicago/Turabian StyleSalari, Valentina, Edoardo Parrella, Francesca Mengoni, Laís Cintra, Giuseppe Bertini, and Paolo Francesco Fabene. 2025. "Phage-Microbiota Crosstalk: Implications for Central Nervous System Disorders" International Journal of Molecular Sciences 26, no. 13: 6183. https://doi.org/10.3390/ijms26136183
APA StyleSalari, V., Parrella, E., Mengoni, F., Cintra, L., Bertini, G., & Fabene, P. F. (2025). Phage-Microbiota Crosstalk: Implications for Central Nervous System Disorders. International Journal of Molecular Sciences, 26(13), 6183. https://doi.org/10.3390/ijms26136183







