Role of Serotonin, Membrane Transporter, and 5-HT2 Receptors in Pathogenesis of Atherosclerotic Plaque Formation in Immature Heterozygous Low-Density Lipoprotein-Receptor-Deficient Mice
Abstract
1. Introduction
2. Results
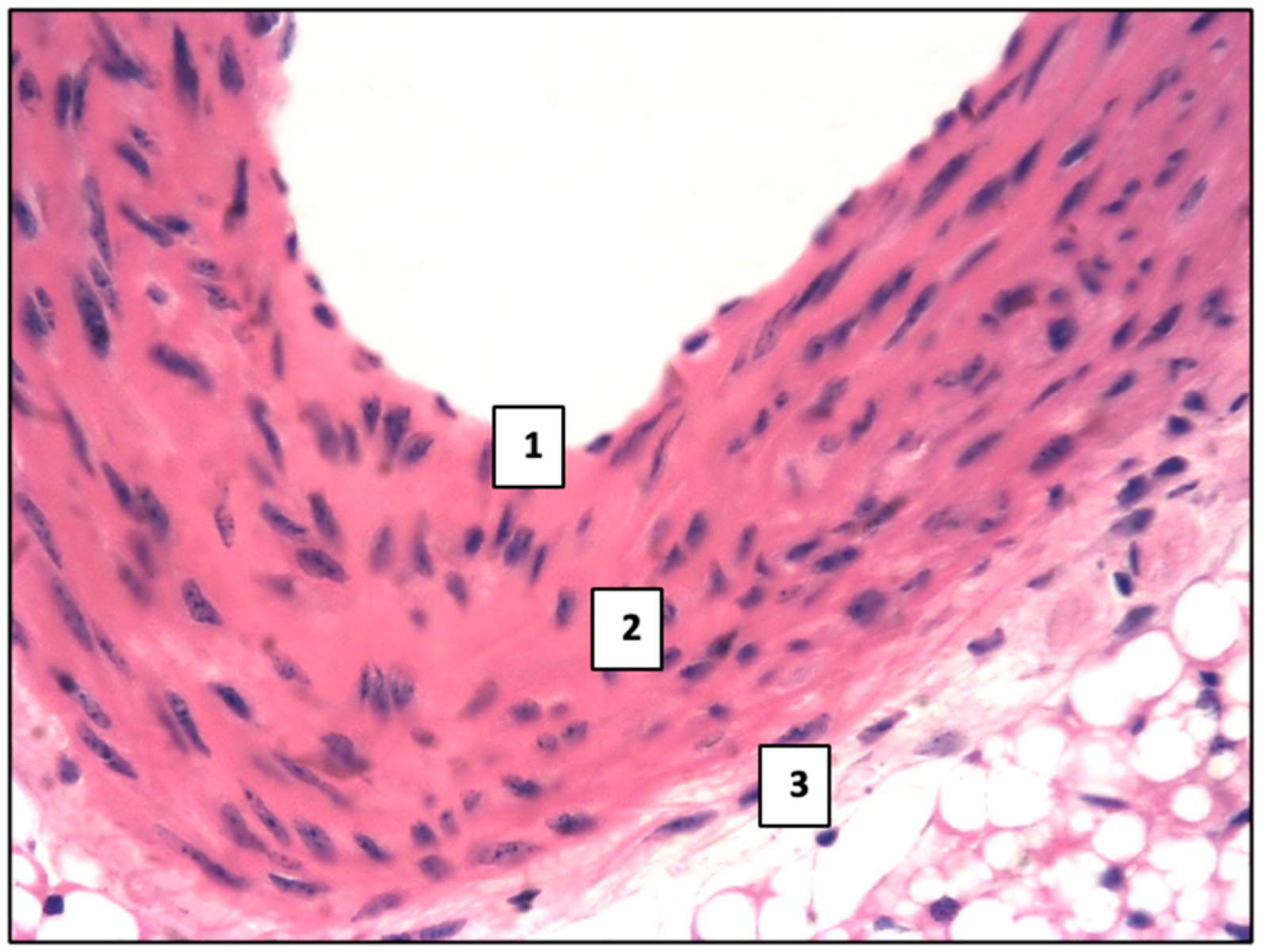
2.1. Control Group
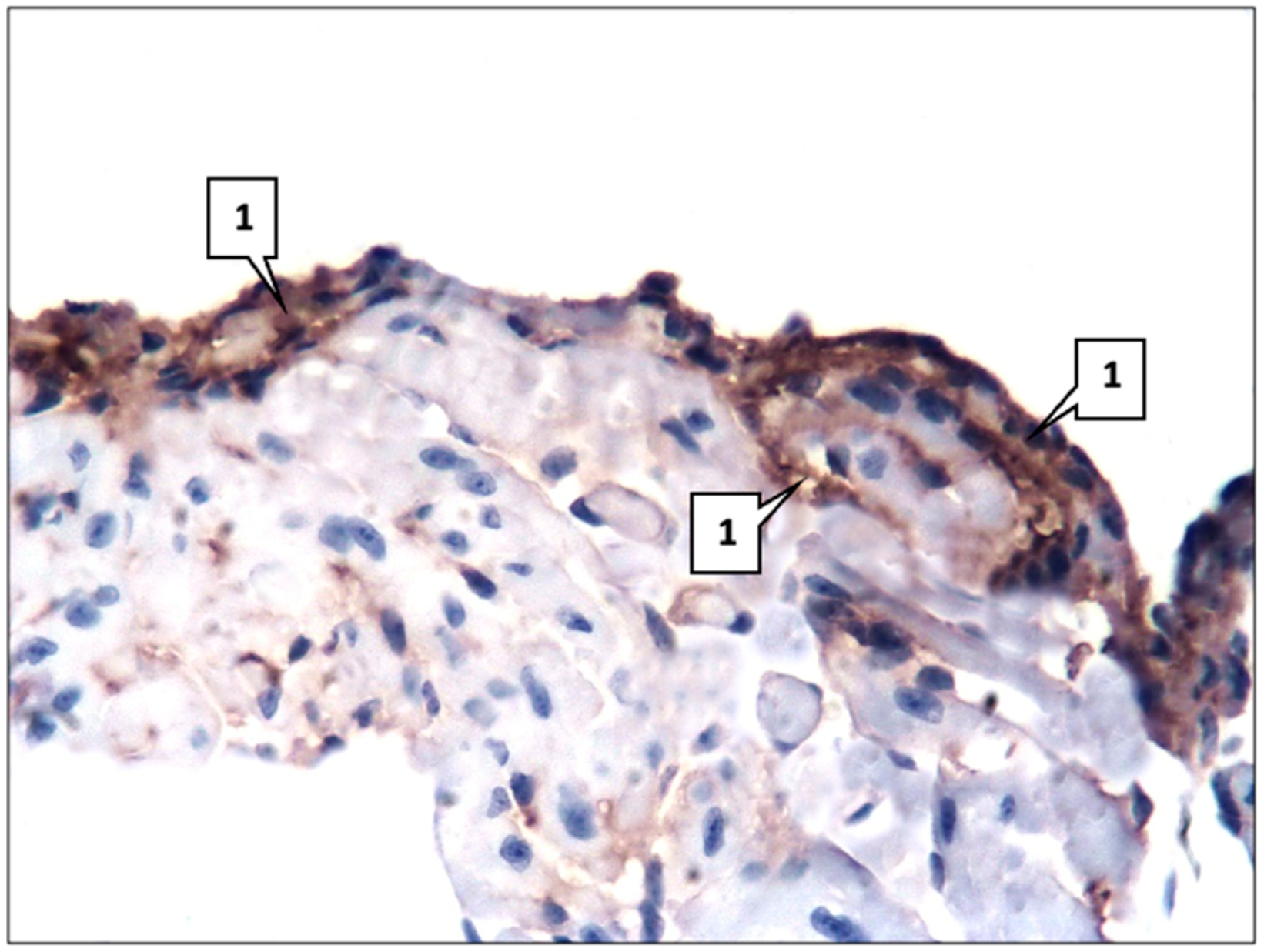
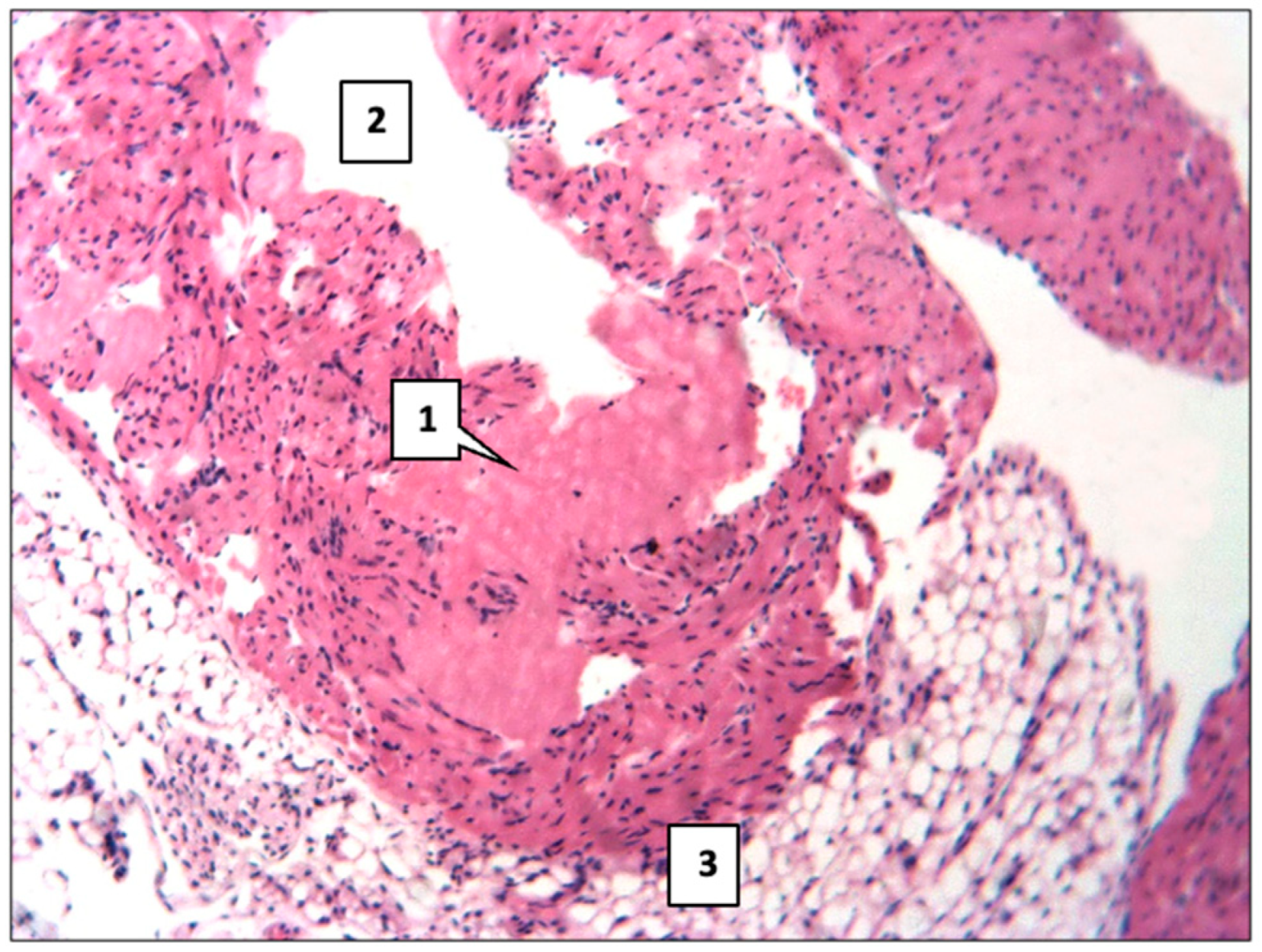
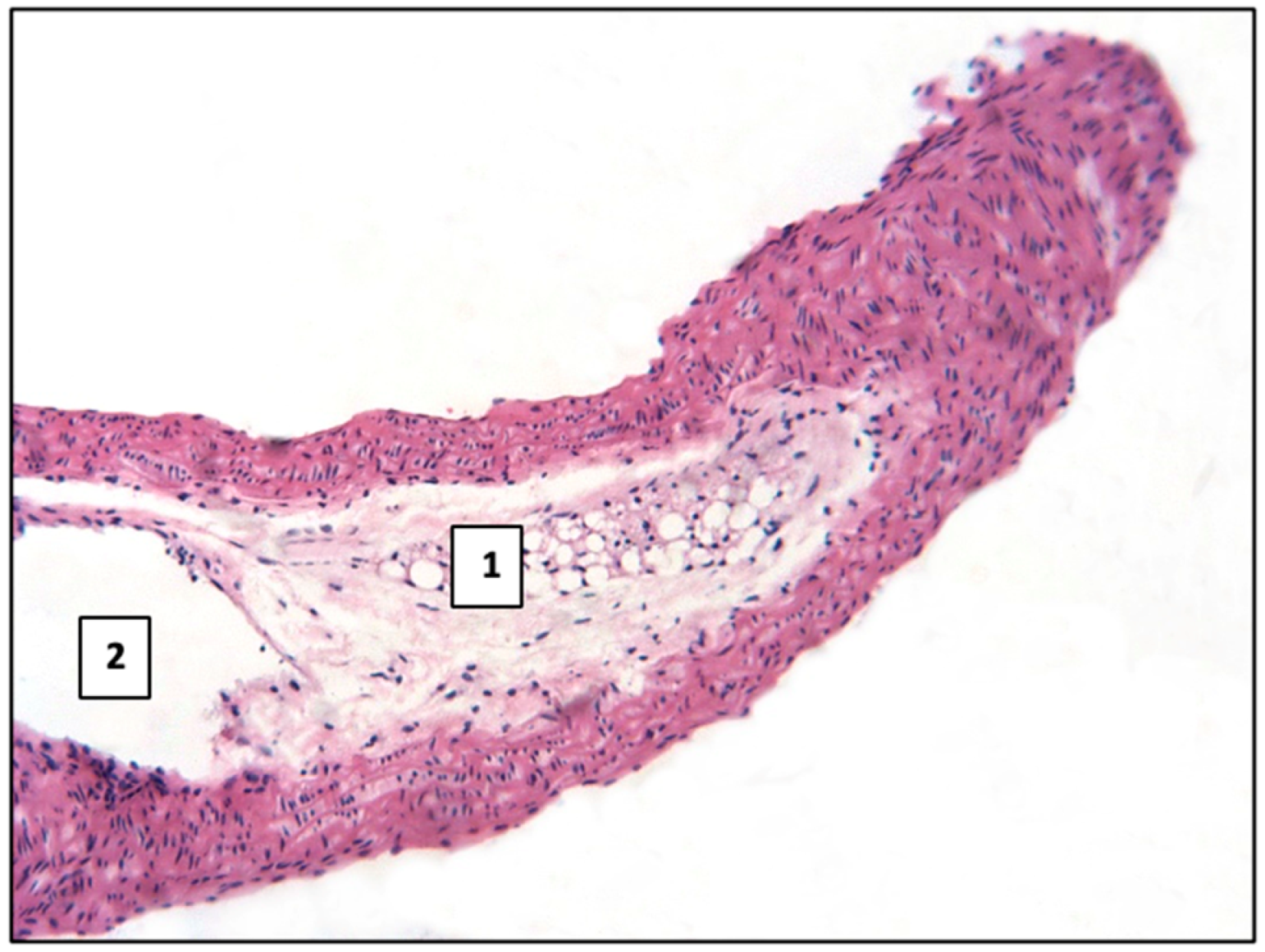
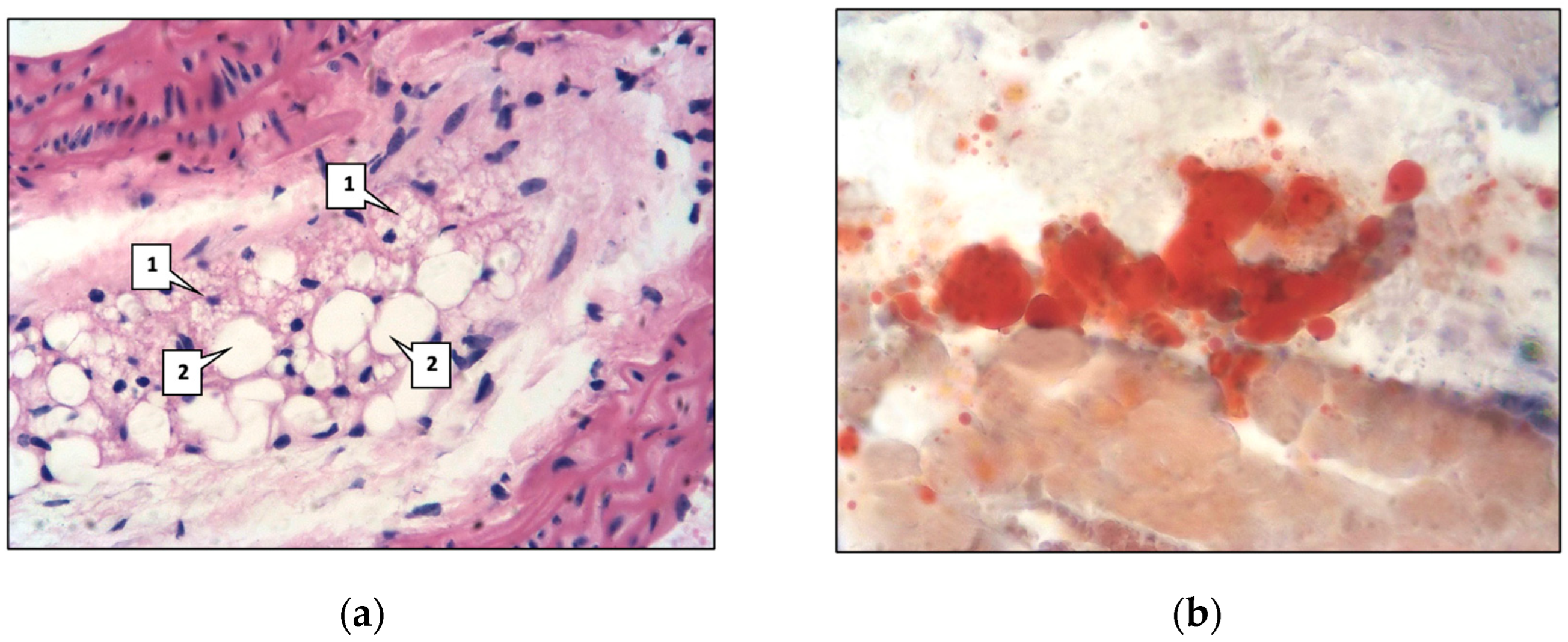
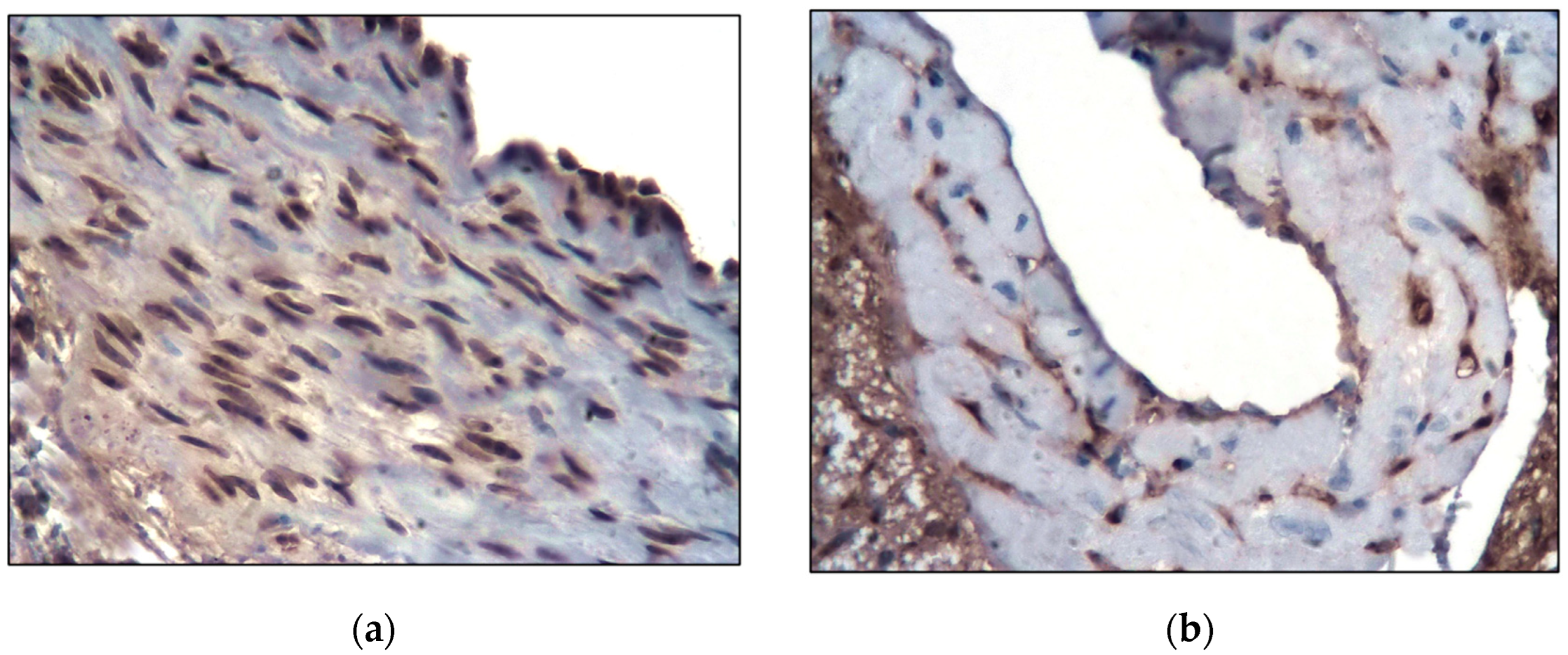
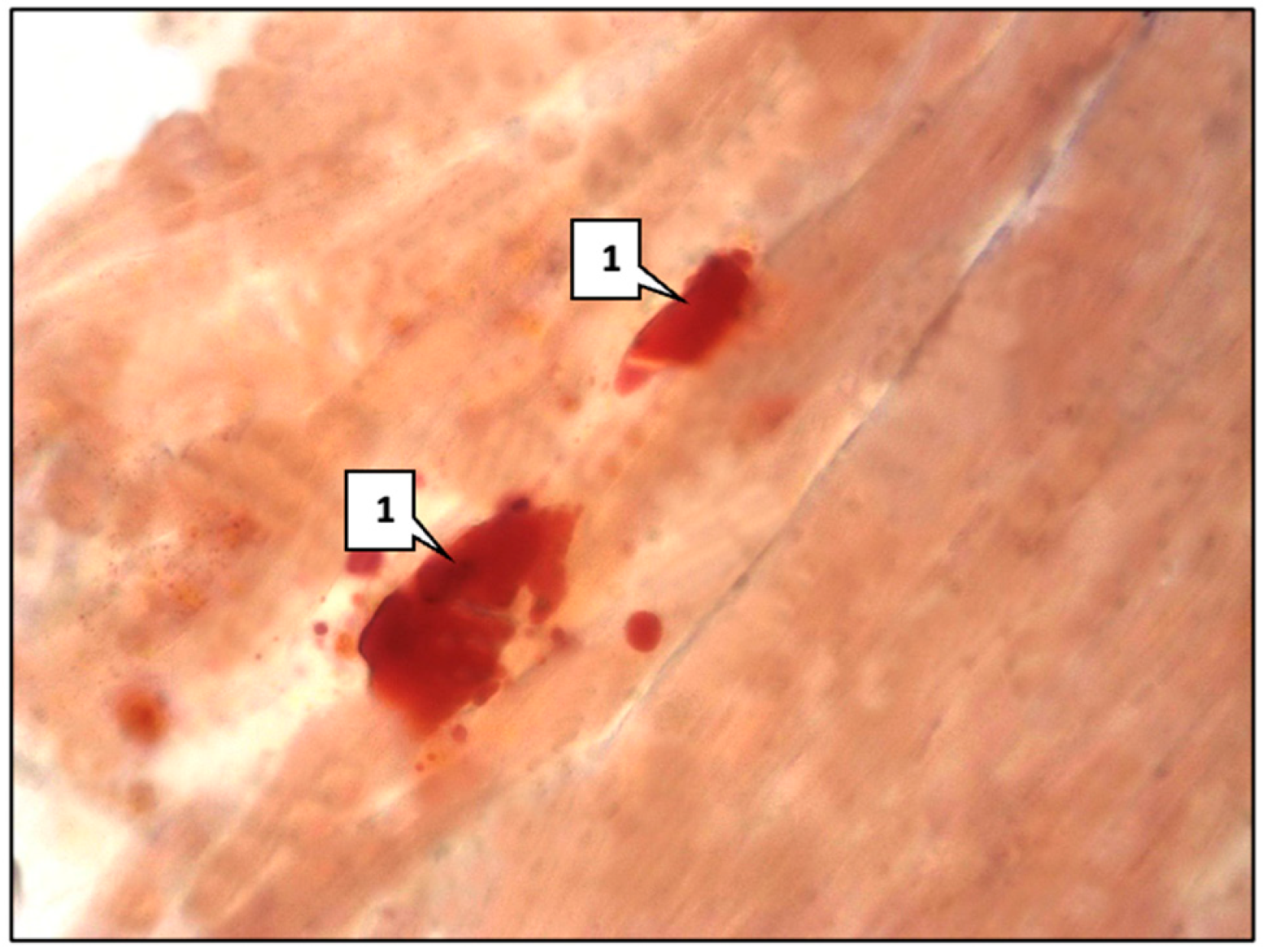
2.2. Main Group
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Morphological and Immunohistochemical Studies
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, P.; Lanas, F.; Roth, G.; Lopez-Jaramillo, P.; Lonn, E.; Miller, V.; Mente, A.; Leong, D.; Schwalm, J.D.; Yusuf, S. Cardiovascular disease in the Americas: The epidemiology of cardiovascular disease and its risk factors. Lancet Reg. Health Am. 2025, 42, 100960. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.E.; Biasucci, G.; Banderali, G.; Vania, A.; Pederiva, C. Diet and Lipid-Lowering Nutraceuticals in Pediatric Patients with Familial Hypercholesterolemia. Children 2024, 11, 250. [Google Scholar] [CrossRef]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lin, H.Y.; Chan, Y.W.; Li, K.H.; To, O.T.; Yan, B.P.; Liu, T.; Li, G.; Wong, W.T.; Keung, W.; et al. Mouse models of atherosclerosis: A historical perspective and recent advances. Lipids Health Dis. 2017, 16, 12. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Do the Apoe-/- and Ldlr-/- Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef]
- Oppi, S.; Lüscher, T.F.; Stein, S. Mouse Models for Atherosclerosis Research-Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar] [CrossRef]
- Sadykova, D.I.; Salakhova, K.R.; Galimova, L.F.; Slastnikova, E.S.; Khaliullina, C.h.D. Familial Hypercholesterolemia in Children. The Current State of the Problem. Curr. Pediatr. 2023, 22, 231–240. [Google Scholar] [CrossRef]
- Mainieri, F.; Tagi, V.M.; Chiarelli, F. Recent advances on Familial Hypercholesterolemia in children and adolescents. Biomedicines 2022, 10, 1043. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Zhang, Y.; Wang, J.; Wang, C.; Liu, Y.; Yang, G.; Yuan, L. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics 2021, 11, 2953–2965. [Google Scholar] [CrossRef]
- Chubykina, U.V.; Ezhov, M.V.; Rozhkova, T.A.; Tamaeva, B.M.; Sokolov, A.A.; Ershova, A.I.; Meshkov, A.N.; Mikhailina, V.I.; Blokhina, A.; Limonova, A.; et al. Compliance of heterozygous familial hypercholesterolemia patients: 5-years follow-up of the RENAISSANCE registry. Russ. Cardiol. Bull. 2023, 18, 35–48. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, 213–256. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R.; Greer, F.R.; Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics 2008, 122, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.; Gauchel, N.; Bode, C.; Duerschmied, D. Serotonin: A platelet hormone modulating cardiovascular disease. J. Thromb. Thrombolysis 2021, 52, 42–47. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Sarkar, P.; Mozumder, S.; Bej, A.; Mukherjee, S.; Sengupta, J.; Chattopadhyay, A. Structure, dynamics and lipid interactions of serotonin receptors: Excitements and challenges. Biophys. Rev. 2020, 13, 101–122. [Google Scholar] [CrossRef]
- Bahr, F.S.; Ricke-Hoch, M.; Ponimaskin, E.; Müller, F.E. Serotonin Receptors in Myocardial Infarction: Friend or Foe? ACS Chem. Neurosci. 2024, 15, 1619–1634. [Google Scholar] [CrossRef]
- Imamdin, A.; van der Vorst, E.P.C. Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1549. [Google Scholar] [CrossRef]
- Neumann, J.; Hofmann, B.; Dhein, S.; Gergs, U. Cardiac Roles of Serotonin (5-HT) and 5-HT-Receptors in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4765. [Google Scholar] [CrossRef]
- Mercado, C.P.; Kilic, F. Molecular mechanisms of SERT in platelets: Regulation of plasma serotonin levels. Mol. Interv. 2010, 10, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ziu, E.; Mercado, C.P.; Li, Y.; Singh, P.; Ahmed, B.A.; Freyaldenhoven, S.; Lensing, S.; Ware, J.; Kilic, F. Down-regulation of the serotonin transporter in hyperreactive platelets counteracts the pro-thrombotic effect of serotonin. J. Mol. Cell. Cardiol. 2012, 52, 1112–1121. [Google Scholar] [CrossRef]
- Fraer, M.; Kilic, F. Serotonin: A different player in hypertension-associated thrombosis. Hypertension 2015, 65, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Sadykova, D.; Nigmatullina, R.; Salakhova, K.; Slastnikova, E.; Galimova, L.; Khaliullina, C.; Valeeva, I. Membrane Transporter of Serotonin and Hypercholesterolemia in Children. Int. J. Mol. Sci. 2024, 25, 767. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, X.; Li, C.; Li, R.; Tong, X.; Zhang, R.; Shan, X.; Yang, J.; Ma, X.; Lu, W.; et al. 5-HT2A Receptor and 5-HT Degradation Play a Crucial Role in Atherosclerosis by Modulating Macrophage Foam Cell Formation, Vascular Endothelial Cell Inflammation, and Hepatic Steatosis. J. Atheroscler. Thromb. 2022, 29, 322–336. [Google Scholar] [CrossRef]
- Frishman, W.H.; Grewall, P. Serotonin and the heart. Ann. Med. 2000, 32, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.; Huang, J.; Huang, N. Structure, Function, and Pharmaceutical Ligands of 5-Hydroxytryptamine 2B Receptor. Pharmaceuticals 2021, 14, 76. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef]
- Parajulee, A.; Kim, K. Structural studies of serotonin receptor family. BMB Rep. 2023, 56, 527–536. [Google Scholar] [CrossRef]
- Shan, J.; Khelashvili, G.; Mondal, S.; Mehler, E.L.; Weinstein, H. Ligand-dependent conformations and dynamics of the serotonin 5-HT(2A) receptor determine its activation and membrane-driven oligomerization properties. PLoS Comput. Biol. 2012, 8, 1002473. [Google Scholar] [CrossRef]
- Sauer, W.H.; Berlin, J.A.; Kimmel, S.E. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003, 108, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Almuwaqqat, Z.; Jokhadar, M.; Norby, F.L.; Lutsey, P.L.; O’Neal, W.T.; Seyerle, A.; Soliman, E.Z.; Chen, L.Y.; Bremner, J.D.; Vaccarino, V.; et al. Association of Antidepressant Medication Type With the Incidence of Cardiovascular Disease in the ARIC Study. J. Am. Heart Assoc. 2019, 8, e012503. [Google Scholar] [CrossRef] [PubMed]
- Silverstein-Metzler, M.G.; Justice, J.N.; Appt, S.E.; Groban, L.; Kitzman, D.W.; Carr, J.J.; Register, T.C.; Shively, C.A. Long-term sertraline treatment and depression effects on carotid artery atherosclerosis in premenopausal female primates. Menopause 2017, 24, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Saleh, C.; Ilia, T.S.; Schöpfer, R.; Seidl, U.; Deraita, J.; Todua-Lennigk, S.; Lieb, J.; Budincevic, H.; Trzcinska, M.; Hovhannisyan, K.; et al. Atherosclerosis and depression: Is carotid intima-media thicker in patients with depression compared to matched control individuals? A systematic review and meta-analysis. J. Psychiatr. Res. 2024, 173, 216–224. [Google Scholar] [CrossRef]
- Francescangeli, J.; Karamchandani, K.; Powell, M.; Bonavia, A. The Serotonin Syndrome: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2019, 20, 2288. [Google Scholar] [CrossRef]
- Chen, S.J.; Cho, R.L.; Yeh, S.H.; Tsai, M.C.; Chuang, Y.P.; Lien, C.F.; Chiu, C.H.; Yeh, Y.W.; Lin, C.S.; Ma, K.H. Pitavastatin attenuates hypercholesterolemia-induced decline in serotonin transporter availability. Lipids Health Dis. 2024, 23, 250. [Google Scholar] [CrossRef]
- Arai, T.; Wang, N.; Bezouevski, M.; Welch, C.; Tall, A.R. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 1999, 274, 2366–2371. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, M.-J.; Cao, Z.; Yang, C.; Wang, J.; Wang, B.; Liu, J.; Wang, Y.; Xian, X.; Zhang, F.; et al. Heterozygous Ldlr-Deficient Hamster as a Model to Evaluate the Efficacy of PCSK9 Antibody in Hyperlipidemia and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5936. [Google Scholar] [CrossRef]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122, 3–14. [Google Scholar] [CrossRef]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- Ezhov, M.V.; Bazhan, S.S.; Ershova, A.I.; Meshkov, A.N.; Sokolov, A.A.; Kukharchuk, V.V.; Gurevich, V.S.; Voevoda, M.I.; Sergienko, I.V.; Shakhtshneider, E.V.; et al. Clinical guidelines for familial hypercholesterolemia. Ateroscleroz 2019, 15, 58–98. [Google Scholar]
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Ragino, Y.u.L.; Volkov, A.M.; Chernyavskyi, A.M. Stages of atherosclerotic plaque development and unstable plaque types: Pathophysiologic and histologic characteristics. Russ. J. Cardiol. 2013, 5, 88–95. [Google Scholar] [CrossRef]
- Ni, W.; Geddes, T.J.; Priestley, J.R.; Szasz, T.; Kuhn, D.M.; Watts, S.W. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br. J. Pharmacol. 2008, 154, 663–674. [Google Scholar] [CrossRef]
- Lairez, O.; Cognet, T.; Schaak, S.; Calise, D.; Guilbeau-Frugier, C.; Parini, A.; Mialet-Perez, J. Role of serotonin 5-HT2A receptors in the development of cardiac hypertrophy in response to aortic constriction in mice. J. Neural. Transm. 2013, 120, 927–935. [Google Scholar] [CrossRef]
- John Jayakumar, J.A.K.; Panicker, M.M. The roles of serotonin in cell adhesion and migration, and cytoskeletal remodeling. Cell Adh. Migr. 2021, 15, 261–271. [Google Scholar] [CrossRef]
- Morotti, A.; Barale, C.; Melchionda, E.; Russo, I. Platelet Redox Imbalance in Hypercholesterolemia: A Big Problem for a Small Cell. Int. J. Mol. Sci. 2022, 23, 11446. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, Y.; Yang, F.; Yang, Y.; Cui, Z.; Shi, D.; Bai, R. Vascular smooth muscle cell phenotypic switching in atherosclerosis. Heliyon 2024, 10, 37727. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lu, Y.; Wang, Z.; Guo, J.; Hou, Y.; Wang, X.; Qin, Z.; Gao, J.; Sun, Z.; Dai, Y.; et al. Atherosclerosis: The Involvement of Immunity, Cytokines and Cells in Pathogenesis, and Potential Novel Therapeutics. Aging Dis. 2023, 14, 1214–1242. [Google Scholar] [CrossRef]
- Déglise, S.; Bechelli, C.; Allagnat, F. Vascular smooth muscle cells in intimal hyperplasia, an update. Front. Physiol. 2023, 13, 1081881. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Nemecek, G.M.; Coughlin, S.R.; Handley, D.A.; Moskowitz, M.A. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc. Natl. Acad. Sci. USA 1986, 83, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Watada, S.; Harada, H.; Matsubara, K.; Obara, H.; Matsumoto, K.; Ando, N.; Kitagawa, Y. Effect of sarpogrelate hydrochloride, a 5-hydroxytryptamine2 receptor antagonist, on allograft arteriosclerosis after aortic transplantation in rats. Transpl. Immunol. 2013, 29, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Sumi, D.; Matsui-Hirai, H.; Fukatsu, A.; Arockia Rani, P.; Kano, H.; Tsunekawa, T.; Iguchi, A. Sarpogrelate HCl, a selective 5-HT2A antagonist, retards the progression of atherosclerosis through a novel mechanism. Atherosclerosis 2003, 168, 23–31. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Mota, K.O.; Elimban, V.; Shah, A.K.; de Vasconcelos, C.M.L.; Bhullar, S.K. Role of Vasoactive Hormone-Induced Signal Transduction in Cardiac Hypertrophy and Heart Failure. Cells 2024, 13, 856. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Setola, V.; Roth, B.L.; Merryman, W.D. Serotonin receptors and heart valve disease—It was meant 2B. Pharmacol. Ther. 2011, 132, 146–157. [Google Scholar] [CrossRef]
- Bender, A.M.; Parr, L.C.; Livingston, W.B.; Lindsley, C.W.; Merryman, W.D. 2B Determined: The Future of the Serotonin Receptor 2B in Drug Discovery. J. Med. Chem. 2023, 66, 11027–11039. [Google Scholar] [CrossRef]
- Nebigil, C.G.; Choi, D.S.; Dierich, A.; Hickel, P.; Le Meur, M.; Messaddeq, N.; Launay, J.M.; Maroteaux, L. Serotonin 2B receptor is required for heart development. Proc. Natl. Acad. Sci. USA 2000, 97, 9508–9513. [Google Scholar] [CrossRef]
- Snider, J.C.; Riley, L.A.; Mallory, N.T.; Bersi, M.R.; Umbarkar, P.; Gautam, R.; Zhang, Q.; Mahadevan-Jansen, A.; Hatzopoulos, A.K.; Maroteaux, L.; et al. Targeting 5-HT2B Receptor Signaling Prevents Border Zone Expansion and Improves Microstructural Remodeling After Myocardial Infarction. Circulation 2021, 143, 1317–1330. [Google Scholar] [CrossRef]
- Fong, F.; Xian, J.; Demer, L.L.; Tintut, Y. Serotonin receptor type 2B activation augments TNF-α-induced matrix mineralization in murine valvular interstitial cells. J. Cell. Biochem. 2021, 122, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Tarbit, E.; Singh, I.; Peart, J.N.; Bivol, S.; Rose’Meyer, R.B. Increased release of serotonin from rat primary isolated adult cardiac myofibroblasts. Sci. Rep. 2021, 11, 20376. [Google Scholar] [CrossRef] [PubMed]
- Mekontso-Dessap, A.; Brouri, F.; Pascal, O.; Lechat, P.; Hanoun, N.; Lanfumey, L.; Seif, I.; Benhaiem-Sigaux, N.; Kirsch, M.; Hamon, M.; et al. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation 2006, 113, 81–89. [Google Scholar] [CrossRef]
- Brenner, B.; Harney, J.T.; Ahmed, B.A.; Jeffus, B.C.; Unal, R.; Mehta, J.L.; Kilic, F. Plasma serotonin levels and the platelet serotonin transporter. J. Neurochem. 2007, 102, 206–215. [Google Scholar] [CrossRef]
- Deveau, C.M.; Rodriguez, E.; Schroering, A.; Yamamoto, B.K. Serotonin transporter regulation by cholesterol-independent lipid signaling. Biochem. Pharmacol. 2021, 183, 114349. [Google Scholar] [CrossRef]
- Ferraro, M.; Masetti, M.; Recanatini, M.; Cavalli, A.; Bottegoni, G. Mapping Cholesterol Interaction Sites on Serotonin Transporter through Coarse-Grained Molecular Dynamics. PLoS ONE 2016, 11, 0166196. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, S.M.; Williams, D.C.; Schloss, P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry 2001, 40, 10507–10513. [Google Scholar] [CrossRef]
- Koterov, A.N.; Ushenkova, L.N.; Zubenkova, E.S.; Wainson, A.A.; Biryukov, A.P. The relationship between the age of the based laboratory animals (mice, rats, hamsters and dogs) and the age of human: Actuality for the age-ralated radiosensitivity problem and the analysis of published data. Med. Radiol. Radiat. Saf. 2018, 63, 5–27. [Google Scholar] [CrossRef]
- GOST 33215-2014; Guidelines for Accommodation and Care of Animals. Environment, Housing and Management. Russian standards Gosstandart: Moscow, Russia, 2014.
- Sarkisov, D.S.; Perov, Y.L. Microscopic equipment. In Manual for Doctors and Laboratory Technicians; Medicine: Moscow, Russia, 1996; 544p. [Google Scholar]
- Petrov, S.V.; Raikhlin, N.T. Guide to Immunohistochemical Diagnostics of Human Tumors; Tatmedia: Kazan, Russia, 2012; 624p. [Google Scholar]
- Stefanov, S.B. Morphometric grid of random step as a means of accelerated measurement of elements of morphogenesis. Cytology 1974, 6, 785–787. [Google Scholar]
- Avtandilov, G.G. Medical Morphometry; Medicine: Moscow, Russia, 1990; 382p. [Google Scholar]



| Control Group | Main Group | p 1 | |
|---|---|---|---|
| SERT + cells | 16.2 ± 1.18 | 25.3 ± 1.97 | <0.05 |
| 5HT2A + cells | 18.9 ± 1.22 | 24.9 ± 1.80 | <0.05 |
| 5HT2B + cells | 21.5 ± 1.46 | 32.4 ± 2.05 | <0.05 |
| Control Group | Main Group | p 1 | |
|---|---|---|---|
| aorta | - | 0.82 ± 0.09 | |
| heart | 0.09 ± 0.02 | 1.7 ± 0.011 | <0.05 |
| Control Group | Main Group | p 1 | |
|---|---|---|---|
| SERT + cells | 27.4 ± 2.13 | 36.3 ± 2.25 | <0.05 |
| 5HT2A + cells | 39.9 ± 2.78 | 48.3 ± 2.99 | <0.05 |
| 5HT2B + cells | 19.5 ± 1.74 | 28.6 ± 2.05 | <0.05 |
| Antigen | Clone | Secondary Antibodies | Working Breeding | Manufacturing Company |
|---|---|---|---|---|
| SERT | polyclonal, code P31645 | Goat Anti-Rabbit IgG (H+L) HRP | 1:200 | Affinity Biosciences (Cincinnati, OH, USA) |
| 5HT2A | polyclonal, code P28223 | Goat Anti-Rabbit IgG (H+L) HRP | 1:250 | Affinity Biosciences (Cincinnati, OH, USA) |
| 5HT2B | polyclonal, code P41595 | Goat Anti-Rabbit IgG (H+L) HRP | 1:200 | Affinity Biosciences (Cincinnati, OH, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadykova, D.; Nigmatullina, R.; Salakhova, K.; Slastnikova, E.; Galimova, L.; Khaliullina, C.; Gafurova, E.; Tsyplakov, D. Role of Serotonin, Membrane Transporter, and 5-HT2 Receptors in Pathogenesis of Atherosclerotic Plaque Formation in Immature Heterozygous Low-Density Lipoprotein-Receptor-Deficient Mice. Int. J. Mol. Sci. 2025, 26, 6184. https://doi.org/10.3390/ijms26136184
Sadykova D, Nigmatullina R, Salakhova K, Slastnikova E, Galimova L, Khaliullina C, Gafurova E, Tsyplakov D. Role of Serotonin, Membrane Transporter, and 5-HT2 Receptors in Pathogenesis of Atherosclerotic Plaque Formation in Immature Heterozygous Low-Density Lipoprotein-Receptor-Deficient Mice. International Journal of Molecular Sciences. 2025; 26(13):6184. https://doi.org/10.3390/ijms26136184
Chicago/Turabian StyleSadykova, Dinara, Razina Nigmatullina, Karina Salakhova, Evgeniia Slastnikova, Liliya Galimova, Chulpan Khaliullina, Elena Gafurova, and Dmitry Tsyplakov. 2025. "Role of Serotonin, Membrane Transporter, and 5-HT2 Receptors in Pathogenesis of Atherosclerotic Plaque Formation in Immature Heterozygous Low-Density Lipoprotein-Receptor-Deficient Mice" International Journal of Molecular Sciences 26, no. 13: 6184. https://doi.org/10.3390/ijms26136184
APA StyleSadykova, D., Nigmatullina, R., Salakhova, K., Slastnikova, E., Galimova, L., Khaliullina, C., Gafurova, E., & Tsyplakov, D. (2025). Role of Serotonin, Membrane Transporter, and 5-HT2 Receptors in Pathogenesis of Atherosclerotic Plaque Formation in Immature Heterozygous Low-Density Lipoprotein-Receptor-Deficient Mice. International Journal of Molecular Sciences, 26(13), 6184. https://doi.org/10.3390/ijms26136184





