DNA Methylation Profile Changes in CpG Islands of Ethylene-Signaling Genes Regulated by Melatonin Were Involved in Alleviating Chilling Injury of Postharvest Tomato Fruit
Abstract
1. Introduction
2. Results
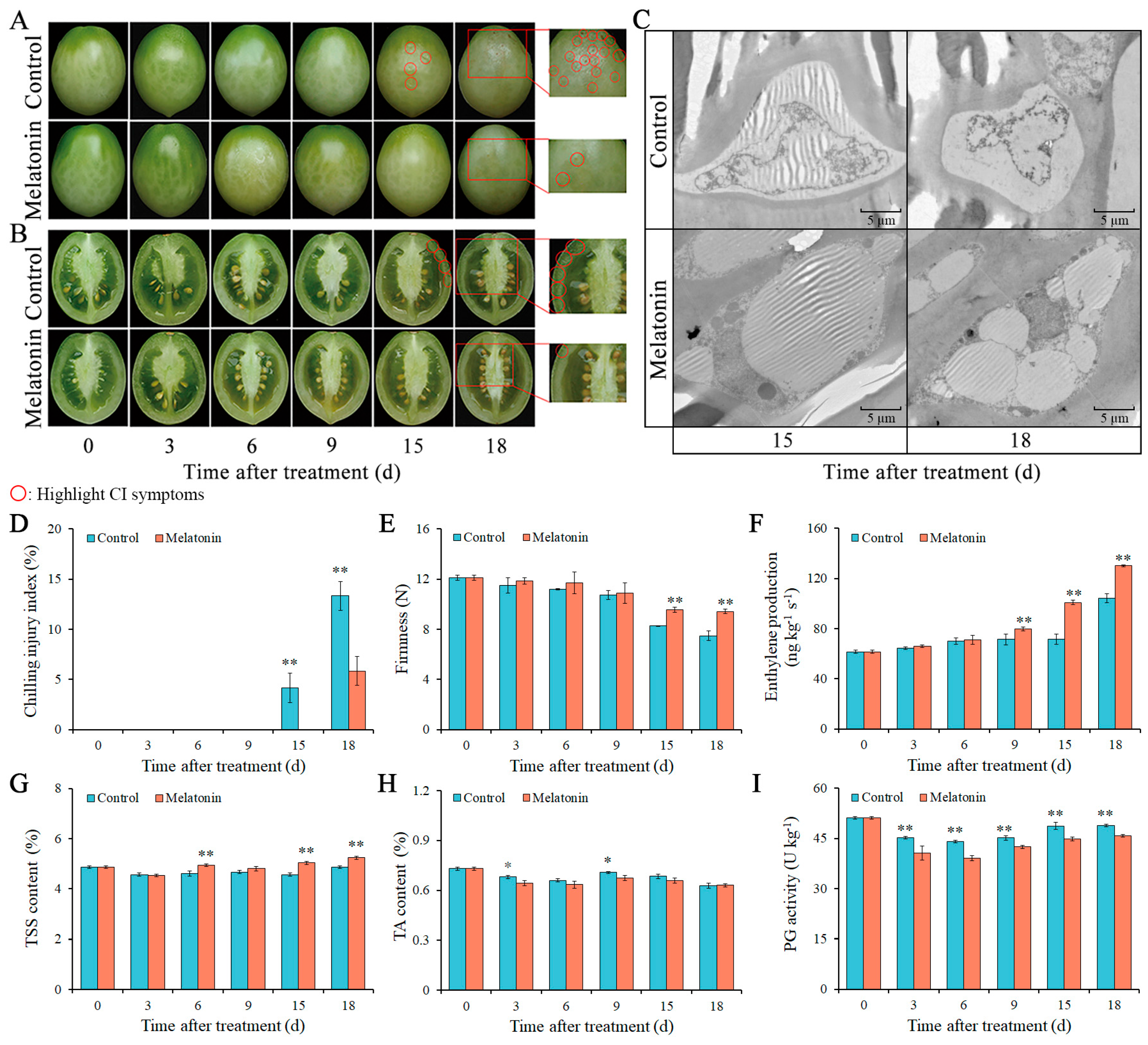
2.1. Effects of MT on CI Symptoms, Cellular Ultrastructure, and CI Index
2.2. Effects of MT on Firmness, Ethylene Production, TSS, TA, and PG Activity
2.3. Electronic Nose Response to Fruit Aroma
2.4. Effects of MT on Phenolic Compounds
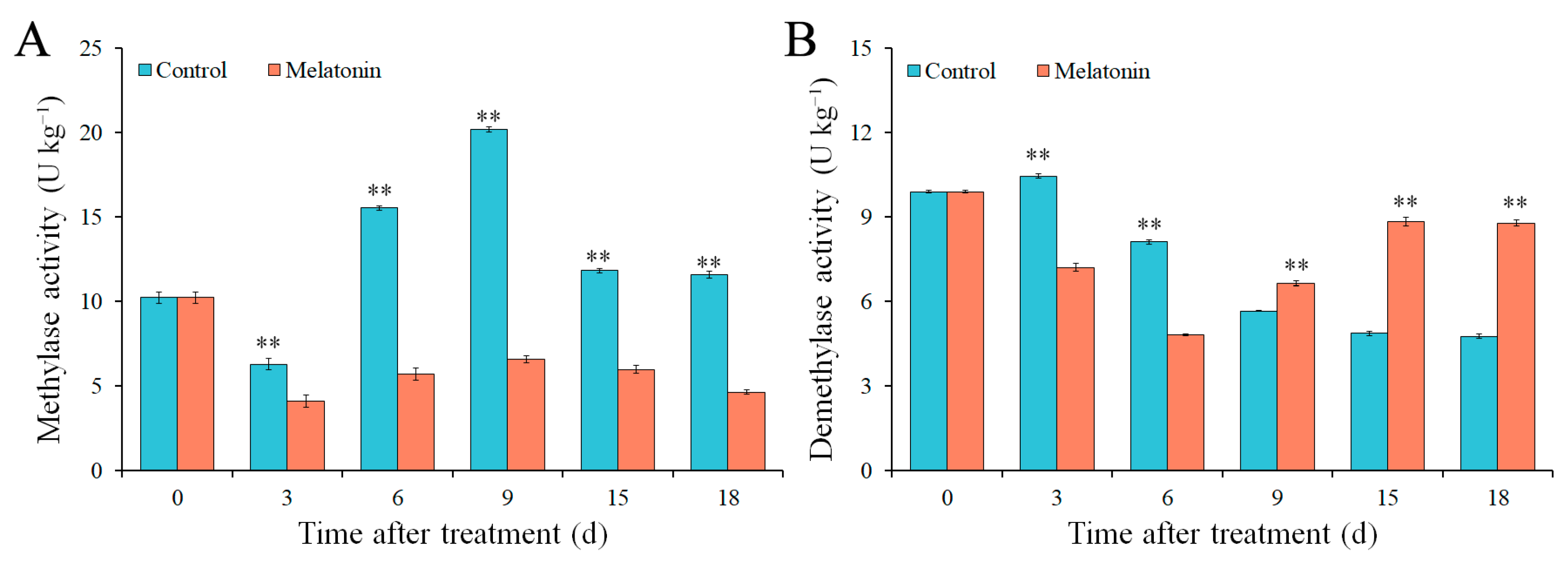
2.5. Effects of MT on Methylase and Demethylase Activity
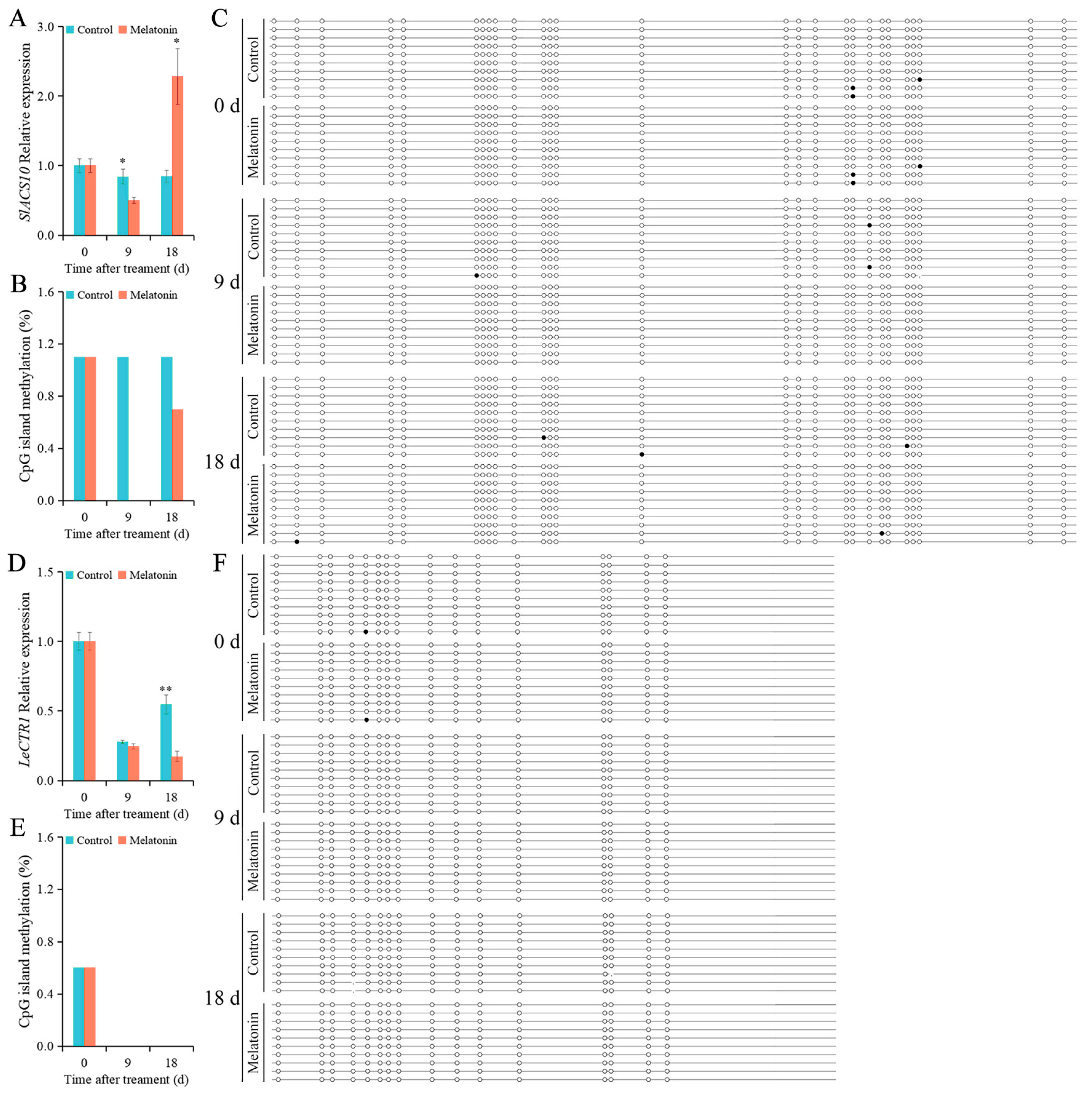
2.6. Effects of MT on the Levels of Genes’ Expression and DNA Methylation
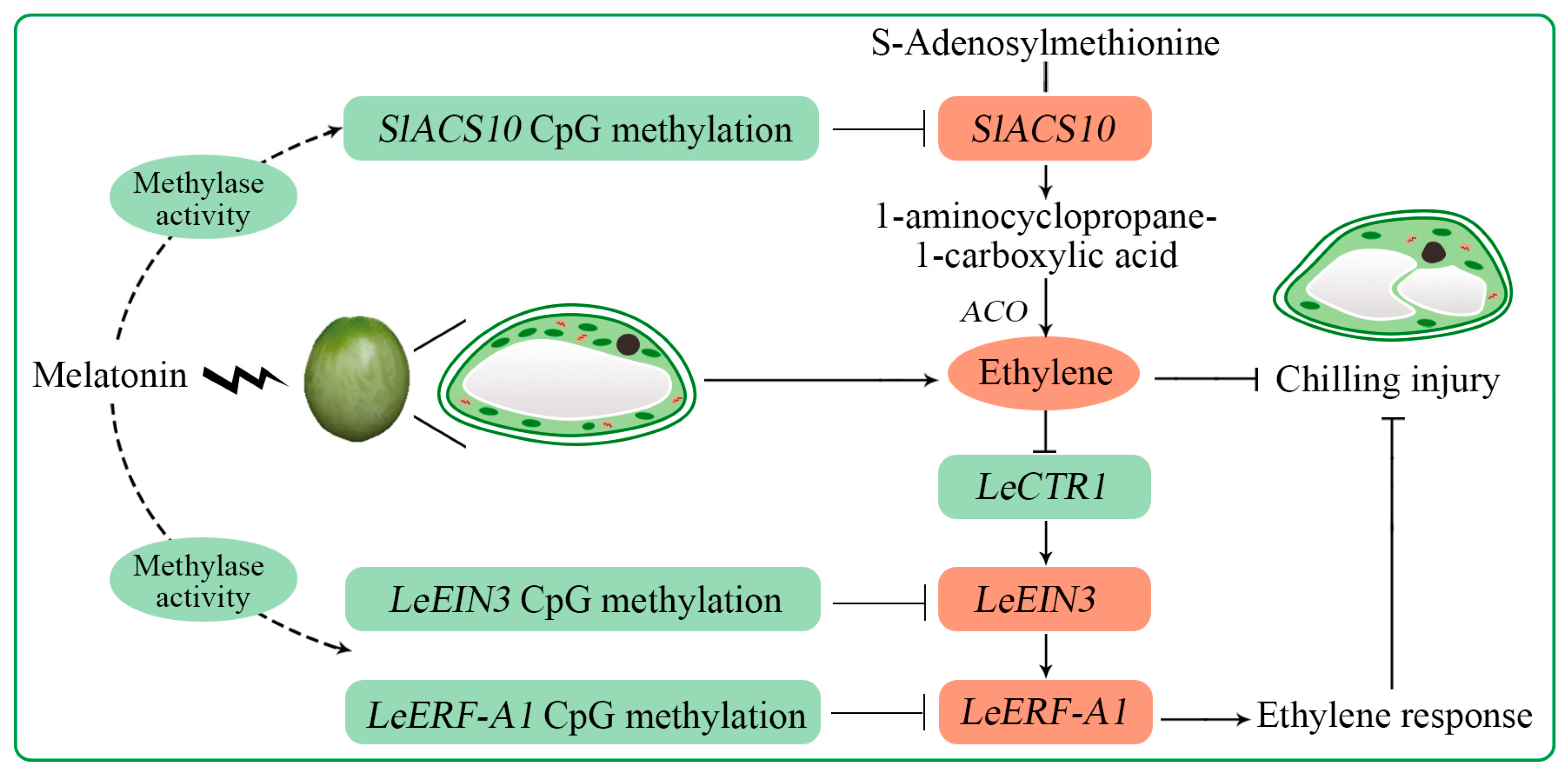
3. Discussion
4. Materials and Methods
4.1. Fruit and Treatment
4.2. Histological Observations of Pericarp
4.3. CI Index
4.4. Firmness, Ethylene Production and Total Soluble Solids, Titratable Acidity, and Polygalacturonase Activity
4.5. Volatile Aroma Evaluation
4.6. Detection of Phenolic Compounds
4.7. Detection of Methylase and Demethylase Activity
4.8. Expression Analysis of Genes Related to Ethylene Signaling
4.9. Bisulfite Sequencing PCR and DNA Methylation Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MT | melatonin |
| CI | chilling injury |
| TSS | total soluble solids |
| TA | titratable acidity |
| PG | polygalacturonase |
| ANOVA | analysis of variance |
| LDA | linear discriminant analysis |
| PCA | principal component analysis |
References
- Sevillano, L.; Sanchez-Ballesta, M.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- McGlasson, W.B.; Scott, K.J.; Mendoza, D.B.J. The refrigerated storage of tropical and subtropical products. Int. J. Air-Cond. Refrig. 1979, 2, 199–206. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, L.; Fan, B.; Liu, K.; Yu, M.; Zheng, Y.; Ding, Y.; Sheng, J. Physiological and genetic properties of tomato fruits from 2 cultivars differing in chilling tolerance at cold storage. J. Food Sci. 2009, 74, C348–C352. [Google Scholar] [CrossRef]
- Hobson, G.E. Low-temperature injury and the storage of ripening tomatoes. J. Hortic. Sci. 1987, 62, 55–62. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Saltveit, M.E. Chilling-injury of harvested tomato (Solanum lycopersicum L.) cv. Micro-Tom fruit is reduced by temperature pre-treatments. Postharvest Biol. Technol. 2012, 63, 123–128. [Google Scholar] [CrossRef]
- Affandi, F.Y.; Verdonk, J.C.; Ouzounis, T.; Ji, Y.; Woltering, E.J.; Schouten, R.E. Far-red light during cultivation induces postharvest cold tolerance in tomato fruit. Postharvest Biol. Technol. 2020, 159, 111019. [Google Scholar] [CrossRef]
- Li, P.; Yin, F.; Song, L.; Zheng, X. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chem. 2016, 202, 125–132. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci. Hortic. 2013, 156, 73–85. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Hot air treatment-induced arginine catabolism is associated with elevated polyamines and proline levels and alleviates chilling injury in postharvest tomato fruit. J. Sci. Food Agric. 2013, 93, 3245–3251. [Google Scholar] [CrossRef]
- Liu, C.; Jahangir, M.M.; Ying, T. Alleviation of chilling injury in postharvest tomato fruit by preconditioning with ultraviolet irradiation. J. Sci. Food Agric. 2012, 92, 3016–3022. [Google Scholar] [CrossRef]
- Min, D.; Li, F.; Zhang, X.; Cui, X.; Shu, P.; Dong, L.; Ren, C. SlMYC2 involved in methyl jasmonate-induced tomato fruit chilling tolerance. J. Agric. Food Chem. 2018, 66, 3110–3117. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Morteza, S.A.; Luo, Z.; Razavi, F. Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food Bioprocess Technol. 2019, 12, 741–750. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Rüdiger, H.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef] [PubMed]
- Pieri, C.; Moroni, F.; Marra, M.; Marcheselli, F.; Recchioni, R. Melatonin is an efficient antioxidant. Arch. Gerontol. Geriatr. 1995, 20, 159–165. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Qian, Y.; Zhu, X.; Tan, D.; Chen, S.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Cao, S.; Song, C.; Shao, J.; Bian, K.; Chen, W.; Yang, Z. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage. J. Agric. Food Chem. 2016, 64, 5215–5222. [Google Scholar] [CrossRef] [PubMed]
- Jannatizadeh, A. Exogenous melatonin applying confers chilling tolerance in pomegranate fruit during cold storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Zhang, Z. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, T.; Zhu, J. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Lang, Z.; Wang, Y.; Tang, K.; Tang, D.; Datsenka, T.; Cheng, J.; Zhang, Y.; Handa, A.K.; Zhu, J. Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc. Natl. Acad. Sci. USA 2017, 114, E4511–E4519. [Google Scholar] [CrossRef] [PubMed]
- Gallusci, P.; Hodgman, C.; Teyssier, E.; Seymour, G.B. DNA methylation and chromatin regulation during fleshy fruit development and ripening. Front. Plant Sci. 2016, 7, 807. [Google Scholar] [CrossRef]
- Henderson, I.R.; Jacobsen, S.E. Epigenetic inheritance in plants. Nature 2007, 447, 418–424. [Google Scholar] [CrossRef]
- Zhou, L.; Tian, S.; Qin, G. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019, 20, 156. [Google Scholar] [CrossRef]
- Telias, A.; Wang, L.; Stevenson, D.E.; Cooney, J.M.; Hellens, R.P.; Allan, A.C.; Hoover, E.E.; Bradeen, J.M. Apple skin patterning is associated with differential expression of MYB10. BMC Plant Biol. 2011, 11, 93. [Google Scholar] [CrossRef]
- Cheng, J.; Niu, Q.; Zhang, B.; Chen, K.; Yang, R.; Zhu, J.; Zhang, Y.; Lang, Z. Downregulation of RdDM during strawberry fruit ripening. Genome Biol. 2018, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, R.; Niu, Q.; Tang, K.; Zhang, B.; Zhang, H.; Chen, K.; Zhu, J.; Lang, Z. Global increase in DNA methylation during orange fruit development and ripening. Proc. Natl. Acad. Sci. USA 2019, 116, 1430. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef]
- Gao, H.; Wang, D.; Cao, M.; Yang, T.; Cao, W.; Yang, Y.; Lu, Z. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2017, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Yu, W.; Sheng, J.; Zhao, R.; Wang, Q.; Ma, P.; Shen, L. Ethylene biosynthesis is involved in regulating chilling tolerance and SLCBF1 gene expression in tomato fruit. Postharvest Biol. Technol. 2019, 149, 139–147. [Google Scholar] [CrossRef]
- Tarun, A.S.; Lee, J.S.; Theologis, A. Random mutagenesis of 1-aminocyclopropane-1-carboxylate synthase: A key enzyme in ethylene biosynthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9796–9801. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Murachi, S.; Okunishi, H.; Shiomi, S.; Nakano, R.; Kubo, Y.; Inaba, A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998, 118, 1295–1305. [Google Scholar] [CrossRef]
- Zuo, J.; Wang, Y.; Zhu, B.; Luo, Y.; Wang, Q.; Gao, L. Comparative analysis of DNA methylation reveals specific regulations on ethylene pathway in tomato fruit. Genes 2018, 9, 266. [Google Scholar] [CrossRef]
- Kieber, J.J.; Rothenberg, M.; Roman, G.; Feldmann, K.A.; Ecker, J.R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 1993, 72, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Ouaked, F.; Rozhon, W.; Lecourieux, D.; Hirt, H. A MAPK pathway mediates ethylene signaling in plants. EMBO J. 2003, 22, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Hutchison, C.E.; Laskey, J.; Kieber, J.J. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 2003, 33, 221–233. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef]
- Tieman, D.M.; Ciardi, J.A.; Taylor, M.G.; Klee, H.J. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J. 2001, 26, 47–58. [Google Scholar] [CrossRef]
- Liu, M.; Gomes, B.L.; Mila, I.; Purgatto, E.; Peres, L.; Frasse, P.; Maza, E.; Zouine, M.; Roustan, J.P.; Bouzayen, M.; et al. Comprehensive profiling of ethylene response factor expression identifies ripening-associated ERF genes and their link to key regulators of fruit ripening in tomato. Plant Physiol. 2016, 170, 1732–1744. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, B.; Xu, W.; Zhu, H.; Chen, A.; Xie, Y.; Shao, Y.; Luo, Y. LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Rep. 2007, 26, 1999–2008. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Ren, H.; Zhang, Z.; Zhang, H.; Wang, J.; Wang, X.; Huang, R. ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis related gene enhances the tolerance under salinity and cold in tobacco. Planta 2007, 226, 815–825. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LEERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Pan, X.; Chen, X.; Zhang, Z.; Lu, X.; Huang, R. Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res. 2011, 20, 857–866. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, T.; Gan, S.; Ren, X.; Fang, L.; Karungo, S.K.; Wang, Y.; Chen, L.; Li, S.; Xin, H. Ethylene positively regulates cold tolerance in grapevine by modulating the expression of Ethylene Response Factor 057. Sci. Rep. 2016, 6, 24066. [Google Scholar] [CrossRef]
- Ouyang, Z.; Liu, S.; Huang, L.; Hong, Y.; Li, X.; Huang, L.; Zhang, Y.; Zhang, H.; Li, D.; Song, F. Tomato SlERF.A1, SlERF.B4, SlERF.C3 and SlERF.A3, members of B3 group of ERF family, are required for resistance to Botrytis cinerea. Front. Plant Sci. 2016, 7, 1964. [Google Scholar] [CrossRef]
- Picton, S.; Gray, J.; Barton, S.; Abubakar, U.; Lowe, A.; Grierson, D. cDNA cloning and characterisation of novel ripening-related mRNAs with altered patterns of accumulation in the ripening inhibitor (rin) tomato ripening mutant. Plant Mol. Biol. 1993, 23, 193–207. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Deng, R.; Shataer, D.; Hong, J.Y.; Wang, L.; Jin, P.; Zhao, Y.T. L-Glutamate treatment alleviates chilling injury of prune (Prunus domestica L.) fruit by regulating ROS homeostasis, GABA shunt, and energy metabolism. Food Chem. 2024, 461, 140899. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wang, C.Y.; Gross, K.C.; Smith, D.L. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 2002, 214, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, M.; Xu, X.; Dong, J.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.; Chen, K.; Fei, Z.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Juan, W.S.; Valdés, H.; Moya-LeÓn, M.A.; Infante, R.; Campos-Vargas, R. The aroma development during storage of castlebrite apricots as evaluated by gas chromatography, electronic nose, and sensory analysis. Postharvest Biol. Technol. 2009, 51, 212–219. [Google Scholar] [CrossRef]
- Duan, W.H.; Ngaffo Mekontso, F.; Li, W.; Tian, J.X.; Li, J.K.; Wang, Q.; Xu, X.B. Alleviation of postharvest rib-edge darkening and chilling injury of carambola fruit by brassinolide under low temperature storage. Sci. Hortic. 2022, 299, 111015. [Google Scholar] [CrossRef]
- Xu, X.; Yin, L.; Ying, Q.; Song, H.; Xue, D.; Lai, T.; Xu, M.; Shen, B.; Wang, H.; Shi, X. High-throughput sequencing and degradome analysis identify miRNAs and their targets involved in fruit senescence of Fragaria ananassa. PLoS ONE 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Sequence (5’-3’) |
|---|---|---|
| GAPDH | Forward Primer Reverse Primer | AGCCACTCAGAAGACCGTTG AGGTCAACCACGGACACATC |
| SlACS10 | Forward Primer Reverse Primer | GCTCAATGCATTTGCAGTCTTG CCACAGGATTCGAGGGGTTAG |
| LeCTR1 | Forward Primer Reverse Primer | GCAGCAGACGGAAGAGAGTT CTGAGCAGGAGCCCAAACA |
| LeEIN3 | Forward Primer Reverse Primer | TTGATCGAAATGGCCCTGCT GGGTGGAGATAACCCCCTTCT |
| SlERF-A1 | Forward Primer Reverse Primer | GGCGAAAAATGGAGCACGAG CCACGAGCAACCTTCTTCCT |
| LeERT10 | Forward Primer Reverse Primer | ATTGAAGCCGCCGTACAGAA CAATCTCACCTCGAAAGCCG |
| Gene | Primer | Sequence (5’-3’) |
|---|---|---|
| SlACS10 | Forward Primer Reverse Primer | GGTTAGGTAGTTGATTGA(C/T)GTTATATT CAAATACCTAAAATTACCCAATAATT |
| LeCTR1 | Forward Primer Reverse Primer | GTATTTGATTTGGATTTGATGGATT TACCAATACATCAATCACAAAATCC |
| LeEIN3 | Forward Primer Reverse Primer | GTGGAGTTTAAGAAGTTGAGTATAAGT CATCATTTTCAACATATACTTCAATAT |
| SlERF-A1 | Forward Primer Reverse Primer | T(C/T)GGAATTTGTGGTTTTATTAGAGA ATTACTTTATTCCAC(G/A)AACAACCTTC |
| LeERT10 | Forward Primer Reverse Primer | TGCAATCTCTATGTGATGAAATCAACT TCATAACTAGTCATTTCAAGTTCAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Shan, S.; Li, J.; Zhang, Z.; Yang, J.; Zhang, W.; Song, H.; Xu, X.; Duan, W. DNA Methylation Profile Changes in CpG Islands of Ethylene-Signaling Genes Regulated by Melatonin Were Involved in Alleviating Chilling Injury of Postharvest Tomato Fruit. Int. J. Mol. Sci. 2025, 26, 6170. https://doi.org/10.3390/ijms26136170
Yan J, Shan S, Li J, Zhang Z, Yang J, Zhang W, Song H, Xu X, Duan W. DNA Methylation Profile Changes in CpG Islands of Ethylene-Signaling Genes Regulated by Melatonin Were Involved in Alleviating Chilling Injury of Postharvest Tomato Fruit. International Journal of Molecular Sciences. 2025; 26(13):6170. https://doi.org/10.3390/ijms26136170
Chicago/Turabian StyleYan, Jingrui, Shuangshuang Shan, Jiangkuo Li, Zhengke Zhang, Jiali Yang, Wanli Zhang, Hongmiao Song, Xiangbin Xu, and Wenhui Duan. 2025. "DNA Methylation Profile Changes in CpG Islands of Ethylene-Signaling Genes Regulated by Melatonin Were Involved in Alleviating Chilling Injury of Postharvest Tomato Fruit" International Journal of Molecular Sciences 26, no. 13: 6170. https://doi.org/10.3390/ijms26136170
APA StyleYan, J., Shan, S., Li, J., Zhang, Z., Yang, J., Zhang, W., Song, H., Xu, X., & Duan, W. (2025). DNA Methylation Profile Changes in CpG Islands of Ethylene-Signaling Genes Regulated by Melatonin Were Involved in Alleviating Chilling Injury of Postharvest Tomato Fruit. International Journal of Molecular Sciences, 26(13), 6170. https://doi.org/10.3390/ijms26136170






