Abstract
Age-related macular degeneration (AMD) is a progressive retinal disorder and a leading cause of irreversible blindness among elderly individuals, impacting millions of people globally. This review synthesizes the current understanding of the cellular and molecular signaling mechanisms driving AMD, with a focus on the distinct pathophysiological features of dry and wet AMD subtypes. Key mechanisms include oxidative stress, inflammation, lipid metabolism dysregulation, and immune dysregulation, all of which converge on the retinal pigment epithelium (RPE) as a central player in disease initiation and progression. In dry AMD, oxidative damage, mitochondrial dysfunction, and lipofuscin accumulation impair RPE function, contributing to drusen formation and geographic atrophy. In wet AMD, vascular endothelial growth factor-mediated angiogenesis, coupled with inflammation and endothelial metabolic reprogramming, drives choroidal neovascularization. This article integrates findings from multiomics approaches and highlights the potential of artificial intelligence in elucidating AMD pathogenesis and advancing personalized therapies. Future research directions emphasize targeting these molecular pathways to develop innovative treatments, offering hope for improved management of this debilitating condition.
1. Introduction
Age-related macular degeneration (AMD) is a leading cause of vision loss in individuals over 55 years of age in developed countries [1]. With an aging global population, the prevalence of AMD is projected to rise, with estimates suggesting that by 2040, approximately 288 million people will be affected worldwide [1], posing significant challenges to healthcare systems and quality of life [2]. The disease manifests in two primary forms: dry AMD, characterized by gradual atrophy of the retinal pigment epithelium (RPE) and photoreceptors, and wet AMD, characterized by aberrant choroidal neovascularization (CNV) [3]. These pathological changes disrupt the retina’s neurovascular–immune microenvironment, resulting in progressive visual impairment.
The pathophysiology of AMD is complex and is driven by oxidative stress, chronic inflammation, lipid metabolism abnormalities, and immune dysregulation [4]. The RPE, a critical layer supporting photoreceptor function and retinal homeostasis, is particularly susceptible. In dry AMD, oxidative damage and lipid-rich drusen impair RPE integrity [4], whereas in wet AMD, inflammatory and angiogenic processes accelerate disease progression [5]. Although treatments such as anti-vascular endothelial growth factor (VEGF) agents for wet AMD and emerging complement inhibitors for dry AMD have advanced care, limitations persist, including inconsistent treatment responses and the absence of effective early-stage interventions.
This review integrates current findings on the cellular and molecular mechanisms of AMD, focusing on the interplay among oxidative stress, inflammation, lipid dysregulation, and angiogenesis. Drawing on insights from multiomics and advanced imaging, this review examines the roles of the RPE, immune system, and metabolic pathways in disease pathogenesis. The review is structured to first explore the RPE and oxidative stress, followed by investigations of inflammation, lipid metabolism, and the angiogenic mechanisms specific to wet AMD. Finally, we outline future directions, emphasizing the potential of multiomics and artificial intelligence to enhance AMD diagnosis and treatment. Our goal is to provide a clear framework for understanding AMD and inspire innovative treatment approaches.
2. Oxidative Stress
2.1. Sources of Oxidative Stress
The retina experiences significant oxidative stress from both endogenous and exogenous sources (Figure 1). Endogenous stressors arise from the unique anatomical and metabolic properties of the retina, which create a microenvironment highly conducive to reactive oxygen species (ROS) generation. Endogenous stressors: (1) High oxygen demand: The outer retina–RPE complex sustains elevated metabolic activity, particularly in photoreceptors, which demand high oxygen levels and consequently produce substantial amounts of ROS [6]. (2) Chronic phototoxicity: Prolonged exposure to ultraviolet/blue light combined with abundant photosensitizers (e.g., retinaldehyde, zeaxanthin) in the neurosensory retina and RPE drives photochemical oxidation. (3) Lipid peroxidation-prone membranes: Photoreceptor outer segments contain high concentrations of polyunsaturated fatty acids (PUFAs). These lipids act as primary targets for ROS-mediated peroxidation, initiating cytotoxic chain reactions that directly impair RPE function [6].
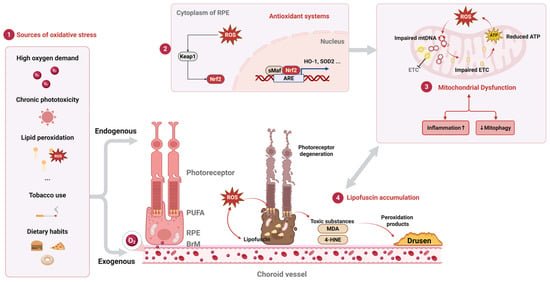
Figure 1.
Oxidative stress pathways and RPE dysfunction in AMD. Schematic depicting key sources of oxidative stress (part 1), antioxidant defense mechanisms (including Keap1/Nrf2/ARE pathway) (part 2), and consequences of oxidative damage in the retinal pigment epithelium (RPE) during AMD pathogenesis (part 3). Mitochondrial dysfunction (part 4): lipofuscin accumulation. ROS, reactive oxygen species; PUFA, polyunsaturated fatty acids; RPE, retinal pigment epithelium; BrM, Bruch’s membrane; Nrf2, nuclear factor erythroid 2-related factor 2; Keap1, Kelch-like ECH-associated protein 1; ARE, antioxidant response element; sMaf, small Maf protein; HO-1, heme oxygenase-1; SOD2, superoxide dismutase 2; mtDNA, mitochondrial DNA; ETC, electron transport chain; ATP, adenosine triphosphate; 4-HNE, 4-hydroxynonenal; MDA, malondialdehyde. Upward arrow (↑) indicates enhancement/increase; downward arrow (↓) indicates reduction/decrease.
Exogenous factors further exacerbate the retina’s oxidative load. Cigarette smoke (Figure 1), a major contributor [7], delivers more than 4700 chemicals—including approximately 1015 free radicals per puff—directly into systemic circulation [8]. These oxidants overwhelm endogenous antioxidant defenses, depleting critical molecules such as ascorbic acid and glutathione while also disrupting protein thiols and inducing oxidative modifications to DNA, lipids, and proteins. Although a direct causal link between smoking and AMD remains unconfirmed, its role in amplifying systemic oxidative stress is well documented [9]. Dietary habits also influence retinal oxidative balance: high-fat diets, particularly those rich in saturated fats, promote lipoprotein oxidation and foster a pro-oxidative microenvironment. While less impactful than smoking, such dietary patterns represent a modifiable risk factor for AMD, underscoring the interplay between lifestyle choices and retinal health [9].
2.2. Antioxidant Systems
The retina employs a sophisticated network of antioxidant defenses to counteract its inherent oxidative burden. These systems spatially and temporally regulate ROS to prevent widespread damage. For example, oxidant-producing enzymes localize near their molecular targets, whereas oxidants such as hydrogen peroxide (H2O2) enter cells through regulated pathways such as aquaporin channels, ensuring controlled activity [10]. The transient nature of ROS—evident in their short half-life and brief production bursts, such as during NADPH oxidase (NOX)-dependent signaling—further confines their effects to specific cellular compartments. This spatial restriction allows the localized neutralization of excess ROS before they inflict systemic harm.
At the cellular level [11], oxidative stress triggers a robust antioxidant response in RPE cells [12], mediated by the Keap1/Nrf2 pathway (Figure 1, part 2) [13]. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) serves as a master regulator of cellular antioxidant defenses. Under basal conditions, Nrf2 is constitutively bound to Kelch-like ECH-associated protein 1 (KEAP1), which targets it for ubiquitin-mediated degradation. However, elevated oxidative stress disrupts this interaction, liberating Nrf2. Subsequently, Nrf2 translocates to the nucleus where it heterodimerizes with transcriptional coactivators, such as small Maf proteins (sMAF). This complex binds to antioxidant response elements (AREs) within the promoter regions of target genes, initiating their transcription. Key antioxidant genes activated by Nrf2 include heme oxygenase-1 (HO-1), which degrades heme to release iron and the antioxidant biliverdin, and superoxide dismutase 2 (SOD2), which catalyzes the conversion of superoxide anion into hydrogen peroxide (H2O2). In AMD, this critical defense mechanism is compromised by the dysregulation of Nrf2 signaling. This dysregulation, often resulting from enhanced KEAP1-mediated ubiquitination or the impaired nuclear translocation of Nrf2, renders RPE cells vulnerable to an unchecked accumulation of ROS [14]. Experimental evidence underscores the critical role of Nrf2: Nrf2-deficient mice exhibit accelerated retinal degeneration, whereas the pharmacological activation of Nrf2—through the use of compounds such as sulforaphane—reduces oxidative injury and preserves retinal integrity, suggesting therapeutic potential for AMD [14].
Notably, the Keap1/Nrf2 pathway intersects with autophagy, a cellular recycling process that degrades oxidatively damaged components. Autophagy serves as a secondary protective mechanism [15], clearing dysfunctional organelles and protein aggregates generated by oxidative stress [16]. Compromised autophagy in RPE cells disrupts this quality control system [17], leading to the accumulation of toxic debris and contributing to AMD-like pathology in preclinical models [18].
2.3. Oxidative Damage in the RPE
RPE cells face substantial oxidative stress due to their unique physiological roles and microenvironment. One primary source is their exposure to high ambient oxygen levels, driven by the retina’s exceptional metabolic demands. The macula, in particular, receives some of the highest blood flows in the body, subjecting the RPE to oxygen partial pressures of 70–90 mm Hg [19]. This oxygen-rich environment inherently elevates the risk of oxidative damage. Additionally, the RPE performs the critical task of phagocytosing approximately 30,000 photoreceptor outer segments daily [20]. During this process, phagosomal NADPH oxidase and the peroxisomal β-oxidation of outer segment lipids generate intracellular H2O2 at levels comparable to those observed in activated macrophages [21], further amplifying oxidative stress [22]. Photo-oxidative stress from processing light for vision is perhaps the most unique and additional source of oxidative stress. Photo-oxidative stress represents another distinct challenge. The role of the RPE in processing light for vision exposes it to continuous photochemical damage, which directly contributes to oxidative injury in the retina, RPE, and choroid [23]. In response to these external stressors, the RPE itself produces high levels of physiological ROS because of its intense metabolic activity. Its dense mitochondrial network, which is essential for meeting energy demands, serves as a major endogenous source of ROS. While mitochondrial ROS participate in normal signaling, aging exacerbates their harmful effects: older mitochondria not only generate more ROS, but also render cells increasingly vulnerable to oxidative damage.
Drusen formation, a hallmark of early AMD, arises from the progressive accumulation of basal linear deposits (BLinD) and basal laminar deposits (BLamD) beneath the RPE. Proteomic analyses identify drusen as extracellular deposits rich in lipids, complement proteins (e.g., C3, C5b-9), and inflammatory mediators such as amyloid-β (Aβ) [24], with lipids constituting more than 40% of the drusen volume [25].
2.3.1. Mitochondrial Dysfunction as a Central Driver of Oxidative Stress
Mitochondria are essential organelles responsible for adenosine triphosphate (ATP) production via oxidative phosphorylation. This process involves electron transfer through multi-subunit complexes (I to IV) of the electron transport chain (ETC), coupled with molecular oxygen reduction. Under physiological conditions, 2–5% of oxygen undergoes incomplete reduction, generating elevated ROS and superoxide [26]. Mitochondrial dysfunction critically underpins oxidative stress in the RPE, serving as a major source of ROS. This dysfunction plays a pivotal role in the pathogenesis of AMD through interconnected mechanisms: oxidative stress, mitochondrial DNA (mtDNA) damage, impaired autophagy/mitophagy, and chronic inflammation [27].
In AMD, ROS production is exacerbated [28], inducing oxidative damage to mtDNA—which lacks protective histones and resides near ROS-generating ETC complexes (Figure 1, part 3) [29]. Such damage is more extensive in AMD patients than in age-matched controls [28], impairing ETC subunit genes, reducing ATP synthesis, and amplifying ROS generation in a self-perpetuating vicious cycle [29]. Furthermore, mtDNA repair mechanisms (e.g., base excision repair, BER) exhibit reduced efficiency in AMD, promoting lesion accumulation [30].
Autophagy, particularly the selective clearance of damaged mitochondria via PINK1-Parkin-mediated mitophagy [31], is also compromised [32]. RPE-specific autophagy knockouts recapitulate AMD phenotypes, confirming this impairment. Mitophagy failure—linked to the dysregulation of regulators like NFE2L2 and PGC-1α—results in the accumulation of dysfunctional mitochondria, lipofuscin, and drusen, all pathological hallmarks of AMD [33]. Concurrently, damaged mitochondria activate the NLRP3 inflammasome [34], triggering the release of pro-inflammatory cytokines (e.g., IL-1β, IL-18) that exacerbate RPE degeneration [35].
2.3.2. Lipofuscin Accumulation
Lipofuscin accumulates in the RPE as a byproduct of light-driven vitamin A recycling in the visual cycle. This fluorescent pigment, which is primarily composed of the bisretinoid N-retinyl-N-retinylidene ethanolamine (A2E) [36], progressively builds up with age and is implicated in AMD pathogenesis (Figure 1, part 4) [37], although its precise role remains under investigation [38]. The essential role of the RPE in the phagocytosis of lipid-rich photoreceptor outer segments becomes compromised over time, leading to the detection of cytotoxic lipofuscin deposits via fundus autofluorescence imaging. A2E, a condensation product of all-trans retinal (atRAL), exacerbates oxidative stress by sensitizing RPE cells to blue light, which generates singlet oxygen and superoxide radicals that overwhelm endogenous antioxidant defenses [39]. These reactive species damage mitochondrial DNA, disrupt electron transport chain function—as evidenced by a 60% reduction in cytochrome c oxidase activity in AMD-affected RPEs [40]—and ultimately trigger apoptosis [41]. Concurrently, the lipid peroxidation of PUFAs generates toxic aldehydes such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA). These byproducts form adducts with proteins and DNA, disrupting cellular homeostasis [42]. Notably, MDA accumulation in AMD modifies endogenous molecules, creating oxidation-specific epitopes that contribute to disease progression. The Y402 polymorphism in complement factor H (CFH), a major genetic risk factor for AMD, markedly impairs the ability of CFH to bind MDA, providing a mechanistic link between genetic susceptibility and oxidative damage [43]. In advanced AMD, these peroxidation products accumulate within drusen and the Bruch’s membrane (BrM), exacerbating local inflammation and impairing nutrient transport to photoreceptors, thereby driving degenerative changes.
3. Inflammation and Immune Dysregulation
Inflammation and immune dysregulation play central roles in AMD, particularly in its early pathogenesis. Drusen, the hallmark extracellular deposits of dry AMD, contain proinflammatory components such as apolipoprotein E, coagulation factors, acute-phase proteins, IgG, and complement activators, which collectively signify localized inflammation as a critical driver of disease initiation [44]. Convergent evidence underscores inflammatory cascades [45] and immune imbalance [46] as key mechanisms promoting drusen formation and AMD progression [47]. These processes involve the accumulation of extracellular deposits, regional immune activation, and the recruitment of microglia and macrophages to subretinal and choroidal regions, amplifying tissue damage.
The aging retina increasingly accumulates oxidative damage, which is marked by elevated protein oxidation and lipid peroxidation byproducts [48]. Under oxidative or metabolic stress, retinal neurons and RPE cells experience structural and functional compromise, triggering a graded immune response (Figure 2). Initially, adaptive mechanisms such as heat shock protein induction and autophagy activation attempt to mitigate damage and restore homeostasis (Figure 2, part 1). However, chronic stress—characteristic of aging—overwhelms these defenses, driving cellular senescence or apoptosis.
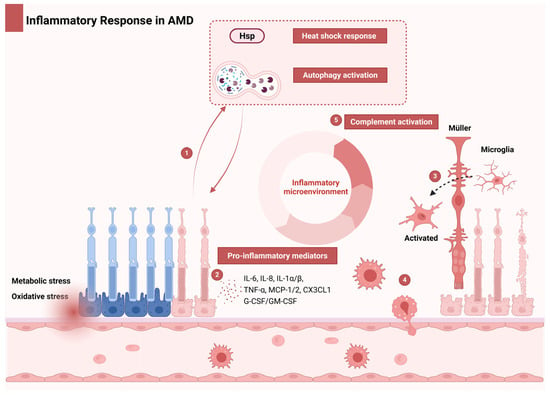
Figure 2.
Inflammatory cascade and immune dysregulation in AMD. Under oxidative/metabolic stress, compromised retinal neurons and RPE cells initiate an inflammatory cascade. Part 1: Early adaptation: HSPs and autophagy provide transient protection. Part 2: Proinflammatory microenvironment: Chronic stress in aging retinas overwhelms protective mechanisms, inducing cellular senescence/apoptosis. Senescent cells establish a self-perpetuating microenvironment via senescence-associated proinflammatory mediators, including IL-6, IL-8, TNF-α, IL-1α/β, MCP-1/2, CX3CL1, and G-CSF/GM-CSF. Part 3: Local amplification: Proinflammatory mediators activate resident microglia, Müller cells, and RPE while recruiting choroidal macrophages. Part 4: Complement dysregulation: Local inflammation amplifies complement system activity. Part 5: Systemic engagement: Exceeding adaptive thresholds triggers systemic innate immune activation and complement amplification. This feedforward loop drives AMD progression through oxidative damage, chronic immune activation, and complement-mediated tissue injury. Abbreviations: AMD, age-related macular degeneration; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; HSP, heat shock protein; IL, interleukin; MCP-1/2, monocyte chemoattractant protein-1/2; RPE, retinal pigment epithelium; TNF-α, tumor necrosis factor-alpha.
3.1. Immune Cell Involvement
Senescent cells secrete proinflammatory mediators, including IL-6, IL-8, TNF-α, IL-1α/β, MCP-1/2, CX3CL1, and granulocyte/macrophage colony-stimulating factor (G-CSF/GM-CSF), which establish a proinflammatory microenvironment (Figure 2, part 2) [49]. These cytokines activate resident retinal immune cells (microglia, Müller cells, and RPE) (Figure 2, part 3) and recruit choroidal macrophages (Figure 2, part 4). Immune cells, particularly retinal microglia and infiltrating macrophages, critically contribute to AMD pathology. Single-cell RNA sequencing studies have identified distinct microglial subpopulations in AMD retinas, revealing a shift toward proinflammatory M1-like states that exacerbate disease progression [50]. Resident microglia, which are activated by drusen components and oxidative stress, adopt this inflammatory phenotype, releasing cytokines such as IL-1β, IL-6, and TNF-α [51]. These mediators impair RPE function and promote CNV in neovascular AMD. Concurrently, peripheral macrophages are recruited to the retina via chemokine ligand 2 (CCL2)-CCR2 signaling, amplifying inflammatory cascades and perpetuating a cycle of tissue damage and immune activation. This interplay between resident and recruited immune cells creates a self-sustaining inflammatory milieu, driving retinal degeneration and advancing AMD pathology.
3.2. Complement System Dysregulation
When tissue stress surpasses local adaptive thresholds, systemic cytokine release mobilizes innate immunity, including complement amplification (Figure 2, part 5), to mediate tissue remodeling through parainflammation—a homeostatic response that becomes dysregulated in aging retinas [52]. This disruption transforms a protective mechanism into a pathological driver, exacerbating AMD progression by sustaining chronic inflammation, promoting further immune cell infiltration, and disrupting retinal–choroidal crosstalk.
Complement system dysregulation is a cornerstone of AMD pathogenesis and is driven by chronic inflammation and innate immune hyperactivity. The alternative complement pathway dominates this process, although all three pathways (classical, lectin, alternative) contribute to host defense under normal conditions (Figure 3). In AMD, oxidative stress and visual cycle byproducts trigger RPE and choroidal cells to downregulate complement inhibitors such as decay-accelerating factor (DAF), membrane cofactor protein (MCP), and CD59, enabling pathological complement activation in host tissues [53]. Histopathological studies have confirmed this mechanism, revealing that the complement components C3 and C5 within drusen and the C5b-9 membrane attack complex (MAC) are deposited in the aged Bruch’s membrane (BrM) and choriocapillaris (CC) of AMD donors [46]. Genetic evidence further solidifies this link: genome-wide association studies (GWAS) implicate over 50 AMD-associated loci, with complement genes (CFH, CFI, CFB, C3, FB/C2) accounting for a significant proportion of disease heritability (Table 1) [54].
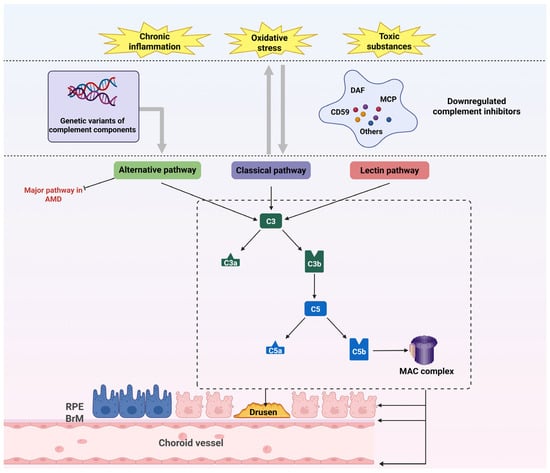
Figure 3.
Complement system dysregulation in AMD. Oxidative stress, chronic inflammation, and visual cycle byproducts downregulate complement inhibitors (DAF, MCP, CD59), enabling uncontrolled complement activation. Genetic susceptibility amplifies complement dysregulation. Subsequent deposition of C3/C5 within drusen and C5b-9 MAC drives structural damage to RPE, BrM, and choriocapillaris. A self-perpetuating cycle emerges wherein complement activation exacerbates oxidative stress, inflammation, and toxin accumulation. Abbreviations: AMD, age-related macular degeneration; BrM, Bruch’s membrane; DAF, decay-accelerating factor; MAC, membrane attack complex; MCP, membrane cofactor protein; RPE, retinal pigment epithelium.

Table 1.
Major pathways involved in AMD, their roles, and associated genes.
The CFH Y402H polymorphism (rs1061170), present in ~50% of AMD patients, exemplifies this dysregulation. This variant reduces CFH binding affinity for glycosaminoglycans and C-reactive protein (CRP) [55], impairing its ability to inhibit alternative pathway C3 convertase [56] and leading to uncontrolled C3b deposition and MAC formation at the RPE-BrM interface (Figure 3) [57]. These events coincide with lipoprotein accumulation in drusen, amplifying oxidative and inflammatory damage [58]. In addition to its regulatory role, CFH exerts cell-autonomous protective effects: endogenous CFH in RPE cells preserves transcriptional and metabolic homeostasis under oxidative stress [59], while exogenous CFH administration mitigates oxidized lipid-induced RPE injury [60]. The CFH H402 variant also disrupts MDA binding and lipid metabolism [43], as demonstrated in aged mice fed high-fat diets that develop AMD-like pathology with genotype-dependent lipoprotein dysregulation in ocular tissues and plasma [61].
Complement amplification is further exacerbated by gain-of-function variants in CFB (enhancing alternative pathway activity) and C3 (rs2230199 R102G, increasing C3b production), which drive MAC-mediated RPE lysis, photoreceptor degeneration, and chronic inflammation (Figure 3) [62]. These findings underscore complement dysfunction as a multifactorial driver of AMD, extending beyond immune activation to disrupt metabolic homeostasis, exacerbate oxidative stress, and synergize with lipid dysregulation. Overall, complement dysregulation connects genetic susceptibility, inflammatory cascades, and tissue remodeling, positioning complement dysregulation as both a biomarker and therapeutic target in AMD.
4. Lipid Metabolism and Drusen Formation
The RPE secretes cholesterol-rich lipoproteins containing apolipoproteins B (ApoB) and E (ApoE) into BrM [63], which is derived from ingested circulating lipoproteins or phagocytosed photoreceptor outer segments (Figure 4, Part 1) [64]. Under normal physiological conditions, the RPE recycles cholesterol to photoreceptors or exports it via reverse cholesterol transport mediated by ApoA-I [65], forming high-density lipoprotein (HDL) particles [66]. However, impaired reverse cholesterol transport drives the RPE to secrete ApoB100-containing lipoproteins into BrM [67], initiating lipid accumulation akin to atherosclerotic processes [24]. Soft drusen and BLinD emerge when aged BM and the choroidal endothelium fail to clear these effluxed metabolites, leading to sub-RPE deposits (Figure 4, Part 1) [64].
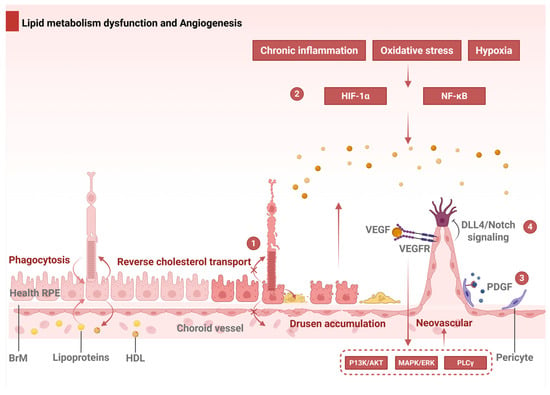
Figure 4.
Dysregulated lipid metabolism and angiogenic signaling in AMD. Part 1: Cholesterol homeostasis and pathological RCT forming sub-RPE deposits. Part 2: Hypoxia/oxidative stress activates HIF-1α/NF-κB, inducing VEGF-driven CNV. Part 3: PDGF amplifies CNV through pericyte recruitment and vessel destabilization. Part 4: VEGF-induced Dll4/Notch signaling regulates endothelial tip–stalk patterning; dysregulation causes aberrant sprouting. Abbreviations: RPE, retinal pigment epithelium; BrM, Bruch’s membrane; RCT, reverse cholesterol transport; VEGF = A, vascular endothelial growth factor A; HDL, high-density lipoprotein; VEGFR, VEGF receptor; PDGF, platelet-derived growth factor; Dll4, Delta-like ligand 4.
Genetic studies underscore lipid metabolism’s centrality to AMD, with variants in LIPC, CETP, ABCA1, and APOE influencing disease risk [63]. The APOE-ε4 allele reduces the drusen burden but exacerbates complement activation, whereas APOE-ε2 promotes cholesterol retention and increases hydraulic resistance in BrM [68]. The oxidative modification of lipoproteins within drusen generates lipid peroxides [69] and hydroxyapatite deposits coated with inflammatory proteins [70], triggering angiogenesis [71] and inflammation [72]. Advanced lipid peroxidation end products (ALEs) further accumulate in drusen, BrM, and lipofuscin [38], destabilizing proteins and inducing apoptosis in photoreceptors and RPE cells40 [36].
Diet critically modulates lipid dynamics in AMD. Omega-3 PUFAs (ω-3 PUFAs), such as docosahexaenoic acid (DHA), which is abundant in photoreceptors, exert anti-inflammatory and antioxidant effects. The AREDS2 trial demonstrated that ω-3 PUFA supplementation with antioxidants reduces advanced AMD progression by 25% [73]. Conversely, diets high in trans and saturated fats increase AMD risk, with BrM lipid profiles reflecting dietary intake rather than photoreceptor-derived sources [64]. Therapeutic strategies targeting lipid deposition, such as intravitreal L-4F (an ApoA-I mimetic), restore the BrM ultrastructure in preclinical models by clearing lipids and complement deposits [74]. However, clinical trials on statins have shown inconclusive benefits, and a Cochrane review revealed insufficient evidence to support their use in AMD prevention [75]. Overall, dysregulated lipid metabolism connects genetic predispositions, oxidative stress, and inflammatory pathways, positioning it as a pivotal therapeutic target in AMD.
5. Angiogenesis
5.1. VEGF Signaling: A Central Driver
VEGF-A drives pathological angiogenesis in wet AMD primarily by binding to VEGF receptor 2 (VEGFR2) on endothelial cells, activating downstream pathways such as the PI3K/Akt, MAPK/ERK, and PLCγ pathways (Figure 4, part 2). These pathways stimulate endothelial proliferation, migration, and lumen formation [76], culminating in CNV [77]. Hypoxia and inflammation further amplify VEGF-A expression through the transcriptional regulators HIF-1α and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), directly linking microenvironmental stress to aberrant vessel growth (Figure 4, part 2) [77]. Additional VEGF family members, including VEGF-C and VEGF-D, exacerbate CNV progression by interacting with VEGFR-2 and VEGFR-3, enhancing vascular permeability and neovessel formation. Elevated VEGF-C/D levels in the RPE and vitreous of AMD patients underscore their pathogenic contributions. Co-regulators such as platelet-derived growth factor (PDGF) (Figure 4, part 3) and angiopoietin-2 (Ang-2) further modulate CNV by recruiting pericytes and destabilizing the mature vasculature, respectively. Concurrently, dysregulated Notch signaling disrupts endothelial cell patterning. VEGF-A induces Delta-like ligand 4 (Dll4) in tip cells, which activates Notch receptors in adjacent stalk cells, suppressing VEGFR2 expression to establish a balanced tip–stalk cell distribution (salt-and-pepper pattern) (Figure 4, part 4). In AMD, aberrant Notch signaling disrupts this balance, resulting in excessive sprouting and disorganized vascular networks.
5.2. Inflammation and the Immune–Vascular Axis
Chronic inflammation is a hallmark of wet AMD pathogenesis. RPE cells secrete VEGF-A under oxidative stress or complement activation (e.g., by C3a and C5a), directly fueling CNV development [78]. Complement fragments C3a and C5a, detected in AMD-associated drusen, are correlated with increased VEGF expression and CNV severity. NLRP3 inflammasome activation in the RPE and immune cells (e.g., microglia and macrophages) drives the release of IL-1β and IL-18, perpetuating a pro-angiogenic microenvironment. Genetic predispositions, such as the CFH Y402H variant, exacerbate systemic inflammation [79] (e.g., elevated IL-6, TNF-α) and AMD risk [77]. Immune cells play dual roles: proinflammatory macrophages secrete VEGF-A to promote CNV, while alternatively activated subsets may mitigate angiogenesis. Single-cell analyses revealed upregulated IL-4/IL-13 signaling in AMD endothelial cells, suggesting that VEGF-independent angiogenic pathways may expand therapeutic targets.
5.3. Metabolic Reprogramming in Endothelial Cells
Metabolic dysregulation in endothelial cells is emerging as a critical contributor to CNV [80]. AMD endothelial cells exhibit altered glycolysis, oxidative phosphorylation, and amino acid metabolism. Glycolysis dominates ATP production, with the YAP-mediated upregulation of PFKFB3 enhancing glycolytic flux and CNV progression. The pharmacological inhibition of glycolysis (e.g., 3PO) reduces CNV in preclinical models. Glutamine and arginine metabolism support TCA cycle activity, redox balance, and nitric oxide synthesis, whereas fatty acid oxidation (FAO) fuels tip cell sprouting—processes inhibited by etomoxir (a CPT1 inhibitor) or pitavastatin. Mitochondrial ROS and calcium signaling further modulate angiogenic responses. Metabolomic profiling identifies distinct lipidomic signatures (e.g., glycerophospholipids, sphingolipids) and altered glucose metabolism (e.g., reduced α-ketoglutarate) in AMD patients, highlighting systemic metabolic dysregulation as both a biomarker and therapeutic avenue.
6. The Collapse of Neurovascular–Immune Microenvironment Crosstalk
6.1. Molecular Mechanisms of Blood–Retinal Outer Barrier Disruption
The outer blood–retina barrier (oBRB) comprises tight junctions (TJs) within the RPE, which act to regulate and filter the molecular movement of solutes and nutrients from the choroid to the subretinal space. The integrity of the oBRB relies on the coordinated function of three interconnected components: the RPE, CC, and BrM [81]. Disruption of the oBRB is particularly relevant to the pathology of AMD [82], which occurs due to photoreceptor/RPE/BrM/CC complex alterations [83].
Within the oBRB, TJs play a critical role in maintaining the barrier between the subretinal space and the CC. However, studies have shown that aging can compromise this barrier, as evidenced by a localized reduction in the TJ protein zona occludens-1 (ZO-1) in the RPE cells of aged mice [84], indicating TJ disruption in the oBRB. This disruption may be driven by oxidative stress, which has been shown to alter the distribution of ZO-1 and occluding [85], as well as cadherin in adherent junctions [86]. Notably, Cho et al. demonstrated that the oxidative stress-induced downregulation of thioredoxin-interacting protein (TXNIP) may contribute to TJ dysfunction by activating Src kinase, leading to the destabilization of ZO-1 in RPE cells [87]. These findings collectively suggest that oxidative stress is a potential driver of TJ disruption in the oBRB rather than merely exacerbating an existing condition, highlighting its central role in the breakdown of retinal barrier function.
In addition to oxidative stress, inflammation has been implicated as a significant contributor to the disruption of TJs in the oBRB. Previous studies have shown that inflammatory mediators, such as TNF-α [88], can directly influence the distribution and expression of key junctional proteins in RPE cells through the activation of p38 MAPK signaling pathways [89]. Moreover, van der Wijk et al. demonstrated that TNF-α could increase the permeability of the BRB in vitro, a process mediated by cAMP but independent of Rho/Rac signaling [90]. Moreover, in the context of an aged or damaged RPE, monomeric CRP can reach the apical side of the RPE, where it amplifies the proinflammatory microenvironment and further exacerbates barrier dysfunction [91]. Together, these findings highlight the multifaceted role of inflammation in driving oBRB disruption, particularly under conditions of RPE compromise.
The accumulation of abnormal substances within RPE cells could also lead to TJ disruption. Oxidized LDL, which is abnormally deposited due to lipid metabolism disorders, can mediate oxidative stress and inflammation in the RPE through the canonical Wnt pathway, leading to the disruption of ZO-1 arrangement at intercellular junctions [92]. Like Alzheimer’s disease, the deposition of Aβ is also a common pathologic feature of AMD [93]. ZO-1 and occludin are markedly attenuated [94] and lose their integrity as TJs in the RPE when intracellular and extracellular Aβ accumulate [95].
Disruption of the RPE-RPE interaction is often a precursor to epithelial–mesenchymal transition (EMT). Persistent activation of the transforming growth factor β (TGF-β) pathway triggers the loss of adhesive properties and polarity in RPE cells, inducing a mesenchymal phenotype through EMT [96]. In healthy eyes, the RPE secretes low levels of VEGF to suppress endothelial cell apoptosis and sustain the integrity of the CC-associated endothelium. Initiated by RPE dysfunction [83], dry AMD is characterized by significant thinning of the CC [97], with degenerative capillary endothelial cells, altered pericyte distribution [98], and reduced endothelial fenestration [99]. In addition to the RPE, intimate pericyte–endothelial connections remain fundamentally important for vascular integrity. Mediated by the angiopoietin-1/Tie2 signaling pathway, these connections could stimulate angiogenesis and shield retinal neurons from ischemic injury [100]. However, these interactions become weak and disrupted during aging, leading to capillary dropout, vascular abnormalities, and oBRB compromise [101]. In contrast, wet AMD initially involves choroidal vascular loss that disrupts the photoreceptor–RPE–Bruch’s membrane–choriocapillaris complex [83]. A hemodynamic model of AMD revealed that the CC underwent reduced perfusion and vascular density, leading to impaired nutrient exchange, oxidative stress, and inflammation [102]. Furthermore, in an inflammation-prone setting with a buildup of complement factors [46], along with pro-inflammatory agents such as CRP [103], the CC is vulnerable to dysfunction, making the adjacent RPE hypoxic. Thus, the hypoxia-induced RPE due to diminished blood flow may enhance VEGF secretion, thereby triggering CNV [104].
BrM is a critical extracellular matrix layer situated between the RPE and the CC and plays a vital role in maintaining the integrity of the oBRB. In AMD, there are several structural and functional changes in the BrM, such as waste accumulation, thickening, and calcification, which disrupt the BRB by impairing permeability and elasticity [105]. Additionally, in wet AMD, the BrM constitutes an important obstacle to pathological CNV [106]. However, an increased expression of MMP-9, which attacks the architecture of the BrM [107], has been demonstrated in AMD [108]. The MMP-9-mediated degradation of BrM structural proteins enables endothelial cell migration in angiogenesis, thereby initiating CNV formation [106].
6.2. Cascade Mechanisms of Photoreceptor Degeneration
Photoreceptor degeneration is a central event in the progression of AMD, leading to irreversible vision loss [109]. This degenerative process is not a singular event, but rather a cascade of interconnected molecular and cellular events initiated by oxidative stress and amplified through inflammatory responses, metabolic dysregulation, and cellular apoptosis.
As established in Section 2, oxidative stress in the RPE, driven by mitochondrial dysfunction and lipid peroxidation, serves as the primary initiator of photoreceptor degeneration in AMD. This oxidative damage not only impairs RPE function but also sets the stage for subsequent inflammatory and degenerative processes. The CCL2-CCR2 axis plays a significant role in the pathogenesis of AMD by driving chronic inflammation and contributing to photoreceptor degeneration. CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), is a key chemokine that recruits CCR2-positive monocytes [110] and microglia [111] to sites of retinal injury or inflammation (Figure 5, part 1) [112]. In AMD, CCL2 is often upregulated in response to oxidative stress [113] and other pathological stimuli [114], leading to the infiltration of these immune cells into the subretinal space.
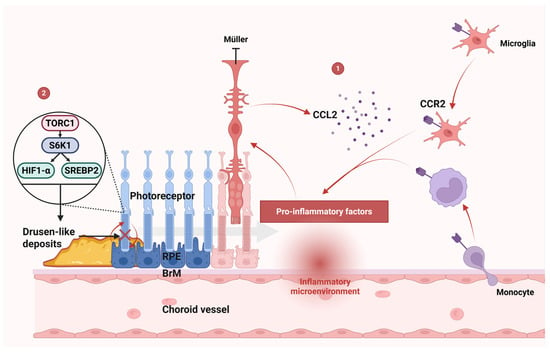
Figure 5.
Mechanisms of photoreceptor degeneration. Part 1: CCL2-CCR2 axis: CCL2 recruits CCR2+ monocytes/microglia to sites of retinal injury. Infiltrating cells release proinflammatory cytokines, driving RPE and photoreceptor degeneration. Müller cell activation amplifies this cascade through CCL2 production, establishing a feedforward inflammatory loop. Part 2: mTORC1 pathway: mTORC1 hyperactivity contributes to drusen-like deposit formation, disrupting RPE-photoreceptor metabolic exchange and accelerating AMD progression. Abbreviations: AMD, age-related macular degeneration; CCL2, C-C motif chemokine ligand 2; CCR2, C-C chemokine receptor type 2; HIF1-α, hypoxia-inducible factor 1-alpha; mTORC1, mechanistic target of rapamycin complex 1; RPE, retinal pigment epithelium; SREBP2, sterol regulatory element-binding protein 2.
Once recruited, CCR2-positive monocytes and microglia release proinflammatory cytokines, exacerbating tissue damage and promoting the degeneration of RPE cells and photoreceptors (Figure 5, part 1) [112]. This inflammatory cascade is further amplified by the activation of Müller cells, which produce CCL2 in response to macrophage infiltration [115]. The expression of CCL2 by Müller cells could further promote the infiltration of monocytes/microglia [116]. In addition, activated Müller cells may also reduce the secretion of neurotrophic factors [115], further compromising photoreceptors’ survival.
Targeting this axis, either by inhibiting CCL2 or blocking CCR2, has emerged as a promising therapeutic strategy to mitigate inflammation, protect retinal cells, and potentially slow the progression of AMD. Previous studies have shown that genetic knockout of the CCR2 gene can impair the recruitment of microglia [110] and reduce photoreceptor apoptosis [111], highlighting the detrimental role of the CCL2-CCR2 axis in AMD progression. Florian Sennlaub et al. reported that retinal tissues constitutively express CX3CR1, which suppresses CCL2 production and inhibits CCR2+ monocyte infiltration, serving as a critical regulator of inflammatory responses and neurodegenerative processes in AMD [112]. By disrupting this chemokine-receptor interaction, it may be possible to reduce the chronic inflammatory environment that drives AMD pathology.
The CCL2-CCR2 axis is just one of several interconnected pathways between oxidative stress and inflammatory pathways. Oxidative stress can directly trigger activation of the NF-κB pathway, resulting in an elevated production of multiple proinflammatory factors and the generation of a pro-inflammatory environment [117]. Moreover, the activation of Cyclic GMP-AMP Synthase–Stimulator of Interferon Genes (cGAS-STING) signaling, which is a key cytosolic DNA sensor system in innate immunity, was also detected in the context of oxidative stress-induced retinal degeneration [118]. DNA from oxidative stress-exposed cells strongly activate STING signaling [119], subsequently inducing type I interferon production through IRF3/IRF7 while simultaneously promoting inflammatory cytokine expression via NF-κB pathway activation [120].
Oxidative stress and inflammation contribute significantly to the progressive dysfunction of RPE cells. Oxidative stress induces damage to mitochondrial DNA integrity, lipid membranes, and cellular proteins within RPE cells [121], impairing their normal function. This mitochondrial damage can lead to a loss of mitochondria, which in turn alters the metabolic and differentiation state of the RPE [122]. Importantly, RPE cells play a critical role in supporting photoreceptor health by facilitating glucose transport via GLUT-1 glucose transporters [123]. However, oxidative stress and inflammation disrupt this supportive function. mTORC1 activation is subsequently required for the survival of metabolically stressed photoreceptors [124]. Activated mTORC1 can stimulate glycolysis, the pentose phosphate pathway, and lipid biosynthesis through the activation of HIF1-α and SREBP2 mediated by S6K1 (Figure 5, part 2) [125]. In retinitis pigmentosa, the activation of mTORC1 in cones promotes long-term cone survival in mice, with an increased utilization of glucose and increased levels of the key metabolite NADPH [124]. However, activated mTORC1 in photoreceptors has been linked to the formation of drusen-like deposits in AMD (Figure 5, part 2) [126]. These deposits interfere with the normal exchange of nutrients and waste products between the RPE and photoreceptors, further exacerbating RPE dysfunction and contributing to the progression of AMD.
The synergistic interplay of oxidative stress, inflammation, and metabolic dysfunction drives the progressive degeneration of photoreceptors through diverse cell death mechanisms, including apoptosis [127], necroptosis [128], ferroptosis [129], and dysregulated autophagy [130]. This culminates in the final step in the degenerative process, leading to permanent structural and functional deficits in the retina.
These advances in elucidating the cellular and molecular pathophysiology of AMD have catalyzed the development of multiple targeted therapeutic strategies. These include both clinically validated interventions and emerging treatment modalities that show considerable promise in preclinical and early-phase clinical studies.
7. Intervention Strategies in the Era of Precision Medicine
Despite these advances, current therapies targeting key pathogenic pathways, notably angiogenesis and complement dysregulation, remain limited by interpatient variability in treatment response and non-negligible adverse effects. To overcome these challenges, innovative therapeutic strategies are rapidly emerging, including epigenetic modulators, gut–retina axis interventions, and regenerative medicine approaches, which hold transformative potential for AMD management.
7.1. Limitations and Breakthroughs of Current Therapies
7.1.1. Wet AMD
In recent years, anti-VEGF therapies have transformed wet AMD treatment, effectively preventing and reducing CNV in patients [131]. Nevertheless, a proportion of wet AMD cases demonstrate resistance to anti-VEGF agents, necessitating repeated intravitreal injections.
A previous study revealed that anti-VEGF therapy-resistant vasculature was supported by pericytes and was immunohistochemically positive for NG2, PDGFRβ, and αSMA in malignant melanoma [132]. In wet AMD, CD34+ progenitor cells exhibit plasticity in vitro, differentiating into either VWF+ endothelial cells or NG2+ pericytes [133]. However, a correlation between the refractory treatment response to anti-VEGF injections and the expansion potential and differentiation characteristics of CD34+ cells was not detected because of the variability in the results collected from wet AMD patients [133].
Bioinformatics and network pharmacology analysis revealed that wet AMD patients with anti-VEGF resistance showed enhanced immune system activation alongside reduced antioxidant capacity [134]. SOD1 was pinpointed as a hub gene in treatment-resistant pathogenesis, which was negatively correlated with immune infiltration [134]. In addition, Zhuang et al. demonstrated through integrated proteomic–metabolomic analysis and volumetric lesion assessment that decreased YIPF3 levels may predict anti-VEGF therapy failure, showing significant associations with limited lesion regression material in wet AMD [135].
Moreover, the compensatory upregulation of alternative pro-angiogenic mediators [136], such as PDGF, fibroblast growth factor, placental growth factor, interleukins, and TGF-β [137], may occur following VEGF blockade [138], which is implicated in the development of anti-VEGF resistance. Faced with the challenge of anti-VEGF resistance, various treatment options have been explored, including steroid injections, laser therapy, shortened anti-VEGF treatment intervals, switching anti-VEGF agents, and topical medications, with varying outcomes [139].
7.1.2. Dry AMD
For dry AMD, contemporary clinical interventions are directed mainly at complement cascade modulation to attenuate the inflammatory-mediated degeneration in advancing GA. Pegcetacoplan, the first treatment approved by the US Food and Drug Administration (FDA) for GA, is a synthetic molecule that selectively inhibits C3, leading to a comprehensive downregulation of the entire complement system [140]. In two multicenter, randomized, double-masked, sham-controlled, phase 3 trials (NCT03525613, OAKS; NCT03525600, DERBY), pegcetacoplan slowed GA lesion growth in both the monthly and bimonthly arms [141]. However, the pegcetacoplan treatment group showed elevated frequencies of ocular complications such as intraocular inflammation, incident neovascular AMD, and anterior ischemic optic neuropathy relative to control arms [141]. In addition, the American Society of Retina Specialists (ASRS) investigated cases of retinal vasculitis and retinal artery occlusion following the treatment of pegcetacoplan, with seven recorded cases occurring within the first half-year post-approval [142]. For some patients, the associated adverse event risks could potentially offset the mild benefits of modestly slowing lesion progression [143].
The treatment effect of pegcetacoplan might depend on the specific GA phenotype. Using a deep learning model to illustrate the treatment effect of pegcetacoplan, Irmela Mantel et al. reported that extrafoveal lesions demonstrated greater treatment effects and therapeutic response in the foveal region compared to peripheral retinal areas [144], which corresponds well with a post hoc analysis of the OAKS and DERBY studies [145]. Moreover, deep learning-based OCT analysis has indicated that the difference in loss between the ellipsoid zone (EZ) and RPE strongly affects disease progression and the therapeutic response to pegcetacoplan, which might become an important criterion for the management of GA secondary to AMD [146].
In addition to pegcetacoplan, avacincaptad pegol, a C5 inhibitor, has also gained approval for treating GA secondary to AMD in adult patients [147]. In a randomized pivotal phase 2/3 trial (NCT02686658, GATHER1), twelve-month data revealed that avacincaptad pegol effectively reduced GA lesion growth rates in AMD [148]. And C5 inhibition may provide superior safety to pegcetacoplan by preserving C3 activity. Clinical data from the GATHER2 trial (NCT04435366) demonstrated significantly lower inflammatory risk with avacincaptad pegol—only one mild intraocular inflammation case reported over 24 months in this randomized, double-masked, sham-controlled phase 3 study [149].
7.2. Translational Potential of Emerging Therapeutic Targets
The interplay between epigenetic and metabolic pathways reveals critical molecular crosstalk, offering novel insights into AMD pathogenesis and treatment targets [150]. Retinal DNA methylation profiling has uncovered 87 significant gene–epigenome interactions in human samples, with specific DNA methylation alterations disrupting glutathione homeostasis and glycolytic flux pathways central to oxidative stress mitigation in early AMD [151]. Genome-wide methylation analyses further revealed an AMD-associated epigenetic dysregulation of SKI and GTF2H4, genes that govern TGF-β-mediated inflammation and transcription-coupled DNA repair mechanisms [152]. Additionally, analyses of metabolite quantitative trait loci (meQTLs) in AMD patients and controls have revealed significant associations involving metabolites and genes such as ASPM and LIPC, underscoring the potential role of glycerophospholipid metabolism in AMD pathogenesis [153]. Collectively, these findings position epigenetic-metabolic crosstalk as a dynamic driver of AMD pathology across disease stages.
Epigenomic studies could inform the development of novel therapeutic strategies. Tiina Suuronen et al. reported that the induction of DNA hypomethylation with 5-aza-2′-deoxycytidine (AZA) could cause the overexpression of clusterin (CLU, 8p21.1) through the hypomethylation at its promoter region, influencing the pathogenesis of AMD through anti-angiogenic and anti-inflammatory effects (Figure 6) [154]. This can lead to the upregulation of clusterin (CLU, located at 8p21.1) by reducing methylation in its promoter region.
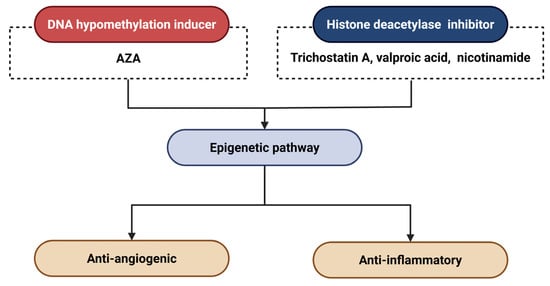
Figure 6.
Epigenetic therapeutic strategies for AMD. Abbreviations: AZA, 5-aza-2′-deoxycytidine.
Histone deacetylase (HDAC) inhibitors represent promising therapeutic candidates for AMD, analogous to DNA hypomethylation-inducing agents, by modulating epigenetic pathways implicated in disease progression. Notably, trichostatin A, a pan-HDAC inhibitor, has been reported to suppress CNV through multiple mechanisms: it attenuates pro-angiogenic signaling, impedes CNV-associated wound healing responses, and inhibits pathological EMT in RPE cells [155]. In addition, pharmacological studies demonstrate that valproic acid [154] and nicotinamide [156] exert anti-angiogenic effects in AMD through HDAC inhibition, whereas sulforaphane confers photoprotection to RPE cells via HDAC suppression [157].
Furthermore, given the promising potential of RNA therapy for treating inherited retinal degeneration, RNA editing may pave the way for groundbreaking clinical advancements in epigenetic studies [158]. In addition to RNA therapy, induced pluripotent stem cells (iPSCs) are also expected to provide tools for performing functional assays to test key findings in epigenetic studies and serve as models for the development of therapeutics [159].
Aside from the advances in epigenetics, recent studies have highlighted the growing importance of systemic interactions in retinal diseases, particularly through the gut-retina axis. Accumulating data indicates possible participation of the gut-retina axis in AMD pathogenesis, driving the exploration of new therapeutic avenues.
A three-arm randomized controlled trial (NCT06391411) reported that micronutrient supplementation with lutein, zeaxanthin, and saffron correlated with enhanced visual acuity alongside a notable decrease in medium-chain fatty acid (MCFA) levels [160]. MCFAs act as important regulators of energy metabolism, membrane trafficking, and gene expression in various anaerobic bacteria. These results suggest that micronutrient supplementation may help restore the gut-retina axis, enhancing ocular outcomes in wet AMD patients [160].
Further research by Zhang et al. revealed metformin’s therapeutic potential in wet AMD through gut microbiota modulation and gut–retina axis regulation [161]. Specifically, metformin administration altered microbial composition, notably enhancing Bifidobacterium and Akkermansia populations, while elevating concentrations of butyrate, SCFAs, and cholic acid in fecal samples [161]. These microbial metabolites have been implicated in mediating the protective effects of metformin. For example, previous studies have shown that butyrate can inhibit neovascularization partially through the TXNIP/VEGFR2 signaling pathway [162]. Similarly, metformin-elevated SCFAs have been shown to stimulate GPR41 and GPR43 receptors on gut epithelial cells, activating mitogen-activated protein kinase (MAPK) signaling and inducing rapid chemokine/cytokine secretion, potentially influencing retinal inflammatory responses and angiogenic regulation [163]. For cholic acid, ursodeoxycholic acid (UDCA) was found to downregulate the expression of VEGF and have inhibitory effects on CNV in a preclinical study [164]. Together, these findings highlight the multifaceted role of gut microbiome-derived metabolites in mediating anti-wet AMD effects through the gut-retina axis.
In addition to the aforementioned therapies, emerging strategies such as dietary modifications, probiotics, fecal microbiota transplantation, and microbiome-targeted interventions may help normalize microbial composition, decrease inflammatory markers, and positively influence AMD outcomes [165].
7.3. Regenerative Medicine and Personalized Models
Stem cells possess the capacity to be directed toward ocular lineages, offering promise for vision rehabilitation and protection. Both embryonic-derived cells (ESCs) and induced pluripotent stem cells (iPSCs), along with tissue-resident adult stem cells, represent optimal cellular substrates for ophthalmologic regenerative applications [166].
Among these candidate cell sources, ESCs currently represent the most clinically advanced platform for ocular regeneration, particularly in RPE replacement therapies. Previous clinical studies involving ESC-derived RPE cell transplantation have shown promising safety profiles along with preliminary evidence of therapeutic potential [167,168,169,170,171], but the requirement of local immunosuppression is still one of the main downsides of this approach. Although the intraocular space has been described as immune-privileged, both surgical intervention and the pathological state of AMD can compromise the blood–retinal barrier’s integrity. Moreover, the RPE cells derived from ESCs exhibit significant histocompatibility complex disparities with host tissues. Despite these challenges, in a phase 1/2a, prospective, single-arm, interventional study (NCT02590692), clinical data demonstrated excellent safety and tolerability profiles of human ESC-RPE transplantation in advanced dry AMD patients through a median 3-year follow-up [172], providing promising evidence for the implant’s long-term viability.
While ESCs show clinical potential, alternative pluripotent stem cell sources face distinct challenges. Clinical trials using iPSC-RPEs, however, have yielded limited success in treating AMD. A single-patient study (UMIN000011929) reported the transplantation of an autologous iPSC-derived RPE patch on the native secreted basement membrane, without improvement in best-corrected visual acuity at the 12-month follow-up [173]. Beyond pluripotent stem cells, tissue-resident adult stem cells present unique advantages for ocular repair, though their clinical translation faces distinct hurdles [166]. These endogenous progenitors (e.g., retinal Müller glia-derived stem cells) have demonstrated multipotent differentiation capacity into functional photoreceptors and RPE cells in preclinical models [174]. Their intrinsic tissue compatibility theoretically eliminates immune rejection concerns associated with ESC/iPSC-derived transplants.
Several important challenges are hindering the clinical application of stem cell transplantation for AMD [166], including cell manufacturing, cell delivery, cell survival, physiological cell behavior, immunogenicity, and tumorigenicity [175]. Addressing these issues is imperative to ensure robust long-term functional integration and therapeutic efficacy.
Compared to single-cell grafts, the transplantation of complex 3D retinal tissues demonstrates superior functional integration potential. In primate models of retinal degeneration, hESC-derived retinal tissue differentiated into multiple retinal cell types and established synaptic connections with host tissue, offering clinically relevant insights for transplantation protocol optimization [176]. In a clinical trial (jRCTa050200027), allogeneic iPSC-derived retinal organoid sheets were safely transplanted in two advanced retinitis pigmentosa patients, demonstrating 2-year graft survival, increased retinal thickness, and stabilized visual function without serious adverse events, supporting further clinical investigation [177]. While clinical organoid research for AMD remains limited, this industry–academia collaboration demonstrates clinically translatable safety and efficacy, providing a technically and economically viable model.
The therapeutic advantages of 3D retinal tissues extend beyond structural integration to their paracrine signaling potential. Notably, 3D human retinal organoids secrete extracellular vesicles (EVs) with enhanced retinal specificity compared to conventional human umbilical cord MSC-derived EVs [178]. Proteomic analyses demonstrate that organoid-derived EVs are enriched in retinal function-related proteins and EV biogenesis proteins, suggesting their dual role in structural repair and microenvironment modulation [178]. This synergy between 3D tissue transplantation and EV-mediated signaling presents a comprehensive strategy for retinal regeneration, addressing both cellular replacement and pathological host environment challenges.
Apart from its therapeutic potential, 3D tissue culture modeling could also offer platforms for the high-throughput testing of therapeutic candidates and studies of gene interactions to improve models of complex genetic diseases [179]. Kevin Achberger et al. first presented the retina-on-a-chip (RoC), an innovative microphysiological platform mimicking human retinal architecture that provided vasculature-like perfusion and permitted the accurate simulation of physiological interactions between mature photoreceptor segments and the RPE in vitro [180]. The RoC has the potential to accelerate pharmaceutical innovation while offering novel perspectives on retinal disease pathogenesis. To create a high-throughput compatible drug screening platform that mimics physiological angiogenesis, Zhao et al. engineered a dual-reporter hPSC system for generating vascularized organoids in vitro [181]. This innovative model enables the quantitative assessment of VEGF inhibitors’ efficacy and the rapid screening of potential angiogenic modulators through bioluminescent detection [181]. The spectrum of therapeutic strategies reviewed here, from conventional to experimental, is comprehensively summarized (Table 2).

Table 2.
Targeted therapeutic approaches for AMD.
8. Future Perspectives: Integrating Multiomics and Artificial Intelligence
AMD is characterized by a multifactorial etiology involving genetic predisposition, metabolic dysregulation, and environmental influences. Despite significant advancements in understanding its pathogenesis, the heterogeneity of AMD and the variability in therapeutic responses remain substantial challenges. Recent progress in artificial intelligence (AI) and multiomics technologies has provided novel insights into the molecular mechanisms underlying AMD, offering transformative potential for both research and clinical practice.
AI-driven personalized approaches, including diagnosis, therapeutic decision-making, and prognostication, are reshaping the management of AMD. Deep learning algorithms have demonstrated exceptional proficiency in analyzing high-dimensional datasets derived from retinal imaging (e.g., optical coherence tomography (OCT)) and multiomics profiles, significantly enhancing diagnostic accuracy, sensitivity, and specificity for AMD [182]. Schranz et al. conducted a multicenter study of 230 wet AMD patients, which revealed significant quantitative associations between AI-quantified OCT fluid compartments and macular neovascularization characteristics, enabling accurate diagnosis and precision-guided therapeutic optimization [183]. Furthermore, long-term clinical outcomes may be improved by using AI algorithms to detect retinal fluid in AMD eyes, as a 10-year follow-on study showed that an AI algorithm achieved higher accuracy compared to retinal specialists, especially for intraretinal fluid [184]. In addition, the utilization of EV protein chips combined with machine learning methods identified ABCA1 as a biomarker for the early diagnosis of AMD, offering a promising new method for liquid biopsy diagnostics and improving the clinical diagnosis and management of AMD [185].
Beyond diagnostics, AI demonstrates superior precision compared to clinical specialists in personalizing anti-VEGF therapeutic selection. Several studies have focused on the AI-based prediction of anti-VEGF treatment outcomes to provide wet AMD patients with personalized medicine, including drug selection [186] and dose determination [187]. In a retrospective study, Moon et al. developed an attention-based generative adversarial network (GAN) model trained on 1684 OCT images to predict VEGF agent-specific anatomical treatment outcomes in patients with wet AMD, demonstrating the algorithm’s superior sensitivity over clinician assessment for short-term response prediction [186]. Notably, AI-driven biomarker discovery has identified novel therapeutic targets, including ferroptosis-associated diagnostic genes (e.g., ABCA1) and senescence-linked immune microenvironment signatures, which are correlated with distinct programmed cell death pathways [188] and disease progression trajectories [189]. Moreover, integrative AI models combining genetic data with imaging biomarkers have elucidated genotype–phenotype interactions, offering mechanistic insights into AMD pathogenesis while identifying candidate therapeutic targets [190].
For the prognostication of AMD progression, advanced techniques such as metadata-enhanced contrastive learning further refine feature extraction from longitudinal OCT datasets, improving risk stratification for GA conversion and wet AMD development [191]. Holland et al. demonstrated that a metadata-enhanced approach outperformed the alternative in AMD stage classification, subtype differentiation, and visual acuity prediction across two longitudinal datasets comprising 170,427 retinal OCT images from 7912 AMD patients [191]. These innovations collectively underscore AI’s transformative potential in transitioning AMD care from empirical protocols to data-driven, patient-specific strategies, although large-scale clinical validation remains imperative.
Building upon AI-driven advancements in AMD management, the synergistic integration of multiomics analytics and machine learning frameworks is emerging as a transformative paradigm for precision medicine. For example, Zhang et al. leveraged a deep subspace nonnegative matrix factorization (DS-NMF) algorithm to deconvolute multiomics profiles, reconstructing disease-associated gene expression trajectories that delineated AMD subtypes characterized by divergent immune pathway activation and macrophage infiltration patterns [192]. They further established a robust diagnostic model through multiple machine learning algorithms, including RF, AdaBoost, KNN, and SVM-RFE, bridging molecular heterogeneity to clinically actionable classification [192]. This multiomics-to-clinic pipeline exemplifies how AI can decode biological complexity to guide subtype-specific therapeutic development. In parallel, Jackson et al. integrated multiomics data with convolutional neural networks (CNNs) to uncover novel genetic and metabolic insights into retinal diseases and their systemic connections [193]. Such integrative approaches not only resolve mechanistic ambiguities, but also prioritize biomarkers with dual diagnostic and therapeutic relevance.
As these technologies continue to mature, they will undoubtedly reshape the landscape of AMD research and therapy, offering hope for improved patient outcomes and a deeper understanding of this complex disease. The convergence of multiomics and AI represents a paradigm shift in the study and management of AMD, heralding a new era of personalized and precision medicine in ophthalmology.
9. Criteria for Inclusion and Exclusion of the Literature
To ensure a comprehensive and unbiased review, a systematic literature search was conducted using the databases PubMed, Scopus, and Web of Science. These databases were selected for their extensive coverage of the medical and scientific literature relevant to AMD. The search utilized keywords including ‘age-related macular degeneration’, ‘AMD’, ‘oxidative stress’, ‘inflammation’, ‘lipid metabolism’, ‘immune dysregulation’, ‘angiogenesis’, and ‘molecular mechanisms’ to capture studies addressing the key pathophysiological processes discussed in this review. Studies were included if they were published in peer-reviewed journals, focused on the cellular and molecular mechanisms of AMD. Exclusion criteria encompassed studies not directly related to AMD, those not written in English, and purely clinical studies lacking mechanistic insights.
Author Contributions
Conceptualization, F.J., J.M. and C.L.; writing—original draft preparation, F.J., J.M. and C.L.; writing—review and editing, F.J., J.M. and C.L.; supervision, Y.Z. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Sichuan Provincial Science and Technology Support Project (no. 2024YFFK0303, Meixia Zhang) and the National Natural Science Foundation of China (grant No. 82301232, Yun Zhang).
Acknowledgments
The authors are grateful to the members of our laboratories for their input and discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMD | Aged-related Macular Degeneration |
| VEGF | Vascular Endothelial Growth Factor |
| CNV | Choroidal Neovascularization |
| RPE | Retinal Pigment Epithelium |
| ROS | Reactive Oxygen Species |
| HO-1 | Heme Oxygenase-1 |
| SOD2 | Superoxide Dismutase 2 |
| Drp1 | Dynamin-1-like protein |
| mtDNA | Mitochondrial DNA |
| GA | Geographic Atrophy |
| A2E | Bisretinoid N-retinyl-N-retinylidene ethanolamine |
| atRAL | All-trans retinal |
| 4-HNE | 4-hydroxynonenal |
| MDA | Malondialdehyde |
| CFH | Complement Factor H |
| BrM | Bruch’s Membrane |
| G-CSF/GM-CSF | Granulocyte/Macrophage Colony-Stimulating Factor |
| DAF | Decay-Accelerating Factor |
| MCP | Membrane Cofactor Protein |
| MAC | Membrane Attack Complex |
| CC | Choriocapillaris |
| CRP | C-Reactive Protein |
| CCL2 | Chemokine Ligand 2 |
| BLinD | Basal Linear Deposits |
| BLamD | Basal Laminar Deposits |
| ApoB | Apolipoproteins B |
| ApoE | Apolipoproteins E |
| HDL | High-Density Lipoprotein |
| ALE | Advanced Lipid Peroxidation End product |
| DHA | Docosahexaenoic Acid |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| PDGF | Platelet-Derived Growth Factor |
| Ang-2 | Angiopoietin-2 |
| FAO | Fatty Acid Oxidation |
| oBRB | Outer Blood–Retina Barrier |
| TJ | Tight Junction |
| ZO-1 | Zona Occludens-1 |
| TXNIP | Thioredoxin-Interacting Protein |
| EMT | Epithelial–Mesenchymal Transition |
| TGF-β | Transforming Growth Factor β |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| cGAS-STING | Cyclic GMP-AMP Synthase–Stimulator of Interferon Genes |
| PUFA | Polyunsaturated Fatty Acid |
| DNMT | DNA Methyltransferase |
| AZA | 5-aza-2′-deoxycytidine |
| HDAC | Histone Deacetylase |
| MCFA | Medium-Chain Fatty Acid |
| CNN | Convolutional Neural Network |
| PIGF | Placental Growth Factor |
| FDA | US Food and Drug Administration |
| ASRS | American Society of Retina Specialists |
| EZ | Ellipsoid Zone |
| meQTLs | Metabolite Quantitative Trait Loci |
| iPSCs | Induced Pluripotent Stem Cells |
| SCFAs | Short-Chain Fatty Acids |
| MAPK | Mitogen-Activated Protein Kinase |
| UDCA | Ursodeoxycholic Acid |
| ESCs | Embryonic-Derived Cells |
| EVs | Extracellular Vesicles |
| hUC | Human Umbilical Cord |
| RoC | Retina-on-a-Chip |
| AI | Artificial Intelligence |
| OCT | Optical Coherence Tomography |
| DS-NMF | Deep Subspace Nonnegative Matrix Factorization |
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Hobby, A.E.; Binns, A.M.; Crabb, D.P. How Does Age-Related Macular Degeneration Affect Real-World Visual Ability and Quality of Life? A Systematic Review. BMJ Open 2016, 6, e011504. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef]
- Guymer, R.H.; Campbell, T.G. Age-Related Macular Degeneration. Lancet 2023, 401, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-Related Macular Degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Handa, J.T. Oxidative Stress as a Therapeutic Target for the Prevention and Treatment of Early Age-Related Macular Degeneration. Surv. Ophthalmol. 2021, 66, 423–440. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Role of Oxidants/Antioxidants in Smoking-Induced Lung Diseases. Free Radic. Biol. Med. 1996, 21, 669–681. [Google Scholar] [CrossRef]
- Rangasamy, T.; Cho, C.Y.; Thimmulappa, R.K.; Zhen, L.; Srisuma, S.S.; Kensler, T.W.; Yamamoto, M.; Petrache, I.; Tuder, R.M.; Biswal, S. Genetic Ablation of Nrf2 Enhances Susceptibility to Cigarette Smoke-Induced Emphysema in Mice. J. Clin. Invest. 2004, 114, 1248–1259. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The Impact of Oxidative Stress and Inflammation on RPE Degeneration in Non-Neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of Autophagy by Reactive Oxygen Species (ROS): Implications for Cancer Progression and Treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Desideri, E.; Cardaci, S.; Rotilio, G.; Ciriolo, M.R. Under the ROS…thiol Network Is the Principal Suspect for Autophagy Commitment. Autophagy 2010, 6, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Monsen, V.T.; Pettersen, K.; Mildenberger, J.; Misund, K.; Kaarniranta, K.; Schønberg, S.; Bjørkøy, G. The Marine N-3 PUFA DHA Evokes Cytoprotection against Oxidative Stress and Protein Misfolding by Inducing Autophagy and NFE2L2 in Human Retinal Pigment Epithelial Cells. Autophagy 2015, 11, 1636–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-Related Retinopathy in NRF2-Deficient Mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated Autophagy in the RPE Is Associated with Increased Susceptibility to Oxidative Stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Viiri, J.; Amadio, M.; Marchesi, N.; Hyttinen, J.M.T.; Kivinen, N.; Sironen, R.; Rilla, K.; Akhtar, S.; Provenzani, A.; D’Agostino, V.G.; et al. Autophagy Activation Clears ELAVL1/HuR-Mediated Accumulation of SQSTM1/P62 during Proteasomal Inhibition in Human Retinal Pigment Epithelial Cells. PLoS ONE 2013, 8, e69563. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy, Exosomes and Drusen Formation in Age-Related Macular Degeneration. Autophagy 2009, 5, 563–564. [Google Scholar] [CrossRef]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.-L.; Thompson, D.A.; et al. Deletion of Autophagy Inducer RB1CC1 Results in Degeneration of the Retinal Pigment Epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative Damage and Age-Related Macular Degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar]
- Ershov, A.V.; Bazan, N.G. Photoreceptor Phagocytosis Selectively Activates PPARgamma Expression in Retinal Pigment Epithelial Cells. J. Neurosci. Res. 2000, 60, 328–337. [Google Scholar] [CrossRef]
- Miceli, M.V.; Liles, M.R.; Newsome, D.A. Evaluation of Oxidative Processes in Human Pigment Epithelial Cells Associated with Retinal Outer Segment Phagocytosis. Exp. Cell Res. 1994, 214, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.J.; Miceli, M.V.; Newsome, D.A. Phagocytosis and H2O2 Induce Catalase and Metallothionein Gene Expression in Human Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1271–1279. [Google Scholar]
- Ham, W.T.; Ruffolo, J.J.; Mueller, H.A.; Clarke, A.M.; Moon, M.E. Histologic Analysis of Photochemical Lesions Produced in Rhesus Retina by Short-Wave-Length Light. Investig. Ophthalmol. Vis. Sci. 1978, 17, 1029–1035. [Google Scholar] [PubMed]
- Curcio, C.A.; Millican, C.L.; Bailey, T.; Kruth, H.S. Accumulation of Cholesterol with Age in Human Bruch’s Membrane. Investig. Ophthalmol. Vis. Sci. 2001, 42, 265–274. [Google Scholar] [PubMed]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen Proteome Analysis: An Approach to the Etiology of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of Reactive Oxygen Species by Mitochondria: Central Role of Complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of Mitochondrial Dysfunction and Their Impact on Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Terluk, M.R.; Kapphahn, R.J.; Soukup, L.M.; Gong, H.; Gallardo, C.; Montezuma, S.R.; Ferrington, D.A. Investigating Mitochondria as a Target for Treating Age-Related Macular Degeneration. J. Neurosci. 2015, 35, 7304–7311. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA Damage as a Potential Mechanism for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef]
- Lin, H.; Xu, H.; Liang, F.-Q.; Liang, H.; Gupta, P.; Havey, A.N.; Boulton, M.E.; Godley, B.F. Mitochondrial DNA Damage and Repair in RPE Associated with Aging and Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3521–3529. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.-C.; Zhou, Z.; Green, D.R.; et al. Noncanonical Autophagy Promotes the Visual Cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Viiri, J.; Kaarniranta, K.; Błasiak, J. Mitochondrial Quality Control in AMD: Does Mitophagy Play a Pivotal Role? Cell Mol. Life Sci. 2018, 75, 2991–3008. [Google Scholar] [CrossRef] [PubMed]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.T.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of NRF-2 and PGC-1α Genes Leads to Retinal Pigment Epithelium Damage Resembling Dry Age-Related Macular Degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Tarallo, V.; Hirano, Y.; Gelfand, B.D.; Dridi, S.; Kerur, N.; Kim, Y.; Cho, W.G.; Kaneko, H.; Fowler, B.J.; Bogdanovich, S.; et al. DICER1 Loss and Alu RNA Induce Age-Related Macular Degeneration via the NLRP3 Inflammasome and MyD88. Cell 2012, 149, 847–859. [Google Scholar] [CrossRef]
- Zhou, J.; Jang, Y.P.; Kim, S.R.; Sparrow, J.R. Complement Activation by Photooxidation Products of A2E, a Lipofuscin Constituent of the Retinal Pigment Epithelium. Proc. Natl. Acad. Sci. USA 2006, 103, 16182–16187. [Google Scholar] [CrossRef]
- Zhang, Q.; Presswalla, F.; Calton, M.; Charniga, C.; Stern, J.; Temple, S.; Vollrath, D.; Zacks, D.N.; Ali, R.R.; Thompson, D.A.; et al. Highly Differentiated Human Fetal RPE Cultures Are Resistant to the Accumulation and Toxicity of Lipofuscin-Like Material. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Fishkin, N.; Zhou, J.; Cai, B.; Jang, Y.P.; Krane, S.; Itagaki, Y.; Nakanishi, K. A2E, a Byproduct of the Visual Cycle. Vision. Res. 2003, 43, 2983–2990. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The Bisretinoids of Retinal Pigment Epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef]
- Nordgaard, C.L.; Karunadharma, P.P.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Mitochondrial Proteomics of the Retinal Pigment Epithelium at Progressive Stages of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2848–2855. [Google Scholar] [CrossRef]
- Suter, M.; Remé, C.; Grimm, C.; Wenzel, A.; Jäättela, M.; Esser, P.; Kociok, N.; Leist, M.; Richter, C. Age-Related Macular Degeneration. The Lipofusion Component N-Retinyl-N-Retinylidene Ethanolamine Detaches Proapoptotic Proteins from Mitochondria and Induces Apoptosis in Mammalian Retinal Pigment Epithelial Cells. J. Biol. Chem. 2000, 275, 39625–39630. [Google Scholar] [CrossRef]
- Oxidation-Specific Epitopes Are Danger-Associated Molecular Patterns Recognized by Pattern Recognition Receptors of Innate Immunity—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21252151/ (accessed on 6 April 2025).
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.N.; Charbel Issa, P.; Cano, M.; Brandstätter, H.; Tsimikas, S.; Skerka, C.; et al. Complement Factor H Binds Malondialdehyde Epitopes and Protects from Oxidative Stress. Nature 2011, 478, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, K.B.; Handa, J.T. Lipids, Lipoproteins, and Age-Related Macular Degeneration. J. Lipids 2011, 2011, 802059. [Google Scholar] [CrossRef]
- Penfold, P.L.; Killingsworth, M.C.; Sarks, S.H. Senile Macular Degeneration: The Involvement of Immunocompetent Cells. Graefes Arch. Clin. Exp. Ophthalmol. 1985, 223, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A Role for Local Inflammation in the Formation of Drusen in the Aging Eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An Integrated Hypothesis That Considers Drusen as Biomarkers of Immune-Mediated Processes at the RPE-Bruch’s Membrane Interface in Aging and Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H. Parainflammation, Chronic Inflammation, and Age-Related Macular Degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Cherepanoff, S.; McMenamin, P.; Gillies, M.C.; Kettle, E.; Sarks, S.H. Bruch’s Membrane and Choroidal Macrophages in Early and Advanced Age-Related Macular Degeneration. Br. J. Ophthalmol. 2010, 94, 918–925. [Google Scholar] [CrossRef]
- O’Koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.W.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 2019, 50, 723–737.e7. [Google Scholar] [CrossRef]
- Guillonneau, X.; Eandi, C.M.; Paques, M.; Sahel, J.-A.; Sapieha, P.; Sennlaub, F. On Phagocytes and Macular Degeneration. Prog. Retin. Eye Res. 2017, 61, 98–128. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, K.B.; Fijalkowski, N.; Cano, M.; Handa, J.T. Decreased Membrane Complement Regulators in the Retinal Pigmented Epithelium Contributes to Age-Related Macular Degeneration. J. Pathol. 2013, 229, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A Large Genome-Wide Association Study of Age-Related Macular Degeneration Highlights Contributions of Rare and Common Variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Skerka, C.; Lauer, N.; Weinberger, A.A.W.A.; Keilhauer, C.N.; Sühnel, J.; Smith, R.; Schlötzer-Schrehardt, U.; Fritsche, L.; Heinen, S.; Hartmann, A.; et al. Defective Complement Control of Factor H (Y402H) and FHL-1 in Age-Related Macular Degeneration. Mol. Immunol. 2007, 44, 3398–3406. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A Common Haplotype in the Complement Regulatory Gene Factor H (HF1/CFH) Predisposes Individuals to Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef]
- Molins, B.; Fuentes-Prior, P.; Adán, A.; Antón, R.; Arostegui, J.I.; Yagüe, J.; Dick, A.D. Complement Factor H Binding of Monomeric C-Reactive Protein Downregulates Proinflammatory Activity and Is Impaired with at Risk Polymorphic CFH Variants. Sci. Rep. 2016, 6, 22889. [Google Scholar] [CrossRef]
- Clark, S.J.; Perveen, R.; Hakobyan, S.; Morgan, B.P.; Sim, R.B.; Bishop, P.N.; Day, A.J. Impaired Binding of the Age-Related Macular Degeneration-Associated Complement Factor H 402H Allotype to Bruch’s Membrane in Human Retina. J. Biol. Chem. 2010, 285, 30192–30202. [Google Scholar] [CrossRef]
- Armento, A.; Honisch, S.; Panagiotakopoulou, V.; Sonntag, I.; Jacob, A.; Bolz, S.; Kilger, E.; Deleidi, M.; Clark, S.; Ueffing, M. Loss of Complement Factor H Impairs Antioxidant Capacity and Energy Metabolism of Human RPE Cells. Sci. Rep. 2020, 10, 10320. [Google Scholar] [CrossRef] [PubMed]
- Borras, C.; Canonica, J.; Jorieux, S.; Abache, T.; El Sanharawi, M.; Klein, C.; Delaunay, K.; Jonet, L.; Salvodelli, M.; Naud, M.-C.; et al. CFH Exerts Anti-Oxidant Effects on Retinal Pigment Epithelial Cells Independently from Protecting against Membrane Attack Complex. Sci. Rep. 2019, 9, 13873. [Google Scholar] [CrossRef]
- Landowski, M.; Kelly, U.; Klingeborn, M.; Groelle, M.; Ding, J.-D.; Grigsby, D.; Bowes Rickman, C. Human Complement Factor H Y402H Polymorphism Causes an Age-Related Macular Degeneration Phenotype and Lipoprotein Dysregulation in Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 3703–3711. [Google Scholar] [CrossRef]
- Calippe, B.; Augustin, S.; Beguier, F.; Charles-Messance, H.; Poupel, L.; Conart, J.-B.; Hu, S.J.; Lavalette, S.; Fauvet, A.; Rayes, J.; et al. Complement Factor H Inhibits CD47-Mediated Resolution of Inflammation. Immunity 2017, 46, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Bowes Rickman, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A Systems Biology Approach towards Understanding and Treating Non-Neovascular Age-Related Macular Degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A. Soft Drusen in Age-Related Macular Degeneration: Biology and Targeting Via the Oil Spill Strategies. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD160–AMD181. [Google Scholar] [CrossRef]
- Malek, G.; Li, C.-M.; Guidry, C.; Medeiros, N.E.; Curcio, C.A. Apolipoprotein B in Cholesterol-Containing Drusen and Basal Deposits of Human Eyes with Age-Related Maculopathy. Am. J. Pathol. 2003, 162, 413–425. [Google Scholar] [CrossRef]
- Fujihara, M.; Cano, M.; Handa, J.T. Mice That Produce ApoB100 Lipoproteins in the RPE Do Not Develop Drusen yet Are Still a Valuable Experimental System. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7285–7295. [Google Scholar] [CrossRef]
- Curcio, C.A.; Johnson, M.; Rudolf, M.; Huang, J.-D. The Oil Spill in Ageing Bruch Membrane. Br. J. Ophthalmol. 2011, 95, 1638–1645. [Google Scholar] [CrossRef]
- Klaver, C.C.; Kliffen, M.; van Duijn, C.M.; Hofman, A.; Cruts, M.; Grobbee, D.E.; van Broeckhoven, C.; de Jong, P.T. Genetic Association of Apolipoprotein E with Age-Related Macular Degeneration. Am. J. Hum. Genet. 1998, 63, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Reffatto, V.; Bundy, J.G.; Kortvely, E.; Flinn, J.M.; Lanzirotti, A.; Jones, E.A.; McPhail, D.S.; Fearn, S.; Boldt, K.; et al. Identification of Hydroxyapatite Spherules Provides New Insight into Subretinal Pigment Epithelial Deposit Formation in the Aging Eye. Proc. Natl. Acad. Sci. USA 2015, 112, 1565–1570. [Google Scholar] [CrossRef]
- Pilgrim, M.G.; Lengyel, I.; Lanzirotti, A.; Newville, M.; Fearn, S.; Emri, E.; Knowles, J.C.; Messinger, J.D.; Read, R.W.; Guidry, C.; et al. Subretinal Pigment Epithelial Deposition of Drusen Components Including Hydroxyapatite in a Primary Cell Culture Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 708–719. [Google Scholar] [CrossRef]
- Cano, M.; Fijalkowski, N.; Kondo, N.; Dike, S.; Handa, J. Advanced Glycation Endproduct Changes to Bruch’s Membrane Promotes Lipoprotein Retention by Lipoprotein Lipase. Am. J. Pathol. 2011, 179, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tian, J.; Yang, Y.; Cutler, R.G.; Wu, T.; Telljohann, R.S.; Mattson, M.P.; Handa, J.T. Oxidized Low Density Lipoproteins Induce a Pathologic Response by Retinal Pigmented Epithelial Cells. J. Neurochem. 2008, 105, 1187–1197. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Rudolf, M.; Curcio, C.A.; Schlötzer-Schrehardt, U.; Sefat, A.M.M.; Tura, A.; Aherrahrou, Z.; Brinkmann, M.; Grisanti, S.; Miura, Y.; Ranjbar, M. Apolipoprotein A-I Mimetic Peptide L-4F Removes Bruch’s Membrane Lipids in Aged Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2019, 60, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, P.; Li, T.; Hatef, E. Statins for Age-related Macular Degeneration. Cochrane Database Syst. Rev. 2016, 2016, CD006927. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen Complement Components C3a and C5a Promote Choroidal Neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Yeo, N.J.Y.; Wazny, V.; Nguyen, N.L.U.; Ng, C.-Y.; Wu, K.X.; Fan, Q.; Cheung, C.M.G.; Cheung, C. Single-Cell Transcriptome of Wet AMD Patient-Derived Endothelial Cells in Angiogenic Sprouting. Int. J. Mol. Sci. 2022, 23, 12549. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Ju, Y.; Gu, P. Metabolic Regulation of Endothelial Cells: A New Era for Treating Wet Age-Related Macular Degeneration. Int. J. Mol. Sci. 2024, 25, 5926. [Google Scholar] [CrossRef]
- Fields, M.A.; Del Priore, L.V.; Adelman, R.A.; Rizzolo, L.J. Interactions of the Choroid, Bruch’s Membrane, Retinal Pigment Epithelium, and Neurosensory Retina Collaborate to Form the Outer Blood-Retinal-Barrier. Prog. Retin. Eye Res. 2020, 76, 100803. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.-W.; Zhang, D.-D.; Fan, Z.-G. Blood-Retinal Barrier as a Converging Pivot in Understanding the Initiation and Development of Retinal Diseases. Chin. Med. J. 2020, 133, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune Regulation in the Aging Retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative Stress Affects the Junctional Integrity of Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef]
- Miura, Y.; Roider, J. Triamcinolone Acetonide Prevents Oxidative Stress-Induced Tight Junction Disruption of Retinal Pigment Epithelial Cells. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 641–649. [Google Scholar] [CrossRef]
- Ji Cho, M.; Yoon, S.-J.; Kim, W.; Park, J.; Lee, J.; Park, J.-G.; Cho, Y.-L.; Hun Kim, J.; Jang, H.; Park, Y.-J.; et al. Oxidative Stress-Mediated TXNIP Loss Causes RPE Dysfunction. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Shirasawa, M.; Sonoda, S.; Terasaki, H.; Arimura, N.; Otsuka, H.; Yamashita, T.; Uchino, E.; Hisatomi, T.; Ishibashi, T.; Sakamoto, T. TNF-α Disrupts Morphologic and Functional Barrier Properties of Polarized Retinal Pigment Epithelium. Exp. Eye Res. 2013, 110, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.R.; Mahaling, B.; Tan, A.; Mehta, M.; Kaur, C.; Hunziker, W.; Kim, J.E.; Barathi, V.A.; Ghosh, A.; Chaurasia, S.S. ITF2357 Regulates NF-κB Signaling Pathway to Protect Barrier Integrity in Retinal Pigment Epithelial Cells. FASEB J. 2024, 38, e23512. [Google Scholar] [CrossRef]
- van der Wijk, A.-E.; Vogels, I.M.C.; van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. TNFα-Induced Disruption of the Blood-Retinal Barrier In Vitro Is Regulated by Intracellular 3′,5′-Cyclic Adenosine Monophosphate Levels. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3496–3505. [Google Scholar] [CrossRef]
- Romero-Vázquez, S.; Adán, A.; Figueras-Roca, M.; Llorenç, V.; Slevin, M.; Vilahur, G.; Badimon, L.; Dick, A.D.; Molins, B. Activation of C-Reactive Protein Proinflammatory Phenotype in the Blood Retinal Barrier in Vitro: Implications for Age-Related Macular Degeneration. Aging 2020, 12, 13905–13923. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.-J.; Kim, K.-W.; Yu, Y.S.; Kim, J.H. Oxidized Low Density Lipoprotein-Induced Senescence of Retinal Pigment Epithelial Cells Is Followed by Outer Blood-Retinal Barrier Dysfunction. Int. J. Biochem. Cell Biol. 2012, 44, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal Degeneration and Alzheimer’s Disease: An Evolving Link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, J.H.; Mook-Jung, I.; Kim, K.-W.; Park, W.J.; Park, K.H.; Kim, J.H. Intracellular Amyloid Beta Alters the Tight Junction of Retinal Pigment Epithelium in 5XFAD Mice. Neurobiol. Aging 2014, 35, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Bruban, J.; Glotin, A.-L.; Dinet, V.; Chalour, N.; Sennlaub, F.; Jonet, L.; An, N.; Faussat, A.M.; Mascarelli, F. Amyloid-Beta(1-42) Alters Structure and Function of Retinal Pigmented Epithelial Cells. Aging Cell 2009, 8, 162–177. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Arya, M.; Sabrosa, A.S.; Duker, J.S.; Waheed, N.K. Choriocapillaris Changes in Dry Age-Related Macular Degeneration and Geographic Atrophy: A Review. Eye Vis. 2018, 5, 22. [Google Scholar] [CrossRef]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and Choriocapillaris in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef]
- Grebe, R.; Mughal, I.; Bryden, W.; McLeod, S.; Edwards, M.; Hageman, G.S.; Lutty, G. Ultrastructural Analysis of Submacular Choriocapillaris and Its Transport Systems in AMD and Aged Control Eyes. Exp. Eye Res. 2019, 181, 252–262. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.E.; Choi, D.-K.; Jang, J.Y.; Jung, J.-J.; Kiyonari, H.; Shioi, G.; Chang, W.; Suda, T.; Mochizuki, N.; et al. Angiopoietin-1 Guides Directional Angiogenesis through Integrin Avβ5 Signaling for Recovery of Ischemic Retinopathy. Sci. Transl. Med. 2013, 5, 203ra127. [Google Scholar] [CrossRef]
- Hughes, S.; Gardiner, T.; Hu, P.; Baxter, L.; Rosinova, E.; Chan-Ling, T. Altered Pericyte–Endothelial Relations in the Rat Retina during Aging: Implications for Vessel Stability. Neurobiol. Aging 2006, 27, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Trivizki, O.; Gregori, G.; Wang, R.K. An Update on the Hemodynamic Model of Age-Related Macular Degeneration. Am. J. Ophthalmol. 2022, 235, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Dridi, S.; Tarallo, V.; Gelfand, B.D.; Fowler, B.J.; Cho, W.G.; Kleinman, M.E.; Ponicsan, S.L.; Hauswirth, W.W.; Chiodo, V.A.; et al. DICER1 Deficit Induces Alu RNA Toxicity in Age-Related Macular Degeneration. Nature 2011, 471, 325–330. [Google Scholar] [CrossRef]
- Seddon, J.M.; McLeod, D.S.; Bhutto, I.A.; Villalonga, M.B.; Silver, R.E.; Wenick, A.S.; Edwards, M.M.; Lutty, G.A. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Pouw, A.E.; Greiner, M.A.; Coussa, R.G.; Jiao, C.; Han, I.C.; Skeie, J.M.; Fingert, J.H.; Mullins, R.F.; Sohn, E.H. Cell–Matrix Interactions in the Eye: From Cornea to Choroid. Cells 2021, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hussain, A.A.; Limb, G.A.; Marshall, J. Age-Dependent Variation in Metalloproteinase Activity of Isolated Human Bruch’s Membrane and Choroid. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2676–2682. [Google Scholar]
- Kamei, M.; Hollyfield, J.G. TIMP-3 in Bruch’s Membrane: Changes during Aging and in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2367–2375. [Google Scholar] [PubMed]
- Wubben, T.J.; Weh, E.; Besirli, C.G. Photoreceptor Degeneration: More Than a Bystander in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2025, 1468, 27–31. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; Wang, J.; Mao, P.; Lv, X.; Yuan, S.; Huang, Z.; Ding, Y.; Xie, P.; Liu, Q. Knockout of Ccr2 Alleviates Photoreceptor Cell Death in Rodent Retina Exposed to Chronic Blue Light. Cell Death Dis. 2016, 7, e2468. [Google Scholar] [CrossRef]
- Guo, C.; Otani, A.; Oishi, A.; Kojima, H.; Makiyama, Y.; Nakagawa, S.; Yoshimura, N. Knockout of Ccr2 Alleviates Photoreceptor Cell Death in a Model of Retinitis Pigmentosa. Exp. Eye Res. 2012, 104, 39–47. [Google Scholar] [CrossRef]
- Sennlaub, F.; Auvynet, C.; Calippe, B.; Lavalette, S.; Poupel, L.; Hu, S.J.; Dominguez, E.; Camelo, S.; Levy, O.; Guyon, E.; et al. CCR2(+) Monocytes Infiltrate Atrophic Lesions in Age-Related Macular Disease and Mediate Photoreceptor Degeneration in Experimental Subretinal Inflammation in Cx3cr1 Deficient Mice. EMBO Mol. Med. 2013, 5, 1775–1793. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tsujikawa, M.; Itabe, H.; Du, Z.-J.; Xie, P.; Matsumura, N.; Fu, X.; Zhang, R.; Sonoda, K.; Egashira, K.; et al. Chronic Photo-Oxidative Stress and Subsequent MCP-1 Activation as Causative Factors for Age-Related Macular Degeneration. J. Cell Sci. 2012, 125, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Gallo, N.B.; Hancox, L.S.; Miller, N.J.; Radeke, C.M.; Maloney, M.A.; Cooper, J.B.; Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; et al. Systems-Level Analysis of Age-Related Macular Degeneration Reveals Global Biomarkers and Phenotype-Specific Functional Networks. Genome Med. 2012, 4, 16. [Google Scholar] [CrossRef]
- Caicedo, A.; Espinosa-Heidmann, D.G.; Piña, Y.; Hernandez, E.P.; Cousins, S.W. Blood-Derived Macrophages Infiltrate the Retina and Activate Muller Glial Cells under Experimental Choroidal Neovascularization. Exp. Eye Res. 2005, 81, 38–47. [Google Scholar] [CrossRef]
- Rutar, M.; Natoli, R.; Provis, J.M. Small Interfering RNA-Mediated Suppression of Ccl2 in Müller Cells Attenuates Microglial Recruitment and Photoreceptor Death Following Retinal Degeneration. J. Neuroinflam. 2012, 9, 221. [Google Scholar] [CrossRef]
- Yadav, U.C.S.; Ramana, K.V. Regulation of NF-κB-Induced Inflammatory Signaling by Lipid Peroxidation-Derived Aldehydes. Oxid. Med. Cell Longev. 2013, 2013, 690545. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Ke, Q.; Nie, Q.; Qi, R.; Zhu, X.; Liu, W.; Hu, X.; Sun, Q.; Fu, J.-L.; Tang, X.; et al. Inhibition of cGAS-STING by JQ1 Alleviates Oxidative Stress-Induced Retina Inflammation and Degeneration. Cell Death Differ. 2022, 29, 1816–1833. [Google Scholar] [CrossRef]
- Gehrke, N.; Mertens, C.; Zillinger, T.; Wenzel, J.; Bald, T.; Zahn, S.; Tüting, T.; Hartmann, G.; Barchet, W. Oxidative Damage of DNA Confers Resistance to Cytosolic Nuclease TREX1 Degradation and Potentiates STING-Dependent Immune Sensing. Immunity 2013, 39, 482–495. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and Function of the cGAS-STING Pathway of Cytosolic DNA Sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Lin, H.; Godley, B.F.; Boulton, M.E. Mitochondrial DNA Damage and Its Potential Role in Retinal Degeneration. Prog. Retin. Eye Res. 2008, 27, 596–607. [Google Scholar] [CrossRef]
- Zhao, C.; Yasumura, D.; Li, X.; Matthes, M.; Lloyd, M.; Nielsen, G.; Ahern, K.; Snyder, M.; Bok, D.; Dunaief, J.L.; et al. mTOR-Mediated Dedifferentiation of the Retinal Pigment Epithelium Initiates Photoreceptor Degeneration in Mice. J. Clin. Invest. 2011, 121, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Swarup, A.; Samuels, I.S.; Bell, B.A.; Han, J.Y.S.; Du, J.; Massenzio, E.; Abel, E.D.; Boesze-Battaglia, K.; Peachey, N.S.; Philp, N.J. Modulating GLUT1 Expression in Retinal Pigment Epithelium Decreases Glucose Levels in the Retina: Impact on Photoreceptors and Müller Glial Cells. Am. J. Physiol. Cell Physiol. 2019, 316, C121–C133. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; Ma, S.; Le, Y.Z.; Hall, M.N.; Rüegg, M.A.; Punzo, C. Activated mTORC1 Promotes Long-Term Cone Survival in Retinitis Pigmentosa Mice. J. Clin. Invest. 2015, 125, 1446–1458. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a Metabolic Gene Regulatory Network Downstream of mTOR Complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Hurley, J.B. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci. 2021, 7, 665–692. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Shi, F.; Xie, H.; Yang, Q.; Liu, D.; Qu, S.; Qin, H.; Zhang, C.; Xu, G.-T.; et al. Activated Microglia-Induced Neuroinflammatory Cytokines Lead to Photoreceptor Apoptosis in Aβ-Injected Mice. J. Mol. Med. 2021, 99, 713–728. [Google Scholar] [CrossRef]
- Peng, J.-J.; Song, W.-T.; Yao, F.; Zhang, X.; Peng, J.; Luo, X.-J.; Xia, X.-B. Involvement of Regulated Necrosis in Blinding Diseases: Focus on Necroptosis and Ferroptosis. Exp. Eye Res. 2020, 191, 107922. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, K.; He, D.; Yang, B.; Tao, L.; Chen, J.; Wu, Y. Induction of Ferroptosis by HO-1 Contributes to Retinal Degeneration in Mice with Defective Clearance of All-Trans-Retinal. Free Radic. Biol. Med. 2023, 194, 245–254. [Google Scholar] [CrossRef]
- Lin, J.B.; Murakami, Y.; Miller, J.W.; Vavvas, D.G. Neuroprotection for Age-Related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100192. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; von Felbert, V.; Middleton, M.; Kato, M.; Ergün, S.; Augustin, H.G.; Schadendorf, D. Resistance to Antiangiogenic Therapy Is Directed by Vascular Phenotype, Vessel Stabilization, and Maturation in Malignant Melanoma. J. Exp. Med. 2010, 207, 491–503. [Google Scholar] [CrossRef]
- Catchpole, T.; Nguyen, T.D.; Gilfoyle, A.; Csaky, K.G. A Profile of Circulating Vascular Progenitor Cells in Human Neovascular Age-Related Macular Degeneration. PLoS ONE 2020, 15, e0229504. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Wu, Y.; Wang, B.; Xi, Y.; Hu, K. Bioinformatics and Network Pharmacology Explore the Role of Immune Cells in the Occurrence of Anti-Vascular Endothelial Growth Factor (VEGF) Resistance in Patients with Neovascular Age-Related Macular Degeneration(nAMD) and the Application of Complementary Medicine Treatment. Ocul. Immunol. Inflamm. 2024, 32, 1945–1960. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, M.; Mi, L.; Zhang, X.; Pu, J.; He, G.; Zhang, L.; Yu, H.; Yao, L.; Chen, H.; et al. Molecular Responses of Anti-VEGF Therapy in Neovascular Age-Related Macular Degeneration: Integrative Insights From Multi-Omics and Clinical Imaging. Investig. Ophthalmol. Vis. Sci. 2024, 65, 24. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.A.; Hanout, M.; Sarwar, S.; Hassan, M.; Do, D.V.; Nguyen, Q.D.; Sepah, Y.J. Platelet Derived Growth Factor Inhibitors: A Potential Therapeutic Approach for Ocular Neovascularization. Saudi J. Ophthalmol. 2015, 29, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Van Bergen, T.; Canning, P.; Lengyel, I.; Feyen, J.H.M.; Stitt, A.W. The Placental Growth Factor Pathway and Its Potential Role in Macular Degenerative Disease. Curr. Eye Res. 2019, 44, 813–822. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, C.; Du, Y.; Lin, X.; Jiang, Y.; Lee, C.; Tian, G.; Mi, J.; Li, X.; Chen, Q.; et al. PDGF-CC Underlies Resistance to VEGF-A Inhibition and Combinatorial Targeting of Both Suppresses Pathological Angiogenesis More Efficiently. Oncotarget 2016, 7, 77902–77915. [Google Scholar] [CrossRef][Green Version]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10, 1049. [Google Scholar] [CrossRef]
- Ammar, M.J.; Hsu, J.; Chiang, A.; Ho, A.C.; Regillo, C.D. Age-Related Macular Degeneration Therapy: A Review. Curr. Opin. Ophthalmol. 2020, 31, 215. [Google Scholar] [CrossRef]
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.-F.; Slakter, J.S.; et al. Pegcetacoplan for the Treatment of Geographic Atrophy Secondary to Age-Related Macular Degeneration (OAKS and DERBY): Two Multicentre, Randomised, Double-Masked, Sham-Controlled, Phase 3 Trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef]
- Witkin, A.J.; Jaffe, G.J.; Srivastava, S.K.; Davis, J.L.; Kim, J.E. Retinal Vasculitis After Intravitreal Pegcetacoplan: Report From the ASRS Research and Safety in Therapeutics (ReST) Committee. J. Vitreoretin. Dis. 2024, 8, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Khanani, I.; Aziz, A.A.; Khanani, Z.A.; Khan, H.; Mojumder, O.; Sulahria, H.; Ali, H.; Khan, H.; Rahimzadeh, T.S.; Vannavong, J.; et al. The Safety of Recently Approved Therapeutics in Age-Related Macular Degeneration. Int. Ophthalmol. Clin. 2025, 65, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mantel, I.; Lasagni Vitar, R.M.; De Zanet, S. Modeling Pegcetacoplan Treatment Effect for Atrophic Age-Related Macular Degeneration with AI-Based Progression Prediction. Int. J. Retin. Vitr. 2025, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B.; Heier, J.S.; Chaudhary, V.; Wykoff, C.C. Treatment of Geographic Atrophy: An Update on Data Related to Pegcetacoplan. Curr. Opin. Ophthalmol. 2024, 35, 64–72. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Mai, J.; Reiter, G.S.; Riedl, S.; Vogl, W.-D.; Sadeghipour, A.; McKeown, A.; Foos, E.; Scheibler, L.; Bogunovic, H. Disease Activity and Therapeutic Response to Pegcetacoplan for Geographic Atrophy Identified by Deep Learning-Based Analysis of OCT. Ophthalmology 2025, 132, 181–193. [Google Scholar] [CrossRef]
- Kang, C. Avacincaptad Pegol: First Approval. Drugs 2023, 83, 1447–1453. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Arshad Khanani, MD: Two-Year Results from GATHER2 Trial. Available online: https://www.hcplive.com/view/arshad-khanani-md-two-year-results-gather2-trial (accessed on 28 February 2025).
- Mondal, A.K.; Gaur, M.; Advani, J.; Swaroop, A. Epigenome–Metabolism Nexus in the Retina: Implications for Aging and Disease. Trends Genet. 2024, 40, 718–729. [Google Scholar] [CrossRef]
- Advani, J.; Mehta, P.A.; Hamel, A.R.; Mehrotra, S.; Kiel, C.; Strunz, T.; Corso-Díaz, X.; Kwicklis, M.; van Asten, F.; Ratnapriya, R.; et al. QTL Mapping of Human Retina DNA Methylation Identifies 87 Gene-Epigenome Interactions in Age-Related Macular Degeneration. Nat. Commun. 2024, 15, 1972. [Google Scholar] [CrossRef]
- Porter, L.F.; Saptarshi, N.; Fang, Y.; Rathi, S.; den Hollander, A.I.; de Jong, E.K.; Clark, S.J.; Bishop, P.N.; Olsen, T.W.; Liloglou, T.; et al. Whole-Genome Methylation Profiling of the Retinal Pigment Epithelium of Individuals with Age-Related Macular Degeneration Reveals Differential Methylation of the SKI, GTF2H4, and TNXB Genes. Clin. Epigenetics 2019, 11, 6. [Google Scholar] [CrossRef]
- Lains, I.; Zhu, S.; Han, X.; Chung, W.; Yuan, Q.; Kelly, R.S.; Gil, J.Q.; Katz, R.; Nigalye, A.; Kim, I.K.; et al. Genomic-Metabolomic Associations Support the Role of LIPC and Glycerophospholipids in Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Suuronen, T.; Nuutinen, T.; Ryhänen, T.; Kaarniranta, K.; Salminen, A. Epigenetic Regulation of Clusterin/Apolipoprotein J Expression in Retinal Pigment Epithelial Cells. Biochem. Biophys. Res. Commun. 2007, 357, 397–401. [Google Scholar] [CrossRef]
- Chan, N.; He, S.; Spee, C.K.; Ishikawa, K.; Hinton, D.R. Attenuation of Choroidal Neovascularization by Histone Deacetylase Inhibitor. PLoS ONE 2015, 10, e0120587. [Google Scholar] [CrossRef]
- Gemenetzi, M.; Lotery, A.J. Epigenetics in Age-Related Macular Degeneration: New Discoveries and Future Perspectives. Cell Mol. Life Sci. 2020, 77, 807–818. [Google Scholar] [CrossRef]
- Tanito, M.; Masutani, H.; Kim, Y.-C.; Nishikawa, M.; Ohira, A.; Yodoi, J. Sulforaphane Induces Thioredoxin through the Antioxidant-Responsive Element and Attenuates Retinal Light Damage in Mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 979–987. [Google Scholar] [CrossRef]
- Fry, L.E.; Peddle, C.F.; Barnard, A.R.; McClements, M.E.; MacLaren, R.E. RNA Editing as a Therapeutic Approach for Retinal Gene Therapy Requiring Long Coding Sequences. Int. J. Mol. Sci. 2020, 21, 777. [Google Scholar] [CrossRef] [PubMed]
- Farkas, M.H.; DeAngelis, M.M. Age-Related Macular Degeneration: From Epigenetics to Therapeutic Implications. Adv. Exp. Med. Biol. 2021, 1256, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Pagliai, G.; Di Gloria, L.; Pallecchi, M.; Barca, F.; Pieri, B.; Bartolucci, G.; Ramazzotti, M.; Amedei, A.; Palendri, G.; et al. Beneficial Effects of Micronutrient Supplementation in Restoring the Altered Microbiota and Gut-Retina Axis in Patients with Neovascular Age-Related Macular Degeneration-A Randomized Clinical Trial. Nutrients 2024, 16, 3971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Xiao, J.; Xie, B.; Barba, H.; Boachie-Mensah, M.; Shah, R.N.; Nadeem, U.; Spedale, M.; Dylla, N.; Lin, H.; et al. Oral Metformin Inhibits Choroidal Neovascularization by Modulating the Gut-Retina Axis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 21. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, M.; Xu, Y.; Huang, S.; Liang, J.; Cao, Y.; Chen, H. Sodium Butyrate Inhibits Neovascularization Partially via TNXIP/VEGFR2 Pathway. Oxid. Med. Cell Longev. 2020, 2020, 6415671. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.; Kim, D.; Jin, M.; Ko, H.J.; Song, Y.H.; Lee, Y.; Ahn, B.-N.; Kim, S.-K.; Lee, Y.; Shin, M.C.; et al. Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 561. [Google Scholar] [CrossRef]
- Kammoun, S.; Rekik, M.; Dlensi, A.; Aloulou, S.; Smaoui, W.; Sellami, S.; Trigui, K.; Gargouri, R.; Chaari, I.; Sellami, H.; et al. The Gut-Eye Axis: The Retinal/Ocular Degenerative Diseases and the Emergent Therapeutic Strategies. Front. Cell. Neurosci. 2024, 18, 1468187. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M. Cell-Based Therapy for Degenerative Retinal Disease. Trends Mol. Med. 2016, 22, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Rep. 2015, 4, 860–872. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.W.; Wang, L.; Li, S.Y.; Zhao, C.J.; Hao, J.; Li, Q.Y.; Zhao, T.T.; Wu, W.; Wang, Y.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium Transplants as a Potential Treatment for Wet Age-Related Macular Degeneration. Cell Discov. 2018, 4, 50. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A Bioengineered Retinal Pigment Epithelial Monolayer for Advanced, Dry Age-Related Macular Degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell–Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; Kashani, A.H.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; et al. Long-Term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy. Ophthalmology 2024, 131, 682–691. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Jayaram, H.; Jones, M.F.; Eastlake, K.; Cottrill, P.B.; Becker, S.; Wiseman, J.; Khaw, P.T.; Limb, G.A. Transplantation of Photoreceptors Derived from Human Muller Glia Restore Rod Function in the P23H Rat. Stem Cells Transl. Med. 2014, 3, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Izrael, M.; Chebath, J.; Molakandov, K.; Revel, M. Clinical Perspective on Pluripotent Stem Cells Derived Cell Therapies for the Treatment of Neurodegenerative Diseases. Adv. Drug Deliv. Rev. 2025, 218, 115525. [Google Scholar] [CrossRef]
- Shirai, H.; Mandai, M.; Matsushita, K.; Kuwahara, A.; Yonemura, S.; Nakano, T.; Assawachananont, J.; Kimura, T.; Saito, K.; Terasaki, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Tissue in Two Primate Models of Retinal Degeneration. Proc. Natl. Acad. Sci. USA 2016, 113, E81–E90. [Google Scholar] [CrossRef] [PubMed]
- Hirami, Y.; Mandai, M.; Sugita, S.; Maeda, A.; Maeda, T.; Yamamoto, M.; Uyama, H.; Yokota, S.; Fujihara, M.; Igeta, M.; et al. Safety and Stable Survival of Stem-Cell-Derived Retinal Organoid for 2 Years in Patients with Retinitis Pigmentosa. Cell Stem Cell 2023, 30, 1585–1596.e6. [Google Scholar] [CrossRef]
- Arthur, P.; Kandoi, S.; Sun, L.; Kalvala, A.; Kutlehria, S.; Bhattacharya, S.; Kulkarni, T.; Nimma, R.; Li, Y.; Lamba, D.A.; et al. Biophysical, Molecular and Proteomic Profiling of Human Retinal Organoid-Derived Exosomes. Pharm. Res. 2023, 40, 801–816. [Google Scholar] [CrossRef]
- Wright, C.B.; Becker, S.M.; Low, L.A.; Tagle, D.A.; Sieving, P.A. Improved Ocular Tissue Models and Eye-On-A-Chip Technologies Will Facilitate Ophthalmic Drug Development. J. Ocul. Pharmacol. Ther. 2020, 36, 25–29. [Google Scholar] [CrossRef]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging Organoid and Organ-on-a-Chip Technology to Generate Complex Multi-Layer Tissue Models in a Human Retina-on-a-Chip Platform. Elife 2019, 8, e46188. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M.; Pan, Z.; Kong, W.; Hong, Z.; Zhang, W.; Sun, B.; Zhang, J.; Wang, X.; Wang, K. A Novel Quantitative Angiogenesis Assay Based on Visualized Vascular Organoid. Angiogenesis 2025, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Kiruthika, M.; Malathi, G. A Comprehensive Review on Early Detection of Drusen Patterns in Age-Related Macular Degeneration Using Deep Learning Models. Photodiagnosis Photodyn. Ther. 2025, 51, 104454. [Google Scholar] [CrossRef]
- Schranz, M.; Bogunovic, H.; Deak, G.; Sadeghipour, A.; Reiter, G.S.; Schmidt-Erfurth, U. Linking Disease Activity with Optical Coherence Tomography Angiography in Neovascular Age Related Macular Degeneration Using Artificial Intelligence. Sci. Rep. 2024, 14, 19278. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.L.; Clemons, T.E.; Domalpally, A.; Elman, M.J.; Havilio, M.; Agrón, E.; Benyamini, G.; Chew, E.Y. Retinal Specialist versus Artificial Intelligence Detection of Retinal Fluid from OCT: Age-Related Eye Disease Study 2: 10-Year Follow-On Study. Ophthalmology 2021, 128, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, Y.; Fang, Y.; Lan, T.; Shi, W. Plasma Extracellular Vesicle-Associated Proteins as Promising Diagnostic Biomarkers of Age-Related Macular Degeneration. Discov. Med. 2024, 36, 2356–2364. [Google Scholar] [CrossRef]
- Moon, S.; Lee, Y.; Hwang, J.; Kim, C.G.; Kim, J.W.; Yoon, W.T.; Kim, J.H. Prediction of Anti-Vascular Endothelial Growth Factor Agent-Specific Treatment Outcomes in Neovascular Age-Related Macular Degeneration Using a Generative Adversarial Network. Sci. Rep. 2023, 13, 5639. [Google Scholar] [CrossRef]
- Yeh, T.-C.; Luo, A.-C.; Deng, Y.-S.; Lee, Y.-H.; Chen, S.-J.; Chang, P.-H.; Lin, C.-J.; Tai, M.-C.; Chou, Y.-B. Prediction of Treatment Outcome in Neovascular Age-Related Macular Degeneration Using a Novel Convolutional Neural Network. Sci. Rep. 2022, 12, 5871. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J. Identification of Ferroptosis-Related Gene in Age-Related Macular Degeneration Using Machine Learning. Immun. Inflamm. Dis. 2024, 12, e70059. [Google Scholar] [CrossRef]
- Luo, S.; Hu, Q.; Jiang, B.; Zhang, Z.; Sun, D. Bioinformatics Analysis for Constructing a Cellular Senescence-Related Age-Related Macular Degeneration Diagnostic Model and Identifying Relevant Disease Subtypes to Guide Treatment. Aging 2024, 16, 8044–8069. [Google Scholar] [CrossRef]
- Sendecki, A.; Ledwoń, D.; Nycz, J.; Wąsowska, A.; Boguszewska-Chachulska, A.; Mitas, A.W.; Wylęgała, E.; Teper, S. A Deep Learning Approach to Explore the Association of Age-Related Macular Degeneration Polygenic Risk Score with Retinal Optical Coherence Tomography: A Preliminary Study. Acta Ophthalmol. 2024, 102, e1029–e1039. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Leingang, O.; Bogunović, H.; Riedl, S.; Fritsche, L.; Prevost, T.; Scholl, H.P.N.; Schmidt-Erfurth, U.; Sivaprasad, S.; Lotery, A.J.; et al. Metadata-Enhanced Contrastive Learning from Retinal Optical Coherence Tomography Images. Med. Image Anal. 2024, 97, 103296. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Chen, J.; Su, S.; Cai, Y.; Yang, X.; Sang, A. Integrating Multi-Omics to Identify Age-Related Macular Degeneration Subtypes and Biomarkers. J. Mol. Neurosci. 2024, 74, 74. [Google Scholar] [CrossRef]
- Jackson, V.E.; Wu, Y.; Bonelli, R.; Owen, J.P.; Scott, L.W.; Farashi, S.; Kihara, Y.; Gantner, M.L.; Egan, C.; Williams, K.M.; et al. Multi-Omic Spatial Effects on High-Resolution AI-Derived Retinal Thickness. Nat. Commun. 2025, 16, 1317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).